Abstract
Progenitor and pluripotent cell types offer promise as regenerative therapies but transfecting these sensitive cells has proven difficult. Herein, a series of linear trehalose-oligoethyleneamine “click” copolymers were synthesized and examined for their ability to deliver plasmid DNA (pDNA) to two progenitor cell types, human dermal fibroblasts (HDFn) and rat mesenchymal stem cells (RMSC). Seven polymer vehicle analogs were synthesized in which three parameters were systematically varied: the number of secondary amines (4–6) within the polymer repeat unit (Tr433, Tr530, and Tr632), the end group functionalities [PEG (Tr4128PEG-a, Tr4118PEG-b), triphenyl (Tr4107-c), or azido (Tr499-d)], and the molecular weight (degree of polymerization of about 30 or about 100) and the biological efficacy of these vehicles was compared to three controls: Lipofectamine 2000, JetPEI, and Glycofect. The trehalose polymers were all able to bind and compact pDNA polyplexs, and promote pDNA uptake and gene expression [luciferase and enhanced green fluorescent protein (EGFP)] with these primary cell types and the results varied significantly depending on the polymer structure. Interestingly, in both cell types, Tr433 and Tr530 yielded the highest luciferase gene expression. However, when comparing the number of cells transfected with a reporter plasmid encoding enhanced green fluorescent protein, Tr433 and Tr4107-c yielded the highest number of HDFn cells positive for EGFP. Interestingly, with RMSC, all of the higher molecular weight analogs (Tr4128PEG-a, Tr4118PEG-b, Tr4107-c, Tr499-d) yielded high percentages of cells positive for EGFP (30–40%).
Keywords: Biocompatibility, DNA, Drug Delivery Nanoparticle, Polyethyleneoxide, Polymerization
1. Introduction
The discovery of induced pluripotent stem cells (iPS) in 2006 [1] has immediately offered the broad scope of opportunities for the search of treatment of various degenerative disorders. The progenitor and pluripotent cells that can differentiate into various cell types could hold a solution for the future regenerative therapies. In order to engineer these cells to have therapeutic potential, a recombinant genetic material needs to be inserted into the intracellular environment. With the completion of mapping the entire human genome [2], nucleic acids have the potential to yield unlimited customized therapies for a broad array of the most devastating human diseases. However, the delivery of nucleic acids both in vivo and ex vivo to therapeutically-relevant cells still remains the major hurdle towards realizing the full utility of this powerful medicinal tool. Nucleic acids are bulky, charged macromolecules that are unstable in the presence of nucleases, and vehicles are required to compact them into nanosized particles, protect them from degradation, carry them through cellular membranes, and release them at the desired locations within target cells. This has been demonstrated with both viruses [3–4] and chemical reagent-based methods relying on calcium phosphate [5–6], lipids [7–9], and macromolecules [10–13]. Chemical materials that fulfill these duties but interfere minimally with native cellular function and viability are continually refined and improved to offer tailored methods for nucleic acid administration; however, all delivery methods developed thus far have limitations in either delivery efficiency or toxicity. At the forefront of nucleic acid delivery systems are carbohydrate-oligoamine copolymers, which demonstrate a high propensity towards nucleic acid delivery while exhibiting low toxicity and high stability in endogenous conditions [14–16]. Indeed, polycationic carbohydrate-based synthetic polymers were recently used by Davis and coworkers for the first demonstration of systemic delivery of siRNA and the demonstration of RNAi in human subjects, a major milestone in nucleic acid-based therapeutics [17].
To continue to progress the field of nucleic acid therapeutics, vehicles must be designed that can deliver nucleic acid both in vivo and ex vivo under a wide variety of conditions, including in the presence of serum proteins and antibiotics. This is a major limitation for many commercially-available transfection reagents formulated with both lipids and polymers. Compatibility with serum and antibiotics is especially important performing ex vivo genetic engineering of therapeutic cells, as these conditions permit cells being transfected to exhibit optimal growth, behave as they would in native environments, and remain free of bacterial contamination. These factors are essential for the success of cell-based therapies after cell reintroduction or engineered tissue implantation has occurred. Major cells of interest to the community include a wide variety of immunological [18–21], progenitor [22–24], and structural tissue-based cells [25–26]. We have examined transfection in cell types of interest to regenerative medicine and cellular based therapies. The first cell type, human dermal fibroblasts, are routinely examined for tissue engineering applications for their ability to rapidly grow into skin grafts in burn patients and perhaps most importantly these cells are on the forefront of cellular therapy for their ability to be induced as pluripotent stem cells exhibiting the proginating ability of embryonic stem cells without the perceived ethical issues. Another cell of therapeutic importance for its ability to differentiate into important bone related cell types are human mesenchymal stem cells. To this end, we have developed a series of trehalose-oligoethyleneamine copolymers and demonstrated their ability to transfect neonatal human dermal fibroblasts (HDFn), which are of high therapeutic importance for their ability to be induced into pluripotent stem (iPS) cells [27]. In addition we demonstrate that these polymers have achieved gene delivery in primary rat mesenchymal stem cells (RMSCs). The polymeric delivery vehicles synthesized and examined herein are potent in cell culture media containing serum and antibiotics, which demonstrates the effectiveness of this series of macromolecules towards their applicability for use as delivery agents in progenitor and pluripotent cells.
As the delivery of nucleic acids in media containing high concentrations of serum and antibiotics is key, we have utilized a polycation motif containing trehalose; our lab has demonstrated previously that this disaccharide helps polyplex stability in serum-containing media [28–30]. Polymers were synthesized in similar degrees of polymerization via step-growth Cu(I)-catalyzed azide-alkyne cycloaddition polymerization, resulting in a series of trehalose-containing copolymers with a range of secondary amines (from 4 to 6) within the polymer backbone (Fig. 1; Tr433, Tr530, Tr632). These glycopolymers were tested for pDNA binding, cytotoxicity, cellular uptake, and transfection efficiency in vitro in primary fibroblast and progenitor cells. We have also created a number of analogs with different end functionalities such as PEG (Fig. 1; Tr4128PEG-a, Tr4118PEG-b), azido-trehalose (Fig. 1; Tr4107-c), and triphenylacetamide groups (Fig. 1; Tr499-d) to explore the role of polymer end groups on the ability of these systems for primary cell therapies. PEG functionalities could provide additional protection against aggregation with serum proteins and other undesired interactions with the components of physiological systems required for progenitor cell therapies. In addition, PEG functionalities can be used in the future as linkers for the incorporation of other functionalities, like targeting ligands, MRI contrast agents, and radiotracers for the development of multifunctional systems for both gene delivery and imaging [31–33]. In addition, non-PEGylated Tr4 analogues were created (Fig. 1; Tr4107-c, Tr499-d) to see the impact of different end-groups on cellular uptake and transfection efficiency. This work contributes to our continuous efforts in designing better nucleic acid carriers based upon structure-bioactivity relationships in order to contribute to therapies aimed at important cellular therapeutic targets currently being used in the clinic. These polymeric vehicles demonstrate highly efficient gene delivery to primary progenitor cell types, both human dermal fibroblasts and rat mesenchymal stem cells in the presence of serum, antibiotics, and growth factors. These cell types are examples of cell types utilized for tissue engineering and cell-based therapy research and development. These materials have promise to enable ex vivo cellular engineering to be carried out more efficiently, with better cellular viability, and free from bacterial contamination, all critical constraints in translating engineered cellular therapies to the clinic.
Fig. 1.
The structures of the synthetic targets to be examined as nucleic acid vehicles for progenitor cell types.
2. Materials and Methods
2.1. General
All reagents and solvents used in the synthesis, if not specified otherwise, were obtained from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific Co. (Pittsburgh, PA) and were used without further purification. Methanol, dichloromethane, and dimethylformamide were purified with MBRAUN MB SPS solvent purification system. PEGs were obtained from NOF America Corporation. Compounds 6a and 6b, partially-Boc-protected tetraethylenepentamine and pentaethylenehexamine, respectively, were synthesized using a previously-published procedure [30–31]. All the reactions were monitored to completion using thin layer chromatography (TLC) with ninhydrin staining for oligoamines and p-anisaldehyde staining for visualizing oligosaccharides along with UV detection when possible. Ultrapure water was used in all synthesis and dialysis purification steps. The dialysis membranes were manufactured by Spectrum Laboratories, Inc. (Rancho Dominguez, CA). The liquid chromatography-mass spectra (LC-MS) were obtained with an Agilent system with a time-of-flight (TOF) analyzer coupled to a Thermo Electron TSQ-LC/MS ESI mass spectrometer. IR spectra were measured with a Perkin Elmer Spectrum One FT-IR spectrometer. 1H and 13C NMR were recorded on a 400MR Varian-400 Hz spectrometer. The 1H NMR data are reported as follows: chemical shift (δ ppm), multiplicity, J coupling constant (Hz), and peak integration. CHCl3 (7.27 ppm) or HOD (4.79 ppm) were used as an internal reference. Cell culture media and supplements were obtained from Gibco/Invitrogen (Carlsbad, CA). JetPEI solution was purchased from Polyplus Transfection™ (New York, NY). RMSC and HDFn cells were purchased from Invitrogen (Carlsbad, CA).
2.2. Monomer synthesis
2.2.1. Synthesis of the trehalose monomer
2,3,4,2′,3′,4′-Hexa-O-acetyl-6,6′-diiodo-6,6′-dideoxy-D-trehalose (2)
Trehalose 1 (10.0 g, 29.2 mmol) was dissolved in anhydrous dimethylformamide (DMF) (300 mL) and cooled to 0 °C. Triphenylphosphine (48.9 g, 186 mmol) and then iodine (38.2 g, 150 mmol) were added to that mixture, which was allowed to stir at 0 °C for 10 min, after which the temperature was raised to 80 °C and the reaction was stirred for additional 3 h. The reaction mixture was concentrated under reduced pressure, cooled to 0 °C and ice-cold water (500 mL) was added under vigorous stirring to precipitate the triphenylphosphine oxide side product, which was removed via filtration. The remaining solution was evaporated under reduced pressure to yield a brown solid. This crude material was dried in high vacuum, then dissolved in anhydrous pyridine (200 mL) and acetic anhydride was added (100 mL). The reaction mixture was stirred at room temperature under N2 for 24 h. Then the mixture was concentrated via rotary evaporation and poured on ice (500 g) under vigorous stirring. The off-white precipitate were isolated by filtration, air dried, and recrystallized from ethanol to yield final product 2, as a white crystalline solid. Yield after recrystallization: 11.2 g (47%). 1H-NMR (CDCl3): δ = 2.02 (s, 6H, COCH3), 2.07 (s, 6H, COCH3), 2.15 (s, 6H, COCH3), 3.07 (dd, J = 2.3, 8.6 Hz, 2H, H–6), 3.23 (dd, J = 2.3, 11.0 Hz, 2H, H–6), 3.95 (td, J = 2.4, 9.5 Hz, 2H, H–5), 4.90 (dd, J = 9.2 Hz, 2H, H–4), 5.20 (dd, J = 3.9, 10.2 Hz, 2H, H–2), 5.42 (d, J = 3.9 Hz, 2H, H–1), 5.48 (dd, J = 9.2, 10.2 Hz, 2H, H–3). 13C-NMR (CDCl3): δ = 2.61 (C–6), 20.79 (CH3CO), 20.85 (CH3CO), 21.35 (CH3CO), 69.45 (C–2), 69.91 (C–3), 70.12 (C–5), 72.50 (C–4), 91.94 (C–1), 169.58 (CH3CO), 169.72 (CH3CO), 170.05 (CH3CO). LC-ESI-MS (m/z): Theoretical (M+H)+ C24H33I2O15 = 814.99; Found = 814.85; Theoretical (M+NH4)+ C24H36I2NO15 = 832.02; Found = 831.87.
2,3,4,2′,3′,4′-Hexa-O-acetyl-6,6′-diazido-6,6′-dideoxy-D-trehalose (3)
To a solution of 2 (11.0 g, 13.5 mmol) dissolved in anhydrous DMF (100 mL), sodium azide (5.30 g, 81.5 mmol) was added. The heterogeneous mixture was then heated, while stirring, to 80 °C and allowed to react for 24 h. The reaction mixture was then cooled, concentrated under reduced pressure and titrated with ice cold water (200 mL) to precipitate the crude product, which was filtered and dried in high vacuum to yield 7.80 g (90%) of pure acetylated title product 3. 1H-NMR (CDCl3): δ = 2.03 (s, 6H, COCH3), 2.07 (s, 6H, COCH3), 2.13 (s, 6H, COCH3), 3.18 (dd, J = 2.5, 13.3 Hz, 2H, H–6), 3.37 (dd, J = 7.3, 13.3 Hz, 2H, H–6), 4.10 (ddd, J = 2.5, 7.3, 10.0 Hz, 2H, H–5), 5.00 (dd, J = 9.3, 10.2 Hz, 2H, H–4), 5.09 (dd, J = 3.9, 10.3 Hz, 2H, H–2), 5.33 (d, J = 3.9 Hz, 2H, H–1), 5.48 (dd, J = 9.3, 10.3 Hz, 2H, H–3). 13C-NMR (CDCl3): δ = 20.60 (CH3CO), 50.95 (C–6), 69.63 (C–3), 69.68 (C–4), 69.79 (C–2), 69.88 (C–5), 92.97 (C–1), 169.57 (CH3CO), 169.60 (CH3CO), 169.92 (CH3CO). FT-IR: (cm−1) 2961, 2097, 1745, 1436, 1368, 1289, 1212. LC-ESIMS (m/z): Theoretical (M+H)+ C24H33N6O15 = 645.20; Found = 645.06. Theoretical (M+NH4)+ C24H36N7O15 = 662.23; Found = 662.09.
2.2.3. Synthesis of the oligoethyleneamine monomers
N1,N5-bis(2-ethylphthalimido)-N1,N2,N3,N4,N5-penta(tert-butoxycarbonyl)- tetraethylenepentamine (7a)
N2,N3,N4-tri(tert-butoxycarbonyl)-tetraethylenepentamine [0] 6a (4.0 g, 8.2 mmol) was dissolved in anhydrous dichloromethane (100 mL) and cooled to 0 °C. Under a N2(g) atmosphere and with stirring, a solution of phthalimidoacetaldehyde [34] (3.8 g, 21 mmol) in dichloromethane (10 mL) was added slowly through a syringe to the solution of partially-protected oligoamine. The resulting solution turned from colorless to slightly yellowish, indicating the formation of an imine. The reaction mixture was stirred at 0 °C for an additional 30 min, and subsequently concentrated into an oil, which was re-dissolved in anhydrous methanol (100 mL). Sodium triacetoxyborohydride (4.2 g, 20 mmol) was added to the reaction flask and the resulting heterogeneous mixture was stirred for 1 h at 0 °C, then overnight at room temperature. The mixture was washed with saturated sodium bicarbonate solution (2 × 100 mL) to neutralize the residual sodium triacetoxyborohydride, and then washed with distilled water (2 × 100 mL). The washed organic layer was dried with sodium sulfate, filtered, and concentrated to yield a yellowish oil. This crude product was re-dissolved in dichloromethane, and Boc-anhydride (3.9 g, 18 mmol) was added to this solution. The reaction was stirred for 6 h at room temperature, after which the solution was concentrated to a viscous oil, which was purified by flash chromatography (ethyl acetate/hexane, 1/1 (v/v)). Fractions containing the product were isolated and concentrated under reduced pressure to yield a colorless oil, which, after stirring with cold diethyl ether, yielded a white amorphous solid. Yield: 3.9 g (46%). 1H-NMR (CDCl3): δ= 1.43–1.45 (m, 45H, (CH3)3CO), 3.23–3.38 (m, 20H, CH2), 3.45–3.53 (m, 2H, CH2), 3.78–3.87 (m, 2H, CH2), 7.65–7.86 (m, 8H, arom.). LC-ESI-MS (m/z): Theoretical (M+H)+ C53H78N7O14 = 1036.56; Found = 1036.64. Theoretical (M+Na)+ C53H77N7NaO14 = 1058.54; Found = 1058.61.
N1,N6-bis(2-ethylphthalimido)-N1,N2,N3,N4,N5,N6-hexa(tert-butoxycarbonyl)-pentaethylenehexamine (7b)
The compound was synthesized using the procedure described for the synthesis of 7a except N2,N3,N4,N5-tetra(tert-butoxycarbonyl)-pentaethylenehexamine 6b (4.2 g, 6.6 mmol) was used in place of 6a using other reagents in corresponding molar ratios. Yield: 3.1 g (40%). 1H-NMR (CDCl3): δ = 1.43–1.46 (m, 54H, (CH3)3CO), 3.22–3.37 (m, 24H, CH2), 3.42–3.55 (m, 2H, CH2), 3.79–3.87 (m, 2H, CH2), 7.66–7.87 (m, 8H, arom.). LC-ESI-MS (m/z): Theoretical (M+H)+ C60H91N8O16 = 1179.65; Found = 1179.71. Theoretical (M+Na)+ C60H90N8NaO16 = 1201.64; Found = 1201.70.
N2,N3,N4,N5,N6-penta(tert-butoxycarbonyl)-hexaethyleneheptamine (8a)
Compound 7a (1.5 g, 1.4 mmol) was dissolved in methanol (20 mL) and reacted with hydrazine monohydrate (1 mL, d=1.032 g/mL, 21 mmol) under reflux conditions for 4 h. The progression of the reaction was monitored by TLC (dichloromethane/methanol/ammonia hydroxide, 100/10/1 (v/v/v)). Upon completion, the reaction mixture was cooled, causing the formation of a white precipitate, the side product 2,3-dihydrophthalazine-1,4-dione. The reaction mixture was concentrated under reduced pressure to yield a white amorphous solid, which was dissolved in dichloromethane (30 mL) and washed with 4 M NH4OH solution (2 × 20 mL) and then with water (2 × 20 mL). The washed organic layer was collected, dried with sodium sulfate, filtered, and concentrated under reduced pressure to yield a colorless oil. An amorphous solid was formed following the addition of 5 mL of diethyl ether and cooling it for 1 h at −24 °C. The crude solid was recrystallized from methyl tert-butyl ether (MTBE). Yield: 0.9 g (82%). 1H-NMR (CDCl3): δ = 1.45 (s, 45H, (CH3)3CO), 2.81 (t, 4H, CH2NH2), 3.31 (q, 20H, CH2NBoc). LC-ESI-MS (m/z): Theoretical (M+H)+ C37H74N7O10 = 776.55; Found = 776.55.
N2,N3,N4,N5,N6,N7-hexa(tert-butoxycarbonyl)-heptaethyleneoctamine (8b)
The compound was synthesized accordingly to the procedure described for the synthesis of 8a where, instead of 7a, 7b (1.2 g, 1.0 mmol) was used with other reagents in corresponding molar ratios. Yield: 0.8 g (93%). 1H-NMR (CDCl3): δ = 1.45 (s, 54H, (CH3)3CO), 2.79–2.84 (m, 4H, CH2NH2), 3.22–3.37 (m, 24H, CH2NBoc). LC-ESI-MS (m/z): Theoretical (M+H)+ C44H87N8O12 = 919.64; Found = 919.64.
N1,N5-bis(2-ethylpropynamido)-N1,N2,N3,N4,N5-penta(tert-butoxycarbonyl)- tetraethylenepentamine (9a)
A solution of N,N′-dicyclohexylcarbodiimide (DCC) (1.6 g, 7.9 mmol) in anhydrous dichloromethane (20 mL) was cooled to 0 °C under nitrogen atmosphere. A solution of propiolic acid (0.6 g, 8.6 mmol) in dichloromethane (3 mL) was then added drop-wise through a syringe. The resulting heterogeneous mixture was stirred at 0 °C for additional 30 min, then a solution of diamine 8a (1.4 g, 1.8 mmol) in dichloromethane (5 mL) was added drop-wise through a syringe. The mixture was stirred for 1 h at 0 °C and then overnight at room temperature. The insoluble side product, 1,3-dicyclohexylurea, was filtered from the reaction mixture. The remaining filtrate was concentrated under reduced pressure to yield a brown oil, which was purified with flash chromatography (ethyl acetate/chloroform, 2/1 (v/v)). The fractions with the desired product were combined and concentrated under reduced pressure to yield an off-white amorphous solid, which was recrystallized from ethyl acetate to yield the title product 9a. Yield: 0.9 g (57%). 1H-NMR (CDCl3): δ = 1.44–1.47 (m, 45H, (CH3)3CO), 2.71–2.76 (bs, 2H, C≡CH), 3.25–3.46 (m, 24H, CH2). FT-IR: 3294, 3210, 2973, 2926, 2101, 1675, 1660, 1519, 1471, 1418, 1362, 1235, 1155. LC-ESI-MS (m/z): Theoretical (M+H)+ C43H74N7O12 = 880.54; Found = 880.48. Theoretical (M+Na)+ C43H73N7NaO12 = 902.52; Found = 902.44.
N1,N6-bis(2-ethylpropynamido)-N1,N2,N3,N4,N5,N6-hexa(tert-butoxycarbonyl) pentaethylenehexamine (9b)
This compound was obtained following a similar procedure as the one reported above for 9a, with 8b (2.0 g, 2.2 mmol) used as a starting material. Yield: 1.3 g (58%). 1H-NMR (CDCl3): δ = 1.44–1.47 (m, 54H, (CH3)3CO), 2.72–2.76 (bs, 2H, C≡CH), 3.25–3.46 (m, 28H, CH2). FT-IR: 3216, 2975, 2932, 2104, 1682, 1658, 1537, 1471, 1415, 1365, 1244, 1158. LC-ESI-MS (m/z): Theoretical (M+H)+ C24H33N6O15 = 1023.63; Found = 1023.55. Theoretical (M+Na)+ C24H36N7O15 = 1045.62; Found = 1045.53.
2.3. Synthesis of Tr433, Tr530, and Tr632
2.3.1. General procedure for the polymerization
The diazido trehalose monomer 3 (0.15–0.60 mmol), the equivalent molar amount of alkyne terminated oligoethyleneamines 9 [29], 9a, or 9b, and tert-butanol (1–2 mL) were mixed in a 5 mL vial and cooled to 0 °C. To the cooled, stirred reaction mixture, 1 M copper sulfate (0.2 eq) and 1 M sodium ascorbate (0.4 eq) aqueous solutions were added, after which water was added to a 1/1 (v/v) total ratio of tert-butanol/water. The mixture was heated to 50 °C and vigorously stirred at that temperature for 2 h. The reaction was terminated by cooling the heterogeneous mixture with an ice bath. After cooling, the reaction mixture separated into a viscous yellow oily layer and a blue aqueous supernatant. The copper-containing supernatant was removed and the remaining viscous oily residue was dissolved in 1 mL of DMSO and precipitated by adding 4 M NH4OH (2 mL) solution. Precipitates were recovered via centrifugation, after which a fresh portion of 4 M NH4OH solution was added to the precipitant and the centrifugation was repeated. This cycle was repeated until no blue color was visually detectable in the NH4OH supernatant, indicating that copper (II) was removed from the “click” polymer product. The off-white precipitate was washed with water (2 × 2 mL) in a similar manner described for NH4OH then the resulting precipitates were dried in high vacuum and carried out to the deprotection steps.
2.3.2. General procedure for the deprotection of Ac and Boc protecting groups
The hydroxyl-protecting acetyl groups were removed from each of the polymers by dissolving each polymer, obtained with the above described method, in 2 mL of sodium methoxide solution in dry methanol (0.05 g/mL). This solution was then stirred overnight at room temperature, dialyzed against methanol with 1000 MWCO membrane to remove NaOMe, and then dried in high vacuum. The resulting deacetylated polymer was suspended in 1 mL of a solution of 4 M HCl in dioxane and stirred at room temperature for 4 h to remove the Boc protecting groups. The HCl and dioxane were removed under reduced pressure yielding a solid product, which was re-dissolved in ultrapure water and purified via dialysis with 3500 MWCO membrane against ultrapure water for 48 h (water changes: 3 × 4 L; at 8, 24, and 36 hours). The final deprotected polymers were lyophilized to dryness to yield a white fluffy solid.
Poly[(trehalose-ditriazolamido)pentaethylenetetraamine] (Tr433)
Yield in 3 steps: 105 mg, (51%). 1H-NMR (D2O): δ = 3.11–3.28 (m, 18H), 3.47–3.52 (m, 2H), 3.75 (t, J = 5.7 Hz, 4H), 3.84 (t, J = 9.4 Hz, 2H), 4.19 (t, J = 7.8 Hz, 2H), proton signals overlapping with HOD peak, 8.49 (s, 2H). FT-IR: (cm−1) 3235, 2921, 1654, 1577, 1510, 1435, 1346, 1246.
Poly[(trehalose-ditriazolamido)hexaaethylenepentaamine] (Tr530)
Yield in 3 steps: 156 mg, (61%). 1H-NMR (D2O): δ = 3.01–3.05 (m, 8H), 3.19–3.27 (m, 10H), 3.36 (t, J = 5.4 Hz, 4H), 3.48 (dd, J = 3.6, 10.0 Hz, 4H), 3.78–3.84 (m, 6H), 4.18 (t, J = 8.0 Hz, 2H), proton signals overlapping with HOD peak, 8.49 (s, 2H). FT-IR: (cm−1) 3280, 1651, 1575, 1512, 1435, 1372, 1244.
Poly[(trehalose-ditriazolamido)heptaethylenehexaamine] (Tr632)
Yield in 3 steps: 96 mg, (79%). 1H-NMR (D2O): δ = 2.88–3.64 (m, 26H), 3.65–4.11 (m, 8H), 4.12–4.23 (m, 2H), proton signals overlapping with HOD peak, 8.51 (s, 2H). FT-IR: (cm−1) 3270, 2920, 2842, 1646, 1572, 1509, 1453, 1433, 1365, 1244.
2.4. Synthesis of Tr4128-PEGa, Tr4118-PEGb, Tr4107-c, and Tr499-d
2.4.1. Synthesis of end-groups
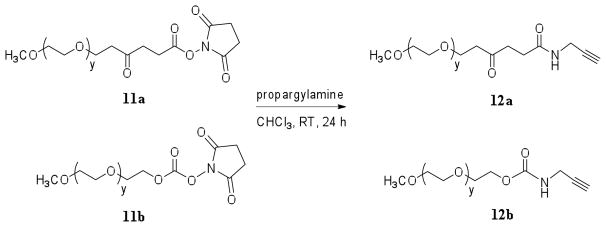
mPEG-CO-CH2CH2-CO-NH-CH2C≡CH (11a)
To a solution of mPEG-NHS (10a) (1.0 g, 0.20 mmol) in 7 mL of chloroform, a solution of propargylamine (0.3 g, 5.4 mmol) dissolved in 3 mL of chloroform was added. The resulting mixture was stirred at room temperature for 24 h. The solvent was then evaporated and the resulting yellowish oily residue was re-dissolved in methanol (10 mL), transferred to a 1000 MWCO membrane and dialyzed against methanol (1 L) for 24 h (methanol changes: 3 × 1 L; at 2, 5, and 22 h). The dialyzed compound was concentrated and the resulting oily residue was precipitated by adding cold diethyl ether (5 mL). The white amorphous precipitates were filtered and dried in high vacuum to yield amorphous alkyne-terminated methoxy-PEG 11a. Yield: 0.8 g (80%). 1H-NMR (CDCl3): δ = 2.20 (t, J = 2.6 Hz, 1H, C≡CH), 2.46 (t, J = 6.8 Hz, 2H), 2.67 (t, J = 6.8 Hz, 2H), 3.34 (s, 3H, OCH3), 3.43 (m, 4H,), 3.61 (m, 364H, O–CH2CH2–O), 3.78 (m, 4H), 4.00 (dd, J = 2.6, 5.3, 2H), 4.21 (m, 2H).
mPEG-O-CO-NH-CH2C≡CH (11b)
The compound was synthesized as described in the procedure above for 10b, except 0.50 g of mPEG-NHS 11a (dissolved in 5 mL of chloroform) and 0.06 g of propargylamine were used. Yield: 0.40 g (80%). 1H-NMR (CDCl3): δ = 2.24 (t, J = 2.5 Hz, 1H, C≡CH), 3.38 (s, 3H, OCH3), 3.46 (m, 4H), 3.64 (m, 364H, O–CH2CH2–O), 3.82 (m, 4H), 3.96 (m, 2H), 4.24 (m, 2H).
2,2,2-Triphenyl-N-(prop-2-yn-1-yl)acetamide (13)
A solution of 2,2,2-triphenylacetic acid (12) (5.00 g, 17.3 mmol) in anhydrous dichloromethane (20 mL) was cooled to 0 °C in nitrogen atmosphere. The solution of DCC in 20 mL of anhydrous dichloromethane was added drop-wise and the reaction mixture was stirred at 0 °C for 1 h. Then the solution of propargylamine (0.87 g, 15.7 mmol) in 10 mL of anhydrous dichloromethane was added to the reaction mixture and it was stirred for an additional hour at 0 °C, then at room temperature overnight. The insoluble white precipitate that formed in the course of the reaction was filtered and the filtrate evaporated under reduced pressure to yield an off-white solid, which was further purified with flash chromatography (ethyl acetate/hexane:1/1). The fraction with the Rf = 0.4 was isolated to obtain the title product 14. Yield: 2.81 g (50%). 1H-NMR (CDCl3): δ = 2.18 (t, J = 2.6 Hz, 1H, C≡CH), 4.12 (dd, J = 2.6, 5.3, 2H, NHCH2), 5.94 (bs, 1H, NH), 7.21–7.36 (m, 15H, arom.). 13C-NMR (CDCl3): δ = 29.97, 67.62, 71.53, 71.93, 127.05, 127.48, 127.89, 128.33, 130.44, 130.85, 143.21, 173.25. LC-ESI-MS (m/z): Theoretical (M+H)+ C23H20NO = 326.15; Found = 326.04. Theoretical (M+NH4)+ C23H23N2O = 343.18; Found = 343.06.
2.4.2. General procedure for the polymerization of the azido-terminated Tr4 block
The diazido trehalose monomer 3, the equivalent molar amount of alkyne terminated oligoethyleneamine 9 [29], and tert-butanol were mixed in a 10 mL vial and cooled to 0 °C. To the cooled, stirred reaction mixture, 1 M copper sulfate (0.2 eq) and 1 M sodium ascorbate (0.4 eq) aqueous solutions were added, after which water was added to a 1/1 (v/v) total ratio of tert-butanol/water (the concentration of each of the monomers in the suspension was about 5wt%). The mixture was heated to 50 °C and vigorously stirred at that temperature for 1 h. Then, an additional 0.2 eq of trehalose monomer 3 was added to the mixture to end-cap the formed polymer product, and it was stirred for 1 h at the same temperature. The reaction was terminated by cooling the heterogeneous mixture in an ice bath. After cooling, the reaction mixture separated into a viscous yellow oily layer and a blue aqueous supernatant. The copper-containing supernatant was removed and the remaining viscous oily residue was dissolved in 1 mL of DMSO and precipitated by adding 4 M NH4OH (2 mL) to the solution. Precipitates were recovered via centrifugation, after which a fresh portion of 4 M NH4OH solution was added to the precipitant and the centrifugation was repeated. This cycle was repeated until no blue color was visually detectable in the NH4OH supernatant, indicating that copper (II) was removed from the “click” polymer product. The off-white precipitate was washed with water (2 × 2 mL) in a similar manner described for NH4OH then the resulting precipitates were dried in high vacuum and carried out to the further end-capping step.
2.4.3. General procedure for the end-capping of Tr4
The batch of the fully protected, azido-trehalose terminated Tr4 polymer from the previous step was separated in 4 parts: A, B, C, and D. Parts A and B were further functionalized with PEG groups resulting in the protected polymers Tr4128PEG-a and Tr4118PEG-b, respectively; part C was end-capped with the 2,2,2-triphenyl-N-(prop-2-yn-1-yl)acetamide (13) and resulted in protected Tr4107-c. Part D was carried out to the deprotection steps without further modifications to result in Tr499-d.
PEGylation of Tr4
Parts A and B were each mixed with the corresponding PEG agent (25 wt%), 11a for Tr4128PEG-a and 11b for Tr4118PEG-b, and tert-butanol/water mixture following the same procedure as described for the polymerization of the Tr433 polymer. Copper(II) sulfate (1 M, 0.3 eq) and sodium ascorbate (1 M, 0.6 eq) were added to the reaction mixture and it was stirred overnight at 70 °C. After this time, the PEGylated product was isolated the same way as described in the procedure for the polymerization of azido-terminated Tr4. The solid product was re-dissolved in methanol, transported to a 3500 MWCO membrane and dialyzed against methanol (1 L) for 12 h (methanol was changed after 6 h). A small amount of part C was also end-capped with the triphenylacetamide 13 in a similar manner to yield protected Tr4-c.
2.4.4. General procedure for the deprotection of Ac and Boc protecting groups
The deprotection of the acetyl and Boc groups was achieved by the same method as described above. Determining the yield, the molecular weight of Tr4 (in both protected and deprotected polymers) was calculated from averaged degree of polymerization of 100. Molecular weight of PEG per polymer chain was averaged to 5000 Da per each PEG end-group.
Tr4128PEG-a
Started with 200 mg of fully protected diazido-terminated Tr4. Yield: 120 mg, (99%). 1H-NMR (D2O): δ = 2.94–3.08 (m, 14H), 3.23 (t, 2H), 3.45–3.60 (m, 6H), 3.74 (s, 3H), 3.81 (t, 2H), 4.16 (t, 2H), proton signals overlapping with HOD peak, 8.41 and 8.44 (2 s, 2H).
Tr4118PEG-b
Started with 200 mg of fully protected diazido-terminated Tr4. Yield: 98 mg, (82%). 1H-NMR (D2O): δ = 2.79–2.93 (m, 14H), 3.08 (t, 2H), 3.32–3.60 (m, 6H), 3.74 (s, 3H), 3.81 (t, 2H), 4.16 (t, 2H), proton signals overlapping with HOD peak, 8.41 and 8.44 (2 s, 2H).
Tr4107-c
Started with 50 mg of fully protected diazido-terminated Tr4. Yield: 19 mg, (73%). 1H-NMR (D2O): δ = 2.94–3.08 (m, 14H), 3.23 (t, 2H), 3.45–3.60 (m, 6H), 3.74 (s, 3H), 3.82 (t, 2H), 4.18 (t, 2H), proton signals overlapping with HOD peak, 8.44 and 8.47 (2 s, 2H).
Tr499-d
Started with 200 mg of fully protected diazido-terminated Tr4. Yield: 97 mg, (92%). 1H-NMR (D2O): δ = 2.87–3.00 (m, 14H), 3.23 (t, 2H), 3.44–3.61 (m, 8H), 3.82 (t, 2H), 4.16 (t, 2H), proton signals overlapping with HOD peak, 8.44 (s, 2H).
2.5. Polymer and polyplex characterization
2.5.1. Gel permeation chromatography (GPC)
The resulting Tr433, Tr530, Tr632, and Tr499-d “click” polymers were characterized by aqueous GPC utilizing an eluent of 1 w% acetic acid/0.1 M NaSO4 (aq) at a flow rate of 0.3 mL/min at 25 °C with an injection volume of 100 μL, Eprogen, Inc. CATSEC columns (100, 300, and 1000 Å), Waters 2489 UV/vis detector (λ = 274 nm), Wyatt Optilab rex refractometer (λ = 658 nm), and Wyatt DAWN Heleos-II multiangle laser light scattering (MALLS) detector (λ = 662 nm). For the polymers Tr4128PEG-a, Tr4118PEG-b, and Tr4107-c, the same GPC methods were used except using GMPWXL (aqueous phase) column and an eluent of 0.5 M sodium acetate (pH 5.5 adjusted with acetic acid) with 20v% acetonitrile (aq) at a flow rate of 0.6 mL/min at 25 °C. Polymer characterization data can be found in Tables 1 and 2 (See Supporting Information for GPC chromatograms).
Table 1.
Gel permeation chromatography (GPC) and static light scattering characterization of the Tr4-Tr6 polymer series.
Weight-average molecular weight (Mw), polydispersity index (Mw/Mn), and degree of polymerization (DPw) as obtained from GPC analysis.
The lowest observed N/P ratio of polymer-pDNA binding obtained from gel electrophoresis assay.
Table 2.
GPC and static light scattering characterization of the longer and end-functionalized Tr4 polymers.
| Polymer | Mw (kDa)a | PDIa | PEGw (%)b | DPwc | N/Pd |
|---|---|---|---|---|---|
| Tr4128PEG-a | 103.6 | 1.8 | 10 | 128 | 2 |
| Tr4118PEG-b | 95.7 | 1.2 | 10 | 118 | 2 |
| Tr4107-c | 77.8 | 1.2 | 0 | 107 | 2 |
| Tr499-d | 72.1 | 1.1 | 0 | 99 | 2 |
Weight average molecular weight (Mw) and polydispersity index (Mw/Mn), as obtained from GPC analysis.
Average PEG content by weight calculated from the weight average molecular weights.
Degree of polymerization of the Tr4 block calculated from the weight average molecular weights.
The lowest observed N/P ratio of polymer-pDNA binding obtained from gel electrophoresis assay.
2.5.2. Gel electrophoresis shift assays
The ability of polymers to bind with pDNA was measured by gel electrophoresis at 65 V for 45 min. Agarose gel (0.6 w/v %) containing ethidium bromide (0.06 w/v %) was prepared in TAE buffer (40 mM Tris-acetate, 1 mM EDTA, pH 8). Each polymer was dissolved in nuclease-free water (Gibco, Carlsbad, CA). Plasmid DNA (10 μL, 0.075 μg/μL) was mixed with the same volume of polymer solution at N/P ratios between 0 and 20 (N = moles of secondary amine groups on polymer backbone; P = moles of phosphate groups on the pDNA) and incubated for 45 min at room temperature before the addition of loading buffer (2 μL of Blue Juice, Invitrogen, Carlsbad, CA). An aliquot (10 μL) of each sample was loaded into the well of the gel. A stable polymer-pDNA complex is shown by the lack of the migration of the pDNA in the electrophoretic field.
2.5.3. Dynamic light scattering and zeta potential measurements
The size and zeta potential of each polyplex type were measured on a Zetasizer (Nano ZS) dynamic light scattering instrument (Malvern Instruments, Malvern UK). The instrument employs a 4.0 mW He-Ne laser operating at 633 nm with a 173° scattering angle. The polyplexes were formed at five different N/P ratios in triplicate (20, 10, 7, 5, and 3) by adding an aqueous solution of a polymer (150 μL) to an aqueous solution of pDNA (150 μL, 0.075 μg/mL), gently mixing with a pipette tip, and incubating at room temperature for 45 min. Prior to the measurement, polyplex solutions were diluted either with nuclease-free water (700 μL) or DMEM supplemented with 10% FBS (700 μL). For the polyplexes formed in water, the particle diameter was analyzed based on the size distribution by both volume and intensity (the readings were very close). For the polyplexes in serum media the particle diameter was analyzed based on the size distribution by only intensity (two large extra peaks at around 10 and 40 nm are always present and correspond to the serum proteins). Zeta potentials were measured using the same polyplex solutions in nuclease-free water only.
2.6.4. Luciferase expression assay
Prior to transfection, either primary neonatal human dermal fibroblast cells (HDFn, Invitrogen, Carlsbad, CA) or rat mesenchymal stem cells (RMSC, Invitrogen, Carlsbad, CA) were plated on 24-well plates at a density of 100,000 cells per well, with approximately 70% confluency. HDFn cells were incubated in Medium 106, supplemented with 2% FBS, hydrocortisone (1 μg/mL), human epidermal growth factor (1 ng/mL), basic fibroblast growth factor (3 ng/mL), and heparin (10 μg/mL), for 24 h at 37 °C in a 5% CO2 environment. RMSC were incubated in Mesanpro RS medium (Invitrogen) supplemented with 2% FBS. For both cell lines, medium was changed 30 minutes prior to transfection. Stock solutions at N/P 20 for each polymer were prepared and diluted to lower N/P ratios (10, 7) so that equal volume of polymer solution (V = 13.33 μL) and pDNA ([pDNA] = 0.075 mg/mL, Vt = 13.33 μL) could be combined to form the polyplex solution for each well (total pDNA per well = 1 μg). Positive controls - Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA), jetPEI™ (Polyplus Transfection™, New York, NY), and Glycofect™ (Techulon Inc., Blacksburg, VA) – were formulated with pDNA based upon their recommended protocols. Solutions of transfection reagent-pDNA (gWiz-Luciferase, Aldevron, Fargo, ND) complexes for each N/P were added in triplicate to the corresponding wells. Plates were swirled to ensure homogenous solution formation and incubated for 4 h, after which more medium (500 μL) was added to each well. Cells were incubated for additional 20 h, followed by a medium change and 24 h of additional incubation time (48 h total). Medium was removed from wells and cells were lysed in 100 μL Cell Lysis Buffer (Promega, Madison, WI). Cell lysates were deposited on 96-well plates and analyzed for luciferase activity. Data was reported in Relative Light Units (RLU) and normalized to cells only control.
2.5.5. MTT assay
Cells were prepared and transfected using the same methodology as reported above under the luciferase assays. However, at the 47 h time point, medium was removed from each well and replaced with medium containing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, [MTT] = 0.5 mg/mL). Cells were incubated for an additional 1 h then washed with PBS and lysed in 250 μL DMSO. Sample cell lysates were analyzed via absorbance vs. negative control lysate absorbance to determine cell viability.
2.5.6. EGFP analysis via flow cytometry
Cells were plated and polyplexes were formed by the same methods reported above with a plasmid encoding enhanced green florescent protein (EGFP-C1). Transfection and medium change conditions are consistent with those reported above; however, cells were only subjected to complexes at a single charge ratio, N/P=20. After 48 h, cells were trypsinized, washed with PBS twice, and suspended in 500 μL of PBS. Flow cytometry analysis of each sample provided the percentage of cells positive for EGFP.
3. Results and discussion
Herein, we present a series of trehalose-oligoethyleneamine linear copolymers that demonstrate the propensity to enable highly efficient transfection of therapeutically-relevant primary progenitor cell types. This work demonstrates highly efficient reagents for primary tissue transfections in the presence of serum, antibiotics, and growth factors, which is currently an area where the most effective transfection reagents fall short or fail. This work also enables further creative design and characterization of the structure-property relationships of carbohydrate-containing copolymer delivery systems investigated in our group [28–30].
To optimize the polymer structure toward achieving the highest pDNA transfection efficiency and lowest toxicity with the primary stem cells (RMSCs) and dermal fibroblasts (HDFn) in the complex growth media, we sought to create several polymer types that varied in the amount of the ethyleneamines within the polymer repeat unit (4, 5, and 6 ethyleneamines). In addition, we sought to discover the role of end group modification and explored PEGylation versus a triphenylamide end-group on the most synthetically-accessible polymer, Tr4. To this end, seven different polymer delivery vehicle analogs were created to examine and optimize the nucleic acid delivery profile to the two progenitor cell types, HDFn and RMSCs. The series of trehalose-oligoethyleneamine copolymers were synthesized via step-growth Cu(I)-catalyzed “click” chemistry to examine how the amine density within a polymer backbone and end group modification influences the efficiency of pDNA delivery to these sensitive and difficult to transfection primary cell types.
3.1. Synthesis and characterization of polymers
The trehalose diazido monomer was synthesized by utilizing the previously-published method [30] with modifications as shown in Fig. 2. We first exchanged both primary hydroxyls with iodo groups by triphenylphosphine-mediated substitution as described in a previously-published procedure [35] and all secondary hydroxyl groups were acetylated with acetic anhydride in pyridine to yield compound 2, which was purified by recrystallization from ethanol. (In the case of incomplete iodine substitution, monoiodotrehalose, along with the peracetylated trehalose as side products, can be removed from the product mixture with column chromatography using a mixture of 5% diethyl ether in dichloromethane as eluent.) Purified compound 2 was then converted to 3 via substitution of the iodo groups with azido groups in a similar manner to previously reported methods [36–37].
Fig. 2.

Synthesis of trehalose monomer 3. Conditions: a) Ph3P, I2, DMF, 80 °C, 3 h; b) Ac2O/Py (1/2), 24 h, RT; c) NaN3, DMF, 80 °C, 24 h.
To create the trehalose copolymer analogs with additional amine units, higher-order oligoamine precursors had to be synthesized, leading to the creation of alkyne-functionalized oligoethyleneamines with 5 and 6 secondary amines, as shown in Fig. 3. Briefly, the primary amines of either tetraethylenepentamine or pentaethylenehexamine were selectively protected with trifluoroacetyl groups, followed by tert-butoxycarbonyl (Boc) protection of the secondary amines, and subsequent deprotection of triflouroacetyl groups in basic conditions, thus liberating the primary amine end groups and obtaining compounds 6a and 6b as we reported before [30]. The additional amines were further introduced via reductive amination, in reaction of 6a and 6b with phthalimidoacetaldehyde 5 [34] and subsequently using sodium triacetoxyborohydride as a mild reducing agent to convert the imine bonds to the amines via a slightly modified procedure from Abdel-Magid et al. [8]. Newly-formed secondary amines were then protected with Boc groups to yield 7a and 7b. The phthalimido groups were subsequently removed by treatment with hydrazine, yielding the terminal diamines 8a and 8b. Alkyne functionalities were further introduced through DCC coupling of the primary amines of 8a and 8b with propiolic acid. In general, synthesis of these protected oligoamines is quite challenging because of multiple synthetic steps, low yields at each step, and tedious purification, as is common with amines.
Fig. 3.
Synthesis of oligoamine monomers 9a and 9b. Conditions: a) TFA, DCM, 12 h, 0 °C→RT [34]; b) 5, DCM, 0 °C→RT, 30 min; c) Na(OAc)3BH, MeOH, 12 h, RT; d) Boc2O, MeOH, 6 h, RT; e) N2H4·H2O, MeOH, 3 h, reflux; f) DCC+propiolic acid, DCM, 0 °C →RT, 12 h.
Polymerization was carried out in a tert-butanol/water mixture (1/1 v/v) in the presence of Cu(I), which was generated in situ via Cu(II) sulfate reduction with sodium ascorbate. This highly-reported “click” procedure [39–41] yielded polymers with protected secondary amines and hydroxyls to avoid chelating of copper. Upon completion of the polymerization reactions, copper(II) was removed via multiple washes of the protected polymers with aqueous NH4OH. Protecting groups were subsequently deprotected with sodium methoxide in methanol and 4 M HCl in dioxane, as shown in Fig. 4. The final materials were dialyzed against water to narrow their respective polydispersity indexes, which are intrinsically larger in step-growth based polymerization procedures. The conditions of polymerization established previously [29] lead to a library of polymers with similar degrees of polymerization. This permits valid comparison within the Tr4-Tr6 series. The weight-average molecular weight and degree of polymerization for each of the purified polymers, along with their respective polydispersity indexes, were determined by gel permeation chromatography analysis and results are summarized in Table 1. Polymer structures were also characterized and confirmed with 1H-NMR and IR (Supporting Information).
Fig. 4.
Synthesis of Tr4-Tr6 polymers. Conditions: a) CuSO4 (0.2 eq), sodium ascorbate (0.4 eq), tBuOH/H2O (1/1), 50 °C, 2 h; b) 0.1 g/mL NaOMe in MeOH, RT, 12 h; c) 4 M HCl in dioxane RT, 4 h.
The role of higher polymer molecular weight and end group functionality was explored by creating longer and also PEGylated analogues of a Tr4 parent polymer (Fig. 1: Tr4128PEG-a, Tr4118PEG-b, Tr4107-c, Tr499-d). The PEG (5 kDa) moiety was selectively incorporated at both ends of the linear trehalose-containing polycation. With this modification, the resulting structure became an A-B-A block copolymer where A is a neutral PEG block and does not contribute to the electrostatic binding with negatively charged phosphates of nucleic acids unlike the B block – polycationic Tr4 (Fig. 1; Tr4128PEG-a, Tr4118PEG-b). We hypothesized that the Tr4 block would be bound to the pDNA is the core of the polyplex, while PEG would be displayed on the outer layer of the particle, therefore partially shielding the positive surface charge and increasing the hydrodynamic radius. To create PEG end-groups, a 5 kDa NHS-activated methoxy-PEG molecules 11a and 11b were functionalized with alkyne end-groups by reaction with an excess of propargylamine, therefore preparing the “click” chemistry-ready conjugation site (Fig. 5). To remove the excess propargylamine, the products were dialyzed against methanol, resulting in a slight loss of material and reducing the yield to 80%. The resulting PEGs were subsequently conjugated with Tr4 using “click” chemistry conditions. It should be noted that the PEGylated Tr4 polymer Tr4128PEG-a contains an amide bond, however, the analogous structure, polymer Tr4118PEG-b, was created with a less stable carbamate bond between the PEG and Tr4 moieties. Different linkages between the PEG and Tr4 blocks were chosen to evaluate whether this slight modification would have an impact on the biological properties of the polymers. It was hypothesized that the carbamate bond in structure Tr4118PEG-b would slowly degrade in physiological conditions, therefore potentially separating the PEG block from the Tr4 block. In addition, as shown in Fig. 6, we synthesized triphenylacetamide-capped Tr4107-c, to analyze and compare the effect of adding a hydrophobic end group to this structure on delivery. Finally, all three end-functionalized Tr4 polymers were compared with parent Tr4, azido-trehalose terminated Tr499-d. GPC analysis of the final polymers (Tr4128PEG-a, Tr4118PEG-b, Tr4107-c, Tr499-d) revealed the molecular weight of the polymers and the loading of the PEG functionality per polymer molecule was calculated (Table 2). Both PEGylated Tr4 polymers, Tr4128PEG-a and Tr4118PEG-b, contained about 10wt% of the incorporated PEG functionality, and the degree of polymerization of the Tr4 block was slightly over 100. It should be mentioned that the relatively narrow polydispersities are likely a result of the polymer purification step, which involves exhaustive dialysis to remove unreacted monomers, oligos, and catalyst. GPC analysis also proved the absence of methoxy-PEG alkyne, showing just one peak on a chromatogram (corresponding to Tr4128PEG-a and Tr4118PEG-b respectively), thus indicating that all residual methoxy-PEG alkyne was removed from the reaction mixture during dialysis.
Fig. 5.
Synthesis of methoxy-PEGs 12a and 12b. MWPEG = 5 kDa.
Fig. 6.

Synthesis of triphenylacetamide 14.
3.2. Polymer-pDNA binding, particle size, and zeta potential
Gel electrophoresis shift assays revealed that all the polymers were able to inhibit pDNA migration in the electrophoretic field at a very low N/P ratio of 2 (Supporting Information). Dynamic light scattering (DLS) data indicates that polyplex sizes (hydrodynamic diameter) for the Tr4-Tr6 series (Tr433, Tr530, Tr632) in water are in a range of 60–80 nm with the zeta potential increasing in correlation with polyplex N/P ratio increase (Supporting Information). After diluting the Tr4-Tr6 polyplex solutions in medium supplemented with 10% FBS, a slight increase in particle size over time was noticed, indicating that the polyplexes could be aggregating or swelling in the presence of serum proteins. Interestingly, for higher N/P ratios, increased particle stability in the presence of serum proteins is observed based on only the small particle size increase after 4 h in serum solution. It is also worth mentioning that at N/P ratios above 20, we started to notice multimodal peak distributions in the DLS analysis of the polyplexes, which suggests that at these high N/P ratios the polyplex solutions are not monodisperse and free trehalose polymer is likely present. Based on these observations, N/P ratios of 7, 10, and 20 were chosen for further cell culture experiments. In case of longer and end-functionalized Tr4 polymers (Fig. 1.; Tr4128PEG-a, Tr4118PEG-b, Tr4107-c, Tr499-d) particle size has a similar trend as in Tr4-Tr6 series, it was found to be decreasing with the increase of the N/P ratio and finally stabilize around 400 nm. Surprisingly, the zeta potential in both PEGylated polyplyplex types (Tr4128PEG-a and Tr4118PEG-b) was positive, indicating that PEG moiety does not completely shield the polyplex surface charge as we initially hypothesized. Detailed DLS data can be found in the Supporting Information.
3.3. Cellular delivery and toxicity studies
Transfection efficiency and cytotoxicity were measured in two therapeutically-relevant cell types — implantable cells used in regenerative therapies, human dermal fibroblasts (HDFn), and progenitor-type cells, rat mesenchymal stem cells (RMSC). Transfection efficiency was determined with luciferase reporter gene expression assays and via enhanced green fluorescent protein (EGFP) detection using flow cytometry. The primary cell lines were transfected with reporter gene plasmids formulated with the corresponding polymers, at N/P ratios of 7, 10, and 20. At 48 h post-transfection, luciferase activity was measured in cell lysates and reported as relative light units (RLU). The results were compared with commercially available transfection reagent positive controls: Lipofectamine™ 2000, jetPEI™, and Glycofect™ Transfection Reagent. In HDFn cells (Fig. 7), polyplexes formed with Tr433 and Tr530 showed similar transfection efficiency at N/P 20 and 10, but, at N/P 7, Tr530 yielded higher transfection efficiency than Tr433. Interestingly, polyplexes with Tr632 generally showed lower transfection efficiency than either with Tr433 or Tr530 and we do not quite understand this effect. At N/P 20 all three polymers showed higher transgene expression than jetPEI™, though jetPEI™ transfection was carried out at a lower N/P ratio (at N/P 5) per manufacturer’s recommendations. As shown, Glycofect™ yields very high total gene expression; however, the protocol for use of Glycofect™ mandates transfection to be carried out in serum-free conditions for the first four hours. In RMSCs (Fig. 7) we saw a similar trend within the Tr4-Tr6 polymer series and we were encouraged to again find extremely high efficacy with Tr433 polyplexes, comparable with jet-PEI™ and Glycofect™, indicating that this polymer may be an effective tool for creating cellular therapies. In general, in both primary cell types, Tr433 (at N/P 20) mediated the highest transgene expression in the complex media, followed by Tr530; Tr632 showed the poorest transfection efficiency. This result could be related to, in part, to the decreased molar amount of polymer used (to formulate the polyplexes as specific N/P ratios), which occurs as the degree of secondary amine stoichiometry increases in the polymer repeat unit. Our previous studies indicate that increasing the density of protonatable secondary amines (from 1–4 per repeat unit) within the polymer repeat unit in a series of glycopolymers we termed poly(glycoamidoamine)s, [42–46] resulted in a significant increase in transfection efficiency, without any significant reduction in cellular viability. However, with a further increase in secondary amines (5 and 6), gene expression plateaued and cytotoxicity increased particularly in linear models.[46] Herein, the transfection efficiency within the current trehalose copolymer series seems to follow an opposite trend, transfection efficiency actually decreases as the number of secondary amines within the polymer repeat unit increases, but still retaining low cytotoxicity. Results of examining the longer PEGylated series of Tr4, (Tr4128PEG-a and Tr4118PEG-b) along with the non-PEGylated structures Tr4107-c (with the triphenylacetamide end-groups) and Tr499-d (with azido-trehalose end-groups), revealed that in the HDFn cell line, transfection (gene expression) was noticed only at N/P ratio of 20 for Tr4128PEG-a, Tr4118PEG-b, and, surprisingly, also for Tr4107-c. However, transfection was not observed for the same polymers at lower N/P ratios of 7 and 10. A relatively high fraction of cell survival was found for these systems, particularly with HDFn cells and slightly lower viability profiles were observed with RMSCs (but higher transfection values were found in this cell type). Interestingly, the non-PEGylated polymer with the triphenyl end groups, Tr4107-c, offered the lowest transfection profile at all N/P ratios in both cell types and we currently do not understand this trend. Diazido-trehalose terminated Tr499-d, however, performed better than the controls at all tested N/P ratios and and generally performed better than the shorter analog, Tr433, at N/P ratios of 7 and 10 in HDFn cells. In RMSCs, all three tested N/P ratios (7, 10, and 20) for the PEGylated (Tr4128PEG-a and Tr4118PEG-b) and the non-PEGylated analogs, Tr4107-c and Tr499-d, exhibited fairly good transfection efficiency; however a slightly higher toxicity (cell survival 80–60%) was observed for these structures (Fig. 8). In general, these longer polymers had a lower overall transfection efficiency profile, and this result could be due to a tighter pDNA complex formation with the longer polymers, potentially hindering the release of pDNA.
Fig. 7.
Luciferase gene expression observed with polyplexes formed with pDNA and corresponding polymers at N/P 7, 10, and 20. Positive controls (Lipofectamine™ 2000, jetPEI™ at N/P 5, and Glycofect™) were formulated with pDNA based upon their recommended protocols. The data are reported as the mean ± standard of deviation of three replicates and normalized to the cell only control.
Fig. 8.
MTT assay. Cell viability is reported as a fraction of cell survival. Polyplexes were formed with pDNA and corresponding polymers at N/P 7, 10, and 20. Positive controls (Lipofectamine™ 2000, jetPEI™ at N/P 5, and Glycofect™) were formulated with pDNA based upon their recommended protocols. The data are reported as the mean ± standard of deviation of three replicates.
To gain information about cell viability under the luciferase transfection conditions, an MTT assay was performed. The assay indicates similar toxicity profiles within the Tr4-Tr6 polymer series. A general trend of toxicity increase with N/P ratio was noticed with all polymer vehicles examined in this study. In particular, at an N/P ratio of 20, increased toxicity was observed for the entire polymer series, in both cell lines (below 60% survival for Tr433 and Tr530) (Fig. 8). Nevertheless, at N/P 7 all the polymers were relatively non-toxic to the tested cell lines with the cellular survival at 80% and above. Finding effective polymers that deliver nucleic acids into cells in a benign manner is a crucial property, particularly for these sensitive cell types that are routinely carried forward for therapeutic and regenerative medicine applications.
To quantify transfection efficiency on a per-cell basis, cells were transfected with a plasmid encoding enhanced green fluorescent protein (EGFP) under the same conditions used in the cellular assays described above. After 48 h, flow cytometry analysis was performed and reported as the percentage of cells positive for EGFP. In general, the percentage of EGFP positive cells was higher with Tr433 (Fig. 9, 16% for HDFn and 20% for RMSC) than Tr530 (Fig. 9, 5% for HDFn and 10% for RMSC) and Tr632 (Fig. 9, 3% for HDFn and 8% for RMSC). These data revealed a trend of decreasing cellular internalization as the number of secondary amines within the polymer repeat unit was increased. Tr433 also revealed a higher number of EGFP positive cells when compared to the positive controls. In fact, the only standard that elicited a significant number of cells positive for EGFP gene expression in both cell lines was Glycofect™, (15% in HDFn and 7% in RMSC). Increasing the molecular weight of Tr4 from DP 33 (Tr433) to DP 99 (Tr499-d) resulted in a decrease in the number of EGFP positive cells with the HDFn cell line; decreased EGFP expression was also noticed with both PEGylated polymers. Interestingly, a slight increase in EGFP expression was noticed with triphenylacetamide terminated Tr4107-c. In rat mesenchymal stem cells, however, a significant increase in the number of EGFP positive cells was found in flow cytometry experiments with all four longer and end-functionalized Tr4 polymers when compared to the shorter Tr4-Tr6 series (Fig. 9). Amazingly, about 40% of RMSCs were positive for EGFP expression with the longer Tr4 derivatives. Moreover, polymer Tr499-d with azidotrehalose end-groups was slightly less efficient for EGFP expression than the other three Tr4 structures end-capped with either PEG or the triphenylacetamide groups. While we do not fully understand this effect, it could be due to the difference in the cell surface, morphology, and physiological behavior of these two cell types. It appears that different molecular weights and PEGylation of the trehalose polymer series have a large influence on the ability to deliver pDNA to stem cells.
Fig. 9.
EGFP expression observed via flow cytometry with polyplexes formed with pDNA and corresponding polymers at N/P 20. Positive controls (Lipofectamine™ 2000, jetPEI™ at N/P 5, and Glycofect™) were formulated with pDNA based upon their recommended protocols. The data is shown is % of EGFP positive cells.
4. Conclusions
For genetic engineering of primary cells for immunological and genetic therapies, cells must be reliably transfected ex vivo under optimal culture conditions, such as in the presence of serum proteins and antibiotics, to insure robust cell health and minimal contamination. Ultimately, transfection solutions need to be developed for gene transfer to sensitive cells in robust cell culture environments; such materials would further enable regenerative medical procedures based on ex vivo engineered cells. In an attempt to achieve this goal, trehalose-oligoethyleneamine “click” copolymers were designed and synthesized to demonstrate the high binding affinity and delivery efficiency associated with an incorporated short polyethyleneimine (PEI) units coupled with the biocompatibility and serum stability provided by trehalose unit. We have demonstrated that higher order trehalose-based polymers, with the increased number of secondary amines (4–6), show promise in transfecting primary neonatal human dermal fibroblasts and rat mesenchymal stem cells in conditions requiring the presence of serum, antibiotics, and growth factors. Tr4 showed the most promising results for delivering pDNA and mitigating transgene expression of reporters in these primary cells. In vitro characterizations of PEGylated and unmodified Tr4 and pDNA complexes provided only limited conclusions about the effect of PEGylation on in vivo transfection. There is a possibility that PEGylated and non-PEGylated trehalose-polycations can have different cellular internalization and intracellular trafficking mechanisms, which could lead to the differences in efficacy noticed herein. The results of in vitro characterization described here suggest that both modified and unmodified Tr4 polymers have a great promise for delivery of nucleic acids into stem-cell based and regenerative therapies. In addition, PEG-functionality can be further modified to yield polyplexes, with incorporated targeting ligands, MRI agents, or radiotracers toward the development of multifunctional vehicles for successful gene delivery treatment and diagnostic applications. Indeed, the future of regenerative therapies is reliant upon multi-functional polymers able to successfully interact with progenitor cells in a benign manner.
Supplementary Material
Acknowledgments
We acknowledge funding of this project provided by the Camille and Henry Dreyfus Foundation. We also would like to thank Dr. Mehdi Ashraf-Khorassani for performing LC-ESI-MS for small molecules, as well as Dr. Larry Sallans and Dr. Stephen Macha, both of University of Cincinnati Mass Spectrometry Facility, for valuable discussions in MS analysis of oligoamines. K.K. would like to thank Antons Sizovs for helpful discussions.
Appendix. Supporting Information
Supplementary data associated with this article can be found, in the online version, at doi:
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science. 2001;291(5507):1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 3.Schaffer DV, Koerber JT, Lim K-i. Molecular engineering of viral gene delivery vehicles. Annu Rev Biomed Eng. 2008;10(1):169–94. doi: 10.1146/annurev.bioeng.10.061807.160514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Breckenridge C, Kaur B, Chiocca EA. Pharmacologic and chemical adjuvants in tumor virotherapy. Chem Rev. 2009;109(7):3125–40. doi: 10.1021/cr900048k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovtun A, Heumann R, Epple M. Calcium phosphate nanoparticles for the transfection of cells. Biomed Mater Eng. 2009;19(2/3):241–7. doi: 10.3233/BME-2009-0586. [DOI] [PubMed] [Google Scholar]
- 6.Epple M, Ganesan K, Heumann R, Klesing J, Kovtun A, Neumann S, et al. Application of calcium phosphate nanoparticles in biomedicine. J Mater Chem. 2010;20(1):18–23. [Google Scholar]
- 7.Bhattacharya S, Bajaj A. Advances in gene delivery through molecular design of cationic lipids. Chem Commun. 2009;31:4632–56. doi: 10.1039/b900666b. [DOI] [PubMed] [Google Scholar]
- 8.Rao N, Gopal V. Cell biological and biophysical aspects of lipid-mediated gene delivery. Biosci Rep. 2006;26(4):301–24. doi: 10.1007/s10540-006-9026-8. [DOI] [PubMed] [Google Scholar]
- 9.Tros de Ilarduya C, Sun Y, Düzgünes N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010;40(3):159–70. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz Mellet C, Benito J, García Fernández J. Preorganized, macromolecular, gene-delivery systems. Chem Eur J. 2010;16(23):6728–42. doi: 10.1002/chem.201000076. [DOI] [PubMed] [Google Scholar]
- 11.Parekh HS. The advance of dendrimers - a versatile targeting platform for gene/drug delivery. Curr Pharm Des. 2007;13(27):2837–50. doi: 10.2174/138161207781757024. [DOI] [PubMed] [Google Scholar]
- 12.Pietersz GA, Choon-Kit T, Apostolopoulos V. Structure and design of polycationic carriers for gene delivery. Mini Rev Med Chem. 2006;6(12):1285–98. doi: 10.2174/138955706778992987. [DOI] [PubMed] [Google Scholar]
- 13.Mok H, Park TG. Functional polymers for targeted delivery of nucleic acid drugs. Macromol Biosci. 2009;9(8):731–43. doi: 10.1002/mabi.200900044. [DOI] [PubMed] [Google Scholar]
- 14.Reineke TM. Poly(glycoamidoamine)s: cationic glycopolymers for DNA delivery. J Polym Sci, Part A: Polym Chem. 2006;44(24):6895–908. [Google Scholar]
- 15.Davis ME, Pun SH, Bellocq NC, Reineke TM, Popielarski SR, Mishra S, et al. Self-assembling nucleic acid delivery vehicles via linear, water-soluble, cyclodextrin-containing polymers. Curr Med Chem. 2004;11(2):179–97. doi: 10.2174/0929867043456179. [DOI] [PubMed] [Google Scholar]
- 16.Sizovs A, McLendon PM, Srinivasachari S, Reineke TM. Carbohydrate polymers for nonviral nucleic acid delivery. Top Curr Chem. 2010:1–60. doi: 10.1007/128_2010_68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–70. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–9. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 19.Lipscomb MF, Masten BJ. Dendritic cells: immune regulators in health and disease. Physiol Rev. 2002;82(1):97–130. doi: 10.1152/physrev.00023.2001. [DOI] [PubMed] [Google Scholar]
- 20.Syme R, Glück S. Generation of dendritic cells: role of cytokines and potential clinical applications. Transfus Apher Sci. 2001;24(2):117–24. doi: 10.1016/s1473-0502(01)00005-2. [DOI] [PubMed] [Google Scholar]
- 21.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annu Rev Immunol. 2000;18(1):245. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 22.McNeish J. Embryonic stem cells in drug discovery. Nat Rev Drug Discovery. 2004;3(1):70–80. doi: 10.1038/nrd1281. [DOI] [PubMed] [Google Scholar]
- 23.Charge SBP, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–38. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 24.Grewal SIS, Moazed D. Heterochromatin and epigenetic control of gene expression. Science. 2003;301(5634):798–802. doi: 10.1126/science.1086887. [DOI] [PubMed] [Google Scholar]
- 25.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9(6):702. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 26.Lechler R, Ng WF, Steinman RM. Dendritic cells in transplantation - friend or foe? Immunity. 2001;14(4):357–68. doi: 10.1016/s1074-7613(01)00116-9. [DOI] [PubMed] [Google Scholar]
- 27.Wernig M, Meissner A, Foreman R, Brambrink T, Manching K, Hochedlinger K, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–24. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 28.Prevette LE, Lynch ML, Kizjakina K, Reineke TM. Correlation of amine number and pDNA binding mechanism for trehalose-based polycations. Langmuir. 2008;24(15):8090–101. doi: 10.1021/la800120q. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasachari S, Liu Y, Prevette LE, Reineke TM. Effects of trehalose click polymer length on pDNA complex stability and delivery efficacy. Biomaterials. 2007;28(18):2885–98. doi: 10.1016/j.biomaterials.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasachari S, Liu Y, Zhang G, Prevette L, Reineke TM. Trehalose click polymers inhibit nanoparticle aggregation and promote pDNA delivery in serum. J Am Chem Soc. 2006;128(25):8176–84. doi: 10.1021/ja0585580. [DOI] [PubMed] [Google Scholar]
- 31.Kojima H, Mukai Y, Yoshikawa M, Kamei K, Yoshikawa T, Morita M, et al. Simple PEG conjugation of SPIO via an Au–S bond improves its tumor targeting potency as a novel MR tumor imaging agent. Bioconjugate Chem. 2010;21(6):1026–31. doi: 10.1021/bc900487p. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y-Y, Liu C, Hong M-H, Zhu S-J, Pei Y-Y. Tumor cell targeting of transferrin–PEG–TNF-α conjugate via a receptor-mediated delivery system:3 design, synthesis, and biological evaluation. Bioconjugate Chem. 2006;18(1):41–9. doi: 10.1021/bc060135f. [DOI] [PubMed] [Google Scholar]
- 33.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Molecular Pharmaceutics. 2009;6(3):659–68. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 34.Veale EB, O’Brien JE, McCabe T, Gunnlaugsson T. The synthesis, N-alkylation and epimerisation study of a phthaloyl derived thiazolidine. Tetrahedron. 2008;64(28):6794–800. [Google Scholar]
- 35.García Fernández J, Ortiz Mellet C, Jiménez Blanco J, Fuentes Mota J, Gadelle A, Coste-Sarguet A, et al. Isothiocyanates and cyclic thiocarbamates of [alpha], [alpha]′-trehalose, sucrose, and cyclomaltooligosaccharides. Carbohydr Res. 1995;268(1):57–71. doi: 10.1016/0008-6215(94)00312-4. [DOI] [PubMed] [Google Scholar]
- 36.Menger FM, Mbadugha BNA. Gemini surfactants with a disaccharide spacer. J Am Chem Soc. 2001;123(5):875–85. doi: 10.1021/ja0033178. [DOI] [PubMed] [Google Scholar]
- 37.Reineke TM, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 1. Carbohydrate size and its distance from charge centers. Bioconjugate Chem. 2002;14(1):247–54. doi: 10.1021/bc025592k. [DOI] [PubMed] [Google Scholar]
- 38.Abdel-Magid AF, Carson KG, Harris BD, Maryanoff CA, Shah RD. Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride. Studies on direct and indirect reductive amination procedures. J Org Chem. 1996;61(11):3849–62. doi: 10.1021/jo960057x. [DOI] [PubMed] [Google Scholar]
- 39.Qin A, Lam JWY, Tang BZ. Click polymerization: progresses, challenges, and opportunities. Macromolecules. 2010;43(21):8693–702. [Google Scholar]
- 40.Golas PL, Matyjaszewski K. Marrying click chemistry with polymerization: expanding the scope of polymeric materials. Chemical Society Reviews. 2010;39(4):1338–54. doi: 10.1039/b901978m. [DOI] [PubMed] [Google Scholar]
- 41.Finn MG, Fokin VV. Click chemistry: function follows form. Chemical Society Reviews. 2010;39(4):1231–2. doi: 10.1039/c003740k. [DOI] [PubMed] [Google Scholar]
- 42.Liu YM, Reineke TM. Poly(glycoamidoamine)s for gene delivery. Structural effects on cellular internalization, buffering capacity, and gene expression. Bioconjugate Chem. 2007;18(1):19–30. doi: 10.1021/bc060029d. [DOI] [PubMed] [Google Scholar]
- 43.Liu YM, Wenning L, Lynch M, Reineke TM. Gene delivery with novel poly(1-tartaramidoamine)s. ACS Symp Ser. 2006:217–27. [Google Scholar]
- 44.Liu YM, Reineke TM. Hydroxyl stereochemistry and amine number within poly(glycoamidoamine)s affect intracellular DNA delivery. J Am Chem Soc. 2005;127(9):3004–15. doi: 10.1021/ja0436446. [DOI] [PubMed] [Google Scholar]
- 45.Liu YM, Wenning L, Lynch M, Reineke TM. New poly(D-glucaramidoamine)s induce DNA nanoparticle formation and efficient gene delivery into mammalian cells. J Am Chem Soc. 2004;126(24):7422–3. doi: 10.1021/ja049831l. [DOI] [PubMed] [Google Scholar]
- 46.Lee CC, Liu Y, Reineke TM. General structure-activity relationship for poly(glycoamidoamine)s: The effect of amine density on cytotoxicity and DNA delivery efficiency. Bioconjugate Chem. 2008;19(2):428–40. doi: 10.1021/bc7001659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.