Abstract
Hereditary tyrosinemia type I (HT1) is caused by deficiency in fumarylacetoacetate hydrolase (FAH), an enzyme that catalyzes the last step of tyrosine metabolism. The most severe form of the disease presents acutely during infancy, and is characterized by severe liver involvement, most commonly resulting in death if untreated. Generation of FAH+/− pigs was previously accomplished by adeno-associated virus-mediated gene knockout in fibroblasts and somatic cell nuclear transfer. Subsequently, these animals were outbred and crossed to produce the first FAH−/− pigs. FAH-deficiency produced a lethal defect in utero that was corrected by administration of 2-(2-nitro-4-trifluoromethylbenzyol)-1,3 cyclohexanedione (NTBC) throughout pregnancy. Animals on NTBC were phenotypically normal at birth; however, animals were euthanized approximately four weeks after withdrawal of NTBC due to clinical decline and physical examination findings of severe liver injury and encephalopthy consistent with acute liver failure. Biochemical and histological analyses, characterized by diffuse and severe hepatocellular damage, confirmed the diagnosis of severe liver injury. FAH−/− pigs provide the first genetically engineered large animal model of a metabolic liver disorder. Future applications of FAH−/− pigs include discovery research as a large animal model of HT1 and spontaneous acute liver failure, and preclinical testing of efficacy of liver cell therapies, including transplantation of hepatocytes, liver stem cells, and pluripotent stem cell-derived hepatocytes.
Keywords: inborn error of metabolism, hepatocyte, porcine, fumarylacetoacetate, succinylacetone
Section 1: Introduction
1.1
Hereditary tyrosinemia type I (HT1; OMIM #276700) is an autosomal-recessive inborn error of metabolism caused by deficiency in fumarylacetoacetate hydrolase (FAH), an enzyme that catalyzes the last step of tyrosine metabolism.[1–4] Absence of FAH causes accumulation of the toxic metabolite fumarylacetoacetate (FAA) in hepatocytes and renal proximal tubules, the two major cell types that express FAH.[5–7] Clinically, individuals with HT1 commonly develop symptoms within the first few weeks of life; however, presentation is often variable, even within a family.[3] Acute onset of HT1 is characterized by severe liver involvement,[8] most frequently leading to death, if untreated. The most common treatment for HT1 is a low-tyrosine diet combined with administration of 2-(2-nitro-4-trifluoromethylbenzyol)-1,3 cyclohexanedione (NTBC)[9], a potent inhibitor of 4-hydroxyphenylpyruvate dioxygenase (Fig. S1).
1.2
Two Fah-knockout mouse models have been previously described: the c14CoS albino mouse and the FahΔexon5 mouse.[10–12] Fah-knockout mice have proven a tremendous resource for translational research related to treatment of a metabolic liver disease by various cell and gene therapy approaches.[13–17] However, as has been demonstrated elegantly by the creation of cystic fibrosis transmembrane conductance regulator (CFTR) knockout pigs,[18] the pig is a more appropriate research model because of its similarity in size, anatomy, and biology to the human.[19] We have previously reported the generation and characterization of heterozygous FAH+/− pigs[20] by using adeno-associated virus (AAV) and homologous recombination to target and disrupt the porcine FAH gene, located on chromosome 7 in the pig genome. An AAV vector was used to deliver a knockout construct targeted to exon 5 of FAH fetal pig fibroblasts with an average knockout targeting frequency of 5.4% achieved. Targeted FAH+/− fibroblasts were used as nuclear donors for somatic cell nuclear transfer (SCNT) to porcine oocytes, and multiple viable FAH+/− pigs were born. FAH+/− pigs were phenotypically normal, but had decreased FAH transcriptional and enzymatic activity compared to FAH+/+ animals. Therefore, the goal of this study was to generate and characterize FAH−/− pigs in order to develop a more relevant preclinical model of an inborn error of metabolism than currently exists.
Section 2: Materials and methods
2.1. Animals and animal care
FAH−/− pigs were produced in a 50% Large White 50% Landrace pig. All animals received humane care in compliance with the regulations of the Institutional Animal Care and Use Committee at Mayo Clinic. All animals were observed at least daily for clinical signs and symptoms consistent with HT1 and acute liver failure. All animals were weighed daily until reaching a weight of 20 kg; animals were subsequently weighed twice weekly. NTBC (Yecuris, Portland, OR) was administered to the animals orally mixed within a portion of daily chow rations. Pregnant sows were given 50–100mg of NTBC per day for the duration of gestation. Weaned piglets were administered 1mg/kg NTBC per day until day 30. The animals were observed until they consumed the entire portion of medicated food.
2.2. PCR genotyping
Pig tissue, from ear or tail, was added to 100μl of lysis buffer (stock lysis solution = 440μl 0.01% SDS; 40μl 10mg/ml proteinase K; 20μl 0.5M EDTA). Following a 90-min incubation at 50°C and 30-min incubation at 95°C, 0.5μl of the lysed tissue were used for each 25μl PCR reaction using BIOLASE DNA Polymerase (Bioline, Taunton, MA) with the following three primers: WT-F: TTTCCTCCGCAGGTGACTAC; MUT-F: GGGAGGATTGGGAAGACAAT; R- GACAACATGCTGCTGGACAC. PCR conditions were as follows: 94°C for 5 min; 35 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s; 72°C for 5 min. PCR generated products of either 168bp (wild-type allele) or 232bp (mutant allele), which were electrophoresed on a 2.5% TAE agarose gel and visualized with ethidium bromide staining.
2.3. FAH protein assays
For western blot analysis, liver samples were homogenized in cell lysis buffer (Cell Signaling, Danvers, MA) and isolated total protein separated by SDS-PAGE, followed by immunoblotting onto a polyvinylidene fluoride membrane (TransBlot Turbo, BioRad, Hercules, CA). The primary antibodies against FAH[21] and beta-Actin (#4970; Cell Signaling, Danvers, MA) were detected with a secondary HRP conjugated anti-rabbit antibody (Cell Signaling, Danvers, MA), and imaged using a chemiluminescent substrate for detection of HRP (Thermo Scientific, Waltham, MA). FAH enzyme assays were carried out on a cytosolic fraction of homogenized liver as described previously.[12] Absorbance at 330nm was measured every 15 sec by spectrophotometry after additional of fumarylacetoacetate substrate.
2.4. Histopathological analysis
For liver and kidney analysis, tissue samples were fixed in 10% neutral buffered formalin (Protocol, Fisher-Scientific, Pittsburgh, PA) and processed for paraffin embedding and sectioning. For hematoxylin and eosin staining, as well as periodic acid-Schiff staining, slides were prepared using standard protocols. FAH immunohistochemistry using a polyclonal rabbit anti-FAH primary antibody[21] was performed with a Bond III automatic stainer (Leica, Buffalo Grove, IL) with a 20 min antigen retrieval step using Bond Epitope Retrieval Solution 2 (Leica, Buffalo Grove, IL) and stained with diaminobenzidine (Leica, Buffalo Grove, IL).
2.5. Blood analysis
Blood was obtained via the right femoral vein using an ultrasound guided percutaneous technique on a monthly basis and juts prior to euthanasia. Serum and plasma were separated for analysis using standard protocols. For amino acid analysis, blood samples were collected and dried on 903 Protein Saver Cards (GE Healthcare, Pittsburgh, PA). Amino acids and succinylacetone were measured in dried blood spots by tandem mass spectrometry as previously described.[22]
2.6. NTBC kinetics
Three wild-type pigs were fed an oral 1mg/kg dose of NTBC. Blood samples were collected every 12 hours and plasma separated for analysis prior to freezing at −20°C. Plasma samples were thawed and vortexed at room temperature. A 100 μL aliquot was removed from each sample and combined with 25 μL of water:acetonitrile (1:1) and 300 μL of internal standard (200 ng/mL of d4-hydroxycoumarin prepared in acetonitrile). A standard curve and quality control samples were similarly prepared with commercially available pig plasma by spiking 25 μL of NTBC (in 1:1 water:acetonitrile) into 100 μL plasma over a range of 2.5 to 2,500 ng/mL. The samples were vortexed for 5 min and centrifuged at 4,000 rpm for 10 min at 10°C. 200 μL of the supernatant was transferred to a new plate and diluted with 100 μL of water:acetonitrile (1:1) and vortexed prior to analysis. Extracted plasma samples were analyzed by LC/MS/MS with a triple quadrupole mass spectrometer in negative electrospray ionization mode. A multiple reaction monitoring method containing mass transitions appropriate for both analyte and internal standard was developed. Reversed phase HPLC was performed with a Waters Atlantis dC18 column (100 × 2.1 mm, 5 micron) using a linear binary gradient of mobile phase A (0.1 mM ammonium acetate in water:methanol (95:5)) and mobile phase B (0.1 mM ammonium acetate in methanol) at a flow rate of 0.750 mL/min. Initial conditions were 30%B, held for 0.20 min then changed to 98%B over 1.80 min and held for 0.50 min. Column re-equilibration was attained by returning to initial conditions for 0.50 min.
Section 3: Results
3.1. NTBC administration during gestation rescues in utero lethality in FAH−/− pigs
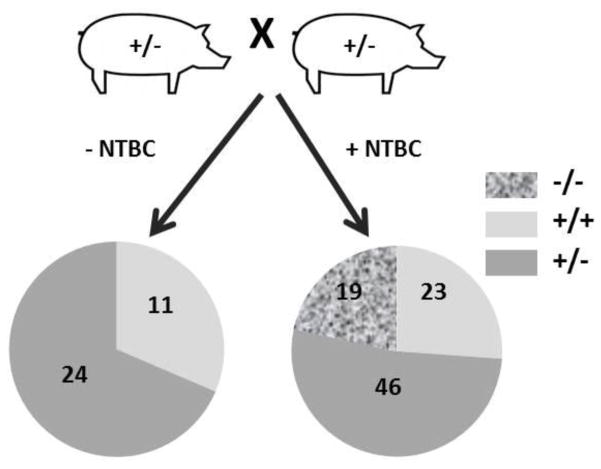
Female FAH+/− pigs, previously generated by SCNT, were allowed to develop to maturity prior to breeding with wild-type male pigs to generate male and female FAH+/− pigs. Upon further out-crossing to wild-type pigs to generate genetic diversity in the herd, male and female FAH+/− pigs were bred together to potentially produce the first FAH−/− pigs (Fig. 1). Surprisingly, in the absence of NTBC during gestation, no FAH−/− piglets were born. Next, females were put on NTBC prior to conception and for the duration of pregnancy (approximately 115 days). Pregnant sows were allowed to farrow naturally by vaginal birth. NTBC administration throughout pregnancy rescued the in utero lethality of FAH deficiency as multiple healthy FAH−/− piglets were born within the predicted Mendelian ratio (Fig. 1).
Fig. 1. FAH-deficiency is an in utero lethal defect in the absence of NTBC.
FAH+/− male and female pigs were bred together, with our without administration of NTBC in the food. No FAH−/− piglets were born in the absence of NTBC administration. 19 out of 88 piglets were FAH−/− when NTBC was administered to the sow throughout pregnancy.
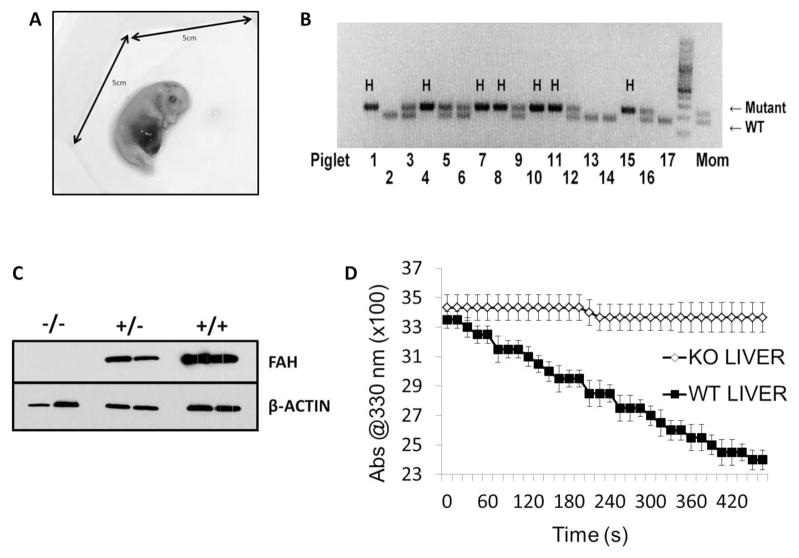
As no remnant or mummified fetuses were delivered by sows not on NTBC, we hypothesized that FAH-deficiency was causing in utero lethality prior to day 35 of gestation.[23] To test this, a pregnant sow on NTBC was euthanized at 30 days of gestation and fetuses were collected for molecular analyses (Fig. 2A). Of the 17 fetuses recovered, 7 were FAH−/− as determined by PCR genotyping (Fig. 2B). Additionally, liver tissue was collected from all animals and FAH expression tested by Western blot analysis, revealing FAH was robustly expressed at the protein level at day 30 of gestation in FAH+/+ and FAH+/− fetal pigs, but not expressed in FAH−/− fetal pigs (Fig. 2C). Finally, in order to categorically rule out presence of another enzyme that may act in a compensatory mechanism in pigs to catalyze the breakdown of FAA, a standard FAH enzyme assay was performed on liver homogenates using FAA as a substrate. Consistent with the western blot analysis, FAA was able to be metabolized with associated decrease in absorbance at 330nm in wild-type siblings but not in FAH−/− animals (Fig. 2D).
Fig. 2. FAH is expressed early during pig fetal development.
(A) Image of fetus harvested at day 30 of gestation. (B) A three-primer PCR assay was deigned to genotype all piglets based on the presence or absence of a wild-type (WT) or mutant FAH allele. In this litter of 17 piglets, seven were FAH−/− (H=Homozygote for mutant allele), four were FAH+/−, and six were FAH+/+. (C) Western blot analysis using an FAH antibody was performed to confirm absence of FAH protein in FAH−/− liver homogenate compared to FAH+/− and FAH+/+ pigs. A β-actin antibody was used as a loading control for all samples. (D) An FAH enzyme assay was performed on FAH−/− and FAH+/+ liver homogenates (n=3 for each genotype). FAH−/− liver homogenate was unable to break down FAA, as measured by a decrease in absorbance at 330 nm, indicating absence of FAH activity.
3.2. Effect of NTBC withdrawal in FAH−/− pigs
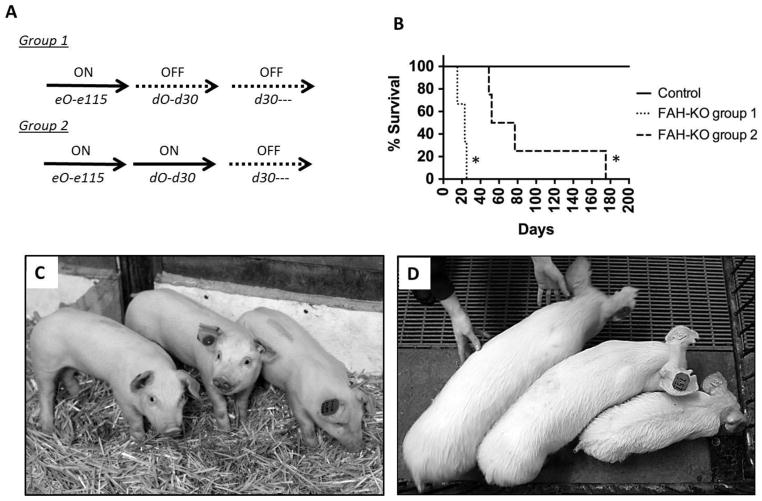
In humans, NTBC has been reported to have an estimated half-life in serum of 54 hours.[24] As NTBC kinetics were unknown in pigs, we tested the half-life of NTBC after a single administration of 1mg/kg in three pigs and determined the half-life to be 20.9 hours (Fig. S2). We next set out to test the effect of NTBC withdrawal in two groups of FAH−/− piglets (Fig. 3A). In the first group of animals, no NTBC was administered to either the piglets or to their nursing sow after birth. These three animals, experienced severe failure to thrive and rapidly succumbed to complications of acute liver failure including severe hypoglycemia, coagulopathy, encephalopathy and infection by 25 days of age (Fig. 3B). In the second group of pigs, four piglets received 1mg/kg NTBC through day 30 of life. NTBC was removed from the diet at this later time point to determine the effect of FAH-deficiency in weaned pigs. In three of these animals, weight gain ceased within seven days of NTBC withdrawal, and was accompanied by a progressive failure to thrive phenotype (Fig. 3, C and D). These three animals were euthanized 19, 22 and 47 days after NTBC withdrawal (49, 52 and 77 days after birth) due to similar clinical findings of hypoglycemia, coagulopathy, and encephalopathy as seen in the Group 1 animals. One animal (L795) continued to slowly gain weight for 112 days prior to cessation of weight gain (Fig. S3). However, despite the modest weight gain, this animal demonstrated signs of encephalopathy and was euthanized 145 days after NTBC withdrawal due to an upper gastrointestinal hemorrhage.
Fig. 3. FAH−/− pigs off NTBC undergo rapid clinical decline.
(A) Schematic of the two groups of FAH−/− pigs used for these studies, indicating whether or not NTBC was administered (ON/OFF NTBC). (B) Kaplan-Meier survival curve for two groups of FAH−/− pigs compared to a control group. * = P<0.01 for differential survival between both FAH−/− groups compared to controls. (C,D) Images of three littermate pigs on NTBC at day 30 on left, compared to immediately prior to euthanasia on right in which the FAH−/− pig on the far right had been off NTBC for 47 days.
3.3. FAH−/− pigs have multiple biochemical abnormalities
We next analyzed several biochemical parameters to further characterize the phenotype of FAH−/− animals (Table 1 and Fig. S3 and S4). At the time of euthanasia, blood serum analysis revealed a pattern of acute liver injury including significant elevations in alkaline phosphatase, aspartate aminotransferase, ammonia, total bilirubin and prothrombin time in FAH−/− animals compared to control animals. Fasting serum glucose levels were significantly decreased compared to controls. Next, amino acid levels were compared to analyze the effect of FAH-deficiency on tyrosine metabolism (Table 1). Serum analysis revealed tyrosine was significantly elevated in FAH−/− animals compared to controls indicating a block in tyrosine metabolism. Finally, elevation in urinary succinylacetone is the primary diagnostic of HT1 in human patients and is caused by a build-up of FAA due to FAH-deficiency. Consistent with this phenotype, succinylacetone levels were significantly increased in FAH−/− animals at the time of euthanasia in both blood and urine samples (Table 1, Fig. S3 and S4).
Table 1.
Biochemical parameters in FAH−/− pigs at time of euthanasia.
| Unit | FAH−/− Group 1 | FAH−/− Group 2 | Control | P Value | |
|---|---|---|---|---|---|
| AST | U/L | 205.5 ± 58.7 | 343.7 ± 154.5 | 61.0 ± 19.3 | 0.0137 |
| Alkaline Phosphatase | IU/L | 1629.5 ± 379.7 | 918.3 ± 282.5 | 330.5 ± 75.2 | 0.0091 |
| Total Bilirubin | mg/dl | 2.7 ± 0.4 | 1.0 ± 0.7 | 0.1 ± 0.1 | 0.0266 |
| Ammonia | μmol/L | 629.0 ± 369.1 | 553.0 ± 561.6 | 64.3 ± 37.3 | 0.0533 |
| INR | - | 1.9 ± 0.1 | 1.8 ± 0.6 | 1.0 ± 0.1 | 0.0112 |
| Glucose | mg/dl | 8.5 ± 0.7 | 28.0 ± 22.6 | 108.5 ± 18.9 | 0.0002 |
| Creatinine (blood) | mg/dl | 0.3 ± 0.0 | 0.4 ± 0.1 | 0.7 ± 0.1 | 0.0008 |
| Creatinine (urine) | mg/dl | 25.2 ± 0.0* | 22.8 ± 18.9 | 47.1 ± 27.7 | 0.1978 |
| Tyrosine | μM | 753.0 ± 188.1 | 826.3 ± 277.5 | 132.0 ± 2.8 | 0.0102 |
| Methionine | μM | 424.5 ± 222.7 | 153.9 ± 138.0 | 44.8 ± 7.7 | 0.2243 |
| Succinylacetone (blood) | μg/ml | 3.0 ± 0.4 | 2.9 ± 0.9 | 0.9 ± 0.2 | 0.0006 |
| Succinylacetone (urine) | μM | 8.3 ± 0.0* | 5.7 ± 4.2 | 0 ± 0.0 | 0.0322 |
Results are expressed as average ± standard deviation. FAH−/− Group 1, n=2; FAH−/− Group 2, n=3; Control, n=4.
n=1.
Experimental results were analyzed for significance by applying a student 2-tailed t-test assuming equal variance by combining results from both FAH−/− groups and comparing to the control group.
3.4. FAH−/− pigs have progressive liver injury
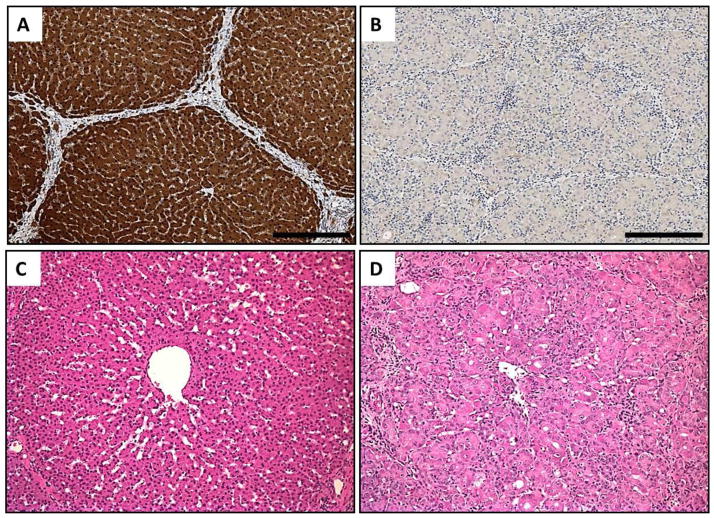
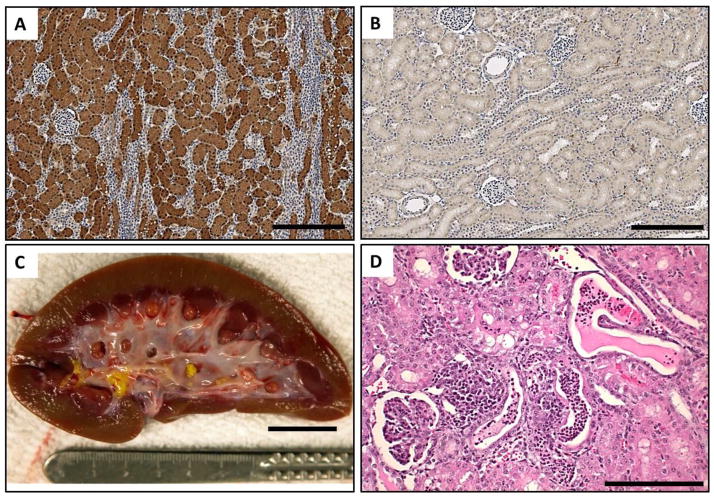
We next analyzed the histology of livers from FAH−/− animals off NTBC. We first confirmed absence of FAH in these livers by IHC. Whereas FAH was detected robustly in the hepatocytes of FAH+/+ or FAH+/− animals, FAH was completely absent in FAH−/− livers (Fig. 4, A and B). Secondly, periodic acid-Schiff (PAS) staining was performed to access glycogen levels in the liver. Consistent with liver failure as the primary cause of death in FAH−/− animals, PAS staining was markedly decreased in FAH+/− livers compared to livers from wild-type siblings (Fig. S5, A to C). Next, a detailed histopathological analysis revealed diffuse hepatocellular injury, with hepatocytes exhibiting necrosis, cytoplasmic ballooning degeneration, karyomegaly and prominent nucleoli in FAH−/− animals only (Fig. 4, C and D; Fig. S5, D to F).
Fig. 4. FAH−/− pigs have severe liver damage after NTBC withdrawal.
(A, B) FAH-immunohistochemistry in liver of FAH+/+ (left) and FAH−/− (right) pigs. FAH (stained brown) is completely absent in FAH−/− hepatocytes. Scale bar = 250 um. (C,D) H&E staining in liver of FAH+/+ (left) and FAH−/− (right) pig. FAH−/− livers show significant hepatocyte injury, with necrosis and inflammation compared to the control liver. Scale bar = 100um.
3.5. FAH−/− pigs have dysmorphic kidney morphology
As FAH is also expressed in renal proximal tubules, we next analyzed the kidney by histology to determine if the kidneys were also damaged in FAH−/− animals. Whereas FAH was detected uniformly in the renal proximal tubules of FAH+/+ or FAH+/− pigs, FAH was completely absent in FAH−/− kidneys (Fig. 5, A and B). Examination of kidneys at the time of euthanasia revealed conspicuous macroscopic changes (2/4 animals from Group 2; 0/3 animals from Group 1) characterized by the presence of a yellow deposit in the renal pelvis that was determined to contain a calcium phosphate peak upon spectral analysis (Fig. 5C). In contrast to liver damage, kidney damage was more variable; however, kidneys from FAH−/− pigs from both groups had areas of focal acute pyelitis but kidney damage was more prominent in animals that did not receive any NTBC from birth (Group 1). In addition to pyelitis, cortical kidney damage included tubular epithelial injury with proteinaceous casts, tubular dilation, and acute pyelonephritis (Fig. 5D).
Fig. 5. FAH−/− pigs have kidney abnormalities after NTBC withdrawal.
(A,B) FAH-immunohistochemistry in kidneys of FAH+/+ (left) and FAH−/− (right) pigs. FAH (stained brown) is completely absent in FAH−/− renal tubular cells. Scale bar = 250 um. (C) Gross morphology of kidney from FAH−/− pig showing presence of a calcium phosphate deposit in the pelvis. Scale bar = 20mm. (D) H&E staining in kidney of FAH−/− pig showing renal tubular injury. Scale bar = 400um.
3.6. FAH−/− pigs have no other major abnormalities
As there have been isolated reports of abnormalities outside the liver and kidney of HT1 patients, we next performed a thorough histopathological analysis of all other major organs of FAH−/− pigs. With the exception of the pancreas, no significant pathology was identified (normal histology not shown). However, at the time of euthanasia, there was a decrease in islet size and islet number in the pancreata of FAH−/− pigs (2/7 pigs; Fig. S6, A and B). To investigate this further, we performed IHC with an antibody against FAH on pancreatic sections. Interestingly, the anti-FAH antibody labeled pancreatic islet cells robustly in FAH+/+ animals, whereas no FAH+ cells were detected in pancreata of FAH−/− pigs (Fig. S6, C and D).
Section 4: Discussion
4.1
As limitations in murine models have become more apparent, a substantial need exists for the creation of improved models of human disease.[25] The advent of SCNT and improved gene targeting strategies have made the pig the preferred choice for generating large animal models of human diseases.[18, 26, 27] Metabolic diseases, or inborn errors of metabolism, are caused by single gene defects in which absence or dysfunction of a protein results in abnormal synthesis or metabolism of a protein, carbohydrate, or fat. While the individual incidence of each disorder is rare, it is estimated that 10% of all pediatric liver transplants are resultant from inborn errors of metabolism.[28] A potential alternative strategy to liver transplantation is hepatocyte transplantation [29]. Since initial preclinical experiments in a rodent model for Crigler-Najjar syndrome type 1[30], a number of small animal models of metabolic liver diseases have been treated by hepatocyte transplantation, including hereditary tyrosinemia type 1[13], phenylketonuria[31], Wilson’s disease[32], progressive familial intrahepatic cholestasis[33], and alpha-1 antitrypsin deficiency. [13, 30, 34]. These experiments in rodents have paved the way for hepatocyte transplantation in humans, including the first published partial correction in a patient with metabolic liver disease in 1998 [35]. A major limitation of hepatocyte transplantation in humans has been a shortage of transplantable cells. Therefore, there has been intense interest in developing new methods to generate hepatocytes from various cell types including fetal and adult liver stem cells [36–38], pluripotent stem cells [39–41], and by direct reprogramming from other lineages.[16, 17] The Fah-knockout mouse has played a critical role in many of these studies by providing an essential test of in vivo efficacy of hepatocyte function and repopulation competency [42]. However, as has been demonstrated recently by generation of CFTR knockout animals [18], pigs provide a more clinically-relevant model of human disease and should accelerate development of regenerative therapies for various human disorders. We believe, therefore, that a large animal knockout model of FAH-deficiency will provide an important preclinical model of metabolic liver disease and prove useful in the evaluation of novel liver cell therapies.
4.2
In this study, we report the creation of the first genetically modified large animal model of a metabolic liver disorder, HT1, in pigs that are deficient in FAH, an enzyme that catalyzes the last step in tyrosine metabolism.[4] Based on the results presented here, we believe FAH−/− pigs off NTBC closely resemble the phenotype of acute-onset HT1 in humans. First, the most common presentation for HT1 in infants is failure to thrive and this phenotype was represented in these pigs as evidenced by cessation of weight gain and muscle wasting when animals were off NTBC. Second, diagnosis of HT1 in the clinic consists of detection of elevated tyrosine and succinylacetone in the blood or urine, of which both parameters were observed in FAH−/− pigs. Confirmation of HT1 diagnosis in the clinic is done by measuring FAH enzyme activity, which was also performed in FAH−/− pigs to confirm FAH-deficiency. Third, liver injury is the key characteristic of acute-onset HT1 in infants. Hepatocyte damage was the predominant histological finding in the liver of these animals, presumably caused by accumulation of FAA within these cells caused by FAH deficiency.[6, 7] Severe liver damage was confirmed biochemically at autopsy by elevation of AST, alkaline phosphatase, ammonia, and total bilirubin in the blood. Fourth, similar to the human phenotype,[3] renal tubular damage was also detected in pigs off NTBC. In HT1, renal tubular dysfunction is caused cell autonomously by accumulation of FAA, as well as non-cell autonomously by circulating succinylacetone.[7] As a result, renal tubular injury is more pronounced in the chronic form of human HT1, and we predict that future studies will reveal more extensive kidney damage in FAH−/− pigs that survive longer than in this study.
4.3
It is important to acknowledge, however, that there are differences between FAH−/− pigs and HT1 patients. First, FAH-deficiency in humans is not believed to cause a lethal defect in utero, even in HT1 patients with no detectable FAH activity.[2, 3] In our early experiments, pregnant sows were not administered NTBC and no FAH−/− progeny were recovered. As calcification occurs around day 35 of development in pigs,[23] and no mummified fetuses were recovered at birth in these litters off NTBC, these results indicated that FAH-deficiency was causing lethality prior to day 35 of gestation. This hypothesis is substantiated by the fact that FAH is expressed early in the developing pig as demonstrated by our western blot and enzyme assays. This is also a notable difference between Fah−/− mice and FAH−/− pigs. In mice, Fah gene expression is detected around day E15.5 of development in the liver.[43] Consequently, FAH-deficiency in mice does not produce an in utero lethal defect and mice are born, albeit with severe liver damage.[12, 44] Less is known about FAH activity in human development; however, FAH protein has been detected by western blot in human fetal liver.[45] Moreover, succinylacetone can be detected in the amniotic fluid of HT1 fetuses at around 15–18 weeks of gestation,[3, 46] indicating FAH is active by at least this time point in human development. Additionally, confirmation of FAH-deficiency in the developing human fetus leading to liver damage has also been shown by detection of succinylacetone and alpha-fetoprotein in the cord blood of newborns with HT1.[47] To that point, there have been two recent reports of administration of NTBC during gestation in patients with HT1.[48, 49] While this approach has potential therapeutic benefit, up to now there has been no suitable research model to test the effect of in utero administration of NTBC to the fetus in an appropriate preclinical model. The results presented here would strengthen the argument that a patient carrying a fetus known to be at high risk of liver damage caused by absence of a functional FAH gene should be given NTBC for the duration of pregnancy.
4.4
It is interesting to note, that in utero lethality in FAH−/− pigs, which is correctable by NTBC, provides unique opportunities to study regenerative therapies targeted to the fetus.[50] As liver injury can be initiated by stopping NTBC at any time in development, FAH−/− pigs could be used to test efficacy of gene therapy and in utero cell transplant approaches at any point during gestation, providing a unique disease model that is currently unavailable. Additionally, FAH-deficiency during development may provide a strong selective advantage for the expansion of transplanted human hepatocytes in utero,[51] potentially allowing rapid repopulation of the FAH−/− liver with cells[42] that could be used for various cell therapy approaches for myriad liver disorders. A comparable strategy has already been demonstrated in the pig for generating exogenic pancreas in an apancreatic pig by way of blastocyst complementation.[52] This success of this procedure was based on the vacant pancreatic niche created by expressing a HES1 transgene driven by the PDX1 promoter which resulted in an apancreatic phenotype. To this point, FAH-deficiency in the developing mouse has been already exploited for liver repopulation with FAH-positive cells,[53] suggesting FAH-deficiency could provide a unique niche in the developing FAH−/− pig fetus for repopulation with FAH-positive cells.
4.5
In conclusion, our data show that FAH−/− pigs represent the first genetically modified large model of a metabolic liver disease. FAH−/− pigs off NTBC die of acute liver failure caused by severe hepatocyte dysfunction due to deficiency of the enzyme FAH. FAH−/− pigs closely resemble the human HT1 phenotype, although differences do exist. We anticipate FAH−/− pigs will provide a tremendous resource for preclinical studies aimed at developing regenerative treatments for metabolic liver disease. Additionally, it can be expected that FAH−/− pigs will provide a clinically relevant model for testing efficacy of various cell therapy approaches, including hepatocytes, liver stem cells, pluripotent stem cell-derived hepatocytes, and induced multipotent progenitor cells.
Highlights.
Pigs homozygous for a targeted FAH knockout allele were generated.
FAH deficiency is an in utero lethal defect in pigs that is correctable with NTBC.
FAH−/− pigs off NTBC die of acute liver failure.
FAH−/− pigs closely model the human disorder of hereditary tyrosinemia type 1.
FAH−/− pigs will provide a unique model for testing efficacy of cell therapies.
Acknowledgments
Financial Support
S. Nyberg was funded by the National Institutes of Health (grant RO1-DK56733), the Marriott Foundation, the Wallace H. Coulter Foundation, the American Society of Transplant Surgeons/Pfizer Collaborative Scientist Grant, and the American Society of Transplant Surgeons/National Kidney Foundation Folkert Belzer Award. M. Grompe was supported by the National Institutes of Health (grant DK048252).
We thank Angela Major of the NIDDK-sponsored Digestive Disease Core Laboratory of the Texas Medical Center (DK56338), LouAnn Gross (Mayo Clinic, Rochester) and Jenny Pattengill (Mayo Clinic, Arizona) for histology support. We thank Denise Rokke (Mayo Clinic, Rochester) for elemental analysis.
List of Abbreviations
- HT1
Hereditary tyrosinemia type I
- FAH
Fumarylacetoacetate hydrolase
- NTBC
2-(2-nitro-4-trifluoromethylbenzyol)-1,3 cyclohexanedione
- FAA
Fumarylacetoacetate
- AAV
Adeno-associated virus
- SCNT
Somatic cell nuclear transfer
- CFTR
Cystic fibrosis transmembrane conductance regulator
Footnotes
Conflict of Interest
None of the authors have a conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Laet C, Dionisi-Vici C, Leonard JV, McKiernan P, Mitchell G, Monti L, de Baulny HO, Pintos-Morell G, Spiekerkotter U. Recommendations for the management of tyrosinaemia type 1. Orphanet journal of rare diseases. 2013;8:8. doi: 10.1186/1750-1172-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grompe M. The pathophysiology and treatment of hereditary tyrosinemia type 1. Semin Liver Dis. 2001;21:563–571. doi: 10.1055/s-2001-19035. [DOI] [PubMed] [Google Scholar]
- 3.Sniderman King L, Trahms C, Scott CR. Tyrosinemia Type 1. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. GeneReviews. Seattle (WA): 1993. [Google Scholar]
- 4.Lindblad B, Lindstedt S, Steen G. On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci U S A. 1977;74:4641–4645. doi: 10.1073/pnas.74.10.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endo F, Sun MS. Tyrosinaemia type I and apoptosis of hepatocytes and renal tubular cells. Journal of inherited metabolic disease. 2002;25:227–234. doi: 10.1023/a:1015646400182. [DOI] [PubMed] [Google Scholar]
- 6.Jorquera R, Tanguay RM. The mutagenicity of the tyrosine metabolite, fumarylacetoacetate, is enhanced by glutathione depletion. Biochemical and biophysical research communications. 1997;232:42–48. doi: 10.1006/bbrc.1997.6220. [DOI] [PubMed] [Google Scholar]
- 7.Kubo S, Sun M, Miyahara M, Umeyama K, Urakami K, Yamamoto T, Jakobs C, Matsuda I, Endo F. Hepatocyte injury in tyrosinemia type 1 is induced by fumarylacetoacetate and is inhibited by caspase inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9552–9557. doi: 10.1073/pnas.95.16.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo P, O’Regan S. Visceral pathology of hereditary tyrosinemia type I. American journal of human genetics. 1990;47:317–324. [PMC free article] [PubMed] [Google Scholar]
- 9.Lindstedt S, Holme E, Lock EA, Hjalmarson O, Strandvik B. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet. 1992;340:813–817. doi: 10.1016/0140-6736(92)92685-9. [DOI] [PubMed] [Google Scholar]
- 10.Gluecksohn-Waelsch S. Genetic control of morphogenetic and biochemical differentiation: lethal albino deletions in the mouse. Cell. 1979;16:225–237. doi: 10.1016/0092-8674(79)90001-1. [DOI] [PubMed] [Google Scholar]
- 11.Russell LB, Russell WL, Kelly EM. Analysis of the albino-locus region of the mouse. I. Origin and viability. Genetics. 1979;91:127–139. doi: 10.1093/genetics/91.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grompe M, al-Dhalimy M, Finegold M, Ou CN, Burlingame T, Kennaway NG, Soriano P. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev. 1993;7:2298–2307. doi: 10.1101/gad.7.12a.2298. [DOI] [PubMed] [Google Scholar]
- 13.Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, Grompe M. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1996;12:266–273. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- 14.Paulk NK, Wursthorn K, Wang Z, Finegold MJ, Kay MA, Grompe M. Adeno-associated virus gene repair corrects a mouse model of hereditary tyrosinemia in vivo. Hepatology. 2010;51:1200–1208. doi: 10.1002/hep.23481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lisowski L, Lau A, Wang Z, Zhang Y, Zhang F, Grompe M, Kay MA. Ribosomal DNA integrating rAAV-rDNA vectors allow for stable transgene expression. Mol Ther. 2012;20:1912–1923. doi: 10.1038/mt.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 17.Zhu S, Rezvani M, Harbell J, Mattis AN, Wolfe AR, Benet LZ, Willenbring H, Ding S. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature. 2014;508:93–97. doi: 10.1038/nature13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB, Jr, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper DK, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133–147. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- 20.Hickey RD, Lillegard JB, Fisher JE, McKenzie TJ, Hofherr SE, Finegold MJ, Nyberg SL, Grompe M. Efficient production of Fah-null heterozygote pigs by chimeric adeno-associated virus-mediated gene knockout and somatic cell nuclear transfer. Hepatology. 2011;54:1351–1359. doi: 10.1002/hep.24490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Montini E, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. Kinetics of liver repopulation after bone marrow transplantation. Am J Pathol. 2002;161:565–574. doi: 10.1016/S0002-9440(10)64212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turgeon C, Magera MJ, Allard P, Tortorelli S, Gavrilov D, Oglesbee D, Raymond K, Rinaldo P, Matern D. Combined newborn screening for succinylacetone, amino acids, and acylcarnitines in dried blood spots. Clin Chem. 2008;54:657–664. doi: 10.1373/clinchem.2007.101949. [DOI] [PubMed] [Google Scholar]
- 23.Christianson WT. Stillbirths, mummies, abortions, and early embryonic death. The Veterinary clinics of North America Food animal practice. 1992;8:623–639. doi: 10.1016/s0749-0720(15)30708-8. [DOI] [PubMed] [Google Scholar]
- 24.Hall MG, Wilks MF, Provan WM, Eksborg S, Lumholtz B. Pharmacokinetics and pharmacodynamics of NTBC (2-(2-nitro-4-fluoromethylbenzoyl)-1,3-cyclohexanedione) and mesotrione, inhibitors of 4-hydroxyphenyl pyruvate dioxygenase (HPPD) following a single dose to healthy male volunteers. British journal of clinical pharmacology. 2001;52:169–177. doi: 10.1046/j.0306-5251.2001.01421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prather RS, Lorson M, Ross JW, Whyte JJ, Walters E. Genetically Engineered Pig Models for Human Diseases. Annual Review of Animal Biosciences. 2013;1(1):203–219. doi: 10.1146/annurev-animal-031412-103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renner S, Fehlings C, Herbach N, Hofmann A, von Waldthausen DC, Kessler B, Ulrichs K, Chodnevskaja I, Moskalenko V, Amselgruber W, Goke B, Pfeifer A, Wanke R, Wolf E. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes. 2010;59:1228–1238. doi: 10.2337/db09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saeki K, Matsumoto K, Kinoshita M, Suzuki I, Tasaka Y, Kano K, Taguchi Y, Mikami K, Hirabayashi M, Kashiwazaki N, Hosoi Y, Murata N, Iritani A. Functional expression of a Delta12 fatty acid desaturase gene from spinach in transgenic pigs. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6361–6366. doi: 10.1073/pnas.0308111101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen K, Horslen S. Metabolic liver disease in children. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2008;14:713–733. doi: 10.1002/lt.21520. [DOI] [PubMed] [Google Scholar]
- 29.Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–449. doi: 10.1097/01.tp.0000231689.44266.ac. [DOI] [PubMed] [Google Scholar]
- 30.Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, Najarian JS. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976;192:892–894. doi: 10.1126/science.818706. [DOI] [PubMed] [Google Scholar]
- 31.Hamman K, Clark H, Montini E, Al-Dhalimy M, Grompe M, Finegold M, Harding CO. Low therapeutic threshold for hepatocyte replacement in murine phenylketonuria. Mol Ther. 2005;12:337–344. doi: 10.1016/j.ymthe.2005.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida Y, Tokusashi Y, Lee GH, Ogawa K. Intrahepatic transplantation of normal hepatocytes prevents Wilson’s disease in Long-Evans cinnamon rats. Gastroenterology. 1996;111:1654–1660. doi: 10.1016/s0016-5085(96)70029-x. [DOI] [PubMed] [Google Scholar]
- 33.De Vree JM, Ottenhoff R, Bosma PJ, Smith AJ, Aten J, Oude Elferink RP. Correction of liver disease by hepatocyte transplantation in a mouse model of progressive familial intrahepatic cholestasis. Gastroenterology. 2000;119:1720–1730. doi: 10.1053/gast.2000.20222. [DOI] [PubMed] [Google Scholar]
- 34.Wilson JM, Johnston DE, Jefferson DM, Mulligan RC. Correction of the genetic defect in hepatocytes from the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1988;85:4421–4425. doi: 10.1073/pnas.85.12.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 36.Huch M, Dorrell C, Boj SF, van Es JH, Li VSW, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. In vitro expansion of single Lgr5(+) liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W, Kulik M, Sherwood S, Tallheden T, Cheng N, Furth ME, Reid LM. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haridass D, Yuan Q, Becker PD, Cantz T, Iken M, Rothe M, Narain N, Bock M, Norder M, Legrand N, Wedemeyer H, Weijer K, Spits H, Manns MP, Cai J, Deng H, Di Santo JP, Guzman CA, Ott M. Repopulation efficiencies of adult hepatocytes, fetal liver progenitor cells, and embryonic stem cell-derived hepatic cells in albumin-promoter-enhancer urokinase-type plasminogen activator mice. Am J Pathol. 2009;175:1483–1492. doi: 10.2353/ajpath.2009.090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada T, Yoshikawa M, Kanda S, Kato Y, Nakajima Y, Ishizaka S, Tsunoda Y. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002;20:146–154. doi: 10.1634/stemcells.20-2-146. [DOI] [PubMed] [Google Scholar]
- 40.Yu Y, Liu H, Ikeda Y, Amiot BP, Rinaldo P, Duncan SA, Nyberg SL. Hepatocyte-like cells differentiated from human induced pluripotent stem cells: relevance to cellular therapies. Stem Cell Research. 2012;9:196–207. doi: 10.1016/j.scr.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Si-Tayeb K, Noto FK, Nagaoka M, Li JX, Battle MA, Duris C, North PE, Dalton S, Duncan SA. Highly Efficient Generation of Human Hepatocyte-Like Cells from Induced Pluripotent Stem Cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li T, Huang J, Jiang Y, Zeng Y, He F, Zhang MQ, Han Z, Zhang X. Multi-stage analysis of gene expression and transcription regulation in C57/B6 mouse liver development. Genomics. 2009;93:235–242. doi: 10.1016/j.ygeno.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruppert S, Kelsey G, Schedl A, Schmid E, Thies E, Schutz G. Deficiency of an enzyme of tyrosine metabolism underlies altered gene expression in newborn liver of lethal albino mice. Genes & development. 1992;6:1430–1443. doi: 10.1101/gad.6.8.1430. [DOI] [PubMed] [Google Scholar]
- 45.Tanguay RM, Valet JP, Lescault A, Duband JL, Laberge C, Lettre F, Plante M. Different molecular basis for fumarylacetoacetate hydrolase deficiency in the two clinical forms of hereditary tyrosinemia (type I) American journal of human genetics. 1990;47:308–316. [PMC free article] [PubMed] [Google Scholar]
- 46.Gagne R, Lescault A, Grenier A, Laberge C, Melancon SB, Dallaire L. Prenatal diagnosis of hereditary tyrosinaemia: measurement of succinylacetone in amniotic fluid. Prenatal diagnosis. 1982;2:185–188. doi: 10.1002/pd.1970020307. [DOI] [PubMed] [Google Scholar]
- 47.Hostetter MK, Levy HL, Winter HS, Knight GJ, Haddow JE. Evidence for liver disease preceding amino acid abnormalities in hereditary tyrosinemia. The New England journal of medicine. 1983;308:1265–1267. doi: 10.1056/NEJM198305263082105. [DOI] [PubMed] [Google Scholar]
- 48.Vanclooster A, Devlieger R, Meersseman W, Spraul A, Kerckhove KV, Vermeersch P, Meulemans A, Allegaert K, Cassiman D. Pregnancy during nitisinone treatment for tyrosinaemia type I: first human experience. JIMD reports. 2012;5:27–33. doi: 10.1007/8904_2011_88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia Segarra N, Roche S, Imbard A, Benoist JF, Greneche MO, Davit-Spraul A, Ogier de Baulny H. Maternal and fetal tyrosinemia type I. Journal of inherited metabolic disease. 2010;33(Suppl 3):S507–510. doi: 10.1007/s10545-012-9569-8. [DOI] [PubMed] [Google Scholar]
- 50.MacKenzie TC, Kobinger GP, Kootstra NA, Radu A, Sena-Esteves M, Bouchard S, Wilson JM, Verma IM, Flake AW. Efficient transduction of liver and muscle after in utero injection of lentiviral vectors with different pseudotypes. Mol Ther. 2002;6:349–358. doi: 10.1006/mthe.2002.0681. [DOI] [PubMed] [Google Scholar]
- 51.Fisher JE, Lillegard JB, McKenzie TJ, Rodysill BR, Wettstein PJ, Nyberg SL. In utero transplanted human hepatocytes allow postnatal engraftment of human hepatocytes in pigs. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2013;19:328–335. doi: 10.1002/lt.23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsunari H, Nagashima H, Watanabe M, Umeyama K, Nakano K, Nagaya M, Kobayashi T, Yamaguchi T, Sumazaki R, Herzenberg LA, Nakauchi H. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4557–4562. doi: 10.1073/pnas.1222902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Espejel S, Roll GR, McLaughlin KJ, Lee AY, Zhang JY, Laird DJ, Okita K, Yamanaka S, Willenbring H. Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. J Clin Invest. 2010;120:3120–3126. doi: 10.1172/JCI43267. [DOI] [PMC free article] [PubMed] [Google Scholar]