Abstract
BACKGROUND
Down syndrome (DS) and Fetal Alcohol Syndrome (FAS) are two leading causes of birth defects with phenotypes ranging from craniofacial abnormalities to cognitive impairment. Despite different origins, we report that in addition to sharing many phenotypes, DS and FAS may have common underlying mechanisms of development.
METHODS
Literature was surveyed for DS and FAS as well as mouse models. Gene expression and apoptosis were compared in embryonic mouse models of DS and FAS by qPCR, immunohistochemical and immunoflurorescence analyses. The craniometry was examined using MicroCT at postnatal day 21.
RESULTS
A literature survey revealed over 20 comparable craniofacial and structural deficits in both humans with DS and FAS and corresponding mouse models. Similar phenotypes were experimentally found in pre- and postnatal craniofacial and neurological tissues of DS and FAS mice. Dysregulation of two genes, Dyrk1a and Rcan1, key to craniofacial and neurological precursors of DS, was shared in craniofacial precursors of DS and FAS embryos. Increased cleaved caspase 3 expression was also discovered in comparable regions of the craniofacial and brain precursors of DS and FAS embryos. Further mechanistic studies suggested overexpression of trisomic Ttc3 in DS embyros may influence nuclear pAkt localization and cell survival.
CONCLUSIONS
This first and initial study indicates that DS and FAS share common dysmorphologies in humans and animal models. This work also suggests common mechanisms at cellular and molecular levels that are disrupted by trisomy or alcohol consumption during pregnancy and lead to craniofacial and neurological phenotypes associated with DS or FAS.
Keywords: Down syndrome, fetal alcohol syndrome, craniofacial, apoptosis, development, mouse models
INTRODUCTION
Trisomy 21 (Ts21) affects ~1 out of 700 live births and individuals with Down syndrome (DS) present with neurological, craniofacial, skeletal, cardiac and immunological impairments (Epstein, 2001; Van Cleve and Cohen, 2006). The presentation and severity of these traits vary widely and affect a large number of tissues in individuals with DS (Wiseman et al., 2009). Fetal alcohol syndrome (FAS) with characteristic facial, neural, and organ deficits is caused by the abusive consumption of alcohol by an expectant mother and affects ~1 out of 1000 live births (Abel and Hannigan, 1995). Incidence of fetal alcohol spectrum disorders (FASD) with variable combination of facial, organ and brain teratology was estimated to be 10 times that of FAS (Hoyme et al., 2005; May et al., 2009; Sokol et al., 2003). Because of the wide range and variation of phenotypes, it is difficult to diagnose FAS early in life (Schaefer and Deere, 2011). Although the upstream initiation of genetically caused DS or environmentally induced FAS are diverse, many downstream phenotypes of the two diseases appear to converge. Individuals with FAS and DS present with similar phenotypes including microcephaly; smaller skull base breadth and canthal distances; structural alterations in cerebellum and hippocampus; and short stature and reduced bone development (Gressens et al., 1992; Riley et al., 2011) (Table 1).
Table 1.
Phenotypic Similarities between Down Syndrome (DS) and Fetal Alcohol Syndrome (FAS)
| Human |
Mouse |
||||
|---|---|---|---|---|---|
| DSa | FAS | DS | FAS | ||
| Craniofacial | Skull Base Breadth | Smaller1,2 | Smaller3,4 | Smaller5 | Smaller6 |
| anthropometry | Upper and lower facial width | Smaller1,2 | Smaller3,4 | Smaller5 | Smaller6 |
| Canthal distances | Smaller1 | Smaller3,4 | Smaller5 | Smaller6 | |
| Minimal frontal breadth | Smaller1 | Smaller3,4 | Larger5 | Smaller6 | |
| Papebral fishers | Smaller2 | Smaller3,4 | N/Ab | Smaller6 | |
| Nasal | Smaller1,2,7 | Smaller3,4 | Smaller5 | Smaller6 | |
| Philtrum length | Smaller2,7 | Smaller3,4 | N/A | Larger6 | |
| Facial height | Smaller1,2 | Smaller4 | N/A | Smaller6 | |
| Facial depth | Smaller1 | Smaller3,4 | N/A | Smaller6 | |
| Maxillary and mandibular arch | Smaller1 | Smaller3,4 | Smaller5 | Smaller | |
| Microcephaly | Present8 | Present9 | Present10 | Present11 | |
| Brain | Cerebellum | Reduced12 | Reduced13 | Reduced, Purkinje cell deficit14,15 | Purkinje cell deficit16,17 |
| Hippocampus | Reduced12 | Reduced13 | Dentate gyrus18 | Dentate gyrus19 | |
| Cortex | Neuronal impairment20 | Neuronal impairment21,22 | Neuronal impairment23 | Neuronal impairment24 | |
| Learning | Impaired12 | Impaired25,26 | Impaired27 | Impaired28 | |
| Memory | Impaired12 | Impaired29 | Impaired27 | Impaired28 | |
| Other structures | Heart | Septal defects30 | Septal defects31 | Septal defects32 | N/A |
| Bone Development | Reduced33 | Reduced34 | Reduced35 | N/A | |
| Stature | Shortened36 | Shortened37 | Shortened35 | N/A | |
| Limbs | Malformed38 | N/A | N/A | Malformed39 | |
| Hearing | Loss40 | Loss41 | Loss42 | N/A | |
| Kindney | Dysplasia43 | Dysplasia44 | N/A | N/A | |
| GI Tract | Pseudoobstruction45 | Pseudoobstruction44 | N/A | N/A | |
References containing phenotypic information
Weijer-man et al., 2010
Information not available.
Mouse models have been effectively used to better understand the deficits associated with DS and FAS. Ts65Dn mice, widely used as a model of DS, contain about half of the genes in three copies that are orthologous to those found on human chromosome 21 (Hsa21) and exhibit neurological, craniofacial, skeletal and cardiac phenotypes associated with DS (Moore and Roper, 2007; Patterson, 2009; Reeves et al., 1995). Craniofacial phenotypes that are similar to humans with DS include reduced mandibular and maxillary regions, rostrocaudal dimensions of the neurocranium, and overall reduction and flattening of the face (Richtsmeier et al., 2000). For FAS, different inbred strains of mice have been shown to vary in both alcohol consumption and the resultant phenotype. C57BL/6 (B6) mice consume ethanol with high avidity and replicate craniofacial features in humans with FAS (Anthony et al., 2010; Khisti et al., 2006; Moore et al., 2007; Ogawa et al., 2005; Sulik, 2005).
Because trisomy of a few genes likely initiates the cellular and morphological deficits leading to DS phenotypes (Lana-Elola et al., 2011), these genes may be involved in critical pathways that influence craniofacial and neurological phenotypes important in DS and other syndromes with similar phenotypes such as FAS. It has been hypothesized that Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Dyrk1a) and Regulator of calcineurin 1 (Rcan1—also called Dscr1), two genes found on Hsa21 and in three copies in Ts65Dn mice, contribute to early craniofacial deficits in DS (Arron et al., 2006). Dyrk1a is a serine-threonine kinase that regulates many downstream proteins and transcription factors including cyclic AMP response element-binding protein (Creb), forkhead in rhabdomyosarcoma (Fkhr), and nuclear factor of activated T cells (Nfat) (Branchi et al., 2004; Arron et al., 2006). Dyrk1a dysregulation affects many functions including cell proliferation, differentiation, and survival (Yabut et al., 2010). Rcan1 inhibits calcineurin, a calcium activated serine/threonine protein phosphatase (Fuentes et al., 2000). The inhibition of calcineurin affects the activity of transcription factors and other proteins. Rcan1 has also been known to play roles in cardiac and skeletal muscle hypertrophy as well as immunodeficiency (Harris et al., 2005; Ram and Chinen, 2011). Overexpression of Dyrk1a and Rcan1 have been linked to the dysregulation of the Nfat transcription factor, and it has been hypothesized that the altered activity of these genes causes significant changes in cell proliferation, differentiation and survival and results in skeletal, neurological and motor function deficits (Asagiri et al., 2005; Arron et al., 2006; Lee et al., 2009; Yabut et al., 2010). We have recently shown that expression of Dyrk1a and Rcan1 are altered in the mandibular precursor of Ts1Rhr mice, another DS mouse model with only 33 trisomic genes that are homologous to those found on Hsa21 (Deitz and Roper, 2011).
Another trisomic gene, Tetratricopeptide repeat protein 3 (Ttc3), has been implicated in neuronal phenotypes associated with DS (Berto et al., 2007). Ttc3 targets v-akt murine thymoma viral oncogene homolog 1 (Akt) for ubiquitination when it is activated (Suizu et al., 2009) and in turn, Akt influences cell proliferation, migration, survival, and differentiation through CREB and NF-jB transcription factors (Peng et al., 2003; Enomoto et al., 2005; Wang and Brattain, 2006; Suizu et al., 2009). A genomic analysis of FAS has shown that Akt is downregulated (Hard et al., 2005) and the decrease in the inactive form of Akt in FAS has been linked to an increase in Phosphatase and tensin homolog (Pten), a protein responsible for the inhibition of the PI3K–Akt cascade (Xu et al., 2003; Green et al., 2007). Together, these results suggest that Akt deficiencies may be linked to cell survival in the development of DS and FAS phenotypes.
Apoptosis may be a common cellular mechanism important in DS and FAS phenotypes. Although actual mechanisms are mostly unknown, an increase in apoptosis has been observed in the mandibular arch of embryos treated with ethanol (Wang and Bieberich, 2010). Investigations at the cellular level have shown that in developing embryos of several different FAS models, ethanol causes high levels of apoptosis in neural crest (NC) (Garic-Stankovic et al., 2005; Wang and Bieberich, 2010; Flentke et al., 2011). Other studies in mouse models suggest that an epigenetic DNA methylation alteration prevails in proapoptotic genes in FAS (Liu et al., 2009). Apoptosis has also been studied in DS, with differing conclusions depending on the study. Increased apoptosis has been observed in immunological tissues of adult mouse models of DS and individuals with DS (Paz-Miguel et al., 1999; Elsayed and Elsayed, 2009), in brains of some individuals with DS (Guidi et al., 2008) as well as fibroblasts from Ts65Dn mice (Contestabile et al., 2009). During development, increased apoptosis has been observed in the hippocampus of individuals with DS, but no differences in apoptotic cells were observed in neuronal precursors of Ts65Dn mice (Chakrabarti et al., 2007; Guidi et al., 2008).
After initiation of DS and FAS by extra chromosomal material or alcohol consumption, respectively, the mechanisms and causal pathways that lead to craniofacial, neurological, cardiac and other phenotypes have largely not been identified. Because of craniofacial phenotypes we have observed in mouse models of DS (Roper et al., 2009) and FAS (Anthony et al., 2010), we hypothesize that there may be common genetic, cellular and molecular mechanisms that are disturbed by trisomy or alcohol exposure during embryogenesis. In this study, we compared common phenotypes from DS and FAS as well as investigate dysregulation in gene expression and apoptosis as general mechanisms that may lead to common phenotypes observed in DS and FAS.
MATERIALS AND METHODS
Mouse Models of DS and FAS
To generate DS model embryos, female B6EiC3Sn a/A-Ts(1716)65Dn (Ts65Dn) and B6.129S4-Gt(ROSA)26Sortm1-Sor/J (B6.R26R) and male C3H/HeJ (C3H) mice from the Jackson Laboratory (Bar Harbor, ME) were used. The B6(R26R)C3F1 mice were bred by crossing B6.R26R females with C3H males. The P21 Ts65Dn mice were generated from Ts65Dn x B6(R26R)C3F1 matings. The Ts65Dn embryos were generated by crossing Ts65Dn females with Wnt1-LacZ males. Nine or 10 days after the observed copulatory plug in the Ts65Dn female, embryonic day 9.5 and 10 (E9.5 and E10) embryos were removed and genotyped by polymerase chain reaction (PCR) and fluorescent in situ hybridization (FISH) on the yolk sac tissue (Roper et al., 2009; Reinholdt et al., 2011).
To analyze gene expression at a sensitive stage of craniofacial development, a whole embryo culture model under strict alcohol and staging control conditions was used. The whole embryo culture model was adopted from our previous studies (Ogawa et al., 2005). In brief, 3–6 somite embryos (at E8.25) in the placenta cone were harvested from C57BL/6 (B6) dams (Harlan, Inc., Indianapolis, IN) in phosphate buffered saline (PBS) containing 4% fetal bovine serum (FBS), and placed into a 20 mL culture bottle containing 70% heat-activated rat serum and 30% PB1 buffer with 20% O2, 5% CO2, and 75% N2. Embryos were then cultured in a medium containing 6 µL/mL 95% ethanol solution for 44 hours (Chen et al., 2011; Ogawa et al., 2005).
For craniofacial craniometry comparison of FAS and DS, we analyzed adolescent mice at postnatal day (P)21. A previously established liquid diet model of alcohol treatment was used for the FAS group (Anthony et al., 2010). In essence, three treatment groups of B6 dams were used: prepregnancy and pregnancy alcohol exposure (alc-alc), pre-pregnancy alcohol exposure and pregnancy isolcaloric pair fed (alc-pf), and chow. All alcohol treatments used alcohol concentrations of 4.8% (v/v) in liquid diet (Purina Micro-stabilized Alcohol Diet [PMI]; Purina Mills Inc., Richmond, IN) as per supplier’s instructions, with 5% (w/v) sucrose. Pf diets used the PMI diet mixture with equal caloric maltose dextran (isocaloric diets) as a substitute for alcohol calories, and the volume of liquid diet intake was restricted to that of a matched dam from the alcohol group throughout all treatments. All liquid diets were formulated at 1 cal/ mL and were administered using a 35-mL drinking tube (Dyets Inc, NY). Prepregnancy alcohol started with 2.4% (v/v) liquid diet for 2 days and then 14 days at 4.8% (v/v) alcohol. Animals were then placed on chow and water diets during the alcohol withdrawal period of 4 days and during the subsequent mating period. At E7, pregnant dams were placed on 4.8% (v/v) alcohol (alc-alc), and alc-pf dams were given volumes of isocaloric diet matched to the respective alcohol-consuming dam through the end of E16. All alc-alc and alc-pf dams were weighed, and total volume (in mL) of a 4.8% (v/v) liquid diet was recorded at 10:00 AM daily during both prepregnancy and pregnancy periods. Drinking measures (in g/kg) were obtained by converting the volume of 4.8% (v/v) alcohol liquid diet, consumed each day, to grams of alcohol consumed, and the resultant value divided by the dam’s body weight (kg). Chow treatment was ad libitum chow and water diet through prepregnancy and pregnancy periods. All alc-alc, alc-pf, and chow groups were fostered by surrogate mothers under chow feeding conditions to avoid potential nursing negligence and maternal factors from treated dams. Birth was designated as P0. To align the potential delayed birth of the alc-alc group, the fertilization age was used with reference to postnatal birth age (e.g. E40 as P21). A separate set of alcohol treated dams (N = 7) was used to collect tail-vein blood (serum) for blood alcohol concentration (BAC), to assure that the alcohol concentration was at the appropriate level for craniofacial malformations to be manifest (Anthony et al., 2010). Samples were collected during prepregnancy on four independent days over an 8 day period. Blood samples were collected on each collection day at 2 and 6 h into the dark cycle (12 and 4 pm). Blood samples (20 µL) were collected in heparinized tubes and plasma was collected through centrifugation and stored at −80°C prior to analysis with Gas Chromatograph (GC, Agilent Technologies; model 6890). Each sample was analyzed in duplicate and reported as mean ± SEM. The BAC achieved 100−200 mg/dL.
MicroCT of Ts65Dn and Alcohol Treated Mice
All microCT images on craniofacial structures from DS and FAS model mice were acquired with 50 kVp and isotropic 46 mm resolution using EVS-R9 system (GE Healthcare, Waukesha, WI) at the Indiana University School of Medicine. The microCT was performed at P21 for alcohol treated and Ts65Dn mice. Alcohol treated mice and controls were anesthetized using isoflourane during the microCT scan (induction chamber of 1.5% and 0.8–1.2 l/min and maintenance at 0.5 l/min for 45 min) in the animal CT suite at the Indiana Institute for Biomedical Imaging Sciences (IIBIS). To use the same microCT facilities with similar parameters for the craniofacial bone analysis, Ts65Dn mice were sedated at P21 and perfused with 4% paraformaldehyde (PFA) and, after death, transferred to the same CT suite for microCT scan.
Gene Expression Analysis of Ts65Dn and Ethanol Exposed Mouse Models
E9.5 and E10 embryos from Ts65Dn mothers were somite matched (24–28 somites) to FAS mouse model counterparts. DS and FAS embryos were dissected into three parts: the first branchial arch (BA1), the remaining head, and the rest of the body. RNA was isolated from these three parts using previously described methods (Deitz and Roper, 2011). qPCR was performed using target (Dyrk1a, Rcan1 and Ttc3) and reference (control) (Ipo8) primers according to the manufacturer’s instructions (Taq-Man Gene Expression Assay, Applied Biosystems, Foster City, CA). The crossing point (Cp) values (done in triplicate) from each target primer were analyzed and normalized to the reference probe using the Applied Biosystems 7300 Real Time PCR System and software (Pfaffl, 2001). Average values for each primer were compared between trisomic and euploid samples or ethanol treated and control samples to compute the fold change in expression.
Immunohistochemical Analysis of Apoptosis in DS and FAS Model
DS and FAS E10 embryos (and controls) were placed in 4% PFA for a minimum of 2 days. One affected and one control embryo were placed together in a 10% gelatin mold and after hardening were placed in 4% PFA for a minimum 2 days. Molds were then trimmed and sectioned at 40 mm on a vibratome (Leica Microsystems). Cleaved caspase-3 (Cell Signaling Technology, Boston) was used as a primary antibody at 1:150 dilution and biotin conjugated goat anti-rabbit secondary antibody at 1:500 dilution (Jackson ImmunoResearch, West Grove, PA). Peroxidase-conjugated streptavidin at a 1:500 dilution was used for detection (Jackson ImmunoResearch). Immunostaining protocol was performed as previously described (Chen et al., 2011). Images for ethanol treated and control embryos were previously published (Chen et al., 2011). The ratio of apoptotic cells in the BA1 and developing brain were quantified at the same time for FAS and control as well as Ts65Dn and euploid embryos.
Immunofluorescent Analysis of Akt in Ts65Dn Embryos
Ts65Dn and control embryos were fixed in 4% PFA, 5% sucrose in 0.1 M phosphate buffer (pH 7.4) for 90 min. Embryos were rinsed in PBS two times and infiltrated with 20% sucrose in 0.1 M phosphate buffer overnight. Embryos were subsequently embedded in a 3:1 mixture of the 20% phosphate buffer solution and Tissue-Tek OCT (Sakura Finetek USA, Inc., Torrance, CA). Molds were filled halfway with OCT mixture, placed into a tray containing a dry ice-70% EtOH mixture, embryos were oriented using a dissecting microscope and the mold completely filled and allowed to freeze. Embryo molds were stored at −80°C and removed to −20°C 1 h before sectioning on a cryostat.
The cryostat sectioned slides were washed in PBS (1×) for 10 min. For permeablization, slides were washed with gentle shaking in PBS with 0.5% Triton X-100 for 15 minutes. Antigen retrieval was carried out in PBS with 1% SDS for 5 min after which slides were washed in PBS with 0.2%Triton X-100 three times for 5 min. Sections on the slides were circled with PAP pen to create a hydrophobic barrier and promptly placed into humidified chamber for 20 min. Goat blocking buffer (5% normal goat serum, 0.025% Triton X-100, in PBS) was added for 60 min at room temperature. After removing the blocking buffer, the pAkt antibody (Cell Signaling) was added at a 1:200 dilution in blocking buffer, and the slides were placed in humidified chamber at 4°C overnight. The next day, the slides were brought to room temperature, excess antibody removed and slides washed two times for 10 min in PBS with 0.2% Triton X-100 at room temperature. An Alexa-fluor secondary antibody (Invitrogen) was applied at a 1:1000 dilution in PBS with 0.2% Triton X-100 and incubated for 60 min. Excess antibody was removed and slides were washed 10 min each in PBS with Triton X-100. Pro gold Dapi/Antifade (Life Technologies, Grand Island, NY) mounting media was added before coverslips were applied and sealed using clear nail polish. Slides were stored at 4°C in the dark until pictures were taken using an Olympus FV-111-MPE confocal multiphoton microscope (Olympus, Center Valley, PA). Percent fluorescence in the nuclei was analyzed using ImageJ (National Institute of Health, Bethesda, MD) (Dunn et al., 2011).
RESULTS
Comparison Between Craniofacial, Neuronal, and Other Abnormalities in DS and FAS Studies
Prior results in our respective laboratories studying DS and FAS suggested similar craniofacial deficits are found in the two syndromes (Anthony et al., 2010; Roper et al., 2009). We evaluated previously published literature to compare anthropometric measurements between the two syndromes. Similarities in over 10 craniofacial measurements, including measurements of the skull base, facial widths and heights, as well as microcephaly, were observed in independent studies of humans with DS and FAS. These craniofacial similarities also extended to their respective mouse models, except differences in the minimal frontal breadth and philtrum length (Table 1 and included references). The brain also presented many of the same general deficits in both DS and FAS including an abnormal dentate gyrus in the hippocampus, reduction of cerebellar size, and overall neuronal impairment of the cortex. Other structures, including heart and bone also displayed similarities between the two syndromes (Table 1).
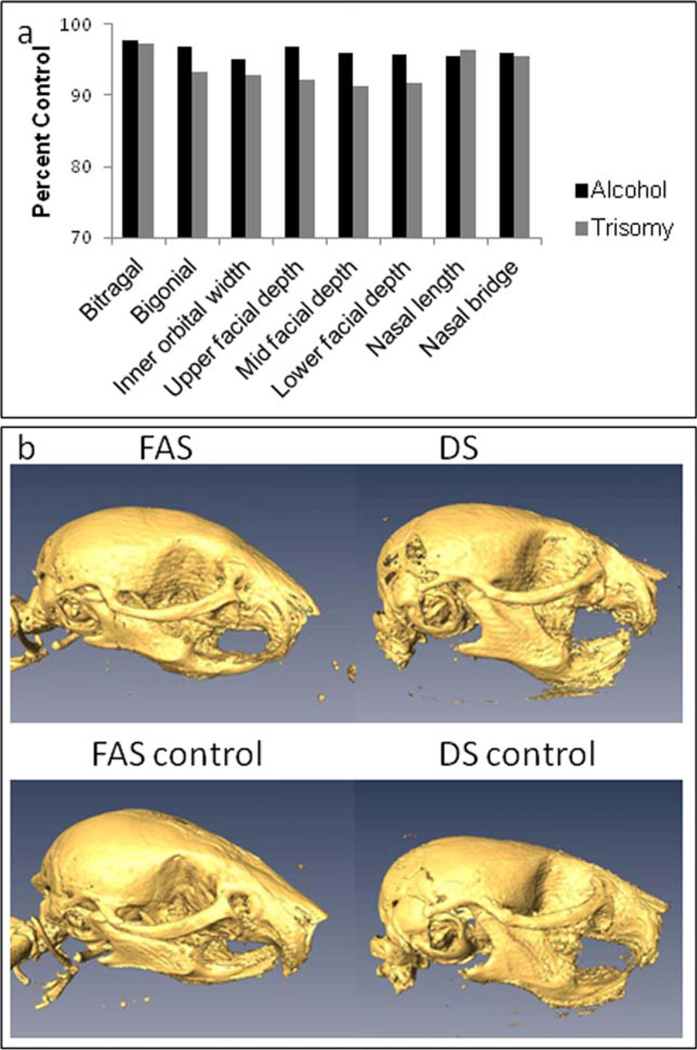
To confirm postnatal craniofacial anomalies previously observed in mouse models of DS and FAS (Richtsmeier et al., 2000; Anthony et al., 2010), we examined eight craniometric measurements from microCT scans of P21 DS and FAS mice. The measurements were chosen to represent phenotypes apparent in individuals with DS and FAS including inner orbital width, facial depth, nasal length, and bitragal width. We observed similar reductions in craniofacial measurements in both DS and FAS mouse models that compare to similar measurements in humans (Fig. 1 and Table 1). Given the comparable craniofacial dysmorphology, we further investigated the cellular basis underlying the common FAS and DS craniofacial and other structural dysmorphology.
Figure 1.
MicroCT analysis of DS and FAS model mice: (a) Measurements taken from P21 alcohol treated or Ts65Dn mice as well as their respective controls. The eight measurements from DS and FAS mouse models show similar craniofacial abnormalities as seen in humans with DS and FAS. Percent control lengths were obtained by dividing alc-alc/alc-pf averages (n= 16 and 18, respectively—data from (Liang et al., 2011)) and Ts65Dn/control averages (n= 3 for both). (b) Facial microCT images of DS or FAS and respective control mice. Note the reduced nasal length and flattened head in both DS and FAS heads. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Altered Dyrk1a and Rcan1 Expression in Mandibular Precursor of DS and FAS Embryos
Because of the common craniofacial dysmorphology seen in young adult mice, we further evaluated craniofacial precursors in DS and FAS embryos. The oral cavity is reduced in individuals with DS and FAS. This is consistent with the smaller mandibular arch reported in both humans with DS and FAS and between respective mouse models (Allanson et al., 1993; Richtsmeier et al., 2000; Moore et al., 2002; Moore et al., 2007; Roper et al., 2009). Because the first branchial arch (BA1) or mandibular precursor is significantly reduced during Ts65Dn mouse development (Roper et al., 2009) and altered expression of Dyrk1a and Rcan1 have been implicated in DS craniofacial deficits (Arron et al., 2006; Park et al., 2009; Deitz and Roper, 2011), we specifically examined these genes in the BA1, as well as head and body, of DS and FAS mouse models. Comparable downregulation of Dyrk1a and upregulation of Rcan1 in the BA1 was found in the developing DS and FAS embryos (Fig. 2), even though copy number was unchanged in FAS embryos. Interestingly, decreased expression of Dyrk1a and increased expression of Rcan1 was observed in the BA1 of both DS and FAS E10 embryos, but similarities in gene dysregulation between DS and FAS embryos (i.e., increased expression of Rcan1 in the head of both DS and FAS embryos) were not seen in the broader regions of head or body in the embryos.
Figure 2.
Gene Expression in DS and FAS Mouse Models: (a) Dyrk1a expression is similarly dysregulated in the mandibular arch (BA1) of Ts65Dn and FAS E10 embryos. The BA1 however presents similar expression which is relevant to craniofacial deficits. Ts65Dn embryos display consistent downregulation in all three tissues. The ethanol treated embryos are less consistent in the Dyrk1a expression patterns and show decreased expression in the BA1, almost normal expression in the head, and over expression in the body. (b) qPCR analysis of Rcan1, like Dyrk1a, also displays dysregulation throughout the embryo at E10 in both DS and FAS models. A similar increased expression in the BA1 suggests possible genetic origins of similar craniofacial deficits. Error bars represent standard error. All tissues from both DS and FAS models contained sample sizes of 5.
Caspase 3 Activity in DS and FAS Craniofacial and Other Neurological Precursors
To test the hypothesis that similar cellular mechanisms are disrupted by trisomy and alcohol exposure during embryonic craniofacial and neurological development, we examined apoptotic cells in somite matched DS and FAS embryos through measurement of cleaved caspase 3 (c-caspase 3). Though apoptosis has been linked to the craniofacial and neurological phenotypes in FAS (Cartwright and Smith, 1995; Sulik, 2005), less is known about its role in developmental phenotypes in DS. E10 somite-matched embryos (24–28 somites) from both DS and FAS models exhibited similar c-caspase three patterning in the mandibular precursor, forebrain, midbrain, and hind-brain (Fig. 3 and Table 2) (Chen et al., 2011). However, a higher ratio of c-caspase 3 marked cells was observed in FAS than DS in all regions examined as compared to their respective control embryos.
Figure 3.
Immunohistochemistry analysis using Caspase 3: (a) Ts65Dn and (b) control E10 embryos were stained for c-Caspase 3. Magnified Ts65Dn and control mandibular arch or BA1 shown in (c) and (e). Larger image of the Ts65Dn and control midbrains shown in (d) and (f). A total of 3 comparisons between 3 trisomic and 3 euploid embryos were used. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table 2.
c-Capsase 3 Expression in Ts65Dn, Ethanol Treated and Respective Control 24–28 Somite Embryos
| Tissue | Trisomic:euploid ratioa |
Ethanol:control ratiob |
|---|---|---|
| First branchial arch | 2.63:1 | 5.47:1 |
| Forebrain | 2.67:1 | 3.29:1 |
| Midbrain | 1.51:1 | 2.52:1 |
| Hindbrain | 1.20:1 | 1.59:1 |
Ts65Dn and controls, n=3.
Ethanol treated and controls, n=4; data as previously published in Chen et al., 2011.
Increased Expression of Ttc3 Linked to Decreased Nuclear Localization of pAkt in Ts65Dn Craniofacial Precursor
Ttc3, a gene that may act upstream in the craniofacial and neurological apoptosis pathway, was also analyzed. We hypothesized that overexpression of Ttc3 and decreased levels of pAkt would lead to the increased apoptosis in the Ts65Dn craniofacial precursors. Ttc3 targets Akt, and Akt is downregulated in alcohol exposed embryos (Hard et al., 2005; Suizu et al., 2009). Using qPCR, we found the expression level of Ttc3 was elevated ~1.5-fold in the mandibular arch, neural tube (NT) of E9.5 and the entire head of E10.5 trisomic embryos (Fig. 4). Subsequent immunofluorescence studies showed a reduced level of pAkt in the nuclei of cells comprising E9.5 Ts65Dn BA1 as compared to euploid control embryos (Fig. 5). Nuclear localization of pAkt was reduced in the cells of the mandibular arch of trisomic (24%) vs. euploid (57%) embryos (Fig. 5b).
Figure 4.
Ttc3 Expression in Ts65Dn embryos: Ttc3 expression in three different tissues of Ts65Dn as compared to euploid embryos analyzed using qPCR. All three tissues display an increase of approximately 1.5 fold over control embryos. Sample size for the head, NT, and BA1 is 6, 4, and 5 respectively.
Figure 5.
Immunofluorescence of pAkt in the mandibular arch of Ts65Dn and control embryos. (a) Red = pAkt, blue = DAPI nuclear staining, and green=co-localization of both DAPI and pAkt in the nucleus. There is a significant increase of nuclear pAkt in euploid BA1 when compared to trisomic BA1. Note that the euploid cells contain much more pAkt within the nucleus than the trisomic cells and that the organization and number of trisomic cells is reduced. (b) Quantification of the overall percent of pAkt fluorescence in trisomic and euploid BA1 nuclei using ImageJ. Error bars indicate standard error. Sample size for euploid analysis was 3 different embryos and 14 sections. Triso-mic analysis contained 4 total embryos and 13 sections. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
We found similar craniofacial anomalies including a reduced mandible, midface hypoplasia and brachycephaly between individuals with DS and FAS as well as corresponding mouse models (Allanson et al., 1993; Richtsmeier et al., 2000; Moore et al., 2002; Moore et al., 2007; Anthony et al., 2010). The most notable differences between syndromes were in the minimal frontal breadth (a larger distance in DS as compared to euploid mice, whereas smaller distances in humans with DS and FAS as well as FAS animal models) and a larger philtrum length (larger length in FAS as compared to normal mice in contrast to the smaller length in humans with DS and FAS—no measurements have been reported in DS mouse models). When we extended our observations to neurological and other structures, DS and FAS as studied in both humans and mouse models shared remarkable similarities in overall phenotypes. Although the magnitude of the quantifiable differences may be distinct with each syndrome, common phenotypes are found in DS and FAS.
When we compared craniofacial abnormalities in P21 mice generated in our colonies, the microCT analysis of both DS and FAS models confirmed similar craniofacial deficits as seen in humans (as well as those previously published in mice). Small scalar differences between the two mouse models may be due to differences in the genetic background of the DS and FAS mouse models. (Note: the Ts65Dn mouse, the most commonly used model of DS, cannot be inbred and is maintained on a B6 3 C3H intercross with about 50% B6 and C3H alleles, on average. These FAS studies utilized the B6 mouse due to its high propensity to drink alcohol and exhibit FAS phenotypes.) Despite these genetic differences, similar phenotypes are seen in DS and FAS models along with comparable phenotypes in the more heterogeneous human genetic background.
Some craniofacial features and molecular mechanisms may be shared between DS, FAS and other syndromes. Apert, 22q11.2 deletion and Treacher Collins syndromes have distinct craniofacial features compared to DS and FAS, and alterations in FGF2, TBX1, and TCOF1, respectively, are thought to cause craniofacial deficits linked to these syndromes (Posnick and Ruiz, 2000; Carinci et al., 2005; Butts, 2009; Conte et al., 2011). Early alterations in neural crest (NC) have been implicated in the craniofacial phenotypes of Treacher Collins, 22q11.2 deletion, Down and Fetal Alcohol syndromes (Walker and Trainor, 2006; Roper et al., 2009; Chen et al., 2011), though extensive comparisons between syndromes have not been done. Ethanol exposure has been linked to holoprosencephaly (Cohen and Shiota, 2002), and alobar holoprosencephaly has been associated with genes on Hsa21 (Muenke et al., 1995). Deficits in sonic hedgehog (Shh) have been linked to abnormal NC in holoprosencephaly, DS and FAS (Aoto et al., 2008; Roper et al., 2009). Our preliminary analyses suggest that similar genetic and molecular mechanisms, including increased apoptosis in the BA1, may be associated with altered craniofacial development in DS and FAS. Hypotheses about commonalities between additional specific phenotypic, genetic and cellular deficits between DS, FAS and other syndromes remain to be tested.
Similar Dyrk1a and Rcan1 Expression in the BA1 in DS and FAS Embryos
Because we observed similar abnormalities in many craniofacial phenotypes, we hypothesized that similar genetic and cellular factors were linked to the development of craniofacial abnormalities in DS and FAS. The altered expression of Dyrk1a and Rcan1, important in a number of developmental processes, in the mandibular arch of both DS and FAS models is intriguing, though the extent of the dysregulation of Rcan1 may be different in DS and FAS development (Fig. 2). In the developing head and body, Dyrk1a and Rcan1 expression is different between DS and FAS embryos, likely because of the differential expression in more heterogeneous tissues. It has been hypothesized that Dyrk1a and Rcan1 are dysregulated by trisomy and lead to DS phenotypes, but we show that that prenatal exposure to alcohol also alters their expression in embryonic craniofacial precursors and may lead to alterations in similar craniofacial phenotypes. Both Dyrk1a and Rcan1 regulate transcription factors such as Nfat and Creb and dysregulation of these genes may lead to possible detrimental downstream effects in cellular and structural abnormalities associated with DS and FAS (Park et al., 2009; Kim and Seo, 2011). Rcan1 has been shown to affect apoptosis in DS brains via caspase 9 and caspase 3 activation (Sun et al., 2011). Because of the multiple pathways regulated by Dyrk1a, it has been implicated in many neurodegenerative disorders (Wegiel et al., 2011; Jones et al., 2012). These similarities in gene dysregulation may also provide future common targets for the treatment of these two syndromes.
Corresponding Apoptosis in DS and FAS Embryos
An increase in cell death during development has been well documented in FAS. Prenatal exposure to alcohol causes apoptosis in NC derived tissues including the mandibular arch as well as the brain and cranial nerves (Dunty et al., 2002; Wang and Bieberich, 2010). In the Ts65Dn DS mouse, NC generation, migration and proliferation are also affected in the mandibular arch (Roper et al., 2009). The studies herein show significant apoptosis in the Ts65Dn mandibular precursor with a greater than two-fold increase in c-caspase 3 as compared to control embryos. Much higher levels of apoptosis (as compared to controls) were seen in alcohol treated embryos and this may be due to developmental differences in DS and FAS or because of the culturing methods of the FAS embryonic model. Likewise, we observed an approximate 1.5–2-fold increase of c-caspase 3 in the fore-, mid-, and hindbrain of the Ts65Dn embryos suggesting an increase in apoptosis may lead to cognitive impairment observed in individuals with DS. Apoptosis has been described in human neural progenitor cells found in ventricular and subventricular zones of the cortex and in the frontal cortex of early postnatal Ts65Dn mice (Lu et al., 2011).
Regulation of pAkt in Ts65Dn Embryos by Ttc3
Trisomic Ttc3 is upregulated in the mandibular arch and may be important in regulating apoptosis of craniofacial precursor cells. Ttc3 effectively degrades activated Akt in the nucleus. pAkt regulates transcription factors such as Creb, p300 and Foxos, having effects on cell survival, proliferation, and differentiation (Huang and Chen, 2005; Cabodi et al., 2009; Carloni et al., 2010). With an increased expression of Ttc3 found in Ts65Dn and its potential effect on pAkt nuclear levels, we observed a decrease in pAkt within the nucleus of cells located in the trisomic mandibular precursor. The decreased pAkt seen in Ts65Dn may correspond to an Akt deficit seen in FAS rat models caused by an increase in the phosphatase Pten (Xu et al., 2003; Green et al., 2007). While the origin of the observed deficit of activated Akt in DS and FAS is dissimilar, the dysregulation of Akt may lead to apoptosis in both DS and FAS. Similar studies examining Akt expression in both DS and FAS mouse models are needed to confirm this hypothesis.
In conclusion, our study indicates that dysmorphologies commonly found in DS and FAS humans are replicated, in part, in corresponding animal models. This initial study also suggests that common cellular and molecular mechanisms are disrupted by trisomy or alcohol consumption during pregnancy and result in similar craniofacial and neurological phenotypes associated with DS and FAS. Further analysis of these disorders using animal models and human studies may provide a context for a better understanding and potential treatments of these disorders.
ACKNOWLEDGMENTS
The authors thank Ms. Lijun Ni for her assistance on the embryonic culture study, and Mr. Huisi Ai for performing MRI imaging scanning.
Supported by AA016698 and U01 AA017123 as part of Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) Consortium to Feng C. Zhou; and by DE021034 to Randall J. Roper.
REFERENCES
- Abel EL, Hannigan JH. Maternal risk factors in fetal alcohol syndrome: provocative and permissive influences. Neurotoxicol Teratol. 1995;17:445–462. doi: 10.1016/0892-0362(95)98055-6. [DOI] [PubMed] [Google Scholar]
- Allanson JE, O’Hara P, Farkas LG, Nair RC. Anthropometric craniofacial pattern profiles in Down syndrome. Am J Med Genet. 1993;47:748–752. doi: 10.1002/ajmg.1320470530. [DOI] [PubMed] [Google Scholar]
- Anthony B, Vinci-Booher S, Wetherill L, et al. Alcohol-induced facial dysmorphology in C57BL/6 mouse models of fetal alcohol spectrum disorder. Alcohol. 2010;44:659–671. doi: 10.1016/j.alcohol.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto K, Shikata Y, Higashiyama D, et al. Fetal ethanol exposure activates protein kinase A and impairs Shh expression in prechordal mesendoderm cells in the pathogenesis of holoprosencephaly. Birth Defects Res A Clin Mol Teratol. 2008;82:224–231. doi: 10.1002/bdra.20447. [DOI] [PubMed] [Google Scholar]
- Arron JR, Winslow MM, Polleri A, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- Asagiri M, Sato K, Usami T, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awan AK, Macafee DA, Hall RI. Intestinal obstruction in an adult with Down’s syndrome. J R Soc Med. 2004;97:334–335. doi: 10.1258/jrsm.97.7.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LL, Moran TH, Richtsmeier JT, et al. Discovery and genetic localization of Down syndrome cerebellar phenotypes using the Ts65Dn mouse. Hum Mol Genet. 2000;9:195–202. doi: 10.1093/hmg/9.2.195. [DOI] [PubMed] [Google Scholar]
- Berto G, Camera P, Fusco C, Imarisio S, Ambrogio C, Chiarle R, Silengo L, Di Cunto F. The Down syndrome critical region protein TTC3 inhibits neuronal differentiation via RhoA and Citron kinase. J Cell Sci. 2007;120(Pt 11):1859–1867. doi: 10.1242/jcs.000703. [DOI] [PubMed] [Google Scholar]
- Bianchi P, Ciani E, Guidi S, et al. Early pharmacotherapy restores neurogenesis and cognitive performance in the Ts65Dn mouse model for Down syndrome. J Neurosci. 2010;30:8769–8779. doi: 10.1523/JNEUROSCI.0534-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazek JD, Billingsley CN, Newbauer A, Roper RJ. Embryonic and not maternal trisomy causes developmental attenuation in the Ts65Dn mouse model for Down syndrome. Dev Dyn. 2010;239:1645–1653. doi: 10.1002/dvdy.22295. [DOI] [PubMed] [Google Scholar]
- Branchi I, Bichler Z, Minghetti L, et al. Transgenic mouse in vivo library of human Down syndrome critical region 1: association between DYRK1A overexpression, brain development abnormalities, and cell cycle protein alteration. J Neuropathol Exp Neurol. 2004;63:429–440. doi: 10.1093/jnen/63.5.429. [DOI] [PubMed] [Google Scholar]
- Burd L, Deal E, Rios R, Adickes E, Wynne J, Klug MG. Congenital heart defects and fetal alcohol spectrum disorders. Congenit Heart Dis. 2007;2:250–255. doi: 10.1111/j.1747-0803.2007.00105.x. [DOI] [PubMed] [Google Scholar]
- Butts SC. The facial phenotype of the velo-cardio-facial syndrome. Int J Pediatr Otorhinolaryngol. 2009;73:343–350. doi: 10.1016/j.ijporl.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Cabodi S, Morello V, Masi A, et al. Convergence of integrins and EGF receptor signaling via PI3K/Akt/FoxO pathway in early gene Egr-1 expression. J Cell Physiol. 2009;218:294–303. doi: 10.1002/jcp.21603. [DOI] [PubMed] [Google Scholar]
- Caley LM, Kramer C, Robinson LK. Fetal alcohol spectrum disorder. J Sch Nurs. 2005;21:139–146. doi: 10.1177/10598405050210030301. [DOI] [PubMed] [Google Scholar]
- Carinci F, Pezzetti F, Locci P, et al. Apert and Crouzon syndromes: clinical findings, genes and extracellular matrix. J Craniofac Surg. 2005;16:361–368. doi: 10.1097/01.scs.0000157078.53871.11. [DOI] [PubMed] [Google Scholar]
- Carloni S, Girelli S, Scopa C, et al. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy. 2010;6:366–377. doi: 10.4161/auto.6.3.11261. [DOI] [PubMed] [Google Scholar]
- Cartwright MM, Smith SM. Increased cell death and reduced neural crest cell numbers in ethanol-exposed embryos: partial basis for the fetal alcohol syndrome phenotype. Alcohol Clin Exp Res. 1995;19:378–386. doi: 10.1111/j.1530-0277.1995.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Cebolla AM, Cheron G, Hourez R, et al. Effects of maternal alcohol consumption during breastfeeding on motor and cerebellar Purkinje cells behavior in mice. Neurosci Lett. 2009;455:4–7. doi: 10.1016/j.neulet.2009.03.034. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L, Galdzicki Z, Haydar TF. Defects in embryonic neurogenesis and initial synapse formation in the forebrain of the Ts65Dn mouse model of Down syndrome. J Neurosci. 2007;27:11483–11495. doi: 10.1523/JNEUROSCI.3406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Dehart DB, Sulik KK. Protection from ethanol-induced limb malformations by the superoxide dismutase/catalase mimetic, EUK-134. FASEB J. 2004;18:1234–1236. doi: 10.1096/fj.03-0850fje. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ozturk NC, Ni L, et al. Strain differences in developmental vulnerability to alcohol exposure via embryo culture in mice. Alcohol Clin Exp Res. 2011;35:1293–1304. doi: 10.1111/j.1530-0277.2011.01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM, Jr, Shiota K. Teratogenesis of holoprosencephaly. Am J Med Genet. 2002;109:1–15. doi: 10.1002/ajmg.10258. [DOI] [PubMed] [Google Scholar]
- Coles CD, Lynch ME, Kable JA, et al. Verbal and nonverbal memory in adults prenatally exposed to alcohol. Alcohol Clin Exp Res. 2010;34:897–906. doi: 10.1111/j.1530-0277.2010.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte C, D’Apice MR, Rinaldi F, et al. Novel mutations of TCOF1 gene in European patients with Treacher Collins syndrome. BMC Med Genet. 2011;12:125. doi: 10.1186/1471-2350-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A, Fila T, Cappellini A, et al. Widespread impairment of cell proliferation in the neonate Ts65Dn mouse, a model for Down syndrome. Cell Prolif. 2009;42:171–181. doi: 10.1111/j.1365-2184.2009.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitz SL, Roper RJ. Trisomic and allelic differences influence phenotypic variability during development of Down syndrome mice. Genetics. 2011;189:1487–1495. doi: 10.1534/genetics.111.131391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol. 2011;300:C723–C742. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunty WC, Jr, Zucker RM, Sulik KK. Hindbrain and cranial nerve dysmorphogenesis result from acute maternal ethanol administration. Dev Neurosci. 2002;24:328–342. doi: 10.1159/000066748. [DOI] [PubMed] [Google Scholar]
- Elliott EJ, Payne J, Morris A, et al. Fetal alcohol syndrome: a prospective national surveillance study. Arch Dis Child. 2008;93:732–737. doi: 10.1136/adc.2007.120220. [DOI] [PubMed] [Google Scholar]
- Elsayed SM, Elsayed GM. Phenotype of apoptotic lymphocytes in children with Down syndrome. Immun Ageing. 2009;6:2. doi: 10.1186/1742-4933-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A, Murakami H, Asai N, et al. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev Cell. 2005;9:389–402. doi: 10.1016/j.devcel.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Epstein CJ. Down Syndrome (Trisomy 21) In: Scriver CR, Beaud WS, Sly WS, Valle D, et al., editors. The metabolic & molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 1223–1256. [Google Scholar]
- Farkas LG, Katic MJ, Forrest CR. Surface anatomy of the face in Down’s syndrome: anthropometric proportion indices in the craniofacial regions. J Craniofac Surg. 2001;12:519–524. doi: 10.1097/00001665-200111000-00003. discussion 525-516. [DOI] [PubMed] [Google Scholar]
- Ferrario VF, Dellavia C, Zanotti G, Sforza C. Soft tissue facial anthropometry in Down syndrome subjects. J Craniofac Surg. 2004;15:528–532. doi: 10.1097/00001665-200405000-00037. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Galofre E. Dendritic spine anomalies in fetal alcohol syndrome. Neuropediatrics. 1987;18:161–163. doi: 10.1055/s-2008-1052472. [DOI] [PubMed] [Google Scholar]
- Fidler DJ, Nadel L. Education and children with Down syndrome: neuroscience, development, and intervention. Ment Retard Dev Disabil Res Rev. 2007;13:262–271. doi: 10.1002/mrdd.20166. [DOI] [PubMed] [Google Scholar]
- Flentke GR, Garic A, Amberger E, et al. Calcium-mediated repression of beta-catenin and its transcriptional signaling mediates neural crest cell death in an avian model of fetal alcohol syndrome. Birth Defects Res A Clin Mol Teratol. 2011;91:591–602. doi: 10.1002/bdra.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes JJ, Genesca L, Kingsbury TJ, et al. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet. 2000;9:1681–1690. doi: 10.1093/hmg/9.11.1681. [DOI] [PubMed] [Google Scholar]
- Garic-Stankovic A, Hernandez MR, Chiang PJ, et al. Ethanol triggers neural crest apoptosis through the selective activation of a pertussis toxin-sensitive G protein and a phospholipase Cbeta-dependent Ca2+ transient. Alcohol Clin Exp Res. 2005;29:1237–1246. doi: 10.1097/01.alc.0000172460.05756.d9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aguero A, Vicente-Rodriguez G, Moreno LA, Casajus JA. Bone mass in male and female children and adolescents with Down syndrome. Osteoporos Int. 2011;22:2151–2157. doi: 10.1007/s00198-010-1443-7. [DOI] [PubMed] [Google Scholar]
- Green ML, Singh AV, Zhang Y, et al. Reprogramming of genetic networks during initiation of the Fetal Alcohol Syndrome. Dev Dyn. 2007;236:613–631. doi: 10.1002/dvdy.21048. [DOI] [PubMed] [Google Scholar]
- Gressens P, Lammens M, Picard JJ, Evrard P. Ethanol-induced disturbances of gliogenesis and neuronogenesis in the developing murine brain: an in vitro and in vivo immunohistochemical and ultrastructural study. Alcohol Alcohol. 1992;27:219–226. [PubMed] [Google Scholar]
- Guidi S, Bonasoni P, Ceccarelli C, et al. Neurogenesis impairment and increased cell death reduce total neuron number in the hippocampal region of fetuses with Down syndrome. Brain Pathol. 2008;18:180–197. doi: 10.1111/j.1750-3639.2007.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Thomas RD, Sreenivas V, et al. Ultrasonographic femurtibial length ratio: a marker of Down syndrome from the late second trimester. Am J Perinatol. 2001;18:217–224. doi: 10.1055/s-2001-15500. [DOI] [PubMed] [Google Scholar]
- Han F, Yu H, Zhang J, et al. Otitis media in a mouse model for Down syndrome. Int J Exp Pathol. 2009;90:480–488. doi: 10.1111/j.1365-2613.2009.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans PS, England R, Prowse S, et al. UK and Ireland experience of cochlear implants in children with Down syndrome. Int J Pediatr Otorhinolaryngol. 2010;74:260–264. doi: 10.1016/j.ijporl.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Hard ML, Abdolell M, Robinson BH, Koren G. Gene-expression analysis after alcohol exposure in the developing mouse. J Lab Clin Med. 2005;145:47–54. doi: 10.1016/j.lab.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Harris CD, Ermak G, Davies KJ. Multiple roles of the DSCR1 (Adapt78 or RCAN1) gene and its protein product calcipressin 1 (or RCAN1) in disease. Cell Mol Life Sci. 2005;62:2477–2486. doi: 10.1007/s00018-005-5085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydar TF, Nowakowski RS, Yarowsky PJ, Krueger BK. Role of founder cell deficit and delayed neuronogenesis in microencephaly of the trisomy 16 mouse. J Neurosci. 2000;20:4156–4164. doi: 10.1523/JNEUROSCI.20-11-04156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer R, Burd L. Review of published studies of kidney, liver, and gastrointestinal birth defects in fetal alcohol spectrum disorders. Birth Defects Res A Clin Mol Teratol. 2009;85:179–183. doi: 10.1002/bdra.20562. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Chen CC. Akt phosphorylation of p300 at Ser-1834 is essential for its histone acetyltransferase and transcriptional activity. Mol Cell Biol. 2005;25:6592–6602. doi: 10.1128/MCB.25.15.6592-6602.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Single alcohol exposure in early life damages hippocampal stem/progenitor cells and reduces adult neurogenesis. Neurobiol Dis. 2007;26:597–605. doi: 10.1016/j.nbd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Incerti M, Vink J, Roberson R, et al. Reversal of alcohol-induced learning deficits in the young adult in a model of fetal alcohol syndrome. Obstet Gynecol. 2010;115(2 Pt 1):350–356. doi: 10.1097/AOG.0b013e3181cb59da. [DOI] [PubMed] [Google Scholar]
- Jones EL, Aarsland D, Londos E, Ballard C. A pilot study examining associations between DYRK1A and alpha-synuclein dementias. Neurodegen Dis. 2012;10:229–231. doi: 10.1159/000334759. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Wolstenholme J, Shelton KL, Miles MF. Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol. 2006;40:119–126. doi: 10.1016/j.alcohol.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Seo SR. The regulator of calcineurin 1 (RCAN1/DSCR1) activates the cAMP response element-binding protein (CREB) pathway. J Biol Chem. 2011;286:37841–37848. doi: 10.1074/jbc.M111.232165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferman JC, Druschel CM, Kupchik GS. Increased prevalence of renal and urinary tract anomalies in children with Down syndrome. Pediatrics. 2009;124:e615–e621. doi: 10.1542/peds.2009-0181. [DOI] [PubMed] [Google Scholar]
- Kurt MA, Davies DC, Kidd M, et al. Synaptic deficit in the temporal cortex of partial trisomy 16 (Ts65Dn) mice. Brain Res. 2000;858:191–197. doi: 10.1016/s0006-8993(00)01984-3. [DOI] [PubMed] [Google Scholar]
- Kvigne VL, Leonardson GR, Neff-Smith M, et al. Characteristics of children who have full or incomplete fetal alcohol syndrome. J Pediatr. 2004;145:635–640. doi: 10.1016/j.jpeds.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Lana-Elola E, Watson-Scales SD, Fisher EM, Tybulewicz VL. Down syndrome: searching for the genetic culprits. Dis Model Mech. 2011;4:586–595. doi: 10.1242/dmm.008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Ha J, Kim HJ, et al. Negative feedback Inhibition of NFATc1 by DYRK1A regulates bone homeostasis. J Biol Chem. 2009;284:33343–33351. doi: 10.1074/jbc.M109.042234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Ai H, Anthony B, et al. Postnatal craniofacial bone dysmorphology and growth pattern affected by prenatal alcohol exposure and diet nutrition. IUPUI Research Day. 2011 [Google Scholar]
- Liu Y, Balaraman Y, Wang G, et al. Alcohol exposure alters DNA methylation profiles in mouse embryos at early neurulation. Epigenetics. 2009;4:500–511. doi: 10.4161/epi.4.7.9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Esposito G, Scuderi C, Steardo L, Delli-Bovi LC, Hecht JL, Dickinson BC, Chang CJ, Mori T, Sheen V. S100B and APP promote a gliocentric shift and impaired neurogenesis in Down syndrome neural progenitors. PLoS One. 2011;6:e22126. doi: 10.1371/journal.pone.0022126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Meintjes EM, Jacobson JL, Molteno CD, et al. An FMRI study of number processing in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2010;34:1450–1464. doi: 10.1111/j.1530-0277.2010.01230.x. [DOI] [PubMed] [Google Scholar]
- Moller RS, Kubart S, Hoeltzenbein M, et al. Truncation of the Down syndrome candidate gene DYRK1A in two unrelated patients with microcephaly. Am J Hum Genet. 2008;82:1165–1170. doi: 10.1016/j.ajhg.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CS, Roper RJ. The power of comparative and developmental studies for mouse models of Down syndrome. Mamm Genome. 2007;18:431–443. doi: 10.1007/s00335-007-9030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ES, Ward RE, Jamison PL, et al. New perspectives on the face in fetal alcohol syndrome: what anthropometry tells us. Am J Med Genet. 2002;109:249–260. doi: 10.1002/ajmg.10197. [DOI] [PubMed] [Google Scholar]
- Moore ES, Ward RE, Wetherill LF, et al. Unique facial features distinguish fetal alcohol syndrome patients and controls in diverse ethnic populations. Alcohol Clin Exp Res. 2007;31:1707–1713. doi: 10.1111/j.1530-0277.2007.00472.x. [DOI] [PubMed] [Google Scholar]
- Muenke M, Bone LJ, Mitchell HF, et al. Physical mapping of the holoprosencephaly critical region in 21q22.3, exclusion of SIM2 as a candidate gene for holoprosencephaly, and mapping of SIM2 to a region of chromosome 21 important for Down syndrome. Am J Hum Genet. 1995;57:1074–1079. [PMC free article] [PubMed] [Google Scholar]
- Myrelid A, Gustafsson J, Ollars B, et al. Growth charts for Down’s syndrome from birth to 18 years of age. Arch Dis Child. 2002;87:97–103. doi: 10.1136/adc.87.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, et al. Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev. 2009;15:209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Kuwagata M, Ruiz J, et al. Differential teratogenic effect of alcohol on embryonic development between C57BL/6 and DBA/2 mice: a new view. Alcohol Clin Exp Res. 2005;29:855–863. doi: 10.1097/01.alc.0000163495.71181.10. [DOI] [PubMed] [Google Scholar]
- O’Leary-Moore SK, Parnell SE, Godin EA, et al. Magnetic resonance microscopy-based analyses of the brains of normal and ethanol-exposed fetal mice. Birth Defects Res A Clin Mol Teratol. 2010;88:953–964. doi: 10.1002/bdra.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Oh Y, Chung KC. Two key genes closely implicated with the neuropathological characteristics in Down syndrome: DYRK1A and RCAN1. BMB Rep. 2009;42:6–15. doi: 10.5483/bmbrep.2009.42.1.006. [DOI] [PubMed] [Google Scholar]
- Patterson D. Molecular genetic analysis of Down syndrome. Hum Genet. 2009;126:195–214. doi: 10.1007/s00439-009-0696-8. [DOI] [PubMed] [Google Scholar]
- Paz-Miguel JE, Flores R, Sanchez-Velasco P, et al. Reactive oxygen intermediates during programmed cell death induced in the thymus of the Ts(1716)65Dn mouse, a murine model for human Down’s syndrome. J Immunol. 1999;163:5399–5410. [PubMed] [Google Scholar]
- Peng X, Haldar S, Deshpande S, et al. Wall stiffness suppresses Akt/eNOS and cytoprotection in pulse-perfused endothelium. Hypertension. 2003;41:378–381. doi: 10.1161/01.hyp.0000049624.99844.3d. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnick JC, Ruiz RL. Treacher Collins syndrome: current evaluation, treatment, and future directions. Cleft Palate Craniofac J. 2000;37:434. doi: 10.1597/1545-1569(2000)037<0434:TCSCET>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ram G, Chinen J. Infections and immunodeficiency in Down syndrome. Clin Exp Immunol. 2011;164:9–16. doi: 10.1111/j.1365-2249.2011.04335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RH, Irving NG, Moran TH, et al. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- Reinholdt LG, Ding Y, Gilbert GJ, et al. Molecular characterization of the translocation breakpoints in the Down syndrome mouse model Ts65Dn. Mamm Genome. 2011;22:685–691. doi: 10.1007/s00335-011-9357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtsmeier JT, Baxter LL, Reeves RH. Parallels of craniofacial maldevelopment in Down syndrome and Ts65Dn mice. Dev Dyn. 2000;217:137–145. doi: 10.1002/(SICI)1097-0177(200002)217:2<137::AID-DVDY1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson R, Cameroni I, Toso L, et al. Alterations in phosphorylated cyclic adenosine monophosphate response element of binding protein activity: a pathway for fetal alcohol syndrome-related neuro-toxicity. Am J Obstet Gynecol. 2009;200:193, e191–e195. doi: 10.1016/j.ajog.2008.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper RJ, Baxter LL, Saran NG, et al. Defective cerebellar response to mitogenic Hedgehog signaling in Down [corrected] syndrome mice. Proc Natl Acad Sci USA. 2006;103:1452–1456. doi: 10.1073/pnas.0510750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper RJ, VanHorn JF, Cain CC, et al. A neural crest deficit in Down syndrome mice is associated with deficient mitotic response to Sonic hedgehog. Mech Dev. 2009;126:212–219. doi: 10.1016/j.mod.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer GB, Deere D. Recognition, diagnosis and treatment of fetal alcohol syndrome. J Ark Med Soc. 2011;108:38–40. [PubMed] [Google Scholar]
- Servais L, Hourez R, Bearzatto B, et al. Purkinje cell dysfunction and alteration of long-term synaptic plasticity in fetal alcohol syndrome. Proc Natl Acad Sci USA. 2007;104:9858–9863. doi: 10.1073/pnas.0607037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow ME, Keiver K. Prenatal ethanol exposure disrupts the histological stages of fetal bone development. Bone. 2007;41:181–187. doi: 10.1016/j.bone.2007.04.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Spohr HL, Willms J, Steinhausen HC. Fetal alcohol spectrum disorders in young adulthood. J Pediatr. 2007;150:175–179. 179, e171. doi: 10.1016/j.jpeds.2006.11.044. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, et al. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Suizu F, Hiramuki Y, Okumura F, et al. The E3 ligase TTC3 facilitates ubiquitination and degradation of phosphorylated Akt. Dev Cell. 2009;17:800–810. doi: 10.1016/j.devcel.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Sulik KK. Genesis of alcohol-induced craniofacial dysmorphism. Exp Biol Med (Maywood) 2005;230:366–375. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- Sun X, Wu Y, Chen B, et al. Regulator of calcineurin 1 (RCAN1) facilitates neuronal apoptosis through caspase-3 activation. J Biol Chem. 2011;286:9049–9062. doi: 10.1074/jbc.M110.177519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterberger U, Lubec G, Dierssen M, et al. The cerebral cortex in fetal Down syndrome. J Neural Transm. 2003;(Suppl67):159–163. doi: 10.1007/978-3-7091-6721-2_14. [DOI] [PubMed] [Google Scholar]
- Van Cleve SN, Cohen WI. Part I: clinical practice guidelines for children with Down syndrome from birth to 12 years. J Pediatr Health Care. 2006;20:47–54. doi: 10.1016/j.pedhc.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Walker MB, Trainor PA. Craniofacial malformations: intrinsic vs extrinsic neural crest cell defects in Treacher Collins and 22q11 deletion syndromes. Clin Genet. 2006;69:471–479. doi: 10.1111/j.0009-9163.2006.00615.x. [DOI] [PubMed] [Google Scholar]
- Wang G, Bieberich E. Prenatal alcohol exposure triggers ceramide-induced apoptosis in neural crest-derived tissues concurrent with defective cranial development. Cell Death Dis. 2010;1:e46. doi: 10.1038/cddis.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Brattain MG. AKT can be activated in the nucleus. Cell Signal. 2006;18:1722–1731. doi: 10.1016/j.cellsig.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Gong CX, Hwang YW. The role of DYRK1A in neurodegenerative diseases. FEBS J. 2011;278:236–245. doi: 10.1111/j.1742-4658.2010.07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijerman ME, van Furth AM, van der Mooren MD, et al. Prevalence of congenital heart defects and persistent pulmonary hypertension of the neonate with Down syndrome. Eur J Pediatr. 2010;169:1195–1199. doi: 10.1007/s00431-010-1200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AD, Mjaatvedt CH, Moore CS. Characterization of the cardiac phenotype in neonatal Ts65Dn mice. Dev Dyn. 2008;237:426–435. doi: 10.1002/dvdy.21416. [DOI] [PubMed] [Google Scholar]
- Wiseman FK, Alford KA, Tybulewicz VL, Fisher EM. Down syndrome-recent progress and future prospects. Hum Mol Genet. 2009;18(R1):R75–R83. doi: 10.1093/hmg/ddp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yeon JE, Chang H, et al. Ethanol impairs insulin-stimulated neuronal survival in the developing brain: role of PTEN phosphatase. J Biol Chem. 2003;278:26929–26937. doi: 10.1074/jbc.M300401200. [DOI] [PubMed] [Google Scholar]
- Yabut O, Domogauer J, D’Arcangelo G. Dyrk1A overexpression inhibits proliferation and induces premature neuronal differentiation of neural progenitor cells. J Neurosci. 2010;30:4004–4014. doi: 10.1523/JNEUROSCI.4711-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]