Abstract
With the evolution of a relatively large brain size in haplorhine primates (i.e., tarsiers, monkeys, apes and humans), there have been associated changes in the molecular machinery that delivers energy to the neocortex. Here we investigated variation in lactate dehydrogenase (LDH) expression and isoenzyme composition of the neocortex and striatum in primates using quantitative Western blotting and isoenzyme analysis of total homogenates and synaptosomal fractions. Analysis of isoform expression revealed that LDH in the synaptosomal fraction from both forebrain regions shifted towards a predominance of the heart-type, aerobic isoforms, LDHB, among haplorhines as compared to strepsirrhines (i.e., lorises and lemurs), while in total homogenate of neocortex and striatum there was no significant difference in the LDH isoenzyme composition between the primate suborders. The largest increase occurred in synapse-associated LDH-B expression in the neocortex, displaying an especially remarkable elevation in the ratio of LDH-B to LDH-A in humans. The phylogenetic variation in LDH-B to LDH-A ratio was correlated with species typical brain mass, but not encephalization quotient. A significant LDHB increase in the sub-neuronal fraction from haplorhine neocortex and striatum suggests a relatively higher rate of aerobic glycolysis that is linked to synaptosomal mitochondrial metabolism. Our results indicate that there is differential composition of LDH isoenzymes and metabolism in synaptic terminals that evolved in primates to meet increased energy requirements in association with brain enlargement.
Keywords: lactate dehydrogenase, strepsirrhines, haplorhines, brain, metabolism, synaptosome
INTRODUCTION
During the course of primate evolution, changes in metabolic genes have accompanied brain size expansion (Horrobin 1998; Grossman et al. 2001; Grossman et al. 2004; Uddin et al. 2008; Goodman and Sterner 2010). There are several strategies for meeting the energetic demands of a large brain and they can be manifested differentially across taxa (Barrickman and Lin 2010, Navarrete et al. 2011). One metabolic strategy involves greater use of lactate as a neuronal fuel. It has been demonstrated that conversion of lactate to and from pyruvate is governed by specific lactate dehydrogenase (LDH) isoforms, thereby forming a highly adaptable metabolic intermediate system (Brooks 2002; Castro et al. 2009). Furthermore, it has been suggested that the brain's ability to produce and use lactate can be locally regulated by changing LDH-A/LDH-B gene activity ratios and controlling subunit composition of LDH isoenzymes, allowing the brain to optimize use of energy resources and metabolism (Ross et al. 2011).
The subunits of lactate dehydrogenase (LDH; L-lactate:NAD+ oxidoreductase, EC 1.1.1.27) exist as two major structural forms, usually referred to as A and B, respectively, which give rise to five different isozymes of the tetrameric molecule in higher vertebrates. The differences in the properties of the LDH isozymes are dependent on their subunit composition and are most exaggerated between the homotetramers A4 (LDH-5) and B4 (LDH-1).
LDH is a key enzyme in the control of energy metabolism, catalyzing the interconversion of pyruvate to lactate and regulating the levels of these metabolites in accordance with oxygen availability. LDH is therefore at the junction between glycolysis, the metabolic pathway that converts glucose into pyruvate, and the tricarboxylic acid cycle, a series of enzyme-catalyzed chemical reactions that form a key part of aerobic respiration in cells. Two proteins, A (so-called “muscle type”) and B (so-called “heart type”), are the products of the genes LDH-A and LDH-B, respectively, and the LDH enzyme may be composed of the following five isoenzymes: B4, B3A, A2B2, BA3 and A4.
According to the compositional ratio of the two subunits in a tetramer and its five isoenzymes in vivo, total LDH activity exhibits distinct physical and catalytic properties. The high concentrations of pyruvate present in anaerobic tissues can be effectively reduced to lactate by the A4 and A3B1 isoenzymes, while the B4 and B3A1 isoenzymes are inhibited by such high levels of pyruvate. On the other hand, the B4 and B3A1 isoenzymes are able to catalyze efficiently the interconversion of low concentrations of pyruvate and lactate, as present in aerobic tissues, because of their high affinity for these substrates. Pyruvate reconverted from lactate is then further converted to acetyl-CoA and irreversibly committed to entering the citric acid cycle.
The observation of variable LDH isoenzyme distributions in different tissues led to hypotheses regarding possible function (Dawson et al. 1964; Fondy and Kaplan 1965). The two most common isoforms, LDH-A and LDH-B, have overlapping tissue expression across mammals, with LDH-B predominantly expressed in the heart and LDH-A expressed in skeletal muscle (Nisselbaum and Bodansky 1959; Plagemann et al. 1960; Vesell 1961; Vesell and Bearn 1961; Charpentier and Goutefongea 1964; Hinks and Masters 1964; Latner and Skillen 1964; Hinks and Masters 1965; Goodman et al. 1969; Koen and Goodman 1969; Beebee and Carty 1982; Milne and Doxey 1987).
The expression profile of these two isoenzymes in brain also shows marked variation across species and more pronounced differences in certain regions of the brain than in others (Lowenthal et al. 1961; Nisselbaum and Bodansky 1961; Syner and Goodman 1966; Goodman et al. 1969; Koen and Goodman 1969). Among haplorhine primates with relatively large brain sizes, the shift from LDH-A to LDH-B is especially pronounced in the neocortex and corpus callosum as compared to smaller brained strepsirrhines (Goodman et al. 1969). For instance, it was shown that LDH-A is richest in the strepsirrhine Lorisoidea, especially in their cerebral cortical regions, which have 3-4 times more LDH-A than the aerobic B type of LDH, whereas LDH-B is more highly expressed in the cerebral cortex of haplorhine species, including humans, other apes, and monkeys (Lowenthal et al. 1961; Nisselbaum and Bodansky 1961; Syner and Goodman 1966; Goodman et al. 1969; Koen and Goodman 1969).
Furthermore, recent studies on the distribution of LDH-A and LDH-B support the theory of metabolic compartmentalization and have reported that there is selective, or even exclusive, enrichment of LDH-B in neurons and LDH-A in astrocytes (Bittar et al. 1996; Laughton et al. 2000; Pellerin et al. 2003). For the most part, however, these studies have determined expression of RNA message for the A and B subunits, rather than determining the actual level and distribution of the isoenzymes in brain cells.
The current research explores variation among species in LDH isoenzyme expression to determine the pattern of metabolic changes in the evolution of the forebrain of primates. Specifically, we examined LDH isoenzyme expression in the synaptosomal, neuronal enriched fraction, as well as in total homogenates from the neocortex and striatum of different primate species.
MATERIALS AND METHODS
Sources of samples
Frozen brain samples from nonhuman primates were acquired from naturally deceased or humanely euthanized animals from various zoos and research facilities. All protocols were approved by the relevant institutional animal care and use committees or scientific advisory committees. All brains were collected within 14 hours of the animal's death. No pathology was observed in the brain on routine examination and no individual showed neurological symptoms before death. Each brain was stored at −80 °C prior to use in this study. The taxonomic composition and full details of the age and sex distribution of the samples are shown in Table 1. For each individual, two independent tissue samples, approximately 100-150 mg each were dissected from the right parietal cortex and striatum without thawing. Both samples from each region for each individual were processed independently and then used for protein extraction and further analysis. At least two independent analyses were performed on isolated synaptosomes and two pieces of the parietal cortex were taken for each experimental setup and for each biochemical analysis.
Table 1.
Characteristics of primate species, brain regions examined, and methods used
| SPECIES | AGE (years) | SEX | Brain Regions | Experimental methods | ||
|---|---|---|---|---|---|---|
| Western Blot | Isoenzyme Analysis | ELISA | ||||
| Striatum | Yes | Yes | Yes | |||
| Lorises | ||||||
| Red slender loris (Loris tardigradus) | 18.4 | M | Neocortex | Yes | Yes | NO |
| Striatum | NO | NO | NO | |||
| Pygmy slow loris (Nycticebus pygmaeus) | 14 | M | Neocortex | Yes | Yes | NO |
| Striatum | NO | NO | NO | |||
| Pygmy slow loris (Nycticebus pygmaeus) | 10 | M | Neocortex | Yes | Yes | Yes |
| Striatum | Yes | Yes | Yes | |||
| Lemurs | ||||||
| Blue-eyed black lemur (Eulemur flavifrons) | 18.1 | F | Neocortex | Yes | Yes | NO |
| Striatum | NO | NO | NO | |||
| Ring-tailed lemur (Lemur catta) | 15 | F | Neocortex | Yes | Yes | NO |
| Striatum | NO | NO | NO | |||
| Ring-tailed lemur (Lemur catta) | 24 | F | Neocortex | Yes | Yes | Yes |
| New World monkeys | ||||||
| White fronted spider monkey (Ateles belzebuth) | 36 | F | Neocortex | Yes | Yes | NO |
| Striatum | NO | NO | NO | |||
| White-faced saki (Pithecia pithecia) | 10 | F | Neocortex | Yes | Yes | Yes |
| Striatum | Yes | Yes | Yes | |||
| Squirrel monkey (Saimiri sciureus) | 30 | F | Neocortex | Yes | Yes | Yes |
| Striatum | Yes | Yes | Yes | |||
| Old World monkeys | ||||||
| Olive baboon (Papio anubis) | 6.5 | F | Neocortex | Yes | Yes | NO |
| Striatum | NO | NO | NO | |||
| Rhesus macaque (Macaca mulatta) | 23.3 | F | Neocortex | Yes | Yes | Yes |
| Striatum | Yes | Yes | Yes | |||
| Rhesus macaque (Macaca mulatta) | 24.5 | M | Neocortex | Yes | Yes | Yes |
| Striatum | Yes | Yes | Yes | |||
| Rhesus macaque (Macaca mulatta) | 13.2 | M | Neocortex | Yes | Yes | Yes |
| Striatum | Yes | Yes | Yes | |||
| Rhesus macaque (Macaca mulatta) | 15.67 | F | Neocortex | Yes | Yes | Yes |
| Striatum | Yes | Yes | Yes | |||
| Pigtailed macaque (Macaca nemestrina) | 15.6 | M | Neocortex | Yes | Yes | NO |
| Striatum | NO | NO | NO | |||
| Hominoids | ||||||
| Chimpanzee (Pan troglodytes) | 31.2 | M | Neocortex | Yes | Yes | NO |
| Striatum | NO | NO | NO | |||
| Chimpanzee (Pan troglodytes) | 22.4 | F | Neocortex | Yes | Yes | Yes |
| Striatum | Yes | Yes | Yes | |||
| Chimpanzee (Pan troglodytes) | 26.4 | M | Neocortex | Yes | Yes | Yes |
| Striatum | Yes | Yes | Yes | |||
| Chimpanzee (Pan troglodytes) | 22.9 | M | Neocortex | Yes | Yes | Yes |
| Striatum | Yes | Yes | Yes | |||
| Chimpanzee (Pan troglodytes) | 31.2 | M | Neocortex | Yes | Yes | Yes |
| Striatum | NO | NO | NO | |||
| Human (Homo sapiens) | 73 | M | Neocortex | Yes | Yes | NO |
| Striatum | NO | NO | NO | |||
| Human (Homo sapiens) | 78 | F | Neocortex | Yes | Yes | NO |
| Striatum | NO | NO | NO | |||
| Human (Homo sapiens) | 46.4 | F | Neocortex | Yes | Yes | Yes |
| Striatum | Yes | Yes | Yes | |||
| Human (Homo sapiens) | 51.2 | M | Neocortex | Yes | Yes | Yes |
| Striatum | Yes | Yes | Yes | |||
| Human (Homo sapiens) | 49.4 | M | Neocortex | Yes | Yes | Yes |
| Striatum | Yes | Yes | Yes | |||
For statistical analyses, the sample was divided into three phylogenetic groups: strepsirrhines (including lemurs and lorises), “monkeys” (including New World monkeys and Old World monkeys), and hominids (including humans and chimpanzees). Even though the New World and Old World monkeys are paraphyletic, we pooled data from these species for certain statistical analyses in order to increase sample size and because they share a somewhat similar brain size. We also analyzed the results divided into the primate suborders: Strepsirrhines and Haplorhines.
Materials
L-lactate (lithium salt) and all other chemicals were purchased from Sigma Chemical (St. Louis, MO, USA). NativePAGE Novex Bis-tris Gel System kit was purchased from Invitrogen (Carlsbad, CA, USA). All reagents and chemicals were of the highest analytical grade quality. Solutions for the protein assay were purchased from Bio-Rad (Hercules, CA, USA).
Preparation of synaptosomal –enriched fraction
Synaptosomal fractions were prepared from the dissected brain samples by two different methods: (1) sucrose density gradient centrifugation, and (2) synaptic vesicle immunoisolation (data not shown in this manuscript). Detailed procedures for preparation of synaptosome-enriched fractions can be found in the Supplemental Methods. These two experimental protocols were compared mainly on the basis of protein yield after the purification procedure. Synaptosomal purity was assessed by Western blot analysis of the presynaptic marker, synaptophysin (SYP), and the glial marker, glial fibrillary acidic protein (GFAP). To quantify the extent of LDH isoenzymes colocalization, we scanned the films and measured the band densities with Scion Image software (Scion Corp., Frederick, MD, USA).
Isoenzyme analysis: electrophoresis and staining
LDH isoenzyme patterns were determined by previously published methods (Leiblich et al. 2006). Briefly, the LDH isoenzymes in the subcellular fractions from the cerebral cortex samples were separated on NativePAGE Novex Bis-tris Gel System (Invitrogen; Carlsbad, CA, USA). Regions of LDH activity were made visible by using a staining solution containing: 0.1 M sodium lactate, 1.5 mM NAD, 0.1 M Tris-HCl (pH 8.6), 10 mM NaCl, 5 mM MgCl2, 0.03 mg/ml phenazinmethosulphate (PMS) and 0.25 mg/ml nitrobluetetrazolium (NBT). The assay relies on the conversion of lactate to pyruvate, with the production of NADH and H+. The NADH then reduces PMS, which in turn reduces NBT to give an insoluble product, diformazan. Protein extracted from human heart and skeletal muscles served as positive controls and were obtained from Novus Biologicals. LDH isoenzyme bands were identified by comparison of retention time to standards. Individual bands were quantified by densitometry. Values for individual isoenzymes are expressed as the percent of total isoenzyme (the sum of the densitometry of all isoenzymes in that sample), and reflect the mean ± standard deviation (SD) of three gels.
Western blot analysis
Samples obtained from all protocols were analyzed by Western blots with 4-12% SDS-PAGE (Invitrogen, NP0322BOX). Western blot assays were performed as described previously (Sherwood et al., 2010). The following primary antibodies were used for immunoblotting: anti-LDH-A (1/1000, Santa Cruz, sc- 27230), anti-LDH-B (1/500, Novus Biologicals, NB100-79987), and anti-LDH (1/1000, Santa Cruz, sc-133123). Antibodies were characterized in detail before being used for research (see the Supplemental Material). The blots were also probed with antibodies against cell-specific marker proteins: anti-GFAP (glial marker, 1/1000, Santa Cruz, sc-9065) and anti-SYP (presynaptic neuronal marker, 1/1000, Abcam, ab14692). For detection of antibodies, appropriate peroxidase-conjugated secondary antibodies were used in conjunction with enhanced chemiluminescence (Amersham Pharmacia Biosciences) to obtain images saved on film. The signals were quantitatively evaluated with Scion Image software. Equal protein loading was confirmed with anti-β-actin antibody (1/1000, Santa Cruz Biotechnology, sc-1616). Protein bands were scanned using an EPSON Perfection 4870 Photo scanner and quantitatively analyzed by Scion Image software.
Representative Western blots and zymograms
The samples for the representative Western blots as well as zymograms presented in figures were comprised of a mixture of total homogenates pooled from the species represented larger phylogenetic group. To account for blot-to-blot variation in exposure and film development, three concentrations of a blotting standard were loaded onto each gel. The standard comprised a mixture of protein samples from two human inferior frontal gyri. The intensity of the bands for each unknown sample was normalized to this standard and quantified with Scion Image software.
Enzyme-linked immunosorbent assay (ELISA)
For the quantitative determination of LDH-A and LDH-B relative concentrations in total brain homogenates and sub-cellular fractions, the quantitative enzyme immunoassay technique was used with commercially available antibodies against two LDH isoforms. Details of the protocol can be found in Supplemental Methods.
Calculation of aerobic status, the percent value of LDH subunits, and statistical analysis
The LDH-B/LDH-A ratio, or aerobic status (Koen 1969; Koen 1971), was calculated as the densitometry of LDH-B expression divided by LDH-A, adjusted by loading control levels. The percent value of LDH subunits in the zymograms was calculated from the measured intensities of the LDH bands. The nonparametric Mann-Whitney U-test was used to determine significant differences between the strepsirrhine and haplorhine groups. The Kruskal–Wallis one-way analysis of variance test was used to examine differences between LDH expressions in the following primates groups: strepsirrhines, “monkeys”, and apes. PAST statistical software (Hammer et al. 2001) was used for analyses. All data in figures and tables are expressed as mean ± S.D. Pearson correlations were conducted on the optical density values from isoenzyme, Western blot, and ELISA analyses to investigate the relationship of protein expression in fractions of the neocortex and striatum. Statistical significance is reported at α ≤ 0.05.
RESULTS
Expression changes of LDH-A and LDH-B in synaptosomal fractions
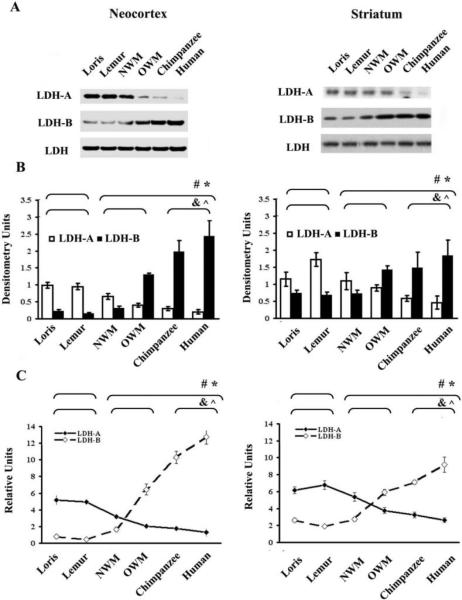
We used Western blotting and ELISA to identify phylogenetic differences in LDH isoform expression profiles in synaptosomal fractions (Fig. 1). The two methods concordantly revealed that LDH-B levels in synaptosomal fractions from the neocortex and striatum varied significantly across species. In contrast, LDH-A levels also varied across species, but showed an opposite pattern of decreasing expression in those species where LDH-B levels were increased. Quantitative Western blot analysis demonstrated that neocortical synaptosomal LDH-B expression level was elevated 2-fold in NWM, 6-fold in OWM, and 12-fold in chimpanzees and humans as compared to strepsirrhines. By contrast, LDH-A level was decreased 1.5-fold in NWM, 2-fold in OWM and 4-fold in chimpanzees and humans relative to strepsirrhines. (Fig. 1A and 1B, left column)
Figure 1.
Comparative analyses of LDH isoenzyme expression levels in synaptosomal fractions from the neocortex and striatum A) A representative immunoblot showing LDH isoenzymes in the neocortical and striatal synaptosomal fractions. An isoform indifferent antibody was utilized to detect the total level of LDH and to serve as an internal loading control. B) The densitometry evaluated level of LDH-A and LDH-B proteins, as determined by Western blot, are shown. C) Bar graphs show relative LDH isoenzymes level as measured by ELISA in neocortical and striatal synaptosomes. * and # symbols indicate P ≤ 0.05 for Mann-Whitney U-test between strepsirrhines and haplorhines for fractional LDH-A and LDH-B levels, respectively. ^ and & indicate P ≤ 0.05 for Kruskal–Wallis test among strepsirrhines, “monkeys”, and chimpanzees and humans for LDH-A and LDH-B, respectively.
In the striatum there were also significant differences in LDH-B and LDH-A synaptosomal protein levels among groups, but the magnitude of this variation was not as remarkable as that observed in the neocortex. LDH-B expression level was elevated approximately 2-fold in OWM, chimpanzees and humans, while LDH-A level was decreased 1.3-fold in NWM, 1.6-fold in OWM, and 2.8-fold in chimpanzees and humans, as compared to strepsirrhines (Fig. 1A and 1B, right column). These results were further corroborated with ELISA (Fig. 1C).
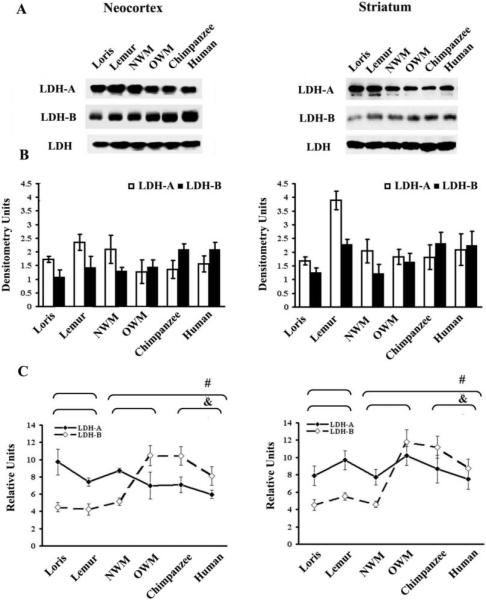
Differences among species were not as pronounced when total tissue homogenates were used in analyses. For example, Western blot analysis of total homogenates from neocortex detected a relatively small difference in LDH-B expression level between the human and chimpanzee groups versus strepsirrhines (1.7-fold increase; P = 0.11, Kruskal–Wallis test) (Fig. 2A and 2B). Higher LDH-B expression level was identified by ELISA in total homogenates of haplorhines neocortex (by 96%) and striatum (by 81%) as compared to strepsirrhine primates (P ≤ 0.05, Mann-Whitney U-tests) (Fig. 2C). Additionally, no significant differences in LDH-A between strepsirrhine and haplorhine groups were found in either neocortex or striatum from total homogenates (P = 0.07 and P = 0.25, Mann-Whitney U-test accordingly for neocortex and striatum) (Fig. 2A and 2B).
Figure 2.
Comparative analyses of LDH isoenzyme expression levels in total homogenates from the neocortex and striatum A) A representative immunoblot showing LDH isoenzymes in the neocortical and striatal total homogenates. An isoform indifferent antibody was utilized to detect the total level of LDH and to serve as an internal loading control. B) The densitometry evaluated level of LDH-A and LDH-B proteins, as determined by Western blot, are shown. C) Bar graphs show relative LDH isoenzymes level as measured by ELISA in neocortical and striatal total homogenates. * and # symbols indicate P ≤ 0.05 for Mann-Whitney U-test between strepsirrhines and haplorhines for fractional LDH-A and LDH-B levels, respectively. ^ and & indicate P ≤ 0.05 for Kruskal–Wallis test among strepsirrhines, “monkeys”, and chimpanzees and humans for LDH-A and LDH-B, respectively.
LDH isoenzyme patterns in the synaptosomal fraction shifted toward aerobic forms of enzyme in the neocortex and striatum of haplorhine primates
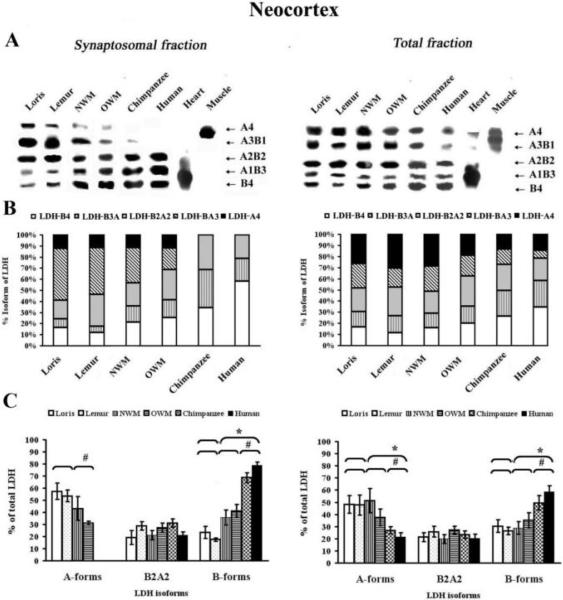
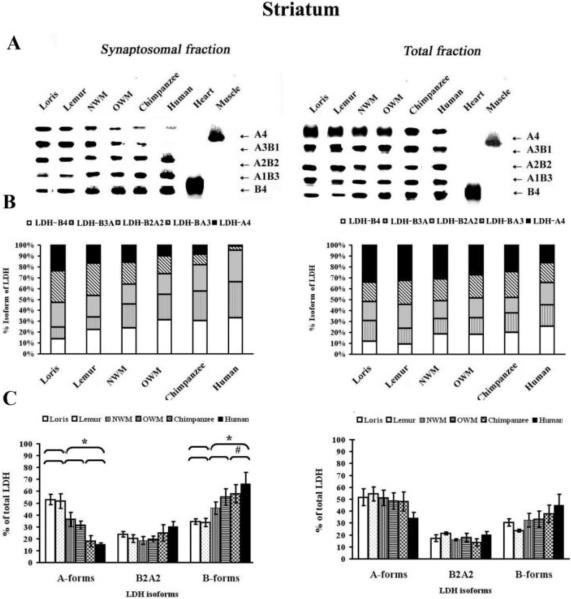
Isoenzyme analysis of synaptosomal fractions and total homogenates revealed five isoenzyme bands corresponding to the five possible tetrameric LDH isoforms (Fig. 3 and 4). Densitometric analysis showed that both aerobic and anaerobic forms were present in synaptosomal fractions from strepsirrhines and New World monkeys and Old World monkeys, whereas the anaerobic forms, LDH-BA3 and LDH-A4, were not found, or found in trace concentrations, in the neocortex (Fig. 3A) and striatum synaptosomes of humans and chimpanzees (Fig. 4A).
Figure 3.
LDH isoenzyme patterns from the synaptosomal neocortical fractions and total homogenates. A) Representative zymogramms from two fractions, synaptosomal and total homogenates isolated from the neocortex. LDH isoenzyme tetrameric composition (right side) indicates the number of A (anaerobic) and B (aerobic) subunits that constitute each isoenzyme. Quantification of the relative amount of protein subunits was achieved by scanning gels as described in experimental procedures. The values obtained by densitometric analysis of the blotted LDH isoenzymes are reported as isoform composition (B) and as proportion of predominantly A or B forms, or A2B2 forms (C). * symbol indicates that the difference in the values of strepsirrhines vs. haplorhines was significant (Mann-Whitney U-test, P ≤ 0.05). # symbol indicates that the Kruskal-Wallis test showed significant differences among the following primates groups: strepsirrhines, “monkeys”, and chimpanzees and humans (P ≤ 0.05).
Figure 4.
LDH isoenzyme patterns from the synaptosomal striatal fractions and total homogenates. A) Representative zymogramms from two fractions, synaptosomal and total homogenates isolated from the primate striatum. LDH isoenzyme tetrameric composition (right side) indicates the number of A (anaerobic) and B (aerobic) subunits that constitute each isoenzyme. Quantification of the relative amount of protein subunits was achieved by scanning gels as described in experimental procedures. The values obtained by densitometric analysis of the blotted LDH isoenzymes are reported as isoform composition (B) and as proportion of predominantly A or B forms, or A2B2 forms (C). * symbol indicates that the difference in the values of strepsirrhines vs. haplorhines was significant (Mann-Whitney U-test, P ≤ 0.05). # symbol indicates that the Kruskal-Wallis test showed significant differences among the following primates groups: strepsirrhines, “monkeys”, and chimpanzees and humans (P ≤ 0.05).
Regional differences were apparent in the levels of synaptosomal LDH isoforms among primate taxa. As expected, synaptosomal fractions from strepsirrhine neocortex (Fig. 3A) predominantly showed expression of the LDH-BA3 (44.3±4.3) isoenzyme, with a weaker expression of LDH-B4 (14.2±2.6) and LDH-B3A1 (6.7±0.9). In contrast, synaptosomal fractions from chimpanzees and humans displayed LDH-B4 (46.3±11.9) and LDH-B3A1 (27.5±6.8) as major forms, with no detectable levels of LDH-A3B1 and LDH-A4 (Fig. 3B). Synaptosomal fractions from both New World and Old World monkeys displayed an equal balance between the percentage of aerobic and anaerobic isoforms (Fig. 3C).
In the synaptosomes derived from the striatum of strepsirrhines, the highest level of expression was for the LDH anaerobic form LDH-B1A3 (29.4±6.3) (Fig. 4A), and the lowest expression was for the LDH aerobic form, LDH-B3A1 (11.0±2.7) (Fig. 4B). LDH isoenzyme composition of chimpanzee and human striatal synaptosome samples showed an increase in the amount of aerobic B forms, LDH-B4 (31.9±5.9) and LDH-B3A1 (29.9±4.2) with a minor amount of anaerobic forms LDH-A3B1 (6.7±0.8) and LDH-A4 (4.9±0.5) (Fig. 4B and 4C).
In total homogenates, the occurrence of the five AB tetramers in all primates was evident, with a predominance of LDH-A4 in strepsirrhine neocortex (28.3±5.2) (Fig. 3) and striatum (33.4±5.5) (Fig. 4). Both New World and Old World monkeys have maintained almost the same level LDH-A4 in the neocortex (22.2±5.6) and striatum (29.3±4.6); chimpanzees and humans, on the other hand, predominantly demonstrated expression of LDH-B4 in neocortex (30.6±5.7) and striatum (22.7±6.2) (Fig. 3 and Fig. 4).
Phylogenetic differences were observed in total neocortical homogenates from chimpanzees and humans for LDH-B4, LDH-B3A (2.1-and 1.6-fold) and LDH-A4 and LDHA3B (2-and 1.9-fold) as compared to strepsirrhines (P ≤ 0.05, Mann-Whitney U-test and P ≤ 0.05, Kruskal–Wallis test accordingly for aerobic and anaerobic LDH isoforms) (Fig. 3). In the striatum, the level of LDH-B4 was increased (1.9-fold) in haplorhines as compared to strepsirrhine primates, but this difference did not reach statistical significance (P = 0.15, Mann-Whitney U-test) (Fig. 4).
The ratios of LDH-B to LDH-A in synaptosomal fractions from neocortex
The polypeptide ratio LDH-B/LDH-A signifies aerobic status (Koen 1969; Koen 1971) and phylogenetic differences in the proportions of two kinds of LDH in primate brain regions were shown previously (Goodman at al. 1969). Here we calculated the polypeptide ratio of single isoforms, LDH-B/LDH-A (Western blot, isoenzyme analysis, and ELISA) for neocortical and striatal brain fractions (see Table 2).
As estimated by these three different methods, LDH-B/LDH-A ratios differed markedly in the synaptosomal neocortical fractions among primate taxa (Table 2). The average ratio, as measured by Western blot, increased 5- to 10-fold across taxa, from 0.195 in strepsirrhines, to 0.484 in NWM, 3.256 in OWM, and 9.194 in chimpanzees and humans. The LDH-B/LDH-A ratio in the synaptosome obtained from the striatum as measured by Western blot also varied 2- to 3- fold with phylogeny, from a ratio of 0.520 in strepsirrhines, to 0.663 in NWM, 1.588 in OWM, and 3.270 in chimpanzees and humans.
Table 2.
Quantification of the relative ratio LDH-B to LDH-A in the primate neocortex and striatum (Western blot, Isoenzyme Analysis, and ELISA of total homogenates and synaptosomal fractions). All data in the tables are expressed as mean ± S.D.
| Species group |
Total homogenate | Synaptosomal fraction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Western Blot | Isoenzyme Analysis | ELISA | Western Blot | Isoenzyme Analysis | ELISA | |||||||
| Neocortex | Striatum | Neocortex | Striatum | Neocortex | Striatum | Neocortex | Striatum | Neocortex | Striatum | Neocortex | Striatum | |
| Lorises | 0.633±0.089 | 0.753±0.073 | 0.631±0.062 | 0.589±0.049 | 0.461±0.052 | 0.571±0.049 | 0.226±0.034 | 0.641±0.076 | 0.408±0.059 | 0.652±0.034 | 0.153±0.019 | 0.428±0.075 |
| Lemurs | 0.608±0.175 | 0.588±0.066 | 0.554±0.057 | 0.435±0.034 | 0.572±0.028 | 0.568±0.054 | 0.164±0.030 | 0.398±0.035 | 0.328±0.031 | 0.652±0.048 | 0.089±0.016 | 0.283±0.032 |
| New World monkeys | 0.619±0.163 | 0.603±0.094 | 0.562±0.075 | 0.635±0.061 | 0.587±0.035 | 0.593±0.080 | 0.484±0.035 | 0.663±0.087 | 0.827±0.080 | 1.260±0.278 | 0.516±0.058 | 0.512±0.061 |
| Old World monkeys | 1.136±0.346 | 0.898±0.097 | 0.944±0.131 | 0.690±0.078 | 1.496±0.364 | 1.155±0.316 | 3.256±0.549 | 1.588±0.101 | 1.324±0.337 | 1.753±0.105 | 3.184±0.251 | 1.572±0.175 |
| Chimpanzee | 1.538±0.308 | 1.283±0.420 | 1.837±0.221 | 0.787±0.138 | 1.472±0.496 | 1.292±0.479 | 6.640±0.768 | 2.541±0.271 | Undefined | 3.209±0.301 | 5.853±0.696 | 2.191±0.426 |
| Human | 1.341±0.351 | 1.078±0.363 | 2.735±0.438 | 1.314±0.335 | 1.355±0.387 | 1.161±0.441 | 11.747±2.570 | 3.998±0.319 | Undefined | 4.264±0.539 | 9.914±1.031 | 3.512±0.436 |
When results from Western blotting and ELISA methods were compared, a high correlation of LDH-B/LDH-A ratio in synaptosomal fractions was obtained from the neocortex (r = 0.998, P = 0.0001) and striatum (r = 0.985, P = 0.0003). It should be noted that we were unable to calculate the ratio of LDH-B to LDH-A by using isoenzyme analysis, due to an inability to determine anaerobic A forms of LDH in the synaptosomal fractions from the neocortex of chimpanzees and humans.
The ratio of LDH-B / LDH-B expression in total homogenates and synaptosomal fractions versus brain size
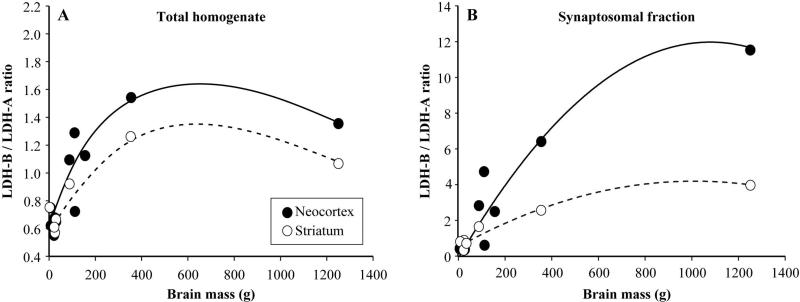
We examined the association between the ratio of LDH-B/LDH-B expression in total homogenates and synaptosomal fractions versus brain size in our comparative sample of primates. For this analysis, we calculated species mean LDH-B/LDH-A ratio based on Western blot and we obtained measures of species average brain mass and encephalization quotient (EQ) from Boddy et al. (2012). For the three individuals in our sample that did not have bran mass or EQ reported in the Boddy et al. (2012) dataset, we used brain masses from other conspecifics that we have in our brain collection and we calculated an average EQ from the congeners in the Boddy et al. (2012) dataset. To model the relationship, we fit separate linear, quadratic, and cubic polynomial regressions to the LDH-B/LDH-A ratio values as a function of brain mass or EQ. Results showed that all three polynomial regressions for LDH-B/LDH-A ratio data against brain mass were significant and quadratic functions had the lowest P values and therefore were selected as the best fit to the data (Fig. 5). The quadratic models for synaptosomal LDH-B/LDHA ratio were more highly correlated with brain mass (neocortex: r2 = 0.925, F2,9 = 55.8, P < 0.001; striatum r2 = 0.970, F2,4 = 65.7, P = 0.001) than the total homogenate values (neocortex: r2 = 0.804, F2,9 = 18.5, P = 0.001; striatum r2 = 0.904, F2,4 = 18.8, P = 0.009). None of the polynomial regressions of any LDH-B/LDH-A ratio data against EQ were significant.
Figure 5.
Analysis of LDH ratio versus brain mass. Graphs show the association between the ratio of LDH-B / LDH-B expression in total homogenates (A) and synaptosomal (B) fractions versus brain mass across primates. A cubic spline is fit to the data from total homogenates and the best fit quadratic function is fit to the data from synaptosomes.
DISCUSSION
Brain tissue has an exceptionally high metabolic rate and, unlike other tissues and organs, cerebral metabolism may not be reduced without causing irreversible brain damage. Thus, it is often asserted that evolutionary increase in brain size requires compensatory metabolic adaptations (Armstrong 1983). Previous studies demonstrated that within the order Primates, there are phylogenetic differences in the relative proportions of LDH isoenzymes among brain regions (Goodman at al. 1969). The current study is the first examination of LDH isoenzyme distribution among different primate species at the neuronal subcellular level in neocortical and striatal tissue.
In humans, the adult brain comprises about 2% of total body weight, but utilizes about 20% of the energy consumed by an individual (Chugani 1987; Jacobs et al. 1995). Prior to adulthood, energy use by the brain is considerably higher, exceeding more than half the body's total energy budget. Keeping the brain supplied with energy is therefore a critical function. Because the proportion of the body's total energetic budget that is allocated to the brain is so large, evolutionary accommodations to alleviate this systemic energy stress are suggested to have occurred, particularly in human evolution where brain size expansion has been extreme (Aiello and Wheeler 1995; Aiello and Wells 2002). Substantial evolution on the haplorhine lineage has taken place in the electron transport chain in the sequence of protein complexes (Grossman et al. 2004) and in their expression level (Uddin et al. 2004). Work on neuron-glia metabolic coupling (Pellerin and Magistretti 2004; Aubert et al. 2005; Pellerin and Magistretti 2012) shows that astrocytes use glucose to produce lactate for release to neurons, which in turn use lactate as their preferred substrate. Our previous work showed that the proportion of glial cells relative to neurons increases in haplorhine primates as overall brain size enlarges, with humans and other apes displaying the highest glia–neuron ratios (Sherwood et al. 2006). Further significant evolutionary changes have taken place through an increase in complexity of the synapse, in terms of the number of its components, the timing of their expression, and the evolution of individual components (Àlex Bayés et al. 2011).
Synaptosomal fractions were analyzed in the current study since there is increasing evidence that lactate is synthesized within astrocytes and oxidative use of lactate for energy is highly localized in synaptic terminals (O'Brien et al. 2007). In our research, synaptosomal fractions were isolated from two regions of the primate forebrain, the neocortex and striatum, and compared with total homogenates from these regions. By using Western blot and ELISA techniques, significant phylogenetic and brain region-specific variation in single LDH-B and LDH-A subunits were observed in synaptic protein-enriched fractions. In particular, the relative enrichment of LDH-B in the synaptosome was detected in a manner that was associated with brain size increase across primate phylogeny.
Differences in the LDH isoenzyme composition of synaptosomes and total homogenates obtained from the primate neocortex and striatum were also determined by isoenzyme analysis. The study of LDH heterogeneity in the total homogenate demonstrated that it consists of five basic LDH isoenzymes: B4, B3A, A2B2, BA3 and A4. By contrast, in synaptosomal fractions all five LDH isoenzymes were detected only in strepsirrhines, New World monkeys and Old World monkeys. In synaptosome-enriched fractions from chimpanzee and humans, LDH-A3B1 was slightly detectable and LDH-A4 was not identified in synaptosomes from the neocortex; in the striatum of chimpanzees and humans only trace amounts of anaerobic LDH isoforms were detected (Fig. 2). Our results are in agreement with previously published results demonstrating selective enrichment of LDH-B in isolated synaptic terminals (McKenna et al. 1993; McKenna et al. 1995; McKenna et al. 1998).
The most pronounced elevation of LDH-B expression was observed in the synaptosomal fractions of chimpanzee and human neocortex, indicating that during evolution an increased demand for efficient energetic allocation has accompanied modifications of the neocortex to a greater extent than for the striatum. This more intensified energetic cost of neocortical synapses in association with brain expansion, relative to those in the striatum, may be due to the larger average somatic size of neocortical neurons as brains get larger (Sherwood et al., 2003; Kreitzer 2009), differences in the intrinsic bioenergetic or metabolic properties of cortical and striatal synaptosomes (Choi et al. 2011), or selectively greater volumetric enlargement of neocortex due to developmental timing (Rapoport 1990, Finlay and Darlington 1995). Further research is needed to characterize the subcellular morphology of neurons in the neocortex and striatum in different primate species that might drive energetic demand.
We have demonstrated the predominance of aerobic isoforms in chimpanzee and human neocortex, while anaerobic isoforms predominate in strepsirrhine neocortex. This suggests that for haplorhine species synaptosomes utilize aerobic respiration as reported previously (Kauppinen and Nicholls 1986; Lores-Arnaiz and Bustamante 2011). This can mostly be explained by the presence of mitochondria in the synaptosomal fraction. It is well known that mitochondria play a key role in meeting the demands of neuronal synapses for energy (ATP) (Lemire et al. 2008). This intimate relationship between lactate and the mitochondria, therefore, likely imparts multiple benefits to a high-energy demanding organ like the brain (Grossman et al. 2001; Herculano-Houzel 2011).
Different brain region-specific cellular mechanisms could be implicated in regulation of intracellular LDH-A and LDH-B localization across primate species that can be exerted at any step in the pathway of LDH isoenzyme gene expression or during protein turnover. LDH subunits are encoded by two separate genes, LDH-A and LDH-B (Bittar et al. 1996), and they are regulated differently and independently (Bunn and Poyton 1996; O'Brien J et al. 2007). First, it is possible that relatively larger neurons in the neocortex of primates with larger brains require specific responses to oxygen availability due to their intrinsic bioenergetic properties involved with propagation of signals across a longer distance (Sherwood et al., 2006). In contrast to the LDH-B gene, the LDH-A gene possesses hypoxia recognition sites in its promoter sequence, responsive to a hypoxia-inducible transcription factor (HIF-1) (Bunn and Poyton 1996) that is known to upregulate LDH-A levels under hypoxic conditions (Semenza et al. 1996). A second mechanism is LDH-B promoter hypermethylation and the consequences of loss of protein expression. Numerous studies have shown that hypermethylation of normally unmethylated CpG dinucleotides located in a gene promoter is associated with gene silencing at the transcriptional level (Gagneux and Varki 2001; Das and Singal 2004). Preliminary data shows differential utilization of conserved CpG methylation of the LDH-B gene between human and dwarf lemur (Duka et al. 2010).
Other key components facilitate lactate uptake and utilization by neurons. One is the high-affinity proton-linked monocarboxylic acid transporter MCT2 that is associated with nerve endings (McKenna et al. 1998; Hertz and Dienel 2005); it is one of a family of 14 that have different substrate affinities and brain distributions (Brooks 2002; Pellerin and Magistretti 2004). Another of this transporter family, MCT1, has been shown to exist in a complex with LDH and a chaperone protein, CD147, in association with COX on the mitochondrial inner membrane (Hashimoto et al. 2006). This finding supports the lactate shuttle and explains the oxidative catabolism of lactate.
LDH has been long known to exhibit different kinetics such that LDH-A more rapidly converts pyruvate to lactate than LDH-B, and LDH-B is more susceptible to inhibition by pyruvate (Dawson et al. 1964). These properties are important in the metabolic support they provide for the operation of the lactate shuttle since pyruvate can be converted to acetyl- CoA, via pyruvate dehydrogenase, which then enters the tricarboxylic acid cycle for aerobic respiration. Conversely, conversion of pyruvate to lactate regenerates NAD+ from NADH, which promotes glycolysis. Thus, the distribution of LDH types reported here can be related to metabolic features of their compartments. It is noteworthy that distinct synaptosome sub-populations are differentially susceptible to bioenergetic failure under conditions of increased energy demand (Choi et al. 2009).
In conclusion, LDH isoforms have distinct expression patterns in neocortex and striatum subcellular fractions of primate species, which is correlated with variation in total brain size. These differences may contribute to divergent lactate dynamics and oxidative capacities in synaptic terminals and may have driven metabolic remodeling from ‘anaerobic-glycolytic’ to ‘aerobic, oxidative phosphorylation enhanced’ metabolism during primate brain evolution.
ACKNOWLEDGMENTS
We thank Dr. Zack Papper for helpful discussion. This work was supported by the National Science Foundation Grant numbers (BCS-0515484, BCS-0549117, BCS-0824531, BCS-0827546, DGE-0801634), National Institutes of Health (NS-42867, RR-00165, HHSN268201100065C) and the James S. McDonnell Foundation (22002078 and 220020293).
Abbreviations
- LDH
lactate dehydrogenase
- NWM
New World monkeys
- OWM
Old World monkeys
- MCT
monocarboxylic acid transporter
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- GFAP
glial fibrilary acidic protein
- SYP
synaptophysin
- PMS
phenazinmethosulphate
- NBT
nitrobluetetrazolium
- PSD
postsynaptic density
- HIF-1
hypoxia-inducible factor 1
REFERENCES
- Aiello LC, Wheeler P. The expensive-tissue hypothesis – the brain and the digestive-system in human and primate evolution. Curr. Anthropol. 1995;36:199–221. [Google Scholar]
- Aiello LC, Wells JCK. Energetics and the evolution of the genus Homo. Ann. Rev. Anthropol. 2002;31:323–338. [Google Scholar]
- Alves PM, McKenna MC, Sonnewald U. Lactate metabolism in mouse brain astrocytes studied by [13C]NMR spectroscopy. Neuroreport. 1995;6:2201–2204. doi: 10.1097/00001756-199511000-00024. [DOI] [PubMed] [Google Scholar]
- Armstrong E. Relative brain size and metabolism in mammals. Science. 1983;220:1302–1304. doi: 10.1126/science.6407108. [DOI] [PubMed] [Google Scholar]
- Aubert A, Costalat R, Magistretti PJ, Pellerin L. Brain lactate kinetics: Modeling evidence for neuronal lactate uptake upon activation. Proc Natl Acad Sci USA. 2005;102:16448–16645. doi: 10.1073/pnas.0505427102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Witzmann FA. Synaptosome proteomics. Subcell. Biochem. 2007;43:77–98. doi: 10.1007/978-1-4020-5943-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrickman NL, Lin MJ. Encephalization, expensive tissues, and energetics: An examination of the relative costs of brain size in strepsirrhines. Am J Phys Anthrop. 2010;143:579–590. doi: 10.1002/ajpa.21354. [DOI] [PubMed] [Google Scholar]
- Bayés A, van de Lagemaat LN, Collins MO, Croning MD, Whittle IR, Choudhary JS, Grant SG. Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nat Neurosci. 2011;14:19–21. doi: 10.1038/nn.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebee TJ, Carty DS. Purification and radioimmunoassay of rat lactate dehydrogenase A and B subunits. Biochem J. 1982;205:313–320. doi: 10.1042/bj2050313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar PG, Charnay Y, Pellerin L, Bouras C, Magistretti PJ. Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J Cereb BloodFlow Metab. 1996;16:1079–1089. doi: 10.1097/00004647-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Boddy AM, McGowen MR, Sherwood CC, Grossman LI, Goodman M, Wildman DE. Comparative analysis of encephalization in mammals reveals relaxed constraints on anthropoid primate and cetacean brain scaling. J Evol Biol. 2012;25:981–994. doi: 10.1111/j.1420-9101.2012.02491.x. [DOI] [PubMed] [Google Scholar]
- Bogin B. Patterns of Human Growth. Cambridge University Press; Cambridge: 1999. [Google Scholar]
- Brandt RB, Laux JE, Spainhour SE, Kline ES. Lactate dehydrogenase in rat mitochondria. Arch Biochem Biophys. 1987;259:412–422. doi: 10.1016/0003-9861(87)90507-8. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Cell-cell and intracellular lactate shuttles. J Physiol. 2009;587:5591–6000. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA. Lactate shuttles in nature. Biochem Soc Trans. 2002;30:258–264. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Castro MA, Beltrán FA, Brauchi S, Concha A metabolic switch in brain: glucose and lactate metabolism modulation by ascorbic acid. J Neurochem. 2009;110:423–440. doi: 10.1111/j.1471-4159.2009.06151.x. [DOI] [PubMed] [Google Scholar]
- Charpentier J, Goutefongea R. Electrophoretic characteristics of lactic dehydrogenase in normal and exudative pig muscle. Nature. 1964;201:1325–1326. doi: 10.1038/2011325a0. [DOI] [PubMed] [Google Scholar]
- Choi SW, Gerencser AA, Nicholls DG. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem. 2009;109:1179–1191. doi: 10.1111/j.1471-4159.2009.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, Gerencser AA, Lee DW, Rajagopalan S, Nicholls DG, Andersen JK, Brand MD. Intrinsic bioenergetic properties and stress sensitivity of dopaminergic synaptosomes. J Neurosci. 2011;31:4524–4534. doi: 10.1523/JNEUROSCI.5817-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT. Positron emission tomography: principles and applications in pediatrics. Mead Johnson Symp Perinat Dev Med. 1987;25:15–18. [PubMed] [Google Scholar]
- Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22:4632–4642. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- Dawson DM, Goodfriend TL, Kaplan NO. Lactic dehydrogenases: functions of the two types rates of synthesis of the two major forms can be correlated with metabolic differentiation. Science. 1964;143:929–933. doi: 10.1126/science.143.3609.929. [DOI] [PubMed] [Google Scholar]
- Duka T, Papper Z, Stimpson CD, Anderson S, Bonar CJ, Raghanti MA, Wildman DE, Sherwood CC. Phylogenetic variation in the methylation of lactate dehydrogenase LDHA and LDHB promoters and isoenzyme expression patterns in the primate cerebral cortex. Abstract Viewer/Itinerary Planner. Society for Neuroscience; San Diego, CA: 2010. [Google Scholar]
- Fang X, Li JJ, Tan W. Using molecular beacons to probe molecular interactions between lactate dehydrogenase and single-stranded DNA. Anal Chem. 2000;72:3280–3285. doi: 10.1021/ac991434j. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Savchenko V, Apparsundaram S, Zwick M, Wright J, Heilman CJ, Yi H, Levey AI, Blakely RD. Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J Neurosci. 2003;23:9697–9709. doi: 10.1523/JNEUROSCI.23-30-09697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Foley RA, Lee PE. Ecology and energetics of encephalization in hominid evolution. Philos Trans R Soc Lod Series B. 1991;334:223–232. doi: 10.1098/rstb.1991.0111. [DOI] [PubMed] [Google Scholar]
- Fondy TP, Kaplan NO. Structural and functional properties of the H and M subunits of lactic dehydrogenases. Ann N Y Acad Sci. 1965;119:888–904. doi: 10.1111/j.1749-6632.1965.tb47450.x. [DOI] [PubMed] [Google Scholar]
- Gagneux P, Varki A. Genetic differences between humans and great apes. Mol Phylogenet Evol. 2001;8:2–13. doi: 10.1006/mpev.2000.0799. [DOI] [PubMed] [Google Scholar]
- Goodman M, Sterner KN. Colloquium paper: phylogenomic evidence of adaptive evolution in the ancestry of humans. Proc Natl Acad Sci U S A. 2010;107:8918–8923. doi: 10.1073/pnas.0914626107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, Syner FN, Stimson CW, Rankin JJ. Phylogenetic changes in the proportions of two kinds of lactate dehydrogenase in primate brain regions. Brain Res. 1969;14:447–459. doi: 10.1016/0006-8993(69)90121-8. [DOI] [PubMed] [Google Scholar]
- Grossman LI, Schmidt TR, Wildman DE, Goodman M. Molecular evolution of aerobic energy metabolism in primates. Mol Phylogenet Evol. 2001;18:26–36. doi: 10.1006/mpev.2000.0890. [DOI] [PubMed] [Google Scholar]
- Grossman LI, Wildman DE, Schmidt TR, Goodman M. Accelerated evolution of the electron transport chain in anthropoid primates. Trends Genet. 2004;20:578–585. doi: 10.1016/j.tig.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Hussien R, Brooks GA. Colocalization of MCT1, CD147 and LDH in mitochondrial inner membrane of L6 muscle cells: Evidence of a mitochondrial lactate. Am J Physiol Endocrinol Metab. 2006;290:1237–1244. doi: 10.1152/ajpendo.00594.2005. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S. Scaling of brain metabolism with a fixed energy budget per neuron: implications for neuronal activity, plasticity and evolution. PLoS One. 2011;6:1–9. doi: 10.1371/journal.pone.0017514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L. The astrocyte-neuron lactate shuttle: a challenge of a challenge. J Cereb Blood Flow Metab. 2004;24:1241–1248. doi: 10.1097/00004647-200411000-00008. [DOI] [PubMed] [Google Scholar]
- Hertz L, Dienel GA. Lactate transport and transporters: general principles and functional roles in brain cells. J Neurosci Res. 2005;79:11–18. doi: 10.1002/jnr.20294. [DOI] [PubMed] [Google Scholar]
- Hinks M, Masters CJ. Developmental changes in ruminant lactate dehydrogenase. Biochemistry. 1964;3:1789–1791. doi: 10.1021/bi00899a035. [DOI] [PubMed] [Google Scholar]
- Hinks M, Masters CJ. The epigenetic control of lactate-dehydrogenase biosynthesis. Life Sci. 1965;4:679–703. doi: 10.1016/0024-3205(65)90008-1. [DOI] [PubMed] [Google Scholar]
- Hoogland G, Blomenröhr M, Dijstelbloem H, de Wit M, Spierenburg HA, van Veelen CW, van Rijen PC, van Huffelen AC, Gispen WH, de Graan PN. Characterization of neocortical and hippocampal synaptosomes from temporal lobe epilepsy patients. Brain Res. 1999;837:55–66. doi: 10.1016/s0921-4534(99)00331-7. [DOI] [PubMed] [Google Scholar]
- Horrobin DF. Schizophrenia: the illness that made us human. Med Hypotheses. 1998;50:269–288. doi: 10.1016/s0306-9877(98)90000-7. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Chugani HT, Allada V, Chen S, Phelps ME, Pollack DB, Raleigh MJ. Developmental changes in brain metabolism in sedated rhesus macaques and vervet monkeys revealed by positron emission tomography. Cereb Cortex. 1995;5:222–233. doi: 10.1093/cercor/5.3.222. [DOI] [PubMed] [Google Scholar]
- Jeannotte AM, Sidhu A. Regulation of the norepinephrine transporter by alpha-synuclein-mediated interactions with microtubules. Eur J Neurosci. 2007;26:1509–1520. doi: 10.1111/j.1460-9568.2007.05757.x. [DOI] [PubMed] [Google Scholar]
- Kauppinen RA, Nicholls DG. Synaptosomal bioenergetics. The role of glycolysis, pyruvate oxidation and responses to hypoglycaemia. Eur J Biochem. 1986;158:159–165. doi: 10.1111/j.1432-1033.1986.tb09733.x. [DOI] [PubMed] [Google Scholar]
- Kaufman AM, Milnerwood AJ, Sepers MD, Coquinco A, She K, Wang L, Lee H, Craig AM, Cynader M, Raymond LA. Opposing roles of synaptic and extrasynaptic NMDA receptor signaling in cocultured striatal and cortical neurons. J Neurosci. 2012;32:3992–4003. doi: 10.1523/JNEUROSCI.4129-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci. 2005;30:142–150. doi: 10.1016/j.tibs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Kline ES, Brandt RB, Laux JE, Spainhour SE, Higgins ES, Rogers KS, Tinsley SB, Waters MG. Localization of L-lactate dehydrogenase in mitochondria. Arch Biochem Biophys. 1986;246:673–680. doi: 10.1016/0003-9861(86)90323-1. [DOI] [PubMed] [Google Scholar]
- Knull HR, Fillmore SJ. Glycolytic enzyme levels in synaptosomes. Comp Biochem Physiol B. 1985;81:349–351. doi: 10.1016/0305-0491(85)90324-4. [DOI] [PubMed] [Google Scholar]
- Koen AL, Goodman M. Lactate dehydrogenase isozymes: qualitative and quantitative changes during primate evolution. Biochemical Genetics. 1969;3:457–447. doi: 10.1007/BF00485606. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC. Physiology and Pharmacology of Striatal Neurons. Annu Rev Neurosci. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Latner AL, Skillen AW. Lactate dehydrogenase isoenzymes in foetal and neonatal tissues. J Embryol Exp Morphol. 1964;12:501–509. [PubMed] [Google Scholar]
- Laughton JD, Charnay Y, Belloir B, Pellerin L, Magistretti PJ, Bouras C. Differential messenger RNA distribution of lactate dehydrogenase LDH-1 and LDH-5 isoforms in the rat brain. Neuroscience. 2000;96:619–625. doi: 10.1016/s0306-4522(99)00580-1. [DOI] [PubMed] [Google Scholar]
- Leiblich A, Cross SS, Catto JW, Phillips JT, Leung HY, Hamdy FC, Rehman I. Lactate dehydrogenase-B is silenced by promoter hypermethylation in human prostate cancer. Oncogene. 2006;25:2953–2960. doi: 10.1038/sj.onc.1209262. [DOI] [PubMed] [Google Scholar]
- Lemire J, Mailloux RJ, Appanna VD. Mitochondrial lactate dehydrogenase is involved in oxidative-energy metabolism in human astrocytoma cells (CCF-STTG1). PLoS One. 2008;3:e1550. doi: 10.1371/journal.pone.0001550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W, Robertson M. Nutritional requirements and human evolution: A bioenergetics model. Am J Hum Bio. 1992;4:179–195. doi: 10.1002/ajhb.1310040204. [DOI] [PubMed] [Google Scholar]
- Leonard W, Robertson M. Evolutionary perspectives on human nutrition: The influence of brain and body size on diet and metabolism. Amer J Hum Bio. 1994;6:77–88. doi: 10.1002/ajhb.1310060111. [DOI] [PubMed] [Google Scholar]
- Leonard WR, Robertson ML, Snodgrass JJ, Kuzawa CW. Metabolic correlates of hominid brain evolution. Comparative biochemistry and physiology. 2003;136:5–15. doi: 10.1016/s1095-6433(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Li RC, Lee SK, Pouranfar F, Brittian KR, Clair HB, Row BW, Wang Y, Gozal D. Hypoxia differentially regulates the expression of neuroglobin and cytoglobin in rat brain. Brain Res. 2006;1096:173–179. doi: 10.1016/j.brainres.2006.04.063. [DOI] [PubMed] [Google Scholar]
- Lores-Arnaiz S, Bustamante J. Age-related alterations in mitochondrial physiological parameters and nitric oxide production in synaptic and non-synaptic brain cortex mitochondria. Neuroscience. 2011;188:117–124. doi: 10.1016/j.neuroscience.2011.04.060. [DOI] [PubMed] [Google Scholar]
- Lowenthal A, Van Sande M, Karcher D. Heterogeneity of lactic and malic dehydrogenase in serum, cerebrospinal fluid, and brain extracts in man and sheep. Ann N Y Acad Sci. 1961;94:988–995. doi: 10.1111/j.1749-6632.1961.tb35590.x. [DOI] [PubMed] [Google Scholar]
- Martin RD. Relative brain size and basal metabolic rate in terrestrial vertebrates. Nature. 1981;293:57–60. doi: 10.1038/293057a0. [DOI] [PubMed] [Google Scholar]
- McGowen MR, Montgomery SH, Clark C, Gatesy J. Phylogeny and adaptive evolution of the brain-development gene microcephalin (MCPH1) in cetaceans. BMC Evol Biol. 2011;11:98. doi: 10.1186/1471-2148-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC, Tildon JT, Stevenson JH, Boatright R, Huang S. Regulation of energy metabolism in synaptic terminals and cultured rat brain astrocytes: differences revealed using aminooxyacetate. Dev Neurosci. 1993;15:320–329. doi: 10.1159/000111351. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Tildon JT, Stevenson JH, Hopkins IB, Huang X, Couto R. Lactate transport by cortical synaptosomes from adult rat brain: characterization of kinetics and inhibitor specificity. Dev Neurosci. 1998;20:300–309. doi: 10.1159/000017325. [DOI] [PubMed] [Google Scholar]
- Milne EM, Doxey DL. Lactate dehydrogenase and its isoenzymes in the tissues and sera of clinically normal dogs. Res Vet Sci. 1987;43:222–224. [PubMed] [Google Scholar]
- Navarrete A, van Schaik CP, Isler K. Energetics and the evolution of human brain size. Nature. 2011;480:91–93. doi: 10.1038/nature10629. [DOI] [PubMed] [Google Scholar]
- Phelan P, Gordon-Weeks PR. Isolation of synaptosomes, growth cones, and their subcellular components. In: Turner AJ, Bachelard HS, editors. Neurochemistry: a practical approach. Oxford University Press; Oxford: 1997. pp. 1–38. [Google Scholar]
- Nisselbaum JS, Bodansky O. Reactions of lactic dehydrogenase from various rabbit organs with antirabbit muscle lactic dehydrogenase. J Biol Chem. 1959;234:3276–3280. [PubMed] [Google Scholar]
- Nisselbaum JS, Bodansky O. Purification and properties of human heart lactic dehydrogenase. J Biol Chem. 1961;236:323–327. [PubMed] [Google Scholar]
- O'Brien J, Kla KM, Hopkins IB, Malecki EA, McKenna MC. Kinetic parameters and lactate dehydrogenase isozyme activities support possible lactate utilization by neurons. Neurochemical Res. 2007;32:597–607. doi: 10.1007/s11064-006-9132-9. [DOI] [PubMed] [Google Scholar]
- Øyvind Hammer Ø , Harper DAT, Ryan PD. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica. 2001;4:1–9. [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L. Lactate as a pivotal element in neuron-glia metabolic cooperation. Neurochem Int. 2003;43:331–338. doi: 10.1016/s0197-0186(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. How to balance the brain energy budget while spending glucose differently. J Physiol. 2003;546:325. doi: 10.1113/jphysiol.2002.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist. 2004;10:53–62. doi: 10.1177/1073858403260159. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Larsen KE, Behr GG, Romero N, Przedborski S, Brundin P, Sulzer D. Expanded CAG repeats in exon 1 of the Huntington's disease gene stimulate dopamine-mediated striatal neuron autophagy and degeneration. Hum Mol Genet. 2001;10:1243–1254. doi: 10.1093/hmg/10.12.1243. [DOI] [PubMed] [Google Scholar]
- Plagemann PG, Gregory KF, Wroblewski F. The electrophoretically distinct forms of mammalian lactic dehydrogenase. J Biol Chem. 1960;235:2282–2287. [PubMed] [Google Scholar]
- Rapoport SI. Integrated phylogeny of the primate brain, with special reference to humans and their diseases. Brain Res Brain Res Rev. 1990;15:267–294. doi: 10.1016/0165-0173(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Ross JM, Öberg J, Brené S, Coppotelli G, Terzioglu M, Pernold K, Goiny M, Sitnikov R, Kehr J, Trifunovic A, Larsson NG, Hoffer BJ, Olson L. High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc Natl Acad Sci U S A. 2010;107:20087–20092. doi: 10.1073/pnas.1008189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Bahn S. Functional genomics and proteomics in the clinical neurosciences. Elsevier; 2006. [Google Scholar]
- Schulte PM. Environmental adaptations as windows on molecular evolution. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:597–611. doi: 10.1016/s1096-4959(00)00357-2. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Duka T, Stimpson CD, Schenker NM, Garrison AR, Schapiro SJ, Baze WB, McArthur MJ, Erwin JM, Hof PR, Hopkins WD. Neocortical synaptophysin asymmetry and behavioral lateralization in chimpanzees (Pan troglodytes). Eur J Neurosci. 2010;31:1456–1464. doi: 10.1111/j.1460-9568.2010.07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, Hof PR. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci U S A. 2006;103:13606–13611. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeal RM, Keefe KA, Wilcox KS. Differences in excitatory transmission between thalamic and cortical afferents to single spiny efferent neurons of rat dorsal striatum. Eur J Neurosci. 2008;28:2041–2052. doi: 10.1111/j.1460-9568.2008.06505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syner FN, Goodman M. Differences in the lactic dehydrogenases of primate brains. Nature. 1966;209:426–428. doi: 10.1038/209426a0. [DOI] [PubMed] [Google Scholar]
- Uddin M, Opazo JC, Wildman DE, Sherwood CC, Hof PR, Goodman M, Grossman LI. Molecular evolution of the cytochrome c oxidase subunit 5A gene in primates. BMC Evol Biol. 2008;8:8. doi: 10.1186/1471-2148-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Wildman DE, Liu G, Xu W, Johnson RM, Hof PR, Kapatos G, Grossman LI, Goodman M. Sister grouping of chimpanzees and humans as revealed by genome-wide phylogenetic analysis of brain gene expression profiles. Proc Natl Acad Sci U S A. 2004;101:2957–2962. doi: 10.1073/pnas.0308725100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesell ES. Significance of the heterogeneity of lactic dehydrogenase activity in human tissues. Ann N Y Acad Sci. 1961;94:877–889. doi: 10.1111/j.1749-6632.1961.tb35581.x. [DOI] [PubMed] [Google Scholar]
- Vesell WS, Bearn G. Isozymes of lactic dehydrogenase in human tissues. J Clin Invest. 1961;40:586–591. doi: 10.1172/JCI104287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KR, Reddigari S, Patel GL. Identification of a nucleic acid helixdestabilizing protein from rat liver as lactate dehydrogenase-5. Proc. Natl. Acad. Sci. USA. 1985;82:5260–5264. doi: 10.1073/pnas.82.16.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Xia X, Kone BC. Expression profile of a human inducible nitric oxide synthase promoter reporter in transgenic mice during endotoxemia. Am J Physiol Renal Physiol. 2005;288:214–220. doi: 10.1152/ajprenal.00258.2004. [DOI] [PubMed] [Google Scholar]
- Yura A, Kiuchi Y, Uchikawa T, Uchida J, Yamazaki K, Oguchi K. Possible involvement of calmodulin-dependent kinases in Ca(2+)-dependent enhancement of [3H]5-hydroxytryptamine uptake in rat cortex. Brain Res. 1996;738:96–102. doi: 10.1016/0006-8993(96)00762-7. [DOI] [PubMed] [Google Scholar]
- Zhong XH, Howard BD. Phosphotyrosine-containing lactate dehydrogenase is restricted to the nuclei of PC12 pheochromocytoma cells. Mol Cell Biol. 1990;10:770–776. doi: 10.1128/mcb.10.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]