Abstract
Background and objective
Systemic inflammation is a well-known risk factor for diseases such as atherosclerosis and is augmented by the presence of obesity. In addition, it has been shown that inflammation may be negatively influenced by certain macronutrients, specifically the omega-3 and omega-6 fatty acids. The primary aim of this study is to determine whether obesity modifies the association between plasma phospholipid polyunsaturated fatty acids (PUFAs) and markers of inflammation and endothelial activation in Multi-Ethnic Study of Atherosclerosis (MESA) participants.
Subjects
A sample of 2848 adults (25% African American, Chinese, Hispanic, and White) randomly selected from the MESA cohort.
Measurements
Relative plasma PUFA concentrations were determined using gas chromatography-flame ionization detection. Levels of three inflammatory markers (high-sensitivity C-reactive protein, interleukin (IL)-6 and tumor necrosis factor-receptor 1) and two endothelial activation markers (soluble intercellular adhesion molecule-1 (sICAM-1) and E-selectin) were determined with enzyme immunoassays. Linear regression analysis was used to evaluate the relationship between these markers and plasma PUFAs.
Results
Obesity modified the associations of linoleic acid (Pint=0.01), dihomo-γ-linolenic (Pint=0.07) and eicosapentaenoic acid (EPA) (Pint=0.04) with sICAM-1 concentrations; in addition, obesity modified the association of IL-6 with dihomo-γ-linolenic (Pint=0.01). In obese individuals, sICAM-1 was inversely related to EPA levels (P=0.02), but directly related to linoleic acid levels (P<0.001). Conversely, sICAM-1 was inversely related to linoleic acid levels in normal weight individuals (P=0.04). IL-6 concentrations were significantly and directly related to dihomo-γ-linolenic acid (DGLA) in normal weight (P=0.01) and obese participants (P<0.001), but the scale of increase across tertiles was greater in obese adults. Main effects of fatty acid and inflammatory marker associations are also reported.
Conclusion
The modifying effect of obesity on the association of plasma PUFAs with IL-6 and sICAM-1 suggests differences in fatty acid metabolism and may also have implications in dietary fatty acid intake for obese individuals, particularly for linoleic and EPAs. Further study is warranted to confirm and explain the strong associations of DGLA with inflammatory and endothelial activation markers.
Keywords: fatty acids, inflammation, epidemiology, omega-3
Introduction
Inflammation and endothelial activation are associated with the development of chronic diseases including coronary heart disease and atherosclerosis.1,2 Indeed, inflammation within the endothelial wall is now considered a key component of atherosclerosis progression.2 A number of environmental factors such as smoking3 and obesity4,5 have long been recognized as promoters of inflammation and disease development; however, it has only recently gained acceptance that long chain polyunsaturated fatty acids (PUFAs) have the potential to reduce inflammation6 as well as the risk of atherosclerosis7 and cardiovascular disease events.8–10
Most commonly associated with fish oil, omega-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been well studied since their association with lower rates of thrombosis and heart disease.11 Plasma levels of these omega-3 PUFAs have since been inversely associated with circulating pro-inflammatory cytokines such as C-reactive protein, interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α), as well as endothelial activation markers.12–14 Further experiments using cell culture models have revealed a number of anti-inflammatory signaling pathways induced by EPA, DHA and their downstream metabolites.15,16 Though less well-studied, the omega-3 PUFAs alpha-linolenic acid (ALA) and docosapentaenoic acid may show similar associations and anti-inflammatory characteristics.
Apart from the omega-3s, the omega-6 fatty acids found in nuts and oils rich in linoleic acid, for example safflower and corn oils, have garnered recent attention for their potential cardiovascular benefits, as well.17–20 Past investigators have largely focused on the relationship between inflammation and plasma omega-6 PUFAs linoleic acid (LA) and arachidonic acid (AA),12–14 but to our knowledge, no studies have examined the relationship of inflammation/endothelial activation with plasma γ-linolenic acid (GLA) or dihomo-γ-linolenic acid (DGLA). Overall, it remains unclear whether certain plasma omega-3 or omega-6 PUFA concentrations predict an inflammatory phenotype while others predict an anti-inflammatory phenotype.21
In addition to the potential influence of specific omega-3 or omega-6 PUFAs on inflammation and endothelial activation, obesity is already a well-known promoter of both phenomena.4,5 Although previous studies have adjusted for body mass index (BMI) in statistical models,12–14 no study has determined whether obesity influences the relationship between plasma PUFAs and markers of inflammation and endothelial activation. The purpose of this study is to determine the extent to which obesity and weight status modify the relationship between plasma phospholipid PUFAs and systemic inflammatory and endothelial activation markers in a large adult population.
Materials and methods
Population
The primary aim of Multi-Ethnic Study of Atherosclerosis (MESA) is to investigate the development and progression of subclinical cardiovascular disease. The study design has been described elsewhere,22 and information about the MESA protocol is available online (www.mesa-nhlbi.org). Briefly, 6814 men and women between the ages of 45 and 84 years without clinical evidence of cardiovascular disease were recruited from six communities in the United States (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; New York, NY; and St Paul, MN). Institutional Review Board approval was obtained at all MESA sites, and all participants gave informed consent. Recruitment and baseline examinations began in July 2000 and were conducted over a 24-month period.
The current analyses consist of a sample of 2848 randomly selected adults from the MESA cohort who gave informed consent (MESA Genetics Candidate Gene Evaluation Cohort). The study population was represented equally by each of the following races/ethnicities: African American (n=703), Asian (of Chinese descent n=712), Hispanic (n=709) and White (n=722) participants.
Measurements
Questionnaire information was obtained regarding age, sex, race/ethnicity, education, and lifestyle factors including smoking status, alcohol consumption and physical activity. Height (m) and weight (kg) were measured according to standard procedures.22 Fasting blood was drawn and serum and EDTA-anticoagulant tubes were collected and processed using a standardized protocol.22 The serum and plasma samples were aliquoted and stored at −70 °C until time of use.
Plasma fatty acid profile
Phospholipid fatty acids were extracted from EDTA plasma (n=2848) using the method previously described by Cao et al.23 In brief, lipids were extracted from the plasma using a chloroform/methanol extraction method and the cholesteryl esters, triglyceride, phospholipids and free fatty acids were separated by thin layer chromatography. Fatty acids from the phospholipids were derivatized to methyl esters and detected by gas chromatography flame ionization detection. The fatty acids detected were expressed as a percent of total fatty acids. The following representative CVs were obtained (n=20): LA, 2.6%; ALA, 2.4%; AA, 2.4%; EPA, 3.3%; docosapentaenoic acid, 2.9% and DHA, 2.7%.
Analysis of inflammatory markers/cytokines adhesion/endothelial activation markers
High-sensitivity C-reactive protein was measured (n=2848) on a BNII nephelometer (N high-sensitivity C-reactive protein; Dade Behring Inc., Deerfield, IL, USA) using a particle enhanced immunonepholometric assay. Interleukin-6 (IL-6; n=2796), tumor necrosis factor-α soluble receptor 1 (sTNF-R1; n=998), soluble intercellular adhesion molecule-1 (sICAM-1; n=1193) and soluble E-selectin (E-selectin; n=998) were measured using the quantitative sandwich enzyme immunoassay technique of ELISA assays (Quantikine HS Human IL-6 Immunoassay, Quantikine Human sTNF RI Immunoassay, Parameter Human sICAM-1 Immunoassay, Parameter Human E-selectin Immunoassay, respectively; R&D Systems, Minneapolis, MN, USA).
Statistical analysis
All analyses were conducted using SAS (version 9.2, SAS Institute Inc., Cary, NC, USA). BMI was calculated as weight (kg) divided by height (m2). Weight status was created as normal weight (BMI <25 kgm−2), overweight (BMI 25<–30 kgm−2) and obese (BMI 30+ kgm−2). Baseline characteristics are presented as means (s.d.) for continuous variables and frequencies (%) for categorical variables and stratified by weight status. Levels of biomarkers with skewed distributions were log transformed prior to analysis and results were back transformed and presented as geometric means. Generalized linear regression analysis evaluated levels of biomarkers across tertiles of plasma phospholipid PUFAs, adjusting for age, gender, race/ethnicity, field center, education, smoking, physical activity, energy intake, HDL, LDL, triglycerides and diagnosed diabetes. Interaction terms were included in the models to determine whether weight status modified the relations between plasma phospholipid PUFAs and biomarkers. Multiple comparisons were evaluated in linear regression analysis using Tukey’s test. Tests for interaction were considered significant at P≤0.10.
Results
Baseline characteristics are shown in Table 1. Average age was 62.1±10.2 years, over 50% were female, and the majority of adults had greater than a high school education. The subcohort is composed of ~25% of each race/ethnicity in the MESA study, including African Americans, Chinese, Hispanics and Whites. Only 13.7% were smokers; however, 67% of adults were current drinkers. Among this sample, 32.3% were of normal weight, 38.7% overweight and 29% were obese.
Table 1.
Unadjusted baseline characteristics of a selected sample of participants enrolled in the multi-ethnic study of atherosclerosis, n=2846
| Characteristics | All (n=2846) | Normal weight (n=921) | Overweight (n=1104) | Obese (n=825) |
|---|---|---|---|---|
| Age, years (s.d.) | 62.1 (10.2) | 62.7 (10.7) | 62.4 (10.2) | 60.8 (9.4) |
| Gender, n (%) | ||||
| Female | 1517 (53.3) | 419 (45.6) | 574 (52.1) | 336 (40.7) |
| Race/ethnicity, n (%) | ||||
| African-American | 703 (24.7) | 120 (13.1) | 273 (24.8) | 310 (37.5) |
| Chinese | 712 (25.0) | 459 (50.0) | 223 (20.2) | 30 (3.6) |
| Hispanic | 709 (24.9) | 112 (12.2) | 315 (28.6) | 282 (34.2) |
| White | 722 (25.4) | 227 (24.7) | 291 (26.4) | 204 (24.7) |
| Education, n (%) | ||||
| <HS | 619 (21.7) | 178 (19.4) | 262 (23.7) | 179 (21.7) |
| HS | 512 (18.0) | 149 (16.2) | 193 (17.5) | 170 (20.6) |
| Some college | 794 (27.9) | 239 (26.0) | 283 (25.6) | 272 (33.0) |
| College, 4 years | 473 (16.6) | 194 (21.1) | 181 (16.4) | 98 (11.9) |
| College, 5+years | 450 (15.8) | 160 (17.4) | 185 (16.8) | 105 (12.7) |
| Current smoker, n (%) | 390 (13.7) | 103 (11.2) | 164 (14.8) | 123 (14.9) |
| Current alcohol, n (%) | 1427 (67.6) | 438 (71.5) | 577 (68.9) | 412 (62.2) |
| Physical activity, Met-min per week (s.d.) | 1454 (2145) | 1428 (1838) | 1585 (2405) | 1307 (2084) |
Abbreviation: HS, high school.
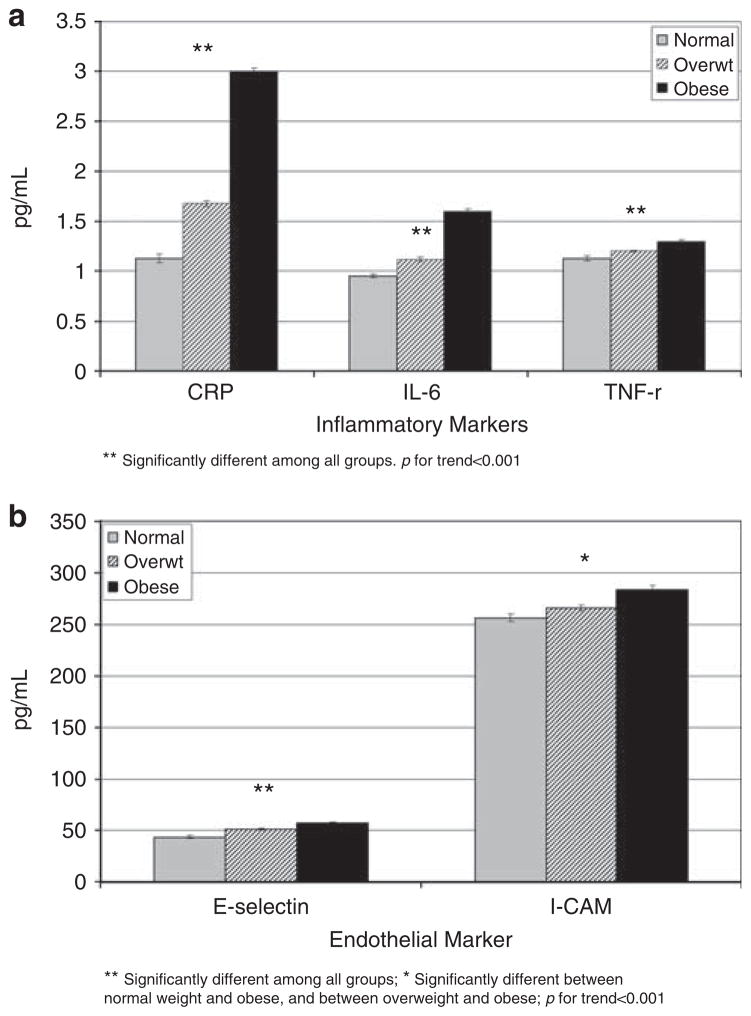
All markers of inflammation and endothelial activation were higher with greater adiposity levels, shown in Figures 1a and b, (all trends, P<0.001).
Figure 1.
(a) Levels of inflammatory markers across weight categories. Models were adjusted for age, gender, race/ethnicity, center, education, smoking, physical activity and energy intake. **Significantly different among all groups. P for trends <0.001. (b) Levels of endothelial markers across weight categories. Models were adjusted for age, gender, race/ethnicity, center, education, smoking, physical activity and energy intake. **Significantly different among all groups. *Significantly different between normal weight and obsese, and between overweight and obsese; P for trends <0.001.
As shown in Table 2, weight status modified the relations between plasma fatty acids and IL-6 and ICAM-1. For the omega-6 PUFAs, sICAM-1 levels were greater across tertiles of LA in obese adults, but the opposite trend was observed in normal weight adults (Pinteraction=0.01). Although levels of sICAM-1 were greater across tertiles of DGLA in all weight classes, the scale of increase across tertiles was greater in obese adults compared with normal or overweight adults (Pinteraction=0.07). For omega-3 PUFAs, sICAM-1 decreased across tertiles of EPA and (EPA+DHA) among obese adults, whereas no relation was observed in those who were normal weight or overweight (Pinteraction=0.04 for EPA; Pinteraction=0.05 for EPA+DHA).
Table 2.
Obesity modifies the relationsa between plasma phospholipid polyunsaturated fatty acids, markers of inflammation and soluble ICAM-1 among a selected sample of participants enrolled in the Multi-Ethnic Study of Atherosclerosis, n=1193
| Biomarker | Weight class | Tertiles of phospholipid fatty acid
|
P-value | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| sICAM-1 (ng ml−1)b | |||||
| Fatty acids | |||||
| LA: 18:2 n6cis (range %) | |||||
| 11.4–36.1 | Normal weight | 271.6 | 255.3 | 255.2 | 0.04 |
| 11.7–32.7 | Overweight | 271.2 | 261.1 | 273.8 | 0.75 |
| 13.1–33.3 | Obese | 263.6 | 279.8 | 304.1 | <0.001 |
| Pinteraction=0.01 | |||||
| DGLA: 20:3 n6 (range %) | |||||
| 0.9–6.2 | Normal weight | 256.3 | 251.0 | 281.2 | 0.008 |
| 1.2–6.6 | Overweight | 256.5 | 273.1 | 274.9 | 0.03 |
| 1.7–6.6 | Obese | 260.8 | 267.6 | 292.1 | 0.02 |
| Pinteraction=0.07 | |||||
| EPA: 20:5 n3 (range %) | |||||
| 0.09–14.5 | Normal weight | 256.5 | 260.4 | 263.2 | 0.41 |
| 0.13–9.1 | Overweight | 264.8 | 263.2 | 277.8 | 0.11 |
| 0.14–5.0 | Obese | 287.8 | 278.7 | 262.6 | 0.02 |
| Pinteraction=0.04 | |||||
| EPA+DHA (range %) | |||||
| 1.5–22.1 | Normal weight | 262.5 | 255.5 | 262.9 | 0.96 |
| 1.4–19.3 | Overweight | 265.3 | 261.6 | 279.3 | 0.10 |
| 1.7–14.4 | Obese | 286.2 | 276.4 | 259.0 | 0.02 |
| Pinteraction=0.05 | |||||
| IL-6 (pgml−1) | |||||
| Fatty acids | |||||
| DGLA: 20:3 n6 (range %) | |||||
| 0.9–6.2 | Normal weight | 0.95 | 0.97 | 1.09 | 0.01 |
| 1.2–6.6 | Overweight | 1.10 | 1.08 | 1.18 | 0.17 |
| 1.7–6.6 | Obese | 1.21 | 1.64 | 1.64 | <0.001 |
| Pinteraction=0.01 | |||||
Abbreviations: DHA, docosahexaenoic acid; DGLA, dihomo-γ-linolenic acid; EPA, eicosapentaenoic acid; IL, interleukin; LA, linoleic acid; sICAM-1, soluble intercellular adhesion molecule-1.
Adjusted for age, gender, race/ethnicity, field center, education, smoking, physical activity, and energy intake, high-density lipoprotein, low-density lipoprotein, triglycerides and diagnosed diabetes.
Black participants were excluded from the sICAM-1 analysis, as discussed in the Strengths and limitations section. Bold values indicate a significant intra-group difference.
Finally, weight status modified the relation between DGLA and IL-6 (Pinteraction=0.01). Similar to sICAM-1, the levels of IL-6 were significantly greater across tertiles of DGLA in normal weight and obese participants, but again the scale of increase across tertiles was greater in obese adults. Although it did not reach significance, IL-6 levels were highest in individuals in the highest tertile of DGLA compared with tertiles 1 and 2 among overweight participants (P=0.17).
No other modifying effect of adiposity reached significance, and main effects data for plasma phospholipid PUFAs relative to markers of inflammation are shown in Tables 3 and 4. Generally, several of the markers of inflammation or endothelial activation were lower in the highest tertiles of omega-6 PUFAs LA and AA and the omega-3 fatty acids ALA, EPA, docosapentaenoic acid, DHA and (EPA+DHA). In contrast, inflammatory markers were higher in the highest tertiles of omega-6 PUFAs GLA and DGLA.
Table 3.
Relationsa,b between plasma phospholipid omega-6 fatty acids (%) and markers of inflammation and endothelial function among a selected sample of participants enrolled in the Multi-Ethnic Study of Atherosclerosis, n=2846c
| Fatty acid | Biomarker | Tertiles of phospholipid fatty acid
|
P-valuea | P-valueb | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| Omega-6 FA | ||||||
| 18:2 n6cis (LA) | hsCRP | 1.89 | 1.66 | 1.66 | 0.004 | 0.02 |
| IL-6 | 1.16 | 1.19 | 1.15 | 0.36 | 0.58 | |
| TNF-r | 1.18 | 1.19 | 1.22 | 0.34 | 0.04 | |
| E-selectin | 50.9 | 51.8 | 46.9 | 0.001 | 0.02 | |
| sICAM-1 | NA | NA | NA | |||
| 18:3 n6 (GLA) | hsCRP | 1.70 | 1.75 | 1.75 | 0.28 | 0.89 |
| IL-6 | 1.10 | 1.22 | 1.20 | <0.001 | 0.003 | |
| TNF-r | 1.16 | 1.22 | 1.23 | 0.02 | 0.03 | |
| E-selectin | 47.6 | 49.6 | 53.3 | 0.02 | 0.02 | |
| sICAM-1d | 268.9 | 264.5 | 273.8 | 0.16 | 0.10 | |
| 20:3 n6 (DGLA) | hsCRP | 1.42 | 1.68 | 2.21 | <0.001 | <0.001 |
| IL-6 | NA | NA | NA | |||
| TNF-r | 1.16 | 1.21 | 1.21 | 0.003 | 0.59 | |
| E-selectin | 48.7 | 49.1 | 51.0 | 0.003 | 0.48 | |
| sICAM-1 | NA | NA | NA | |||
| 20:4 n6 (AA) | hsCRP | 1.71 | 1.71 | 1.77 | 0.60 | 0.73 |
| IL-6 | 1.22 | 1.13 | 1.15 | 0.08 | 0.07 | |
| TNF-r | 1.20 | 1.19 | 1.20 | 0.92 | 0.92 | |
| E-selectin | 50.9 | 49.2 | 48.8 | 0.49 | 0.51 | |
| sICAM-1d | 270.6 | 269.0 | 261.0 | 0.18 | 0.32 | |
Abbreviations: AA, arachidonic acid; DGLA, dihomo-γ-linolenic acid; FA, fatty acid; GLA, γ-linolenic acid; hsCRP, high-sensitivity C-reactive protein; IL, interleukin; LA, linoleic acid; sICAM-1, soluble intercellular adhesion molecule-1; TNF, tumor necrosis factor receptor. NA: not applicable as joint effects were reported in Table 2.
Adjusted for age, gender, race/ethnicity, field center, education, smoking, physical activity and energy intake.
Adjusted further for body mass index, high-density lipoprotein, low-density lipoprotein, triglycerides and diagnosed diabetes.
Sample size for CRP: n=2848; IL-6: n=2796; TNF-r: n=998; E-selectin: n=998; sICAM: n=1193.
Black participants were excluded from the sICAM-1 analysis, as discussed in the Strengths and limitations section. Bold values indicate a significant intra-group difference.
Table 4.
Relationsa,b between plasma phospholipid omega-3 fatty acids (%) and markers of inflammation and endothelial function among a selected sample of participants enrolled in the Multi-Ethnic Study of Atherosclerosis, n=2846c
| Fatty acid | Biomarker | Tertiles of phospholipid fatty acid
|
P-valuea | P-valueb | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| Omega-3 FA | ||||||
| 18:3 n3 (ALA) | hsCRP | 1.82 | 1.70 | 1.68 | 0.08 | 0.25 |
| IL-6 | 1.17 | 1.16 | 1.17 | 0.67 | 0.84 | |
| TNF-r | 1.18 | 1.21 | 1.20 | 0.80 | 0.88 | |
| E-selectin | 48.7 | 50.2 | 49.9 | 0.72 | 0.64 | |
| sICAM-1d | 262.8 | 266.6 | 272.5 | 0.13 | 0.009 | |
| 20:5 n3 (EPA) | hsCRP | 1.78 | 1.79 | 1.63 | 0.22 | 0.17 |
| IL-6 | 1.21 | 1.17 | 1.12 | 0.05 | 0.02 | |
| TNF-r | 1.25 | 1.18 | 1.16 | <0.001 | <0.001 | |
| E-selectin | 47.1 | 48.6 | 51.7 | 0.05 | 0.05 | |
| sICAM-1d | NA | NA | NA | |||
| 22:5 n3 (DPA) | hsCRP | 2.08 | 1.70 | 1.48 | <0.001 | <0.001 |
| IL-6 | 1.26 | 1.19 | 1.06 | <0.001 | <0.001 | |
| TNF-r | 1.25 | 1.17 | 1.17 | 0.58 | 0.68 | |
| E-selectin | 47.7 | 49.4 | 52.0 | 0.12 | 0.03 | |
| sICAM-1d | 269.4 | 265.6 | 267.3 | 0.74 | <0.001 | |
| 22:6 n3 (DHA) | hsCRP | 1.79 | 1.82 | 1.60 | 0.04 | 0.22 |
| IL-6 | 1.24 | 1.20 | 1.06 | <0.001 | <0.001 | |
| TNF-r | 1.24 | 1.19 | 1.16 | 0.05 | 0.02 | |
| E-selectin | 48.9 | 48.1 | 50.2 | 0.58 | 0.36 | |
| sICAM-1d | 268.9 | 261.0 | 273.4 | 0.08 | 0.46 | |
Abbreviations: ALA, alpha linolenic acid; DHA, docosahexaenoic acid; DPA, docosapenaenoic acid; EPA, eicosapentaenoic acid; FA, fatty acid; hsCRP, high-sensitivity C-reactive protein; IL, interleukin; LA, linoleic acid; sICAM-1, soluble intercellular adhesion molecule-1; TNF, tumor necrosis factor receptor. NA: not applicable as joint effects were reported in Table 2.
Adjusted for age, gender, race/ethnicity, field center, education, smoking, physical activity and energy intake.
Adjusted further for body mass index, high-density lipoprotein, low-density lipoprotein, triglycerides and diagnosed diabetes.
Sample size for CRP: n=2848; IL-6: n=2796; TNF-r: n=998; E-selectin: n=998; sICAM: n=1193.
Black participants were excluded from the sICAM-1 analysis, as discussed in the Strengths and limitations section. Bold values indicate a significant intra-group difference.
Discussion
In the present cross-sectional study, obesity was found to modify the associations of several PUFAs (LA, DGLA, EPA and EPA+DHA) with sICAM-1 or IL-6. More significant associations were generally observed between PUFAs and concentrations of inflammatory markers in obese compared with normal weight or overweight adults. Additionally, we confirm previous findings that (1) obese individuals have elevated markers of inflammation and endothelial activation 24,25 and (2) modest but significant differences in PUFA composition were evident among weight classes.26,27
Obesity, plasma phospholipid composition, and markers of inflammation and endothelial activation
Obese individuals had significantly lower levels of omega-3 PUFAs (ALA, docosapentaenoic acid and DHA) and the omega-6 PUFA, LA—but the presence of obesity resulted in significantly higher levels of omega-6 PUFAs GLA, DGLA and AA compared with normal or overweight adults (data not shown). Though variations in diet and fatty acid absorption may contribute to the above observations, differences in the activities of fatty acid metabolic enzymes have been reported in an animal model of obesity28 as well as in obese human subjects29 and may account for such variations in plasma PUFA composition. Overall, the origin(s) of altered PUFA composition in obese adults is unclear, but may be the product of diet, metabolism and/or absorption differences.
In addition to modifying PUFA composition, the presence of obesity altered the relations of fatty acids with sICAM-1 and IL-6. Although normal weight individuals with relatively high plasma LA levels showed significantly lower levels of sICAM-1, the reverse was found in obese individuals. In addition, high plasma levels of EPA and (EPA+DHA) were associated with lower levels of sICAM-1 among obese participants, yet no association was observed in non-obese individuals. As sICAM-1 is both a marker of endothelial activation and a predictor of future cardiovascular disease events,30 these findings may have implications for PUFA intake in obese versus non-obese individuals, if confirmed.
PUFAs and markers of inflammation and endothelial activation Omega-6 PUFAs
Expectedly, omega-6 fatty acids were found to be differentially associated with markers of inflammation and endothelial activation; however, the pattern of these associations was not expected. Indeed, GLA and DGLA have been documented for their conversion into the series-1 prostanoids31 and suppression of inflammatory leukotriene (LT) formation,32 yet they were positively associated with inflammatory and endothelial activation markers with a more significant association found in obese adults. Converse to GLA and DGLA, plasma LA and AA were found to be inversely associated with inflammatory markers. Given that AA has been characterized for inducing inflammation through its metabolism into inflammatory LTs, an inverse association with IL-6 was not expected. However, the formation of pro-inflammatory LTs from AA is a tightly regulated process,33 thus moderate elevations in AA levels may have little to no net effect on LT production and resulting inflammation. Alternatively, the metabolism of AA into the anti-inflammatory lipoxin A4 34 and the reported suppressive effect of AA on the transcription factor nuclear factor-κB35 may explain the above observation. Indeed, the inhibition of nuclear factor-κB or generation of lipoxin A4 may suppress the production/release of a number of cytokines, including IL-6.36,37
Finally, LA has previously been recognized for its benefits in reducing both total and LDL cholesterol,38,39 yet its inconsistent associations with inflammatory markers12–14 make it unclear whether relatively higher plasma levels in the present study population are beneficial in the context of inflammation. Similar to Kalogeropoulos et al.,14 our findings provide evidence for a potential benefit as individuals in the highest tertile of plasma LA had the lowest levels of high-sensitivity C-reactive protein and E-selectin. Overall, further research of omega-6 fatty acids and their metabolism is warranted to more fully explain their putative roles in inflammation and endothelial activation.
Omega-3 PUFAs
As expected, plasma EPA and (EPA+DHA) were found to be inversely associated with inflammation and endothelial activation markers, and previous studies have outlined a host of mechanisms by which EPA may influence inflammatory pathways.40 Apart from these well-reviewed mechanisms, EPA also generates non-classical eicosanoids—among them, the powerful anti-inflammatory E-series resolvins.41. The resolvins were recently discovered and subsequently shown to reduce leukocytes-endothelial interaction, 42 suppress production of inflammatory stimuli,43 and promote the resolution of inflammation.44 In addition, EPA may also directly downregulate inflammatory genes by suppressing nuclear factor-κB activity as shown in cell culture models,45,46 potentially through activation of PPARs.47 Ultimately, it is likely that EPA influences inflammation and endothelial activation through some combination of the above factors. In agreement with a number of previous studies,13,14 we found that plasma levels of DHA were negatively associated with markers of inflammation, IL-6 and TNF-R1. Similar to EPA, DHA generates powerful anti-inflammatory non-classic eicosanoids, including the D-series resolvins, maresins and protectins,48 which may account for the negative correlations observed here.
Strengths and limitations
This study is the largest to date that examines plasma PUFAs relative to selected biomarkers. Plasma PUFAs were measured to avoid the problems of dietary questionnaires, that is, inaccurate self-reporting and assumptions of uniform in vivo fatty acid metabolism. This analysis is limited by the cross-section study design, which eliminates the possibility of determining temporality of relationships. We also examined the effect modification of weight status as determined by BMI—future studies may benefit from measuring visceral fat, that is, central adiposity or percentage of body fat. Based on a previous report,49 we did not include Black participants in sICAM-1 data analysis as a common single nucleotide polymorphism polymorphism in Blacks results in an underestimation of this analyte. Finally, we controlled for factors that may influence inflammation and endothelial activation, but the possibility of residual confounders cannot be excluded.
Conclusions
It has largely been accepted that higher levels of omega-3 PUFAs lead to reduced risk of cardiovascular disease, high plasma EPA and (EPA+DHA) levels with concomitantly lower plasma LA levels appear especially important among obese individuals in reducing the likelihood of high levels of sICAM-1. The current cross-sectional study offers insight, but prospective studies are needed to determine whether higher plasma or cell membrane levels of omega-3 fatty acids and other PUFAs directly influence inflammation and subsequent risk for disease development, particularly in obese individuals. Finally, further study is also warranted to confirm and explain the strong associations of DGLA with inflammatory and endothelial activation markers.
Acknowledgments
We thank the other investigators, staff and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This study was supported by the following contracts, N01-HC-95159 through N01-HC-95169 from the National Heart, Lung and Blood Institute.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Shah PK. Plaque disruption and thrombosis. Potential role of inflammation and infection. Cardiol Clin. 1999;17:271–281. doi: 10.1016/s0733-8651(05)70074-6. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J. 2005;26:1765–1773. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]
- 4.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 5.Cottam DR, Mattar SG, Barinas-Mitchell E, Eid G, Kuller L, Kelley DE, et al. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg. 2004;14:589–600. doi: 10.1381/096089204323093345. [DOI] [PubMed] [Google Scholar]
- 6.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 7.Lindqvist HM, Sandberg AS, Fagerberg B, Hulthe J. Plasma phospholipid EPA and DHA in relation to atherosclerosis in 61-year-old men. Atherosclerosis. 2009;205:574–578. doi: 10.1016/j.atherosclerosis.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 8.GISSI-Prevenzione Investigators. Dietary supplementation with n–3 polyunsaturated fatty acids and vitamin E in 11 324 patients with myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 9.Sun Q, Ma J, Campos H, Rexrode KM, Albert CM, Mozaffarian D, et al. Blood concentrations of individual long-chain omega-3 fatty acids and risk of nonfatal myocardial infarction. Am J Clin Nutr. 2008;88:216–223. doi: 10.1093/ajcn/88.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 11.Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;2:117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- 12.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 13.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 14.Kalogeropoulos N, Panagiotakos DB, Pitsavos C, Chrysohoou C, Rousinou G, Toutouza M, et al. Unsaturated fatty acids are inversely associated and n-6/n-3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clin Chim Acta. 2010;411:584–591. doi: 10.1016/j.cca.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009;81:187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian H, Lu Y, Sherwood AM, Hongqian D, Hong S. Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions. Invest Ophthalmol Vis Sci. 2009;50:3613–3620. doi: 10.1167/iovs.08-3146. [DOI] [PubMed] [Google Scholar]
- 17.Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr. 2001;20:5–19. doi: 10.1080/07315724.2001.10719008. [DOI] [PubMed] [Google Scholar]
- 18.Moussavi N, Gavino V, Receveur O. Could the quality of dietary fat, and not just its quantity, be related to risk of obesity? Obesity (Silver Spring) 2008;16:7–15. doi: 10.1038/oby.2007.14. [DOI] [PubMed] [Google Scholar]
- 19.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119:902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 20.Laaksonen DE, Nyyssönen K, Niskanen L, Rissanen TH, Salonen JT. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med. 2005;165:193–199. doi: 10.1001/archinte.165.2.193. [DOI] [PubMed] [Google Scholar]
- 21.Browning LM, Krebs JD, Moore CS, Mishra GD, O’Connell MA, Jebb SA. The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab. 2007;9:70–80. doi: 10.1111/j.1463-1326.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 23.Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006;52:2265–2272. doi: 10.1373/clinchem.2006.072322. [DOI] [PubMed] [Google Scholar]
- 24.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto K, Sera Y, Abe Y, Tominaga T, Horikami K, Hirao K, et al. High serum concentrations of soluble E-selectin correlate with obesity but not fat distribution in patients with type 2 diabetes mellitus. Metabolism. 2002;51:932–934. doi: 10.1053/meta.2002.33354. [DOI] [PubMed] [Google Scholar]
- 26.Fernández-Real JM, Broch M, Vendrell J, Ricart W. Insulin resistance, inflammation, and serum fatty acid composition. Diabetes Care. 2003;26:1362–1368. doi: 10.2337/diacare.26.5.1362. [DOI] [PubMed] [Google Scholar]
- 27.Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr. 2005;82:1178–1184. doi: 10.1093/ajcn/82.6.1178. [DOI] [PubMed] [Google Scholar]
- 28.Blond JP, Henchiri C, Bézard J. Delta 6 and delta 5 desaturase activities in liver from obese Zucker rats at different ages. Lipids. 1989;24:389–395. doi: 10.1007/BF02535146. [DOI] [PubMed] [Google Scholar]
- 29.Araya J, Rodrigo R, Pettinelli P, Araya AV, Poniachik J, Videla LA. Decreased liver fatty acid delta-6 and delta-5 desaturase activity in obese patients. Obesity (Silver Spring) 2010;18:1460–1463. doi: 10.1038/oby.2009.379. [DOI] [PubMed] [Google Scholar]
- 30.Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 31.Levin G, Duffin KL, Obukowicz MG, Hummert SL, Fujiwara H, Needleman P, et al. Differential metabolism of dihomo-gamma-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: implications for cellular synthesis of prostaglandin E1 and prostaglandin E2. Biochem J. 2002;365:489–496. doi: 10.1042/BJ20011798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iversen L, Fogh K, Kragballe K. Effect of dihomogammalinolenic acid and its 15-lipoxygenase metabolite on eicosanoid metabolism by human mononuclear leukocytes in vitro: selective inhibition of the 5-lipoxygenase pathway. Arch Dermatol Res. 1992;284:222–226. doi: 10.1007/BF00375798. [DOI] [PubMed] [Google Scholar]
- 33.Leslie CC. Regulation of arachidonic acid availability for eicosanoid production. Biochem Cell Biol. 2004;82:1–17. doi: 10.1139/o03-080. [DOI] [PubMed] [Google Scholar]
- 34.Rao NL, Dunford PJ, Xue X, Jiang X, Lundeen KA, Coles F, et al. Anti-inflammatory activity of a potent, selective leukotriene A4 hydrolase inhibitor in comparison with the 5-lipoxygenase inhibitor zileuton. J Pharmacol Exp Ther. 2007;321:1154–1160. doi: 10.1124/jpet.106.115436. [DOI] [PubMed] [Google Scholar]
- 35.Stuhlmeier KM, Kao JJ, Bach FH. Arachidonic acid influences proinflammatory gene induction by stabilizing the inhibitor-kappaBalpha/nuclear factor-kappaB (NF-kappaB) complex, thus suppressing the nuclear translocation of NF-kappaB. J Biol Chem. 1997;272:24679–24683. doi: 10.1074/jbc.272.39.24679. [DOI] [PubMed] [Google Scholar]
- 36.Ji Q, Zhang L, Jia H, Xu J. Pentoxifylline inhibits endotoxin-induced NF-kappa B activation and associated production of proinflammatory cytokines. Ann Clin Lab Sci. 2004;34:427–436. [PubMed] [Google Scholar]
- 37.Sodin-Semrl S, Taddeo B, Tseng D, Varga J, Fiore S. Lipoxin A4 inhibits IL-1 beta-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. J Immunol. 2000;164:2660–2666. doi: 10.4049/jimmunol.164.5.2660. [DOI] [PubMed] [Google Scholar]
- 38.Rassias G, Kestin M, Nestel PJ. Linoleic acid lowers LDL cholesterol without a proportionate displacement of saturated fatty acid. Eur J Clin Nutr. 1991;45:315–320. [PubMed] [Google Scholar]
- 39.Hodson L, Skeaff CM, Chisholm WA. The effect of replacing dietary saturated fat with polyunsaturated or monounsaturated fat on plasma lipids in free-living young adults. Eur J Clin Nutr. 2001;55:908–915. doi: 10.1038/sj.ejcn.1601234. [DOI] [PubMed] [Google Scholar]
- 40.Calder P. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343–352. doi: 10.1007/s11745-003-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serhan CN, Arita M, Hong S, Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39:1125–1132. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- 42.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 44.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo CJ, Chiu KC, Fu M, Lo R, Helton S. Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NF kappa B activity. J Surg Res. 1999;82:216–221. doi: 10.1006/jsre.1998.5524. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Joshi-Barve S, Barve S, Chen LH. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr. 2004;23:71–78. doi: 10.1080/07315724.2004.10719345. [DOI] [PubMed] [Google Scholar]
- 47.Di Nunzio M, Danesi F, Bordoni A. n-3 PUFA as regulators of cardiac gene transcription: a new link between PPAR activation and fatty acid composition. Lipids. 2009;44:1073–1079. doi: 10.1007/s11745-009-3362-y. [DOI] [PubMed] [Google Scholar]
- 48.Norling LV, Serhan CN. Profiling in resolving inflammatory exudates identifies novel anti-inflammatory and pro-resolving mediators and signals for termination. J Intern Med. 2010;268:15–24. doi: 10.1111/j.1365-2796.2010.02235.x. [DOI] [PubMed] [Google Scholar]
- 49.Register TC, Burdon KP, Lenchik L, Bowden DW, Hawkins GA, Nicklas BJ, et al. Variability of serum soluble intercellular adhesion molecule-1 measurements attributable to a common polymorphism. Clin Chem. 2004;50:2185–2187. doi: 10.1373/clinchem.2004.036806. [DOI] [PubMed] [Google Scholar]