Abstract
Objective
Depressive disorders are important causes of disease burden and are associated with substantial economic costs. Therefore, it is important to design a health care system that can effectively manage depression at sustainable costs. This paper computes the benefit-to-costs ratio of the current Dutch health care system for depression, and investigates whether offering more online preventive interventions improves the cost-effectiveness overall.
Methods
A health economic (Markov) model was used to synthesize clinical and economic evidence and to compute population-level costs and effects of interventions. The model compares a base-case scenario without preventive telemedicine and alternative scenarios with preventive telemedicine. The central outcome is the benefit-to-cost ratio, also known as return-on-investment (ROI).
Results
In terms of ROI, a health care system with preventive telemedicine for depressive disorders offers better value for money than a health care system without internet-based prevention. Overall, the ROI increases from €1.45 ($1.72) in the base-case scenario to €1.76 ($2.09) in the alternative scenario where preventive telemedicine is offered. In a scenario where the costs of offering preventive telemedicine are balanced by cutting back on the expenditure for curative interventions, ROI increases to €1.77 ($2.10), while keeping the health care budget constant.
Conclusion
In order for a health care system for depressive disorders to remain economically sustainable, its cost-benefit ratio needs to be improved. Offering preventive telemedicine at a large scale is likely to introduce such an improvement.
Keywords: depressive disorder, prevention, e-health, cost-benefit analysis
OBJECTIVE
Reducing the disease burden due to depressive disorders at affordable costs is of great significance to public health. After all, depression is the single leading cause of non-fatal disease burden [1–3] and has substantial economic consequences [4–7].
Cushioning the adverse effects of depression requires a health care system well equipped to manage the disorder. To that end, the mix of interventions for depression that are offered needs to be acceptable for both health care users and health care providers. In addition, the interventions need to be effective in generating the required health gains, and economically sustainable over time.
It is a difficult task to identify that particular combination of interventions that meets all these criteria within the extensive range of available interventions, offered in multiple formats to different target groups.
The task of identifying an ‘optimal’ health care system becomes even more daunting, when the acceptability and cost-effectiveness of a newly designed health care system has to be compared with the cost-effectiveness of the current health care system. In particular, we need to know how a (hypothetical) health care system based on widespread implementation of preventive telemedicine would compare to the current health care regime without preventive telemedicine. Would such a health care system produce larger health gains? And how would the new system compare with the current health care regime in terms of its benefit-to-cost ratio?
To facilitate decision making, we developed a health-economic simulation model for depression, DEPMOD. DEPMOD assesses the population-level cost-benefit ratio of an alternative health care system relative to the current one. Although availability of data prompted us to apply DEPMOD to the population aged 18–65, we expect that DEPMOD is also relevant to older populations. Especially since the older population has an elevated risk for depression [8], and evidence seems to suggest an increased risk of additional adverse outcomes for older people with depression [9]. The older population might be under pressure to be economically productive, even beyond the current age of retirement, due to the current economic downturn in graying societies. At the same time, increased life expectancy, common in high-income countries, is associated with an increase in the number of depressed older people. In sum, graying societies, increased demand for mental health care, rising health care expenditure, and dwindling labor forces for mental health, underscore the importance for the health care system to be re-assessed and geared towards offering more cost-effective interventions. Implementing interventions that can be offered over the Internet seems to be a promising approach, because these interventions are likely to be scalable, effective and cost-effective. DEPMOD simulates the possible consequences of offering internet interventions for major depression.
Experience with the Australian Assessing Cost-Effectiveness (ACE) models for heart disease, mental disorders and prevention [10–12], and the WHO’s CHOICE models (CHOosing Interventions that are Cost-Effective)[13, 14] indicates that health economic models may have value for policy making. DEPMOD was specifically designed for the Dutch health care system, using Dutch population-based cohort data on depressive disorder [15] and standard cost prices pertinent to the Dutch health care system [16]. It also models impacts of several preventive e-health interventions that were recently developed, evaluated and disseminated in the Netherlands. That said, DEPMOD can be used for other countries and populations, provided that data requirements are met.
The aim of this paper is to briefly describe DEPMOD and then apply DEPMOD by modeling the current package of health care interventions and an extended package where preventive telemedicine is added, to address the question whether preventive telemedicine offers good value for money.
We define telemedicine (e-health) as psychological self-help interventions that are delivered over the Internet, either with or without minimal therapist support. Meta-analyses of randomized trials have demonstrated the effectiveness of both prevention of depressive disorder [17,18] and (preventive) e-health interventions [19,20]. In addition, telemedicine is very scalable because of the widespread usage of the Internet. Here it should be noted that older people are the fastest growing group of new internet users. One of the main reasons to be on the Internet is that older people have questions about health. By implication, there is a good match between older people’s internet usage and e-mental health. Though not explicitly modeled here, evidence suggests that depression prevention is also effective in the older population [21]
This paper sets out to synthesize the relevant clinical and economic evidence in a health economic modeling study.
METHODS
Comparing scenarios: usual care vs more preventive telemedicine
DEPMOD is used to compute the cost-benefit ratio by comparing ‘usual care’ with an alternative scenario where ‘usual care’ is augmented with preventive telemedicine (scenario A). In addition, a scenario B is analyzed where the costs of offering additional preventive telemedicine are compensated for by reducing the healthcare budget for curative interventions, thereby keeping the overall costs of the new scenario under the current budgetary ceiling.
The ‘usual care’ scenario which forms the basis for the comparisons, is an evidence-based health care system that is fully in agreement with the Dutch clinical guidelines for the treatment of depression. Because it is somewhat better than current Dutch health care system, we refer to it as ‘enhanced usual care’, see table 1. This (long) list of evidence-based interventions was then used to select only those interventions that were acceptable from a patients’ point of view and appropriate from a health care professionals’ point of view. To that end, focus groups were used and a panel of 17 health care users were to judge to what extent they would be willing to accept and actively engage in each of the interventions, whereas a panel of 10 health care professionals judged to what extent the interventions were appropriate to offer for the various manifestations of depressive disorder. Both panels showed a relatively high degree of consensus with regard to their preferences (Cronbach’s α=0.79 for care users and α=0.70 for care providers). Taking these preferences into account, the long list of evidence-based interventions was reduced to a much shorter list of interventions that are not only evidence-based, but also preference-based (see table 1).
Table 1.
Selected evidence-based and preference-based interventions by depression severity level: Costs (in 2009 Euro and Dollar), Compliance with therapy (%) and Effect, as odds ratio (OR) or as standardized effect size, d, all representing average values.
| INTERVENTION BY DEPRESSION SEVERITY LEVEL | Costs | Compliance rate | Effect |
|---|---|---|---|
| SUBCLINICAL DEPRESSION | €($) | % | OR |
|
| |||
| Self-help book1 | 348(413) | 52 | 0.66 |
| Group course: 8–10 sessions2 | 506(601) | 64 | 0.57 |
| E-health intervention (unsupported) 3 | 178(211) | 56 | 0.55 |
| MILD DEPRESSION | €($) | % | d |
|
| |||
| E-health intervention (supported) 4 | 313(372) | 43 | 0.32 |
| “Interapy”: online psychotherapy, 10 sessions CBT5 | 2154(2558) | 44 | 0.70 |
| Individual psychotherapy, primary care, 8 sessions6 | 1296(1539) | 56 | 0.69 |
| MODERATE DEPRESSION | €($) | % | d |
|
| |||
| E-health intervention (supported) 7 | 313(372) | 43 | 0.32 |
| “Interapy”: online psychotherapy, 14 sessions CBT8 | 2154(2558) | 44 | 0.70 |
| Individual psychotherapy, primary care, 8 sessions9 | 1296(1539) | 56 | 0.69 |
| SEVERE DEPRESSION | €($) | % | d |
|
| |||
| individual psychotherapy, out-patient care, 8–24 sessions10 | 1447(1719) | 68 | 0.70 |
| Anti-depressants, 3–6 months via GP11 | 235(279) | 44 | 0.72 |
| Anti-depressants, 3–6 months, with additional psychological support12 | 289(343) | 56 | 0.72 |
| Combination therapy (medication and psychotherapy) 13 | 1215(1443) | 65 | 1.05 |
| RECURRENT DEPRESSION | €($) | % | OR |
|
| |||
| Clinical management with maintenance medication, 12 months14 | 537(638) | 42 | 0.75 |
| Preventive Cognitive Therapy: 8 group sessions15 | 406(482) | 63 | 0.73 |
| Supported self-help PCT: via the internet16 | 403(479) | 46 | 0.73 |
Taken from [22]
Taken from [25]
Taken from [25]
Taken from [28]
Taken from [32]
Our reanalysis of the meta-analysis by Vittengl et al. (2007), [33]
Our reanalysis of the meta-analysis by Vittengl et al. (2007), [33]
See 15. Hypothetical effect size on the assumption that supported e-health is as effective as face-to-face delivered prevention of recurrence, albeit associated with a lower adherence rate.
The list of evidence-based and preference-based interventions forms the basis for performing scenario analysis and is likely to be more cost-effective than usual care, where not every intervention is evidence-based or meets with approval by both care users and health care providers.
Table 2 describes the scenarios that were analyzed by DEPMOD. First, the Base-Case Scenario of evidence-based and preference-based care without prevention, where coverage rates and adherence rates were elicited from the focus groups. The alternative scenario (Scenario A) is essentially the same as the base-case scenario, except that now prevention and (preventive) telemedicine is offered. To be more specific, prevention consists of face-to-face interventions with an arbitrarily low coverage rate set at 2%. Preventive e-health interventions are offered at a coverage rate of 15%, which is likely to be attainable in practice [34]. E-health interventions for prevention of relapse and recurrence are assumed to be a somewhat lower with a coverage set at 10%. Finally, the Scenario B offers telemedicine as in scenario A, while cutting back on other treatment costs, thus keeping the overall costs balanced. Coverage rates in both alternative scenarios are hypothetical and can be used to conduct ‘what if’-analyses around potentially interesting healthcare systems.
Table 2.
Modeled scenarios: coverage rates (%) for each of the interventions by depression severity level.
| INTERVENTION BY DEPRESSION SEVERITY LEVEL | Base case | Alternative A | Alternative B |
|---|---|---|---|
| SUBCLINICAL DEPRESSION | |||
|
| |||
| Self-help book | 0 | 2 | 2 |
| Group course: 8–10 sessions | 0 | 2 | 2 |
| E-health intervention (unsupported) | 0 | 15 | 15 |
| MILD DEPRESSION | |||
|
| |||
| E-health intervention (supported) | 2 | 2 | 1.5 |
| “Interapy”: online psychotherapy, 10 sessions CBT | 2 | 2 | 1.5 |
| Individual psychotherapy, primary care, 8 sessions | 17 | 17 | 12.4 |
| MODERATE DEPRESSION | |||
|
| |||
| E-health intervention (supported) | 2 | 2 | 1.5 |
| “Interapy”: online psychotherapy, 14 sessions CBT | 2 | 2 | 1.5 |
| Individual psychotherapy, primary care, 8 sessions | 16 | 16 | 12 |
| SEVERE DEPRESSION | |||
|
| |||
| individual psychotherapy, out-patient care, 8–24 sessions | 18 | 18 | 13.5 |
| Anti-depressants, 3–6 months via GP | 20 | 20 | 15 |
| Anti-depressants, 3–6 months, with additional psychological support | 20 | 20 | 15 |
| Combination therapy (medication and psychotherapy) | 16 | 16 | 12 |
| RECURRENT DEPRESSION | |||
|
| |||
| Clinical management with maintenance medication, 12 months | 0 | 2 | 2 |
| Mindfulness-based PCT: 8 group sessions | 0 | 2 | 2 |
| Supported self-help PCT: via the internet | 0 | 10 | 10 |
The remainder of the method section describes DEPMOD, which is based on methods as described by Briggs et al (2006) and Drummond et al (2005) [35,36].
DEPMOD
Conceptually, DEPMOD combines the epidemiology of major depression and simulates how a health care system impacts on the incidence (via prevention), prevalence (via treatment) and recurrence (via relapse prevention). Generating health impacts entails costs. Both the costs and the health gains are evaluated by DEPMOD.
The epidemiology of depression is modeled as a series of transitions between different health states (healthy, depressed, death), taking into account both severity of depression (subclinical, mild, moderate and severe depression) and the number of depressive episodes (recurrences).
The simulated health care system consists of a mix of preventive interventions, curative interventions (for mild, moderate and severe depression) and interventions to prevent recurrences, as outlined in table 1 and 2.
The purpose of DEPMOD is to calculate the total health care expenditure and health gains under the current health care system, and to compare the current scenario with the alternative scenarios. The following sections describe the model, the data, and the underlying assumptions in more detail.
Epidemiology
DEPMOD is restricted to depressive disorder, as defined by the Diagnostic and Statistical Manual, fourth edition (DSM-IV) [37]. DEPMOD assumes a population of 10 million people, aged 18–65 years. Estimates of incidence (238,350 new cases per year), episode duration (six months on average), prevalence (588,600 acute cases annually), and recurrence rates of depressive disorder (45% of the currently depressed people have a history of previous episodes) were obtained from the Netherlands Mental Health Survey and Incidence Study (NEMESIS), a population-based psychiatric epidemiological cohort study [15]. Depression-specific mortality rates were assessed meta-analytically [38]. DEPMOD takes into account that the risk of yet another depressive episode increases with the number of previous episodes.
Health care system
A health care system consists of preventive interventions to reduce incidence, treatment of mild, moderate and severe depression to reduce disease burden and relapse prevention in recovered patients to reduce risk of relapse and recurrence. One could think of primary prevention, cure and relapse prevention as a system of health care ‘echelons’ along the disease continuum. Each echelon consists of a mix of interventions.
Each intervention is described by its impact on health (Cohen’s d), coverage rate (% of population receiving the intervention), adherence rate (extent to which patients comply with the intervention), and cost (per intervention per patient). Effects were based on meta-analyses where possible, and RCT’s or estimates otherwise (see table 1). Costs were estimated by mapping the amount of time of health care professionals per intervention multiplied by hourly rates.
The sum of all cost and total health gains are calculated at the level of the population. Costs are restricted to direct medical cost (in euro, €, for the reference year 2009; converted to US$ using purchasing power parities) [39]. Unit cost prices are obtained from the Dutch Guideline for Health Economic Evaluations [16]. Health gains are expressed as a reduction in the disease burden due to depression (i.e., fewer disability adjusted life years, DALYs).
Assessing health gains
Health care interventions aim to reduce the number of disability adjusted life years (DALYs) in the population. DALY is a measure of disease burden in a population, taking into account two components of disease burden: morbidity and mortality. Morbidity is related to time spent in a health state characterized by a lowered quality of life due to disability. Mortality comes into the equation when illness is associated with premature death. For a description of the use of DALYs in economic modeling, see Drummond et al [36].
In DEPMOD, DALY reductions are achieved in two ways. First, by preventing people from becoming depressed through primary prevention. Second, by treating people with depression, thereby lowering their disease burden.
Cost-effectiveness analysis
To allow for parameter uncertainty in costs and effects, the model randomly draws a value from the distributions assigned to the parameters and computes the outcome for that configuration of parameter values. This procedure is repeated 1000 times over all parameters simultaneously. In each run, the outcomes (costs and health gains for each scenarios) are computed and stored in DEPMOD’s memory. Then following Briggs et al (2006), all 1000 simulated outcomes are evaluated simultaneously [35], thus explicitly accounting for uncertainty in the input parameters.
After generating 1000 values of costs and DALYs for the current and alternative health care systems, costs and effects are discounted when the time horizon exceeds one year. Discounting rates (1.5% for the effects and 4.0% for the costs, as per the pertinent economic guideline) are automatically subjected to further sensitivity analyses. In a next step, differences in the costs (incremental costs) and differences in DALYs (incremental effects) across both scenarios are obtained and an estimate of the incremental cost-effectiveness ratio (ICER) is computed, that is: ICER = (C1−C0)/(E1−E0), were C are costs, E are effects and subscripts 1 and 0 refer to the alternative and base case scenario, respectively. The ICER is one of the key outcomes of an economic evaluation, see [35]. The time horizon is 5 years, but this could be changed to a minimum of 1 year. Finally, the return on investment (ROI) of each scenario is calculated by dividing DALY health gains, conservatively valued at €20,000 ($23,750) per DALY, by total cost.
In health economic modeling, making assumptions is inevitable. Whenever assumptions were made, we use conservative assumptions as to decrease the risk of outcomes being overly optimistic. It is important to understand how the assumptions affect the outcome of the model. Text Box 1 presents DEPMOD’s main assumptions, their justifications and their possible impact on the findings.
Text Box 1. DEPMOD’s assumptions and their consequences.
| Assumption | Justification | Implication |
|---|---|---|
| The 1-year incidence is constant at 238,350 cases per year. Prevalence is 588,600 annually in the adult Dutch population of 10 million people. | Data obtained from the Netherlands Mental Health Survey and Incidence Study (NEMESIS), a population-based psychiatric epidemiological cohort study [15]. | Prevalence determines the cost and effects. The ratio incidence/prevalence determines the relative importance of prevention. |
| Episode duration is 6 months on average. | After Spijker et al. [40] | Taking episode duration into account affects health benefits. A shorter duration means less potential to generate health benefits. |
| It is possible to have up to 5 recurring episodes of depression. After the fifth recurrence, a patient is assumed to be chronically depressed. Recurrence rates of depressive disorder are 50%, 70%, 80%, 85% and 90% for the first to the fifth episode. | Relapse rates are higher after a previous depressive episode | Increasing risk of recurrence results in patients making heavier demands on the health care system, which emphasizes the importance of preventing recurrence from a cost-effectiveness point of view. |
| Effects are normally distributed. | After Briggs et al. (2006) [35]. | Uncertainty around the effect parameters is symmetrical |
| Costs are gamma distributed. | After Briggs et al. (2006) [35] | Uncertainty around the cost parameters is skewed to the right. |
| Costs include only direct medical costs (in this paper). | Production losses are not relevant for retired people. Direct non-medical costs are only a fraction from direct medical costs. | The model’s output is from the perspective of healthcare providers, not the patient, and not from parties such as employers. |
| The willingness to pay (WTP) for averting 1 DALY is €20,000 ($23,755). | WTP for averting 1 DALY can be as much as €80,000 ($95,020). A relative low number of €20,000 ($23,755) was chosen to be more conservative. | A healthcare system is deemed cost-effective when the price per DALY averted is less than the WTP ceiling of €20,000 ($23,755). |
| Effects of CBT are maintained over at least one year after treatment, but effects of pharmaceutical interventions decline almost instantly after discontinuation. | Based on analysis after [22] | Longer lasting prophylactic effects for CBT than for pharmaceutical interventions, amounts to in increased cost-effectiveness of CBT relative to anti-depressant medication. |
| CBT offered during the acute stage of depression, introduces a prophylactic effect. | Based on analysis after [22] | The presence of a prophylactic effect makes it more desirable to treat acute cases of depression with CBT because it may help to avoid new onsets of the disorder in the future. |
RESULTS
Alternative A
The first comparison (the Base-Case Scenario versus the Alternative Scenario A) evaluates the added value of offering preventive interventions in terms of improvement of the cost-benefit ratio of the health care system. Cost and effects are modeled out over a period of 5 years. The key findings are:
A health care system with indicated prevention and relapse prevention costs 5% more than a system without preventive telemedicine.
Health gains are 27% higher in the scenario with preventive e-health.
In an evidence-based and preference-based system without preventive e-health, an amount of €13,775, standard deviation €724 ($16,361, standard deviation $860) is required for averting one DALY of disease burden. However, the costs per averted DALY drop to a more favorable €11,361, standard deviation €534 ($13,494, standard deviation $634) when e-health is offered. This means that the costs for averting one DALY decline strongly as a result of web-based prevention, showing that the health care system in its entirety becomes more cost-effective, even though costs increase due to additional investments in preventive telemedicine.
When averting one DALY is economically valued at €20,000 ($23,755), the ROI in the base scenario (without prevention) amounts to €1.45 per Euro invested in health care, standard deviation €0.08 ($1.72 per Dollar invested, standard deviation $0.09). The ROI improves when prevention is added (alternative A) to the system and becomes €1.76, standard deviation €0.08 ($2.09, standard deviation $0.10).
In sum, the data suggest that preventive telemedicine pays off by making the health care system more cost-effective, even though offering preventive telemedicine introduces costs of its own. This finding is robust, because it is hardly affected by uncertainty in the cost and effect parameters. This can be seen in figure 1, where alternative A is achieved by shifting the Base-Case scenario to the right (increased DALY gain), while only slightly shifting the Base-Case scenario upwards (increased cost).
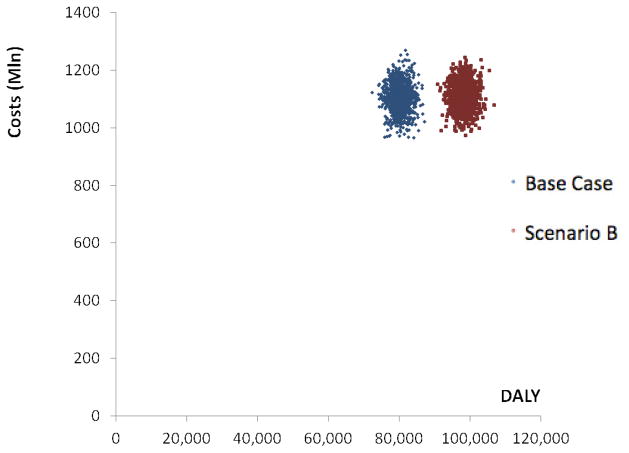
Figure 1.
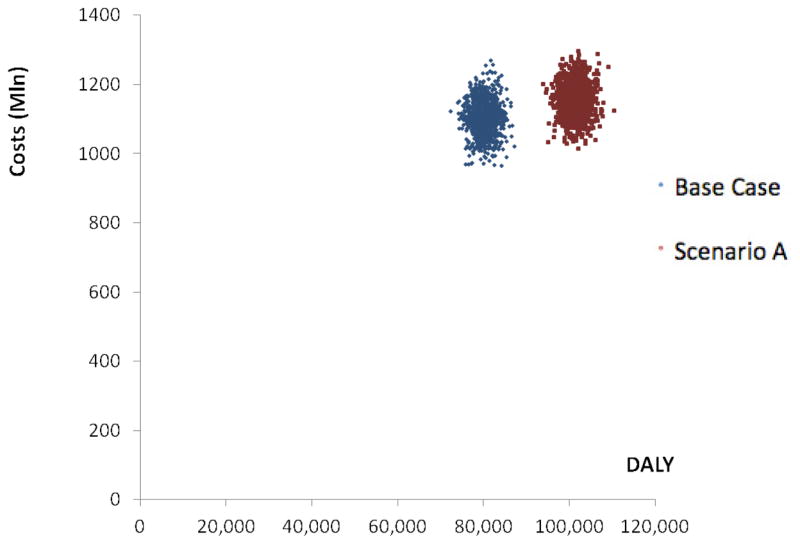
Simulation output costs and DALY averted in the Base Case versus scenario A
Alternative B
The next scenario introduces the same increase in preventive telemedicine, but decreases the coverage of (curative) interventions offered in the base scenario by 5% to keep the total cost of the health care system balanced. Again, the alternative scenario is compared with the base case scenario, and is modeled out over a period of 5 years. Findings are as before, yet slightly more favorable:
Because of the decreased treatment costs in the alternative scenario with 5%, total costs do not change.
Due to the relative cost-effectiveness of preventive e-health, health gains increase with 23%.
As before, it costs €13,775, standard deviation €724 ($16,361, standard deviation $860) to reduce the disease burden of depression by one DALY in an evidence-based and preference-based system without preventive e-health. Under the alternative scenario B this becomes €11,279, standard deviation €529 ($13,397 standard deviation $628) per averted DALY.
Following the same line of reasoning, the ROI increases from €1.45, standard deviation €0.08 ($1.72, standard deviation $0.09) in the base-case scenario to a higher €1.77, standard deviation €0.08 ($2.10, standard deviation $0.10) in Alternative B.
The corollary is that offering preventive e-health interventions makes the health care system more cost-effective, since a larger health gain is achieved, while keeping costs equal. Figure 2 shows that the DALY gains in scenario B are higher, while costs in both scenarios are comparable. These findings appear to be robust as they are unaffected by uncertainty in the model (see the non-overlapping uncertainty intervals in figure 2).
Figure 2.
Simulation output costs and DALY averted in the Base Case versus scenario B
CONCLUSION
Main findings
The main finding of this study is that e-health interventions that aim to prevent onset of first and later episodes of depression can help to make the health care system for depressed patients more cost-effective overall. That is, a health care system for depressive disorders that is both evidence-based and preference-based (i.e., evidence-based interventions that are met with approval of both health care users and health care providers) represents a good return on investment: modeled out over a period of 5 years, every Euro (Dollar) spent would generate health gains worth €1.45 ($1.72) assuming that averting one DALY is conservatively valued at €20,000 ($23,755). However, the same health care system with realistic levels of preventive telemedicine implemented, and fewer curative interventions would produce an even better payout of €1.77 ($2.10) of health-related value for every Euro (Dollar) invested.
Although the model is based on a population aged 18–65, we believe comparable results are likely to be obtained for older populations. Evidence suggests that offering telemedicine to older people is promising. A review on telecare for elderly people with chronic diseases, finds that patients were generally satisfied, accepted the technology and enjoyed self-monitoring [41]. Also, evidence specifically on treating depression in older people with telemedicine is promising. E-health interventions proved to be effective in treating depressive symptoms in older people [19,42], and in a sample of mainly older people, telemedicine was successfully used to adapt a collaborative care model for depression [43]. Besides, from a demographic perspective, the current generation represented by our data are the elderly of the future. And in that future we may have to substantially rely on health technologies that are less labor intensive than our current health care models.
Strengths and Limitations
One of the benefits of a simulation model is that it helps to organize vast fields of knowledge across several disciplines. In the case of DEPMOD these disciplines encompass psychiatric epidemiology and health economics, while the evidence that supports effect-parameters is drawn from randomized clinical trials, meta-analyses, and evidence-based clinical guidelines. It also proved possible to elicit patients’ preferences for certain interventions and to incorporate these preferences in the model. The model makes all information available in a dynamic form, which makes it possible to conduct ‘if – then’ analyses. This could be of assistance when exploring options for health care policies.
Our study has a number of limitations that need to be acknowledged. In health economic modeling, much depends on the assumptions made in the model. Whenever we had to make an assumption we tried to make a conservative one; that is an assumption that is likely to portray a not overly optimistic outcome scenario. For example, we used the more conservative value of €20,000 ($23,755) for averting one DALY and not the more generous €50,000 ($59,388), which is frequently suggested in the literature. Although we accounted for parameter uncertainty to some extent by using extensive sensitivity analyses, we emphasize that the value of our model lies in the comparative analysis of different health care scenarios, rather than the interpretation of absolute values.
Another limitation is that the model is based on a population aged 18–65 years. Data available on the population older than 65 is relatively scant, although evidence seems to suggest that the older population is willing to use and receptive to telemedicine interventions in general and depression oriented telemedicine in particular [19, 41–43]. We recommend increasing this knowledge base in order to assess the full impact of preventive telemedicine in this age group, since the older population segment is becoming increasingly important in terms of health care demand and corresponding costs. Although our model is based on Dutch data, DEPMOD can be used in other countries as well. With the appropriate data on epidemiology, effectiveness of interventions and costs, DEPMOD could be adapted to different contexts and population segments. Thus, diverse populations could be investigated by running DEPMOD separately for each population segment.
It should also be noted that implementing telemedicine on a large scale entails costs of its own. DEPMOD did not include the costs of making such a transition from one health care system to another. However, DEPMOD did compare the benefit-to-cost ratios of two health care systems after full implementation, i.e. when the systems find themselves in a ‘steady state’ balance. It is worth noting that implementation, especially in the presence of a culturally diverse population, is challenging in its own right.
For these reasons, DEPMOD is best seen as an explorative decision support tool. It is able to give almost instant feedback on policy-makers attempts to select the economically more attractive scenario in the context of constrained decision making under uncertainty in a complex environment. We recommend that DEPMOD be used in an iterative consensus building process that encompasses all pertinent stakeholders (such as health care users, health care providers, and policy makers). In any case, we would advise against using DEPMOD as an autopilot for policy making.
DEPMOD can also be employed for setting research agendas, because it helps to identify those parameters that have an impact on health gains and costs. If any of these parameters is surrounded by a non-trivial amount of uncertainty, then it is recommended to conduct empirical research with the aim of reducing uncertainty in that parameter. Finally, we wish to emphasize that ante hoc modeling requires empirical validation later. It is thus recommended that studies be conducted to test the hypotheses suggested by the modeling study.
Implications
Our modeling work shows that preventive interventions, and especially preventive e-health interventions, have potential to improve the cost-effectiveness of the health care system. This finding is consistent with other modeling studies on prevention [12, 44] and e-health [45]. Given the rising demand for health care and the corresponding increase in health care expenditure, preventive telemedicine could play an important role, especially so in graying societies where access to the Internet is available to almost all citizens.
Acknowledgments
Development of the health-economic simulation model (DEPMOD) was financially supported by The Netherlands Organisation for Health Research and Development (ZonMW), Grant # 50-50110-96-634.
Drs. Reynolds and Schulz receive financial support from the NIMH (P30 MH90333)
Footnotes
Competing interests: No disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ustun TB, Ayuso-Mateos JL, Chatterji S, et al. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–92. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- 2.Saarni SI, Suvisaari J, Sintonen H, et al. Impact of psychiatric disorders on health-related quality of life: general population survey. Br J Psychiatry. 2007;190:326–32. doi: 10.1192/bjp.bp.106.025106. [DOI] [PubMed] [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berto P, D’Ilario D, Ruffo P, et al. Depression: cost-of-illness studies in the international literature: a review. J Ment Health Policy Econ. 2000;3:3–10. doi: 10.1002/1099-176x(200003)3:1<3::aid-mhp68>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg PE, Birnbaum HG. The economic burden of depression in the US: societal and patient perspectives. Exp Opin Pharmacother. 2005;6:369–76. doi: 10.1517/14656566.6.3.369. [DOI] [PubMed] [Google Scholar]
- 6.Smit F, Cuijpers P, Oostenbrink J, et al. Excess costs of common mental disorders: population-based cohort study. J Ment Health Policy Econ. 2006;9:193–200. [PubMed] [Google Scholar]
- 7.Vasiliadis HM, Dionne PA, et al. The excess healthcare costs associated with depression and anxiety in elderly living in the community. American Journal of Geriatric Psychiatry. 2012 doi: 10.1016/j.jagp.2012.12.016. Epub. [DOI] [PubMed] [Google Scholar]
- 8.Licht-Strunk E, van der Windt DAWM, et al. The prognosis of depression in older patients in general practice and the community. A systematic review. Family Practice. 2007;24:168–180. doi: 10.1093/fampra/cml071. [DOI] [PubMed] [Google Scholar]
- 9.Byers AL, Covinsky KE, Barnes DE, et al. dysthymia and depression increase risk of dementia an mortality among older veterans. American Journal of Geriatric Psychiatry. 2012;20(8):p664–672. doi: 10.1097/JGP.0b013e31822001c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vos T, Haby MM, Magnus A, et al. Assessing cost-effectiveness in mental health: helping policy-makers prioritize and plan health services. Aust NZ J Psychiat. 2005;39:701–712. doi: 10.1080/j.1440-1614.2005.01654.x. [DOI] [PubMed] [Google Scholar]
- 11.Andrews G, Issakidis C, Sanderson K, et al. Utilising survey data to inform public policy: comparison of the cost-effectiveness of treatment of ten mental disorders. Br J Psychiatry. 2004;184:526–33. doi: 10.1192/bjp.184.6.526. [DOI] [PubMed] [Google Scholar]
- 12.Mihalopoulos C, Vos T, Pirkis J, Smit F, Carter R. Do indicated preventive interventions represent good value-for-money? Aust NZ J Psychiat. 2011;45:36–44. doi: 10.3109/00048674.2010.501024. [DOI] [PubMed] [Google Scholar]
- 13.Hutubessy R, Chisholm D, Edejer TTT. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;1:8. doi: 10.1186/1478-7547-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chisholm D, Sanderson K, Ayoso-Mateos JL, et al. Reducing the global burden of. Br J Psychiatry. 2004;184:393–403. doi: 10.1192/bjp.184.5.393. [DOI] [PubMed] [Google Scholar]
- 15.Bijl RV, De Graaf R, Ravelli A, et al. Gender and age specific first incidence of DSM-III-R psychiatric disorders in the general population. Results from the Netherlands Mental Health Survey and Incidence Study (Nemesis) Soc Psychiatry Psychiatr Epidemiol. 2002;37:372–379. doi: 10.1007/s00127-002-0566-3. [DOI] [PubMed] [Google Scholar]
- 16.Oostenbrink JB, Bouwmans CAM, Koopmanschap MA, et al. Handleiding voor kostenonderzoek, methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg. College voor zorgverzekeringen; 2004. [Google Scholar]
- 17.Cuijpers P, van Straten A, Smit F, et al. Preventing the onset of depressive disorders: A meta-analytic review of psychological interventions. Am J Psychiatry. 2008;165:1272–80. doi: 10.1176/appi.ajp.2008.07091422. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz RF, Cuijpers P, Smit F, et al. Prevention of major depression. Ann Rev Clin Psychol. 2010;6:181–212. doi: 10.1146/annurev-clinpsy-033109-132040. [DOI] [PubMed] [Google Scholar]
- 19.Spek V, Cuijpers P, Nylicek I, et al. Internet-based cognitive behaviour therapy for symptoms of depression and anxiety: a meta-analysis. Psychological Medicine. 2007;37:319–328. doi: 10.1017/S0033291706008944. [DOI] [PubMed] [Google Scholar]
- 20.Andersson G, Cuijpers P. Internet-Based and Other Computerized Psychological Treatments for Adult Depression: A Meta-Analysis. Cognitive Behaviour Therapy. 2009;38:196–205. doi: 10.1080/16506070903318960. [DOI] [PubMed] [Google Scholar]
- 21.van’t Veer-Tazelaar PJ, van Marwijk HWJ. Prevention of Late-Life Anxiety and Depression Has Sustained Effects Over 24 Months: A Pragmatic Randomized Trial. American Journal of Geriatric Psychiatry. 2011;19(3):230–9. doi: 10.1097/jgp.0b013e3181faee4d. [DOI] [PubMed] [Google Scholar]
- 22.Willemse GRWM, Smit F, Cuijpers P, et al. Minimal contact psychotherapy for sub-threshold depression in primary care: a randomised trial. Br J Psychiatry. 2004;185:416–421. doi: 10.1192/bjp.185.5.416. [DOI] [PubMed] [Google Scholar]
- 23.Cuijpers P. A psychoeducational approach to the treatment of depression: a meta-analysis of Lewinsohn’s “Coping with Depression” course. Behavior Therapy. 1998;29:521–533. [Google Scholar]
- 24.Cuijpers P, van Straten A, Andersson G. Internet-administered cognitive behavior therapy for health problems: A systematic review. Journal of Behavioral Medicine. 2008;31:169–177. doi: 10.1007/s10865-007-9144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruwaard J, Schrieken B, Schrijver M, Broeksteeg J, Dekker J, Vermeulen H, Lange A. Web-based Therapist-assisted cognitive behavioural treatment of mild to moderate depression: a randomised trial. 2009 doi: 10.1080/16506070802408086. (downloaded from http://www.interapy.nl/content/pdf/interapy-2009-depression-cbt.pdf) [DOI] [PubMed]
- 26.Cuijpers P, Van Straten A, Warmerdam AM, et al. Characteristics of effective psychological treatments of depression: a metaregression analysis. Psychotherapy Research. 2007;18(2):225–236. doi: 10.1080/10503300701442027. [DOI] [PubMed] [Google Scholar]
- 27.Cuijpers P, Straten A, Andersson G, et al. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. Journal of Consulting and Clinical Psychology. 2008;76(6):909–922. doi: 10.1037/a0013075. [DOI] [PubMed] [Google Scholar]
- 28.Ekers D, Richards D, Gilbody S. A meta-analysis of randomised trials of behavioural treatment of depression. Psychological Medicine. 2008;38:611–623. doi: 10.1017/S0033291707001614. [DOI] [PubMed] [Google Scholar]
- 29.Arroll B, MacGillivray S, Ogston S, et al. Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: a meta-analysis. Ann Fam Med. 2005;3:449–456. doi: 10.1370/afm.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the food and drug administration. Plos Medicine. 2008;5(2):e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuijpers P, Van Straten A, Warmerdam MA, et al. Psychotherapy versus combination of psychotherapy and pharmacotherapy in the treatment of depression: a meta-analysis. Depression and Anxiety. 2009;26:279–288. doi: 10.1002/da.20519. [DOI] [PubMed] [Google Scholar]
- 33.Vittengl JR, Clark LA, Dunn TW, et al. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy’s effects. Journal of consulting and clinical psychology. 2007;75(3):475–488. doi: 10.1037/0022-006X.75.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riper H, Smit F, Van der Zanden R, et al. E-mental health: high tech, high touch, high trust. Utrecht: Trimbos Instituut; 2007. [Google Scholar]
- 35.Briggs A, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford, England: Oxford University Press; 2006. [Google Scholar]
- 36.Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. Oxford, England: Oxford University Press; 2005. [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: Author; 2000. [Google Scholar]
- 38.Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Dis. 2002;72:227–236. doi: 10.1016/s0165-0327(01)00413-x. [DOI] [PubMed] [Google Scholar]
- 39. [accessed June 29, 2012];OECD STATS: PPPs and exchange rates. [OECD website]. Available at http://stats.oecd.org/Index.aspx?DataSetCode=SNA_TABLE4.
- 40.Spijker J, De Graaf R, Van Bijl R, et al. Duration of major depressive episodes in the general population: results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS) Br J Psychiatry. 2002;181:208–213. doi: 10.1192/bjp.181.3.208. [DOI] [PubMed] [Google Scholar]
- 41.Botsis T, Hartvigsen G. Current status and future perspectives in telecare for elderly people suffering from chronic diseases. J Telemed Telecare. 2008;14:195–203. doi: 10.1258/jtt.2008.070905. [DOI] [PubMed] [Google Scholar]
- 42.Spek V, Nyklicek I, Smits N, et al. Internet-based cognitive behavioural therapy for subthreshold depression in people over 50 years old: a randomized controlled clinical trial. Psychol Med. 2007;37(12):1797–1806. doi: 10.1017/S0033291707000542. [DOI] [PubMed] [Google Scholar]
- 43.Fortney JC, Pyne JM, Edlund MJ, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. 2007;22:1086–1093. doi: 10.1007/s11606-007-0201-9. http://bjp.rcpsych.org/content/181/3/208.long-fn-1#fn-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Den berg M, Smit F, Vos T, et al. Cost-effectiveness of opportunistic screening and minimal contact psychotherapy to prevent depression in primary care patients. PloS ONE. 2011;6(8) doi: 10.1371/journal.pone.0022884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smit F, Lokkerbol J, Riper H, et al. Modeling the cost-effectiveness of health care systems for alcohol use disorders: how implementation of eHealth interventions improves cost-effectiveness. J Med Internet Res. 2011;13(3):e56. doi: 10.2196/jmir.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]