Summary
The lactate dehydrogenase-A (LDH-A) enzyme catalyzes the inter-conversion of pyruvate and lactate, is upregulated in human cancers and is associated with aggressive tumor outcomes. Here we use a novel inducible murine model and demonstrate that inactivation of LDH-A in mouse models of NSCLC driven by oncogenic K-RAS or EGFR leads to decreased tumorigenesis and disease regression in established tumors. We also show that abrogation of LDH-A results in reprogramming of pyruvate metabolism, with decreased lactic fermentation in vitro, in vivo, and ex vivo. This was accompanied by re-activation of mitochondrial function in vitro but not in vivo or ex vivo. Finally, using a specific small molecule LDH-A inhibitor, we demonstrated that LDH-A is essential for cancer initiating cell survival and proliferation. Thus, LDH-A can be a viable therapeutic target for NSCLC including cancer stem cell-dependent drug resistant tumors.
Introduction
The enhancement of aerobic glycolysis i.e. the Warburg effect (lactate production in the presence of adequate oxygen) (Warburg, 1930, 1956) not only provides cancer cells with a survival advantage, but also links it to invasive ability (Gatenby and Gillies, 2004). This effect has been seen in different types of tumors and the concomitant increase in glucose uptake is exploited clinically for detection of tumors by 2-fluorodeoxyglucose-based positron emission tomography (FDG-PET) (Gillies, 2001; Hsu and Sabatini, 2008).
Non-small cell lung cancer (NSCLC) is highly glycolytic, accounts for >85% of all lung cancers (Gazdar and Minna, 1999) and is the leading cause of cancer deaths. Mutations at codon 12, which constitute the majority of K-RAS mutations (Forbes et al., 2006) promote fermentative glycolysis in NSCLC (Vizan et al., 2005). Enhanced fermentative glycolysis in a hypoxic tumor microenvironment results in increased acid production (Ebert et al., 1996), which in turn lowers extracellular pH and has been linked to the metastatic ability of cancer cells (Fischer et al., 2007; Walenta et al., 1997).
K-RAS mutations have been frequently reported in NSCLC, particularly in adenocarcinoma (Riely et al., 2009). Mutations in EGFR are also common in NSC adenocarcinomas, and are generally exclusive with K-RAS mutations. Somatic activating mutations in EGFR produce a constitutively active tyrosine-kinase (L858R) and tumors bearing these mutations respond to the EGFR tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib (Amann et al., 2005; Kobayashi et al., 2005). Clinically, despite the dramatic responses to such inhibitors, most patients relapse, owing to acquiring the resistance mutations (T790M, and L747S) (Costa et al., 2007). Any potential therapeutic opportunity that can address highly glycolytic NSCLC will have a significant clinical impact.
Tumor initiating cells (TICs) or cancer stem cells (CSCs) comprise a small population of tumor-forming, self-renewing, cancer cells within a tumor and are associated with aggressive disease and poor prognosis (Ailles and Weissman, 2007). It has been suggested that CSCs cluster in hypoxic niches, rely on fermentative glycolysis and have decreased mitochondrial respiration similar to progenitor cells during normal development (Hill et al., 2009; Zhou et al.). Glycolysis is known to be indispensable for progenitor proliferation and biosynthesis (Agathocleous et al., 2012).
Targeting tumor metabolism via anti-glycolytic therapies may offer a therapeutic opportunity as it represents a key converging step for multiple deregulated signaling pathways in cancer cells. The lactate dehydrogenase-A gene (LDH-A), a target for HIF-1α and MYC, is of particular interest because it catalyzes the NADH-dependent reduction of pyruvate to lactate, a step essential for regenerating the NAD+, which is needed for maintaining glycolysis and other metabolic activities. Enhanced expression of LDH-A has been associated with evolution of aggressive and metastatic cancers in a variety of tumor types (Koukourakis et al., 2005). Since therapies to address highly glycolytic NSCLC tumors are limited, development of LDH-A inhibitors may have a significant impact on this patient population.
Here, we detail the generation of a tamoxifen regulated cre-recombinase conditional mouse model of LDH-A deletion (Cretm-LDH-Afl/fl). To investigate the role of LDH-A in NSCLC, we have used Ccsp-rtTA/(tetO)7-K-RAS4bG12D bitransgenic mice (Fisher et al., 2001) and Ccsp-rtTA/(tetO)-EGFR L858R-T790M (Regales et al., 2007) to generate Cretm-LDH-Afl/fl;Ccsp-rtTA/(tetO)7-K-RAS4bG12D and Cretm-LDH-Afl/fl;Ccsp-rtTA/(tetO)-EGFR-L858R-T790M respectively. These Cretm-LDH-Afl/fl;Ccsp-rtTA/(tetO)7-K-RAS4bG12D and Cretm-LDH-Afl/fl;Ccsp-rtTA/(tetO)-EGFR-L858R-T790M mice serve as preclinical models in which tumors can be initiated in lung tissue by induction of mutant K-RAS or EGFR-L858R-T790M by doxycycline while LDH-A can be removed in all adult tissues including the lung. This model has enabled us to test the impact of LDH-A inhibition in established tumors. We show that LDH-A expression is required for the development of K-RAS dependent tumors. Importantly, we also show that blockade of LDH-A is therapeutically efficacious in established mutated K-RAS and EGFR-L858R-T790M dependent tumors. Indeed, established tumors regress with reduced LDH-A.
We have also examined the impact of LDH-A suppression on cancer metabolism in vitro. We have previously shown that LDH-A abrogation leads to increased production of reactive oxygen species (ROS) and apoptosis in cancer cells, likely the result of increased respiration (Xie et al., 2009). Here, using stable isotope tracers coupled with metabolomic analysis (stable isotope-resolved metabolomics or SIRM), we demonstrated that LDH-A suppression in cultured cancer cells enhances the Krebs cycle, oxygen consumption and mitochondrial ROS production (Seth et al., 2011). Moreover, using SIRM, we have investigated the metabolic effect of LDH-A attenuation in vivo in our new mouse model and ex vivo in freshly prepared human NSCLC tissue slices. Similar to the in vitro result, glycolytic production of 13C-lactate from 13C6-glucose was attenuated in LDH-A suppressed mouse lung tumors and LDH-A inhibitor-treated human tumor slices, but the Krebs cycle activity was not activated either in vivo or ex vivo.
It has been speculated that cancer stem cells exhibit the Warburg phenotype and may benefit from anti-glycolytic therapies. We demonstrate that oncogenic K-RAS–expressing cancer stem cells are highly susceptible to loss of LDH-A expression. We also validate these findings using an LDH-A specific inhibitor.
Results
Generation and characterization of Cretm-LDH-Afl/fl mice
Since LDH-A deletion is embryonic lethal (data not shown), we have generated conditional LDH-A transgenic mice controlled by tamoxifen-regulated cre-recombinase (Cretm) for inducible inactivation the LDH-A gene. The schematic (Figures S1 A–H) describes LDH-A gene inactivation.
Loss of LDH-A results in hemolytic anemia
Within 6 weeks of tamoxifen treatment, Cretm-LDH-Afl/fl mice but not LDH-Afl/fl developed severe but non lethal hemolytic anemia as evidenced by reduced numbers of red blood cells (RBCs) in the peripheral blood (Figure S2A). Blood smears stained with May-Grunwald-Giemsa marked polychromasia indicating release of early RBCs from the marrow in response to peripheral hemolysis (Figure S2C). The spleens of transgenic animals were greatly enlarged due to marked expansion of red pulp from hyperplastic erythroid precursors, and increased hemosiderin deposits characteristic of RBC phagocytosis were evident (Figures S2B, C). Flow cytometry analysis further confirmed the expansion of CD71+Ter119+ immature erythroblasts in the bone marrow, spleen and peripheral blood of Cretm-LDH-Afl/fl animals (Figure S2D). Notably, tamoxifen-treated Cretm-LDH-Afl/+ animals developed a partial phenotype (Figures S2A–D), as evident in both morphologic and flow-cytometric analyses, demonstrating that LDH-A is haplo-insufficient with respect to erythropoiesis.
Reduction in LDH-A protein levels results in decreased tumorigenesis in a K-RAS driven NSCLC model
In order to generate mice that have all the alleles, Cretm-LDH-Afl/fl mice were mated with Ccsp-rtTA/(tetO)7-K-RAS4bG12D (hereafter referred to as K-RAS) to generate Cretm-LDH-Afl/fl;Ccsp-rtTA/(tetO)7-K-RAS4bG12D (homozygous for LDH-A, hereafter referred as Cretm-LDH-Afl/fl; K-RAS) or Cretm-LDH-Afl/+;Ccsp-rtTA/(tetO)7-K-RAS4bG12D (heterozygous for LDH-A, hereafter referred to as Cretm-LDH-Afl/+;K-RAS). In these mice, addition of doxycycline turns on the oncogenic K-RAS expression in a lung specific manner and intraperitoneal injection of tamoxifen turns off LDH-A expression in all the adult organs. Similarly, control LDH-Afl/fl-Ccsp-rtTA/(tetO)7-K-RAS4bG12D mice lacking the Cretm gene were generated (hereafter referred to as LDH-Afl/fl;K-RAS).
To assess the role of LDH-A in the initiation of oncogenic K-RAS tumors, we took 5 week old Cretm-LDH-Afl/fl;K-RAS and Cretm-LDH-Afl/+;K-RAS mice and initiated doxycycline treatment for an additional two weeks to activate mutant K-RAS (Figure 1A). Following that, we initiated intraperitoneal tamoxifen injection (10 mg ml−1) in corn oil every day for 5 days. We validated success of cre-recombination by RT-PCR analysis by assessing loss of exon 2 and sequencing the truncated LDH-A protein (Figures S1I–K). The control was the identical experiment where we injected corn oil as a vehicle with the same schedule.
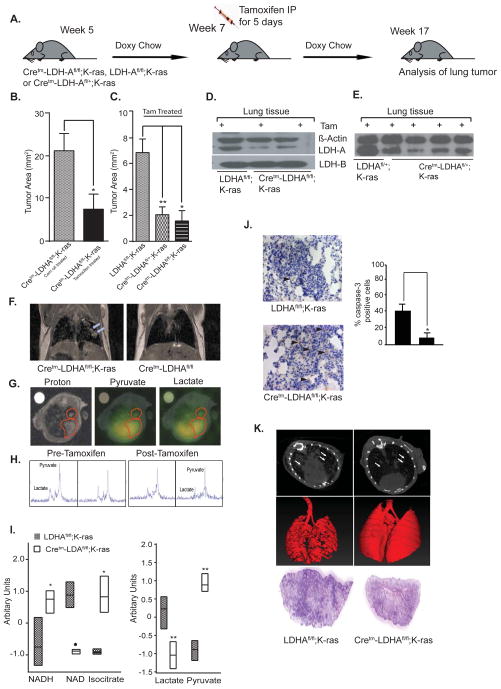
Fig. 1. LDH-A expression is required for initiation of oncogenic K-RAS-induced tumorigenesis.
(A) Schematic representation of an experiment where LDH-A knockdown was initiated 14 days after K-RAS induction by doxycycline diet. (B) Tumor areas of Cretm-LDH-Afl/fl;K-RAS mice treated with tamoxifen (n=7) or corn oil (n=6). (C) Tumor areas of Cretm-LDH-Afl/fl;K-RAS (n=3), Cretm-LDH-Afl/+;K-RAS (n=5) and LDH-Afl/fl;K-RAS (Cretm negative) mice (n=4) treated with tamoxifen (data in B and C are represented as mean ± SD, *= p < 0.05, **= p <0.01 as calculated by two tailed t test). (D) Representative western blot analysis of tumor (along with non-malignant cells) for LDH-A, LDH-B, and beta-actin from LDH-Afl/fl;K-RAS and Cretm-LDH-Afl/fl;K-RAS mice. (E) Representative western blot analysis of tumor (along with non-malignant cells) for LDH-A, and beta-actin from LDH-Afl/fl;K-RAS and Cretm-LDH-Afl/+;K-RAS mice (F) Coronal proton density weight images showing comparison of lungs in Cretm-LDH-Afl/fl;K-RAS mouse on doxycycline diet (left) and Cretm-LDH-Afl/fl mouse (right). Tumors are indicated by arrows in image on left. (G) Proton (left), pyruvate (center), and lactate (right) images acquired in Cretm-LDH-Afl/fl;K-RAS mice prior to tamoxifen administration. Red outlines are drawn around lung lesions. (H) Spectra acquired before (two left panels) and after (two right panels) tamoxifen withdrawal, with signals from pyruvate (pyr) and lactate (lac) labeled at left. (I) Metabolic profiling analysis of NADH, NAD+, isocitrate, lactate, and pyruvate from LDH-Afl/fl;K-RAS v/s. Cretm-LDH-Afl/fl;K-RAS (see Fig. S3 for other pathway specific metabolites). (J) Representative caspase-3 staining of tumors from Cretm-LDH-Afl/fl;K-RAS (top panel), and LDH-Afl/fl;K-RAS (bottom panel); quantitation of caspase 3 staining is shown on the right (means ± SD of n= 4 mice each). (K) Axial CT images and anterior/posterior (A/P) reconstruction from chest/lung and H&E staining of a lung section from LDH-Afl/fl;K-RAS (left panel) and Cretm-LDH-Afl/fl;K-RAS treated with tamoxifen (right panel).
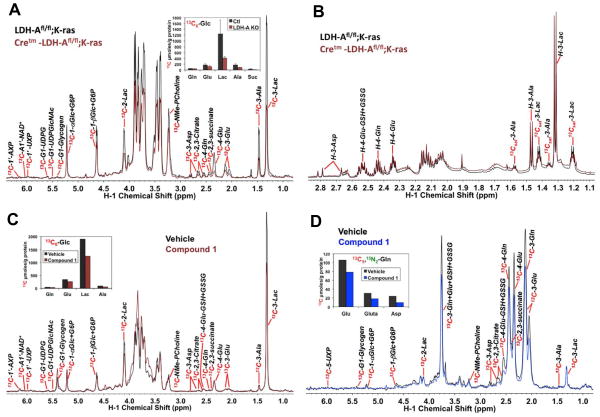
As shown in Figure 1, reduced LDH-A expression resulted in significant reduction in tumor area, suggesting that fermentative glycolysis is a key bioenergetic pathway in oncogenic K-RAS driven tumors. The data also show that deletion of just one allele of LDH-A results in substantial tumor reduction in this model (Figures 1B, C). The decreased expression of LDH-A in lung tumors was verified by western blot analysis. No changes were detected in LDH-B expression when LDH-A gene was deleted (Figures 1D, E). To validate the effects of LDH-A abrogation in vivo, a single Cretm-LDH-Afl/fl;K-RAS mouse was imaged using proton MRI (Figure 1F) and hyperpolarized 13C MRSI (Figures 3F–H) before and after inactivation of LDH-A by tamoxifen administration. As shown (Figures 1F–H), the solid lesions exhibited reductions in the lactate-to-pyruvate ratio levels in the tumor just 10 days after final tamoxifen treatment. For the larger lesion, the ratio fell from 0.47±0.01 to 0.37±0.05, while for the smaller lesion the ratio fell from 0.50±0.04 to 0.31±0.10. The spectra illustrating this reduction in the smaller lesion are shown (Figure 1H). Furthermore, abrogation of LDH-A in tumors led to decreased lactate levels, an increased NAD+/NADH ratio, and increased pyruvate and isocitrate levels (Figure 1I), all consistent with decreased mitochondrial oxidation.
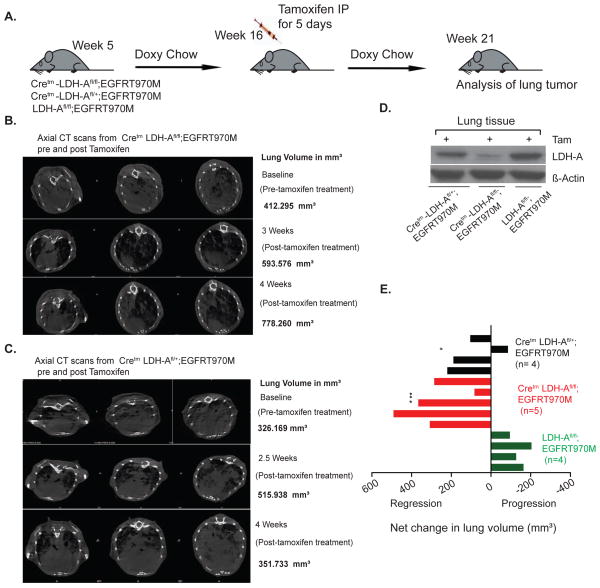
Fig. 3. Reduction in LDH-A levels results in reduced tumorigenesis of established tumors in EGFR- L858R-T970M driven NSCLC model.
(A) Schematic representation of an experiment where LDH-A knockdown was initiated ~80 days after EGFR-T970M mutation induction by doxycycline diet. (B & C) Representative axial CT scans from pre and post Tamoxifen tumor volume of Cretm-LDH-Afl/fl;EGFR-T970M, Cretm-LDH-Afl/+;EGFR-T970M mice. (D) Representative western blot analysis of tumors (along with non-malignant cells) from LDH-Afl/fl;K-RAS, Cretm-LDH-Afl/+;K-RAS, and Cretm-LDH-Afl/fl;K-RAS mice for LDH-A, and beta-actin. (E) Graphical representation of volumetric changes in lung volume pre and post tamoxifen Cretm-LDH-Afl/fl;EGFR-T970M (n=5), Cretm-LDH-Afl/+; EGFRT-970M (n=4), and LDH-Afl/fl; EGFRT970M (n=4) mice that were treated with tamoxifen. Statistical data values are significant as tested by Dunnett’s multiple comparisons test between all three genotypes (* WT to heterozygous and *** WT to homozygous).
Cleaved caspase-3 immunohistochemical analysis (IHC) showed increased apoptosis in K-RAS driven tumors that have reduced LDH-A expression (Figure 1J). We also observed reduced disease in lungs of tamoxifen-treated Cretm-LDH-Afl/fl;K-RAS mice using high resolution axial CT images of lung/chest (Figure 1K).
Reduction in LDH-A protein levels results in regression of established tumors in a K-RAS driven NSCLC model
To assess whether inhibition of LDH-A in an established tumor can impact tumor progression, five week old Cretm-LDH-Afl/fl;K-RAS and Cretm-LDH-Afl/+;K-RAS mice were maintained on a doxycycline diet for an additional 9 weeks. Following that, we initiated intraperitoneal tamoxifen injections (10 mg ml−1) in corn oil every day for 5 days (Figure 2A).
Fig. 2. Reduction in LDH-A levels results in reduced tumorigenesis of established tumors in a K-RAS-driven NSCLC model.
(A) Schematic representation of an experiment where LDH-A knockdown was initiated ~83 days after K-RAS induction by doxycycline diet. (B & C) Tumor areas of Cretm-LDH-Afl/fl;K-RAS (n=12), Cretm-LDH-Afl/+;K-RAS (n=7), and LDH-Afl/fl;K-RAS (n=6) mice that were treated with tamoxifen. Data is representated as mean ± SD, **=p, 0.01, *= p < 0.05 (two tailed t test) (D) Representative western blot analysis of tumors (along with non-malignant cells) from LDH-Afl/fl;K-RAS, Cretm-LDH-Afl/+;K-RAS, and Cretm-LDH-Afl/fl;K-RAS mice for LDH-A, LDH-B, and beta-actin. (E) Axial CT images and anterior/posterior (A/P) reconstruction from chest/lung from LDH-Afl/fl;K-RAS mice (left panel) and Cretm-LDH-Afl/fl;K-RAS mice treated with tamoxifen (right panel) with tumors indicated by arrows (F) Representative coronal and transverse images of lung/chest from same pre and post tamoxifen treated Cretm-LDH-Afl/fl;K-RAS mice (n=5) in comparison to LDH-Afl/fl;K-RAS mice (n=3).
Deletion of just one allele of LDH-A results in substantial tumor reduction in this GEM model (Figures 2B, C). The decreased expression of LDH-A in lung tumors was verified by western blot analysis and no changes were detected in LDH-B expression when LDH-A gene was deleted (Figure 2D). The degree of LDH-A attenuation is consistent with the homozygous and heterozygous knockdown. Furthermore, to quantify the response of LDH-A ablation on tumor growth in Cretm-LDH-Afl/fl;K-RAS in real time, we scanned Cretm-LDH-Afl/fl;K-RAS and LDH-Afl/fl;K-RAS mice pre and the post-tamoxifen treatment. We observed marked regression in tumor growth 10 day post last tamoxifen treatment as measured by coronal and transverse section CT imaging (Figures 2F, G) and representative volumetric assessment of the total lung volume (Movie S1). These results suggest that targeting LDH-A in a K-RAS driven tumor results in tumor regression and targeting LDH-A in lung cancer might have therapeutic efficacy.
Reduction in LDH-A protein levels results in decreased tumorigenesis and regression of established tumors in EGFR resistant NSCLC model
It is possible that other aggressive lung tumors such as those that are resistant to EGFR inhibitors would be amenable to therapeutic targeting via LDH-A. To test this hypothesis, we generated a Cretm-LDH-Afl/fl;Ccsp-rtTA/(tetO)-EGFR-L858R-T790M mouse model (referred as Cretm-LDH-Afl/fl;EGFR-T790M). These mice express EGFR mutation L858R as well as T790M mutation. Similar to the K-RAS model, the EGFR-L858R-T790M expression can be activated in lung epithelial cells by doxycycline and LDH-A expression (whole body) can be modulated by administering tamoxifen.
Five week old Cretm-LDH-Afl/fl;EGFR-T790M, Cretm-LDH-Afl/+;EGFR-T790M and LDH-Afl/fl;EGFR-T790M mice were maintained on a doxycycline diet for additional 8 weeks, as shown (Figure 3A). The response of LDH-A suppression on tumor growth in Cretm-LDH-Afl/+;EGFR-T790M was quantified in real time, we performed MRI scans of Cretm-LDH-Afl/fl;EGFR-T790, Cretm-LDH-Afl/+;EGFR-T790 and LDH-Afl/fl;EGFR-T790 mice pre and post tamoxifen treatment. We show marked regression in tumor growth 20 days post after the last tamoxifen treatment as measured by coronal and transverse section imaging by CT scan (Figures 3B, C) and volumetric assessment of total lung volume. The graphical representation of volumetric analysis is shown as net change in lung volume (Figure 3E). The decreased expression of LDH-A in lung tumors was verified by western blot analysis (Figure 3D). The data also show that deletion of LDH-A results in substantial tumor reduction similar to the K-RAS model. These results suggest that targeting LDH-A in EGFRT-790M driven tumors results in tumor regression. However, unlike the K-RAS tumors where heterozygous ablation of LDH-A was therapeutically efficacious, the EGFR-T790M driven tumors showed a smaller decrease and 1 out of 4 tumors showed increase in tumor growth in the 4th week of monitoring.
Genetic and pharmacologic targeting of LDH-A suppress cancer stem cell function
We next asked whether CSCs are susceptible to reduction in LDH-A levels. To investigate the in vivo role of LDH-A in tumor initiating capacity in oncogenic K-RAS tumors, we isolated tumor cells from Cretm-LDH-Afl/fl;K-RAS or Cretm-LDH-Afl/fl;K-RAS mice that were treated with tamoxifen. As shown in Figure 4, when introduced via tail vein in C57BL6 mice, tumor cells with diminished LDH-A expression colonized the lung at a significantly decreased rate compared to tumor cells that have normal levels of LDH-A expression (Figure 4B). Injection of A549 cells expressing control or LDH-A shRNA in tail vein metastasis assay in athymic mice gave similar results (Figure 4A). Reduction in LDH-A expression in both A549 and Cretm-LDH-Afl/fl;K-RAS was confirmed by western immunoblotting (Figures 4C, D). These findings are consistent with an in vitro matrigel invasion assay with NSCLC A549 cells expressing LDH-A shRNA (Figure 4C). Collectively these data suggest that blockade of fermentative glycolysis diminishes tumor initiating capacity, possibly via blocking cancer stem cells.
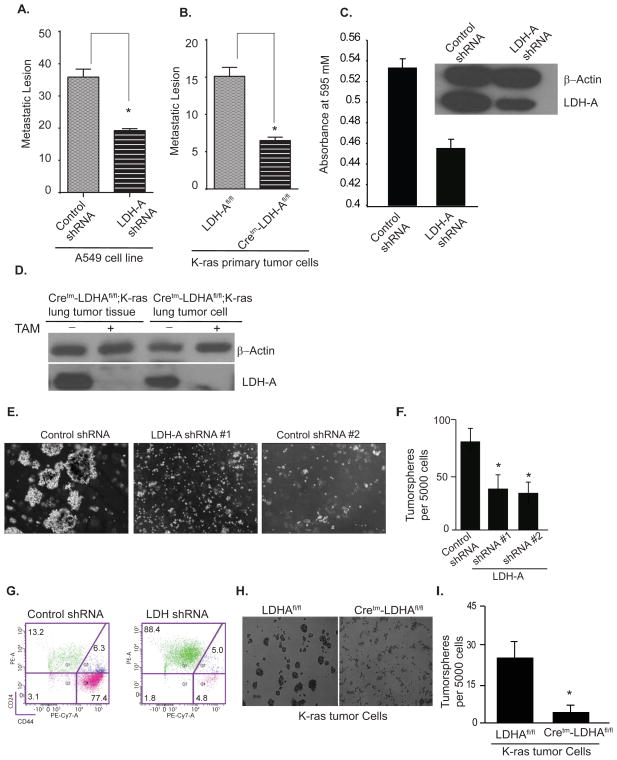
Fig. 4. LDH-A knockdown results in reduction of oncogenic K-RAS-induced cancer stem cells.
Top Panel: Cancer-initiating cells within oncogenic K-RAS-expressing tumors are susceptible to LDH-A abrogration. (A) Cancer-initiating lesions from lungs of syngeneic mice (n=5) that were injected via tail vein with A549 cells expressing either control shRNA or LDH-A shRNA. Cancer-initiating lesions in (A) and (B) were scored after 24 days. (B) Cancer-initiating lesions from lungs of syngeneic mice (n=5) that were injected via tail vein with isolated tumor cells from tamoxifen-treated Cretm-LDH-Afl/fl;K-RAS (n=3) or LDH-Afl/fl;K-RAS (n=2) mice. Data is expressed as ± SEM (**=p < 0.01). (C) In vitro matrigel invasion assay with A549 cells expressing either control shRNA or LDH-A shRNA. Absorbance at 595 nm reflects the number of migrated cells. (D) Western immunoblotting for LDH-A and beta-actin of cells used in in vitro invasion and in vivo cancer initiation assays.
Bottom Panel: Stem cell populations within oncogenic ras-expressing cell lines are reduced by LDH-A shRNA. (E) A549 human NSCLC cells expressing control shRNA or two different LDH-A shRNAs were analyzed in tumorsphere formation assay and (F) Quantification of the results expressed as mean ± SD (*=p < 0.05) (G) FACS analysis of stem cell populations using CD44 and CD24 antibodies in Ras-transformed HMLE (HMLER) and shRNA-LDH-A-HMLER. (H) Tumorsphere formation assay using tumor cells isolated from Cretm-LDH-Afl/fl;K-RAS or LDH-Afl/fl;K-RAS mice and (I) Graphical quantification of the results expressed as mean ± SD (*=p <0.05).
Next, to assess the role of LDH-A on CSCs in vitro, we next down-regulated LDH-A by shRNA in A549 cells and seeded them in a tumorsphere formation assay. As shown (Figures 4E, F), down-regulation of LDH-A expression significantly reduced the ability of A549 cells to form tumorspheres. To assess whether these results can be recapitulated in another oncogenic RAS cell line, we utilized a well-established RAS -induced mammary tumorigenesis system (HMLER) that has been used for CSCs analysis (Al-Hajj et al., 2003; Mani et al., 2008). We found that down regulation of LDH-A in this system results in a significant decrease in CSCs as assessed by tumorsphere assay and by the expression of CD24/44 markers (Figure 4G). We further showed that tumor cells isolated from lungs of tamoxifen-treated Cretm-LDH-Afl/fl;K-RAS mice formed significantly fewer tumorspheres than lung cells from LDH-Afl/fl;K-RAS mice, suggesting that LDH-A abrogation impacts cancer stem cells in vivo (Figures 4H, I).
To validate these findings with LDH-A suppression, we used a specific inhibitor of LDH-A (Compound 1) developed at GlaxoSmithKline (Figure 5A). Compound 1 inhibited recombinant human LDH-A enzyme with an Ki = 4.8 ± 1.1 nM and showed ~10-fold selectivity over LDH-B (Figure 5B) and results in apoptosis (Billiard et al., 2013). Compound 1 decreased cellular lactate production in HepG2 hepatocellular carcinoma cells with an EC50 = 588 ± 200 nM (Figure 5C), increased ROS production (Figure 5D), decreased extracellular acidification rate (Figure 5E), and increased oxygen consumption rate (Figure 5F).
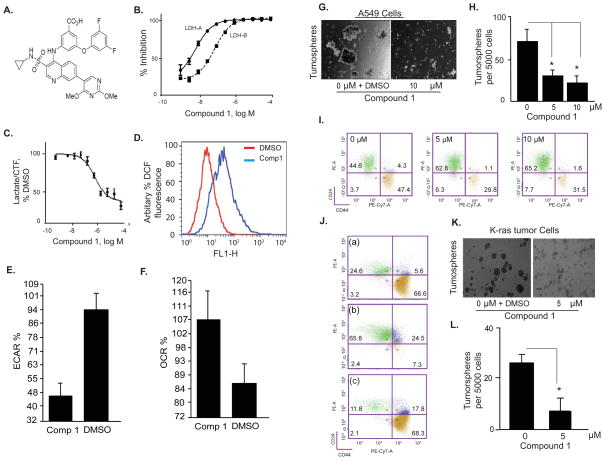
Fig. 5. Small molecule LDH-A inhibitor reduces the number of Ras-induced tumor stem cells.
Left Panel: (A) Chemical structure of the Compound 1. (B) Compound 1 inhibits activity of recombinant human LDH-A (IC50=4.8±1.1 nM) and LDH-B (IC50=53.1±0.9 nM) enzymes. The signal obtained in absence of LDH was set as 100% inhibition and the signal obtained in absence of Compound 1 was set as 0% inhibition (DMSO control, not included on the graphs). Data are means ± SD of two readings, representative of 6 independent experiments. (C) Compound 1 inhibits lactate production in HepG2 human hepatocellular carcinoma cells. Lactate concentration was normalized to cell viability assessed by CellTiter-Fluor™ assay (CTF), and the lactate/CTF ratio obtained in DMSO-treated cells was set at 100%. (D) Compound 1 (10 μM) increases ROS activity in A549 cells as measured by DCF fluorescence (representative of two independent experiments). (E) Compound 1 (10 μM) increases oxygen consumption rate (OCR) and (F) decreased extracellular acidification rate (ECAR) in A549 cells. XF-24 seahorse cell analyzer was used to obtain the data (means ± SD of n=2).
Right Panel: (G & H) Tumorsphere formation assay with A549 cells using vehicle control or 10 μM Compound 1 with graphical quantitation of the results expressed as mean ± SD (*=p< 0.05). (I) FACS analysis using CD44 and CD24 antibodies for stem cell population in ras-transformed HMLE (HMLER) treated with 0, 5 and 10 μM Compound 1 for 30 hours. (J) Decrease in the stem cell population in HMLER by compound1 treatment is specific as shows that CD24/44 positive cells re-appear after withdrawal of the inhibitor (a) plated in absence of compound 1, (b) switched to media containing compound 1 for 24 hours, and (c), switched back to media without compound 1 for additional 24 hours. (K & L) Tumorsphere formation assay with tumor cells isolated from LDH-Afl/fl;K-RAS mice treated with DMSO or 5 μM Compound 1, with graphical quantitation of results expressed as mean +/SD (p < 0.05).
We also found that down regulation of LDH-A activity by Compound 1 reduced the ability of A549 cells to form tumorspheres (Figures 5G, H) and decreased the number of CD24/44 positive cells in HMLER system (Figure 5I) and in stem cells isolated from HMLER (Figure S5A). To investigate whether the observed decrease in CSCs is specific, we cultured HMLER cells with 10 μM Compound 1 for 48 hours before re-plating them in presence or absence of Compound 1. Figure 5J shows that CD24/44 positive cells re-appear after withdrawal of the inhibitor suggesting that Compound 1 affects CSC phenotype. Finally, treatment with Compound 1 significantly reduced the number of tumorspheres generated by tumor cells isolated from lungs of Ccsp-rtTA/(tetO)7-K-RAS4bG12D mice (Figures 5K, L).
To investigate the specificity of LDH-A inhibitor targeting stem v/s. non-stem, we performed proliferation assay, which showed that the LDH-A inhibitor does not exclusively target stem cells, but the impact on stem cells is significant compared to non-stem cells (S. Figure 5B). To extend our results, we also used another previously published stem cell system i.e. MCF10A cells expressing shPTEN or over-expressing PIK3CA (Hanai et al., 2013) to demonstrate that an LDH-A inhibitor reduced the stem cell population as assessed by the tumorsphere assay (Figure 6C).
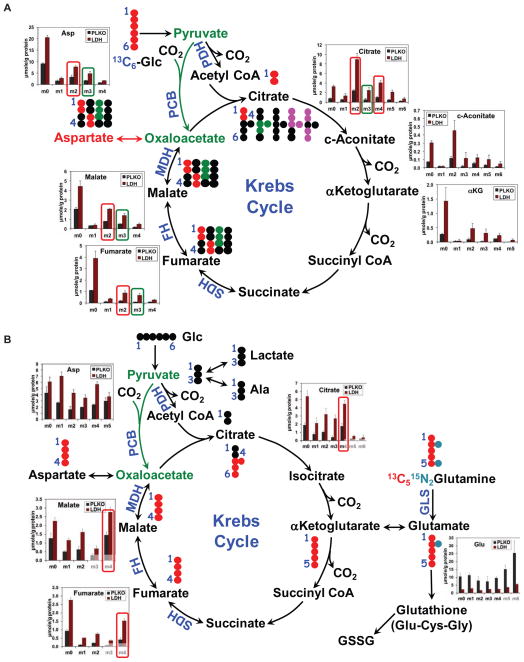
Fig. 6. Enhanced utilization of labeled glucose and glutamine via the Krebs cycle in LDH-A suppressed A549 cells.
(A & B) A549 cells transduced with an empty vector (PLKO) or shRNA targeting LDH-A (LDH) were grown in 13C6-glucose (A) or 13C5,15N2-Gln (B) tracers for 24 h, as described in Methods. The polar extracts were analyzed by GC-MS for the distribution of various 13C isotopologues (same metabolites with different numbers of 13C atoms) of the Krebs cycle metabolites. Also shown in panels A and B are the atom-resolved Krebs cycle tracings with each respective tracer. The m2 (13C2) or m4 (13C4) isotopologues derived from either tracer via the Krebs cycle without input from pyruvate carboxylation (PC) are denoted with red rectangles while the m3 (13C3) isotopologues derived from 13C6-glucose via PC are highlighted with green rectangles. Metabolite concentrations are means ± SD of two or three replicates. Solid and dashed arrows: single and multiple step reactions; single- and double-headed arrows: irreversible and reversible reactions, respectively; αKG: αketoglutarate; ●: 12C;
 ,
,
 : 13C in the first and second turn without PC input;
: 13C in the first and second turn without PC input;
 : 13C with PC input.
: 13C with PC input.
Abrogation of LDH-A results in reactivation of mitochondrial function in cancer cells in vitro
In order to test the hypothesis that reduction in aerobic glycolysis as a result of LDH-A abrogation leads to reactivation of mitochondrial metabolism, we performed 13C6-glucose (Glu) and 13C5,15N2-glutamine (Gln) tracer experiments with A549 cells expressing control shRNA or LDH-A shRNA. We found by GC-MS increased amounts of total and 13C-enriched Krebs cycle metabolites (citrate, cis-aconitate, αKG, fumarate, malate, and aspartate) derived from either [U-13C] glucose (Figure 6A) or [U-13C]-glutamine, (Figure 6B). In particular, the increased synthesis of doubly 13C labeled (13C2- or m2-) and quadruply 13C labeled (13C4- or m4-) citrate from 13C6-glucose in LDH-A suppressed cells reflect enhanced Krebs cycle activity through the first and second turns, respectively (cf. atom-resolved pathway tracing in Figure 6A). Increased flux through the Krebs cycle in these cells was also evidenced by the enhanced accumulation of the 13C4-isotopologues of citrate, fumarate and malate derived from fully labeled glutamine during the first turn of the cycle (cf. Figure 6B). In contrast, 13C enrichment of Glu was substantially attenuated (e.g. 13C515N-Glu or m6-Glu in Figure 6B) by LDH-A suppression in A549 cells grown with 13C5,15N2-Gln. The GC-MS result was corroborated by the 1D HSQC NMR data, as shown (Figure S6), where the abundance of the 13C-3/13C-4-Glu and 13C-4-Glu-glutathione isotopomers was lower in LDH-A suppressed than in control cells. Even more attenuated was the abundance of 13C-4-Gln, which could be accounted for by decreased uptake and/or increased utilization of labeled Gln through the Krebs cycle; the latter is consistent with increased synthesis of labeled products of 13C5,15N2-Gln oxidation through the Krebs cycle (cf. Figure 6B). We further noted that despite the reduction in levels of all 13C labeled Glu isotopologues in LDH-A suppressed cells (Figure 6B), their fractional enrichment in these cells was comparable to that in control cells (Figure S6). The lack of change in 13C fractional distribution together with decreased abundance of Glu and Gln is consistent with both reduced uptake of glutamine and increased glutaminolysis via the Krebs cycle induced by LDH-A knockdown. Enhanced pyruvate carboxylation observed could represent another anaplerotic path enhanced in LDH-A suppressed cells perhaps to compensate for the decreased Gln uptake. Overall decreased synthesis of glutathiones (reduced and oxidized forms) and increased mitochondrial metabolism is expected to lead to increased levels of ROS.
The above findings are in line with our hypothesis that ROS generated in response to LDH-A inhibition as a result of increased flux of pyruvate into the mitochondria. We have also demonstrated that these ROS are indeed mitochondrial in origin (data not shown). The static metabolic profiles from Cretm-LDH-Afl/fl;K-RAS or Cretm-LDH-Afl/+;K-RAS tumors (Figure 1I, H and Figure S3) corroborated the 13C tracer analysis of increased Krebs cycle activity as well as reduced glutathione synthesis and increased NAD+/NADH ratio in LDH-A suppressed cells.
Our mouse model provides a unique opportunity to investigate metabolic reprogramming in tumors in vivo in live animals via SIRM, using 13C6 glucose as tracer in the Cretm-LDH-Afl/fl;K-RAS and LDH-Afl/fl;K-RAS mice post tamoxifen treatment. Figure 7A shows 13C abundance data of lactate and other key metabolites in lung tumors derived from labeled glucose for 4 separate experiments. Relative to the control lung tumors (LDH-Afl/fl;K-RAS), LDH-A suppressed lung tumor (Cretm-LDH-Afl/fl;K-RAS) had reduced synthesis of 13C-lactate (cf. 13C-3-Lac) (p<0.03) which is consistent with reduced LDH-A activity. LDH-A knockdown lung tumors also led to reduced 13C incorporation from labeled glucose into the Krebs cycle metabolites, including Asp, Glu, Gln, succinate, and citrate, although these effects did not reach statistical significance by Wilcoxon analysis (Figure 7, & Figure S4A). Nonetheless, this in vivo result contrasts with the enhanced Krebs cycle activity induced by LDH-A knockdown in cell culture (Figure 6). Metabolic distinctions between in vivo and in vitro systems have been observed for other tumors (Marin-Valencia et al., 2012), which could result from tumor micro-environmental factors absent in cell culture systems, including interaction with stromal cells and variable nutrient supply and oxygenation. Figure 7B shows the corresponding 1H NMR spectra for one of the tumor pairs in Figure 7A. Unlabeled lactate (cf. H-3-Lac) and Ala (H-3-Ala) did not change while 13C-Lac and Ala (cf. 13Csat-3 peaks) depleted with LDH-A knockdown. This result is consistent with reduced glycolytic flux from glucose to lactate, while lactate production from non-glucose sources may not have changed in response to LDH-A suppression.
Fig. 7. LDH-A suppression in Cretm-LDH-Afl/fl;K-RAS, mouse lung tumors and its pharmacological inhibition in primary human lung tumor tissue blocked glucose oxidation via glycolysis without activating the Krebs cycle.
(Top panel) Four each LDH-Afl/fl;K-RAS (Ctl) and Cretm-LDH-Afl/fl;K-RAS (LDH-A KO) mice were tail vein injected with 20 mg of 13C6-glucose in sterile PBS 3 times at 15 minute intervals prior to dissection for lung tumor tissues (one of the LDH-A KO mice had insufficient tissue for metabolite analysis). Polar metabolites were extracted from frozen pulverized tissue powder and analyzed by 1D 1H and 1H-{13C} HSQC NMR as described for Warburg slices in Methods. (A) Displays the HSQC spectra for one pair of control versus LDH-A knockdown mouse tumors, where the intensity of each assigned metabolite 1H signal reflect the 13C abundance of the attached carbon. Also shown is the average content (mean±SE) of selected 13C-labeled glycolytic and Krebs cycle metabolites (as 13C μmole/g protein) obtained from the HSQC data (4 each for Ctl and LDH-A KO). 13C-metabolites were quantified from the peak areas of respective HSQC resonances using the 13C-3-Lac resonance as a calibrator. The 13C abundance of 3-Lac was obtained from the 1H NMR spectra as in (B) calibrated against the DSS standard. Lac: lactate; NMe-PCholine: N-methyl protons of phosphocholine; Glc: glucose; G6P: glucose-6-phosphate; UDP-GlcNAc: UDP-N-acetylglucosamine; UXP: uracil nucleotides; AXP: adenine nucleotides. LDH-A KO induced reduced production of 13C-lactate from 13C6-glucose, which is consistent with decreased glycolytic flow while it also elicited attenuated synthesis of 13C-Ala, -Glu, -succinate, -citrate, and –Asp but this attenuation did not reach statistical significance (cf. Fig. S4). Suffice to say, Krebs cycle activity was not activated by LDH-A suppression. (B) Shows the 1H spectrum of one of the five pairs of tumors. 13Csat-3-Lac: the 13C satellite peaks of the H3 of lactate, the pattern of which indicate fully 13C labeled lactate; GSH+GSSG: reduced and oxidized glutathiones. Reduced production of 13C-lactate and -Ala with little changes in the levels of the unlabeled counterparts is evident.
(Bottom panel) Two tissue slices were procured from one resected human NSCLC tumor, each treated with 13C6-glucose and 13C5,15N2-glutamine, and processed as described in Materials and Methods. (C and D) display respectively the 1D 1H-{13C} HSQC spectra of 13C6-glucose and 13C5,15N2-glutamine treated slices with or without LDH-A inhibitor (10 μM Compound 1). Also shown is the content of selected 13C-labeled glycolytic and Krebs cycle metabolites (as 13C μmole/g protein, determined as in (A)) obtained from the HSQC data. LDH-A inhibitor-induced reduction in 13C-lactate production from 13C6-glucose is evident in (C) while decreased synthesis of Krebs cycle metabolites (succinate, citrate, and Asp) and glutathiones from 13C5,15N2-glutamine in response to LDH-A inhibition is shown in (D). Also clear from (D) is the lack of change in 13C-lactate production from 13C5,15N2-glutamine with LDH-A inhibition.
To test how human lung tumor tissue metabolism responded to LDH-A attenuation, we employed ex vivo cancerous lung tissues freshly resected and thinly sliced from a human NSCLC patient (akin to “Warburg” slices). Similar tissue slices from other patients maintain metabolic activity (e.g. lactate and ATP synthesis) for up to 24 h under our culture conditions (data not shown). When tissue slices each were incubated with LDH-A inhibitor (Compound 1) + 13C6-glucose or with inhibitor + 13C5,15N2-glutamine for 24 h, 13C-lactate production from labeled glucose (Figure 7C) was reduced while that from labeled glutamine was not (Figure 7D), which indicates a block in the LDH-A activity by the inhibitor without altering glutamine metabolism to lactate, corroborating with the LDH-A knockdown mouse tumor result described above (cf. Figure 7B). However, we noted reduced 13C incorporation from labeled glutamine into the Krebs cycle metabolites such as succinate, citrate, and Asp (cf. Figure 7D) in response to LDH-A inhibition, which is consistent with the data obtained from LDH-A knockdown mice but deviates from the in vitro cell culture result (cf. Figure 6).
Discussion
This study allows us to reach a number of important conclusions: 1) reduced LDH-A expression in the adult mouse results in non-lethal anemia, but no other obvious toxicities; 2) reduced LDH-A expression in highly glycolytic tumors that expresses oncogenic K-RAS and/or EGFR L858R mutation in addition to resistant mutation T790M results in decreased tumorigenesis and regression of established tumors and as such may provide therapeutic benefit; 3) both homozygous and heterozygous deletion of LDH-A provides therapeutic efficacy; 4) LDH-A attenuation leads to reduced glycolytic flux in vivo, ex vivo, and in vitro but reactivation of the TCA cycle and anaplerosis occurs only in vitro, implicating the important role of the microenvironment in tumor metabolic reprogramming; 5) LDH-A inhibition impacts CSCs, a population of cells not targeted by most current therapies.
We show that loss of a single allele of LDH-A also results in decreased tumorigenesis. Therefore, it is likely that partial inhibition by a small molecule inhibitor directed against LDH-A may have potential anti-tumor activity. Of interest, we did not see compensation by increased expression of LDH-B, though we cannot rule out that the activity of this enzyme was not increased. These effects of LDH-A ablation on oncogenic K-RAS driven tumor growth were shared by tumors carrying EGFR L858R and T790M mutations, which render them insensitive to EGFR inhibitors.
Our data also show that LDH-A plays a critical role in tumor progression. Previous work from others and us has shown that that LDH-A suppressed cancer cell lines exhibit reduced tumor progression in xenograft models (Fan et al., 2011a; Fantin et al., 2006; Wang et al., 2011). These studies represent an important milestone in validating LDH-A as a therapeutic target, but they suffer from well-known artifacts of utilizing xenografts (Frese and Tuveson, 2007). Here we circumvented these issues by using a genetically engineered mouse (GEM) model. In this model, we temporally ablated LDH-A expression, enabling the investigation of LDH-A’s role in tumor regression as well as in by-stander effect on non-tumor cells, which better approximates the use of a LDH-A inhibitor in a clinical setting. LDH-A ablation led to regression of an established tumor without serious systemic toxicity.
To our knowledge, this report is the first to show that abrogation of a metabolic enzyme in an established tumor model results in reduction of the tumor burden. In fact, metabolically targeted therapies generally delay tumor progression (Patra et al., 2013) or are cytostatic without causing regression (Bonnet et al., 2007; Hatzivassiliou et al., 2005).
In recent years, we have come to appreciate how substrate utilization is dictated by oncogenic enzymes (Nilsson et al., 2012). Based on our work, it is reasonable to hypothesize that all RAS transformed tumors and tumors with EFGR L858R mutations including those develop resistance to EGFR inhibitor therapy may respond to LDH-A inhibition.
By using labeled tracers and SIRM analysis, we were able to define metabolic reprogramming in lung tumor cells following LDH-A abrogation, i.e. increased flow of carbon from both glucose and glutamine through the Krebs cycle but reduced synthesis of glutathiones from glutamine. These reprogrammed events presumably underlie the enhanced oxygen consumption rate (OCR) and increased generation of ROS, previously observed by us (Xie et al., 2009). However, the mitochondrial perturbations in cultured tumor cells induced by LDH-A ablation was not recapitulated in vivo in our mouse model or ex vivo in human lung tumor slices, except for the reduced synthesis of glutathione’s. Such discrepancies between in vivo and in vitro results have been observed in other tumor systems (e.g. glioma) (Marin-Valencia et al., 2012), and highlight the importance of tumor microenvironment and possibly systemic influence on tumor metabolic reprogramming, which in turns underlies inhibition of tumor growth or development.
Several publications described novel inhibitors of LDH-A, but most of the inhibitors are either inactive in cell based assays (Dragovich et al., 2013; Kohlmann et al., 2013) or have other problems (Le et al., 2010) as detailed in (Ward et al., 2012). The inhibitor used here (Billiard et al., 2013) is active in cell based assays at low μM concentrations in number of cell lines (Figure 5, and data not shown) and phenocopies the CSC results generated with either a cell line expressing LDH-A shRNA or tumors isolated from Cretm-LDH-Afl/fl;K-RAS animals. Our attempts to use the LDH-A inhibitor for in vivo work, either in xenografts or GEMs, were limited by its pharmacokinetic properties.
Although it has been speculated that CSCs reside in hypoxic niche (Lock et al., 2013; Zhang et al., 2012) but relatively little is known about the metabolic properties of these cells. We show that reduced LDH-A expression impacts CSCs using established in vivo, in vitro, and ex vivo assays. Some of these findings were further validated with our novel LDH-A specific inhibitor. As in the case for non CSCs, these effects are likely to be mediated via perturbations to the mitochondrial Krebs cycle, as CSCs produce more ROS compared to non-stem counterpart. The ROS production by CSCs was further enhanced by LDH-A suppression (Figures S7A, B). Thus, CSCs could be more vulnerable to LDH-A blockade due to the overall lower capacity of their Krebs cycle (with limitation at the succinate dehydrogenase or SDH step of Complex II), despite a low glycolytic capacity, relative to the non-CSCs (cf. Figure S7C). Complex II is prone to producing superoxide, and its over burden in LDH-A suppressed CSCs may account for the observed excess production of ROS. The Krebs cycle together with the electron transport chain would be less able to oxidize the excess pyruvate or regenerate NAD+ in the cytoplasm via the malate/Asp shuttle to sustain glycolysis. The finding that LDH-A inhibition/ablation impacts cancer stem cells, a population of cells not targeted by a vast majority of current therapies, sets the stage for pre-clinical combinatorial trials with currently approved therapies for NSCLC.
Experimental Procedures
Cells and cell culture
A549 and HepG2 cells were purchased from the American Type Culture Collection and maintained in Ham’s F-12 medium (Mediatech, Inc) with 10% FCS and penicillin/streptomycin (P/S). Immortalized human breast epithelial cells (HMLE), were gifts from Dr. Weinberg (Whitehead Institute). All cell lines were grown as described earlier (Hanai et al., 2013).
Flow cytometry analysis and Fluorescence-Activated Cell Sorting (FACS)
HMLER cells were FACS-sorted into stem cell population (S: CD44 high and CD24 low) and non stem cell population (NS: CD44 low and CD24 high) as described earlier (Hanai et al., 2013).
Tumorsphere formation assay
Single cells were plated in 6-well ultra-low attachment plates (Corning) at a density of ~20,000 viable cells/ml and were processed as described earlier (Hanai et al., 2013). Two tailed t test was used to evaluate the statistical significance of the results. All values are expressed as mean ± S.E for this assay.
Oxygen consumption and PPR analysis by XF24 seahorse platform
The XF24 Extracellular Flux analyzer (Seahorse Biosciences, Billerica, MA) was used to measure oxygen consumption (OCR) and extracellular acidification rate (ECAR). Results were expressed as % ECAR or % OCR.
Steady State Metabolic analysis
Metabolic analysis was performed by BIDMC mass spectrometry core facility. Sample preparation was done according to a procedure developed by the core. Data were analyzed by the web server software located at http://www.metaboanalyst.ca/MetaboAnalyst/faces/Home.jsp. The normalized data were plotted using PRISM software.
Tamoxifen Treatment
All animal studies and procedures were approved by BIDMC Institutional Animal Care and Use Committee and were conducted in accordance with the BIDMC policy on the Care, Welfare and Treatment of Laboratory Animals. As described in the results section, the mice were treated with tamoxifen (10 mg ml−1 in corn oil) or corn oil (only for fig. 3B) (Sigma) by intraperitoneal injection. A total of 76 mice were used in all the experiments.
Doxycycline Treatment
Doxycycline chow (Harlan, Teklad) was used as described previously (Fisher et al., 2001).
PCR genotype and western blot analysis
PCR primers developed by SANGER were used to detect modified LDH-A allele. Western blot analysis was carried out to further validate loss of LDH-A protein.
Statistical Analysis
Means, medians, Welch two tailed t-tests, Wilcoxon rank-sum test and descriptive-statistics were calculated as appropriate using Prism software package or Kaleidagraph (Synergy Software). The Error bars represented in each figure are mean +/− SD or +/− SEM and appropriate analysis is indicated in figure legends. P value p< 0.05 was considered statistically significant.
Tumor initiating cell assay
Athymic mice were injected via tail vein with either A549 cells (104 in 100μl) that were either expressing control shRNA or LDH-A shRNA. For tumor initiating cell assay in syngenic mice, tumor cells (104 in 100μl) isolated from either corn oil or tamoxifen treated Cretm-LDH-Afl/fl;Ccsp-rtTA/(tetO)7-K-RAS4bG12D mice were injected via tail vein. Animals were monitored for 21 days post injection and euthanized as per established guidelines. Total number of animals used for these assays were 20.
Tumor area measuring and Lung metastasis assay
Lung organs from 2mice bearing RAS-driven tumor were removed and washed three times with PBS. Formalin-fixed, paraffin-embedded mouse lung tissue sections were prepared at 5 μm. Each slide 2 or 3 sections cut from different distance were collected and stained by H&E method. H&E stained slides were scanned and tumor area were quantified with Image J (NIH software).
LDH enzymatic and Lactate production assay
These assays were performed as described earlier (Billiard et al., 2013).
Hyperpolarizer and proton imaging
All imaging procedures were approved by our Institutional Animal Care and Use Committee (IACUC) using methods described previously (Seth et al., 2011). 13C magnetic resonance spectroscopic imaging (MRSI) was performed using two-dimensional chemical shift imaging (2D CSI) as described (Kohler et al., 2007).
Micro CT studies
Animals were anesthetized with 2% isoflurane/balance O2. Imaging was performed using the CT component of a NanoPET/CT (Bioscan, Washington, DC) scanner and image analysis was performed using VivoQuant software (inviCRO, Boston, MA) using a Hounsfield Unit windowing technique, normalized for all scans. Volumetric segmentation was performed using a neighborhood threshold.
SIRM analysis
SIRM studies with 13C-enriched tracers were carried out as previously described for both cells in culture and in mice (Fan et al., 2011b; Fan et al., 2012). Metabolites were analyzed by GC-MS and NMR according to standardized protocols as previously described (Lane et al., 2008), using in-house databases of standards for identification (Fan and Lane, 2008). Isotopomer distributions and fractional enrichments were calculated as previously described (Lane et al., 2008).
Warburg Slices
“Warburg slices” are thin slices (<1 mm thick) of tissue, two of which were immediately cut from a freshly resected human NSCLC tumor using a Weck microtome and were maintained in DMEM medium supplemented with 10% FBS, 1X penicillin and streptomycin plus 0.2% 13C6-glucose and 2 mM glutamine or 0.2% glucose and 2 mM 13C5,15N2-glutamine under 5% CO2 at 37°C for 24 h without or with 20 μM Compound 1. The tissues were harvested by rinsing briefly in cold PBS, blotted dry and weighed before flash frozen in liquid N2. Metabolites were extracted and analyzed by 1H{13C} HSQC NMR as described previously (Fan et al., 2011b; Fan et al., 2012).
Supplementary Material
Highlights.
An inducible LDH-A mouse model has been developed and characterized.
Reduced LDH-A results in regression of established tumors in NSCLC mouse models.
Cancer initiating cells are susceptible to LDH-A inhibition.
Pharmacologic inhibition of LDH-A mimics the effects of genetic attenuation.
Acknowledgments
This work was supported in part by Department of Defense award PC094151 (to P.S), grants for target award 2012-01-0602 (to P.S), NIH grants 5R01CA152330, 1R01GM098453 (to P.S), 1P01CA163223-01A1 (to A.N.L and T.F), 1U24DK097215-01A1 (to R.M.H, T.F, and A.N.L) and administrative supplement RM-11-024 (to P.S & T.F), R21EB01447 (to A.G) and startup funds from the Department of Medicine, BIDMC (to P.S). We would like to thank H. Varmus for providing the K-RAS animals, K. Wong for the EGFR-T790M mice, J. Tan and R. Balasubramaniam for processing the mouse and human tissue slice samples for SIRM analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agathocleous M, Love NK, Randlett O, Harris JJ, Liu J, Murray AJ, Harris WA. Metabolic differentiation in the embryonic retina. Nat Cell Biol. 2012;14:859–864. doi: 10.1038/ncb2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–466. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann J, Kalyankrishna S, Massion PP, Ohm JE, Girard L, Shigematsu H, Peyton M, Juroske D, Huang Y, Stuart Salmon J, et al. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res. 2005;65:226–235. [PubMed] [Google Scholar]
- Billiard J, Dennison JB, Briand J, Annan RS, Chai D, Colon M, Dodson CS, Gilbert SA, Greshock J, Jing J, et al. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 2013;1:19. doi: 10.1186/2049-3002-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Costa DB, Kobayashi S, Tenen DG, Huberman MS. Pooled analysis of the prospective trials of gefitinib monotherapy for EGFR-mutant non-small cell lung cancers. Lung Cancer. 2007;58:95–103. doi: 10.1016/j.lungcan.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragovich PS, Fauber BP, Corson LB, Ding CZ, Eigenbrot C, Ge H, Giannetti AM, Hunsaker T, Labadie S, Liu Y, et al. Identification of substituted 2-thio-6-oxo-1,6-dihydropyrimidines as inhibitors of human lactate dehydrogenase. Bioorg Med Chem Lett. 2013;23:3186–3194. doi: 10.1016/j.bmcl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Ebert BL, Gleadle JM, O’Rourke JF, Bartlett SM, Poulton J, Ratcliffe PJ. Isoenzyme-specific regulation of genes involved in energy metabolism by hypoxia: similarities with the regulation of erythropoietin. Biochem J. 1996;313(Pt 3):809–814. doi: 10.1042/bj3130809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Hitosugi T, Chung TW, Xie J, Ge Q, Gu TL, Polakiewicz RD, Chen GZ, Boggon TJ, Lonial S, et al. Tyrosine phosphorylation of lactate dehydrogenase A is important for NADH/NAD(+) redox homeostasis in cancer cells. Mol Cell Biol. 2011a;31:4938–4950. doi: 10.1128/MCB.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan TW, Lane AN, Higashi RM, Yan J. Stable isotope resolved metabolomics of lung cancer in a SCID mouse model. Metabolomics. 2011b;7:257–269. doi: 10.1007/s11306-010-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan TW, Lorkiewicz PK, Sellers K, Moseley HN, Higashi RM, Lane AN. Stable isotope-resolved metabolomics and applications for drug development. Pharmacol Ther. 2012;133:366–391. doi: 10.1016/j.pharmthera.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan TWM, Lane AN. Structure-based profiling of Metabolites and Isotopomers by NMR. Progress in NMR Spectroscopy. 2008;52:69–117. [Google Scholar]
- Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- Fisher GH, Wellen SL, Klimstra D, Lenczowski JM, Tichelaar JW, Lizak MJ, Whitsett JA, Koretsky A, Varmus HE. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–3262. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes S, Clements J, Dawson E, Bamford S, Webb T, Dogan A, Flanagan A, Teague J, Wooster R, Futreal PA, et al. Cosmic 2005. Br J Cancer. 2006;94:318–322. doi: 10.1038/sj.bjc.6602928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- Gazdar AF, Minna JD. Molecular detection of early lung cancer. J Natl Cancer Inst. 1999;91:299–301. doi: 10.1093/jnci/91.4.299. [DOI] [PubMed] [Google Scholar]
- Gillies RJ. The tumour microenvironment: causes and consequences of hypoxia and acidity. Introduction. Novartis Found Symp. 2001;240:1–6. [PubMed] [Google Scholar]
- Hanai JI, Doro N, Seth P, Sukhatme VP. ATP citrate lyase knockdown impacts cancer stem cells in vitro. Cell Death Dis. 2013;4:e696. doi: 10.1038/cddis.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Hill RP, Marie-Egyptienne DT, Hedley DW. Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol. 2009;19:106–111. doi: 10.1016/j.semradonc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- Kohler S, Yen Y, Wolber J, Chen AP, Albers MJ, Bok R, Zhang V, Tropp J, Nelson S, Vigneron D, et al. In Vivo C13 Metabolic Imaging at 3T with Hyperpolarized C13-1-Pyruvate. Magn Reson Med. 2007;58:65–69. doi: 10.1002/mrm.21253. [DOI] [PubMed] [Google Scholar]
- Kohlmann A, Zech SG, Li F, Zhou T, Squillace RM, Commodore L, Greenfield MT, Lu X, Miller DP, Huang WS, et al. Fragment growing and linking lead to novel nanomolar lactate dehydrogenase inhibitors. J Med Chem. 2013;56:1023–1040. doi: 10.1021/jm3014844. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis. 2005;22:25–30. doi: 10.1007/s10585-005-2343-7. [DOI] [PubMed] [Google Scholar]
- Lane AN, Fan TW, Higashi RM. Isotopomer-based metabolomic analysis by NMR and mass spectrometry. Methods Cell Biol. 2008;84:541–588. doi: 10.1016/S0091-679X(07)84018-0. [DOI] [PubMed] [Google Scholar]
- Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock FE, McDonald PC, Lou Y, Serrano I, Chafe SC, Ostlund C, Aparicio S, Winum JY, Supuran CT, Dedhar S. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene. 2013;32:5210–5219. doi: 10.1038/onc.2012.550. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–837. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson LM, Forshell TZ, Rimpi S, Kreutzer C, Pretsch W, Bornkamm GW, Nilsson JA. Mouse genetics suggests cell-context dependency for Myc-regulated metabolic enzymes during tumorigenesis. PLoS Genet. 2012;8:e1002573. doi: 10.1371/journal.pgen.1002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, Chandel N, Laakso M, Muller WJ, Allen EL, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regales L, Balak MN, Gong Y, Politi K, Sawai A, Le C, Koutcher JA, Solit DB, Rosen N, Zakowski MF, et al. Development of new mouse lung tumor models expressing EGFR T790M mutants associated with clinical resistance to kinase inhibitors. PLoS One. 2007;2:e810. doi: 10.1371/journal.pone.0000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:201–205. doi: 10.1513/pats.200809-107LC. [DOI] [PubMed] [Google Scholar]
- Seth P, Grant A, Tang J, Vinogradov E, Wang X, Lenkinski R, Sukhatme VP. On-target inhibition of tumor fermentative glycolysis as visualized by hyperpolarized pyruvate. Neoplasia. 2011;13:60–71. doi: 10.1593/neo.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizan P, Boros LG, Figueras A, Capella G, Mangues R, Bassilian S, Lim S, Lee WN, Cascante M. K-ras codon-specific mutations produce distinctive metabolic phenotypes in NIH3T3 mice [corrected] fibroblasts. Cancer Res. 2005;65:5512–5515. doi: 10.1158/0008-5472.CAN-05-0074. [DOI] [PubMed] [Google Scholar]
- Walenta S, Salameh A, Lyng H, Evensen JF, Mitze M, Rofstad EK, Mueller-Klieser W. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol. 1997;150:409–415. [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Loo TY, Shen JG, Wang N, Wang DM, Yang DP, Mo SL, Guan XY, Chen JP. LDH-A silencing suppresses breast cancer tumorigenicity through induction of oxidative stress mediated mitochondrial pathway apoptosis. Breast Cancer Res Treat. 2011;131:791–800. doi: 10.1007/s10549-011-1466-6. [DOI] [PubMed] [Google Scholar]
- Warburg o. The Metabolism of Tumors. London: Arnold Constable; 1930. [Google Scholar]
- Warburg O. ORIGIN OF CANCER CELLS. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Ward RA, Brassington C, Breeze AL, Caputo A, Critchlow S, Davies G, Goodwin L, Hassall G, Greenwood R, Holdgate GA, et al. Design and synthesis of novel lactate dehydrogenase A inhibitors by fragment-based lead generation. J Med Chem. 2012;55:3285–3306. doi: 10.1021/jm201734r. [DOI] [PubMed] [Google Scholar]
- Xie H, Valera VA, Merino MJ, Amato AM, Signoretti S, Linehan WM, Sukhatme VP, Seth P. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol Cancer Ther. 2009;8:626–635. doi: 10.1158/1535-7163.MCT-08-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Wang X, Gan L, Yu G, Chen Y, Liu K, Li P, Pan J, Wang J, et al. Inhibition of LDH-A by lentivirus-mediated small interfering RNA suppresses intestinal-type gastric cancer tumorigenicity through the downregulation of Oct4. Cancer Lett. 2012;321:45–54. doi: 10.1016/j.canlet.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, Cavanaugh C, Blau CA, Horwitz MS, Hockenbery D, Ware C, et al. HIF1alpha induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J. 31:2103–2116. doi: 10.1038/emboj.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.