Abstract
Background
Smoking cessation is a key component of secondary cardiovascular disease prevention. Varenicline, a partial α4β2 nicotinic acetylcholine receptor agonist, is effective for smoking cessation in healthy smokers, but its efficacy and safety in smokers with cardiovascular disease are unknown.
Methods and Results
A multicenter, randomized, double-blind, placebo-controlled trial compared the efficacy and safety of varenicline with placebo for smoking cessation in 714 smokers with stable cardiovascular disease. Participants received varenicline (1 mg twice daily) or placebo, along with smoking-cessation counseling, for 12 weeks. Follow-up lasted 52 weeks. The primary end point was carbon monoxide–confirmed continuous abstinence rate for weeks 9 through 12 (last 4 weeks of treatment). The continuous abstinence rate was higher for varenicline than placebo during weeks 9 through 12 (47.0% versus 13.9%; odds ratio, 6.11; 95% confidence interval [CI], 4.18 to 8.93) and weeks 9 through 52 (19.2% versus 7.2%; odds ratio, 3.14; 95% CI, 1.93 to 5.11). The varenicline and placebo groups did not differ significantly in cardiovascular mortality (0.3% versus 0.6%; difference, −0.3%; 95% CI, −1.3 to 0.7), all-cause mortality (0.6% versus 1.4%; difference, −0.8%; 95% CI, −2.3 to 0.6), cardiovascular events (7.1% versus 5.7%; difference, 1.4%; 95% CI, −2.3 to 5.0), or serious adverse events (6.5% and 6.0%; difference, 0.5%; 95% CI, −3.1 to 4.1). As a result of adverse events, 9.6% of varenicline and 4.3% of placebo participants discontinued study drug.
Conclusions
Varenicline is effective for smoking cessation in smokers with cardiovascular disease. It was well tolerated and did not increase cardiovascular events or mortality; however, trial size and duration limit definitive conclusions about safety.
Clinical Trial Registration Information
URL: http://www.clinicaltrials.gov/ct2/show/NCT00282984. Unique identifier: NCT00282984
Keywords: cardiovascular diseases, cerebrovascular disorders, peripheral vascular diseases, smoking, trials
Cigarette smoking is a major risk factor for cardiovascular disease (CVD).1 Among smokers with coronary heart disease, smoking cessation is associated with a 36% reduction in risk of all-cause mortality,2 making smoking cessation fundamental to secondary prevention of CVD.3 Although an acute myocardial infarction (MI) or other hospitalization for CVD motivates many smokers to quit in the short term, most resume smoking.4 The result is a large number of smokers with stable CVD who need to quit. Identifying more effective tobacco-dependence treatment for patients with CVD is a high priority for CVD prevention.5
Pharmacotherapy is a standard component of evidencebased smoking cessation treatment.5 Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist,6,7 is effective for smoking cessation in healthy smokers.7,8 It outperformed bupropion in 3 randomized, double-blind, controlled studies9–11 and may be superior to nicotine replacement therapy.12,13 Varenicline is among the first-line pharmacotherapies recommended by the 2008 US Public Health Service clinical practice guideline for tobacco dependence.5 However, evidence of the efficacy of varenicline is derived from studies in generally healthy smokers. The efficacy of varenicline for treating smokers with comorbid illnesses such as CVD has not been studied.
The safety of varenicline in CVD patients has also not been evaluated. The sympathomimetic cardiovascular effects of nicotine are mediated primarily by binding to α3β4 nicotinic acetylcholine receptors,14 which increases heart rate, myocardial contractility, and blood pressure. This increases myocardial work and causes coronary vasoconstriction, reducing myocardial blood supply.15 Because of its relative selectivity for α4β2 nicotinic acetylcholine receptors, varenicline is predicted to have no significant cardiovascular effects, but this has not been tested in people with CVD. We conducted a randomized, controlled trial to test the safety and efficacy of varenicline for smoking cessation in patients with stable CVD.
The trial also provided an opportunity to observe any occurrence of psychiatric adverse events (AEs) that have been reported in the postmarketing of varenicline.7 Cases of abnormal behavior, depression, suicidal ideation, and suicide in smokers taking varenicline have been reported,16 causing concerns about the drug and leading the Food and Drug Administration to issue a public health advisory recommending that physicians inform patients taking varenicline to watch for these symptoms.17 This study provided an opportunity to assess the frequency of these symptoms in the context of a double-blind, placebo-controlled trial in smokers with CVD.
Methods
Study Design
A multicenter, randomized, double-blind, placebo-controlled trial of varenicline for smoking cessation in patients with stable CVD was conducted at 39 sites in 15 countries between February 2006 and August 2008. Participants were randomly assigned to take varenicline or placebo for 12 weeks and were followed up to week 52 in a blinded posttreatment phase. The Institutional Review Board at each site approved the study, and participants provided written informed consent.
Study Population
Participants were adults (35 to 75 years of age) who had smoked an average of ≥10 cigarettes daily in the year before enrollment, wanted to stop smoking but had not tried to quit in the past 3 months, and had stable, documented CVD (other than hypertension) that had been diagnosed for > 2 months. Eligible CVD diagnoses included a history of MI, coronary revascularization, angina pectoris (confirmed by procedure report), peripheral arterial vascular disease (confirmed by physical examination or procedure report), or cerebrovascular disease (stroke or transient ischemic attack confirmed by neurological evaluation or procedure report). Participants were excluded if, in the past 2 months, they had undergone a cardiovascular procedure (eg, percutaneous transluminal coronary angioplasty) or had cardiovascular instability (including MI or unstable angina). Other cardiovascular exclusions were uncontrolled hypertension, significant neurological sequela of cerebrovascular disease, peripheral vascular disease with prior amputation, or severe congestive heart failure (New York Heart Association class III or IV).18 Other exclusion criteria included moderate or severe chronic obstructive pulmonary disease; uncontrolled gastrointestinal, hepatic, or endocrine disease (eg, hemoglobin A1c >9); severe renal impairment; cancer; diagnosis of depression; treatment with antidepressants in the past year; history of psychosis, panic disorder, or bipolar disorder; drug or alcohol abuse or dependence in the past year; or smoking cessation medication use (nicotine replacement therapy, bupropion, clonidine, or nortriptyline) in the past month.
Interventions
Eligible participants were randomly assigned, stratified by study site, to varenicline (0.5 mg once daily for 3 days, 0.5 mg twice daily for 4 days, and then 1.0 mg twice daily for a total of 12 weeks) or to an identical placebo regimen. The study sponsor conducted the randomization centrally using a computer-generated list that prespecified the order of treatment allocation. Study sites obtained treatment group assignments with a Web-based or telephone system.
Participants started study drug the day after randomization. The target quit date was 8 days later. During the 12 weeks of treatment, participants had weekly clinic visits that included 10 minutes of smoking counseling following clinical practice guidelines19 and 1 telephone call made 3 days after the quit date. After the drug treatment ended, participants made 7 clinic visits (weeks 13, 16, 24, 32, 40, 48, and 52) and received 5 telephone calls (weeks 14, 20, 28, 36, and 44) that provided additional brief smoking counseling.
Assessments
Participants were screened at 2 visits. Data collected included medical and smoking history, Fagerström Test for Nicotine Dependence,20 physical examination, vital signs (blood pressure, heart rate, temperature, height, and weight), blood chemistries and hematology, ECG, urinalysis, and urine drug screen. Assessments at each visit included vital signs, smoking status (any cigarette smoking in the past 7 days and since the last clinic visit), exhaled CO level, medication compliance, use of other smoking cessation medications or other tobacco products, and occurrence of AEs. The same measures (excluding exhaled CO and vital signs) were assessed at each telephone call during follow-up. At weeks 12 and 52, the physical examination, ECG, and blood tests were repeated. AEs were collected through 30 days after drug treatment ended; serious AEs (SAEs) were collected for the full 52 weeks.
Outcome Measures
The primary study end point was the 4-week continuous abstinence rate (CAR) during the last 4 weeks of study drug treatment (weeks 9 to 12). Continuous abstinence was defined as self-reported abstinence from any tobacco- or nicotine-containing product since the last visit, but a subject with CO > 10 ppm was classified as a smoker regardless of self-reported abstinence. The key secondary end point was the CAR from week 9 through 52. Other secondary end points were CAR for weeks 9 to 24 and 7-day point prevalence of tobacco abstinence at weeks 12 (end of drug treatment), 24, and 52. Point prevalence abstinence was defined as self-reported abstinence from any tobacco- or nicotine-containing product in the past 7 days that was not contradicted by expired air CO > 10 ppm.
Any AE that resulted in death, was life-threatening, required inpatient hospitalization or prolongation of hospitalization, produced persistent or significant disability or incapacity, or was a congenital anomaly was considered an SAE. The verbatim terms used by the investigators to report AEs were recorded, and a computerized program coded them to preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA version 11.0).21 Preferred terms were grouped into high-level group terms and summarized into system organ class categories. Reported or observed cardiovascular events or deaths resulting from any cause were reviewed separately and adjudicated under blinded conditions by an independent event committee made up of 3 board-certified cardiologists who used a standard events manual. Events reviewed included nonfatal or fatal MI, hospital admission for chest pain, hospitalization for angina pectoris, need for coronary revascularization, resuscitated cardiac arrest, hospitalization for congestive heart failure, fatal or nonfatal stroke or transient ischemic attack, new diagnosis of or admission for a procedure to treat peripheral vascular disease, and death resulting from any cause. Cardiovascular events and all-cause deaths were summarized by treatment group.
Statistical Analysis
The sample of 700 randomized subjects was estimated to provide ≥84% power to detect a group difference in the key secondary end point and 99% power to detect a group difference in the primary end point if the true 4-week CARs for placebo and varenicline conditions were 18% and 40%, respectively, and the week 9 to 52 CARs were 10% and 18%, respectively. Efficacy outcomes were assessed with an intention-to-treat analysis that included all randomized participants. Individuals who discontinued study participation or were lost to follow-up were counted as smokers from the time of study discontinuation. Participants whose self-reported nonsmoking was contradicted by an expired air CO > 10 ppm at any visit were counted as smokers. For calculating CARs, a participant who missed a visit but had a CO-validated report of continuous abstinence at the next visit at which smoking status was available was considered a nonsmoker. However, to be considered abstinent at week 52, a participant had to attend the visit and have CO-confirmed tobacco abstinence. No imputation for missing self-report data was made for calculating 7-day point prevalence.
Primary and secondary end points were analyzed with a logistic regression model that included treatment group and study site as independent variables. Small sites were pooled to achieve model convergence. Hypothesis testing was performed with the likelihood ratio χ2 statistic. The type I family-wise error rate of 0.05 for the primary and key secondary end points was preserved with the use of a stepdown procedure. Posthoc subgroup analysis of CARs at weeks 9 to 12 was done by race (white, nonwhite), gender, age (≤55 and >55 years), cigarettes per day (≤20 and >20), Fagerström score (0 to 5 and >5), and diagnosis (cardiac and noncardiac disease). To do this, the logistic regression model described above was repeated for each individual subgroup.
Safety outcomes were assessed among participants who took at least 1 dose of study drug. Rates of AEs and SAEs, treatment discontinuation as a result of AEs, deaths, adjudicated cardiovascular events, and changes in blood pressure, heart rate, and body weight were summarized by treatment group.
Results
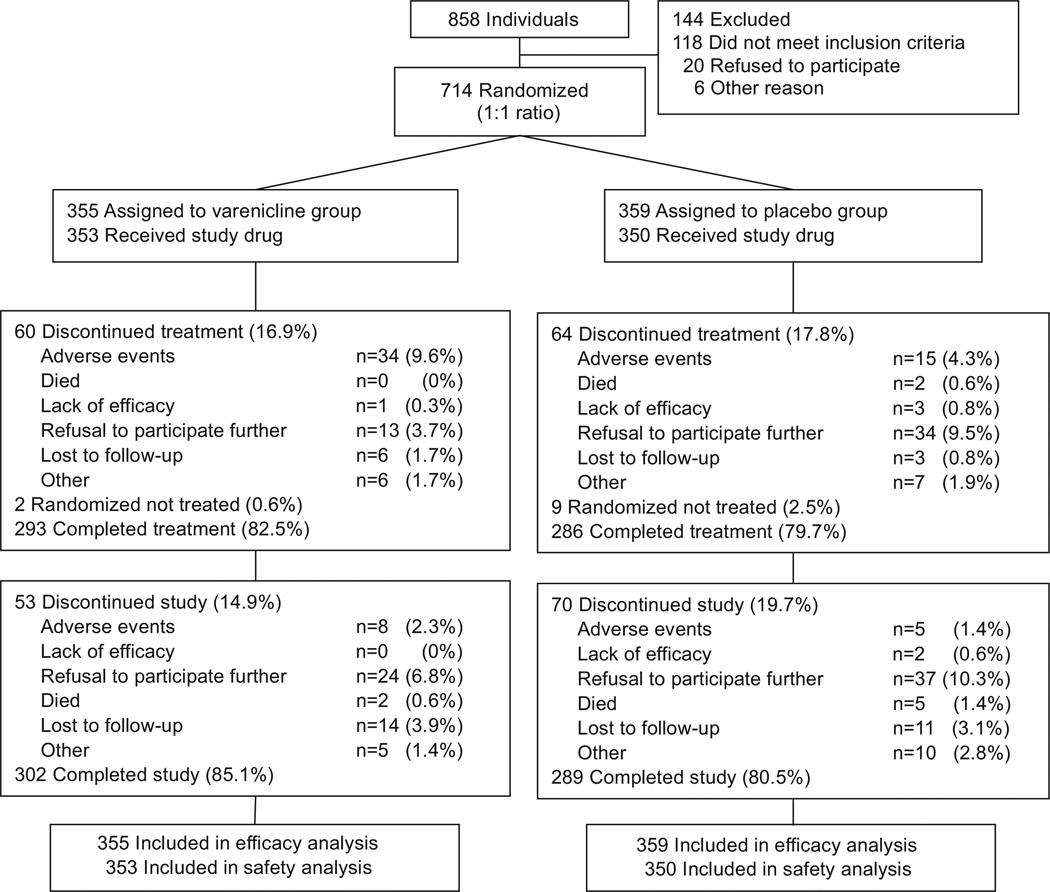
Figure 1 displays the flow of participants through the study. Of 858 smokers who were screened, 714 (83.2%) were eligible for the study, were enrolled, and were randomly assigned to the varenicline (n=355) or placebo (n=359) groups. Overall, 591 (82.8%) of the randomized participants completed the study, including 302 (85.1%) assigned to varenicline and 289 (80.5%) assigned to placebo. Baseline characteristics of the randomized participants were comparable between treatment groups (Table 1).
Figure 1.
Participant disposition. Flow of participants through the study.
Table 1.
Baseline Characteristics of Study Participants
| Varenicline (n=355) |
Placebo (n=359) |
|
|---|---|---|
| Demographic characteristics | ||
| Age, mean (SD), y | 57.0 (8.6) | 55.9 (8.3) |
| Gender (male), n (%) | 267 (75.2) | 295 (82.2) |
| Race, n (%) | ||
| White | 285 (80.3) | 290 (80.8) |
| Black | 3 (0.8) | 2 (0.6) |
| Asian | 30 (8.5) | 31 (8.6) |
| Other | 37 (10.4) | 36 (10.0) |
| Body mass index, mean (SD), kg/m2* | 27.5 (4.4) | 27.9 (4.5) |
| Smoking history | ||
| Time smoking cigarettes, mean (range), y | 40 (5–63) | 39 (12–60) |
| Cigarettes/d (past month), mean (range) | 22.1 (10–60) | 22.9 (10–80) |
| Noncigarette tobacco use in past month, n (%) | 13 (3.7) | 26 (7.2) |
| Fagerströ m test for nicotine dependence score, mean (SD)† | 5.6 (2.1) | 5.7 (2.0) |
| Any previous serious attempts to quit, n (%) | 304 (85.6) | 310 (86.4) |
| Medical history, n (%) | ||
| Cardiac disease | ||
| Angina pectoris | 189 (53.2) | 172 (47.9) |
| MI | 163 (45.9) | 188 (52.4) |
| Prior coronary revascularization | 164 (46.2) | 185 (51.5) |
| Congestive heart failure | 16 (4.5) | 14 (3.9) |
| Atrial fibrillation | 10 (2.8) | 15 (4.2) |
| Cerebrovascular disease | ||
| Stroke | 16 (4.5) | 24 (6.7) |
| Transient ischemic attack | 20 (5.6) | 21 (5.8) |
| Vascular disease | ||
| Hypertension | 195 (54.9) | 202 (56.3) |
| Peripheral arterial disease | 82 (23.1) | 97 (27.0) |
| Prior peripheral revascularization | 37 (10.4) | 42 (11.7) |
| Aortic aneurysm | 0 (0.0) | 2 (0.6) |
| Diabetes mellitus | 47 (13.2) | 60 (16.7) |
n=352 (varenicline); n=348 (placebo).
n=354 (varenicline); n=358 (placebo). Scores range from 0 to 10, with higher scores indicating greater nicotine dependence.
Efficacy
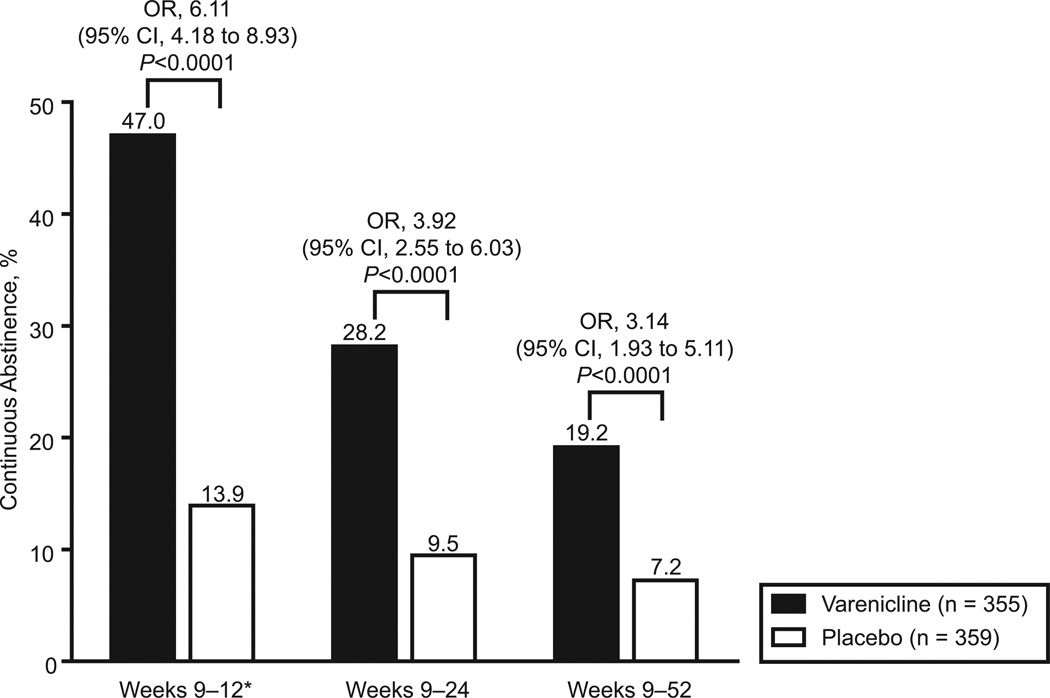
Figure 2 displays the smoking cessation outcomes among all randomized participants after adjustment for study site. The primary outcome measure, CO-validated CAR during the last 4 weeks of drug treatment (weeks 9 to 12), was higher in the varenicline group than in the placebo group (47.0% versus 13.9%; odds ratio [OR], 6.11; 95% confidence interval [CI], 4.18 to 8.93; P<0.0001). The analysis unadjusted for study site produced similar results (OR, 5.39; 95% CI, 3.74 to 7.76), indicating that sites had no significant impact on efficacy. Analyses adjusting for country or region yielded similar results (data not shown). The superiority of varenicline over placebo in verified CARs persisted to the end of the study (Figure 2). The CARs for weeks 9 to 52, the key secondary end point, were 19.2% for varenicline and 7.2% for placebo (OR, 3.14; 95% CI, 1.93 to 5.11; P<0.0001). There was no statistically significant treatment-by-site interaction in these analyses.
Figure 2.
Tobacco CARs. Proportion of participants who reported abstinence from tobacco smoking, confirmed by exhaled CO ≤10 ppm, at all visits during the time period. *Primary study end point (weeks 9 to 12 are the last 4 weeks of study drug treatment).
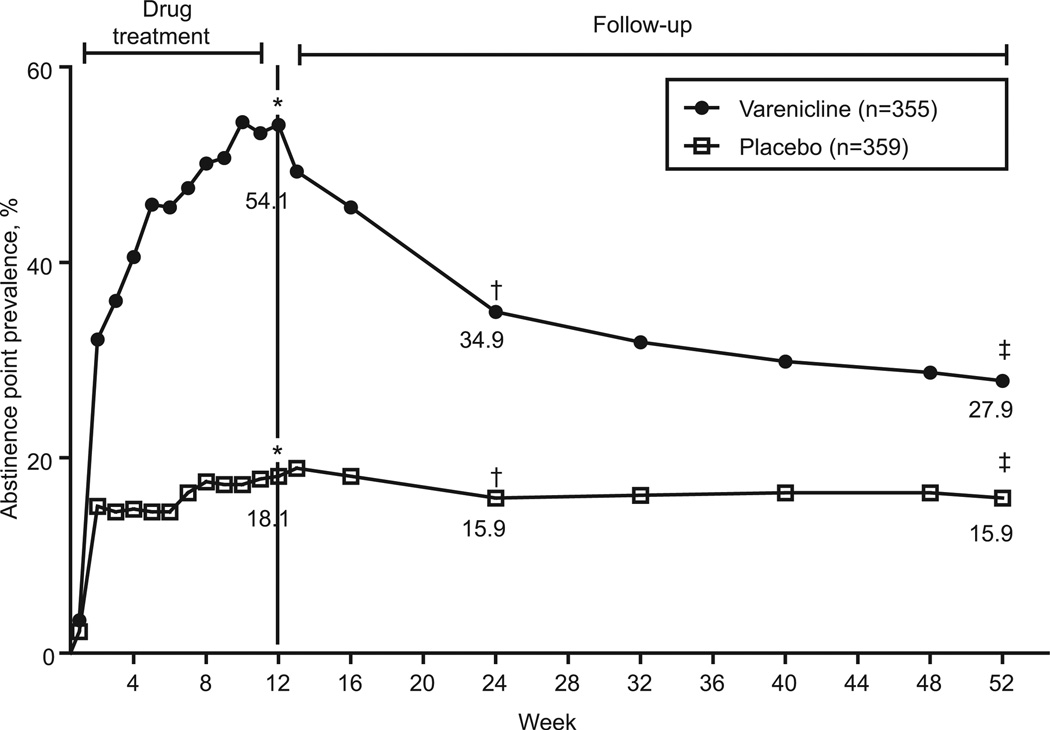
CO-verified 7-day point-prevalence tobacco abstinence rates were higher in the varenicline group than in the placebo group at all study visits during and after drug treatment (Figure 3). The 7-day point prevalence rates rose throughout the 12-week treatment for participants assigned to varenicline. This did not occur in the placebo group. At the end of drug treatment, validated 7-day point prevalence tobacco abstinence was achieved by 54.1% of participants in the varenicline group and 18.1% in the placebo group (OR, 6.05; 95% CI, 4.23 to 8.65; P<0.0001; Figure 3). The difference between groups narrowed after drug treatment ended, but verified 7-day point prevalence abstinence remained higher for the varenicline group throughout the follow-up. Sevenday abstinence rates for varenicline and placebo were 34.9% and 15.9% (OR, 2.98; 95% CI, 2.07 to 4.29; P<.0001) at week 24 and 27.9% and 15.9% (OR, 2.10; 95% CI, 1.45 to 3.05; P=0.0001) at the end of the study (week 52).
Figure 3.
Seven-day point prevalence tobacco abstinence rates. CO-validated abstinence from any tobacco product in the past 7 days. For varenicline (closed circles) vs placebo (open squares): *week 12: OR, 6.05; 95% CI, 4.23 to 8.65; P<0.0001; †week 24: OR, 2.98; 95% CI, 2.07 to 4.29; P<0.0001; ‡week 52: OR, 2.10; 95% CI, 1.45 to 3.05; P<0.0001.
The superiority of varenicline over placebo in continuous abstinence was statistically significant in posthoc analyses of subgroups defined by age, Fagerström score, daily cigarette consumption, and presence of coronary heart disease. Significant effects of varenicline were seen in subgroups of male and white participants. Female and nonwhite participant samples were too small to permit significance testing, but abstinence rates were consistent with those of the overall analysis.
Safety
Safety analyses included the 703 of 714 participants who took ≥1 dose of study drug. Table 2 summarizes all treatment-emergent AEs observed during or within 30 days after the end of drug treatment and all SAEs for 52 weeks. Varenicline was well tolerated; 9.6% of participants discontinued varenicline as a result of an AE. Nausea, the most commonly reported symptom in the varenicline group, occurred in 29.5% of patients. More participants in the varenicline group than in the placebo group reported nausea, vomiting, insomnia, and abnormal dreams.
Table 2.
Treatment-Emergent AEs*
| Varenicline (n=353), n (%) |
Placebo (n=350), n (%) |
Difference Between Groups, % |
95% CI for Difference |
|
|---|---|---|---|---|
| Total AEs† | 949 | 656 | ||
| Participants with ≥1 AE | 288 (81.6) | 227 (64.9) | 16.7 | 10.3–23.2 |
| Participants who stopped drug because of AE | 34 (9.6) | 15 (4.3) | 5.3 | 1.6–9.1 |
| Participants with ≥1 serious AE | 23 (6.5) | 21 (6.0) | 0.5 | −3.1–4.1 |
| Deaths (all causes) | 2 (0.6) | 5 (1.4) | −0.8 | −2.3–0.6 |
| Most common AEs‡ | ||||
| Nausea | 104 (29.5) | 30 (8.6) | 20.9 | 15.3–26.5 |
| Headache | 45 (12.7) | 39 (11.1) | 1.6 | −3.2–6.4 |
| Insomnia | 42 (11.9) | 23 (6.6) | 5.3 | 1.1–9.6 |
| Vomiting | 29 (8.2) | 4 (1.1) | 7.1 | 4.0–10.1 |
| Abnormal dreams | 28 (7.9) | 6 (1.7) | 6.2 | 3.1–9.4 |
| Fatigue | 25 (7.1) | 14 (4.0) | 3.1 | −0.3–6.5 |
| Nasopharyngitis | 23 (6.5) | 30 (8.6) | −2.1 | −6.0–1.8 |
| Constipation | 23 (6.5) | 7 (2.0) | 4.5 | 1.6–7.5 |
| Diarrhea | 22 (6.2) | 18 (5.1) | 1.1 | −2.3–4.5 |
| Dizziness | 22 (6.2) | 16 (4.6) | 1.7 | −1.7–5.0 |
| Dyspepsia | 19 (5.4) | 12 (3.4) | 2.0 | −1.1–5.0 |
| Psychiatric AEs§ | ||||
| Sleep disorders or disturbances (abnormal dreams, insomnia, nightmare, sleep disorder) | 78 (22.1) | 34 (9.7) | 12.4 | 7.1–17.7 |
| Anxiety disorders or symptoms (anxiety, generalized anxiety disorder, neurosis, phobia, stress) | 12 (3.4) | 16 (4.6) | −1.2 | −4.1–1.7 |
| Depressed mood disorders or disturbances (depression, depressed mood, depressive symptom, dysthymia) | 11 (3.1) | 8 (2.3) | 0.8 | −1.6–3.2 |
| Bipolar disorder | 1 (0.3) | 0 (0) | 0.3 | −0.4–1.0 |
| Other mood disorders or disturbances (apathy, listlessness, dysphoria, mood alteration, mood swings, emotional disorder) | 9 (2.5) | 3 (0.9) | 1.7 | −0.2–3.6 |
| Suicidal and self-injurious behaviors | 0 (0) | 0 (0) | … | … |
| Change in physical activity (restlessness) | 3 (0.8) | 3 (0.9) | −0.01 | −1.4–1.4 |
| Sexual dysfunction (decreased libido) | 2 (0.6) | 1 (0.3) | 0.3 | −0.7–1.2 |
| Delirium (confusion) | 1 (0.3) | 0 (0) | 0.3 | −0.4–1.0 |
| Disturbances in behavior (aggression) | 0 (0) | 1 (0.3) | −0.3 | −1.0–0.4 |
| Cognitive and attention disorders | 0 | 0 | … | … |
| Dissociative disorders | 0 | 0 | … | … |
| Disturbances in thinking and perception | 0 | 0 | … | … |
AEs that began or increased in severity during treatment or up to 30 days after the last administration of the investigational product. SAEs that occurred at any time are reported. Except for the number of AEs, participants are counted only once per treatment in each row.
Multiple AEs of the same type in an individual participant were counted only once.
Occurring in ≥5% of participants in either group.
Includes all AEs reported in the MedDRA System Organ Class of Psychiatric Disorders. Each row represents a higher-level group term, which is a combination of individual symptom terms. Symptoms actually reported are in parentheses.
SAEs occurred in 23 participants (6.5%) in the varenicline group and 21 (6.0%) in the placebo group (difference, 0.5%; 95% CI, −3.1 to 4.1). Depression, suicidality, and abnormal behavior were not reported as SAEs, although 1 SAE that occurred 10 months after the end of study drug was an overdose of benzodiazepines in an intoxicated participant.
Two participants in the varenicline group (0.6%) and 5 participants in the placebo group (1.4%) died (difference, −0.8%; 95% CI, −2.3 to 0.6; Table 2). In the varenicline group, 1 death was cardiovascular (acute MI in a 63-year-old man after treatment ended) and 1 was not (pancreatic cancer in a 75-year-old man). In the placebo group, 2 deaths were cardiovascular (acute MI in a 73-year-old man and a 51-yearold man, both after treatment stopped) and 3 were not (diabetic coma in a 63-year-old man during drug treatment, esophageal cancer in a 61-year-old man, and bladder cancer in a 59-year-old man, both after treatment).
Table 2 also displays AEs that were classified as psychiatric disorders.21 Other than sleep disorders, reported by 22.1% of patients in the varenicline group and 9.7% of patients in the placebo group, psychiatric AEs were uncommon (<5%) and did not differ significantly between groups. Any depressed mood disorder or symptom was reported by 3.1% of participants in the varenicline group (versus 2.3% for placebo). Anxiety disorders or symptoms were reported by 3.4% (varenicline) and 4.6% (placebo). Other mood disorders were reported by 2.5% (varenicline) and 0.9% (placebo). No participant in the varenicline group reported suicidal ideation or attempt, a change in behavior, or a cognitive or attention disorder.
Table 3 displays all adjudicated cardiovascular events and all deaths during the trial. The proportion of participants with an adjudicated cardiovascular event was 7.1% in the varenicline group and 5.7% in the placebo group (difference, 1.4; 95% CI, −2.3 to 5.0). The cardiovascular death rate was 0.3% for varenicline and 0.6% for placebo (difference, −0.3; 95% CI, −1.3 to 0.7). Groups did not differ in blood pressure or resting heart rate change from baseline to the end of drug treatment (Table 4).
Table 3.
Adjudicated Cardiovascular Events and All Deaths*
| Varenicline (n=353), n (%)† |
Placebo (n=350), n (%)† |
Difference Between Groups, % |
95% CI for Difference |
|
|---|---|---|---|---|
| Any adjudicated cardiovascular event‡ | 25 (7.1) | 20 (5.7) | 1.4 | −2.3–5.0 |
| Coronary artery disease | ||||
| Nonfatal MI | 7 (2.0) | 3 (0.9) | 1.1 | −0.6–2.9 |
| Need for coronary revascularization | 8 (2.3) | 3 (0.9) | 1.4 | −0.4–3.2 |
| Hospitalization for angina pectoris | 8 (2.3) | 8 (2.3) | −0.02 | −2.2–2.2 |
| Hospitalization for congestive heart failure | 0 (0.0) | 2 (0.6) | −0.6 | −1.5–0.3 |
| Cerebrovascular disease | ||||
| Nonfatal stroke | 2 (0.6) | 1 (0.3) | 0.3 | −0.7–1.2 |
| Transient ischemic attack | 1 (0.3) | 1 (0.3) | −0.0 | −0.8–0.8 |
| Peripheral vascular disease | ||||
| New diagnosis or admission for a procedure to treat peripheral vascular disease | 5 (1.4) | 3 (0.9) | 0.6 | −1.0–2.1 |
| Death | ||||
| All causes | 2 (0.6) | 5 (1.4) | −0.8 | −2.3–0.6 |
| Cardiovascular death | 1 (0.3) | 2 (0.6) | −0.3 | −1.3–0.7 |
| Noncardiovascular death | 1 (0.3) | 3 (0.9) | −0.6 | −1.7–0.5 |
Number (percent) of participants as per the decision of the cardiovascular event adjudication committee. Among cardiovascular events reported by study investigators, 9 varenicline and 2 placebo events were adjudicated as not meeting the criteria for the reported cardiovascular event.
Participants with multiple cardiovascular events of the same type are counted only once per row.
Excludes 4 deaths (1 in the varenicline group and 3 in the placebo group) that were adjudicated as noncardiovascular deaths.
Table 4.
Change in Vital Signs From Baseline to End of Treatment*
| Varenicline (n=304) |
Placebo (n=284) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline† | Week 12‡ | Difference (Baseline to Week 12‡) |
Baseline† | Week 12‡ | Difference (Baseline to Week 12‡) |
Difference Between Groups at Week 12‡ |
95% CI for Difference |
|
| Systolic blood pressure, mean (SD), mm Hg | 126.8 (16.2) | 125.9 (15.3) | −0.8 (15.2) | 126.8 (15.5) | 125.5 (16.1) | −1.3 (13.3) | 0.5 | −1.8 –2.9 |
| Diastolic blood pressure, mean (SD), mm Hg | 76.9 (9.6) | 77.9 (9.4) | 1.1 (9.6) | 77.2 (9.5) | 76.7 (9.5) | −0.5 (9.1) | 1.6 | 0.04–3.1 |
| Pulse rate, mean (SD), bpm | 72.5 (11.3) | 69.9 (11.1) | −2.4 (9.9) | 73.0 (11.8) | 70.1 (11.7) | −2.8 (9.9) | 0.4 | −1.3–2.0 |
Limited to participants with measurements at both baseline and Week 12.
Average of 2 baseline measurements.
Week 12 (end of treatment).
Discussion
In this randomized, double-blind, placebo-controlled trial, varenicline was efficacious for smoking cessation among patients with stable CVD. Varenicline more than tripled the tobacco CAR after 12 weeks of treatment compared with placebo. At that point, more than half of the smokers in the varenicline group (54.1%) were tobacco abstinent compared with 18.1% in the placebo group. Although many participants resumed smoking after treatment ended, the superiority of varenicline over placebo was maintained for 1 year. Varenicline was well tolerated and was not associated with increases in cardiovascular events, deaths, blood pressure, or heart rate. Rates of psychiatric AEs, about which concern has been raised in postmarketing surveillance,7,16,17 were low and similar in the varenicline and placebo groups.
The trial results are comparable to those of 2 previous phase III varenicline trials that had very similar protocols but enrolled generally healthy smokers.9,10 In the present trial, the OR for continuous tobacco abstinence at the end of treatment (OR, 6.11; 95% CI, 4.18 to 8.93) was higher than in the earlier trials (OR, 3.85; 95% CI, 2.70 to 5.50), but the confidence limits of these ORs overlapped. The efficacy of varenicline in this trial is also comparable to the pooled results of all other randomized, controlled trials enrolling generally healthy smokers. In a meta-analysis of 4 trials that used 6-month follow-up data, the pooled OR for varenicline compared with placebo was 3.1 (95% CI, 2.5 to 3.8) for point prevalence abstinence.5 Another meta-analysis of 7 randomized trials reported a pooled risk ratio for continuous abstinence at 6 months of 2.33 (95% CI, 1.95 to 2.80).8
Most clinical trials of smoking cessation pharmacotherapy were conducted in generally healthy smokers; less is known about the efficacy and safety of these medications in outpatients with CVD. The efficacy of varenicline in the present study contrasts with the mixed results of previous trials of transdermal nicotine in CVD patients. In 2 randomized, placebo-controlled trials, transdermal nicotine was safe and effective in smokers with CVD at the end of treatment, but efficacy disappeared after treatment stopped in the only trial that followed up patients for 24 weeks.3,22 Bupropion was safe and efficacious at a 1-year follow-up in a randomized, controlled study of smokers with stable CVD.23 Although smoking cessation counseling was provided to all participants in these trials, the long-term cessation rate for placebo groups was low (9% to 11%). It was also low in the placebo group in our trial (7%). The limited long-term success of counseling in smokers with CVD illustrates the importance of offering effective pharmacotherapy for these smokers, who have a substantial but potentially preventable risk of cardiovascular mortality.2
The time course of response to varenicline in this study was similar to the distinctive pattern observed in previous trials.9,10 The point prevalence tobacco abstinence rate rose during varenicline treatment, declining only after treatment stopped. This pattern is consistent with the hypothesized mechanism of action of varenicline as a partial agonist that reduces the rewarding effects associated with smoking.7 Because smoking is less rewarding when varenicline is being taken, the urge to smoke decreases over time, making it easier to quit. The pattern of varenicline response suggests that a longer course of varenicline might produce higher success rates or at least delay and blunt the relapse to smoking after treatment ends. A 6-month treatment period increased cessation rates in healthy smokers who had quit at 3 months in a previous trial24 and might have the same result in CVD patients.
The safety of varenicline in smokers with CVD has not previously been explored. This study provides reassurance to physicians that varenicline appears to be safe to use in smokers with stable CVD. We detected neither hemodynamic effects nor increases in cardiac end points or mortality. The incidence of psychiatric AEs was low and similar in the varenicline and placebo groups. However, the study protocol excluded smokers with diagnosed depression or who took antidepressant medication. Therefore, the study cannot address the safety or efficacy of varenicline in smokers with comorbid depression, which occurs at a higher rate in smokers with CVD than in the general population of smokers.19
Our study has other limitations. First, the statistical power to detect small changes in cardiovascular outcomes is limited by the sample size, trial duration, and relatively low rates of these events. The confidence limits indicate that a difference between varenicline and placebo of > 0.7% in cardiovascular deaths and > 5.0% in cardiovascular events is unlikely. Furthermore, the sample size was comparable to22,23 or larger than25 previous studies that assessed the safety of nicotine replacement therapy or bupropion in smokers with CVD. Second, the results cannot be generalized to smokers with recent or acute CVD events. However, the lack of effect of varenicline on blood pressure and heart rate suggests that its use might safely be considered in this population. Third, interpretation of the psychiatric symptom data would have been strengthened by systematic assessment of these symptoms during the trial. Nonetheless, spontaneously reported mood-related AEs occurred at a low rate that was similar in the varenicline and placebo groups. The sample size cannot rule out a small effect, but this trial excludes, with 95% confidence, a ≥3.2% absolute difference in the incidence of depressed mood disorders between the varenicline and placebo groups.
Conclusions
Varenicline is an effective smoking cessation therapy for patients with CVD, although many smokers in both groups relapsed after drug treatment ended, as typically occurs in smoking cessation trials. In this trial, varenicline treatment did not increase the risk of cardiovascular events, SAEs, or psychiatric side effects. However, trial size and duration preclude a definitive conclusion about the safety of varenicline. The rapid reduction in the risk of recurrence, disease progression, and cardiovascular complications argues for the priority of smoking cessation in the management of any smoking patient with CVD.26 The availability of effective pharmacotherapies for smoking cessation enhances the cardiovascular clinician’s ability to intercede successfully with this fundamental risk factor.
Supplementary Material
CLINICAL PERSPECTIVE.
Smoking cessation is a key component of secondary cardiovascular disease (CVD) prevention because smokers who quit after the diagnosis of CVD have a rapid reduction in their risk of recurrence, disease progression, and cardiovascular mortality. Despite these facts, treating tobacco dependence often has a low priority in cardiology practice. The availability of more effective treatments for smoking cessation provides an opportunity to engage cardiovascular clinicians in treating tobacco use. Varenicline, a partial α4β2 nicotinic acetylcholine receptor agonist, is effective for smoking cessation in healthy smokers, but its efficacy and safety in smokers with CVD were untested. In this randomized, double-blind, placebo-controlled trial of 714 smokers with stable CVD, varenicline more than tripled the rate of continuous tobacco abstinence compared with placebo at the end of 12 weeks of treatment (47.0% versus 13.9%; odds ratio, 6.11; 95% confidence interval, 4.18 to 8.93). The benefit of varenicline persisted even though many patients resumed smoking after treatment stopped. The rate of continuous tobacco abstinence to 1 year was 19.2% in the varenicline group versus 7.2% in the control subjects (odds ratio, 3.14; 95% confidence interval, 1.93 to 5.11). Varenicline was well tolerated in smokers with CVD. It was not associated with increases in blood pressure, heart rate, new cardiovascular events, or cardiovascular or all-cause mortality, although the extent of drug exposure for safety assessment was limited. The rates of psychiatric adverse events, about which concern has been raised in postmarketing surveillance of varenicline, were low and comparable between the varenicline and placebo groups. These data provide a strong evidence base to support the use of varenicline for outpatient smokers with stable CVD.
Acknowledgments
This trial was conducted in 15 countries: Argentina, Australia, Brazil, Canada, Czech Republic, Denmark, France, Germany, Greece, Mexico, Netherlands, Republic of Korea, Taiwan, the United Kingdom, and the United States. We thank the supervising clinicians for their involvement in the trial. A full list of supervising clinicians is provided in the online-only Data Supplement.
Sources of Funding
This study was funded by Pfizer Inc. Editorial support for the development of this manuscript was provided by Alexandra Bruce, PhD, of UBC Scientific Solutions and was funded by Pfizer Inc.
Footnotes
Disclosures
Drs Rigotti, Pipe, Benowitz, and Tonstad have consulted for Pfizer. Dr Rigotti has been the site principal investigator for clinical trials of smoking cessation medications funded by Pfizer, sanofi-aventis, and Nabi Biopharmaceuticals. Dr Pipe has received educational and research support in the past from Bristol- Myers Squibb, Johnson & Johnson, GlaxoSmithKline, and Merrell-Dow. Drs Benowitz and Tonstad served on the scientific planning committee for this study and have been paid consultants to Pfizer and other pharmaceutical companies that are developing and/or marketing smoking cessation medications. Dr Benowitz has been a paid expert witness in litigation against tobacco companies. At the time of the study, his family owned a small amount of Pfizer stock, but no longer does. Dr Tonstad has been the site principal investigator for clinical trials of smoking cessation medication and other medications funded by Pfizer and other pharmaceutical companies. Dr Arteaga is a statistical director at Pfizer Inc, supporting the varenicline studies. Dr Garza is a senior medical director of clinical research and development at Pfizer Inc, and the medical monitor for this study. The other authors report no conflicts.
References
- 1.Centers for Disease Control and Prevention, US Department of Health and Human Services. [Accessed January 30, 2009];The health consequences of smoking: a report of the Surgeon General. Available at: http://www.cdc.gov/tobacco/data_statistics/sgr/sgr_2004/chapters.htm.
- 2.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290:86–97. doi: 10.1001/jama.290.1.86. [DOI] [PubMed] [Google Scholar]
- 3.Smith SC, Jr, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pasternak RC, Pearson T, Pfeffer MA, Taubert KA. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 4.Quist-Paulsen P, Gallefoss F. Randomised controlled trial of smoking cessation intervention after admission for coronary heart disease. BMJ. 2003;327:1254–1257. doi: 10.1136/bmj.327.7426.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Mullen PD, Orleans CT, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. [Accessed March 12, 2009];Treating tobacco use and dependence: 2008 update. clinical practice guideline: U.S. Department of Health and Human Services, Public Health Service. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat2.chapter.28163.
- 6.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, III, O’Neill BT. Varenicline: an α4β2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 7.Hays JT, Ebbert JO. Varenicline for tobacco dependence. N Engl J Med. 2008;359:2018–2024. doi: 10.1056/NEJMct0800146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2008:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 10.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 11.Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR. Smoking cessation with varenicline, a selective α4β2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166:1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- 12.Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB, Jr, Gong J, Williams KE, Reeves KR. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax. 2008;63:717–724. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, Sutherland G. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103:146–154. doi: 10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- 14.Di Angelantonio S, Matteoni C, Fabbretti E, Nistri A. Molecular biology and electrophysiology of neuronal nicotinic receptors of rat chromaffin cells. Eur J Neurosci. 2003;17:2313–2322. doi: 10.1046/j.1460-9568.2003.02669.x. [DOI] [PubMed] [Google Scholar]
- 15.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis. 2003;46:91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration Centre for Drug Evaluation and Research. [Accessed March 12, 2009];The smoking cessation aids varenicline (marketed as Chantix) and bupropion (marketed as Zyban and generics): suicidal ideation and behavior: drug safety newsletter volume 2 (1) Available at: http://www.fda.gov/CDER/dsn/2009_v2_no1/postmarketing.htm#varenicline_bupropion.
- 17.US Food and Drug Administration, US Department of Health and Human Services, Centre for Drug Evaluation and Research. [Accessed March 12, 2009];Public health advisory: important information on Chantix (varenicline) (update May 16, 2008) Available at: http://www.fda.gov/cder/drug/advisory/varenicline.htm.
- 18.Heart Failure Society of America. [Accessed March 12, 2009];The stages of heart failure: NYHA classification. Available at: http://www.abouthf.org/questions_stages.htm.
- 19.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Jaén CR, Kottke TE, Lando HA, Mecklenburg RE, Mullen PD, Nett LM, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME for the US Department of Health and Human Services, Public Health Service. [Accessed March 12, 2009];Treating tobacco use and dependence. Available at: http://www.surgeongeneral.gov/tobacco/treating_tobacco_use.pdf.
- 20.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 21. [Accessed December 15, 2008];MedDRA: the Medical Dictionary for Regulatory Activities. Available at: http://www.meddramsso.com/MSSOWeb/index.htm.
- 22.Joseph AM, Norman SM, Ferry LH, Prochazka AV, Westman EC, Steele BG, Sherman SE, Cleveland M, Antonuccio DO, Hartman N, McGovern PG. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med. 1996;335:1792–1798. doi: 10.1056/NEJM199612123352402. [DOI] [PubMed] [Google Scholar]
- 23.Tonstad S, Farsang C, Klaene G, Lewis K, Manolis A, Perruchoud AP, Silagy C, van Spiegel PI, Astbury C, Hider A, Sweet R. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. Eur Heart J. 2003;24:946–955. doi: 10.1016/s0195-668x(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 24.Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 25.Nicotine replacement therapy for patients with coronary artery disease: Working Group for the Study of Transdermal Nicotine in Patients With Coronary Artery Disease. Arch Intern Med. 1994;154:989–995. [PubMed] [Google Scholar]
- 26.Forrester JS, Merz CN, Bush TL, Cohn JN, Hunninghake DB, Parthasarathy S, Superko HR. 27th Bethesda Conference: matching the intensity of risk factor management with the hazard for coronary disease events: Task Force 4: efficacy of risk factor management. J Am Coll Cardiol. 1996;27:991–1006. doi: 10.1016/0735-1097(96)87732-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.