Abstract
Purpose
The aim of this study was to determine the survival rates over time of implant-supported ceramic-ceramic and metal-ceramic prostheses as a function of core-veneer thickness ratio, gingival connector embrasure design, and connector height.
Materials and Methods
An IRB-approved, randomized, controlled clinical trial was conducted as a single-blind pilot study involving 55 patients missing three teeth in either one or two posterior areas. These patients (34 women; 21 men; age range 52–75 years) were recruited for the study to receive a 3-unit implant-supported fixed dental prosthesis (FDP). Two implants were placed for each of the 72 FDPs in the study. The implants (Osseospeed, Astra Tech), which were made of titanium, were grit blasted. A gold-shaded, custom-milled titanium abutment (Atlantis, Astra Tech), was secured to each implant body. Each of the 72 FDPs in 55 patients were randomly assigned based on one of the following options: (1) A. Material: ceramic-ceramic (Yttria-stabilized zirconia core, pressable fluorapatite glass-ceramic, IPS e.max ZirCAD and ZirPress, Ivoclar Vivadent) B. metal-ceramic (palladium-based noble alloy, Capricorn, Ivoclar Vivadent, with press-on leucite-reinforced glass-ceramic veneer, IPS InLine POM, Ivoclar Vivadent); (2) occlusal veneer thickness (0.5, 1.0, and 1.5 mm); (3) curvature of gingival embrasure (0.25, 0.5, and 0.75 mm diameter); and (4) connector height (3, 4, and 5 mm). FDPs were fabricated and cemented with dual-cure resin cement (RelyX, Universal Cement, 3M ESPE). Patients were recalled at 6 months, 1 year, and 2 years. FDPs were examined for cracks, fracture, and general surface quality.
Results
Recall exams of 72 prostheses revealed 10 chipping fractures. No fractures occurred within the connector or embrasure areas. Two-sided Fisher’s exact tests showed no significant correlation between fractures and type of material system (p = 0.51), veneer thickness (p = 0.75), radius of curvature of gingival embrasure (p = 0.68), and connector height (p = 0.91).
Conclusions
Although there were no significant associations between connector height, curvature of gingival embrasure, core/veneer thickness ratio, and material system and the survival probability of implant-supported FDPs with zirconia as a core material, the small number of fractures precludes a definitive conclusion on the dominant controlling factor.
Keywords: Clinical, design parameters, implants
The use of ceramic as a primary material in fixed dental prostheses (FDPs) to replace missing teeth has gained popularity over the past 20 years. The total ceramic prosthesis has gained acceptance in the dental community because of increased demand by the public; however, the physical properties of ceramics limit their use and account for significantly more technical complications and lower success rates than metal-ceramic prostheses do. Meta-analyses of clinical data for metal-ceramic FDPs have shown survival levels of 96 to 98% at 5 years, 901 to 92%2 at 10 years, and 741 to 75%2 at 15 years. Since ceramic-ceramic systems are relatively new, few randomized, controlled clinical studies have evaluated their success. Systematic reviews of clinical studies reveal that the survival rate for ceramic-ceramic FDPs is 88.6% compared with 94.4% for metal-ceramic FDPs within a 5-year observation period.3 Different ceramic-ceramic systems have been introduced with cores based on alumina, lithium disilicate glass-ceramic, and zirconia, or glass-infiltrated ceramics. Zirconia substructures are advertised as the strongest and toughest of all the dental framework ceramics. A systematic analysis of zirconia-based FDPs shows a survival rate of 94.3%.4 However, including technical complications such as chipping of the veneer ceramic, this survival rate decreases to 76.4% over a 5-year period.4 Heinzte and Rousson5 compared the survival of zirconia-supported (90%) and metal-supported (97%) FDPs after 3 years through a systematic review. The study concluded that veneer chipping was a major cause of failure. Long-term survival of zirconia frameworks at 10 years has been shown to be 91.5%6 with evidence of marginal deficiency and veneer chipping. Data for prosthesis design parameters based on clinical studies are too limited for clinicians to apply general principles to all-ceramic systems. Guidelines for the selection and design of ceramic-ceramic FDPs are based on manufacturers’ recommendations, which have not been confirmed by clinical research. Thus, there is limited guidance on how to minimize catastrophic failures of these restorations.
The dental implant is also becoming a widely accepted treatment option for the partially and completely edentulous patient. These restorations are somewhat more complex in that implants are firmly osseointegrated into bone, and they cannot be displaced as much under loading compared with restorations supported by the periodontal ligament of teeth. Another biological complication is the possibility of peri-implantitis.
Dental implants are steadily becoming the treatment of choice for supporting metal-ceramic and ceramic-ceramic partial dentures. Analysis of data from multiple clinical studies shows that the cumulative success rates for implant-supported FDPs are 95.2% and 86.7% over periods of 5 years and 10 years, respectively.7 More recently, a systematic analysis was conducted to determine the survival of ceramic-ceramic FDPs produced by CAD/CAM processes and conventionally constructed implant-supported prostheses. The survival rates at 5 years were 100% for the implant and 100% for the prosthesis.8 For FDP restorations, survival ranges varied between 81.4 and 95.6% for the implants and between 72.2 and 100% for the prosthesis. Conversely, conventional tooth-supported FDPs have been reported with a survival level of up to 93.8%8 over a period of 5 years.
Based on an in vitro study, Vult von Steyern et al9 analyzed the fracture resistance of all-ceramic prostheses supported by abutment teeth and by implants. They concluded that implant-supported ceramic prostheses fractured at higher loads than those supported by natural teeth because of the lack of the periodontal ligament.
The objectives of this study were to determine the survival rates over time of implant-supported ceramic-ceramic and metal-ceramic prostheses as a function of material system, core/veneer thickness ratio, radius of curvature of the connector at the gingival embrasure, and connector height. The following hypotheses were proposed:
Posterior yttria-stabilized zirconia-core, 3-unit implant-supported FDPs with a fracture toughness of 9 MPa·l36;m1/2 will withstand occlusal forces of up to 1000 N when the core thickness is as low as 0.5 mm.
A minimum connector height of 3 mm and a minimum radius of curvature of 0.25 mm at the connector embrasure at the gingival area of a 3-unit implant-supported FDP based on an yttria-stabilized zirconia core ceramic will resist fracture under occlusal loading as high as 1000 N.
MATERIALS AND METHODS
Study design
A randomized, controlled clinical trial was conducted to determine the survival of implant-supported FDPs as a function of several design parameters. This single-blind pilot study involved 55 participants needing 72 FDPs to replace three posterior teeth. The participants’ teeth were randomly assigned to receive either a metal-ceramic or a ceramic-ceramic FDP. These were further subdivided into the following design parameters: (1) veneer ceramic thickness, (2) connector radius of curvature, and (3) connector height (Table 1). The overall thickness of the core and veneer thickness was 2.0 mm; thus, the ratio of the veneer thickness to core thickness varied between 0.5 and 1.5 mm.
Table 1.
Randomization groups
| Material | Ceramic Thickness (mm) |
Ratio of radius of curvature (mm)/Connector height (mm) |
|---|---|---|
| Metal-ceramic or all-ceramic (zirconia-based core ceramic) | 0.5 (24 FPDs) | 0.25/3 (8 FPDs); 0.25/4 (8 FPDs); 0.25/5 (8 FPDs) |
| 1.0 (24 FPDs) | 0.5/3 (8 FPDs); 0.5/4 (8 FPDs); 0.5/5 (8 FPDs) | |
| 1.5 (24 FPDs) | 0.75/3 (8 FPDs); 0.75/4 (8 FPDs); 0.75/5 (8 FPDs) | |
Materials used
Implants: Commercially pure titanium, grit blasted with titanium dioxide and fluoride particles (Osseospeed, Astra Tech, Molndal, Sweden)
Custom abutments: Gold-shaded, custom-milled titanium abutments (Atlantis, Astra Tech, Waltham, MA)
Metal-ceramic (MC) FDP: Noble Pd-Au-Ag alloy (Capricorn; Ivoclar, Vivadent, Schaan, Liechtenstein)/press-on leucite-reinforced glass ceramic veneer (IPS InLine POM; Ivoclar Vivadent)
Ceramic-ceramic (CC) FDP: Yttria-stabilized zirconia core (IPS e.max ZirCAD; Ivoclar Vivadent) and a hot, isostatically pressed, fluorapatite glass-ceramic veneer (IPS e.max ZirPress; Ivoclar Vivadent)
Study population
Participants were recruited through broadcast e-mail, flyer, and newspaper advertisements. Participants were selected based on the following criteria:
Aged between 21 to 75 years, and no contraindications to dental treatment.
Good overall dental health, no active caries, no periodontal disease, and periodontal pocket depths not greater than 4 mm.
Missing at least three posterior teeth
Fixed teeth opposing the edentulous area and a full complement of teeth or restored teeth in all other areas
Adequate bone height and width at areas of proposed implant sites
Adequate interocclusal distance to accommodate the prosthesis
Good oral hygiene and compliance with oral hygiene instructions as determined by the amount of plaque present on tooth surfaces
Compliance with appointments and willing to pay $2625 for a 3-unit, implant-supported FDP
Study intervention
Seventy two FDPs, on 55 enrolled participants (no participant having more than two FDPs), who needed replacement of posterior teeth, were randomly assigned to receive either a metal-ceramic or a ceramic-ceramic FDP. Groups were further subdivided according to design parameters (Table 1). For participant allocation, a computer-generated random number table was formulated to facilitate assignment to a specific group. Participants were treated at the University of Florida College of Dentistry (UFCD) by an experienced clinician and prosthodontist between 2008 and 2012. The UFCD Institutional Review Board approved the research protocol for treating human subjects. All participants were required to sign an informed consent form prior to initiating the study. The following baseline information was collected:
General medical history and physical examination
Maximum bite force using a gnathodynamometer
Participants were evaluated for bone height and width through computerized tomography. They were treated by an oral surgeon if bone augmentation was required prior to placement of implants. Healing time was related to the extent of bone augmentation, usually ranging from 4 to 6 months. Each of two implants was placed using a surgical guide. Final impressions were made after 8 weeks of healing using the open tray technique. Final impressions were made with vinylpolysiloxane (VPS) impression material (Aquasil; Dentsply Caulk, Miliford, DE) and poured in Type IV stone (Die-Keen; Whip Mix Corp, Louisville, KY). Soft tissue material (Gingitech; Ivoclar Vivadent) was poured in the impressions. Mounted casts were sent for abutment fabrication, allowing 2 mm of interocclusal clearance for the FDP material. Custom abutments were made to allow for accurate representation of occlusion. For ceramic-ceramic FDPs, core substructures were milled from pre-sintered blanks after they were designed by CAD software (InEOS, Sirona, Salzburg, Austria). For metal-ceramic FDPs, wax patterns for metal substructures were waxed and measured to exact dimensions. The metal was cast using the lost wax technique. Framework substructures were fit intraorally along with the custom abutments to determine the accuracy of mounting. An occlusal record was confirmed using acrylic resin (Duralay Resin; Reliance Dental Mfg., Alsip, IL). Corresponding veneer porcelains were applied and sintered according to the manufacturer’s instructions. Completed FDPs were seated prior to cementation. Occlusal adjustments were made with an ultrafine grit diamond bur (Brassler USA, Savannah, GA). Adjusted FDPs were polished with abrasive wheels and diamond paste. FDPs were cemented with resin cement (RelyX Unicem Self-Adhesive Universal Resin Cement; 3M ESPE, St. Paul, MN). After cementation, occlusal markings in centric occlusion and excursive pathways were observed and photographed for baseline records.
Post-cementation analysis
Participants were recalled at 6 months, 1 year, and 2 years after cementation. They were asked to report any unusual event related to their prosthesis. They were immediately recalled before the designated recall time if they experienced a chipping event or larger fracture. VPS impressions were made of each quadrant at each recall visit, and photographs were taken of occlusal markings. If a clinical fracture was detected, the FDP surfaces were cleaned with a water spray, rinsed with ethanol, and air dried. This procedure was repeated twice. A VPS impression of each fracture surface was made twice to fabricate acrylic replicates for fractographic analysis. Replicates of the fractured FDPs were examined by SEM to determine the origin of fracture and to calculate the stress at fracture. Fractures were classified clinically according to (1) Class 1 (if refinishing was required); (2) Class 2 (if a repair was warranted); and (3) Class 3 (if replacement of the FDP was indicated).
Statistical methods
Two-sided Fisher’s exact tests were performed with material as the independent variable and fracture class as the dependent variable to determine the statistical significance of associations between fracture and design parameters of material, including veneer/core thickness ratio, curvature of gingival connector embrasure, and connector height. Because the event rate is low, the repeated measures aspect (72 teeth in 55 participants) can be ignored without major adverse effects on the validity of the test. A total of 10 failures occurred in 9 participants, indicating that clustering within participants was effectively absent.
RESULTS
Seventy-two FDPs were cemented in 55 participants and recalled for 2 years (men = 21, women = 34; age range 52–75). Participant recruitment, retention, and allocation history are detailed in Figure 1. Ten chipping fractures were observed in the 72 FDPs. Representative clinical fracture images are shown for Class 1–3 fractures (Figs 2–4). Allocation of chipping fractures by fracture classification, material, veneer thickness, connector height, and curvature of gingival connector embrasure are detailed in Tables 2–5. Only one FDP had a Class 3 fracture that required replacement. One of the participants with a Class 2 fracture opted out of the study and requested replacement with a metal-ceramic FDP (1.0 mm veneer thickness). The Fisher’s exact test revealed no significant correlation between fractures and the type of material system (p = 0.51), veneer thickness (p = 0.75), radius of curvature of the gingival connector embrasure (p = 0.68), and connector height (p = 0.91). Because of the low number of fractures, the statistical significance of effects was inconclusive.
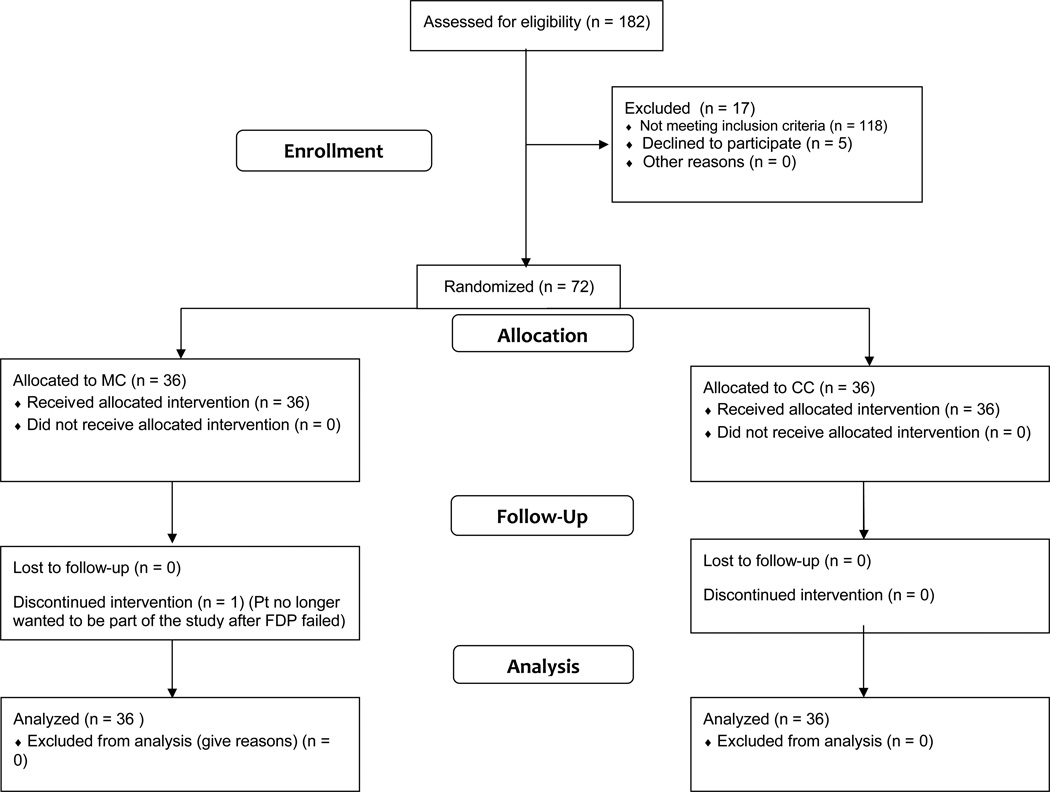
Figure 1.
Study design.
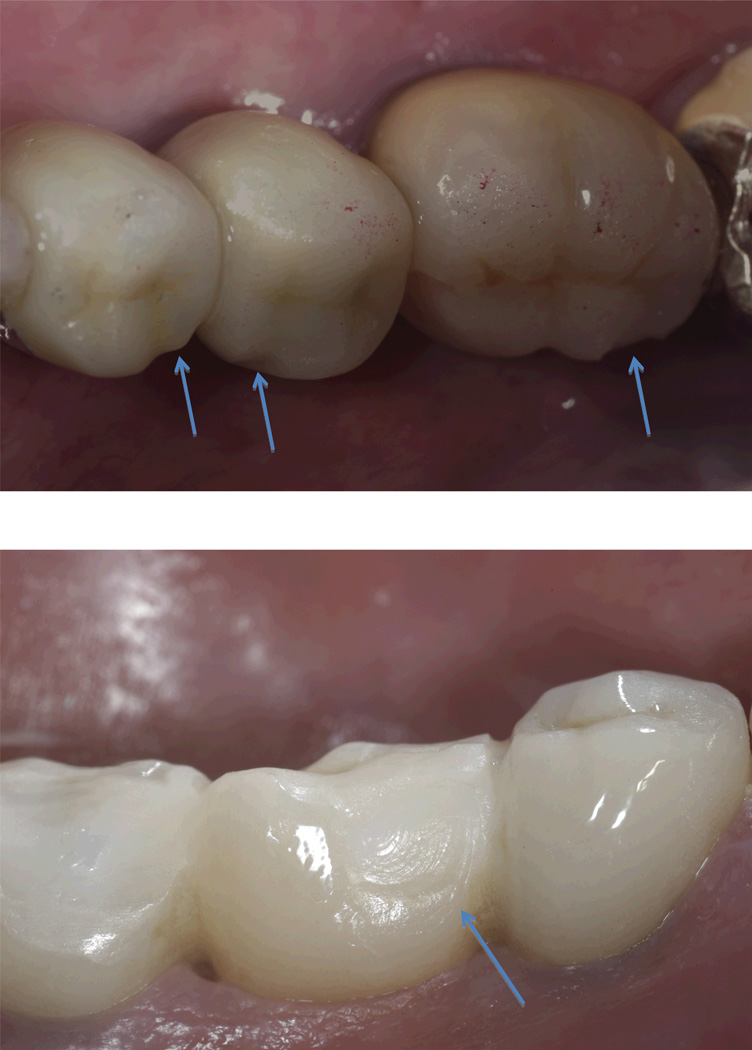
Figure 2.
Representative Class 1 fracture of MC and CC FDPs that were polished and not repaired or replaced.
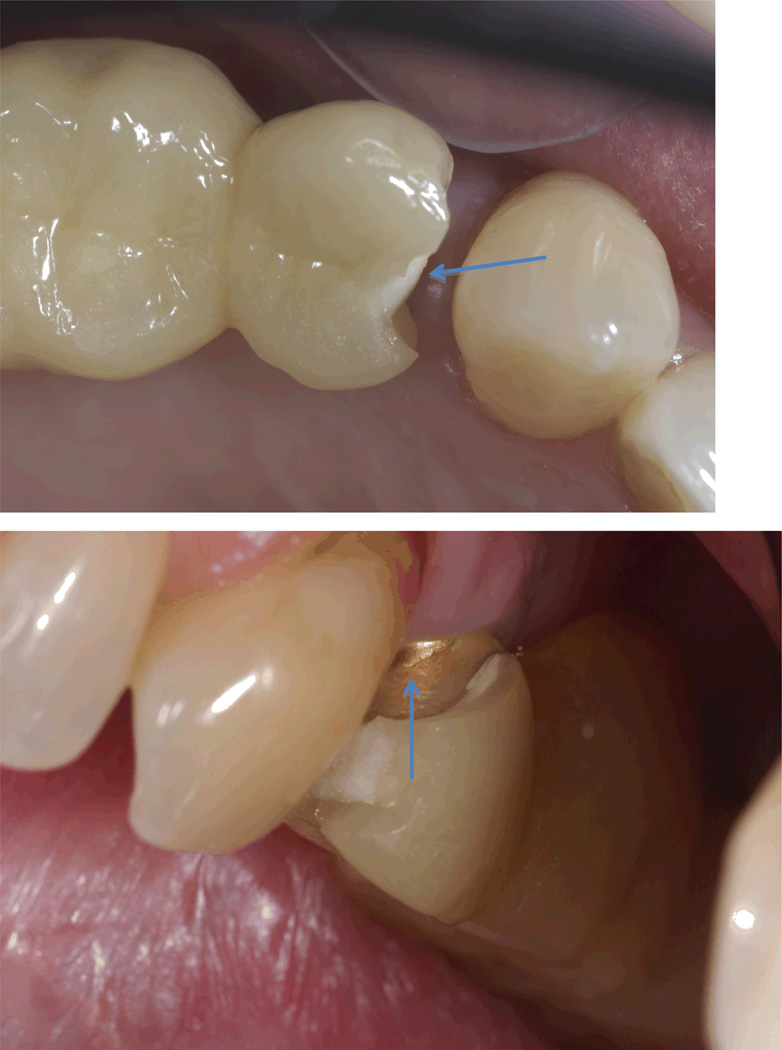
Figure 4.
Representative Class 3 fracture of CC FDP that extended to the zirconia core (top). Another view showing the Atlantis abutment where zirconia core fractured.
Table 2.
Distribution of failures by fracture classification (Entries for # of FDPs are Number of Fractures/Number of teeth)
| Classification of fractures |
# of FDPs | MC | CC |
|---|---|---|---|
| Class 1 | 6/72 | 1/6 | 5/6 |
| Class 2 | 3/72 | 3/3 | 0/3 |
| Class 3 | 1/72 | 0/1 | 1/1 |
Table 5.
Distribution of failures by curvature of gingival embrasure (# of FDPs = # of FDPs with diameter / total number of #FDPs)
| Diameter of curvature of gingival embrasure |
# of FDPs |
|---|---|
| 0.5 | 5/72 |
| 1.0 | 3/72 |
| 1.5 | 2/72 |
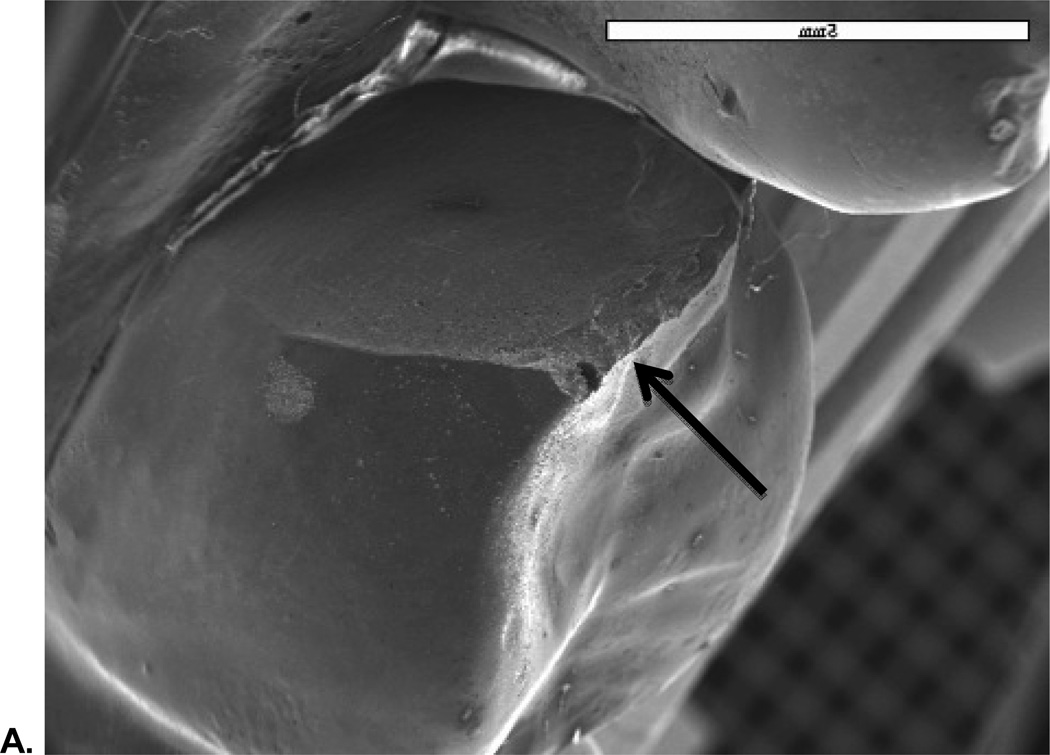
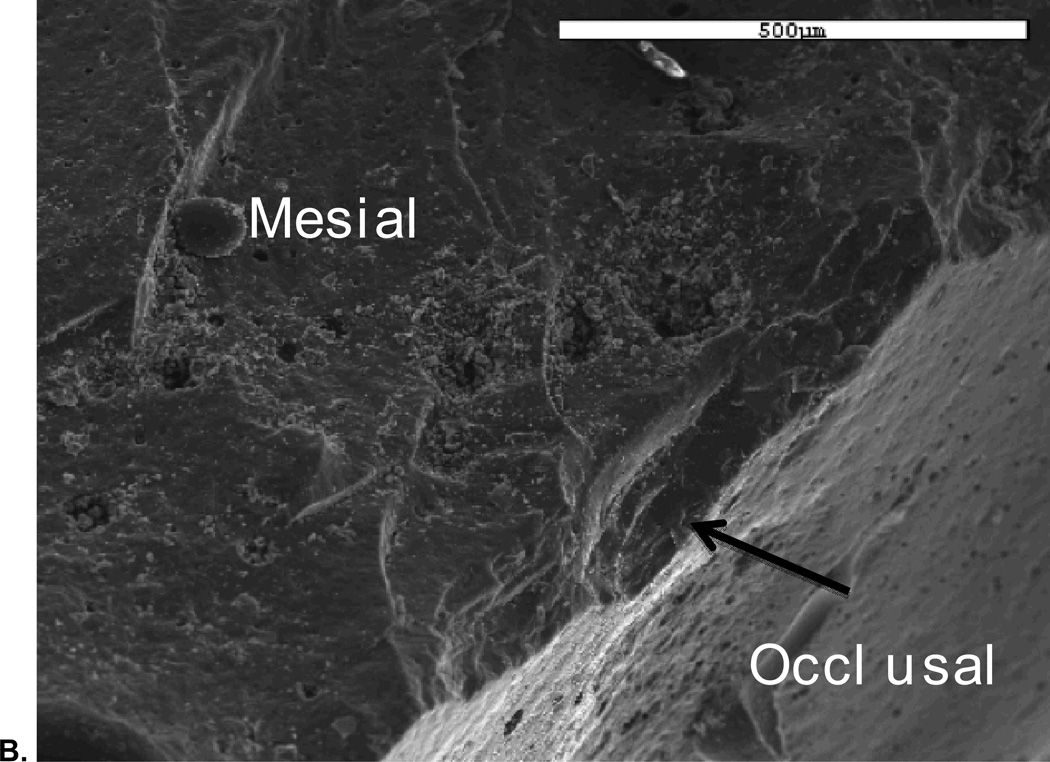
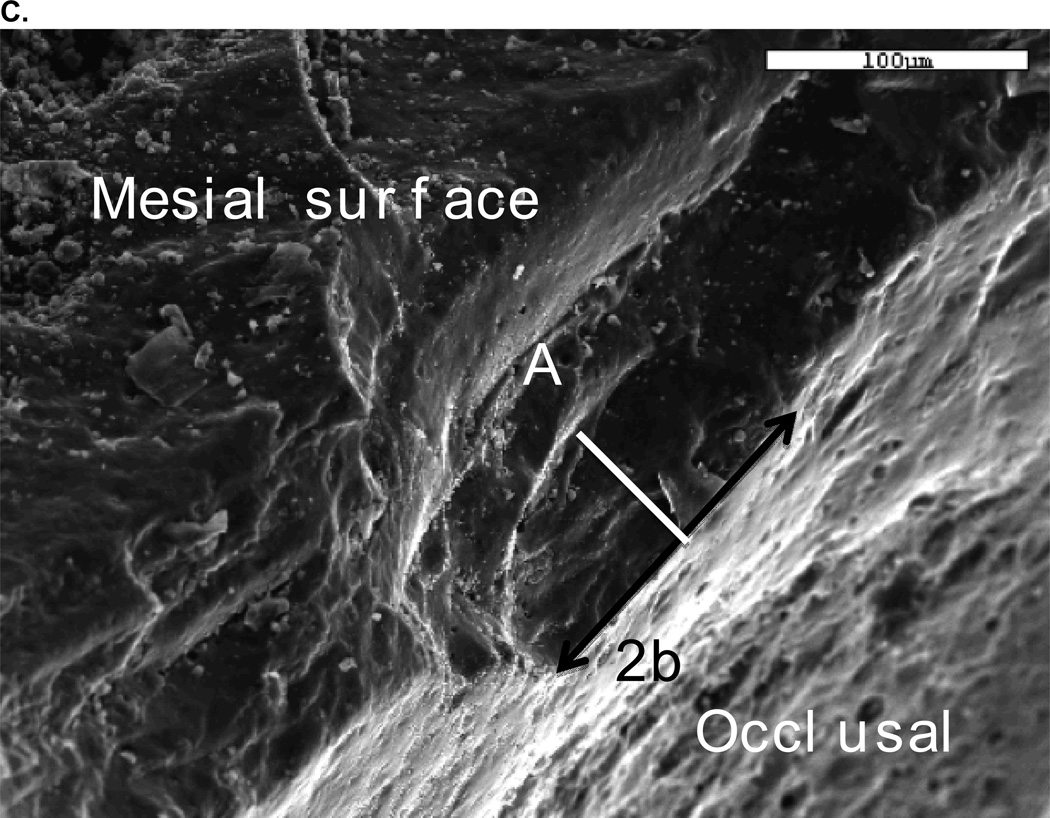
SEM analysis revealed that all fractures originated in the occlusal area. Figure 5 shows a representative SEM image of a Class 2 fracture, which reveals the site and size of the critical flaw area.
Figure 5.
A. SEM image showing fracture originating from occlusal area;
B. Image of critical flaw at higher magnification with view from mesio buccal occlusal area.
C. Fracture stress of 108.5 MPa in veneer determined from flaw size 2b (128 µm) and a (60 µm), Y (1.24) and KIc using σF = Y√c / KIC.
DISCUSSION
Failure in this study is defined as a deviation from shape, size, and quality of the original prosthesis. Therefore, even minor chipping in the veneer ceramic was considered as a chipping fracture and not a technical complication as has often been cited in the literature.10 To date, only 10 of the 72 FDPs exhibited chipping fractures, and only one was replaced because of a fracture that progressed to the core ceramic. This represents an 86% success rate and a 98% survival rate (since 71 of the cemented FDPs did not require replacement).
SEM analysis performed on the replicates of the fractured specimens revealed that all of the fractures originated from along the perimeter of the occlusal surfaces. This is consistent with findings from another clinical study on zirconia FDPs.11 None of the fractures occurred within the connector areas or originated from the gingival embrasure areas of the FDPs. Therefore, both of our hypotheses are accepted, since the minimal dimensions of a 0.5 mm core thickness, a 3 mm connector height, and a 0.25 mm radius of curvature of gingival connector embrasure for MC and CC prostheses withstood occlusal forces in vivo.
The lower success rates of ceramic-ceramic restorations raise the question of manufacturing and biomaterial factors, which could affect the longevity of these restorations. The effects on restoration survival of material properties, connector design, framework and crown design, loading conditions, core/veneer ceramic compatibility, and patient factors are not well understood and should receive more attention from the research community.
In this clinical study, we examined the effect of framework material, core/veneer thickness ratio, connector height, and curvature of the gingival connector embrasure. The yttria-stabilized tetragonal zirconia polycrystalline (Y-TZP) ceramic used in this study represents one of the most recent additions to the core materials for ceramic-ceramic FDPs. Compared with other ceramics used for ceramic-ceramic prostheses, such as lithium-disilicate-based glass-ceramics and zirconia-reinforced, glass-infiltrated alumina, Y-TZP has demonstrated the highest flexural strength and fracture toughness.12,13 Because of these properties, Y-TZP also allows smaller connectors and thinner crowns to be used (Table 6) compared with other ceramic-ceramic products. Placement of ceramic-ceramic posterior prostheses is sometimes difficult when the connector dimensions need to be compromised by the space availability in the posterior region. In a previous clinical study,14 failures occurred in FDPs with smaller-than-recommended connector dimensions. The connector sizes are limited by other factors such as tooth height, occlusion, and esthetics. The potential for zirconia prostheses to be made with smaller connector sizes makes Y-TZP ceramics highly attractive for posterior applications compared with other ceramic-ceramic materials.
Table 6.
Properties of current dental core ceramics
| Ceramic Type | Commercial name |
Flexural strength (MPa) |
Fracture toughness (MPa•m1/2) |
Recommended connector thickness (mm) |
Clinical failure rate |
|---|---|---|---|---|---|
| Glass-infiltrated alumina | In-Ceram | 236–60048–51 | 3.1–4.648,52 | 4 × 3 | 10–1220,21 (5y) |
| Lithium disilicate glass-ceramic | Empress 2, e.Max Press | 300–40053 | 2.8–3.553,54 | 4–5 × 3–455 | 1414 (4y) |
| Densely sintered high-purity alumina | Procera® AlCeram System | 487–69956 | 4.5–6.013 | 3 × 2 (anterior only) | |
| Glass-infiltrated alumina with 35% partially Stabilized zirconia | In-Ceram Zirconia System | 421– 80051,52,57 | 6–858 | 4–5 × 3–4 (posterior only)58 | |
| Yttria-stabilized tetragonal zirconia polycrystalline ceramic | Cercon, DC-Zirkon (Dentsply); Lava (3M ESPE); e.Max Zircad (Ivoclar Vivadent); DCS-Precident (Bien-Air Group/DCS Dental AG) | 900– 150013,59 | 9–1012,13 | 2.8 × 2.727 |
Y-TZP ceramics do not contain a glass phase at grain boundaries and they are sintered with minimal voids, flaws, and cracks, thereby increasing their effective strength. The absence of a glass phase also makes these ceramics resistant to subcritical crack propagation during exposure to water in saliva. Transformation toughening, which occurs because of a tetragonal to monoclinic transformation, is considered beneficial because this phase change at the tip of microcracks is accompanied by volumetric expansion and subsequent compressive stresses around crack tips. This volumetric expansion can result in partial closure of the crack and prevent its propagation through the structure.15
The average clinical survival rate for ceramic-ceramic FDPs (zirconia, glass ceramic, and glass-infiltrated ceramic) is approximately 88% over 5 years of service. The failure rate is approximately twice the failure rate for metal-ceramic FDPs.3 Fischer et al16 analyzed the long-term failure probability and loading capacity of core ceramic FDPs (with no veneer ceramics) made with IPS Empress (a leucite-based glass-ceramic), Empress 2 (a lithium-diisilicate-based glass-ceramic), In-Ceram Alumina (a glass-infiltrated alumina ceramic), and ZrO2 core ceramics using the NASA post-processor CARES/Life (Ceramic Analysis and Reliability Evaluation of Structure Life Prediction) analysis. They predicted that zirconia FDPs should exhibit very high mechanical long-term reliability and a 0% failure probability after 10 years of static loading. They concluded that IPS Empress and In-Ceram Alumina were not suitable for use as posterior FDP materials and that IPS Empress 2 was more suitable for molar sites with a failure rate three times lower than that for In-Ceram Alumina. They also noted a favorable increase in lifetime predictability with proper connector area design.
Teixeira et al17 analyzed strength-probability-time (SPT) diagrams for porcelain, alumina, and Y-TZP ceramics. Their results suggest that Y-TZP showed a high resistance to subcritical crack growth compared with that of the other two ceramics. Clinical studies show that framework fractures, although not rare (6.5% over 5 years),3,18 were more common for In-Ceram Alumina, In-Ceram Zirconia, and glass-ceramic FDPs. The annual failure rate of frameworks made from these materials ranged from 1.88 to 4.24% compared with a failure rate ranging from 0 to 0.48% for zirconia frameworks over a period of 5 years.3 Based on lifetime prediction analysis and limited clinical data, there is clear evidence that zirconia ceramics are more fracture resistant than other ceramics used for all-ceramic FDPs.
Connectors are defined as the component of an FDP that unites the retainer and the pontic.19 Since connectors represent areas of high stress concentration, in vitro studies have demonstrated that this area is one of the weakest parts of the core structure. The principle of “bigger is better” applies to all connector designs, although the constraints of anatomy and esthetics need to be considered. Almost all earlier clinical studies on all-ceramic products (lithium-disilicate-based glass-ceramic and glass-infiltrated alumina) revealed fractures within the connector areas with failure rates ranging from 10 to 12% over 5 years.14,20–24 Results from another study revealed that the connector diameter should be no less than 4 mm when using glass-ceramic cores for posterior applications.25 This study indicated that stresses in the connector area decreased by approximately 40 to 50% when the connector height increased from 3 to 4 mm. Several finite element analyses confirmed that the occlusal-gingival height, shape of the connectors, and span of the pontic area may be controlling factors in the survival of all-ceramic restorations.16,26–28 The connector dimensions and design have a strong influence on the stresses generated within the framework. During functional loading, the pontics and connectors sustain compressive stress while the connector areas sustain tensile stress in the gingival embrasure region. Because of the relatively low fracture toughness and low tensile strength of glass-ceramics, the area of the greatest tensile stress concentration tends to be the weakest point of the framework. Oh and Anusavice29 and Oh et al30 demonstrated that the curvature of the gingival embrasure affected the fracture resistance of lithium-based glass ceramic FDPs. They concluded that smaller radii of curvatures increased stress concentrations along the area that served as the sites of fracture initiation. Increasing the radius of curvature from 0.25 to 0.90 mm at the gingival embrasure more than doubled the fracture resistance. Another study31 revealed that increasing the radius from 0.6 to 0.9 mm of the gingival embrasure increased the fracture strength of a Y-TZP framework with a 3 × 3 mm connector by 20%. Other studies have shown that the direction of curvature of the framework along the connector areas can also affect the fracture resistance.31–33
The connector dimension is also critical for predicting the survival of implant-supported all-ceramic FDPs. Bahat et al31 reported that the minimum dimensions for a Y-TZP framework for a three-unit FDP should be 3 mm high and 2 mm wide. Larsson et al34 concluded that the fracture resistance of a Y-TZP framework was significantly increased as the connector diameters increased. An increase in fracture load of approximately 200 N occurred for connector enlargement from 3.0 to 3.5 mm, and a 300 N increase occurred for an enlargement from 3.5 to 4.0 mm. A 4-mm diameter was recommended for Y-TZP connectors to be used for long-span FDPs or for replacing molar teeth. Mokhtarikhoee et al35 found a 24% increase in the stress along the connector areas after a reduction from 5 to 3 mm in the buccolingual width of the connectors. Clearly, in vitro studies show that connector dimensions and design can play a crucial role in the survival of FDPs.
For this clinical study, no fractures were observed in the connector areas with a minimum height of 3 mm. Also, none of the fractures originated from the gingival embrasure, even with a gingival embrasure radius of 0.25 mm.
The final process in the fabrication of a typical all-ceramic FDP is the application of the veneer porcelain to achieve proper esthetics. The hot-pressing technology for veneering ceramics involves a lost-wax technique vs. the conventional manually layered porcelain. A recent clinical study was performed to compare the longevity and clinical performance of ceramic veneers produced on zirconia, alumina, and metal frameworks.36 It reported that veneers on zirconia fractured three times more often than veneers on metal. This finding raises a question regarding the longevity and clinical performance of zirconia FDPs. Specifically, 56% of the zirconia veneered prostheses exhibited some degree of chipping fracture, while only 28% of the metal-ceramic prostheses exhibited chipping fractures. These findings are consistent with the results from other clinical studies.7,37–40 Several theories can be formulated as to the cause of these fractures:
Inadequate prosthesis design – The supporting structure or framework is critical to prevent veneer fractures. As with metal frameworks, all-ceramic frameworks should provide adequate support in all areas, particularly areas of load. One study indicated that an increase in core thickness significantly improved the reliability of the prosthesis.41
Quality of the porcelain layering or layering technique – Pores represent stress concentrations within the restoration. Micro-CT analysis of ceramic crowns revealed numerous pores within the veneer porcelain for CAD/CAM frameworks as well as heat-pressed veneers.42 In contrast, another study showed that pressed veneers performed better than hand-layered veneers, with less surface degradation, fewer chipping fractures, and fewer bulk fractures.36
Coefficient of thermal expansion (CTE) and cooling schedule – Zirconia tends to cool rather slowly, and this may cause undue residual tensile stresses in the veneering glasses that may lead to chipping fractures. DeHoff et al43 emphasized the risk of excessive residual stresses with ceramic core and veneer combinations as a function of the CTE mismatch. They concluded that some residual stresses exceeded the flexural strength of these materials. Discrepancies in the CTE can also adversely affect the bond strength of the veneer porcelain to zirconia.44 Manufacturers recommend a slow cooling rate to offset this discrepancy, and this method has been shown to also decrease the interfacial strength between the veneer and zirconia core.45 A decrease in the bond strength between the two materials can cause catastrophic delamination of the veneer. Swain46 developed a mathematical model, which incorporates the effects of cooling rate, ceramic thickness, and CTE compatibility to minimize the chipping fracture phenomenon prevalent in ceramic-ceramic prostheses, zirconia-ceramic types in particular.
Loading orientation – Depending on the position in the mouth, a ceramic restoration is better equipped to accept vertical forces, which produce primarily compressive stresses rather than lateral or oblique forces, which result in tensile stresses.
Veneer thickness – Finally, the thickness of the veneering porcelain is also a contributing factor to the success of the restoration. Adequate thickness of the veneer is necessary to allow for proper bulk and strength of the ceramic prosthesis; however, studies have shown that cracking is associated with increased thickness of the veneering porcelain (with corresponding reduction in core ceramic thickness), faster cooling rates, and thermal incompatibility of the ceramic layers.46,47
In this clinical study, the effect of veneer thickness on the failure probability of the FDP was not clearly evident (Table 3). Although not statistically significant, it should be noted that ceramic-ceramic FDP veneers exhibited more chipping failures for a veneer thickness of 1.5 mm, while metal-ceramic had more fractures with a 0.5 mm thick veneer. These fractures may be related to the amount of support of the core material as well as the load orientation of the occlusal forces. Another possibility is the thermal incompatibility of the veneer porcelain supported by zirconia.
Table 3.
Distribution of failures by material and veneer thickness (# of FDPs = # of Fractures/# of FDPs of either MC or CC)
| Material | Veneer thickness (mm) | # of FDPs |
|---|---|---|
| MC | 0.5 | 3/36 |
| 1.0 | 1/36 | |
| 1.5 | 0/36 | |
| Total for MC | 4/36 | |
| CC | 0.5 | 2/36 |
| 1.0 | 0/36 | |
| 1.5 | 4/36 | |
| Total for CC | 6/36 |
CONCLUSIONS
The objective of this study was to determine whether certain design parameters affected the survival of a high-strength core ceramic. Although there were no apparent associations of connector height, curvature of gingival embrasure, core/veneer thickness ratio, and material system on the survival probability of implant-supported FDPs when using zirconia as a core material, the low number of fractures preclude the determination of statistical significance of fracture differences between material types (ceramic-ceramic vs. metal-ceramic). For this reason, plus the fact that all of the chipping failures were observed along the periphery of the restorations, further research is warranted to determine the effect of load orientation and framework design.
Figure 3.
Representative Class 2 fracture of MC repaired with composite.
Table 4.
Distribution of failures by connector height (# of FDPs = # of FDPs with connector height in mm / total number of FDPs)
| Connector Height (mm) | # of FDPs |
|---|---|
| 3 | 3/72 |
| 4 | 4/72 |
| 5 | 3/72 |
Acknowledgements
We would like to acknowledge the laboratory expertise of R.B. Lee and B. Nicholas as well as the clinical assistance of K. Raulerson.
Supported by NIH-NIDCR grants K23DE18414 and DE06672, NIH-NCATS grant 1UL1TR000064, Ivoclar Vivadent, and Astra Tech.
Footnotes
Previously presented at the American Association for Dental Research Meeting, Tampa, FL, March, 2012
The authors deny any conflicts of interest.
REFERENCES
- 1.Creugers NH, Kayser AF, van 't Hof MA. A meta-analysis of durability data on conventional fixed bridges. Community Dent Oral Epidemiol. 1994;22(6):448–452. doi: 10.1111/j.1600-0528.1994.tb00795.x. [DOI] [PubMed] [Google Scholar]
- 2.Scurria MS, Bader JD, Shugars DA. Meta-analysis of fixed partial denture survival: prostheses and abutments. J Prosthet Dent. 1998;79(4):459–464. doi: 10.1016/s0022-3913(98)70162-3. [DOI] [PubMed] [Google Scholar]
- 3.Sailer I, Pjetursson BE, Zwahlen M, Hammerle CH. A systematic review of the survival and complication rates of all-ceramic and metal-ceramic reconstructions after an observation period of at least 3 years. Part II: Fixed dental prostheses. Clin Oral Implants Res. 2007;18(Suppl 3):86–96. doi: 10.1111/j.1600-0501.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- 4.Schley JS, Heussen N, Reich S, Fischer J, Haselhuhn K, Wolfart S. Survival probability of zirconia-based fixed dental prostheses up to 5 yr: a systematic review of the literature. Eur J Oral Sci. 2010;118(5):443–450. doi: 10.1111/j.1600-0722.2010.00767.x. [DOI] [PubMed] [Google Scholar]
- 5.Heintze SD, Rousson V. Survival of zirconia- and metal-supported fixed dental prostheses: a systematic review. Int J Prosthodont. 2010;23(6):493–502. [PubMed] [Google Scholar]
- 6.Sax C, Hammerle CH, Sailer I. 10-year clinical outcomes of fixed dental prostheses with zirconia frameworks. Int J Comput Dent. 2011;14(3):183–202. [PubMed] [Google Scholar]
- 7.Pjetursson BE, Bragger U, Lang NP, Zwahlen M. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns (SCs) Clin Oral Implants Res. 2007;18(Suppl 3):97–113. doi: 10.1111/j.1600-0501.2007.01439.x. [DOI] [PubMed] [Google Scholar]
- 8.Harder S, Kern M. Survival and complications of computer aided-designing and computer-aided manufacturing vs. conventionally fabricated implant-supported reconstructions: a systematic review. Clin Oral Implants Res. 2009;20(Suppl 4):48–54. doi: 10.1111/j.1600-0501.2009.01778.x. [DOI] [PubMed] [Google Scholar]
- 9.Vult von Steyern P, Kokubo Y, Nilner K. Use of abutment-teeth vs. dental implants to support all-ceramic fixed partial dentures: an in-vitro study on fracture strength. Swed Dent J. 2005;29(2):53–60. [PubMed] [Google Scholar]
- 10.Anusavice KJ. Standardizing failure, success, and survival decisions in clinical studies of ceramic and metal-ceramic fixed dental prostheses. Dent Mater. 2012;28(1):102–111. doi: 10.1016/j.dental.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Amleh B, Lyons K, Swain M. Clinical trials in zirconia: a systematic review. J Oral Rehabil. 2010;37(8):641–652. doi: 10.1111/j.1365-2842.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- 12.Tinschert J, Zwez D, Marx R, Anusavice KJ. Structural reliability of alumina-, feldspar-, leucite-, mica- and zirconia-based ceramics. J Dent. 2000;28(7):529–535. doi: 10.1016/s0300-5712(00)00030-0. [DOI] [PubMed] [Google Scholar]
- 13.Christel P, Meunier A, Heller M, Torre JP, Peille CN. Mechanical properties and short-term in-vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J Biomed Mater Res. 1989;23(1):45–61. doi: 10.1002/jbm.820230105. [DOI] [PubMed] [Google Scholar]
- 14.Esquivel-Upshaw JF, Young H, Jones J, Yang M, Anusavice KJ. Four-year clinical performance of a lithia disilicate-based core ceramic for posterior fixed partial dentures. Int J Prosthodont. 2008;21(2):155–160. [PubMed] [Google Scholar]
- 15.Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20(1):1–25. doi: 10.1016/s0142-9612(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 16.Fischer H, Weber M, Marx R. Lifetime prediction of all-ceramic bridges by computational methods. J Dent Res. 2003;82(3):238–242. doi: 10.1177/154405910308200317. [DOI] [PubMed] [Google Scholar]
- 17.Teixeira EC, Piascik JR, Stoner BR, Thompson JY. Dynamic fatigue and strength characterization of three ceramic materials. J Mater Sci Mater Med. 2007;18(6):1219–1224. doi: 10.1007/s10856-007-0131-4. [DOI] [PubMed] [Google Scholar]
- 18.Sailer I, Philipp A, Zembic A, Pjetursson BE, Hammerle CH, Zwahlen M. A systematic review of the performance of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin Oral Implants Res. 2009;20(Suppl 4):4–31. doi: 10.1111/j.1600-0501.2009.01787.x. [DOI] [PubMed] [Google Scholar]
- 19.The glossary of prosthodontic terms. J Prosthet Dent. 2005;94(1):10–92. doi: 10.1016/j.prosdent.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Olsson KG, Furst B, Andersson B, Carlsson GE. A long-term retrospective and clinical follow-up study of In-Ceram Alumina FPDs. Int J Prosthodont. 2003;16(2):150–156. [PubMed] [Google Scholar]
- 21.Vult von Steyern P, Jonsson O, Nilner K. Five-year evaluation of posterior all-ceramic three-unit (In-Ceram) FPDs. Int J Prosthodont. 2001;14(4):379–384. [PubMed] [Google Scholar]
- 22.Sorensen JA, Choi C, Fanuscu MI, Mito WT. IPS Empress crown system: three-year clinical trial results. J Calif Dent Assoc. 1998;26(2):130–136. [PubMed] [Google Scholar]
- 23.Sorensen JA, Kang SK, Torres TJ, Knode H. In-Ceram fixed partial dentures: three-year clinical trial results. J Calif Dent Assoc. 1998;26(3):207–214. [PubMed] [Google Scholar]
- 24.Marquardt P, Strub JR. Survival rates of IPS empress 2 all-ceramic crowns and fixed partial dentures: results of a 5-year prospective clinical study. Quintessence Int. 2006;37(4):253–259. [PubMed] [Google Scholar]
- 25.Kamposiora P, Papavasiliou G, Bayne SC, Felton DA. Stress concentration in all-ceramic posterior fixed partial dentures. Quintessence Int. 1996;27(10):701–706. [PubMed] [Google Scholar]
- 26.Filser F, Kocher P, Weibel F, Luthy H, Scharer P, Gauckler LJ. Reliability and strength of all-ceramic dental restorations fabricated by direct ceramic machining (DCM) Int J Comput Dent. 2001;4(2):89–106. [PubMed] [Google Scholar]
- 27.Luthy H, Filser F, Loeffel O, Schumacher M, Gauckler LJ, Hammerle CH. Strength and reliability of four-unit all-ceramic posterior bridges. Dent Mater. 2005;21(10):930–937. doi: 10.1016/j.dental.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Kelly JR, Tesk JA, Sorensen JA. Failure of all-ceramic fixed partial dentures in vitro and in vivo: analysis and modeling. J Dent Res. 1995;74(6):1253–1258. doi: 10.1177/00220345950740060301. [DOI] [PubMed] [Google Scholar]
- 29.Oh WS, Anusavice KJ. Effect of connector design on the fracture resistance of all-ceramic fixed partial dentures. J Prosthet Dent. 2002;87(5):536–542. doi: 10.1067/mpr.2002.123850. [DOI] [PubMed] [Google Scholar]
- 30.Oh W, Gotzen N, Anusavice KJ. Influence of connector design on fracture probability of ceramic fixed-partial dentures. J Dent Res. 2002;81(9):623–627. doi: 10.1177/154405910208100909. [DOI] [PubMed] [Google Scholar]
- 31.Bahat Z, Mahmood DJ, Vult von Steyern P. Fracture strength of three-unit fixed partial denture cores (Y-TZP) with different connector dimension and design. Swed Dent J. 2009;33(3):149–159. [PubMed] [Google Scholar]
- 32.Tsumita M, Kokubo Y, Vult von Steyern P, Fukushima S. Effect of framework shape on the fracture strength of implant-supported all-ceramic fixed partial dentures in the molar region. J Prosthodont. 2008;17(4):274–285. doi: 10.1111/j.1532-849X.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- 33.Kokubo Y, Tsumita M, Sakurai S, Torizuka K, Vult von Steyern P, Fukushima S. The effect of core framework designs on the fracture loads of all-ceramic fixed partial dentures on posterior implants. J Oral Rehabil. 2007;34(7):503–507. doi: 10.1111/j.1365-2842.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 34.Larsson C, Holm L, Lovgren N, Kokubo Y, Vult von Steyern P. Fracture strength of four-unit Y-TZP FPD cores designed with varying connector diameter. An in-vitro study. J Oral Rehabil. 2007;34(9):702–709. doi: 10.1111/j.1365-2842.2007.01770.x. [DOI] [PubMed] [Google Scholar]
- 35.Mokhtarikhoee S, Jannesari A, Behroozi H. Effect of connector width on stress distribution in all ceramic fixed partial dentures (a 3D finite element study) Conf Proc IEEE Eng Med Biol Soc. 2008;2008:1829–1832. doi: 10.1109/IEMBS.2008.4649535. [DOI] [PubMed] [Google Scholar]
- 36.Christensen RP, Ploeger BJ. A clinical comparison of zirconia, metal and alumina fixed-prosthesis frameworks veneered with layered or pressed ceramic: a three-year report. J Am Dent Assoc. 2010;141(11):1317–1329. doi: 10.14219/jada.archive.2010.0076. [DOI] [PubMed] [Google Scholar]
- 37.Larsson C, Vult von Steyern P, Sunzel B, Nilner K. All-ceramic two- to five-unit implant-supported reconstructions. A randomized, prospective clinical trial. Swed Dent J. 2006;30(2):45–53. [PubMed] [Google Scholar]
- 38.Sailer I, Gottnerb J, Kanelb S, Hammerle CH. Randomized controlled clinical trial of zirconia-ceramic and metal-ceramic posterior fixed dental prostheses: a 3-year follow-up. Int J Prosthodont. 2009;22(6):553–560. [PubMed] [Google Scholar]
- 39.Sailer I, Feher A, Filser F, Gauckler LJ, Luthy H, Hammerle CH. Five-year clinical results of zirconia frameworks for posterior fixed partial dentures. Int J Prosthodont. 2007;20(4):383–388. [PubMed] [Google Scholar]
- 40.Roediger M, Gersdorff N, Huels A, Rinke S. Prospective evaluation of zirconia posterior fixed partial dentures: four-year clinical results. Int J Prosthodont. 2010;23(2):141–148. [PubMed] [Google Scholar]
- 41.Silva NR, Bonfante EA, Rafferty BTM, Zavanelli RA, Rekow ED, Thompson VP, et al. Modified Y-TZP Core Design Improves All-ceramic Crown Reliability. J Dent Res. 2010 doi: 10.1177/0022034510384617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura T, Wakabayashi K, Kawamura Y, Kinuta S, Mutobe Y, Yatani H. Analysis of internal defects in all-ceramic crowns using micro-focus X-ray computed tomography. Dent Mater J. 2007;26(4):598–601. doi: 10.4012/dmj.26.598. [DOI] [PubMed] [Google Scholar]
- 43.DeHoff PH, Anusavice KJ, Gotzen N. Viscoelastic finite element analysis of an all-ceramic fixed partial denture. J Biomech. 2006;39(1):40–48. doi: 10.1016/j.jbiomech.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Saito A, Komine F, Blatz MB, Matsumura H. A comparison of bond strength of layered veneering porcelains to zirconia and metal. J Prosthet Dent. 2010;104(4):247–257. doi: 10.1016/S0022-3913(10)60133-3. [DOI] [PubMed] [Google Scholar]
- 45.Gostemeyer G, Jendras M, Dittmer MP, Bach FW, Stiesch M, Kohorst P. Influence of cooling rate on zirconia/veneer interfacial adhesion. Acta Biomater. 2010;6(12):4532–4538. doi: 10.1016/j.actbio.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 46.Swain MV. Unstable cracking (chipping) of veneering porcelain on all-ceramic dental crowns and fixed partial dentures. Acta Biomater. 2009;5(5):1668–1677. doi: 10.1016/j.actbio.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Guazzato M, Walton TR, Franklin W, et al. Influence of thickness and cooling rate on development of spontaneous cracks in porcelain/zirconia structures. Aust Dent J. 2010;55:306–310. doi: 10.1111/j.1834-7819.2010.01239.x. [DOI] [PubMed] [Google Scholar]
- 48.Seghi RR, Denry IL, Rosenstiel SF. Relative fracture toughness and hardness of new dental ceramics. J Prosthet Dent. 1995;74:145–150. doi: 10.1016/s0022-3913(05)80177-5. [DOI] [PubMed] [Google Scholar]
- 49.Guazzato M, Albakry M, Swain MV, et al. Mechanical properties of In-Ceram Alumina and In-Ceram Zirconia. Int J Prosthodont. 2002;15:339–346. [PubMed] [Google Scholar]
- 50.Giordano RA, 2nd, Pelletier L, Campbell S, et al. Flexural strength of an infused ceramic, glass ceramic, and feldspathic porcelain. J Prosthet Dent. 1995;73:411–418. doi: 10.1016/s0022-3913(05)80067-8. [DOI] [PubMed] [Google Scholar]
- 51.Chong KH, Chai J, Takahashi Y, et al. Flexural strength of In-Ceram alumina and In-Ceram zirconia core materials. Int J Prosthodont. 2002;15:183–188. [PubMed] [Google Scholar]
- 52.Wagner WC, Chu TM. Biaxial flexural strength and indentation fracture toughness of three new dental core ceramics. J Prosthet Dent. 1996;76:140–144. doi: 10.1016/s0022-3913(96)90297-8. [DOI] [PubMed] [Google Scholar]
- 53.Schweiger MHW, Frank M, Drescher H, et al. IPS Empress 2: a new pressable high-strength glass-ceramic for esthetic all-ceramic restorations. Quintessence Dent Technol. 1999;22:143–151. [Google Scholar]
- 54.Quinn JB, Sundar V, Lloyd IK. Influence of microstructure and chemistry on the fracture toughness of dental ceramics. Dent Mater. 2003;19:603–611. doi: 10.1016/s0109-5641(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 55.Sorensen JA, Cruz M, Mito WT, et al. A clinical investigation on three-unit fixed partial dentures fabricated with a lithium disilicate glass-ceramic. Pract Periodontics Aesthet Dent. 1999;11:95–106. quiz 08. [PubMed] [Google Scholar]
- 56.Zeng K, Oden A, Rowcliffe D. Flexure tests on dental ceramics. Int J Prosthodont. 1996;9:434–439. [PubMed] [Google Scholar]
- 57.Seghi RR, Sorensen JA. Relative flexural strength of six new ceramic materials. Int J Prosthodont. 1995;8:239–246. [PubMed] [Google Scholar]
- 58.Guazzato M, Proos K, Quach L, et al. Strength, reliability and mode of fracture of bilayered porcelain/zirconia (Y-TZP) dental ceramics. Biomaterials. 2004;25:5045–5052. doi: 10.1016/j.biomaterials.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 59.Luthardt RG, Holzhuter MS, Rudolph H, et al. CAD/CAM-machining effects on Y-TZP zirconia. Dent Mater. 2004;20:655–662. doi: 10.1016/j.dental.2003.08.007. [DOI] [PubMed] [Google Scholar]