Abstract
Excessive alcohol consumption, characteristic of alcohol use disorders, results in neurodegeneration and behavioral and cognitive impairments that are hypothesized to contribute to the chronic and relapsing nature of alcoholism. Therefore, the current study aimed to advance the preclinical development of transdermal delivery of cannabidiol (CBD) for the treatment of alcohol-induced neurodegeneration. In experiment 1, 1.0%, 2.5% and 5.0% CBD gels were evaluated for neuroprotection. The 5.0% CBD gel resulted in a 48.8% reduction in neurodegeneration in the entorhinal cortex assessed by Fluoro-Jade B (FJB), which trended to statistical significance (p = 0.069). Treatment with the 5.0% CBD gel resulted in day 3 CBD plasma concentrations of ~100.0 ng/mL so this level was used as a target concentration for development of an optimized gel formulation. Experiment 2 tested a next generation 2.5% CBD gel formulation, which was compared to CBD administration by intraperitoneal injection (IP; 40.0 mg/kg/d). This experiment found similar magnitudes of neuroprotection following both routes of administration; transdermal CBD decreased FJB+ cells in the entorhinal cortex by 56.1% (p < 0.05), while IP CBD resulted in a 50.6% (p < 0.05) reduction in FJB+ cells. These results demonstrate the feasibility of using CBD transdermal delivery systems for the treatment of alcohol-induced neurodegeneration.
Keywords: alcoholism, ethanol, cannabidiol, neuroprotection, neurotoxicity, transdermal
1. Introduction
Approximately 8.5% of the U.S. population currently meets the diagnostic criteria for an alcohol use disorder (AUD; Hasin et al., 2007). Although four pharmacotherapy based interventions are approved in the U.S. for the treatment of AUDs, these drugs have had limited efficacy in the patient population (Litten et al., 2012). Additionally, these medications primarily target the motivational properties of alcohol, while the neurodegenerative effects of alcohol that are hypothesized to impair behavioral control and decision making, are not managed by these specific treatments. Therefore, identification of novel targets and development of new therapeutic agents is critical to improve pharmacotherapy based treatment strategies for AUDs.
Neuroprotective agents are hypothesized to have high therapeutic utility for the treatment of AUDs (Crews, 1999). Excessive alcohol intake, characteristic of AUDs, results in neurodegeneration and cognitive and behavioral impairment, effects which are hypothesized to influence the transition to addiction (Koob and Le Moal, 1997; Crews, 1999; Sullivan and Pfefferbaum, 2005). Imaging studies have identified gross anatomical abnormalities throughout the brains of human alcoholics including widespread disruption of white matter tracts, atrophied cortical gray matter and increased cerebral spinal fluid filled space (Pfefferbaum et al., 1992; Mechtcheriakov et al., 2007; Demirakca et al., 2011). These effects have also been observed in postmortem studies showing significant cortical neuronal loss in alcoholic brains (Harper and Kril, 1989; Kril et al., 1997), which is consistent with studies demonstrating long term or permanent deficits in function (Stavro et al., 2012). Some brain structures appear to be more susceptible to the neurodegenerative effects of alcohol, including the frontal lobe (Kril et al., 1997; Pfefferbaum et al., 1997; Qin and Crews, 2012), temporal lobe (Sullivan et al., 1995) and hippocampus (Sullivan et al., 1995). The aforementioned brain regions are involved in problem solving, attention, information processing, learning and memory and behavioral control, therefore it is not surprising that these functions are impaired in AUDs (Stavro et al., 2012). Importantly, a recent study described an association between reductions in cortical gray matter and risk for relapse (Rando et al., 2011), further substantiating the role of alcohol-induced neurodegeneration in AUDs. Therefore, elucidating the mechanism(s) underlying alcohol-induced neurodegeneration and developing neuroprotective pharmacotherapies could improve prevention and treatment strategies for AUDs.
Studies have suggested that chronic alcohol exposure is associated with induction of neuroinflammatory mediators and/or oxidative stress, which leads to neurodegeneration (Crews and Nixon, 2009; Qin and Crews, 2012). Consistent with this hypothesis, a variety of antioxidants, including α-tocopherol, butylated hydroxytoluene (BHT) and cannabidiol (CBD) have been effective in reducing binge alcohol induced neurodegeneration (Hamelink et al., 2005; Crews et al., 2006). Neuroprotection mediated by antioxidant treatment is associated with inhibition of NF-κB-DNA binding, reductions of COX-2 expression and microglial activation (Crews et al., 2006), all of which support the hypothesis that neuroinflammatory signaling and/or oxidative stress contribute to alcohol-induced neurodegeneration (Crews and Nixon, 2009). These studies have clearly demonstrated that antioxidants protect against alcohol-induced neurodegeneration, therefore further development of these agents for clinical use is warranted.
CBD is a main constituent of cannabis sativa. Unlike the more commonly recognized constituent, (-)-Δ9-tetrahydrocannabinol, CBD does not exhibit psychotropic effects as it is not an agonist at cannabinoid 1 receptors (Pertwee, 2008). In fact, CBD is very well tolerated in humans (Cunha et al., 1980). CBD has a plethora of actions, including anticonvulsive, anxiolytic, anti-relapse and neuroprotective properties (Hampson et al., 1998; Mechoulam et al., 2002; Ren et al., 2009), which make it an ideal candidate for treating multiple pathologies associated with AUDs. CBD was initially shown to be neuroprotective in an in vitro model of excitotoxicity by scavenging reactive oxygen species (Hampson et al., 1998). Indeed, comparison of CBD with well-known antioxidants including BHT and α-tocopherol, showed that CBD has a higher antioxidant capacity (Hampson et al., 1998). Extending these findings, another study demonstrated that CBD was neuroprotective in the modified Majchrowicz binge model of alcohol-induced neurodegeneration, presumably through its antioxidant activity (Hamelink et al., 2005).
Although CBD is efficacious in preclinical models and is safe for human use (Cunha et al., 1980), its clinical use has been minimal because of poor oral bioavailability and low aqueous solubility. Estimated oral bioavailability of CBD is roughly 6% (Agurell et al., 1981; Ohlsson et al., 1986); therefore, it is difficult and expensive to achieve suitable plasma levels for clinical efficacy. These drug delivery obstacles may be circumvented by alternative delivery routes, such as transdermal delivery (Paudel et al., 2010). Additionally, transdermal delivery is advantageous because it promotes patient compliance, as this route of administration is non-invasive and pain free compared to injectable formulations, which is especially important in the alcohol dependent population (Swift et al., 2011). Therefore, the current study investigated the utility of CBD transdermal systems for preventing alcohol-induced neurodegeneration using a well-established model of and AUD, the modified Majchrowicz binge model.
2. Materials and Methods
2.1. Housing and Animals
Adult male Sprague Dawley rats weighing approximately 275-300 grams on arrival (n = 148, Charles River, Raleigh, NC) were used in these studies. All treatment protocols followed the Guide for the Care and Use of Laboratory Animals (NRC, 1996) and were approved by the University of Kentucky Institutional Animal Care and Use Committee. Rats were singlely housed in Plexiglas cages in an AAALAC approved University of Kentucky vivarium on a 12 h light/dark cycle with access to rat chow and water ad libitum unless noted. During acclimation, rats were handled daily for at least three days to familiarize rats to experimenters.
2.2. Ethanol Treatment
Rats were exposed to ethanol following the modified Majchrowicz binge model (Majchrowicz, 1975) as reported previously (Morris et al., 2010). This model maintains intoxicating blood ethanol concentrations (BECs) typical of AUDs (Urso et al., 1981), with minimal mortality and a well-defined pattern of neurodegeneration (Collins et al., 1996; Kelso et al., 2011). Rat chow was removed from home cages and rats were administered either ethanol (25% w/v) in nutritionally complete Vanilla Ensure Plus® (Abbott Laboratories, Columbus OH) or an isocaloric diet consisting of dextrose, water and Vanilla Ensure Plus® every 8 h for 4 days by intragastric gavage. Ethanol rats initially received a 5 g/kg priming dose, with subsequent doses based off the following intoxication scale: 0, Normal (5 g/kg); 1, slightly ataxic and hypoactive (4 g/kg); 2, ataxic with elevated abdomen and intact righting reflex (3 g/kg); 3, delayed righting reflex and lack of abdominal elevation (2 g/kg); 4, lack of righting reflex with intact eye blink reflex (1 g/kg); 5, unresponsive including loss of eye blink reflex (0 g/kg). BECs were measured in plasma from tail blood collected 90 minutes after the 7th dose of ethanol (day 3). Approximately 150 μL of blood was collected into microcentrifuge tubes containing heparin (5μL; AAP pharmaceuticals, Schaumberg, IL), centrifuged at 1800 × g for 5 min, and stored at −20°C. BECs were determined in triplicate using a AM1 alcohol analyzer (Analox, Lunenburg, MA) calibrated to a 300 mg/dL external standard.
2.3. Cannabidiol Regimen
CBD was synthesized by AllTranz Inc. and formulated for either intraperitoneal (IP) injection or transdermal gel application. CBD (6 mg/mL) and vehicle solutions for IP injections were prepared daily prior to the morning dose. IP solutions were comprised of 76% sterile saline, 21% cremophor and 3% absolute ethanol. The 1%, 2.5%, 5% (w/w) CBD gels and vehicle gels were prepared and loaded into syringes for gel application. The active and vehicle gels prepared by AllTranz Inc. were composed of ethanol, propylene glycol, sterile water, Transcutol®, preservatives and a crosslinked polyacrylate polymer adjusted to the appropriate pH with triethanolamine to provide suitable rheological properties and pH dependent CBD stability. The optimized formulation described in experiment 2 utilized only a 2.5% (w/w) CBD gel that contained decreased levels of ethanol and an increase in water content. Rats receiving gels had hair removed on their dorsal side using clippers prior to binge treatment and 24 hours before the first gel application. Rats received CBD or vehicle starting after the third dose of ethanol by either daily gel application (11:00 am) or IP injection (20 mg/kg) twice daily (11:00 am and 11:00 pm; see Figure 1A). This IP dose was chosen based off a previous study demonstrating CBD mediated neuroprotection using a similar binge model (Hamelink et al., 2005). Gels (750 μL) were applied to a 35 cm2 area and rubbed into the skin for 30 sec with a finger covered by a nitrile glove.
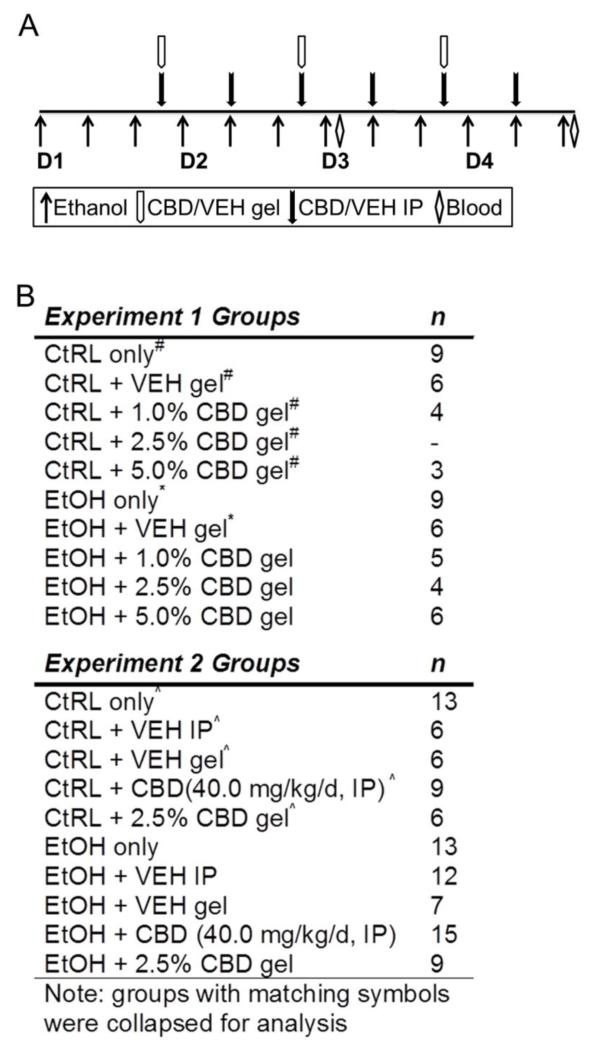
Figure 1.
Treatment regimen for cannabidiol neuroprotection studies. Rats were administered ethanol according to a 4-day binge paradigm (A). In addition to receiving ethanol, rats were co-administered CBD by IP injection twice daily (filled arrows) or topical gel formulation daily (open arrows). Plasma samples were collected on day 3 and prior to euthanasia from tail vein blood or trunk blood, respectively, for determination of blood ethanol concentrations and plasma CBD concentrations. Experiment groups with number of animals (B). Groups with matching symbols were collapsed prior to analysis.
2.4. Cannabidiol Quantification
To determine plasma CBD concentrations, additional tail blood was collected on day 3 and trunk blood was collected during euthanasia. Approximately 250 μL of blood was collected and placed into silanized microcentrifuge tubes containing heparin, centrifuged at 10,000 × g for 3 min and plasma was stored at −70°C until quantification by LC-MS. CBD was extracted according to previously described methods (Paudel et al., 2010). Briefly, CBD was extracted from 50 μL of plasma using 500 μL of acetonitrile (ACN):ethyl acetate (1:1, v/v). Samples were vortexed for 1 min, centrifuged for 20 min at 10,000 × g and supernatants were placed into siliconized test-tubes and evaporated under nitrogen at 37°C. Samples were reconstituted with 100 μL of ACN, vortexed for 1 min and sonicated for 5 min before transfer to HPLC vials with silanized low volume HPLC inserts and placed in a Waters Alliance® 2695 HPLC system. CBD was resolved using a Waters Symmetry® C18 reversed phase column (5 μm, 2.1 × 150 mm; Milford, MA) fitted with a Sentry Symmetry® C18 (3.5 μm, 2.1 × 10 mm) guard column and a mobile phase consisting of ammonium acetate (2mM):ACN (30:70 or 35:65 v/v) at a flow rate of 0.25 mL/min. Electrospray ionization in negative mode was performed for CBD detection (m/z 313, retention time 7.7 or 9.8 min) with either a Waters Micromass ZQ™ 2000 mass spectrometer or a Waters Micromass Quattro Micro™ API system (Milford, MA).
2.5. Fluoro-Jade B Staining
Following binge treatment, rats were euthanized by an overdose of sodium pentobarbital (Nembutal®, MWI Veterinary Supply, Nampa, ID or Fatal Plus®, Vortech Pharmaceuticals, Dearborn, MI) then perfused transcardially using 0.1 M phosphate buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde (PFA). Brains were extracted, post fixed in 4% PFA at 4°C overnight and stored in PBS at 4°C until sectioning. Brains were cut in a 1:12 series on the coronal plane at 40 μm using a vibrating microtome (Leica Microsystems, Wetzlar, Germany) and stored in cyroprotectant at −20°C. Fluoro-JadeB (FJB) was chosen over amino-cupric silver staining to assess neurodegeneration because it is more cost effective, less time consuming and more consistent (Schmued and Hopkins, 2000). Additionally, similar magnitudes of effect are observed following either FJB or silver staining (Cippitelli et al., 2012), suggesting that FJB is an appropriate alternative to silver stain. FJB staining was performed according to the manufacturer’s instructions (Millipore, Billerica, MA) as previously described (Obernier et al., 2002; Leasure and Nixon, 2010). A 1:12 series for each animal was washed (3 × 5 min in TBS) then mounted on Superfrost Plus® slides (Fisher Scientific, Pittsburgh, PA) and allowed to air dry overnight. Sections were then rehydrated (5 min, 1% sodium hydroxide in 80% ethanol; 2 min, 70% ethanol; 2 min, ddH2O), incubated in 0.06% potassium permanganate for 10 min while gently shaking, rinsed in ddH2O for 2 min and stained with 0.001% (w/v) FJB in 0.1% (v/v) acetic acid for 20 minutes while gently shaking in the dark. Sections were further rinsed (3 × 1 min) with ddH2O in the dark, dried on a covered slide warmer and cover-slipped in Cytoseal® (Richard Allen Scientific, Kalamazoo, MI). FJB positive (+) cells were quantified at 200× or 400× magnification using an Olympus BX-51 microscope equipped for epifluorescence with a 488λ cube for blue excitation. The entorhinal cortex was defined using a rat brain atlas (Paxinos and Watson, compact 6th edition, 2009) and FJB+ cells were counted in the entorhinal cortex from −3.60 mm through −6.12 mm from bregma and averaged as the number of FJB+ cells/section. Although, neurodegeneration can be detected throughout the cortico-limbic pathway, only the entorhinal cortex was quantified as a screen for CBD effects because this brain region has the most reproducible injury severity. Stereology was not used because the entorhinal cortex does not have readily identifiable boundaries necessary for implementing stereological procedures and tissue thickness is difficult to accurately measure with the low background characteristic of FJB staining. Strict criteria were used to identify FJB+ cells: cells were included if they were in cortical layers II or III, displayed a pyramidal cell body characteristic of neurons, and/or had observable proximal dendrites. FJB+ cells were rarely observed in control rats (< 1 cell/section) regardless of CBD treatment and were not significantly different, therefore were collapsed into a single control group for each study.
2.6. Statistical analysis
Statistics were performed using GraphPad Prism (Graphpad version 4.03, La Jolla, CA, USA). Average intoxication behavior was analyzed by Kruskal-Wallis tests followed by Dunn’s post-hoc tests when appropriate. Average daily dose, BECs, and CBD plasma concentrations were analyzed by ANOVAs followed by Bonferroni post-hoc tests when appropriate. FJB data was analyzed using ANOVAs followed by planned post-hoc t-tests. Significant variability in FJB cell counts was expected based on previous experience with the binge model; therefore, experiments were designed a priori with the intention of collapsing ethanol and ethanol + vehicle rats in order to reduce the number of animals used while maintaining power. Additionally, the experiments were designed a priori to collapse control groups as FJB is rarely observed (< 1 cell/section) in these rats. Values are presented as mean ± standard error of the mean and analyses were considered significant at p < 0.05.
3. Results
3.1. Experiment 1: Determination of a neuroprotective target CBD plasma concentration following of CBD transdermal delivery
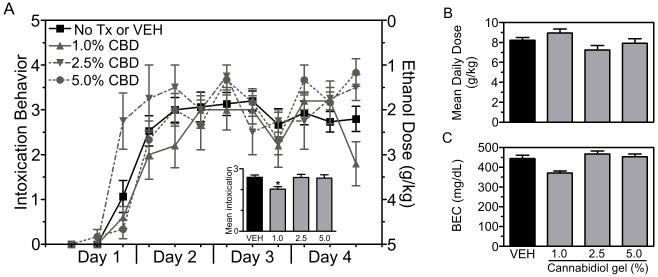
Experiment 1 tested the neuroprotective effects of 1.0% (n = 5), 2.5% (n = 4) and 5.0% (n = 6) CBD gels. First, in order to rule out potentially confounding effects of CBD or vehicle treatment on ethanol pharmacokinetics and intoxication; intoxication behavior, ethanol dose and BECs were compared across treatment groups. Rats treated with ethanol only (n = 9) and ethanol plus vehicle gel (n = 6) were indistinguishable across all measured variables, therefore these groups were collapsed. Regardless of treatment, all rats displayed similar intoxication behavior across the four-days of binge treatment (Figure 2A). The grand mean intoxication behavior was 2.5 ± 0.1 out of 5, which is indicative of rats being intoxicated to the level where they displayed a delayed righting reflex and ataxia. Analysis of mean intoxication behavior (Figure 2A inset) revealed a main effect of treatment [H(3) = 8.258; p < 0.05] and post-hoc tests indicated a significant difference between the ethanol/ethanol + vehicle and ethanol + 1.0% CBD groups (p < 0.05). Although a significant difference in intoxication was observed between these two groups, the effect was not large enough to result in different amounts of ethanol administered. The grand mean ethanol dose for rats in this experiment was 8.2 ± 0.2 g/kg/day, which was not different between groups (Figure 2B). Accordingly, the grand mean peak BEC was 436.9 ± 11.1 mg/dL. Although one-way ANOVA revealed a significant effect of treatment [F(3,29) = 3.085; p = 0.045], post-hoc analysis failed to reveal a significant difference between groups (Figure 2C). These data indicate that transdermal vehicle or transdermal CBD did not alter the intoxicating effects or pharmacokinetics of ethanol. Additionally, these binge data are similar to previous reports using the modified Majchrowicz binge model (Morris et al., 2010).
Figure 2.
Binge treatment data for experiment 1. Rats were treated according to the modified Majchrowicz binge paradigm and administered nothing, vehicle, 1%, 2.5% or 5.0% CBD gel formulations. Ethanol only and ethanol + vehicle groups were statically similar and therefore collapsed (black bars). Behavioral intoxication scores were similar across groups regardless of treatment (A, left axis), therefore each group received similar doses of alcohol (A, right axis). Although mean intoxication score for the 1.0% CBD gel group was significantly lower compared to the vehicle group (A inset), the average daily doses and blood ethanol concentrations did not differ between treatment groups (B-C). Collectively, the binge treatment data shows that CBD gel or vehicle gel treatment did not alter the pharmacokinetics or intoxicating effects of ethanol. *, p < 0.05. Tx = treatment.
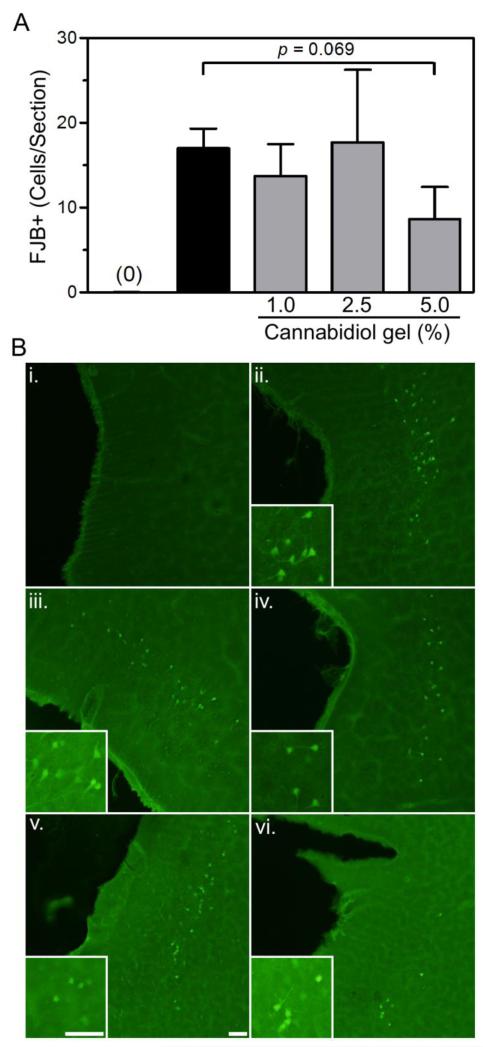
Substantial FJB+ staining was observed in the entorhinal cortex following 4-days of binge ethanol treatment (Figure 3). These cells were typically found in cortical layers II and III adjacent to the rhinal fissure and extending ventrally. FJB+ cells were rarely observed in control rats and control groups did not differ significantly, therefore, all controls were collapsed (n = 22). Ethanol only and ethanol + vehicle gel rats displayed statistically similar FJB+ cell counts, therefore these groups were collapsed prior to analysis. One-way ANOVA revealed a main effect of treatment [F(4,47) = 13.71, p < 0.0001]. Post-hoc tests indicated that rats treated with 1.0% or 2.5% CBD gels had similar FJB+ cell counts as ethanol/ethanol+veh gel rats. However, rats treated with 5.0% CBD gels had a 48.8% reduction in the number of FJB+ cells, which trended to statistical significance (p = 0.069).
Figure 3.
Treatment with 5.0% CBD gel resulted in a reduction in Fluoro-Jade B positive (FJB+) cells in the entorhinal cortex following binge ethanol treatment. Quantification of FJB+ cells in the entorhinal cortex (A). Control rats typically had < 1 FJB+ cell/section therefore were collapsed across treatment groups. Additionally, ethanol and ethanol + vehicle treated rats were indistinguishable, therefore collapsed. Representative images for each treatment group (B): control, i; ethanol, ii; ethanol + vehicle, iii; ethanol + 1.0% CBD, iv; ethanol + 2.5% CBD, v; ethanol + 5.0% CBD, vi. Scale bars = 50 μm.
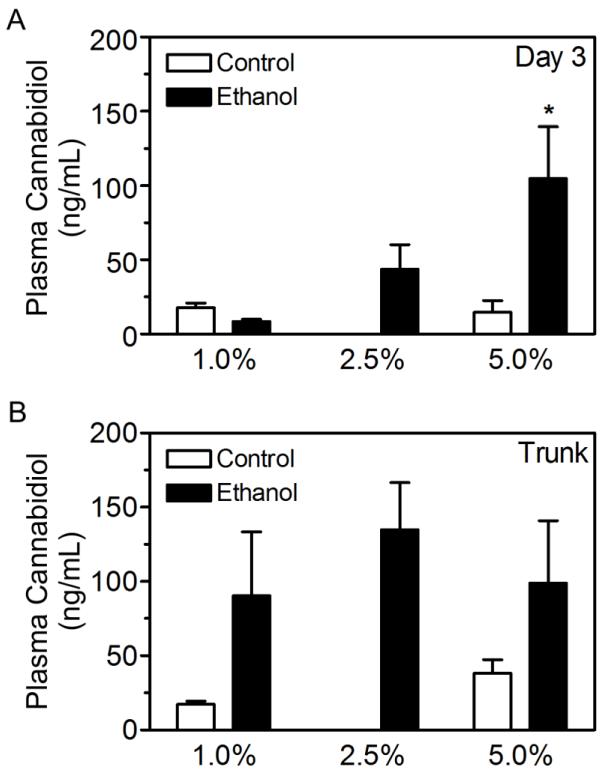
CBD plasma concentrations were analyzed at the beginning of day 3 and at euthanasia (Figure 1A). Control rats treated with 2.5% CBD gel were not included in this experiment therefore a two-way ANOVA was not performed. However, a one-way ANOVA of ethanol groups revealed a main effect of CBD gel percentage (Figure 4A; [F(2,12) = 4.492; p < 0.05]). Post-hoc analysis showed that 5.0% CBD gels resulted in significantly higher CBD plasma concentrations compared to the 1.0% CBD gel group (p < 0.05). However, at euthanasia, CBD plasma concentrations were similar between ethanol groups (Figure 4B; [F(2,13) = 0.29; p > 0.05]).
Figure 4.
CBD plasma concentrations following application of transdermal gel formulations containing 1.0%, 2.5% or 5.0% CBD. CBD plasma levels were quantified in plasma from tail vein blood collected 3 days into binge treatment (A). CBD plasma levels were quantified in plasma from trunk blood collected at euthanasia (B). *, p < 0.05 compared to ethanol + 1.0% CBD.
3.2. Experiment 2: Neuroprotective effects of an optimized CBD transdermal delivery system and IP CBD delivery
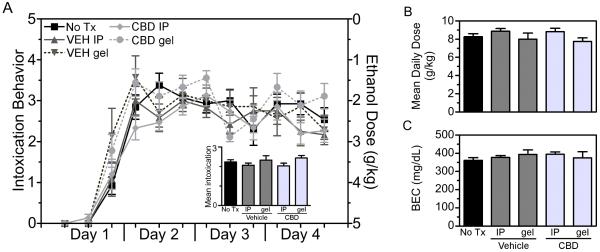
Ethanol intoxication measures in this experiment were similar to experiment 1 and the intoxicating effects of ethanol were similar between ethanol only (n = 13), vehicle IP (n = 12), CBD IP (n = 15), vehicle gel (n = 7) and CBD gel (n = 9) groups across the 4-days of binge treatment (Figure 5A). The grand mean intoxication score was 2.2 ± 0.1 out of 5 (Figure 5A insert); thus rats in this experiment were intoxicated to the level of delayed righting reflexes and ataxia. Additionally, each treatment group in this study received similar doses of ethanol, which on average were 8.4 ± 0.2 g/kg/day (Figure 5B). The grand mean peak BEC for this experiment was 380.4 ± 7.8 mg/dL, which did not differ between groups (Figure 5C), confirming that the drug treatments had no effect on the intoxicating effects or pharmacokinetics of ethanol.
Figure 5.
Binge treatment data for experiment 2. Rats were treated according to the modified Majchrowicz binge paradigm and administered CBD or vehicle by a second generation transdermal gel or IP injection. Behavioral intoxication scores were similar across groups regardless of treatment (A, left axis), therefore each group received similar doses of ethanol (A, right axis). Average daily doses and blood ethanol concentrations did not differ between treatment groups (B-C). Collectively, the binge treatment data shows that CBD or vehicle treatment by either transdermal gels or IP did not alter the pharmacokinetics or intoxicating effects of ethanol.
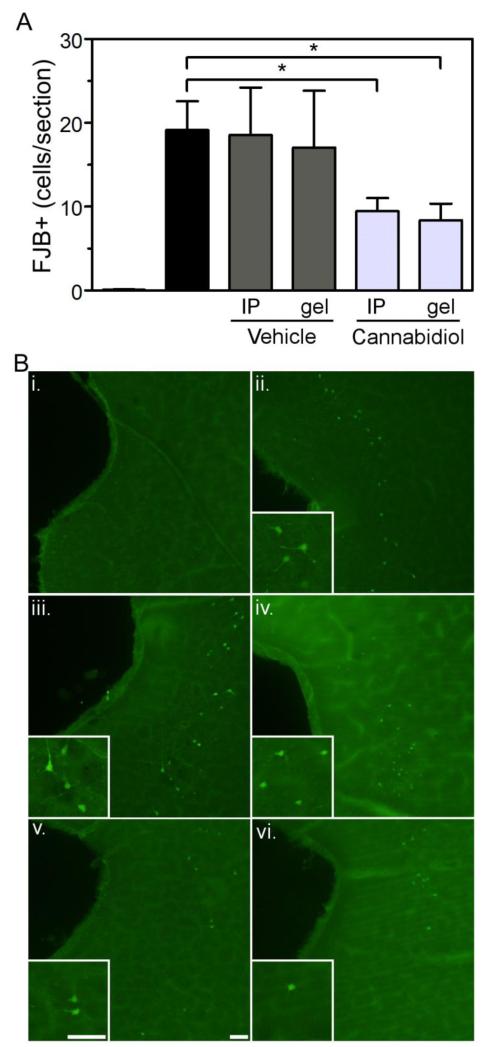
Four days of binge ethanol exposure resulted in neurodegeneration as indicated by the presence of FJB+ cells along the entorhinal cortex. The severity of ethanol-induced damage in the entorhinal cortex was similar between experiment 1 (Figure 3) and experiment 2 (Figure 6). Similar to Experiment 1, controls (n = 40) were statistically similar and therefore collapsed across drug treatment. In contrast to the analysis conducted in experiment 1, ethanol only and ethanol + vehicle groups were not collapsed because the vehicles in this study were delivered by different routes of administration. One-way ANOVA revealed a main effect of treatment [Fig. 6; F(5,84) = 10.63; p < 0.0001]. Post-hoc analysis indicated that administration of CBD by IP administration significantly reduced FJB+ cells in the entorhinal cortex by 50.6% compared to the ethanol only group (p < 0.05). Similarly, transdermal administration of CBD significantly reduced FJB+ cells in the entorhinal cortex by 56.1% compared to the ethanol only group (p < 0.05). Although IP and transdermal CBD administration reduced FJB+ cells by 49.0% and 51.0% compared to their respective vehicle controls, this effect did not reach statistical significance (p > 0.05).
Figure 6.
Treatment with CBD by either IP injection or a second generation transdermal CBD gel resulted in a reduction in Fluoro-Jade B positive (FJB+) cells in the entorhinal cortex following binge ethanol treatment. Quantification of FJB+ cells in the entorhinal cortex (A). Control rats typically had < 1 FJB+ cell/section therefore were collapsed across treatment groups. Representative images for each treatment group (B). control, i; ethanol, ii; ethanol + VEH IP, iii; ethanol + VEH gel, iv; ethanol + 2.5% CBD gel, v; ethanol + CBD IP, vi. *, p < 0.05 compared to ethanol. Scale bars = 50 μm.
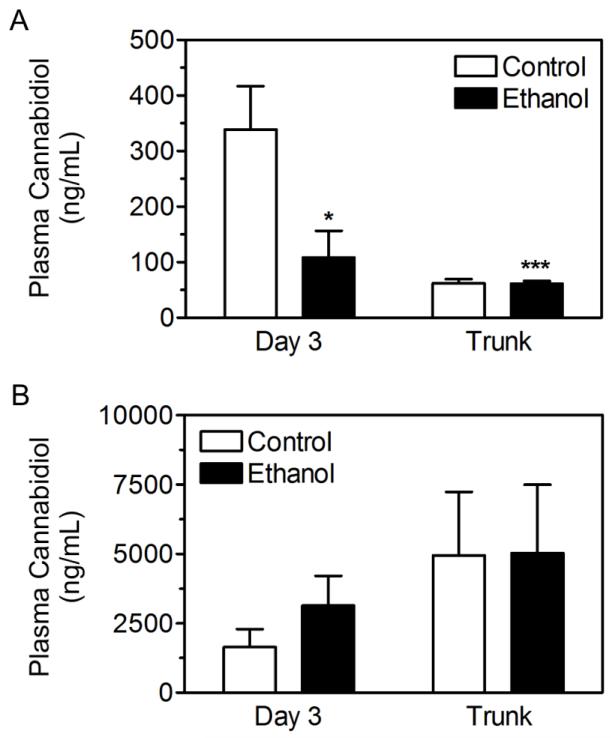
The mean plasma concentration from the 5% CBD gel group in experiment 1 (Figure 4) was used as a target concentration for experiment 2 as this group displayed promising neuroprotective effects. Therefore, a plasma concentration of ~100 ng/mL was targeted following transdermal CBD treatment using a second generation gel formulation from AllTranz Inc. Although the new formulation in experiment 2 only contained 2.5% CBD; day 3 target plasma concentrations of ~100 ng/mL was attainable on average (Figure 7A). Two-way ANOVA revealed main effects of diet [F(1,25) = 7.480; p < 0.05] and time point [F(1,25) = 14.75; p < 0.001], with a signification interaction [F(1,25) = 7.398; p < 0.05]. Post-hoc analysis revealed that CBD plasma levels were significantly lower in binge ethanol treated rats at the day 3 time point compared to controls (p < 0.01). Additionally, control CBD plasma levels during euthanasia were significantly lower than at day 3 (p < 0.001). CBD plasma levels following IP administration (40.0 mg/kg/d) were substantially higher than concentrations achieved following transdermal application (Figure 7B) and were indistinguishable between control and ethanol treated rats.
Figure 7.
CBD plasma concentrations following application of a second generation transdermal CBD gel formulation or after IP injection of CBD. CBD plasma levels were quantified following transdermal application (A) or following IP injection (B) in plasma collected from tail vein blood on day 3 and trunk blood during euthanasia. **, p <0.01, ***, p < 0.001 compared to day 3 control.
4. Discussion
The current study examined the neuroprotective effects of transdermal CBD systems in an accepted model of an AUD that produces substantial neurodegeneration in the cortico-limbic pathway. The first experiment was a pilot study to determine CBD plasma concentrations necessary to observe neuroprotection following transdermal CBD treatment. The 5% gel formulation in this experiment produced promising neuroprotective effects, a 48.8% decrease, while the 1.0% and 2.5% gels were ineffective (Figure 3). The mean day 3 CBD plasma concentration for the 5% CBD gel group was ~ 100 ng/mL and was used as a target concentration because neuroprotection outcomes were promising for this group (Figure 4). In experiment 2, an optimized formulation was developed by AllTranz Inc. to efficiently deliver CBD at the target plasma concentration while using less CBD (Figure 7). Importantly, the neuroprotective effects of transdermal delivery of CBD were comparable to the magnitude of neuroprotection observed following IP injection (Figure 6). Although the degree of neuroprotection appeared to be modest, a 50-60% reduction in FJB+ cells in the entorhinal cortex is similar to previous studies testing neuroprotective agents using the same 4-day binge model (Hamelink et al., 2005; Crews et al., 2006; Cippitelli et al., 2010; Cippitelli et al., 2012). Therefore, these results justify further preclinical development of transdermal CBD for the treatment of alcohol-induced neurodegeneration. Furthermore, preclinical development of neuroprotective agents for the treatment of AUDs is warranted because alcohol-induced brain damage is hypothesized to be critical in promoting impairments in executive self-regulatory behavior, thus contributing to the downward spiral to addiction (Koob and Le Moal, 1997; Crews, 1999).
Interestingly, this study showed that transdermal and IP delivery of CBD produced similar magnitudes of neuroprotection although IP administration resulted in substantially higher CBD plasma levels. Although a full dose-response experiment was not conducted, the current data could suggest that the maximum effective concentration (ECmax) of CBD was achieved following both routes of administration. However, an earlier study by Hamelink et al. failed to observe neuroprotection in the same binge model following IP administration of CBD at 20.0 mg/kg/d (Hamelink et al., 2005), a dose likely to result in plasma concentrations higher than the levels reached following transdermal delivery in the current study. Therefore, it is unlikely that CBD plasma concentrations following transdermal delivery were above the ECmax. Alternatively it is possible that neuroprotection observed following transdermal CBD and IP CBD are mediated though different mechanisms. It has been suggested that the neuroprotective effects of CBD observed during binge alcohol induced neurodegeneration are due to its high antioxidant capacity (Hampson et al., 1998; Hamelink et al., 2005), however, CBD has a plethora of pharmacological targets that may afford neuroprotection. For example, CBD is an inhibitor of endocannabinoid cellular reuptake and metabolism and an agonist at adenosine A2A, serotonin 5-HT1A and transient receptor potential cation channel VI (TRPV1) receptors, all targets implicated in neuroprotection (Bisogno et al., 2001; Karanian et al., 2005; Castillo et al., 2010; Muzzi et al., 2012). Interestingly, many of the receptor mediated effects of CBD follow an inverted u-shaped curve, which is also evident for many of the neuroprotective and anti-inflammatory effects of CBD (Guimaraes et al., 1990; Malfait et al., 2000; Mechoulam et al., 2002; Mishima et al., 2005; Castillo et al., 2010). In fact, a study by Mishima et al., found that CBD prevented cerebral infarction via 5-HT1A receptors at 1.0 and 3.0 mg/kg, but not 0.1 or 10 mg/kg (Mishima et al., 2005). Therefore, it is possible that CBD plasma concentrations achieved following transdermal delivery are conducive to receptor mediated (possibly 5-HT1A) neuroprotection, while higher IP doses, although out of the range for receptor mediated neuroprotection, have effects primarily though antioxidant effects. Alternatively, the neuroprotection observed following transdermal CBD and IP CBD could be related to the different pharmacokinetic profiles expected following each route of administration. It is well known that cannabinoids rapidly distribute to fatty tissue including the brain (Harvey, 2007) and although CBD concentrations were not measured in the brain, it would be interesting to determine how transdermal and IP delivery at these doses differentially affect the brain penetrance of CBD. For example, a recent study found that Cmax and estimated exposure (AUC) in the brain was higher following oral administration compared to IP, which suggests that different routes of administration and their resulting pharmacokinetic profiles affect CBD accumulation in the brain (Deiana et al., 2012). Therefore, an alternative interpretation to explain the similar magnitudes of neuroprotection following transdermal and IP administration of CBD could be that transdermal administration at these doses optimizes brain distribution of CBD.
Importantly, we observed a positive relationship between CBD gel percentage and day 3 CBD plasma concentrations in ethanol treated rats, while CBD plasma concentrations were similar across the 1.0%, 2.5% and 5.0% CBD groups during euthanasia (Figure 4). Although CBD plasma levels were similar at euthanasia, only 5.0% CBD resulted in promising neuroprotective effects (Figure 3). These observations highlight the importance of administering CBD at therapeutic levels early during binge ethanol treatment. CBD treatment was initiated following the third dose of ethanol (Figure 1A), similar to other studies demonstrating neuroprotection following antioxidant treatment (Hamelink et al., 2005; Crews et al., 2006). Neuroprotective agents are likely to be more efficacious when administered at these early timepoints because cellular stress and neurodegeneration can be detected following as few as 1 or 2 days of binge ethanol treatment (Crews et al., 2000; Hayes et al., 2013). For example, unpublished observations show significant impairments in mitochondrial bioenergetics following 2 days of binge treatment (Nixon et al., 2009). Impairment in mitochondrial function is likely a causal factor contributing to alcohol-induced neurodegeneration as these impairments result the production of oxidative stress (Nixon et al., 2009). As CBD is thought to be neuroprotective partially through antioxidant properties, it is possible that CBD attenuates oxidative stress caused by impairments in the mitochondrial electron transport chain. Collectively, these results suggest that neuroprotective agents, including transdermal CBD, need to be administered at therapeutic levels before ethanol-induced neurotoxic events are irreversible.
Enhanced neuroprotection might be observed by administering CBD as a pretreatment in addition to treatment during binge exposure; however this strategy was not implemented in order to mimic a feasible human application for transdermal CBD. For example, an individual could apply a CBD patch if a relapse event occurred and not prophylactically as a pretreatment study would mimic. However, a prophylactic strategy should not be dismissed and may enhance the value of transdermal CBD for the treatment of a variety of other pathologies associated with AUDs in addition to alcohol-induced neurodegeneration. Alcoholism is a cyclical disease consisting of periods of binge intake, acute physical withdrawal, protracted withdrawal and ultimately relapse, which all may be treated by extended release formulations of CBD (Mechoulam et al., 2002; Ren et al., 2009; Scuderi et al., 2009). For example CBD has anticonvulsant effects (acute withdrawal), anxiolytic effects (protracted withdrawal/relapse), reduces drug seeking behavior in rodents (craving/relapse) and has neuroprotective properties (binge intoxication). Therefore, a prophylactic strategy for transdermal CBD treatment could be beneficial if future studies demonstrate efficacy for these other pathologies associated with AUDs. Furthermore, transdermal delivery of other medications, such as naltrexone and acamprosate, could enhance the utility of pharmacotherapy based treatments for alcohol dependence in general. Transdermal delivery is a controllable extended release formulation (Paudel et al., 2010), therefore improves patient compliance because medications can be administered less frequently. Additionally, transdermal products are non-invasive which promotes patient friendly usage, in contrast to injectable formulations. These are important considerations for treating AUDs as compliance has been low for currently approved mediations (Swift et al., 2011).
Although the results of the current study are promising, there are developmental hurdles that need to be overcome in order to translate these findings into a feasible treatment for AUDs. For example, plasma concentrations achieved by the first generation gel formulation in experiment 1 were consistently higher in ethanol treated rats (Figure 4), while the second generation gel formulation resulted in lower CBD plasma concentrations in ethanol treated rats compared to controls at day 2 (Figure 7). Although the reason for the discrepancy between experiment 1 and experiment 2 is unknown, this observation may be related to intrinsic differences in the transdermal flux of CBD between the two formulations. It is also possible that the high BECs achieved during binge ethanol treatment may interfere with the pharmacokinetics of transdermal CBD. For example, studies have shown that forced ethanol consumption in rodents, producing BECs greater than 100 mg/dL, can result in moisture loss in the stratum corneum (Brand and Jendrzejewski, 2008). Dehydration of the stratum corneum could theoretically affect CBD transdermal flux. Furthermore, it is well-known that ethanol interferes with the metabolism of drugs (Weathermon and Crabb, 1999). For example, acute ethanol exposure commonly inhibits hepatic metabolism, while chronic ethanol exposure enhances drug metabolism and clearance (Lieber, 1997). Although it is currently unknown whether altered transdermal flux or metabolism of CBD occurs following binge ethanol treatment, and the current studies were not designed to examine full pharmacokinetic profiles following transdermal delivery of CBD, these considerations are important for future drug development efforts. Even though binge ethanol treatment resulted in alterations in CBD plasma concentrations following transdermal application, one could still argue that transdermal delivery in an alcoholic population may be advantageous compared to oral delivery. Chronic alcohol dependence has dual effects on hepatic metabolism: during ethanol exposure ethanol inhibits hepatic enzyme activity, while enzyme activity can be induced in the absence of ethanol. These contrasting effects on hepatic metabolism can lead to significant variation in systemic blood levels after oral dosing of drugs subjected to high first pass metabolism, such as CBD. Transdermal CBD gels would bypass the first pass effect and thus would be less influenced by the effects of ethanol on hepatic metabolism, leading to more stable systemic blood levels. Even in light of these technological issues, neuroprotection was observed following transdermal CBD delivery. Therefore, future drug development studies are warranted and should be focused on further understanding and optimizing transdermal CBD systems in intoxicated rodents.
Highlights.
Alcohol-induced neurodegeneration is a novel target for alcoholism pharmacotherapy.
Transdermal cannabidiol prevents alcohol-induced neurodegeneration similar to i.p.
Transdermal systems for treating alcoholism may improve patient compliance.
Acknowledgments
We extend our gratitude to Stan Banks, Ph.D. for his assistance in the preparation of this report. This work was supported by funding from the National Institute of Alcohol Abuse and Alcoholism grants AA016959 (KN), AA016499 (KN), and AA019853 (DJL), the National Institute on Drug Abuse grants DA016176 (DJL) and R43DA032161 (ALS) and the Kentucky Science and Technology Corporation grant KSTC-184-512-07-629 (ALS). Sponsors had no contribution to the execution, interpretation or preparation of the current report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Audra Stinchcomb and Dana Hammell are significant shareholders in AllTranz Inc., a transdermal specialty pharmaceutical company developing cannabinoid-based products.
References
- Agurell S, Carlsson S, Lindgren JE, Ohlsson A, Gillespie H, Hollister L. Interactions of delta 1-tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentography. Experientia. 1981;37:1090–2. doi: 10.1007/BF02085029. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–52. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand RM, Jendrzejewski JL. Chronic ethanol ingestion alters xenobiotic absorption through the skin: potential role of oxidative stress. Food Chem Toxicol. 2008;46:1940–8. doi: 10.1016/j.fct.2008.01.034. [DOI] [PubMed] [Google Scholar]
- Castillo A, Tolon MR, Fernandez-Ruiz J, Romero J, Martinez-Orgado J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol Dis. 2010;37:434–40. doi: 10.1016/j.nbd.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Frankola K, Goldstein A, Thorsell A, Singley E, Eskay RL, Heilig M. Alcohol-induced neurodegeneration, suppression of transforming growth factor-beta, and cognitive impairment in rats: prevention by group II metabotropic glutamate receptor activation. Biol Psychiatry. 2010;67:823–30. doi: 10.1016/j.biopsych.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hamelink C, Brunnquell M, Thorsell A, Heilig M, Eskay RL. Binge-like ethanol consumption increases corticosterone levels and neurodegneration whereas occupancy of type II glucocorticoid receptors with mifepristone is neuroprotective. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00451.x. doi:10.1111/j.1369-1600.2012.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcohol Clin Exp Res. 1996;20:284–92. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, Zou J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006;30:1938–49. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT. Alcohol and Neurodegeneration. CNS Drug Reviews. 1999;5:379–94. [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–23. [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–27. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, Sanvito WL, Lander N, Mechoulam R. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21:175–85. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- Deiana S, Watanabe A, Yamasaki Y, Amada N, Arthur M, Fleming S, Woodcock H, Dorward P, Pigliacampo B, Close S, Platt B, Riedel G. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Delta(9)-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology (Berl) 2012;219:859–73. doi: 10.1007/s00213-011-2415-0. [DOI] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kammerer N, Welzel-Marquez H, Hermann D, Heinz A, Mann K. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol Clin Exp Res. 2011;35:1678–85. doi: 10.1111/j.1530-0277.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- Guimaraes FS, Chiaretti TM, Graeff FG, Zuardi AW. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl) 1990;100:558–9. doi: 10.1007/BF02244012. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Ther. 2005;314:780–8. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–73. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Kril J. Patterns of neuronal loss in the cerebral cortex in chronic alcoholic patients. J Neurol Sci. 1989;92:81–9. doi: 10.1016/0022-510x(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Harvey DJ. Absorption, Distribution, and Biotransformation of the Cannabinoids. In: Nahas GG, Sutin KM, Harvey DJ, Aqurell S, editors. Marihuana and Medicine. Humana Press Inc; New Jersey: 1999. pp. 91–103. [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hayes DM, Deeny MA, Shaner CA, Nixon K. Determining the Threshold for Alcohol-Induced Brain Damage: New Evidence with Gliosis Markers. Alcohol Clin Exp Res. 2013;37:425–34. doi: 10.1111/j.1530-0277.2012.01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanian DA, Brown QB, Makriyannis A, Kosten TA, Bahr BA. Dual modulation of endocannabinoid transport and fatty acid amide hydrolase protects against excitotoxicity. J Neurosci. 2005;25:7813–20. doi: 10.1523/JNEUROSCI.2347-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso ML, Liput DJ, Eaves DW, Nixon K. Upregulated vimentin suggests new areas of neurodegeneration in a model of an alcohol use disorder. Neuroscience. 2011;197:381–93. doi: 10.1016/j.neuroscience.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–98. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Nixon K. Exercise neuroprotection in a rat model of binge alcohol consumption. Alcohol Clin Exp Res. 2010;34:404–14. doi: 10.1111/j.1530-0277.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta. 1997;257:59–84. doi: 10.1016/s0009-8981(96)06434-0. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R, Noronha A. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol. 2012;17:513–27. doi: 10.1111/j.1369-1600.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–54. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, Feldmann M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;97:9561–6. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42:11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J. A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:610–4. doi: 10.1136/jnnp.2006.095869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, Fujiwara M. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke. 2005;36:1077–82. doi: 10.1161/01.STR.0000163083.59201.34. [DOI] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA, Nixon K. Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcoho. 2010;44:89–98. doi: 10.1016/j.alcohol.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzi M, Felici R, Cavone L, Gerace E, Minassi A, Appendino G, Moroni F, Chiarugi A. Ischemic neuroprotection by TRPV1 receptor-induced hypothermia. J Cereb Blood Flow Metab. 2012;32:978–82. doi: 10.1038/jcbfm.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Pandya JD, Butler TR, Liput DJ, Morris SA, Prendergast MA, Sullivan PG. Binge ethanol impairs neuronal mitochondrial bioenergetics - reversal by antioxidants and uncouplers. Alcohol Clin Exp Res. 2009;33 (supplement) [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002;26:547–57. [PubMed] [Google Scholar]
- Ohlsson A, Lindgren JE, Andersson S, Agurell S, Gillespie H, Hollister LE. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed Environ Mass Spectrom. 1986;13:77–83. doi: 10.1002/bms.1200130206. [DOI] [PubMed] [Google Scholar]
- Paudel KS, Hammell DC, Agu RU, Valiveti S, Stinchcomb AL. Cannabidiol bioavailability after nasal and transdermal application: effect of permeation enhancers. Drug Dev Ind Pharm. 2010;36:1088–97. doi: 10.3109/03639041003657295. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–89. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–9. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J Neuroinflammation. 2012;9:5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, Sinha R. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry. 2011;168:183–92. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Whittard J, Higuera-Matas A, Morris CV, Hurd YL. Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J Neurosci. 2009;2009;29:14764–9. doi: 10.1523/JNEUROSCI.4291-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ, Fluoro-Jade B. a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–30. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G. Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders. Phytother Res. 2009;23:597–602. doi: 10.1002/ptr.2625. [DOI] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2012;18:203–213. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995;19:110–22. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–94. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Swift R, Oslin DW, Alexander M, Forman R. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J Stud Alcohol Drugs. 2011;72:1012–8. doi: 10.15288/jsad.2011.72.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sci. 1981;28:1053–6. doi: 10.1016/0024-3205(81)90752-9. [DOI] [PubMed] [Google Scholar]
- Weathermon R, Crabb DW. Alcohol and medication interactions. Alcohol Res Health. 1999;23:40–54. [PMC free article] [PubMed] [Google Scholar]