Abstract
Nanotechnology introduces a new field that requires novel approaches and methods for hazard and risk assessment. For an appropriate scientific platform for safety assessment, nanoscale properties and functions of engineered nanomaterials (ENMs), including how the physicochemical properties of the materials related to mechanisms of injury at the nano-bio interface, must be considered. Moreover, this rapidly advancing new field requires novel test strategies that allow multiple toxicants to be screened in robust, mechanism-based assays in which the bulk of the investigation can be carried out at the cellular and biomolecular level whilst maintaining limited animal use and is based on the contribution of toxicological pathways to the pathophysiology of disease. First, a predictive toxicological approach for the safety assessment of ENMs will be discussed against the background of a ‘21st-century vision’ for using alternative test strategies (ATSs) to perform toxicological assessment of large numbers of untested chemicals, thereby reducing a backlog that could otherwise become a problem for nanotechnology. An ATS is defined here as an alternative/reduction alternative to traditional animal testing. Secondly, the approach of selecting pathways of toxicity to screen for the pulmonary hazard potential of carbon nanotubes and metal oxides will be discussed, as well as how to use these pathways to perform high-content or high-throughput testing and how the data can be used for hazard ranking, risk assessment, regulatory decision-making and ‘safer-by-design’ strategies. Finally, the utility and disadvantages of this predictive toxicological approach to ENM safety assessment, and how it can assist the 21st- century vision, will be addressed
Keywords: alternative test strategies, nanomaterials, predictive toxicology, safety assessment
Introduction
With the advent of nanotechnology, powerful tools have become available to develop new materials (e.g., building from the atom upwards or through breakdown of bulk materials).[1] Not only has this disruptive new technology promoted the manufacturing of novel products in which nanoscale properties are used to achieve novel types of quantum mechanical operations, optics, catalysis, electronics, energy generation, enhanced material strength, lightweight composites, and conductivity, but it has also introduced synthetic materials that are capable of interacting with biological molecules and cellular processes, many of which exhibit intrinsic nanoscale features or functions. Thus, engineered nanomaterials (ENMs) have lead to the possibility of manipulating biological structure and function in a fundamentally new way, along with the possibility of introducing new types of hazards at the nano-bio interface. Because the unique physicochemical properties of ENMs are quite different from and potentially more complex than chemicals or medicinal agents, this technology produces a new set of challenges for academia, industry and regulatory agencies to develop appropriate safety assessment and risk management strategies. The physicochemical properties of these materials, including their size, shape, surface area, aspect ratio, surface functionalization, coating and charge, zeta potential, crystallinity, dissolution, wettability and redox potential, in determining biological injury must be considered for the safety assessment. In determining nanomaterial safety, the rapid growth of the industry and the many new materials being introduced through innovation, including passive and active structures that are expected to evolve to nanosystems by design, must also be considered.
Although there are rapid advances in research and knowledge gathering with regard to ENM safety, nanotechnology is based on novel scientific principles that should be considered for hazard assessment. Because of the paucity of information and the uncertainty about how to deal with complex three-dimensional ENM structures, the initial trend has been to regard these materials as “new chemical substances” and to base their safety assessment on established methods for chemical toxicity. This approach could ease the burden of having to establish new safety protocols and regulatory procedures to facilitate international trade and cooperation, but categorizing ENMs as new chemical substances has resulted in concern that new hazards, which may emerge at the nano-bio interface, may be overlooked.[2] From experimental observations to date, it is clear that safety assessments indeed need to be adapted to include the contribution of nanoscale properties and function to the pathways of toxicity (POTs), and that this task cannot be accomplished by using descriptive animal studies that evaluate one material at a time.[2-4] Moreover, the advance of the new field of nanotoxicology requires new test strategies that allow multiple toxicants to be assessed in robust, mechanism-based screening platforms in which the bulk of the investigation can be carried out at the cellular and biomolecular level whilst maintaining limited use of animals to ensure that the hazard assessment also addresses pathophysiology of disease (Fig. 1).[3-6] At the University of California Center for the Environmental Implications of Nanotechnology (UC CEIN) and the UCLA Center for Nano Biology Predictive Toxicology we refer to this as a predictive toxicology approach, which is defined as hazard assessment of ENMs, based on POTs at the cellular level that can also be used to compare, rank and predict the possibility of disease.[7] Implementation of a predictive toxicological approach, which can also be applied to chemicals, is dependent on rapid and high-throughput methods and careful selection at the cellular level of the POTs that play a role in the pathogenesis of disease. Once the screening mechanism is validated by animal studies, the bulk of the investigation can proceed in vitro (Fig. 1). The overall effect is to limit animal use. The linkage of ENM physicochemical characteristics to mechanism-based biological outcomes at the biomolecular and cellular levels allows the establishment of structure-activity relationships (SARs), which provides a robust platform on which to base safety assessment.[5] SAR analysis also allows quantitative assessment of the nanomaterial hazard, development of computerized prediction making models, establishment of read-across categories, and safer-by-design approaches for the synthesis of new materials.[8-10] The disadvantage of in vitro high-throughput screening (HTS) and predictive toxicological approaches is that the assays and testing methods often do not conform with the protocols being used by Organization for Economic Cooperation and Development (OECD).[11, 12] Validation of alternative test strategies (ATS) could be a lengthy procedure.
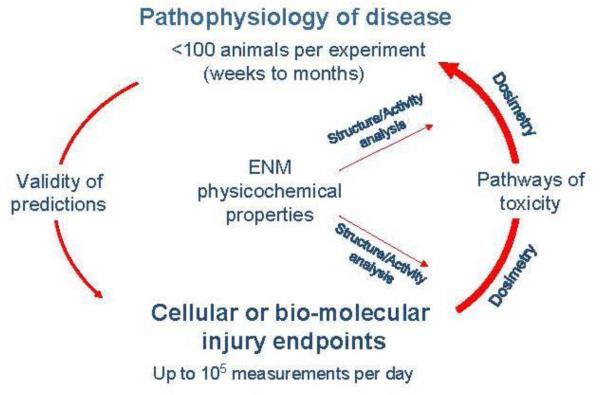
Fig. 1.
Principal components of the proposed predictive toxicological paradigm for engineered nanomaterial hazard assessment. A predictive toxicological approach is defined as establishing and using in vitro mechanisms and pathways of toxicity for predicting the pathophysiology of disease based on defined engineered nanomaterial physiochemical properties that engage the same toxicity pathways in vitro and in vivo. The in vivo outcome is used to validate the in vitro screening approach as being “predictive” and therefore appropriate for screening large batches of materials to obtain quantitative structure-activity relationships that also be apply to the in vivo outcomes. Accordingly, high-content and high-throughput screening in vitro can be used to determine priority of the in vivo approach, where fewer observations can be made owing to cost and time considerations. While the in vitro observations at this stage are not sufficient to drive the safety assessment, an established link to in vivo outcomes could accelarate the in vivo testing becauase of this approach, allowing the use of fewer animals.
In this review, a predictive toxicological approach for safety assessment of ENMs will be discussed against the background of the “21st-century vision” for using ATSs to perform toxicological assessment of a number of untested chemicals, thereby reducing the backlog that has accumulated.[13, 14] Here, ATS is defined as an alternative to animal experiments or refinement/reduction alternatives to traditional animal testing. The method for the selection of a POT to screen for the pulmonary hazard potential of carbon nanotubes (CNTs) and metal oxides (MOxs) will also be reviewed, as well as how to use POTs to perform HTS and how the data can be used for hazard ranking, risk assessment, regulatory decision-making and safer-by design strategies. Finally, the utility and drawbacks of this approach, and how it can assist the 21st-century vision will be considered.
The 21st century vision for toxicological testing of chemicals
As a result of the accumulation of a large number of untested chemicals, and recognizing the scientific opportunities introduced by “omics” technologies, imaging and computational platforms, the National Research Council (NRC) of the US National Academies of Science has recommended a new approach to toxicity testing as outlined in the 2007 report, Toxicity Testing in the 21st Century: A Vision and a Strategy.[14] This strategy proposes a shift from primary in vivo studies in animals to in vitro platforms that can be used for high content data generation and computational toxicological modeling. The NRC vision stresses the importance of performing high content toxicological analysis that assesses multiple chemicals to decrease time and cost burdens and provide strong endorsement of the POT concept. The overall shift from predominantly in vivo to in vitro data generation is a paradigm shift towards a systems biology or toxicology approach.[13, 15]
The US Environmental Protection Agency (EPA) has pursued the NRC recommendations through the establishment of the ToxCastTMTM program (Fig. 2).[16-18] This effort includes the introduction of more than 600 commercial assays using primary cell cultures, virtual organs, and zebrafish embryos to perform biochemical assays for protein function, multi-cell interactions, cell-based reporter assays, transcriptomics and developmental analysis. The goal of the program, which has screened ~1800 chemicals to date, is to obtain sufficient in vitro data to perform bioactivity profiling and collect phenotypic signatures that can be applied to chemical safety assessment.[19] Moreover, the ToxCastTM program has been integrated into a wider Tox-21 initiative, which also includes the National Toxicology Program at the NIEHS, the National Institutes of Health Chemical Genomics Center at the NIH and the US Federal Drug Administration.[20] Through the implementation of high throughput screening (HTS) efforts and validation of ATS approaches, Tox-21 is attempting to facilitate movement from in vivo to in vitro testing for chemical safety evaluation with data incorporation into a streamlined risk assessment process.
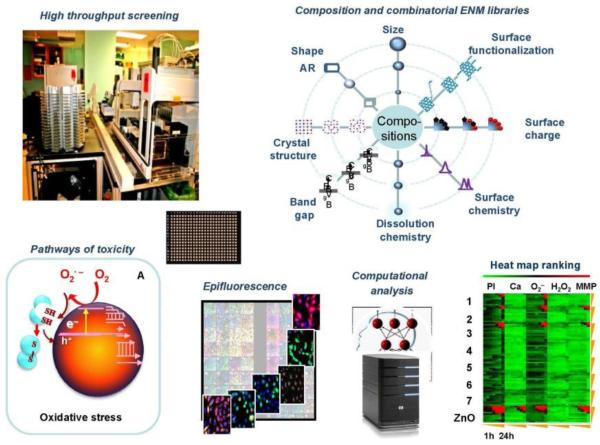
Figure 2.
Tools and infrastructure requirements for developing predictive toxicology and structure-activity relationship (SAR) analysis through high-content and high-throughput screening (HTS) at UCLA. Initially, high-content screening and HTS based on pathways of toxicity that can be assessed in single- and multiparameter assays were performed using material compositions that are representative of the major categories of engineered nanomaterials being produced commercially (e.g. metals, metal oxides, carbon nanotubes and different types of silica). HTS requires the use of robotized equipment and methodology that invovles epifluorescence spectroscopy, UV-visual spectroscopy, luminescence and multiplex cytokine/chemokine assays. The data analysis framework includes in silico tools for data analysis, data transforation, machine learning and modeling of the HTS data. In the example shown, a multiparameter assay for oxidative stress was used to collect epifluorescence data that are displayed in the heatmap, which ranks the data according to a green/red color index. With the accumulation of knowledge about different ENM compositions that use toxicological pathways, we also acquired or synthesized combinatorial ENM libraries in which individual or groups of properties are accentuated to determine their contribution to the toxicological outcome. This allows the development of SAR analyses using computational methods.
HTS was originally developed as a discovery tool for drug development, allowing screening of >100,000 compounds in a single day.[21] It is important to note that HTS by Tox-21 is not currently used as a primary screening tool for safety assessment of toxic substances but aimed, instead, on large-scale data collection to perform toxicological profiling, using robotic testing platforms. This requires large test batteries to characterize thousands of chemicals, because each test only reflects a limited amount of information about the possible adverse biological impact of a given structure. However, it is envisaged that the use of large data bases could be combined with increasingly sophisticated in silico and machine learning platforms to identify within these toxicological signatures the mechanisms of action and new biological markers for hypotheses development and predictive toxicological modeling. From the UCLA perspective, this could also provide an excellent opportunity for facilitating the use of POTs to pursue predictive toxicological approaches. It is important to keep in mind the scale of the results from HTS in large screening programs (e.g., ToxCastTM or Tox-21) when comparing this to the more limited HTS for ENMs (see below). There is currently no need to assess hundreds to thousands of materials in a single screen.
Integration of predictive toxicological approaches for ENMs with the 21st-century vision for the use of ATS for toxic substances
Although the original NRC strategy was principally aimed at addressing large numbers of untested industrial chemicals, the concept also applies to the rapidly expanding number of ENMs. For instance, through control of CNT synthesis, it is possible to derive >10,000 variations by changing wall number, tube length, surface functionalization, coatings, electronic properties, impurities, and other factors. To improve the scope of ENM safety assessment, the UCLA Center for Nano Biology and Predictive Toxicology has introduced a predictive toxicological approach for CNT safety assessment (Fig. 1). To initiate such an approach without sufficient variation in materials and properties to begin screening, we used in-house synthesis and acquisition of compositional and combinatorial ENM libraries to achieve adequate variation of physicochemical properties for rapid throughput screening and high-content data generation and the nano-bio interface.[5] Combinatorial libraries include systematic variation and accentuation of physicochemical properties to explore how those specific properties or array of properties shape biological outcome, as reported by a POT (Fig. 2).[5] This investigation was facilitated by the implementation of high content and HTS approaches to compare batches of materials, ranging from a few (4-8) to large numbers (up to 25) in each comparative analysis.[5, 6] For the purpose of this review, we define high content screening as an analysis that can be performed in 96 well plates (or through automated image analysis), whereas HTS is performed by robotic equipment in 384 well plates. While less than the hundreds to thousands of chemicals being analyzed in the ToxCastTM program, 75,000 data points can be generated in a single comparative HTS assay using multi-parameter cellular responses at hourly intervals (for several hours).[22, 23] We also use multiple concentrations of each ENM to cover a wide dose range. To date, our choice of POTs has been shaped by proteomics discovery or cell biology integrated with pathophysiology of disease (discussed below). A short synopsis of potentially useful mechanistic injury pathways in use to develop predictive toxicological paradigms appears in Fig. 3. Although the volume of screening falls far below the levels of discovery based on toxicogenomics, proteomics, metabalomics and virtual tissue studies in Tox-21, our predictive toxicological efforts have, nonetheless, allowed us to perform comparative analysis of relatively large number of ENMs that can now be used for in vivo and in risk translation studies.[5]
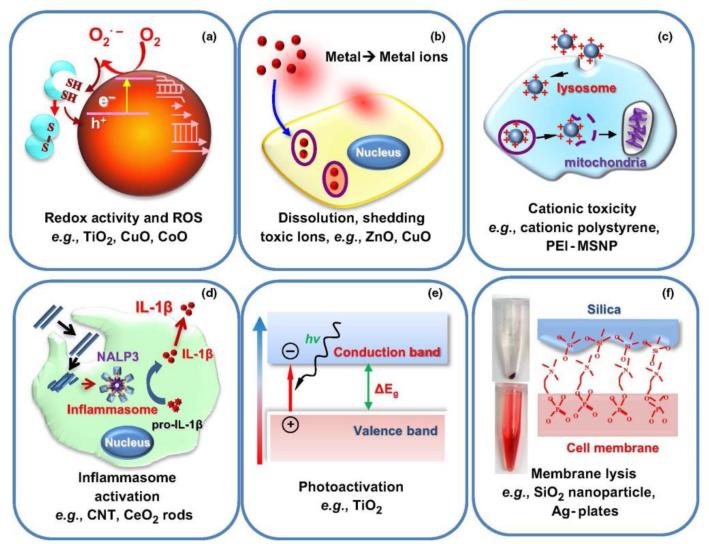
Figure 3.
Pathways of toxicity (POTs) that can be used for in vitro screening, but also have a role in the pathophysiology of disease. POTs are carefully chosen to allow in vitro screening to be predictive of in vivo outcome, thereby enabling large numbers of engineered nanomaterials (ENMs) to be simultaneously assayed, ranked and then used for focused in vivo investigation (see also Fig. 1). (a) induction of oxidative stress by redox active ENMs capable of reactive oxygen species (ROS) generation by the material itself or as a consequence of interaction at the nano-bio interface; (b) material dissolution or shedding of toxic metal ions by metal and metal oxide nanoparticles; (c) cationic injury to the surface membrane or the lysosome; (d) activation of the NRLP3 inflammasome by long aspect ratio materials, which also induce pulmonary fibrosis; (e) photoactivity and generation of electron hole pairs, leading to ROS production; (f) membrane lysis by reactive surface chemistry of a variety of materials.
The final requirement for a predictive logical approach includes the use of a data repository and in silico tools for data analysis, data transformation, machine learning and modeling.[5, 6, 9, 10] Because HTS data are highly dimensional, we use dimension reduction and feature analysis tools to project the high content data onto lower dimension spaces. One visual approach is cluster analysis to extract similarity patterns and perform ENM grouping based on homologous biological outcomes that can be linked to ENM physicochemical properties.[9] Heat-maps provide ordered representations of data that facilitate the identification of similarity patterns in large datasets. A complementary approach is the use of topological preservation methods that are capable of maintaining, in the projected space, the distances measured in the original feature space. The self-organizing map (SOM) algorithm is a valuable computational tool for data transformation.[10] Compared with heat maps, SOM projections facilitate the discovery of imbedded relationships between variables by providing topologically ordered representations of the input space. The data clustering can be visualized over the SOM grid by displaying the distances between cluster centers (distance matrices) or by projecting features onto the different component planes that allow for a more detailed evaluation of the parameters in these planes. In addition to the ranking and prioritization of ENM toxicological potential, UC CEIN utilizes a number of computational, statistical and mathematical programs to establish quantitative structure-activity relationships (QSARs). QSARs are defined as statistical models that relate structural or property descriptors of a chemical to its biological activity.[8, 24] Relative to the chemical world, where SARs can be developed based on the availability of large data bases for both physicochemical properties and toxicology, nano-SAR development uses a different approach in which the nanoscale properties of ENMs must be considered in addition to chemical makeup. As a result of our HTS data, we have been able to develop the 1st generation of multivariate nano-SARs (e.g., concentration, exposure times, sub-lethal and lethal response outcomes). This approach has helped us to establish a relationship between metal oxide nanoparticle parameters that generate oxidative stress and acute lung inflammation, as discussed below.[23] In addition, a predictive toxicological paradigm for CNTs, dependent on the long aspect ratio (LAR) and surface properties that culminate in lysosomal injury and pulmonary fibrosis, will be discussed below.[25-28]
Use of a predictive toxicological paradigm for HTS and SAR analysis of metal oxide (MOx) nanoparticles, premised on oxidative stress and pulmonary inflammation
MOx nanoparticles are widely used in cosmetics, sunscreens, catalysts, textiles and for energy production (e.g., solar batteries). While no human disease has been reported as a result of engineered MOx exposure, it is known that welders inhaling metal fumes (including zinc oxide, magnesium oxide, chromium oxide or cadmium oxide) can develop metal fume fever, an acute inflammatory respiratory illness that is characterized by transient neutrophil inflammation in the lung, usually without chronic consequences.[29] The standard approach for assessing engineered MOx nanoparticle toxicity includes one or more cellular assays combined with rodent exposures, either inhalation studies (acute, subacute or subchronic inhalation) or bolus installation [oropharyngeal or intratracheal (IT)] routes.[4, 30-33] The cellular endpoints that are most frequently used include cytotoxicity (e.g., LDH release, propidium iodide uptake or the MTT assay), apoptosis, ROS production, cytokine production, altered cell growth, and genetic toxicity (e.g., DNA damage and repair, mammalian cell micronucleus testing orcomet assay).[30] The pulmonary response outcomes usually focus on analysis of BAL fluid to measure total and polymorphonuclear (PNM) cell counts, protein, LDH and cytokine levels, and lung histology (using haematoxylin and eosin or more specialized staining).[31] There are also a number of OECD guidelines for oral, dermal and reproductive toxicity, teratogenicity, genotoxicity, neurotoxicity and endocrine tests that can be performed for in vivo chemical analysis.[32] Outside of the pulmonary field, most of these assessment methods have to be properly integrated into ENM toxicity assessment.
Although frequent attempts have been made to reconcile cellular with in vivo outcomes, data regarding the effectiveness of comparisons carried out without using POTs are conflicting. For instance, Becher et al. compared the in vitro pro-inflammatory effects (IL-6, TNF-α and MIP-2) of ceramic (stone) nanoparticles in macrophages and epithelial cells with acute pulmonary inflammation (PMN cell counts in the BAL fluid) of rats receiving IT instillation, and showed good data correlation.[33] In contrast, Sayes et al. failed to demonstrate a relationship between in vitro and in vivo results using nano-and micron-size particles (carbonyl iron, crystalline silica, amorphous silica, nano-ZnO and fine-sized ZnO) in a well-designed dose-response design.[34] The in vitro endpoints included the assessment of LDH release, metabolic activity (MTT assay) and cytokine production (IL-6, TNF-α and MIP-2) in rat L2 lung epithelial cells and primary rat alveolar macrophages as well as co-cultures of these cells, while the in vivo endpoints (at 24 hr and up to 90 days post-instillation) looked at PMN cell counts and LDH values in the BAL fluid of rats receiving IT instillation of the particles.[34] The authors concluded that there was a poor correlation between in vitro and in vivo effects when comparisons were made at the highest doses. However, Oberdörster et al. re-analyzed the Sayes data by converting the particle mass data to particle surface area dose (SAD) and then performed the comparison at the steepest slope of the dose-response curves. For the 24 hr (most complete) data set, this analysis showed that there was indeed a good correlation between MIP-2 levels in vitro vs. the PMN response or LDH levels in the lung. The conclusion was that it is possible to show in vitro/in vivo predictions with a SAD normalization metric. The Oberdörster laboratory went on to demonstrate in their own cell-free and cell-based assays that measurement of ROS production, LDH release and activation of the IL-8 gene showed good correlation with the PMN levels in the BAL fluid of rats being challenged by IT instillation of 7 distinct particle types (Au, nano-TiO2, fine TiO2, NH2-PS, Ag, elemental carbon, Cu).[35] In addition, Donaldson's laboratory demonstrated that IL-8 production in A549 cells, exposed to a panel of low toxicity (e.g., TiO2, carbon black) versus highly reactive α-quartz and metal (e.g., Ni, Co) nanoparticles, showed good correlation to PMN cell counts in the BAL fluid of Wistar rats.[36, 37] The latter report also demonstrated that expressing the particle SAD against PMN cell counts yields a shallow dose-response curve for low toxicity materials, while highly reactive materials yielded a steep, left-shifted dose-response curve. This led to the proposal that SAD expressed per unit PMN cell number can be used as a metric for surface reactivity or “surface specific responsiveness”.
Given the uncertainty about the prediction making capabilities of cellular tests for ATS, we aimed to determine whether analysis of in vitro and in vivo response parameters could be used to define a POT that is premised on intrinsic MOx physicochemical properties associated with cellular injury and whether the analysis can explain the pathophysiology of acute pulmonary inflammation (Fig. 1).[23] Our goals included the development of a HTS platform to advance the safety assessment of these materials and to establish SARs that predict biological hazard premised on fundamental MOx nanoscale properties. Based on a literature survey and our own exploration of the pro-inflammatory effects of ultrafine air pollution particles and oxide nanoparticles in generating reactive oxygen species (ROS) and acute pulmonary inflammation, we developed the hierarchical oxidative stress framework to study the pro-oxidant effects of MOx nanoparticles according to three tiers of oxidative stress.[2, 38] The hierarchical oxidative stress response pathway was based on proteomics and cell-based discoveries.[2, 39] The first tier, as a result of low levels of ROS production, induces protective cellular responses that require the activation of the Nrf2 transcription factor. Nrf2 controls more than 200 genes involved in antioxidant and anti-inflammatory defense. If this homeostatic mechanism fails to restore redox equilibrium, the 2nd tier of oxidative stress initiates the activation of pro-inflammatory signaling pathways (e.g., MAP kinase and NF-B pathways) that control the activation of pro-inflammatory responses. This leads to the production of sets of cytokines and chemokines that are characteristic of specific cellular phenotypes. For instance, we have demonstrated that ZnO nanoparticles initiate oxidative stress effects in the lung epithelial cells and macrophages (as well as representative cell lines) as a result of activating cytokine and chemokine genes that play a role in acute neutrophilic inflammation in the lung (i.e., mimicking metal fume fever).[40] In the third tier, even higher levels of oxidative stress induce mitochondrial perturbation and triggering of organelle-specific ROS generation, decrease of mitochondrial membrane potential and the initiation of mitochondrion-mediated apoptosis or necrosis pathways. Based on the hierarchical paradigm, we set out to establish a series of single-parameter assays to cover the full range of biological responses in each tier; this helped to confirm the importance of oxidative stress as an important POT for MOx's (as well as other categories of ENMs that operate through different physicochemical properties).[38, 40] However, because it is laborious to perform an extensive series of assays for each comparative analysis, we decided to use an assay that could integrate sublethal and lethal oxidative stress responses into a multi-parameter HTS assay.[22, 23, 41, 42] Following experimentation with a series of fluorescent dyes with compatible wavelengths that could be combined into a dye cocktail, we have been able to develop an assay that assesses ROS generation (MitoSox Red), intracellular calcium flux (Fluo-4), mitochondrial membrane depolarization (JC-1), and leakage of the surface membrane (propidium iodide uptake) in dead cells, using automated epifluorescence microscopy that is performed at hourly intervals for 8 and then 24 hrs.[22, 23, 41, 42] Since the critical steps in the assay (e.g., cell seeding, liquid handling, nanoparticle addition at 10 different doses over a 3-log range, capture of epifluorescence images, image analysis, etc) are automated, we could complete the assay in a single day compared to the 2-3 weeks to complete the entire series of individual assays. Full details about HTS assay development have been published elsewhere.[43]
Utilizing the multi-parameter assay for oxidative stress, it became possible to contemporaneously assess the cellular responses of 24 MOx's.[23] This has allowed us to generate >70,000 data points, using side-by-side comparison of bronchial epithelial and macrophage cell lines.[23] Using a heat map to display the data, we could begin to make predictions about the likely in vivo effects of seven nanoparticles (Co3O4, Cr2O3, Ni2O3, Mn2O3, CoO, CuO, ZnO), showing multi-parameter oxidative stress responses in vitro versus particles that did not generate a response. This allowed us to use in vitro hazard ranking to plan in vivo testing in mice receiving oropharyngeal particle instillation. In a matter of two comparative experiments, we could demonstrate excellent agreement between the in vitro results and acute inflammatory effects in the lung, as demonstrated by assessment of PMN counts, LDH and cytokine levels (MCP-1 and IL-6) in the BAL fluid.[23] Moreover, the multi-parameter results showed good correlation with single parameter assays such as MTS, LDH release and measurement of cellular ATP levels. Subsequent in silico analysis using mathematical and statistical methods to calculate a parameter for predicted probability of toxicity that could be used for mathematical regression analysis; this enabed accurate prediction of in vivo toxicological outcome for five materials (Co3O4, Cr2O3, Ni2O3, Mn2O3 and CoO) based on bandgap energy.[23, 44, 45] Moreover, in the analysis of nanoscale properties, the conduction band energy (Ec) levels of these materials showed overlap with a cellular energy equivalent known as the redox potential (-4.12 to -4.84 eV). A possible explanation for the SAR linking the Ec values of specific materials to electron exchange with cellular redox potential is that semiconductors require energy equivalence to function, including for industrial applications.[23, 46] Two of these couples, oxidized/reduced cytochrome c and NADP/NADPH, could be shown to participate in electron exchange with the 5 particles showing overlapping Ec. For two of the seven materials involved in vivo toxicity (ZnO and CuO), the cause was a high rate of dissolution, rather than Ec. The ability to rank MOx's into distinguishable hazard categories by a predictive and HTS paradigm holds the promise of replacing “one-material-at-a-time” toxicological testing with an approach that can rapidly place MOx's into testable hazard categories. The utility of the approach is demonstrated by our ability to perform a complete predictive toxicological analysis for 24 materials in a matter of months rather than the 2-3 years it would have taken with conventional approaches. This work is ongoing and needs to address the role of particle size, MOx doping, and heterojunctions in establishing bandgap and Fermi energy levels.
A predictive toxicological approach for pulmonary hazard ranking, grouping and SAR analysis of CNTs based on aspect ratio and surface catalytic properties
Thed toxicological approach described in this section was developed through an understanding of the pathophysiology of disease rather than the use of “omics” discoveries. Experimental in vitro and in vivo studies have shown that CNT exposure can induce various deleterious biological effects, including granulomatous inflammation (in the lung), coronary artery dysfunction, pulmonary fibrosis, oxidative stress, DNA fragmentation/mutation and disruption of the mitotic spindle (with possible mutagenic effects).[47-59] The most immediate concern to public health has been the potential impact on the lungs of people working with CNTs.[48] Although not a single incident of human CNT toxicity has been reported to date, the possibility that CNTs could act as “fiber-like substances” that induce chronic lung inflammation, fibrosis and mesothelial injury suggesting that they could be the next asbestos.[55, 56] While my own view is that the studies depicting the fiber paradigm are premised on a select number of longer length tube formulations that agglomerate into carbon fibers (but not representative of most CNT formulations), a key question becomes whether it is possible to rank pulmonary hazard potential by using mechanistic cellular studies that reflect the potential of chronic lung inflammation and fibrosis.[25, 28] Which cellular responses should be assessed and how can these adverse effects be related to physicochemical properties such as the state of aggregation/dispersion, hydrophobicity, wall number, aspect ratio, surface functionalities, electronic properties, impurities and coating of the tubes?
Pulmonary fibrosis is a pathological outcome to multiple afferent stimuli, including exposure to inhaled toxic substances such as air pollution particles, fibers and metals. There is cumulative evidence that cooperation between targeted macrophages and epithelial cells play a central role in the pathogenesis of pulmonary fibrosis.[53, 59] Cooperation between IL-1β producing macrophages and epithelial cells in the alveoli and airways leads to epithelial-mesenchymal transition (EMT), which is a graded phenotypic transition towards myofibroblast or fibroblast characteristics.[25-28, 59] Moreover, chemokine release by injured epithelial cells recruits more macrophages and contributes to the production of TGF-β1 and proteases, which further promote EMT. The differentiating myofibroblasts and fibroblasts migrate to small airways and alveoli where they deposit collagen and extracellular matrix in quantities proportional to the severity of the afferent stimulus. Platelet-derived growth factors (PDGFs) produced by mesenchymal cells and macrophages are important pro-survival factors that assist in the replication and migration of the cells undergoing EMT. TGF-β1 is an important growth factor that contributes to collagen synthesis and establishing a matrix synthesis phenotype in the injured lung.
Against this background, we set out to determine whether it is possible to study representative macrophage and epithelial cells ex vivo to determine whether exposure to potentially hazardous MWCNTs could elicit IL-1β, TGF-β and PDGF production. These responses could then be compared to similar biomarkers in a murine model for subchronic lung inflammation and fibrosis.[25-28] Utilizing a series of MWCNT “libraries” in which key properties were accentuated, we showed that quantification of IL-1β production in THP-1 cells (or alveolar macrophages), TGF-β1 production in immortalized bronchial epithelial (BEAS-2B) cells and synergistic PDGF-AA production in a co-culture of these cells, could mimic the response generation in the lungs of mice receiving oropharyngeal installation.[25-28] The only difference is that the appearance of the in vivo biomarkers in the BAL fluid occurs at different time intervals, which reflectled the progression of a subchronic disease process. IL-1β production pre-stages (at 40 hr) TGF-β1 and PDGF-AA release, which followed days later. Interestingly, the tube characteristics associated with the biomarker generation at cellular and organ levels showed excellent correlation with the ability of the same materials to induce pulmonary fibrosis.[25, 26] These include CNT characteristics such as the state of aggregation, hydrophobicity, surface coating, and surface functionalization.
The relationship between specific tube characteristics and IL-1β production in macrophages was further exploited using THP-1 cells, which serve as a model for exploring a mechanistic injury pathway that has been ascribed to LAR materials such as CNTs and metal wires.[25-28, 57, 60] This mechanistic response involves lysosomal damage, which serves as a precursor for IL-1β production in phagocytic cells (Fig. 4).[25-28, 57, 59, 60] Lysosomal damage leads to cathepsin B release, which along with other stimuli is involved in the assembly of the NLRP3 inflammasome subunits. Among these, the caspase 1 subunit is responsible for pro-IL-1β cleavage and IL-1β release. We confirmed the relationship between hazardous CNT properties and NLRP3 inflammasome activation using THP-1 cells in which the NLRP3 or ASC subunits are knocked down. Moreover, IL-1β generation showed good correlation to confocal imaging of THP-1 and pulmonary alveolar macrophages, where cathepsin B release can be followed using the fluorescent substrate, Magic Red.[25, 26, 28] We demonstrated that this injury response is dependent on the suspension stability of the tubes in acidic lysosomal fluid. For tubes dispersed by surrogate lung lining fluid (containing BSA and a phospholipid detergent), the instability of the coat under acidic lysosomal conditions leads to tube aggregation and damage to the lysosomal membrane.[26] If, however, the tubes are coated by a triblock copolymer (Pleutonic F108), which remains stably attached under acidic conditions, the associated lysosomal damage, IL-1β production and pulmonary damage are no longer observed.
The provisional SAR analysis obtained from these studies implicate at least two integrated sets of tube characteristics: one set determining cellular uptake and subcellular localization and the second being dependent on the surface catalytic properties of the tubes leading to lysosomal damage. To date, our data indicate that tube aggregation/dispersion, dimensions (including length and aspect ratio), and surface coating play an important role in cellular uptake and subcellular localization, while surface catalytic activity (propagated by defects, metal contaminants, surface groups, etc.) determines catalytic damage to the lysosomal membrane. Most of the in-house exploration has focused on MWCNTs, we have also done an extensive analysis of a variety of covalent surface-functionalized MWCNTs and SWCNTs manufactured by different processes to induce lung injury that could be predicted from cellular analysis.
All considered, the analysis of a specific set of cellular responses (IL-1β, TGF-β and PDGF-AA) ex vivo, in combination with specific cellular injury response mechanisms (lysosomal damage, NLRP3 inflammasome activation), appears to accurately predict in vivo response generation as determined by assessment of similar biomarkers, histological assessment of pulmonary fibrosis and assays for collagen deposition. To date, enzyme-linked immunoassays to detect IL-1β, TGF-β and PDGF-AA production in THP-1, BEAS-2B cells or co-culture supernatants have provided sufficient throughput capacity to compare up to a dozen or so different tube formulations in a single analysis, without having to resort to an automated HTS assay. If, however, there is widespread agreement that the IL-1β and growth factor platform constitutes a valid predictive platform for CNTs, we could develop a multiplex HTS assay to assess larger CNT batches for safety analysis.
Integration of predictive toxicology and ATS with qualitative and quantitative risk assessment approaches
The introduction of alternative, mechanistic and predictive approaches could play a role in regulating ENM safety; however, decision-making in this area has historically been slow to adapt to new scientific discovery because of the: (i) statutory limitations that apply to regulatory agencies, (ii) difficulty for industry to deviate from standard protocols because of cost and time considerations, and (iii) duration of the approval and validation process at the international level, where it is not easy to change agreed-upon protocols.[11, 13] Risk assessment will need to evolve to accommodate predictive toxicological approaches and ATS, which because of the complexity of “omics” platforms, robotics, HTS, and the need for computational analysis of large databases is often interpreted by the risk assessment community as only an in vitro enterprise with no clear connectivity to traditional risk assessment methods. However, the bottleneck that has been created using traditional safety assessment methods to deal with large numbers of chemicals or untested toxicants has generated a great deal of concern, in addition to impacting efforts to advance new technologies to the marketplace.[13] Consequently, there appears to be growing awareness among regulators and industries worldwide that the status quo has to change and that it is time to consider a role for ATS and predictive toxicological modeling. Among the adaptations that are taking place at the U.S. EPA (ToxCastTM) and the U.S. National Institute of Occupational Safety and Health (NIOSH) is the use of exposure information collected at occupational research and development facilities to estimate lung burdens that can be related to animal and in vitro studies.[61-66] For instance, modeled alveolar surface area concentrations (μg/cm2), representative of short-term (24 hr) and long-term (lifetime) occupational exposures are used for comparison to in vitro (μg/cm2) metrics in the tissue culture dish.[61, 62, 65] Calculation of the pulmonary alveolar SAD is accomplished by using tools such as multi-path particle dosimetry (MPPD) modeling to estimate the deposited mass dose in the alveolar region; this dose is converted to unit SAD that can be compared to similar dose metrics in vitro.[61] While this concept makes good sense when applying daily or short-term deposited dose, the use of chronic or lifelong exposure data could lead to the application of excessively high in vitro doses.[62] Nonetheless, NIOSH has demonstrated the utility of surface area dose (SAD) extrapolations in performing short-and long-term rodent exposures to convert the animal lung burdens to equivalent dose metrics in humans, using the morphometric approach of Stone et al.[67] According to these calculations, the comparable epithelial surface areas of the mouse, rat, and human lung are 0.05, 0.4, and 102 m2 per lung, respectively.
In an attempt to integrate the predictive toxicological approach with more traditional risk assessment in which we can use inter-species dose-response extrapolations, it was necessary to consider the relevance of bolus instillation approaches during performance of in vivo testing and hazard ranking, because this exposure method delivers a high dose rate and high lung burdens, which could overwhelm normal clearance mechanisms.[68] This could lead to lung overload, with the potential to introduce additional pathophysiological mechanisms that do not focus on the original POT that is being pursued. Moreover, oropharyngeal aspiration and IT instillation can also result in a non-uniform particle distribution in the lung,[69] requiring particle suspension in biologically compatible media that often result in significant agglomeration.[70] However, in spite of these issues, Henderson et al. has reported that IT studies provide useful hazard identification, hazard ranking, and mechanistic information that are comparable to biological response outcomes in inhalation studies, provided that lung burdens are kept the same.[71] Historically, bolus exposures to crystalline silica in animals have been shown to predict human responses while IT instillation has been found to be acceptable by the European Union in studies looking at the biopersistence of fibers linked to lung pathology.[72] Moreover, oropharyngeal aspiration in mice can deliver uniform particle deposition throughout the lung if the nanoparticles are suspended in surrogate alveolar lining fluid.[73, 74] NIOSH investigators have also demonstrated that bolus dosing can achieve qualitatively similar pulmonary responses during short-term inhalation exposures, provided that lung burdens are similar, particle overload is avoided and the particles are well dispersed in a biocompatible dispersant.[47, 75-77]
To integrate our predictive toxicology approach with risk assessment, we are collaborating with NIOSH on a tiered approach to reduce the expense of traditional 90 day inhalation studies for establishing occupational exposure limits (OEL's). In this tiered approach, which can also be used for regulatory decision-making, in vitro assays are developed to be predictive of specific POTs, allowing ENMs to be ranked and grouped for selecting representative materials within a group or mechanistic category for further testing in short-term animal studies (Tier 1). These studies are then carried out using oropharyngeal or IT instillation methods to validate the mechanism of injury, the material potency and demonstrating a relationship to pathological outcomes (Tier 2). Tier 3 inhalation studies are even more focused test the most potent ENMs within each physicochemical and/or mechanistic category. Measurement of lung burden in the inhalation studies can be used to reconcile with particle deposition in the human lung in a more traditional risk assessment approach.[25-28, 75-77]
Quantitative/absolute and quantitative/relative risk estimates can be used to inform ENM risk assessment. Quantitative risk assessment (QRA) is the estimation of the severity and likelihood of adverse responses associated with exposure to a hazardous agent.[78, 79] When sufficient dose-response data are available, QRA's can be developed by using data from benchmark materials that have been extensively investigated for comparative analysis to ENMs.[64-66, 80] Benchmark materials (e.g., particles) are well-characterized substances for which the occupational health risks have been estimated and OELs developed, e.g., silica and asbestos. However, ENMs often do not have sufficient data to develop absolute risk estimates, and under these circumstances, categorization approaches may be of use, e.g., considering the similarity of physicochemical properties, biological modes of action, SARs and comparative potency analyses.[66, 81, 82] An important role for ATS is to provide high-throughput data (e.g., short-term in vivo and in vitro) to predict the effects in the workplace. The benefits of characterization using a predictive toxicological approach and HTS include more efficient use of data, reduced animal use and costs, increased sample size, greater robustness of results and increased biological plausibility for other materials in the same mode of action category.[82] Read-across is a powerful tool for toxicological predictions based on similarities of chemical structure, which in the case of ENMs should focus on physicochemical similarities and SARs. In addition, ATS can be a useful tool to develop control banding, which has been used by the pharmaceutical industry as a pragmatic approach to manage the exposure risk of multiple toxicants in the absence of firm toxicological and exposure data.[66, 83, 84] In essence, this risk assessment approach is premised on the likely severity of the hazard and the anticipated probability of exposure. Both hazard and exposure are integrated into a limited number of bands or levels, which combines to yield control or risk bands. The hazard ranking used in a predictive toxicology approach could play an important role in hazard banding.
Use of predictive toxicology and SAR analysis of safer design of ENMs: implications for nanomedicine
An important advantage of high content data generation and development of SARs is the identification of specific physicochemical properties that contribute in a major way to hazard outcome, with the possibility to change, passivate, or eliminate these properties while maintaining a commercially useful function. We have demonstrated that a reduction of ZnO nanoparticle dissolution by Fe-doping can reduce toxicity as determined by the oxidative stress paradigm.[22] However, whether this will be useful in a product such as a sunscreen lotion, will require that material to maintain its UV reflection properties without discoloration of the skin. While the former requirement is met by Fe-doping, the doped formulation undergoes discoloration and is therefore not useful as a commercial procedure for this application. However, other dopants could be tried to accomplish both requirements. One also has to consider whether a decreased rate of dissolution could inadvertently cause more harm in the environment because a less soluble particle could be transported over long distances than a soluble material.
Another demonstration of a possible safer by design principle is the passivation of SWCNT and MWCNT surfaces with the polymer, Pluronic F108.[26, 85] Coating of the tube surface with this triblock copolymer, leads to surface stabilization and passivation in the acidic lysosomal environment, thereby preventing catalytic damage to the lysosomal membrane. This eliminates triggering of IL-1β production and the subsequent series of events that leads to pulmonary fibrosis.[26] While clearly impractical as a safer by design tool for many industrial processes, CNT coating with polymers could be used for the development of nanotherapeutics and imaging agents that make use of the potentially novel properties of these materials in a biological environment.[86]
While these represent early examples of what can be achieved by safer design strategies, the use of SARs to identify the opportunities for improved design is likely to multiply with the introduction of HTS and the computational methods accompanying ATS. This introduces unique opportunities for industry to use SAR analysis as an integral and up front component of material design, as well as for the development of biologically useful materials. The principles of a safer-by-design approach would be of particular use in the field of nanomedicine, where the introduction of progressively sophisticated, therapeutic and diagnostic nanomaterials requires rigorous safety assessment without a clear set of guidelines from regulatory agencies as to how to deal specifically with nanoscale principles to be comparable with the SARs being used for medicinal chemistry.
Potential disadvantages of ATS and predictive toxicology approaches
With so much effort going into the establishment of a 21st-century framework, this paradigm shift requires a large investment in intellect, funding, time and regulatory reframing. Consequently, it requires an honest assessment of the potential disadvantages of such an approach.[87-89] There is always a concern that the emphasis on studying cells is a reductionist approach, which cannot simulate the complexities of an in vivo system. Traditional toxicity testing is premised on determining whether, over a specific dose range relevant to real-life exposures, toxic substances can result in measurable health effects outcomes in an intact animal. In contrast, in vitro and ATS approaches seek to determine whether and by what mechanism a toxic substance may lead to the induction of cellular and biomolecular responses. While these in vitro changes could play a role in the pathophysiology of disease, there are many instances in which cellular responses do not predict the outcome in an intact animal, because multiple cell types, other tissue components and systemic factors determine disease outcome. However, it is also true that a cell is comprised of subcellular machinery wherein nuclei, organelles, cytosolic components, proteins, lipids, and nucleic acids are functionally integrated into cellular networks that contribute in a major way to tissue and organ function. From this perspective, the cellular network can inform about the function of tissue and organ networks which are capable of predicting a disease outcome. Moreover, the rapid advances taking place in systems biology is spearheading the development of tools that are improving the prediction making capabilities of genomics, proteomics, metabalomics, and cellomics, with the assistance of new data processing and decision making technologies. While the contribution of systems biology to systems toxicology is being carried out at different levels of stringency, the contribution of predictive nanotoxicology has been to provide key toxicological pathways that can be studied by high content and HTS approaches.[5, 6] This has allowed comparative analysis of large batches of materials by SARs that relate to the pathophysiology of disease. We foresee that the additional discovery platforms based on omics technologies, imaging, data transformation and prediction tools will further widen the scope of a predictive toxicological approach.
Perturbation of cellular processes, as determined by in vitro analysis, does not include analysis of chronic disease outcomes. This is especially true for adverse health effects where no thresholds have been established, such as carcinogenic, mutagenic or reproductive toxicological effects. However, in the example of the subchronic pulmonary toxicity by LAR materials (such as CNTs), we have demonstrated that a mechanistic injury response can be studied by acute assays which can be used to predict progressive lung injury culminating in chronic inflammation and fibrosis.[25-28] Moreover, it is possible that oxidative stress and inflammation could be predictive of a variety of chronic disease outcomes in response to environmental factors. We also know that for chronic disease processes there are a number of clinical biomarkers that can be assessed instantaneously during disease progression, because these reflect disease mechanisms that contribute to pathogenesis of disease, irrespective of stage. Thus, despite the fact that this remains an unsolved problem, it is possible that in vitro test strategies will evolve to make predictions about chronic disease outcomes.
A cell-based approach does not consider exposure analysis, variation in exposure conditions and dosimetry in the same way that in vivo studies can. Thus, information about real-life exposure scenarios and dosimetry is required for risk analysis. Given the immature state-of-the-art of this technology to assess ENM exposures and provide reali-life exposure data, it is currently only possible to perform quantitative risk assessment for a limited number of materials. While advances in this field will require scientific advancement of exposure assessment, fate and transport determination and toxicokinetics, limited exposure data and a tiered platform can be used to integrate predictive toxicological paradigms into risk assessment, as discussed above.
The mechanistic toxicology approach for ENMs at UCLA may not apply to all toxic substances when using mechanism of action (MOA) as a criterion for screening. For instance, data analysis in the ToxCast™ Phase I library showed that the HTS have limited applicability for predicting in vivo chemical hazards using standard statistical classification mechanisms.[90] This could reflect a lack of statistical approaches (which do not incorporate knowledge of biological or adverse outcome pathways);[19] however, another possibility is that a large number of chemicals may be nonselective in their biological impact and that imposing a MOA approach to these chemicals may not be a helpful screening exercise. In similar fashion, it is possible that ENMs may trigger non-specific cellular responses that are not indicative of adverse health outcomes, including triggering adaptive responses that involve natural coping mechanisms that are not necessarily representative of a toxicological response. There have been several nanotoxicology reports of cellular studies that were not comparable with in vivo response outcomes.[34] However, our proposal to develop predictive toxicological paradigms in which in vitro meausres are consistent with in vivo toxicological outcomes that reflect the pathophysiology of disease has been useful in analyzing several material categories for which a POT could be identified.
As robotic technology is required for HTS, infrastructure investment and increased data handling capabilities are needed to evaluate the data. Moreover, it is unknown how many pathways of toxicity may emerge and how many HTS screening methods need to be developed to deal with the full range of exposures and ENM toxicological scenarios.[13] It is possible that the complexity of the robotic screening process can be reduced by developing miniaturized impedance, spectrophotometry or lab-on a-chip methodologies to perform high content screening.
Screening hundreds or thousands of materials to delineate impacts on numerous cellular injury pathways is likely to generate both false positive and false negative results.[13, 87] This could lead to potentially useful materials being mistakenly labeled as toxic or reporting that a toxic material is harmless. Both scenarios are problematic in that false positives could impact commerce by requiring material recalls, while false negatives could perceivably have future health impacts. To limit the number of false positives or negatives, the HTS approach should be implemented with rigorous set-up protocols that prevent false reporting, including the use of complementary assays that can be used before identifying a material as toxic.[43] Our use of limited animal testing provides an additional opportunity to identify false positives and negatives. It is known, however, that animal use do not always identify toxicological scenarios in humans, as reflected by some drug failures in the pharmaceutical industry.[13]
Validation of test strategies for regulatory purposes is an important challenge for implementing a paradigm shift.[17] There are numerous statutory, legal, historical, and cultural considerations for using preferred and time-honored safety assessment approaches, including reliance on rodent models. While the 21st-century paradigm for chemicals strongly argues for in vitro testing approaches, this transition will not take place overnight.
Conclusion
It is important to develop the safety screening of ENMs based on the relationship between their physicochemical properties and in vitro and in vivo nano-bio interactions that impact POTs, which can be used for rapid-throughput and high-content studies. This also allows the development of predictive toxicological approaches that can be used to accelerate the screening of ENMs, hazard ranking, development of SARs and more focused and reduced animal use. Through the use of dose-response extrapolations involving the calculation of SADs in vitro and in the lung, it is possible to integrate predictive toxicological approaches to risk assessment. This can be done in a tiered manner to limit the number of materials that need to be assessed at each tier for decision-making. We propose that predictive toxicological approaches could be interfaced with the ATS approaches in ToxcastTM and Tox-21, in which the broad-based discovery of signatures of toxicity by a systems biology approach is used to introduce new mechanisms and pathways on which to base predictive screening.
Acknowledgements
This study was primarily supported by the US Public Health Service Grant U19 ES019528 (UCLA Center for Nanobiology and Predictive Toxicology). This work was also supported by the National Science Foundation and the Environmental Protection Agency under Cooperative Agreement Number DBI-0830117. Any opinions, findings, conclusions or recommendations expressed herein are those of the author and do not necessary reflect the views of the National Science Foundation or the Environmental Protection Agency. This work has not been subjected to an Environmental Protection Agency peer and policy review.
Footnotes
Conflict of interest statement
No conflicts of interest to declare
References
- 1.Roco MM,C, Hersam M. Springer; Boston and Berlin: 2010. Nanotechnology Research Directions for Societal Needs in 2010. [Google Scholar]
- 2.Nel A, et al. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–7. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 3.Meng H, et al. A predictive toxicological paradigm for the safety assessment of nanomaterials. ACS Nano. 2009;3(7):1620–7. doi: 10.1021/nn9005973. [DOI] [PubMed] [Google Scholar]
- 4.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nel A, et al. Nanomaterial Toxicity Testing in the 21st Century: Use of a Predictive Toxicological Approach and High-Throughput Screening. Acc Chem Res. 2012 doi: 10.1021/ar300022h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia T, et al. Implementation of a Multidisciplinary Approach to Solve Complex Nano EHS Problems by the UC Center for the Environmental Implications of Nanotechnology. Small. 2012 doi: 10.1002/smll.201201700. [DOI] [PubMed] [Google Scholar]
- 7.Clark KA, White RH, Silbergeld EK. Predictive models for nanotoxicology: current challenges and future opportunities. Regul Toxicol Pharmacol. 2011;59(3):361–3. doi: 10.1016/j.yrtph.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Puzyn T, Leszczynska D, Leszczynski J. Toward the development of “nano-QSARs”: advances and challenges. Small. 2009;5(22):2494–509. doi: 10.1002/smll.200900179. [DOI] [PubMed] [Google Scholar]
- 9.Liu R, et al. Classification NanoSAR development for cytotoxicity of metal oxide nanoparticles. Small. 2011;7(8):1118–26. doi: 10.1002/smll.201002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rallo R, et al. Self-organizing map analysis of toxicity-related cell signaling pathways for metal and metal oxide nanoparticles. Environ Sci Technol. 2011;45(4):1695–702. doi: 10.1021/es103606x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Judson R, et al. Perspectives on validation of high-throughput assays supporting 21st century toxicity testing. ALTEX. 2013;30(1):51–6. doi: 10.14573/altex.2013.1.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.OECD Important Issues on Risk Assessment of Manufactured Nanomaterials. Series on the Safety of Manufactured Nanomaterials. 2012 [Google Scholar]
- 13.Hartung T. Toxicology for the twenty-first century. Nature. 2009;460(7252):208–12. doi: 10.1038/460208a. [DOI] [PubMed] [Google Scholar]
- 14.Committee on Toxicity Testing and Assessment of Environmental Agents, N.R.C. Toxicity Testing in the 21st Century: A Vision and Strategy. Washington, DC: 2007. [Google Scholar]
- 15.Hartung T, et al. Systems toxicology. ALTEX. 2012;29(2):119–28. doi: 10.14573/altex.2012.2.119. [DOI] [PubMed] [Google Scholar]
- 16.Dix DJ, et al. The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci. 2007;95(1):5–12. doi: 10.1093/toxsci/kfl103. [DOI] [PubMed] [Google Scholar]
- 17.Judson RS, et al. In vitro screening of environmental chemicals for targeted testing prioritization: the ToxCast project. Environ Health Perspect. 2010;118(4):485–92. doi: 10.1289/ehp.0901392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavlock R, et al. Update on EPA's ToxCast program: providing high throughput decision support tools for chemical risk management. Chem Res Toxicol. 2012;25(7):1287–302. doi: 10.1021/tx3000939. [DOI] [PubMed] [Google Scholar]
- 19.Dix DJ, et al. Incorporating biological, chemical, and toxicological knowledge into predictive models of toxicity. Toxicol Sci. 2012;130(2):440–1. doi: 10.1093/toxsci/kfs281. author reply 442-3. [DOI] [PubMed] [Google Scholar]
- 20.Collins FS, Gray GM, Bucher JR. Toxicology. Transforming environmental health protection. Science. 2008;319(5865):906–7. doi: 10.1126/science.1154619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt CW. TOX 21: new dimensions of toxicity testing. Environ Health Perspect. 2009;117(8):A348–53. doi: 10.1289/ehp.117-a348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George S, et al. Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping. ACS Nano. 2010;4(1):15–29. doi: 10.1021/nn901503q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, et al. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano. 2012;6(5):4349–68. doi: 10.1021/nn3010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu R, et al. Nano-SAR Development for Bioactivity of Nanoparticles with Considerations of Decision Boundaries. Small. 2013:n/a–n/a. doi: 10.1002/smll.201201903. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, et al. Dispersal State of Multiwalled Carbon Nanotubes Elicits Profibrogenic Cellular Responses That Correlate with Fibrogenesis Biomarkers and Fibrosis in the Murine Lung. Acs Nano. 2011;5(12):9772–9787. doi: 10.1021/nn2033055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, et al. Pluronic F108 Coating Decreases the Lung Fibrosis Potential of Multiwall Carbon Nanotubes by Reducing Lysosomal Injury. Nano Letters. 2012 doi: 10.1021/nl300895y. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, et al. Quantitative Techniques for Assessing and Controlling the Dispersion and Biological Effects of Multiwalled Carbon Nanotubes in Mammalian Tissue Culture Cells. Acs Nano. 2010;4(12):7241–7252. doi: 10.1021/nn102112b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li R, et al. Surface Charge and Cellular Processing of Covalently Functionalized Multiwall Carbon Nanotubes Determine Pulmonary Toxicity. ACS Nano. 2013;7(3):2352–2368. doi: 10.1021/nn305567s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia T, Li N, Nel AE. Potential Health Impact of Nanoparticles. Annual Review of Public Health. 2009;30:137–150. doi: 10.1146/annurev.publhealth.031308.100155. [DOI] [PubMed] [Google Scholar]
- 30.Oberdörster G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. Journal of Internal Medicine. 2010;267(1):89–105. doi: 10.1111/j.1365-2796.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- 31.Oberdorster G, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.OECD Overview of the Organization for Economic Cooperation and Development's strategies related to nanomaterials. Available from: http://www.oecd.org/science/nanosafety/
- 33.Becher R, et al. Rat lung inflammatory responses after in vivo and in vitro exposure to various stone particles. Inhal Toxicol. 2001;13(9):789–805. doi: 10.1080/08958370118221. [DOI] [PubMed] [Google Scholar]
- 34.Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci. 2007;97(1):163–80. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- 35.Rushton EK, et al. Concept of Assessing Nanoparticle Hazards Considering Nanoparticle Dosemetric and Chemical/Biological Response Metrics. Journal of Toxicology and Environmental Health, Part A. 2010;73(5-6):445–461. doi: 10.1080/15287390903489422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duffin R, et al. Proinflammogenic effects of low-toxicity and metal nanoparticles in vivo and in vitro: highlighting the role of particle surface area and surface reactivity. Inhal Toxicol. 2007;19(10):849–56. doi: 10.1080/08958370701479323. [DOI] [PubMed] [Google Scholar]
- 37.Monteiller C, et al. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup Environ Med. 2007;64(9):609–15. doi: 10.1136/oem.2005.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia T, et al. Comparison of the Abilities of Ambient and Manufactured Nanoparticles To Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Letters. 2006;6(8):1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 39.Xiao GG, et al. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J Biol Chem. 2003;278(50):50781–90. doi: 10.1074/jbc.M306423200. [DOI] [PubMed] [Google Scholar]
- 40.Xia T, et al. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. Acs Nano. 2008;2(10):2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.George S, et al. Use of a High-Throughput Screening Approach Coupled with In Vivo Zebrafish Embryo Screening To Develop Hazard Ranking for Engineered Nanomaterials. Acs Nano. 2011;5(3):1805–1817. doi: 10.1021/nn102734s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.George S, et al. Role of Fe Doping in Tuning the Band Gap of TiO(2) for the Photo-Oxidation-Induced Cytotoxicity Paradigm. Journal of the American Chemical Society. 2011;133(29):11270–11278. doi: 10.1021/ja202836s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damoiseaux R, et al. No time to lose-high throughput screening to assess nanomaterial safety. Nanoscale. 2011;3(4):1345–1360. doi: 10.1039/c0nr00618a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel T, et al. Toxicity Profiling of Engineered Nanomaterials via Multivariate Dose Response Surface Modeling. Annals of Applied Statistics. 2012;6(4):1707–1729. doi: 10.1214/12-AOAS563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel TT,D, Rallo R, George S, Xia T, Nel AE. Hierarchical rank aggregation with applications to nanotoxicology. Journal of Agricultural, Biological and Environmental Statistics. 2013 doi: 10.1007/s13253-013-0129-y. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burello E, Worth AP. A theoretical framework for predicting the oxidative stress potential of oxide nanoparticles. Nanotoxicology. 2011;5(2):228–35. doi: 10.3109/17435390.2010.502980. [DOI] [PubMed] [Google Scholar]
- 47.Porter DW, et al. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269(2-3):136–47. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 48.NIOSH Current Intelligence Bulletin: Occupational Exposure to Carbon Nanotubes. 2011 Available from: www.cdc.gov/niosh/docket/review/docket161A/
- 49.Johnston HJ, et al. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics. Nanotoxicology. 2010;4(2):207–46. doi: 10.3109/17435390903569639. [DOI] [PubMed] [Google Scholar]
- 50.Kagan VE, et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: role of iron. Toxicol Lett. 2006;165(1):88–100. doi: 10.1016/j.toxlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Mercer RR, et al. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294(1):L87–97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- 52.Muller J, et al. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: toxicological aspects. Chem Res Toxicol. 2008;21(9):1698–705. doi: 10.1021/tx800101p. [DOI] [PubMed] [Google Scholar]
- 53.Ryman-Rasmussen JP, et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009;4(11):747–51. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shvedova AA, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289(5):L698–708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 55.Poland CA, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3(7):423–8. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 56.Donaldson K, et al. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palomaki J, et al. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 2011;5(9):6861–70. doi: 10.1021/nn200595c. [DOI] [PubMed] [Google Scholar]
- 58.Sargent LM, et al. Single-walled carbon nanotube-induced mitotic disruption. Mutat Res. 2012;745(1-2):28–37. doi: 10.1016/j.mrgentox.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonner JC. Mesenchymal cell survival in airway and interstitial pulmonary fibrosis. Fibrogenesis Tissue Repair. 2010;3:15. doi: 10.1186/1755-1536-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamilton RF, et al. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part Fibre Toxicol. 2009;6:35. doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gangwal S, et al. Informing selection of nanomaterial concentrations for ToxCast in vitro testing based on occupational exposure potential. Environ Health Perspect. 2011;119(11):1539–46. doi: 10.1289/ehp.1103750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oberdorster G. Nanotoxicology: in vitro-in vivo dosimetry. Environ Health Perspect. 2012;120(1):A13. doi: 10.1289/ehp.1104320. author reply A13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen BT, et al. Multi-walled carbon nanotubes: sampling criteria and aerosol characterization. Inhal Toxicol. 2012;24(12):798–820. doi: 10.3109/08958378.2012.720741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuempel ED, Castranova V. Hazard and risk assessment of workplace exposure to engineered nanoparticles: Methods, issues, and carbon nanotube case study. In: Ramachandran G, editor. Assessing Nanoparticle Risk to Human Health. Elsevier, Inc; Oxford: 2012. pp. 65–97. [Google Scholar]
- 65.Kuempel ED, Geraci CL, Schulte PA. Risk assessment and risk management of nanomaterials in the workplace: translating research to practice. Ann Occup Hyg. 2012;56(5):491–505. doi: 10.1093/annhyg/mes040. [DOI] [PubMed] [Google Scholar]
- 66.Kuempel ED, et al. Development of risk-based nanomaterial groups for occupational exposure control. Journal of Nanoparticle Research. 2012;14(9):1–15. doi: 10.1007/s11051-012-1029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stone KC, et al. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 1992;6(2):235–43. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- 68.Oberdorster G, Ferin J, Morrow PE. Volumetric loading of alveolar macrophages (AM): a possible basis for diminished AM-mediated particle clearance. Exp Lung Res. 1992;18(1):87–104. doi: 10.3109/01902149209020653. [DOI] [PubMed] [Google Scholar]
- 69.Brain JD, et al. Pulmonary distribution of particles given by intratracheal instillation or by aerosol inhalation. Environ Res. 1976;11(1):13–33. doi: 10.1016/0013-9351(76)90107-9. [DOI] [PubMed] [Google Scholar]
- 70.Warheit DB, et al. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: differential responses related to surface properties. Toxicology. 2007;230(1):90–104. doi: 10.1016/j.tox.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Henderson RF, et al. A comparison of the inflammatory response of the lung to inhaled versus instilled particles in F344 rats. Fundam Appl Toxicol. 1995;24(2):183–97. doi: 10.1006/faat.1995.1022. [DOI] [PubMed] [Google Scholar]
- 72.Bernstein D, et al. Testing of fibrous particles: short-term assays and strategies. Inhal Toxicol. 2005;17(10):497–537. doi: 10.1080/08958370591001121. [DOI] [PubMed] [Google Scholar]
- 73.Rao GV, et al. Efficacy of a technique for exposing the mouse lung to particles aspirated from the pharynx. J Toxicol Environ Health A. 2003;66(15):1441–52. doi: 10.1080/15287390306417. [DOI] [PubMed] [Google Scholar]
- 74.Sager TM, et al. Improved method to disperse nanoparticles for in vitro and in vivo investigation of toxicity. Nanotoxicology. 2007;1(2):118–129. [Google Scholar]
- 75.Mercer RR, et al. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part Fibre Toxicol. 2010;7:28. doi: 10.1186/1743-8977-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shvedova AA, et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L552–65. doi: 10.1152/ajplung.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Porter DW, et al. Acute pulmonary dose-responses to inhaled multi-walled carbon nanotubes. Nanotoxicology. 2012 doi: 10.3109/17435390.2012.719649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Piegorsch WW, Bailer AF. Analyzing Environmental Data. John Wiley & Sons; Chicester, West Sussex: 2005. Quantitative risk assessment with stimulus-response data. [Google Scholar]
- 79.NRC Science and decisions: advancing risk aasssessment. Committee on improving risk analysis approaches used by the U.S. EPA, Board on Environmental Studies and Toxicology, Division on Eath and Life Studies. 2009.
- 80.EPA, U. Benchmark dose technical guidance. Washington, DC: 2012. [Google Scholar]
- 81.Kuempel ED, Geraci CL, Schulte PA. Risk assessment approaches and research needs for nanoparticles: an examination of data and information from current studies. Proceedings of the NATO Advanced Research Workshop on Nanotechnology: Toxicological Issues and Environmental Safety. In: Simeonova P, Opopol N, Luster M, editors. Nanotechnology: toxicological issues and environmental safety. New York; Springer: 2007. pp. 119–145. [Google Scholar]
- 82.OECD Guidance on grouping of chemicals. Series on Testing and Assessment. 2007.
- 83.Zalk DM, Nelson DI. History and evolution of control banding: a review. J Occup Environ Hyg. 2008;5(5):330–46. doi: 10.1080/15459620801997916. [DOI] [PubMed] [Google Scholar]
- 84.Brouwer DH. Control banding approaches for nanomaterials. Ann Occup Hyg. 2012;56(5):506–14. doi: 10.1093/annhyg/mes039. [DOI] [PubMed] [Google Scholar]
- 85.Mutlu G.k.M., et al. Biocompatible Nanoscale Dispersion of Single-Walled Carbon Nanotubes Minimizes in vivo Pulmonary Toxicity. Nano Letters. 2010;10(5):1664–1670. doi: 10.1021/nl9042483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hersam MC. Progress towards monodisperse single-walled carbon nanotubes. Nat Nanotechnol. 2008;3(7):387–94. doi: 10.1038/nnano.2008.135. [DOI] [PubMed] [Google Scholar]
- 87.Bus JS, Becker RA. Toxicity testing in the 21st century: a view from the chemical industry. Toxicol Sci. 2009;112(2):297–302. doi: 10.1093/toxsci/kfp234. [DOI] [PubMed] [Google Scholar]
- 88.Stokstad E. Putting chemicals on a path to better risk assessment. Science. 2009;325(5941):694–5. doi: 10.1126/science.325_694. [DOI] [PubMed] [Google Scholar]
- 89.EDF Challenges and Limitations of High Throughput In Vitro Testing. Available from: http://www.edf.org/health/section-6-challenges-and-limitations-high-throughput-vitro-testing.
- 90.Thomas RS, et al. A comprehensive statistical analysis of predicting in vivo hazard using high-throughput in vitro screening. Toxicol Sci. 2012;128(2):398–417. doi: 10.1093/toxsci/kfs159. [DOI] [PubMed] [Google Scholar]