Abstract
We assessed bilateral motor and sensory function in individuals with upper limb dystonia due to unilateral perinatal stroke and explored interrelationships of motor function and sensory ability. Reach kinematics and tactile sensation were measured in seven participants with dystonia and nine healthy volunteers. The dystonia group had poorer motor (hold time, reach time, shoulder/elbow correlation) and sensory (spatial discrimination, stereognosis) outcomes than the control group on the non-dominant side. On the dominant side, only sensation (spatial discrimination, stereognosis) was poorer in the dystonia group compared to the control group. In the dystonia group, although sensory and motor outcomes were uncorrelated, dystonia severity was related to poorer stereognosis, longer hold and reach times, and decreased shoulder/elbow coordination. Findings of bilateral sensory deficits in dystonia may be explained by neural reorganization. Visual compensation for somatosensory changes in the non-stroke hemisphere may explain the lack of bilateral impairments in reaching.
Keywords: sensory function, motor skills, dystonia, hemiplegia, stroke, cerebral palsy
Introduction
Perinatal stroke is an etiological diagnosis that encompasses cerebrovascular events of ischemic or hemorrhagic nature that occur in 1 out of 1,500 to 5,000 infants around the time of birth1. Consequences often include focal seizures, cognitive impairment, and motor disability 2. Those who have a persistent motor disability on one side of their body as a result of a perinatal stroke are often diagnosed phenotypically as having hemiplegic cerebral palsy 1,3, one of the symptoms being unilateral dystonia. According to the latest consensus in the field, dystonia is defined as “involuntary sustained or intermittent muscle contractions that cause twisting and repetitive movements, abnormal postures, or both” 4. Dystonia can severely compromise volitional motor control, causing limitations in performing daily activities that persist into adulthood and lead to reduced societal participation5.
The diagnosis of dystonia and the determination of its effect on motor and sensory abilities are far easier if it is the sole or dominant movement disorder present, but more challenging when it coexists along with other forms of hypertonia or involuntary movement disorders. In children with perinatal stroke, dystonia is frequently associated with spasticity, which is more prevalent and easily identified, often obscuring the recognition of dystonia 6,7. As a result, dystonia can go undiagnosed and therapeutic approaches can be ill-suited to address the particular features of dystonia8. Therefore, this study focuses on describing sensory and motor features associated with dystonia due to perinatal stroke in order to increase knowledge and awareness of dystonia and its functional consequences in those with childhood-onset brain injuries.
The available literature has shown that children with arm dystonia are hypo-responsive to sensory stimulation, as evidenced by higher thresholds of tactile discrimination in the dominant hand when compared with healthy children 9,10. Abnormally slow reaching movements 11 and more curved movement paths compared to healthy children and children with predominantly spastic cerebral palsy are also reported 7. However, characteristics of each hand in individuals with dystonia as one component of their movement disorder and the possible link between sensory and motor components have not been thoroughly investigated.
The contribution of sensory deficits to motor performance has been demonstrated in children with spastic cerebral palsy and adults with focal hand dystonia, which may provide some clues regarding common mechanisms of dystonia. In children with hemiplegia from various etiologies, deficits in spatial discrimination and stereognosis in the non-dominant arm are related to poor anticipatory control when lifting objects with different surface properties 12 and to lower dexterity when performing a grasping task without visual guidance 13. In adults with focal hand dystonia, the severity of dystonia correlates with sensory deficits and with abnormalities of the hand area in the somatosensory homunculus14,15. These studies suggest that repeated, stereotyped movements are related to maladaptive cortical changes such as de-differentiation of the somatosensory hand representation, and to disrupted sensory discrimination, sensorimotor feedback, and fine motor control. In children with dystonia, reduced perceptual-motor experiences during development as a result of early brain injury may cause maladaptive changes to be even more pronounced.
Although the non-dominant arm is usually more impaired in hemiplegia, sensory and motor deficits are also seen in the dominant arm 13,16. In the case of unilateral brain injury, the dominant arm may be impacted by disinhibition of the intact hemisphere by the injured hemisphere 17. In addition, the influence of unstable postural control on arm kinematics 18, and the presence of bilateral brain injuries in some individuals with a hemiplegic phenotype 19, may confound the understanding of which factors account for bilateral deficits. Therefore, in the current study, we only investigated patients with unilateral brain lesions and provided sufficient trunk support during the performance of motor tasks. The purposes of this study were: 1) to assess bilateral motor performance and tactile sensation in individuals with unilateral dystonia due to unilateral perinatal stroke; and 2) to investigate the relation between sensory responses and motor outcomes and between the severity of dystonia and all sensory and motor outcomes.
We tested the hypothesis that higher spatial and temporal discrimination thresholds and lower stereognosis scores would be seen in both hands in individuals with childhood-onset dystonia compared to healthy volunteers. We also expected that motor function would be bilaterally affected in patients, although not as prominently on the side ipsilateral to the brain injury, resulting in poorer intra-limb coordination, longer movement times and abnormal hand orientation during a reach-to-lift task. Sensory and motor deficits were hypothesized to correlate with dystonia severity and with each other.
Methods
Participants
A total of 20 participants were recruited into two groups for this study. The group with dystonia included 11 participants with a diagnosis of unilateral perinatal stroke and evidence of dystonia affecting one arm (mean age= 15 ±4 years). Participants' demographics are shown in Table 1. The control group included 9 healthy volunteers with no neurological disorders. The patients were recruited from local outpatient physiatry and neurology clinics and healthy volunteers were recruited from the community.
Table 1.
Participants' demographics.
| Subject | Group | Gender | Age (years) | Dominant side | Etiology | MACS | BFM scores | Affected joints** |
|---|---|---|---|---|---|---|---|---|
| 1 | Dystonia | M | 8.1 | Left | Left MCA stroke |
II | D: 2 ND: 9 |
Shoulder Elbow Wrist Fingers |
| 2 | Dystonia* | F | 11.2 | Left | Left MCA stroke |
I | D: 1 ND: 12 |
Wrist Fingers |
| 3 | Dystonia* | M | 13.6 | Left | Left MCA stroke |
II | D: 3 ND: 16 |
Elbow Wrist Fingers |
| 4 | Dystonia | F | 16.8 | Left | Left MCA/ ACA stroke |
I | D: 1 ND: 6 |
Wrist |
| 5 | Dystonia* | M | 17.1 | Left | Left MCA stroke |
III | D: 1 ND: 16 |
Shoulder Elbow Wrist Fingers |
| 7 | Dystonia* | M | 19.1 | Left | Left MCA stroke |
II | D: 2 ND: 12 |
Shoulder Elbow Wrist Fingers |
| 8 | Dystonia* | M | 19.3 | Left | Left MCA stroke |
II | D: 1 ND: 16 |
Elbow Wrist Fingers |
| N/A | Control | 8F, 1M | 17 ±5 | 8 Right | N/A | N/A | D: 1±0.6 ND: 1±0.7 |
N/A |
Legend:
= associated spasticity; M= Male; F= Female; MCA= Middle cerebral artery; ACA= Anterior cerebral artery; MACS= The manual ability classification system;
=presence of dystonia; BFM= Burke-Fahn-Marsden Dystonia scale; D= Dominant side; ND= Non-dominant side; N/A= Not Applicable
Other inclusion criteria were as follows: 7-40 years of age, good general health, ability to understand and comply with instructions, and bilateral wrist passive range of motion of at least 15 degrees of extension and 15 degrees of flexion from neutral. Adult participants were required to be able to provide their own consent. The participants included in the dystonia group also had to demonstrate dystonia in one wrist, diagnosed by a pediatric physiatrist (K.E.A.) based on the Hypertonia Assessment Tool 20, that began before the age of 13 years. Dystonia could be present in other joints as well. The exclusion criteria were as follows: botulinum toxin injection in the flexor carpi radialis and/or extensor carpi radialis in the last 6 months; and concurrent use of medicines for muscle tone (e.g.: baclofen, trihexyphenidyl, dantrolene sodium, tizanidine, or carbidopa/levodopa).
All the participants in the dystonia group were diagnosed with cerebral palsy21, and were classified by the Manual Ability Classification System (MACS) 22 as I to III by K.E.A. To ensure the homogeneity of the sample, data from 4 of these participants were further excluded from the statistical analysis due to magnetic resonance imaging findings of bilateral brain injuries. In all the remaining participants, the etiology of cerebral palsy was unilateral perinatal middle cerebral artery stroke. All participants were born full term with the exception of participant 5, who was born at 36 weeks of gestation. The participants' ages were not different across groups based on the Mann-Whitney U test (p=0.60). Although the gender distribution was not homogeneous when compared the groups using the Fisher exact test (p= 0.035), this is not expected to have an effect on the outcomes of interest for this study. The presence of associated spasticity in some of the participants is reported on Table 1.
The severity of dystonia was measured by the Burke-Fahn-Marsden (BFM) 23 dystonia scale, which was administered by S.N.K and L.O. and scored through consensus by three trained examiners (A.C.C., L.O., D.D.). The Burke-Fahn-Marsden scale rates the severity of dystonia and provoking factors. Separate scores for each arm can be obtained (maximum=16), as well as a total body score from the sum of the individual scores (maximum =120). Good reliability has been previously reported both for the total and for the arm scores 24. The Burke-Fahn-Marsden arm scores were compared between groups using the Mann-Whitney U test. No differences were found on the dominant side (p=0.053), and a significant group difference was found on the non-dominant side (p= 0.001), which was expected.
The study was approved by the Institutional Review Board of the National Institutes of Health (clinicaltrials.gov identifier: NCT01432899). Written informed consent was provided by the adult participants and by the parents/guardians of child participants. Children also provided written assent. This study is one component of a larger protocol including electrophysiological and biomechanical assessments.
Materials and procedures
Sensory tests
The tactile spatial discrimination threshold was tested using Johnson, Van Boven, Phillips (JVP) domes 25. This device consists of a series of plastic domes with ridges spaced at 3.0, 2.0, 1.5, 1.2, 1.0 and 0.75 mm. During testing, 1 dome was pressed against the index fingertip for 1 second. The test started with the 1.5 mm dome, which was presented 20 times in a randomized sequence of orientation. The participants were required to state whether the ridges were aligned “along” or “across” the finger without the use of vision. SDT was defined as the ridge spacing eliciting 15 correct responses out of 20. If none of the domes resulted in exactly 15 correct responses, SDT was mathematically estimated according to the operation manual based on the success rates of the dome sizes tested 25. In this test, a higher SDT is associated with poorer tactile spatial sensitivity of the tested area.
For the assessment of the temporal discrimination threshold, an electrical stimulator (Nihon-Kohden) was used to apply square pulses through two digital ring electrodes placed on the index finger 15. Each participant's threshold for perceiving a single electrical stimulus was determined by delivering pulses with increasing current from 0 mA in increments of 0.2 mA. The single-stimulus threshold was defined as the lowest intensity current at which the participant reported perception of 10 out of 10 consecutive stimuli. The stimulation level for the subsequent paired-stimulus temporal discrimination threshold test was set at 120% of this value. Six paired-stimulus sequences were then applied, alternating between sequences with increasing or decreasing inter-stimulus intervals in increments of 10 ms, based on the method of limits 26. Each participant was asked to report if 1 or 2 pulses were perceived after each paired-stimulus was presented. In each sequence, the inter-stimulus interval was either increased until the subject perceived 2 stimuli instead of 1, or decreased until the subject perceived 1 stimulus instead of 2. For each sequence, the midpoint between the last inter-stimulus interval and the previous one was recorded, and the temporal discrimination threshold was computed as the mean value of the three increasing and the three decreasing threshold values. Similar to spatial discrimination threshold, higher temporal discrimination threshold is associated with poorer sensitivity of the tested area.
Stereognosis, or the ability to identify objects by touch alone, was tested by presenting participants with 8 familiar objects 13, including a pen, pencil, key, coin, paper clip, bolt, cotton ball, and rubber band. These items were mounted to a solid surface in a way that the items could be manually explored without requiring the participants to manipulate the item in their hand independently. The participants were allowed to touch the object for as long as they needed and then asked to name it. A curtain was placed to block the view of the hand and object during the exploration. The number of correctly named objects was recorded for analysis.
All the tests of tactile sensation were performed with both hands. These tests were previously shown to be valid and reliable measures of hand sensation in individuals with and without sensory deficits 13,27-29. Spatial discrimination threshold and stereognosis testing was performed by A.C.C. and temporal discrimination threshold testing was by S.N.K.
Motor tests
In preparation for the reach-to-lift task, reflective markers were taped to the skin on various anatomical landmarks on the head, trunk, arms and hands. The segment definitions followed the American Society of Biomechanics recommendations for upper extremity motion analysis 30. A rod (diameter = 1 inch; height = 6 inches) was placed vertically on a table at the participant's midline and at a distance corresponding approximately to the full active extension of the arm in front of the body. The participant's trunk was restrained by shoulder straps to isolate arm movement. The participants performed the task five times with each arm and the preferred/dominant arm was tested first. In the control group, hand dominance was identified by means of the Edinburgh inventory 31, and in the dystonia group it was defined as the less affected arm. The participants initiated the task with their hands on their laps. Upon hearing a tone, they were instructed to lift one hand, reach forward, grasp the rod, and lift it off the table. Movement kinematics was recorded by an optical motion capture system (Vicon, Los Angeles, USA).
The beginning of a reach was defined as the time point when hand velocity increased beyond 0.05 m/s. Since hand velocities in children with motor disabilities are known to be multi-phasic 16, we considered that the hand was touching the rod at the time of the first minimum in velocity when the hand was clearly near the rod. The interval between the beginning of the reach and the hand touching the rod was referred to as Reach time.
Intra-limb coordination was assessed by calculating the Pearson's correlation coefficient between shoulder flexion and elbow extension trajectories during reach 32.
The rod was defined as lifted when its distance from the table surface became higher than 5 mm. The Hold time was defined as the interval between the hand touching the rod and the rod lift 12. To account for the participants who were not able to lift the rod, the Hold time values were transformed so values equal to 0 indicated that the participant was not able to lift the rod after several seconds trying, and higher values indicated that the participant lifted the rod shortly after touching it, according to the equation: Transformed hold time= log10((Hold time)-1+1).
Finally, the Hand orientation error was calculated as the angle of the vector perpendicular to the palm at rod contact relative to the average of the same vector over all HV for that hand. This measure illustrates the variation in the orientation of the palm relative to the object axis.
For descriptive purposes, the grasp strategy was categorized as: 1) direct, when no adjustment was performed before grasp; 2) adjusted, when the participant adjusted the hand position after touching the rod, and 3) no grasp, when the participant failed to enclose the rod in the hand 33,34.
Statistical analysis
With the exception of Reach time, variables were not normally distributed, according to Shapiro-Wilk's test. Therefore, non-parametric techniques were used. The mean values of Reach time, Hand orientation error, Shoulder/elbow correlation and transformed Hold time across trials were computed for analysis.
The Mann-Whitney U test was used to compare the groups (Dystonia × Control) on motor (Shoulder/elbow correlation, Reach time, transformed Hold time, and Hand orientation error) and sensory measures (Spatial discrimination threshold, Temporal discrimination threshold, Stereognosis) of each hand. The link between motor and sensory measures in the dystonia group was tested using the Spearman rho test. For all tests, a significance level of p<0.05 was used.
Results
Dominant side
Compared to the control group, the dystonia group had higher spatial discrimination threshold (mean rank DYSTONIA=11.21; mean rankCONTROL=6.39; p=0.042), where higher scores indicate worse sensory abilities, and lower performance in the Stereognosis test (mean rankDYSTONIA=4.86; mean rankCONTROL= 11.33; p=0.005) (Table 2). The motor measures (Shoulder/elbow correlation, Reach time, transformed Hold time, and Hand orientation error) on the dominant hand were not different across groups (Figure 1). On this side, the grasp strategy was predominantly direct for both groups (CONTROL=100%; DYSTONIA=95%).
Table 2.
Median values of sensory measures.
| SDT (mm) | TDT (ms) | Stereognosis (# correct) | ||||
|---|---|---|---|---|---|---|
| DYSTONIA | CONTROL | DYSTONIA | CONTROL | DYSTONIA | CONTROL | |
| Dominant | 3.2* (1.1-5.0) |
1.4 (1.2-2.3) |
50 (21.7-105.0) |
38.3 (20.0-66.7) |
5* (0-8) |
8 (7-8) |
|
| ||||||
| Non-Dominant | 6.4* (1.8-11.3) |
1.5 (1.1-1.8) |
61.7 (21.7-170.0) |
35.0 (20.0-48.3) |
1.5* (0-8) |
8 (7-8) |
Legend: SDT: spatial discrimination threshold; TDT: temporal discrimination threshold; D: dominant side; ND: non-dominant side. Values in parentheses are minimum and maximum.
: p<0.05.
Figure 1.
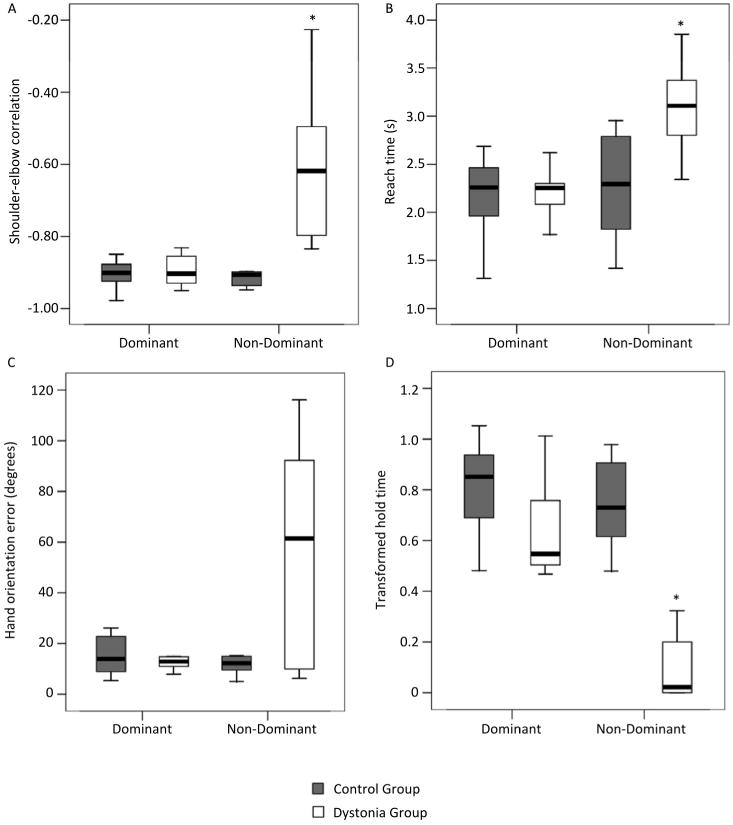
Box plot showing Shoulder-elbow correlation (A), Reach time (B), Hand orientation error (C) and transformed Hold time (D) of the reach-to lift movements on the dominant and non-dominant side. Values are median (central thick lines), 25% and 75% quartile ranges around the median (box height) and upper and lower limits (whiskers); * indicates significant difference between groups.
Non-Dominant side
On the non-dominant side, the dystonia group had significantly higher spatial discrimination threshold (mean rankDYSTONIA=12.64; mean rankCONTROL= 5.28; p=0.0001) and lower stereognosis performance in comparison with HV (mean rankDYSTONIA=4.79; mean rankCONTROL= 11.39; p=0.003) (Table 2). Regarding motor performance, the dystonia group had longer Reach (mean rankDYSTONIA=12.00; mean rankCONTROL= 5.78; p=0.008) and Hold times (mean rankDYSTONIA=4.0; mean rankCONTROL= 12.0; p<0.001), and lower Shoulder/elbow correlation (mean rankDYSTONIA=12.86; mean rankCONTROL=5.11; p<0.001) compared to the control group (Figure 1). Hand orientation error was not different across groups. However, healthy volunteers performed predominantly direct grasps (99%), while patients performed adjusted grasps in 68.7% of the trials, and failed to grasp in 25% of the trials.
Correlation among measures in the dystonia group
The three sensory measures were correlated with each other. Three motor measures (Shoulder/elbow correlation, Reach time and Hold time) were inter-correlated as well (Table 3). Sensory measures of the dystonia group were not correlated with any of the motor measures. However, greater dystonia severity was directly related with poorer stereognosis, longer Hold and Reach times, and decreased Shoulder/elbow correlation, as shown in Table 3.
Table 3.
Correlation between sensory and motor tests for the dystonia group only.
| TDT | Stereognosis | Shoulder/elbow correlation | Reach time | Hand orientation error | Hold Time | BFM arm score | |
|---|---|---|---|---|---|---|---|
| SDT | 0.731 | 0.669 | 0.357 | 0.432 | -0.033 | -0.359 | 0.411 |
| 0.005 | 0.009 | - | - | - | - | - | |
|
| |||||||
| TDT | -0.603 | 0.209 | 0.218 | -0.226 | -0.052 | 0.003 | |
| 0.029 | - | - | - | - | - | ||
|
| |||||||
| Stereognosis | -0.522 | -0.520 | -0.292 | 0.395 | -0.597 | ||
| - | - | - | - | 0.024 | |||
|
| |||||||
| Shoulder/elbow correlation | 0.591 | 0.169 | -0.726 | 0.640 | |||
| 0.026 | - | 0.003 | 0.014 | ||||
|
| |||||||
| Reach Time | 0.371 | -0.819 | 0.812 | ||||
| - | 0.000 | 0.000 | |||||
|
| |||||||
| Hand orientation error | -0.479 | 0.452 | |||||
| - | - | ||||||
|
| |||||||
| Hold Time | 0.852 | ||||||
| 0.000 | |||||||
Legend: SDT: spatial discrimination threshold; TDT: temporal discrimination threshold; For each variable, upper line= Spearman test ρ value; lower line= p value; - = non-significant.
Discussion
In this study, tactile sensory responses and the performance in a reach-to-lift task were tested in participants with dystonia due to unilateral perinatal stroke. The results provide evidence of bilateral sensory deficits in spatial discrimination and stereognosis, and of unilateral impairments in intra-limb coordination, movement time and ability to complete the grasp task. We also described the significant correlation of dystonia severity with sensory and motor components of hand function.
Sensory features
The values of spatial discrimination threshold observed in both hands of healthy volunteers were close to the means expected for healthy individuals (1.4mm) 35,36. In contrast, in the majority of patients the values of spatial discrimination threshold bilaterally were above the limit tested by the instrument. Previous studies have identified bilateral sensory deficits in children with predominantly spastic cerebral palsy and did not specifically verify that those with hemiplegia had purely unilateral brain injury 37. In patients with dystonia, sensory aspects have been previously investigated only on the dominant hand in patients whose impairment severity in the non-dominant hand precluded testing 10.
Stereognosis was also bilaterally affected in the patient group, which is consistent with studies showing that this may be one of the most impaired sensory aspects in hemiplegia 38. Recognizing objects by touch is a complex ability that requires the use of clues from texture, temperature, size, shape, weight, and other features of the object 39. Therefore, the presence of tactile impairments such as spatial and temporal discrimination, and of motor deficits, is expected to affect stereognosis 13,40. The role of tactile and motor factors in stereognosis was supported by the correlation analysis as well.
Although temporal discrimination threshold was correlated with spatial discrimination threshold and stereognosis, the trend towards poorer temporal discrimination in patients than controls did not reach statistical significance as it did for spatial discrimination and stereognosis. One functional magnetic resonance imaging study implicates particular regions in the bilateral frontal cortex (pre-supplementary motor cortex, and anterior cingulate gyrus) that are specifically activated in a temporal discrimination task and are not activated in a spatial discrimination task 41. If these frontal brain areas are critical to temporal discrimination, the lack of significant group differences in temporal discrimination threshold and the presence of group differences in spatial discrimination threshold in our study may be partly explained by greater preservation of these critical brain areas in our patient group. However, in another functional magnetic resonance study, the pre-supplementary motor cortices were bilaterally active during a spatial discrimination task, suggesting the functional neuroanatomy of spatial and temporal discrimination tasks may not be distinct in all circumstances 42. Another factor contributing to the lack of difference between subject groups on temporal discrimination threshold could be the high inter-individual variability in the patients with dystonia and the small sample size.
The mechanisms underlying the presence of bilateral sensory deficits in patients with unilateral lesions are not completely understood. Although it has been shown that damage in the thalamocortical pathways correlates with contralateral deficits in touch and proprioception 19, current knowledge does not support the presence of ipsilateral connections in these pathways.
We speculate that developmental factors may have played a role in the sensory findings of our study. It is known that maturation and experience are involved in tactile sensation, resulting in changes in spatial discrimination during childhood 35 and enhanced spatial discrimination in people who are blind 43. Therefore, a reduced number of perceptual-motor experiences throughout development may have contributed to reorganization of the sensorimotor cortex. Accumulating evidence from animal research has demonstrated the deleterious consequences of non-use and of stereotypical movement patterns early in life to cortical reorganization and hand sensorimotor function44. However, human studies that directly investigate the development and organization of the somatosensory cortex in childhood-onset dystonia are necessary to clarify this issue.
Motor features
The reach-to-lift variables and dystonia severity were only different across groups on the non-dominant side. Although some studies have shown that the dominant arm may be impaired as well16,45, these studies mainly address patients with spastic cerebral palsy. In addition, methodological differences complicate comparisons with our results. Differences or uncertainty about the laterality of brain injury 16,45 and lack of feet and trunk support during testing in Van der Heide et al. 46 may account for findings of longer reaching duration and latency to lift objects in patients' dominant arm. Other outcomes assessed by these studies were related to the arm mechanics, such as jerk and number of movement units, which were not the focus of the current study. Our results for the dominant side point to similarities between patients and controls regarding overall performance of a visually-guided reaching task. However, the quality of movement may be different and should be addressed in future studies.
The group differences on the non-dominant side included reduced Shoulder-elbow correlation, and longer Reach and Hold times in the dystonia group compared to the healthy volunteers. The emergence of spatial coupling between shoulder and elbow is typically expected to occur within the first two years of life and illustrates more efficient arm trajectories 47. Reduced Shoulder-elbow correlation suggests that the patients may have failed to develop typically early in life. This finding is consistent with characteristics of dystonia such as problems with gradation of movement and positioning of the limb in space 48. Difficulties in activating the appropriate muscles 49 with the right timing 11 may be additional factors contributing to uncoordinated movements, with possible consequences for functional performance.
In this study the patients had slower Reach and Hold times on the non-dominant side compared to healthy volunteers. The longer time needed to complete a grasp task has been frequently reported in the literature regarding patients with spastic cerebral palsy compared to typically developing children 16. Although noted less frequently, difficulties with the reach component have also been reported, and are usually related to greater impairment severity50. The effect of severity was confirmed in the current study by the correlation analysis. Another possible cause of slower movements is the presence of dystonia in this study's participants. Inappropriate timing of agonist/antagonist muscles contractions in dystonia is thought to introduce greater noise and variability into the arm mechanics, causing the patients to reduce speed as a compensatory strategy 49. Five of the seven participants with dystonia additionally exhibited spasticity, which is known to contribute to movement slowness as well7.
We expected to find increased hand orientation error in the dystonia group due to known difficulties with forearm supination in patients with cerebral palsy 51. Despite the lack of significant group differences, it is clear that some participants were able to orient the hand according to the object features while others were not (Figure 1C). However, the number of participants in this study did not allow a subgroup analysis to test differences in hand orientation according to the participants' impairment severity and its impact on hand function.
Correlation between sensory and motor outcomes
The correlation analysis failed to show that sensory measures were correlated with reach-to-lift variables. However, it did demonstrate that greater dystonia severity was correlated with poorer stereognosis, longer Reach and Hold times, and decreased Shoulder-elbow correlation.
Based on previous findings that tactile discrimination is relevant to the performance of specific adjustments required to lift an object 12, we hypothesized that reach-to-lift variables would be correlated with sensory measures. One possible explanation for the lack of correlation is that tactile discrimination skills are not as critical to the performance of a simple reach-to-lift task if visual feedback or guidance is available. Support for this idea is provided by Bleyenheuft et al., who found no correlation between tactile spatial discrimination and dexterity in a pegboard task in healthy children 52 and children with spastic hemiplegia 53. These authors suggest that the acquisition of motor components is more critical than of sensory abilities to meet the pegboard task's demands. Another factor could be that the participants used visual guidance to compensate for their sensory loss. Accordingly, sensory deficits have been related to increased activity in visual areas during the performance of manual tasks in adults with focal hand dystonia 54 and to increased activity in areas dedicated to visuospatial and somatosensory attention in children with hemiplegic cerebral palsy 55 compared to healthy individuals.
The association between performance in the stereognosis test and the arm scores measured by the Burke-Fahn-Marsden scale indicates that participants with more severely impaired arms are less able to recognize objects by touch. The relation between stereognosis and manual ability has been reported in children with spastic hemiplegia 56, with improvements in active hand range of motion resulting in better stereognosis 57. The present study demonstrates that the presence of involuntary movements or postures may limit the motor exploration needed to recognize objects as well. The additional finding that the three sensory measures were inter-related suggests that in the same subject, more than one tactile ability may be impaired, and that a combination of sensory and motor factors may be involved in the ability to recognize objects. Indeed, it has been shown that motor constraints associated with sensory loss limit the acquisition of relevant information and the performance of exploratory procedures required to recognize objects, such as enclosure and contour-following 40. In addition, the three sensory tasks may share similar neuroanatomical substrates, including regions in parietal cortex, frontal cortex, cerebellum, and basal ganglia 41,42, supporting the presence of correlations among them.
The association between Shoulder-elbow correlation and Reach and Hold time with Burke-Fahn-Marsden arm scores indicates that joint incoordination and the time to reach for and lift a target are directly proportional to the degree of arm impairment. It was previously reported that the disturbance in arm kinematics during reaching is related to dystonia severity in children with dyskinetic cerebral palsy 9, but previous studies did not address the impact of dystonic symptoms on the grasp component. Our results demonstrate that impairment severity is also related to hand function in patients with unilateral dystonia, which has important functional implications.
Taken together, the findings of the current study describe the nature of sensory and motor deficits and the role of impairment severity in individuals who present with dystonia due to unilateral brain injury. Limitations of this study include the small sample size and the choice of a motor task that may not be challenging enough to be affected by sensory loss when visual input is available. Nevertheless, clear evidence of unilateral motor and bilateral sensory deficits in a group with unilateral brain lesions points to anatomical differences between sensory and motor brain representation or reorganization after injury. Previous work showing bilateral sensory deficits in patients with a hemiplegic phenotype did not exclude those with bilateral injuries, in contrast to our study. To isolate the impact of dystonia in particular on the sensory and motor features described in this study, future work could incorporate additional age-matched cohorts with unique etiologies and symptoms, including a perinatal stroke group with only spasticity, one with only dystonia, and a group of individuals with a genetic form of dystonia. Additionally, we could evaluate children before and after taking a medication such as trihexyphenidyl or levodopa to determine which aspects of the movement disorder are altered.
Given that sensory re-training improves sensory discrimination, fine motor skills and functional independence in adults with focal hand dystonia 58, it is possible that engaging patients with childhood-onset dystonia in a variety of sensorimotor activities with task-relevant sensory stimulation may improve their functioning as well. Moreover, appropriate interventions to help improve hand function should be planned as early as possible based on the patient's degree of impairment. Although preliminary evidence suggests that constraint-induced therapy may increase the activation of the somatosensory cortex 59, the extent to which these changes also result in improved motor performance, especially in the population with dystonia, still needs to be investigated.
Acknowledgments
We thank Dr. Ching-yi Shieh for support with statistical analysis. Many thanks also to Lindsey Curatalo and Laurie Ohlrich for support with data collection and analysis, and to Dr. Nelci Adriana C.F. Rocha for comments on the manuscript.
Financial disclosure: This work was supported by the Intramural Research Program of the National Institutes of Health Clinical Center, the National Institute of Neurological Disorders and Stroke, and the National Council for Scientific and Technological Development (CNPq), Brazil (scholarship to A.C.C).
Footnotes
Author contributions: ACC, one of the first authors who contributed equally to this work, co-wrote the first draft of the paper, and participated in data acquisition, analysis and interpretation of results. SNK, one of the first authors who contributed equally to this work, was involved with study design, co-wrote the first draft of the paper, and participated in data acquisition, analysis and interpretation of results. MH, a mentor who contributed equally to this work, was involved with study design, interpretation of results, and critical review of the paper. KEA, a mentor who contributed equally to this work, was involved with study design, interpretation of results, and critical review of the paper. DLD, a mentor who contributed equally to this work, was involved with study design, interpretation of results, and critical review of the paper.
Declaration of conflicting interests: The authors declare no conflicting interests.
Ethical approval: The study was approved by the Institutional Review Board of the National Institutes of Health (clinicaltrials.gov identifier: NCT01432899).
Contributor Information
Ana Carolina de Campos, Email: decamposa@mail.nih.gov.
Sahana N. Kukke, Email: sahana.kukke@nih.gov.
Mark Hallett, Email: hallettm@ninds.nih.gov.
Katharine E. Alter, Email: kalter@cc.nih.gov.
Diane L. Damiano, Email: damianod@cc.nih.gov.
References
- 1.Lynch JK. Epidemiology and classification of perinatal stroke. Semin Fetal Neonatal Med. 2009;14(5):245–249. doi: 10.1016/j.siny.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Ganesan V. Outcome and rehabilitation after childhood stroke. Handb Clin Neurol. 2013;112:1079–1083. doi: 10.1016/B978-0-444-52910-7.00025-8. [DOI] [PubMed] [Google Scholar]
- 3.Kirton A, deVeber G. Cerebral palsy secondary to perinatal ischemic stroke. Clin Perinatol. 2006;33(2):367–386. doi: 10.1016/j.clp.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Sanger TD, Delgado MR, Gaebler-Spira D, Hallett M, Mink JW. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111(1):e89–97. doi: 10.1542/peds.111.1.e89. [DOI] [PubMed] [Google Scholar]
- 5.Gimeno H, Gordon A, Tustin K, Lin JP. Functional priorities in daily life for children and young people with dystonic movement disorders and their families. Eur J Paediatr Neurol. 2013;17(2):161–168. doi: 10.1016/j.ejpn.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Sanger TD. Toward a definition of childhood dystonia. Curr Opin Pediatr. 2004;16(6):623–627. doi: 10.1097/01.mop.0000142487.90041.a2. [DOI] [PubMed] [Google Scholar]
- 7.Gordon LM, Keller JL, Stashinko EE, Hoon AH, Bastian AJ. Can spasticity and dystonia be independently measured in cerebral palsy? Ped Neurol. 2006;35(6):375–381. doi: 10.1016/j.pediatrneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Boardman JP, Ganesan V, Rutherford MA, Saunders DE, Mercuri E, Cowan F. Magnetic resonance image correlates of hemiparesis after neonatal and childhood middle cerebral artery stroke. Pediatrics. 2005;115(2):321–326. doi: 10.1542/peds.2004-0427. [DOI] [PubMed] [Google Scholar]
- 9.Sanger TD. Arm trajectories in dyskinetic cerebral palsy have increased random variability. J Child Neurol. 2006;21(7):551–557. doi: 10.1177/08830738060210070201. [DOI] [PubMed] [Google Scholar]
- 10.Sanger TD, Kukke SN. Abnormalities of Tactile Sensory Function in Children With Dystonic and Diplegic Cerebral Palsy. J Child Neurol. 2007;22(3):289–293. doi: 10.1177/0883073807300530. [DOI] [PubMed] [Google Scholar]
- 11.Kukke SN, Sanger TD. Contributors to excess antagonist activity during movement in children with secondary dystonia due to cerebral palsy. J Neurophys. 2011;105(5):2100–2107. doi: 10.1152/jn.00998.2009. [DOI] [PubMed] [Google Scholar]
- 12.Gordon AM, Duff SV. Relation between clinical measures and fine manipulative control in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 1999;41(9):586–591. doi: 10.1017/s0012162299001231. [DOI] [PubMed] [Google Scholar]
- 13.Krumlinde-Sundholm L, Eliasson AC. Comparing tests of tactile sensibility: aspects relevant to testing children with spastic hemiplegia. Dev Med Child Neurol. 2002;44(9):604–612. doi: 10.1017/s001216220100264x. [DOI] [PubMed] [Google Scholar]
- 14.Byl NN, Nagarajan SS, Merzenich MM, Roberts T, McKenzie A. Correlation of clinical neuromusculoskeletal and central somatosensory performance: variability in controls and patients with severe and mild focal hand dystonia. Neural Plast. 2002;9(3):177–203. doi: 10.1155/NP.2002.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bara-Jimenez W, Shelton P, Hallett M. Spatial discrimination is abnormal in focal hand dystonia. Neurology. 2000;55(12):1869–1873. doi: 10.1212/wnl.55.12.1869. [DOI] [PubMed] [Google Scholar]
- 16.Ronnqvist L, Rosblad B. Kinematic analysis of unimanual reaching and grasping movements in children with hemiplegic cerebral palsy. Clin Biomech. 2007;22(2):165–175. doi: 10.1016/j.clinbiomech.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu T, Hosaki A, Hino T, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125(8):1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- 18.van der Heide JC, Fock JM, Otten B, Stremmelaar E, Hadders-Algra M. Kinematic characteristics of postural control during reaching in preterm children with cerebral palsy. Ped Res. 2005;58(3):586–593. doi: 10.1203/01.pdr.0000176834.47305.26. [DOI] [PubMed] [Google Scholar]
- 19.Hoon AH, Jr, Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009;51(9):697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jethwa A, Mink J, Macarthur C, Knights S, Fehlings T, Fehlings D. Development of the Hypertonia Assessment Tool (HAT): a discriminative tool for hypertonia in children. Dev Med Child Neurol. 2010;52(5):e83–87. doi: 10.1111/j.1469-8749.2009.03483.x. [DOI] [PubMed] [Google Scholar]
- 21.Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N. Proposed definition and classification of cerebral palsy, April 2005 - Introduction. Dev Med Child Neurol. 2005;47(8):571–576. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 22.Eliasson AC, Krumlinde-Sundholm L, Rosblad B, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48(7):549–554. doi: 10.1017/S0012162206001162. [DOI] [PubMed] [Google Scholar]
- 23.Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35(1):73–77. doi: 10.1212/wnl.35.1.73. [DOI] [PubMed] [Google Scholar]
- 24.Krystkowiak P, du Montcel ST, Vercueil L, et al. Reliability of the Burke-Fahn-Marsden scale in a multicenter trial for dystonia. Mov Disord. 2007;22(5):685–689. doi: 10.1002/mds.21392. [DOI] [PubMed] [Google Scholar]
- 25.Van Boven RW, Johnson KO. The limit of tactile spatial resolution in humans: grating orientation discrimination at the lip, tongue, and finger. Neurology. 1994;44(12):2361–2366. doi: 10.1212/wnl.44.12.2361. [DOI] [PubMed] [Google Scholar]
- 26.Gescheider GA. The classical psychophysics methods. 3. London: Lawrence Erlbaum Associates; 1997. [Google Scholar]
- 27.Gerr FE, Letz R. Reliability of a Widely Used Test of Peripheral Cutaneous Vibration Sensitivity and a Comparison of 2 Testing Protocols. Brit J Ind Med. 1988;45(9):635–639. doi: 10.1136/oem.45.9.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klingels K, De Cock P, Molenaers G, et al. Upper limb motor and sensory impairments in children with hemiplegic cerebral palsy. Can they be measured reliably? Disabil Rehabil. 2010;32(5):409–416. doi: 10.3109/09638280903171469. [DOI] [PubMed] [Google Scholar]
- 29.Bleyenheuft Y, Thonnard JL. Tactile spatial resolution measured manually: a validation study. Somatosens Mot Res. 2007;24(3):111–114. doi: 10.1080/08990220701496639. [DOI] [PubMed] [Google Scholar]
- 30.Wu G, van der Helm FC, Veeger HE, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38(5):981–992. doi: 10.1016/j.jbiomech.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 31.Oldfield RC. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 32.Lacquaniti F, Soechting JF. Coordination of Arm and Wrist Motion during a Reaching Task. J Neurosci. 1982;2(4):399–408. doi: 10.1523/JNEUROSCI.02-04-00399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett TM, Needham A. Developmental differences in infants use of an object's shape to grasp it securely. Dev Psychobiol. 2008;50(1):97–106. doi: 10.1002/dev.20280. [DOI] [PubMed] [Google Scholar]
- 34.Barrett TM, Traupman E, Needham A. Infants' visual anticipation of object structure in grasp planning. Infant Behav Dev. 2008;31(1):1–9. doi: 10.1016/j.infbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Bleyenheuft Y, Cols C, Arnould C, Thonnard JL. Age-related changes in tactile spatial resolution from 6 to 16 years old. Somatosens Mot Res. 2006;23(3-4):83–87. doi: 10.1080/08990220600816440. [DOI] [PubMed] [Google Scholar]
- 36.Van Boven RW, Johnson KO. The limit of tactile spatial resolution in humans: grating orientation discrimination at the lip, tongue and finger. Neurology. 1994;44:2361–2366. doi: 10.1212/wnl.44.12.2361. [DOI] [PubMed] [Google Scholar]
- 37.Wingert JR, Burton H, Sinclair RJ, Brunstrom JE, Damiano DL. Tactile sensory abilities in cerebral palsy: deficits in roughness and object discrimination. Dev Med Child Neurol. 2008;50(11):832–838. doi: 10.1111/j.1469-8749.2008.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper J, Majnemer A, Rosenblatt B, Birnbaum R. The determination of sensory deficits in children with hemiplegic cerebral palsy. J Child Neurol. 1995;10(4):300–309. doi: 10.1177/088307389501000412. [DOI] [PubMed] [Google Scholar]
- 39.Yekutiel M, Jariwala M, Stretch P. Sensory deficit in the hands of children with cerebral palsy: a new look at assessment and prevalence. Dev Med Child Neurol. 1994;36(7):619–624. doi: 10.1111/j.1469-8749.1994.tb11899.x. [DOI] [PubMed] [Google Scholar]
- 40.Lederman SJ, Klatzky RL. Haptic identification of common objects: effects of constraining the manual exploration process. Percept Psychophys. 2004;66(4):618–628. doi: 10.3758/bf03194906. [DOI] [PubMed] [Google Scholar]
- 41.Pastor MA, Day BL, Macaluso E, Friston KJ, Frackowiak RS. The functional neuroanatomy of temporal discrimination. J Neurosci. 2004;24(10):2585–2591. doi: 10.1523/JNEUROSCI.4210-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, Mariola E, Stilla R, et al. Tactile discrimination of grating orientation: fMRI activation patterns. Hum Brain Mapp. 2005;25(4):370–377. doi: 10.1002/hbm.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldreich D, Kanics IM. Tactile acuity is enhanced in blindness. J Neurosci. 2003;23(8):3439–3445. doi: 10.1523/JNEUROSCI.23-08-03439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coq JO, Strata F, Russier M, et al. Impact of neonatal asphyxia and hind limb immobilization on musculoskeletal tissues and S1 map organization: Implications for cerebral palsy. Exp Neurol. 2008;210(1):95–108. doi: 10.1016/j.expneurol.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Rigoldi C, Molteni E, Rozbaczylo C, et al. Movement analysis and EEG recordings in children with hemiplegic cerebral palsy. Exp Brain Res. 2012;223(4):517–524. doi: 10.1007/s00221-012-3278-2. [DOI] [PubMed] [Google Scholar]
- 46.van der Heide JC, Fock JM, Otten B, Stremmelaar E, Hadders-Algra M. Kinematic characteristics of reaching movements in preterm children with cerebral palsy. Ped Res. 2005;57(6):883–889. doi: 10.1203/01.PDR.0000157771.20683.14. [DOI] [PubMed] [Google Scholar]
- 47.Konczak J, Dichgans J. The development toward stereotypic arm kinematics during reaching in the first 3 years of life. Exp Brain Res. 1997;117(2):346–354. doi: 10.1007/s002210050228. [DOI] [PubMed] [Google Scholar]
- 48.Pavone L, Burton J, Gaebler-Spira D. Dystonia in Childhood: Clinical and Objective Measures and Functional Implications. J Child Neurol. 2013;28(3):340–350. doi: 10.1177/0883073812444312. [DOI] [PubMed] [Google Scholar]
- 49.Malfait N, Sanger TD. Does dystonia always include co-contraction? A study of unconstrained reaching in children with primary and secondary dystonia. Exp Brain Res. 2007;176(2):206–216. doi: 10.1007/s00221-006-0606-4. [DOI] [PubMed] [Google Scholar]
- 50.Domellof E, Rosblad B, Ronnqvist L. Impairment severity selectively affects the control of proximal and distal components of reaching movements in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 2009;51(10):807–816. doi: 10.1111/j.1469-8749.2008.03215.x. [DOI] [PubMed] [Google Scholar]
- 51.Mackey AH, Walt SE, Stott NS. Deficits in upper-limb task performance in children with hemiplegic cerebral palsy as defined by 3-dimensional kinematics. Arch Phys Med Rehab. 2006;87(2):207–215. doi: 10.1016/j.apmr.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 52.Bleyenheuft Y, Wilmotte P, Thonnard JL. Relationship between tactile spatial resolution and digital dexterity during childhood. Somatosens Mot Res. 2010;27(1):9–14. doi: 10.3109/08990220903471831. [DOI] [PubMed] [Google Scholar]
- 53.Bleyenheuft Y, Thonnard JL. Tactile spatial resolution in unilateral brain lesions and its correlation with digital dexterity. J Rehab Med. 2011;43(3):251–256. doi: 10.2340/16501977-0651. [DOI] [PubMed] [Google Scholar]
- 54.Hinkley LB, Dolberg R, Honma S, Findlay A, Byl NN, Nagarajan SS. Aberrant Oscillatory Activity during Simple Movement in Task-Specific Focal Hand Dystonia. Front Neurol. 2012;3:1–11. doi: 10.3389/fneur.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van de Winckel A, Verheyden G, Wenderoth N, et al. Does somatosensory discrimination activate different brain areas in children with unilateral cerebral palsy compared to typically developing children? An fMRI study. Res Dev Disabil. 2013;34(5):1710–1720. doi: 10.1016/j.ridd.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 56.Arnould C, Penta M, Thonnard JL. Hand impairments and their relationship with manual ability in children with cerebral palsy. J Rehab Med. 2007;39(9):708–714. doi: 10.2340/16151977-0111. [DOI] [PubMed] [Google Scholar]
- 57.Dahlin LB, Komoto-Tufvesson Y, Salgeback S. Surgery of the spastic hand in cerebral palsy. Improvement in stereognosis and hand function after surgery. J Hand Surg Brit Eur. 1998;23(3):334–339. doi: 10.1016/s0266-7681(98)80053-3. [DOI] [PubMed] [Google Scholar]
- 58.Byl NN, Nagajaran S, McKenzie AL. Effect of sensory discrimination training on structure and function in patients with focal hand dystonia: a case series. Arch Phys Med Rehab. 2003;84(10):1505–1514. doi: 10.1016/s0003-9993(03)00276-4. [DOI] [PubMed] [Google Scholar]
- 59.Cope SM, Liu XC, Verber MD, Cayo C, Rao S, Tassone JC. Upper limb function and brain reorganization after constraint-induced movement therapy in children with hemiplegia. Dev Neurorehab. 2010;13(1):19–30. doi: 10.3109/17518420903236247. [DOI] [PubMed] [Google Scholar]


