Abstract
Background
Infection is common following stroke and is independently associated with worse outcome. Clinical studies suggest that infections occur more frequently in those individuals with stroke-induced immunologic dys-function. This study sought to explore the contribution of immunomodulatory cytokines and hormones to lymphocyte function and infection risk.
Methods
Patients (N = 112) were enrolled as soon as possible after the onset of ischemic stroke. Blood was drawn to assess plasma cortisol, IL-10, IL-1ra, lymphocyte numbers, and lymphocyte function at 72 h after stroke onset; infections were censored through 21 days after stroke onset.
Results
Infection occurred in 25% of patients. Stroke severity was the most important predictor of infection risk. Increased plasma cortisol, IL-10, and IL-1ra, as well as decreased lymphocyte numbers, at 72 h after stroke onset were associated with risk of subsequent infection. After controlling for stroke severity, only IL-1ra was independently associated with infection risk, and the degree of risk was consistent throughout the post-stroke period. Infection, but not IL-1ra itself, was associated with worse outcome at 3 months.
Conclusions
In this study cohort, increased plasma IL-1ra was independently associated with the risk of post-stroke infection. Further studies are needed to validate this finding, which could have important implications for stroke therapy.
Keywords: Stroke, IL-1ra, Infection, IL-10, Cortisol
Introduction
Infections, primarily pneumonias (PNAs) and urinary tract infections (UTIs), are common following stroke and are independently associated with worse outcome [1–3]. Stroke severity [as determined by the National Institutes of Health Stroke Scale (NIHSS) Score] appears to be the most important predictor of infection risk [1, 4, 5]. Recent experimental data, however, suggest that ischemic brain injury may lead to a “systemic immunodepression” that predisposes to infection [6]. In animal studies, this immunodepression is characterized by impaired lymphocyte function, which appears to be sympathetically mediated [6]. Clinical studies also demonstrate that increasing infarct volume is associated with greater immunologic dysfunction, including decreased lymphocyte numbers (especially CD4+ cells), decreased HLA-DR expression by monocytes, and decreased production of tumor necrosis factor (TNF)-α ex vivo [7–11]. Other studies, however, have found that surviving T cells function normally ex vivo [12]. Even if T cell function were inherently normal, circulating inhibitors of lymphocyte function might impact their function in vivo. For instance, increased plasma concentrations of plasma IL-10, an immunomodulatory cytokine that negatively impacts lymphocyte function, are associated with increased risk of infection [13, 14]. Another cytokine that has the potential to suppress the immune response and increase the risk of infection is IL-1 receptor antagonist (IL-1ra). Plasma concentrations of IL-1ra are known to be increased following acute stroke, but the possibility that endogenous production of IL-1ra after stroke may contribute to infection risk has not been explored [15–17]. In this study, we attempted to identify clinical risk factors for infection and immunomodulatory cytokines and hormones associated with the likelihood of developing infection in the post-stroke period.
Materials and Methods
Research Subjects
This study was part of a larger prospective study that followed immune responses over the course of the year after stroke onset; the study was approved by the Institutional Review Board and all patients or their surrogates provided informed consent. Patients with ischemic stroke admitted to either Harborview Medical Center or the University of Washington Medical Center from September 2005 to May 2009 who were at least 18 years of age, who could be enrolled within 72 h of symptom onset and were felt not likely to die from their stroke were eligible. Those patients with ongoing therapy for malignancy, known history of HIV, Hepatitis B or C, history of brain tumor, anemia (hematocrit <35 on admission), and those taking immunomodulatory drugs were excluded. Blood was drawn 1 day after stroke onset (when possible) and at 3, 7, 30, and 90 days after stroke onset and processed immediately after collection. Plasma and serum were frozen at −80° until use.
Clinical and Infection Data
demographic and clinical data were collected on all the patients. Stroke severity was determined by the NIHSS score and outcome by the modified Rankin Scale (mRS), the Glasgow Outcome Scale-Extended (GOSE), and the Stroke Impact Scale (SIS). In hospital infection was defined as clinical symptoms of an infection (fever and/or pyuria for UTI and fever and/or productive cough and radiographic evidence of consolidation for PNA) and positive gram stain or culture data (for both PNA and UTI). For patients discharged from the hospital before 21 days, historical information about infection was obtained at 30 day follow up. Total infarct volume on initial diffusion weighted MRI imaging was calculated by the ABC/2 method [18].
Tests of Lymphocyte Function
Mononuclear cells were isolated over a ficoll gradient and frozen in liquid nitrogen until use. ELISPOT assays were done to detect the secretion of interferon (IFN)-γ (R&D Systems). Cells were cultured in 96-well plates (Multi-Screen®-IP; Millipore) at a concentration of 1 × 106 per ml with media alone or with phytohemagglutinin (PHA; 2.5 μg/well) and incubated for 24 h. Experiments were performed in triplicate; spots were counted using a semi-automated system (MetaMorph®). Data are presented as the relative increase in the number of cells secreting IFN-γ to PHA over that seen with media alone.
Laboratory Studies
Lymphocyte counts were determined by the clinical hematology laboratory. Concentrations of circulating cytokines (IL-1ra and IL-10) were measured with a cytometric bead-based system (Fluorokine® MAP; R&D Systems). Values below the limit of detection (0.3 pg/ml for IL–10 and 11 pg/ml for IL-1ra) are noted as “not detected” (nd) or assigned the value of 0.3 or 11 pg/ml for IL-10 and IL-1ra, respectively. Plasma cortisol and adrenocorticotropic hormone (ACTH) concentrations were determined by the hospital laboratory using standard methodology.
Statistics
Descriptive data are presented as median and interquartile range (IQR) for continuous variables and percents for categorical variables. Group comparisons were performed using the Mann–Whitney U test or the χ2 test statistic as appropriate. Logistic regression was used to test the association between infection in the first 15 days and poor outcome at 3 months, both unadjusted and adjusted for age and stroke severity (as measured by the highest NIHSS in the first 72 h). Logistic regression was also used to estimate odds ratio (OR) and 95% confidence interval (CI) for clinical and laboratory variables measured in the first 72 h that predict the probability of infection within the first 15 days. Results are presented both unadjusted and adjusted stroke severity. Significance was set at P < 0.05. No formal adjustment is made to P values to account for the number of variables tested; results should, therefore, be interpreted cautiously in light of the multiple comparisons performed.
Results
We enrolled a total of 114 patients with acute ischemic stroke from September 2005 to May 2009. One patient had an ongoing infection (cellulitis) at the time of stroke onset and another died within the first week of the study; these two patients are excluded from further analyses. The median age of the remaining 112 patients was 57 (44–67) years, the median NIHSS score was 11 (4–19), and 65% were male. Of the 112 enrolled patients, the etiology of stroke was cardioembolic/atrial fibrillation in 30 (27%), atherosclerotic/artery-artery emboli in 16 (14%), lacunar in 11 (10%), and unknown in 25 (22%); a variety of etiologies accounted for the remaining strokes. Figure 1 depicts the cumulative numbers and types of infection over the first 21 days of the study. The majority of PNAs occurred early (by day 5) while there was a later spike in the number of UTIs; after 15 days, there were no further infections in the censure period. Escherichia coli was the most common pathogenic organism and accounted for 39% of the UTIs (72% of all UTIs were due to Gram-negative organisms). Gram-negative organisms (Klebsiella, Proteus, Morganella, Pseudomonas, and Haemophilus) also accounted for 38% of the PNAs. Given the inclusion and exclusion criteria for this study, as well as the need for consent, the study population differs from that of the total stroke population admitted to the hospital. Review of our stroke database shows that over the same period of time as the study was conducted, a total of 841 patients with the primary diagnosis of ischemic stroke were admitted to the hospital; the median age was 61 years, 59% were male, and the median NIHSS score was 7, suggesting that the patients who consented for participation in the study were slightly younger with more severe strokes. The prevalence of infection among these 841 patients was 13% for UTI and 14% for PNA; the diagnosis of infection in these individuals, however, was ascertained by discharge diagnoses and not rigorous surveillance, as in our study population. In the study cohort, an infection in the first 15 days after stroke was associated with an increased likelihood of poor outcome, as defined by mRS > 3 at 3 months; OR = 19.00 (5.01, 72.00); P < 0.001. After controlling for age and stroke severity, infection was still associated with an increased risk of poor outcome at 3 months, defined either as mRS > 3 [OR = 6.12 (1.30, 28.88); P = 0.022] or by GOSE ≤ 3 [OR = 5.42 (1.16, 25.21); P = 0.031].
Fig. 1.
Cumulative infections over the study period
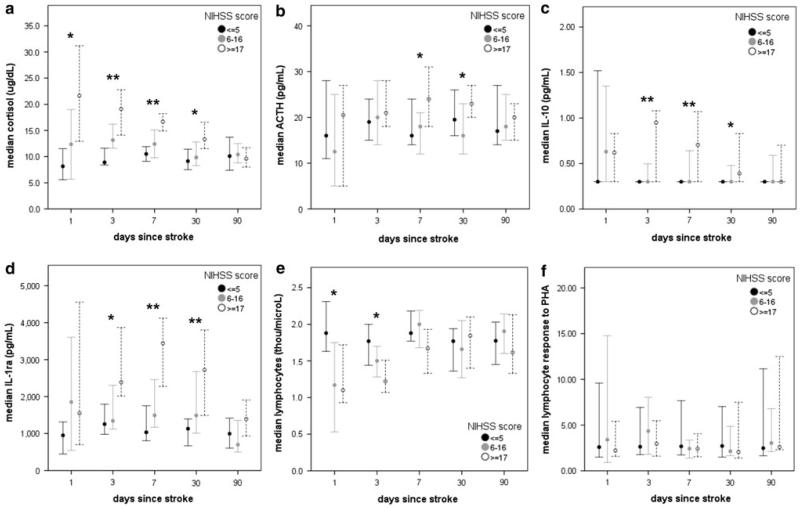
For the purposes of this study, patients were divided into tertiles based on stroke severity. As might be expected, patients with more severe strokes had higher plasma concentrations of cortisol, IL-10, IL-1ra, and lower lymphocyte numbers. The decrease in lymphocyte numbers was short lived, but the differences in the cytokines and hormones that could affect lymphocyte function persisted to 30 days after stroke onset (Fig. 2). None of these variables, when measured at 72 h after stroke onset, was independently associated with stroke outcome.
Fig. 2.
Changes in plasma concentrations of cortisol (a), ACTH (b), IL-10 (c), and IL-1ra (d) over the course of time from stroke onset. Patients with more severe strokes (NIHSS score ≥ 17) had higher concentrations of these immunomodulatory hormones and cytokines that persisted for weeks after stroke onset. There was also an early decrease in lymphocyte numbers (e) among patients with severe stroke, but no difference in the response to PHA (f). Statistics are by Kruskal–Wallis H test; *P < 0.05, **P < 0.01
Table 1 depicts the baseline characteristics among those patients who developed an infection in the first 15 days after stroke onset and those that remained infection free. Patients who developed an infection had larger infarcts and more severe strokes. These individuals were more likely to have decreased level of consciousness, facial weakness, and dysarthria, and as might be expected, were more likely to have been intubated and have a feeding tube placed. Further, there were numerous differences in the laboratory studies done at 72 h among those patients who became infected and those that remained infection free. Of note, however, is that despite the early decrease in lymphocyte numbers among those who developed infection, lymphocyte responses to PHA at 72 h were similar among infected and non-infected patients. The relative contribution of these clinical and laboratory variables to the risk of developing an infection is presented in Table 2. Of these variables, stroke severity is the most potent predictor of infection; after controlling for the highest NIHSS score in the first 72 h, only the plasma concentration of IL-1ra remains predictive of infection risk. In Fig. 1, however, it can be seen that a few individuals already had evidence of infection by day 3, the time the blood was drawn for these laboratory studies. These analyses were thus repeated and patients with infection from day 1 to 8 were sequentially excluded to determine if either overt or preclinical infection might bias the associations of these variables with infection risk (Table 3); the effect of IL-1ra remained consistent over time.
Table 1.
Baseline characteristics of the study population (N = 112 with 28 infections)
| Clinical variables | Infected by day 15 N = 28 |
Infection free at day 15 N = 84 |
P |
|---|---|---|---|
| NIHSSa | 22 (16–27) | 8 (3–16) | <0.001 |
| LOC (item 1a on NIHSS) | 1 (0–2) | 0 (0–1) | 0.005 |
| Facial weakness (item 4 on NIHSS) | 0 (0–0) | 0 (0–0) | 0.001 |
| Dysarthria (item 10 in NIHSS) | 1 (0–2) | 0 (0–1) | 0.002 |
| Age | 58 (47–70) | 55 (43–66) | NS |
| Gender (female) | 10/28 | 29/84 | NS |
| Infarct volume (ml) | 92 (17–339) | 9 (1–47) | <0.001 |
| IV tPA (%) | 10/28 (36) | 18/84 (21) | 0.131 |
| Endovascular therapy (%) | 5/28 (18) | 10/84 (12) | NS |
| Hemicraniectomy (%) | 6/28 (21) | 3/84 (4) | 0.003 |
| Intubated at 72 h (%) | 13/28 (45) | 9/84 (11) | <0.001 |
| Tube feeding at 72 h (%) | 10/28 (36) | 7/84 (8) | <0.001 |
| Urinary catheter at 72 h (%) | 11/28 (39) | 35/84 (42) | NS |
| Cortisol (μg/dl)a | 19 (14–26) | 12 (9–16) | <0.001 |
| ACTH (pg/ml) | 23 (18–34) | 19 (13–31) | 0.115 |
| IL-10 (pg/ml)a | 0.49 (nd–1.21) | nd (nd–0.67) | 0.007 |
| IL-1raa (pg/ml) | 2613 (1700–5404) | 1376 (958–2432) | 0.001 |
| Lymphocytes (thou/ml)b | 1.2 (1.0–1.6) | 1.5 (1.2–1.9) | 0.015 |
| PHA responsea | 3.3 (1.4–10.3) | 2.6 (1.5–6.8) | NS |
All significant values are bolded
NIHSS National Institutes of Health Stroke Scale; LOC level of consciousness; IV Tpa intravenous tissue plasminogen activator; ACTH adrenocorticotrophic hormone; IL interleukin; IL-1ra IL-1 receptor antagonist; PHA phytohemagglutinin
Highest value in the first 72 h;
Lowest value in the first 72 h;
*Patients who were on the drug before admission or started on the drug by 72 h after admission
Table 2.
Clinical and laboratory predictors of infection to 15 day after stroke onset (N = 112 with 28 infections)
| Clinical variables | Number of infections | Unadjusted |
Adjusted for NIHSS |
||
|---|---|---|---|---|---|
| OR (CI) | P | OR (CI) | P | ||
| NIHSSa (per point) | 28 | 1.16 (1.09, 1.23) | <0.001 | – | – |
| LOC (per point on item 1a of the NIHSS) | 28 | 4.27 (2.25, 8.09) | <0.001 | 1.91 (0.83, 4.37) | NS |
| Facial weakness (per point on item 4 of the NIHSS) | 28 | 2.54 (1.38, 4.68) | 0.003 | 0.96 (0.45, 2.06) | NS |
| Dysarthria (per point on item 10 of the NIHSS) | 28 | 2.74 (1.24, 6.05) | 0.012 | 0.88 (0.32, 2.46) | NS |
| Age (per year) | 28 | 1.02 (0.99, 1.05) | NS | 1.01 (0.97, 1.05) | NS |
| Gender (female) | 28 | 1.05 (0.43, 2.58) | NS | 1.23 (0.44, 3.48) | NS |
| Infarct volume (per 10 ml) | 28 | 1.08 (1.04, 1.13) | <0.001 | 1.01 (0.96, 1.06) | NS |
| IV tPA | 28 | 2.04 (0.80, 5.18) | NS | 1.48 (0.50, 4.34) | NS |
| Endovascular therapy | 28 | 1.81 (0.55, 5.95) | NS | 0.72 (0.18, 2.83) | NS |
| Hemicraniectomy | 28 | 7.36 (1.70, 31.83) | 0.008 | 1.86 (0.36, 9.57) | NS |
| Intubated | 28 | 7.13 (2.58, 19.66) | <0.001 | 2.06 (0.60, 7.08) | NS |
| Tube feeding | 28 | 5.50 (1.81, 16.74) | 0.003 | 1.34 (0.36, 4.95) | NS |
| Urinary catheter | 28 | 5.58 (2.18, 14.30) | 0.001 | 1.39 (0.42, 4.60) | NS |
| Cortisola (per 10 μg/dl) | 27 | 3.13 (1.67, 5.86) | <0.001 | 1.50 (0.73, 3.06) | NS |
| ACTHa (per 10 pg/ml) | 25 | 1.19 (0.94, 1.50) | 0.138 | 1.17 (0.89, 1.53) | NS |
| IL-10a (per pg/ml) | 25 | 4.46 (1.65, 12.10) | 0.003 | 2.00 (0.66, 6.09) | NS |
| IL-1raa (per 1000 pg/ml) | 25 | 1.25 (1.06, 1.48) | 0.010 | 1.18 (1.02, 1.38) | 0.029 |
| Lymphocytesb (per thou/ml) | 25 | 0.34 (0.12, 0.90) | 0.031 | 0.78 (0.27, 2.24) | NS |
| PHA responsea (per relative unit) | 25 | 1.03 (1.00, 1.06) | 0.099 | 1.03 (0.99, 1.06) | NS |
All significant values are bolded
NIHSS National Institutes of Health Stroke Scale; LOC level of consciousness; IV tPA intravenous tissue plasminogen activator; ACTH adrenocorticotrophic hormone; IL interleukin; IL-1ra IL-1 receptor antagonist; PHA phytohemagglutin
Highest value in the first 72 h;
Lowest value in the first 72 h
Table 3.
Affect on infection on the association of laboratory variables with infection risk
| Cortisol (per 10 μg/dl) |
ACTH (per 10 pg/ml) |
IL-10 (per pg/ml) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
|||||||
| OR | P | OR | P | OR | P | OR | P | OR | P | OR | P | |
| All patients: | 3.13 (1.67–5.86) | <0.001 | 1.50 (0.73–3.06) | NS | 1.19 (0.94–1.50) | 0.138 | 1.17 (0.89–1.53) | NS | 4.46 (1.65, 12.10) | 0.003 | 2.00 (0.66, 6.09) | NS |
| Excluding patients with infections through day | ||||||||||||
| 1 | 3.35 (1.75–6.40) | <0.001 | 1.62 (0.78–3.37) | 0.194 | 1.20 (0.95–1.52) | 0.123 | 1.18 (0.90–1.54) | NS | 4.86 (1.76–13.38) | 0.002 | 2.21 (0.72–6.79) | NS |
| 2 | 3.80 (1.92–7.54) | <0.001 | 1.78 (0.82–3.82) | 0.143 | 1.21 (0.95–1.54) | 0.118 | 1.20 (0.89–1.60) | NS | 6.45 (2.19–19.00) | 0.001 | 2.76 (0.82–9.35) | 0.102 |
| 3 | 3.60 (1.82–7.12) | <0.001 | 1.68 (0.78–3.64) | 0.184 | 1.21 (0.95–1.54) | 0.12 | 1.20 (0.89–1.60) | NS | 5.91 (1.90–18.36) | 0.002 | 2.32 (0.62–8.76) | NS |
| 4 | 3.37 (1.67–6.79) | 0.001 | 1.47 (0.64–3.38) | NS | 1.22 (0.96–1.55) | 0.111 | 1.20 (0.90–1.62) | NS | 5.12 (1.59–16.56) | 0.006 | 1.85 (0.46–7.49) | NS |
| 5 | 3.18 (1.52–6.66) | 0.002 | 1.41 (0.58–3.43) | NS | 1.18 (0.92–1.53) | 0.197 | 1.17 (0.87–1.56) | NS | 4.91 (1.39–17.39) | 0.014 | 1.82 (0.42–7.94) | NS |
| 6 | 3.18 (1.52–6.66) | 0.002 | 1.41 (0.58–3.43) | NS | 1.18 (0.92–1.53) | 0.197 | 1.17 (0.87–1.56) | NS | 4.91 (1.39–17.39) | 0.014 | 1.82 (0.42–7.94) | NS |
| 7 | 2.97 (1.42–6.23) | 0.004 | 1.08 (0.42–2.78) | NS | 1.17 (0.90–1.52) | NS | 1.16 (0.86–1.57) | NS | 5.80 (1.58–21.31) | 0.008 | 2.05 (0.44–9.63) | NS |
| 8 | 2.93 (1.39–6.18) | 0.005 | 1.10 (0.43–2.83) | NS | 1.20 (0.92–1.56) | 0.171 | 1.18 (0.87–1.61) | NS | 5.20 (1.38–19.50) | 0.015 | 1.85 (0.39–8.82) | NS |
|
| ||||||||||||
| IL-1ra (per 1000 pg/ml) |
Lymphocytes (per 1000/μl) |
Response to PHA |
||||||||||
| Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
|||||||
| OR | P | OR | P | OR | P | OR | P | OR | P | OR | P | |
|
| ||||||||||||
| All patients | 1.25 (1.06–1.48) | 0.010 | 1.18 (1.02–1.38) | 0.029 | 0.34 (0.12–0.90) | 0.031 | 0.78 (0.27–2.24) | NS | 1.03 (1.00–1.06) | 0.099 | 1.02 (0.99–1.06) | NS |
| Excluding patients with infections through day | ||||||||||||
| 1 | 1.22 (1.03–1.45) | 0.019 | 1.17 (1.00–1.36) | 0.044 | 0.30 (0.10–0.84) | 0.022 | 0.70 (0.23–2.09) | NS | 1.02 (0.98–1.06) | NS | 1.02 (0.98–1.06) | NS |
| 2 | 1.26 (1.05–1.51) | 0.014 | 1.22 (1.04–1.45) | 0.017 | 0.30 (0.10–0.91) | 0.034 | 0.84 (0.24–2.87) | NS | 1.03 (0.98–1.07) | NS | 1.03 (0.98–1.08) | NS |
| 3 | 1.25 (1.05–1.49) | 0.014 | 1.22 (1.04–1.44) | 0.017 | 0.32 (0.10–0.98) | 0.046 | 0.90 (0.26–3.08) | NS | 1.03 (0.99–1.07) | 0.187 | 1.03 (0.98–1.08) | 0.181 |
| 4 | 1.22 (1.03–1.43) | 0.018 | 1.20 (1.02–1.42) | 0.025 | 0.38 (0.12–1.15) | 0.087 | 1.10 (0.32–3.86) | NS | 1.03 (0.99–1.08) | 0.145 | 1.04 (0.99–1.09) | 0.146 |
| 5 | 1.22 (1.04–1.44) | 0.014 | 1.21 (1.03–1.43) | 0.022 | 0.39 (0.11–1.34) | 0.136 | 1.12 (0.30–4.21) | NS | 1.03 (0.99–1.07) | 0.186 | 1.03 (0.99–1.08) | 0.173 |
| 6 | 1.22 (1.04–1.44) | 0.014 | 1.21 (1.03–1.43) | 0.022 | 0.39 (0.11–1.34) | 0.136 | 1.12 (0.30–4.21) | NS | 1.03 (0.99–1.07) | 0.186 | 1.03 (0.99–1.08) | 0.173 |
| 7 | 1.24 (1.05–1.46) | 0.012 | 1.25 (1.05–1.49) | 0.014 | 0.42 (0.12–1.45) | 0.17 | 1.53 (0.38–6.16) | NS | 1.03 (0.98–1.07) | NS | 1.03 (0.98–1.08) | 0.187 |
| 8 | 1.23 (1.05–1.45) | 0.011 | 1.24 (1.04–1.48) | 0.015 | 0.46 (0.13–1.62) | NS | 1.62 (0.40–6.66) | NS | 1.03 (0.99–1.08) | 0.167 | 1.04 (0.99–1.09) | 0.158 |
All significant values are bolded
Results are expressed as the odds ratio (OR) and 95% confidence interval and either unadjusted or adjusted for baseline stroke severity
Considering the laboratory values at 72 h after stroke onset, there are clear correlations between stroke severity and cortisol (r2 = 0.581, P < 0.001), IL-10 (r2 = 0.427, P < 0.001), IL-1ra (r2 = 0.366, P < 0.001), and lymphocyte numbers (r2 = −0.387, P < 0.001), but no relationships between stroke severity and plasma ACTH or lymphocyte responses to PHA. All patients who developed PNA had NIHSS scores of at least 20 (Fig. 3). If one restricts analyses to only those patients with severe stroke (NIHSS ≥ 20), there are no differences in the baseline demographics of those who develop PNA and those who do not (data not shown); those that developed PNA, however, had higher concentrations of IL-10 and IL-1ra (Table 4). Among this group of patients with severe stroke, early lymphocyte responses to PHA (as detected by the number of cells secreting IFN-γ ex vivo) were attenuated in those that later developed infection.
Fig. 3.
Individual patient data for each laboratory variable (Y-axis) are depicted as a function of stroke severity (X-axis) and infection status. No PNAs (black circles) occurred in patients with an NIHSS score < 20
Table 4.
Laboratory tests (by hour 72) associated with the risk of PNA to day 15 after stroke among patients with an NIHSS score ≥ 20
| Variable | PNA(+) N = 12 |
No infectiona
N = 14 |
P |
|---|---|---|---|
| Infarct volume (ml) | 150 (13, 344) | 144 (79, 308) | NS |
| Cortisol (μg/dl)b | 22 (17, 32) | 19 (14, 25) | NS |
| ACTH (pg/ml) | 23 (19, 28) | 20 (16, 30.0) | NS |
| IL-10 (pg/ml)b | 1.07 (0.49, 1.68) | nd (nd, nd) | 0.007 |
| IL-1raa (pg//ml) | 3695 (2379, 4338) | 2116 (1128, 4732) | 0.046 |
| lymphocytes (thou/μl)c |
1.0 (0.7, 1.3) | 1.2 (0.9, 1.8) | 0.096 |
| PHA responseb | 1.6 (1.2, 6.6) | 5.5 (2.3, 13.1) | 0.047 |
All significant values are bolded
NIHSS National Institutes of Health Stroke Scale; ACTH adrenocorticotrophic hormone; IL interleukin; IL-1ra IL-1 receptor antagonist; PHA phytohemagglutinin
Laboratory data were missing for one patient without infection
Highest value in the first 72 h
Lowest value in the first 72 h
Discussion
This study aimed to identify cytokines and hormones associated with the risk of infection after stroke. Participants tended to be slightly younger with more severe strokes than the general stroke population at our institution. This fact, however, does not alter the primary findings of this study, which is that stroke severity is the most important variable in determining infection risk and that infection is independently associated with worse stroke outcome. These observations, however, are not novel [1–3]. Further, the relationships between infection risk and plasma cortisol, IL-10, and lymphocyte numbers have been reported previously [9–11, 14]. The unique finding in this study is the relationship between plasma IL-1ra and infection risk. IL-1ra, as the name implies, inhibits the action of IL-1 [19]. Experimental data generally support a beneficial role for IL-1 receptor antagonism in stroke; infectious outcomes in these animal models of stroke are generally not reported [20]. The long-term use of IL-1 receptor antagonists for the treatment of autoimmune diseases is associated with increased risk of infection, but there is little information about short-term risks of IL-1 receptor antagonism [21]. In a phase II clinical stroke [ischemic and intraparenchymal hemorrhage (IPH)] trial of an IL-1ra (anakinra), however, there were 9 “infectious episodes” among the 17 patients allocated to placebo and 16 “infectious episodes” among the 17 patients allocated to treatment with the IL-1ra, accounting for 25% and 44% of adverse events, respectively. No statistics are provided, but the explanation offered for the observed increased rate of infection in the active treatment group is that more patients with IPH were randomized into this group, but the baseline NIHSS scores were identical between the two treatment arms [22].
We found a strong relationship between infection risk and plasma concentrations of cortisol and IL-10; both are highly correlated with stroke severity and neither was independently associated with infection risk. In addition, lymphocyte numbers were decreased in patients who developed infection; the degree of lymphopenia was again strongly related to stroke severity. We did not find, however, evidence of impaired lymphocyte responses to PHA among those individuals who developed infection (although responses were attenuated in patients with severe stroke who developed PNA). In this study, lymphocyte function was assessed ex vivo by individual cell responses to the mitogen PHA in an ELSPOT assay. For this assay, cells are washed in phosphate buffered saline and cultured in lymphocyte proliferation media; our findings thus suggest that there is no intrinsic cellular dysfunction. In vivo, however, it is possible that the endogenous inhibitors of lymphocyte function, like cortisol, IL-1ra, or IL-10, impair lymphocyte function. Further, the ELISPOT assay is an incredibly sensitive method for determining the number of cells secreting a given cytokine, but it does not provide information about the amount of cytokine secreted. It is therefore plausible that the lymphocytes were still capable of responding to PHA by secreting IFN-γ, but the absolute amount of IFN-γ secreted may have been decreased.
In other studies that address the risk of post-stroke infection, the period of censure for infection varies from one week to several weeks after stroke onset [1-3, 8, 9, 11, 14, 23]. In this study, we found that infections continued to accumulate over the course of 15 days and then reached a plateau; our analyses thus focused on those variables that predicted risk over the first 15 days after stroke onset. An important limitation of this study is the fact that the majority of the laboratory assays were done at 72 h after stroke onset. It is certainly possible that important changes in immune function predisposing to infection may have occurred immediately after stroke onset and resolved by the time of our analyses. Of the variables examined, however, it was only the change in lymphocyte numbers that was transient—changes in cortisol, IL-10, and IL-1ra cortisol persisted for weeks. In comparison to many of other studies that address laboratory-based risk factors for infection after stroke, this study is larger and contains a diverse group of patients with mild to severe strokes. Further, we attempted control for the effect of infection (overt or pre-clinical) on the laboratory variable being analyzed to be certain that the variable is not merely a marker of infection; most studies have not taken this precaution. This detail is important given that overt signs of clinical infection often lag behind the initial bacterial inoculum, and infections are characterized by an initial period of inflammation followed by that of a compensatory immunodulatory response [24]. The fact that plasma cortisol, IL-10, and IL-1ra, as assessed at 72 h after stroke onset, can predict the onset of infection several days later suggests that these hormones and cytokines are not merely markers of infection, but rather predictive of infection.
We found that IL-1ra was the only independent predictor of infection risk after stroke in this study. This does not mean, however, that elevated cortisol, elevated IL-10, or decreased lymphocyte numbers do not contribute to infection risk. In fact, changes in these hormones and cytokines may be the mechanism by which severe strokes mediate the increased risk of infection. For instance, the sympathetic response after stroke appears to mediate important immunologic changes in animals as well as in clinical studies [6, 23]. Thus, severe stroke may predispose to the important hormonal and autonomic responses that predispose to infection. Controlling for stroke severity (as done in Table 2) may not be appropriate and tends to minimize the effect of these immunomodulatory molecules on infection risk.
In summary, we find that stroke severity is the most important predictor of infection risk following stroke. Patients with more severe strokes may be at higher risk of infection due to a greater risk of aspiration and exposure to more indwelling catheters, but experimental data also suggest that strokes induce systemic changes in the immune response that predispose to infection. Elevated plasma concentrations of cortisol, IL-10, and IL-1ra are associated with increased risk of infection, although only IL-1ra imparts a risk that appears to be independent of stroke severity. The potential role for IL-1ra in mediating post-stroke infection data will need to be confirmed in larger patient cohorts, but could have implications for stroke therapy.
Acknowledgments
This study was funded by NINDS 5R01NS0-49197.
Footnotes
Disclosures
The authors have no financial disclosures.
References
- 1.Aslanyan S, Weir CJ, Diener HC, Kaste M, Lees KR. Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. Eur J Neurol. 2004;11:49–53. doi: 10.1046/j.1468-1331.2003.00749.x. [DOI] [PubMed] [Google Scholar]
- 2.Hong KS, Kang DW, Koo JS, et al. Impact of neurological and medical complications on 3-month outcomes in acute ischaemic stroke. Eur J Neurol. 2008;15:1324–31. doi: 10.1111/j.1468-1331.2008.02310.x. [DOI] [PubMed] [Google Scholar]
- 3.Vermeij FH, Scholte op Reimer WJ, de Man P, et al. Stroke-associated infection is an independent risk factor for poor outcome after acute ischemic stroke: data from the Netherlands Stroke Survey. Cerebrovasc Dis. 2009;27:465–71. doi: 10.1159/000210093. [DOI] [PubMed] [Google Scholar]
- 4.Indredavik B, Rohweder G, Naalsund E, Lydersen S. Medical complications in a comprehensive stroke unit and an early supported discharge service. Stroke. 2008;39:414–20. doi: 10.1161/STROKEAHA.107.489294. [DOI] [PubMed] [Google Scholar]
- 5.Walter U, Knoblich R, Steinhagen V, Donat M, Benecke R, Kloth A. Predictors of pneumonia in acute stroke patients admitted to a neurological intensive care unit. J Neurol. 2007;254:1323–9. doi: 10.1007/s00415-007-0520-0. [DOI] [PubMed] [Google Scholar]
- 6.Prass K, Meisel C, Hoflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–36. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harms H, Prass K, Meisel C, et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS ONE. 2008;3:e2158. doi: 10.1371/journal.pone.0002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haeusler KG, Schmidt WU, Fohring F, et al. Cellular immunodepression preceding infectious complications after acute ischemic stroke in humans. Cerebrovasc Dis. 2008;25:50–8. doi: 10.1159/000111499. [DOI] [PubMed] [Google Scholar]
- 9.Hug A, Dalpke A, Wieczorek N, et al. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009;40:3226–32. doi: 10.1161/STROKEAHA.109.557967. [DOI] [PubMed] [Google Scholar]
- 10.Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke. 2009;40:1262–8. doi: 10.1161/STROKEAHA.108.532085. [DOI] [PubMed] [Google Scholar]
- 11.Vogelgesang A, Grunwald U, Langner S, et al. Analysis of lymphocyte subsets in patients with stroke and their influence on infection after stroke. Stroke. 2008;39:237–41. doi: 10.1161/STROKEAHA.107.493635. [DOI] [PubMed] [Google Scholar]
- 12.Vogelgesang A, May VE, Grunwald U, et al. Functional status of peripheral blood T-cells in ischemic stroke patients. PLoS ONE. 2010;5:e8718–20. doi: 10.1371/journal.pone.0008718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 14.Chamorro A, Amaro S, Vargas M, et al. Interleukin 10, monocytes and increased risk of early infection in ischaemic stroke. J Neurol Neurosurg Psychiatry. 2006;77:1279–81. doi: 10.1136/jnnp.2006.100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beamer NB, Coull BM, Clark WM, Hazel JS, Silberger JR. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann Neurol. 1995;37:800–5. doi: 10.1002/ana.410370614. [DOI] [PubMed] [Google Scholar]
- 16.Christensen H, Boysen G, Christensen E, Johannesen HH, Bendtzen K. Plasma cytokines in acute stroke. J Stroke Cerebrovasc Dis. 2002;11:72–9. doi: 10.1053/jscd.2002.126688. [DOI] [PubMed] [Google Scholar]
- 17.Emsley HC, Smith CJ, Gavin CM, et al. Clinical outcome following acute ischaemic stroke relates to both activation and autoregulatory inhibition of cytokine production. BMC Neurol. 2007;7:5. doi: 10.1186/1471-2377-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–10. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arend WP. Interleukin-1 receptor antagonist. Adv Immunol. 1993;54:167–227. doi: 10.1016/s0065-2776(08)60535-0. [DOI] [PubMed] [Google Scholar]
- 20.Banwell V, Sena ES, Macleod MR. Systematic review and stratified meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke. J Stroke Cerebrovasc Dis. 2009;18:269–76. doi: 10.1016/j.jstrokecerebrovasdis.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis. 2009;68:25–32. doi: 10.1136/ard.2007.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emsley HC, Smith CJ, Georgiou RF, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–72. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chamorro A, Amaro S, Vargas M, et al. Catecholamines, infection, and death in acute ischemic stroke. J Neurol Sci. 2007;252:29–35. doi: 10.1016/j.jns.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Ward NS, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med. 2008;29:617–25. doi: 10.1016/j.ccm.2008.06.010. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]