Abstract
The goals of this study were to characterize the changes in chondroitin sulfate proteoglycans and hyaluronan in lungs in acute response to gram-negative bacterial infection and to identify cellular components responsible for these changes. Mice were treated with intratracheal (IT) live Escherichia coli, E. coli lipopolysaccharide (LPS), or PBS. Both E. coli and LPS caused rapid selective increases in mRNA expression of versican and hyaluronan synthase (Has) isoforms 1 and 2 associated with increased immunohistochemical and histochemical staining for versican and hyaluronan in the lungs. Versican was associated with a subset of alveolar macrophages. To examine whether macrophages contribute to versican and hyaluronan accumulation, in vitro studies with primary cultures of bone marrow-derived and alveolar macrophages were performed. Unstimulated macrophages expressed very low levels of versican and hyaluronan synthase mRNA, with no detectible versican protein or hyaluronan product. Stimulation with LPS caused rapid increases in versican mRNA and protein, a rapid increase in Has1 mRNA, and concomitant inhibition of hyaluronidases 1 and 2, the major hyaluronan degrading enzymes. Hyaluronan could be detected following chloroquine pre-treatment, indicating rapid turnover and degradation of hyaluronan by macrophages. In addition, the effects of LPS, the M1 macrophage classical activation agonist, were compared to those of IL-4/IL-13 or IL-10, the M2a and M2c alternative activation agonists, respectively. Versican and Has1 increased only in response to M1 activation. Finally, the up-regulation of versican and Has1 in the whole lungs of wild-type mice following IT LPS was completely abrogated in TLR-4−/− mice. These findings suggest that versican and hyaluronan synthesis may play an important role in the innate immune response to gram-negative lung infection.
Keywords: Lung, Macrophage, Versican, Hyaluronan, TLR-4
1. Introduction
In the acute response to bacterial lung infection, gram-negative bacteria are recognized by toll-like receptors (TLRs) on the surface of alveolar macrophages triggering a cascade of events that leads to pulmonary inflammation and, ultimately, bacterial clearance and healing. TLR activation stimulates resident macrophages to secrete a variety of chemokines, cytokines and other molecules that induce recruitment of neutrophils and monocytes from the bloodstream. This leukocyte response is essential to the propagation and resolution of inflammation. We are interested in the potential for proteoglycans and related molecules secreted by macrophages to have a role in promoting the acute inflammatory response to bacterial infections in the lung.
Proteoglycans are important biological modifiers that influence both homeostasis and the response to injury (Esko and Lindahl, 2001; Parish, 2006; Gill et al., 2010). In the lung, proteoglycans also have a role in organ development and contribute to the innate immune response to infection (Gill et al., 2010; Tanino et al., 2012). Following the treatment of lungs with lipopolysaccharide (LPS), the glycosaminoglycan composition changes from predominantly heparan sulfate in healthy lungs to predominantly chondroitin and dermatan sulfate in inflamed lungs (Karlinsky, 1982; Blackwood et al., 1983). Changes in the composition of proteoglycans and their glycosaminoglycan chains, resulting in increases in versican, decorin and biglycan, are also documented in both animal models and human conditions of chronic lung disease (Karlinsky, 1982; Blackwood et al., 1983; Bensadoun et al., 1996, 1997; Malmstrom et al., 2002; de Medeiros Matsushita et al., 2005). Hyaluronan is a glycosaminoglycan that is not attached to a core protein, but binds to a number of chondroitin sulfate proteoglycans, including versican, to form large molecular weight complexes (Day and de la Motte, 2005). Similarly to versican, hyaluronan also is increased in chronic lung diseases (Hallgren et al., 1989; Nettelbladt and Hallgren, 1989; Nettelbladt et al., 1989; Jiang et al., 2005) and has been shown to have an important role in airway mucosal defense (Forteza et al., 2001).
Temporal and spatial changes in the expression of specific chondroitin sulfate proteoglycans and hyaluronan in the lungs during the innate immune response have not been systematically studied. Thus, the goals of this study were to characterize the changes in chondroitin sulfate proteoglycans and hyaluronan in lungs of mice with gram-negative bacteria and to identify specific cellular components that contribute to these changes. While multiple cell types are involved in the inflammatory response of the lungs (Strieter et al., 2002), this study focuses on the contribution of macrophages and epithelial cells as these are the cells that first encounter airborne pathogens and have been shown to have key roles in the initiation and regulation of inflammation in the lungs (Berg et al., 1993; Koay et al., 2002; Skerrett et al., 2004).
2. Results
2.1. Versican mRNA is Selectively Increased in Lungs of E. coli- and LPS-treated mice
To determine if acute inflammation alters the composition of chondroitin sulfate proteoglycans in the lungs, mice were exposed to IT live E. coli or PBS for 6 h and RNA was isolated from whole lung homogenates. Lungs from mice with E. coli pneumonia had a 33.2 ± 3.7-fold increase in versican mRNA, but no changes to the amounts of decorin or biglycan mRNA, as compared to mice treated with PBS (Fig. 1A). To identify changes in proteoglycan expression over time, subsequent studies used LPS to model gram-negative lung infection. LPS was used because this TLR-4 agonist provides a robust, more predictable and consistent activation of the innate immune system. Changes in the amount of mRNA for versican, decorin, and biglycan were measured using RNA isolated at 2, 6, and 24 h after treatment with LPS or PBS (Fig. 1B). There was significantly more versican mRNA in lungs of mice challenged with LPS vs. PBS at all three timepoints. LPS instillation caused a 4.4 ± 1.6-fold increase in versican at 2 h as compared to control lungs, a 33.2 ± 2.1-fold increase at 6 h, and a 4.45 ± 1.0-fold increase at 24 h. There were no changes in versican mRNA expression in the lungs of PBS-treated mice. There were also no changes in decorin or biglycan mRNA expression in the lungs of either LPS- or PBS-treated mice. These findings show that versican expression is rapidly and selectively upregulated in lungs in response to E. coli and LPS.
Fig. 1.
Versican mRNA is increased in lungs of E. coli- and LPS-treated mice. Changes in the relative amounts of mRNA for the chondroitin sulfate proteoglycans, biglycan, decorin, and versican, were determined using mRNA collected from whole lung homogenates and quantitative real time PCR. A, Comparison of mRNA recovered from lungs of mice treated with PBS or 1 µg/g E. coli were made at 6 h. B, Comparison of mRNA recovered from lungs of mice treated with PBS or 1 µg/g LPS were made at 2, 6, and 24 h. Values are the mean ± SEM with a minimum n = 3 for each group studied. The expression of mRNA for each proteoglycan studied is expressed as a relative fold increase in mRNA over the 0 h control. An asterisk (*) shows groups that are significantly different (p ≤ 0.05) when mice treated with PBS and LPS were compared.
2.2. Hyaluronan synthase mRNA is increased in lungs of E. coli- and LPS-treated mice
RNA collected from lungs of mice treated with live E. coli or LPS also was analyzed for changes in expression levels of the hyaluronan synthase isoforms. In mice treated with live E. coli for 6 h, there were significant increases in both Has1 (10.6 ± 1.3-fold increase) and Has2 (8.9 ± 1.0 fold-increase) mRNA, as compared to mice treated with PBS (Fig. 2A). In contrast, there was no change in the amount of Has3 mRNA (1.7 ± 0.6-fold increase). In mice treated with LPS, the expression of Has1 and Has2 mRNA was greatest at 2 h after IT instillation of LPS, but returned to basal levels at 24 h (Fig. 2B). When compared to control, LPS instillation caused 91.0 ± 12.8- and 10.5 ± 0.8-fold increases in Has1, and 21.6 ± 2.7- and 8.9 ± 0.6-fold increases in Has2 at 2 and 6 h, respectively. In contrast, Has3 mRNA was unchanged at 2 and 6 h after LPS instillation, but was significantly increased by 5.9 ± 1.0-fold at 24 h over the control (Fig. 2B). There were no changes in mRNA expression for any of the Has isoforms in PBS-treated mice. These findings show that Has1 and Has2 expression are rapidly and specifically increased, while Has3 expression is delayed in lungs in response to E. coli and LPS.
Fig. 2.
Hyaluronan synthase mRNA is increased in lungs of E. coli- and LPS-treated mice. Changes in the relative amounts of mRNA for the 3 isoforms of hyaluronan synthase, Has1, Has2 and Has3, were determined using mRNA collected from whole lung homogenates and quantitative real time PCR. A, Comparison of mRNA recovered from lungs of mice treated with PBS or 1 µg/g E. coli were made at 6 h. B, comparison of mRNA recovered from lungs of mice treated with PBS or 1 µg/g LPS were made at 2, 6, and 24 h. Values are the mean±SEMwith aminimum n=3 for each group studied. The expression of mRNA for each isoform is expressed as a relative fold increase in mRNA over the 0 h control. An asterisk (*) shows groups that are significantly different (p ≤ 0.05) when mice treated with PBS and LPS were compared.
2.3. Versican and hyaluronan staining are increased in lungs of LPS-treated mice
To determine changes in their spatial localization in lungs, IHC for versican and affinity histochemistry for hyaluronan were performed on tissue samples obtained 6 h after the instillation of PBS or LPS. In the lungs of mice treated with PBS, a low level of positive staining for versican and hyaluronan was observed in the extracellular matrix of large airways and vessels (Table 1). The positive staining for versican and hyaluronan tended to be localized to the basal lamina of epithelial cells and in close proximity to the musculature of the vessels or airways. In these same mice, there was little to no staining for versican or hyaluronan in the lung parenchyma or pulmonary veins (Fig. 3). In mice treated with LPS, there was a clear increase in the positive staining for versican and hyaluronan around pulmonary veins (Fig. 3C and D), and an increase in the number of animals with positive staining for versican in the alveolar septa (Table 1). The majority of the positive staining for versican and hyaluronan appeared to be associated with the extracellular matrix, but intracellular staining of endothelial cells and/or lung fibroblasts could not be ruled out and needs to be investigated in future studies. Photomicrographs for the PBS- (Fig. 3A and B) and LPS-treated lungs (Fig. 3C and D) are representative images and show the same vessel in adjacent tissue sections. Photomicrographs of the PBS-treated lungs are of lower magnification to highlight the minimal amount of versican and hyaluronan in normal lungs. When lungs were evaluated for versican immunostaining, 4/6 (66%) of the mice treated with LPS had alveolar macrophages that stained positive and 3/6 (50%) had white blood cells that stained positive (Table 1 and Fig. 3D and E). In contrast, there was no immunostaining of versican in white blood cells or alveolar macrophages in mice treated with PBS (Table 1 and Fig. 3B). Colloidal carbon was co-instilled with LPS to identify the gross and microscopic locations where LPS was deposited in lungs. The uptake of colloidal carbon by alveolar macrophages was used to identify the cells that were exposed to LPS. Positive staining for hyaluronan was not observed in macrophages or in white blood cells in adjacent tissue sections from the same animals (Fig. 3C and Table 1). Thus, in contrast to control mice, significant positive staining for versican or hyaluronan was detected in the lungs by 6 hours after intratracheal administration of LPS, indicating that versican and hyaluronan are early responses to infection.
Table 1.
Spatial location of positive staining for HA and versican in lungs of mice (6 h).
| Bronchioles | Bronchiole vessels | Alveolar septa | Pulmonary veins | Alveolar macrophages | White blood cells | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBS | LPS | PBS | LPS | PBS | LPS | PBS | LPS | PBS | LPS | PBS | LPS | |
| HA | 4/4 | 6/6 | 4/4 | 6/6 | 1/4 | 4/6 | 1/4 | 5/6 | 0/4 | 0/6 | 0/4 | 0/6 |
| Versican | 2/4 | 5/6 | 3/4 | 6/6 | 1/4 | 5/6 | 0/4 | 5/6 | 1/4 | 4/6 | 0/4 | 3/6 |
Fig. 3.
Versican and hyaluronan staining are increased in lungs of LPS-treated mice. IHC for versican and affinity histochemistry for hyaluronan localization in lung tissue from PBS- or LPS-treated mice. A & B, Lungs from mice treated for 6 h with PBS, 1 µg/g LPS (C & D), or 1 µg/g LPS (E) were stained with an antibody specific for the glycosaminoglycan-β domain of versican (B & D) or with b-HABP protein (A & C). Representative micrographs are shown. Gray arrows indicate alveolar macrophages, identified by colloidal carbon uptake, that do not show positive staining for hyaluronan (C) or versican (D & E). The black arrows indicate alveolar macrophages that also show positive staining for versican (D & E). The black arrow head indicates a circulating white blood cell that shows positive staining for versican (D).
2.4. Versican mRNA and protein are increased in BMDMs treated with LPS
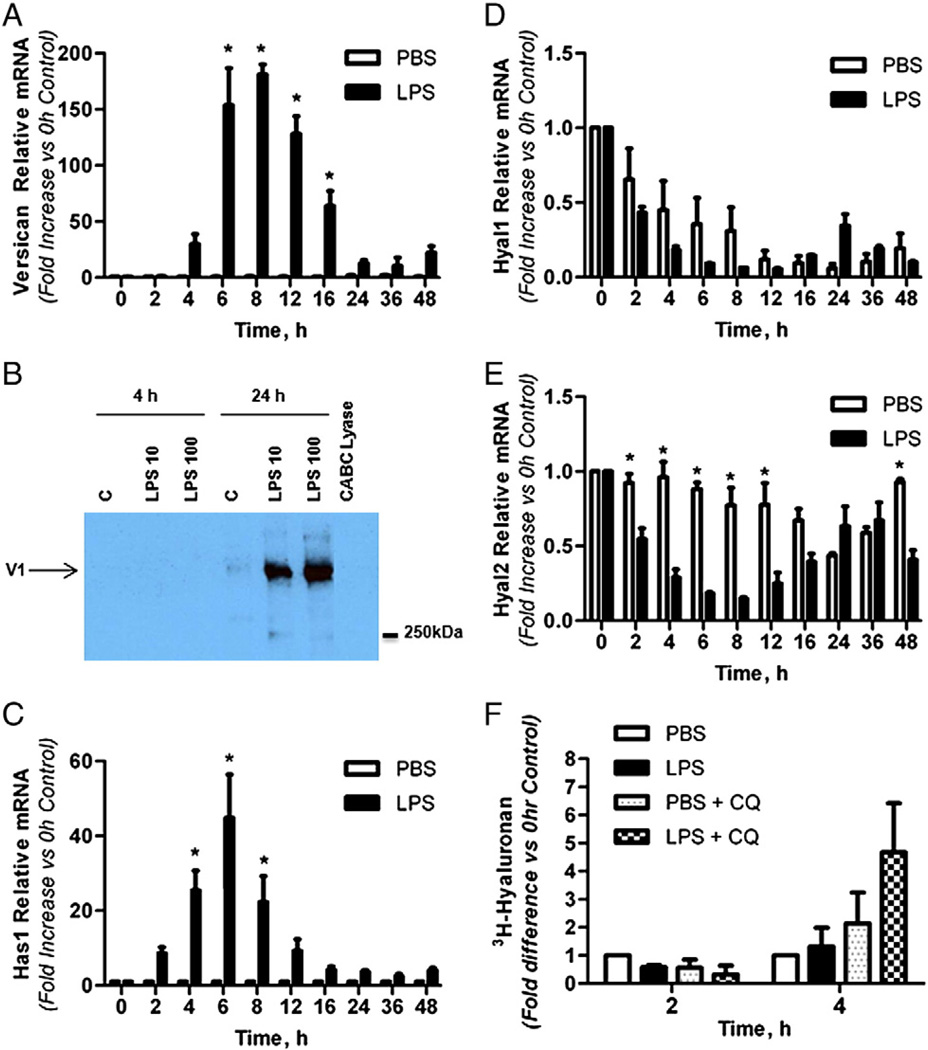
The finding of versican in a subset of alveolar macrophages exposed to LPS was examined in in vitro studies initially using BMDMs, as sufficient numbers of these macrophages are easily obtained for mRNA and protein analyses. Versican mRNA was detected at low levels in untreated macrophages (Ct=33.6 ± 0.3 for 15 ng cDNA, n=6), and expression levels did not change in control cells over the time course of these experiments. However, versican mRNA was markedly increased in response to LPS treatment (Fig. 4A). Versican mRNA was significantly increased from 6 to 16 h after treatment with 10 ng/ml of LPS, with a maximal increase of 180.9 ± 9.3-fold after 8 h, as compared to the PBS control (n = 3, p < 0.0001), and returned to basal levels after 24 h. Treatment with a higher dose of LPS (100 ng/ml) had similar impacts on the fold-increase and time course of versican mRNA expression (data not shown).
Fig. 4.
Versican and hyaluronan are increased in BMDMs treated with LPS. Quantitative real time-PCR showing the relative expression of versican mRNA (A), Has1mRNA (C), Hyal1mRNA (D), and Hyal2mRNA (E) collected from BMDMs treated in vitro with PBS (Control) or LPS (10 ng/ml) for up to 48 h (n=5). B, Western immunoblotting of media from BMDMs treated with PBS (Control), 10 ng/ml LPS or 100 ng/ml LPS for 4 or 24 h. F, [3H]-glucosamine incorporation into hyaluronan from BMDMs treated with PBS or 100 ng/ml LPS, with or without 0.5 mM chloroquine for 2 or 4 h. Values are means ± SEM. Asterisks show groups different from the controls (* p < 0.05).
Proteoglycans were isolated from both the media and cell-associated compartments of BMDMs and evaluated by Western immunoblotting for versican protein (Fig. 4B). Using an antibody specific for the versican glycosaminoglycan-β domain, no versican was detected in the media from cells treated with PBS or from cells treated for 4 h with LPS. However, versican protein was strongly induced after 24 h of treatment with either 10 or 100 ng/ml LPS. Using the same glycosaminoglycan-β antibody, no versican protein was detected in the cell-associated compartment at either 4 or 24 h (data not shown). Also, no versican protein was detected in either compartment using an antibody specific for the versican glycosaminoglycan-α domain at 4 or 24 h (data not shown). These data indicate that the >250 kDa protein detected using the glycosaminoglycan-β antibody is the V1 isoform of versican which contains the glycosaminoglycan-β domain, but not the glycosaminoglycan-α domain of versican (Wight, 2002). Thus, LPS induces synthesis of versican mRNA and secretion of the V1 isoform of versican by BMDMs.
2.5. Has1mRNA and hyaluronan synthesis are increased in BMDMs treated with LPS
The effects of LPS treatment on expression of the hyaluronan synthases (Has 1, 2 and 3), their product (hyaluronan), and the major enzymes that degrade hyaluronan (hyaluronidases 1 and 2), were also examined in vitro using BMDMs. Has1 mRNA was present at very low levels in untreated macrophages (Ct = 38.1 ± 0.3 for 15 ng cDNA, n = 6) and expression levels did not change in control cells over the time course of these experiments. However, Has1mRNA was markedly increased in response to LPS treatment (Fig. 4C). Has1mRNAwas significantly increased from 4 to 8 h after treatment with 10 ng/ml of LPS, with a maximal increase of 44.8 ± 11.6-fold after 6 h, as compared to the PBS control (n = 3, p < 0.0001), and returned to basal levels after 16 h. Treatment with a higher dose of LPS (100 ng/ml) had similar impacts on the fold-increase and time course of versican mRNA expression (data not shown). Neither Has2 nor Has3 mRNA were detected in control or LPS-treated BMDMs over the time course of these experiments (data not shown).
Hyaluronidase 1 and 2 mRNA were present at high levels in untreated macrophages (Hyal1 Ct = 28.2 ± 0.1 and Hyal2 Ct=23.3±0.3 for 15 ng cDNA, n = 3). Hyaluronidase 1 mRNA levels decreased in both control and LPS-treated cells over the time course of these experiments (p < 0.001 at all times vs. control), with no significant differences between the effects of PBS and LPS (Fig. 4D). In contrast, hyaluronidase 2 mRNA levels did not change in control cells over the time course of these experiments, while LPS caused a transient decrease in hyaluronidase 2 that was maximally inhibited to 14.5% of control levels at 8 h and was significantly lower from 2 to 12 h (p < 0.0001 at 4, 6, and 8 h vs. control), after which hyaluronidase 2 levels returned to basal levels (Fig. 4E). It should be noted that while hyaluronidase 1 and 2 levels were significantly lower relative to control as early as 2 h after exposure to LPS, abundant message was still detected for both hyaluronidases at all times.
Hyaluronan synthesis by BMDMs was examined by quantifying hyaluronidase-sensitive [3H]-glucosamine incorporation. Two considerations were incorporated into this assay. First, because of the timing of the mRNA responses of Has1 and Hyal2, which were maximally highest and lowest, respectively, within hours after exposure to LPS, we chose to evaluate [3H]-hyaluronan accumulation at the 2 and 4 h timepoints. Second, because no hyaluronan product could be detected by hyaluronan ELSA (data not shown) we considered that hyaluronan might be rapidly internalized and degraded by macrophages. Therefore, chloroquine treatment was used to inhibit hyaluronan degradation in lysosomal and endosomal cellular compartments (Mapleson and Buchwald, 1981). A low level of [3H]-hyaluronan was measured in the media of control cells (744 ± 290 dpm/200 µl media) and no effect of LPS treatment could be detected by evaluating [3H]-hyaluronan in the media at 2 or 4 h (Fig. 4F). However, when cells were pre-treated with chloroquine prior to LPS treatment, a 4.67 ± 1.75-fold increase in [3H]-hyaluronan production was measured at 4 h, as compared to without chloroquine pre-treatment. No [3H]-hyaluronan was detected in the cellular fraction with or without chloroquine treatment (data not shown).
Thus, LPS specifically and temporally stimulates Has1 while concurrently diminishing both hyaluronidases 1 and 2. Further inhibition of pH-sensitive hyaluronan degradation with chloroquine allowed us to demonstrate increased hyaluronan synthesis by BMDMs in response to LPS treatment. However, hyaluronan was not found to accumulate in either intracellular or extracellular compartments, suggesting that hyaluronan product is rapidly degraded and utilized for metabolic processes by the macrophages.
2.6. Versican and Has1 mRNA are increased in alveolar macrophages treated with LPS
In a limited number of experiments, murine alveolar macrophages were isolated and treated with LPS to verify that these lung macrophages have responses similar to BMDMs macrophages. In response to 4 h-treatment with LPS, versican mRNA increased 30.2 ± 3.2-fold (n = 4, p < 0.001) and Has1 mRNA increased 116.8 ± 24.0-fold vs. PBS control treatment (n = 4, p < 0.001) in alveolar macrophages (Fig. 5). Thus, alveolar macrophages and BMDMs respond similarly to LPS with respect to versican and Has1 mRNA regulation.
Fig. 5.
Versican and Has1 mRNA are increased in alveolar macrophages treated with LPS. Quantitative real time-PCR showing the relative expression of versican and Has1 mRNA in alveolar macrophages treated in vitro with PBS (Control) or LPS (100 ng/ml) for 4 h (n = 4). Values are means ± SEM. Asterisks show groups different from the controls (* p < 0.05).
2.7. Versican and HAS1 mRNA are not increased in epithelial cells treated with LPS
In addition to macrophages, airway epithelial cells are important in the acute response to lung infection. Thus, we also examined the effects of LPS on murine tracheal epithelial cell expression of versican and hyaluronan synthase. Unlike the response by macrophages, LPS (10 or 100 ng/ml) had no significant effect on either versican (Fig. 6A) or Has1 (Fig. 6B) mRNA at 4 or 24 h vs. control cells. Interestingly, whereas LPS had no effects on Has2 or Has3 mRNA in either bone marrow-derived or alveolar macrophages, this TLR4 agonist caused a significant increase in both Has2 and Has3 mRNA levels in epithelial cells (Fig. 6B). Has2 increased by 4.0 ± 0.7-fold (p < 0.0001) and 7.9 ± 0.9-fold (p < 0.0001) in response to 10 ng/ml LPS vs. control at 4 and 24 h, respectively. Has3 increased by 2.3 ± 0.4-fold and 6.0 ± 0.6-fold (p < 0.0001) vs. control at 4 and 24 h, respectively. Similar responses were obtained with a higher concentration of LPS (100 ng/ml). These studies indicate that macrophages and epithelial cells have distinct responses to LPS in terms of versican and hyaluronan synthase regulation.
Fig. 6.
Versican and Has1 mRNA are not increased in epithelial cells treated with LPS. Quantitative real time-PCR showing the relative expression of versican (A) or Has1, Has2, and Has3 (B) mRNA in tracheal epithelial cells treated in vitro with PBS (Control), 10 ng/ml LPS or 100 ng/ml LPS for 4 or 24 h (n=3). Values are means± SEM. Asterisks show groups different from the controls (* p < 0.05).
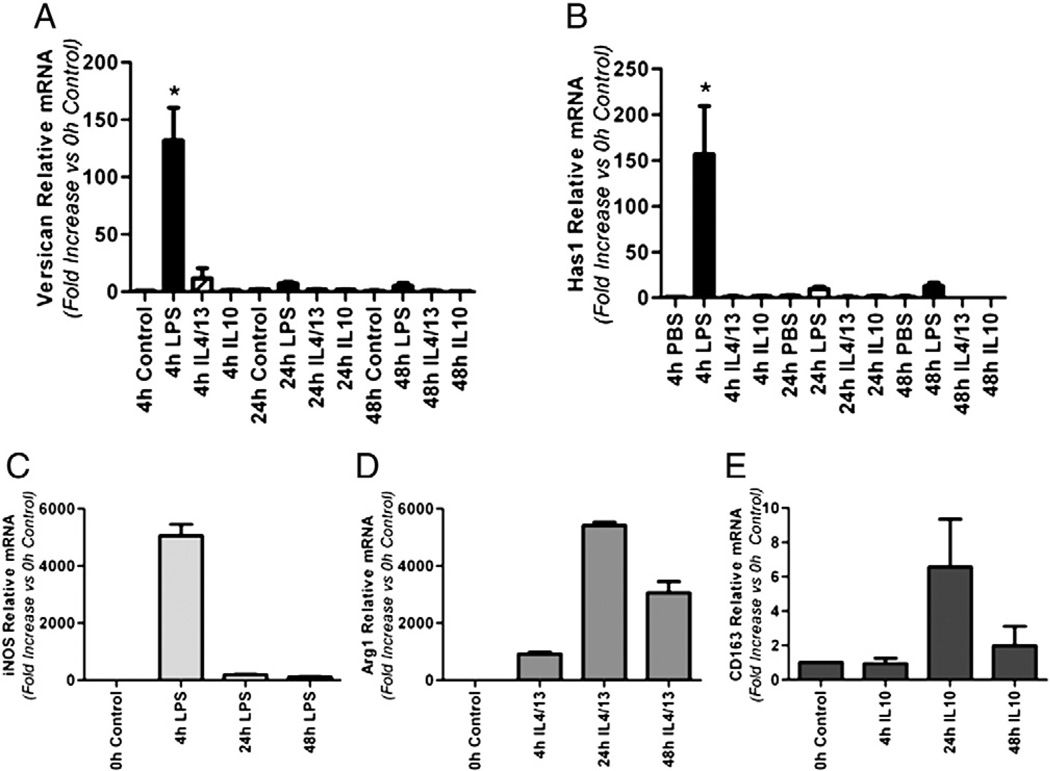
2.8. Versican and Has1 mRNA are Increased by M1 stimulation of BMDMs
As shown in Fig. 3 and Table 1, positive immunostaining for versican was found in the majority of, but not in all, alveolar macrophages in mice treated with LPS. In vitro studies have shown that alveolar macrophages are functionally heterogeneous (Brannen and Chandler, 1988) and exhibit bothM1 andM2 polarization markers (Duan et al., 2012). Therefore, we chose to examine whether versican expression might correlate withM1 or M2 macrophage polarization. BMDMs were treated for 4, 24 or 48 h with (i) LPS, the classical M1 macrophage agonist; (ii) a combination of IL-4 and IL-13, alternative M2a macrophage agonists; or (iii) IL-10, the deactivated M2c macrophage agonist (Mantovani et al., 2004) (Fig. 7). Versican mRNA was rapidly and strongly induced (132.0 ± 28.6-fold, p < 0.0001 vs. control cells) only in response to the M1 agonist, LPS (Fig. 7A). Has1 mRNA also was rapidly and strongly induced (156.6 ± 52.7-fold, p < 0.0001 vs. control cells) only in response to the M1 agonist (Fig. 7B). Neither the combination of IL-4 and IL-13, nor IL-10 had any effect on versican or Has1 mRNA expression even at the later time point of 48 h. None of these agonists stimulated Has2 or Has3 mRNA expression in BMDMs over the time course of these experiments (data not shown). iNOS served as the positive control for M1 activation (Fig. 7C); Arg1 as the positive control for M2a activation (Fig. 7D); and CD163 as the positive control for M2c activation (Nielsen et al., 2006) (Fig. 7E). Thus, upregulation of both versican and Has1 genes reflect classical M1 activation.
Fig. 7.
Versican and Has1 mRNA are increased by M1, but not by M2, stimulation of BMDMs. Quantitative real time-PCR showing the relative expression of versican (A), Has1 (B), iNOS (C) and Arg1 (D) in macrophages treated with PBS (control), 10 ng/ml LPS, 10 ng/ml IL-4 plus 10 ng/ml IL-13, or 10 ng/ml IL4 for 4, 24 or 48 h (n=3). Values are means±SEM. Asterisks show groups different from the controls (* p < 0.05).
2.9. Versican and Has1 are regulated through TLR-4
Two approaches were taken to evaluate the role of toll-like receptor 4, the canonical LPS receptor, in mediating the versican and Has1 response to LPS. First, we considered that standard LPS preparations primarily consist of the polysaccharide Lipid A, or endotoxin, but may be contaminated with bacterial lipopeptides. While endotoxins modulate the immune response via TLR-4, lipopeptides interact with TLR-2. Therefore, mice were exposed to a preparation of ultra-pure LPS which only activates the TLR-4 pathway (Hajjar et al., 2012). Following IT administration of ultra-pure LPS, RNA was isolated from whole lung homogenates. Treatment with ultra-pure LPS (1 µg/g) caused a 25.6 ± 0.9-fold increase in versican (p < 0.001) and a 21.9 ± 3.1-fold increase in Has1 (p < 0.001) as compared to untreated lungs (Fig. 8A), comparable to results with IT administration of standard LPS (Figs. 1B & 2B). No further increases were observed with a higher dose of ultra-pure LPS (2 µg/g). There were no changes to the amounts of decorin or biglycan mRNA in the lungs of mice treated with IT ultra-pure LPS (data not shown), similar to findings with standard LPS (Fig. 1B). Second, we evaluated the effects of IT ultra-pure LPS on versican and Has1 gene expression in the lungs of WT vs TLR-4−/− mice. The ability of IT LPS to stimulate both versican (Fig. 8B) and Has1 (Fig. 8C) was completely abrogated in TLR-4−/− mice. These findings show that TLR-4 is critical for up-regulation of both versican and Has1 by LPS.
Fig. 8.
Versican and Has1are regulated through TLR-4, changes in the relative amounts of mRNA for versican and Has1 were determined using mRNA collected from whole lung homogenates and quantitative real time PCR. A, Comparison of mRNA recovered from lungs of mice treated with PBS, 1 µg/g or 2 µg/g of ultra-pure LPS were made at 4 h. B, Comparison of versican mRNA recovered from lungs of WT or TLR-4−/− mice treated with PBS or 1 µg/g ultra-pure LPS were made at 4 h. C, Comparison of Has1 mRNA recovered from lungs of WT or TLR-4−/− mice treated with PBS or 1 µg/g ultra-pure LPS were made at 4 h. Values are the mean ± SEM with a minimum n= 3 for each group studied. The expression of mRNA is expressed as a relative fold increase in mRNA over the 0 h control. * Shows groups that are significantly different (p ≤ 0.05) when mice treated with PBS and LPS were compared. # shows groups that are significantly different (p ≤ 0.05) when WT vs TLR4−/− mice were compared.
3. Discussion
The goals of this work were to characterize the changes to chondroitin sulfate proteoglycans and hyaluronan in the lungs of mice with gram-negative pneumonia and to identify specific cellular components that contribute to these changes. These extracellular matrix components have been shown to be key mediators in events associated with the inflammatory response (Day and de la Motte, 2005; Wight, 2008). Of particular interest are the roles of macrophages and epithelial cells, as these are the cells that first encounter airborne pathogens and have key roles in the innate immune response of the lungs (Berg et al., 1993; Koay et al., 2002; Skerrett et al., 2004). Live E. coli instilled into the airways of mice induced rapid and selective increases in whole lung mRNA expression of versican and two hyaluronan synthases, Has1 and Has2. To measure changes in the expression of these genes over time and to reduce animal-to-animal variability resulting from live bacteria, we chose to study mice instilled with LPS, a component of the cell wall of gram-negative bacteria that signals through TLR-4 (Poltorak et al., 1998). As with live E. coli, LPS induced rapid and selective increases in whole lung mRNA expression of versican, Has1 and Has2. Whereas there was minimal immunostaining for versican and hyaluronan in the extracellular matrix surrounding bronchioles and bronchiole vessels in mice treated with PBS, there was little to no positive staining of the lung parenchyma or alveolar macrophages. In contrast, immunohistochemistry showed that there was increased accumulation of both molecules as early as 6 h after intratracheal administration of LPS, indicating that versican and hyaluronan expression are early responses to infection. In mice treated with LPS, immunostaining for versican and hyaluronan was increased in areas where neutrophil recruitment had occurred with positive staining around pulmonary veins, in the alveolar septa and versican was present in a subset of alveolar macrophages. These observations were examined in more detail in in vitro studies, which identified increased versican and hyaluronan synthesis in BMDMs treated with LPS and, similarly, in alveolar macrophages treated with LPS. In contrast, primary cultures of murine airway epithelial cells had significant increases in Has2 and Has3 expression, but no changes in versican or Has1 expression. Macrophages did not express Has2 or Has3 under any condition studied. Furthermore, the upregulation of versican and Has1 expression in macrophages was found to be a selective response to LPS, the M1 classical activation agonist, and not to IL-4/IL-13 or IL-10, M2 alternative activation agonists. Finally, no upregulation of versican or Has1 in response to LPS was observed in the lungs of mice lacking TLR-4.
Versican is a protein with a modular design and considerable complexity due to its large size, four known isoforms, and numerous binding partners, including hyaluronan (Wight, 2002; Wu et al., 2005). Because the versican knockout is embryonically lethal, much of what is known about the function of versican comes from studies performed in vitro. These studies show that versican is able to promote leukocyte aggregation (Zheng et al., 2004; Potter-Perigo et al., 2010), angiogenesis (Zheng et al., 2004), endothelial cell migration (Cattaruzza et al., 2002), proliferation, and apoptosis (Sheng et al., 2005). Hyaluronan is a polysaccharide that also has important roles in the tissue response to injury, including angiogenesis, migration, proliferation, and wound healing (Jiang et al., 2005; Bollyky et al., 2012), and is abundant in inflamed tissues (Edelstam et al., 1992). In vivo studies show that versican and hyaluronan are abundantly present in the lung early in gestation, but decrease markedly during fetal lung maturation, indicating that these molecules are important during early lung development (Faggian et al., 2007). Our immunohistochemical findings indicate minimal amounts of positive immunostaining for versican or hyaluronan in lungs of adult mice. However, both versican and hyaluronan are increased in a number of chronic lung diseases including fatal asthma, pulmonary fibrosis, emphysema, acute lung injury, and lymphangioleimyomatosis (Bensadoun et al., 1996, 1997; de Medeiros Matsushita et al., 2005; Merrilees et al., 2008). The interactions between versican and hyaluronan are complex. These molecules are essential for organization and viscoelasticity of the extracellular matrix and help to define cellular phenotype by facilitating processes of migration, proliferation, and differentiation (Yamagata et al., 1989; Yamagata and Kimata, 1994; Evanko et al., 1999; Toole, 2004; Meran et al., 2007; Hattori et al., 2011).
In this study, we report that versican and hyaluronan are increased in the lung during acute inflammation associated with E. coli pneumonia and LPS. We also report that this up-regulation requires TLR-4, as neither versican nor Has1 are increased in response to LPS in the lungs of TLR-4−/− mice. Important studies have shown that proteoglycans (Kim et al., 2009; Frey et al., 2013) and hyaluronan (Jiang et al., 2005) can serve as TLR ligands to modulate the immune response. Our study demonstrates that versican and hyaluronan synthesis are outcomes of TLR-4 activation. Taken together, these studies suggest an autocrine system in which bacterial activation of TLR-4 stimulates synthesis of versican and hyaluronan, products which can themselves interact with TLR-4 to further modulate the inflammatory response.
Our in vitro findings of increased versican mRNA and protein synthesis by macrophages in response to LPS are consistent with our in vivo studies demonstrating increased versican immunostaining in alveolar macrophages of mice exposed to LPS. In contrast, despite significant increases in Has1 mRNA in macrophages treated with LPS, the product of this synthase, hyaluronan, was not detectable by ELSA (data not shown) and could only be detected by manipulation of hyaluronan degradation by chloroquine. Furthermore, while our in vivo studies demonstrate increased hyaluronan by histochemistry in the lungs of mice exposed to LPS, this hyaluronan staining does not localize to alveolar macrophages, which is consistent with our observation that macrophages treated with LPS in vitro rapidly degrade hyaluronan. The apparent discrepancies between expression of Has1 mRNA and hyaluronan synthesis reflect some of the biological and technical challenges to studying the role of hyaluronan in disease. Has expression, hyaluronan synthesis, and hyaluronan accumulation are influenced by a variety of factors, including transcriptional and post-translational regulation of the synthase genes, abundance of UDP-sugar substrates for the hyaluronan synthase enzymes (Jokela et al., 2008; Tammi et al., 2011), and clearance via oxidative or enzymatic degradation of hyaluronan product (Cheng et al., 2013). In addition, the ability to detect hyaluronan by ELSA is molecular mass-dependent and more accurate for higher mass versus lower mass hyaluronan (Yuan et al., 2013).
Our difficulties detecting hyaluronan product either in vivo in alveolar macrophages or in vitro in BMDM cultures could be due to any combination of these factors. Macrophages are highly metabolic cells that rapidly deplete glucose from the media and macrophage glucose consumption is further enhanced by LPS (Fukuzumi et al., 1996), potentially limiting the availability of UDP-glucosamine for hyaluronan synthesis. At the same time, macrophages are highly degradative cells that endogenously express high levels of reactive oxygen species-generating enzymes and hyaluronidases, and both reactive oxygen species-generating enzymes and hyaluronidases are abundantly present under inflammatory conditions in the lung (Monzon et al., 2010; Cheng et al., 2013). Finding that Has1 expression was highest and hyaluronidase 1 and 2 expression were lowest concurrently within hours after exposure of macrophages to LPS suggested an optimal window for measuring hyaluronan. Both hyaluronidase 1 and 2 are hyaluronan-degrading enzymes that function in the acidic environments of lysosomes and of unique endocytic vesicles at the plasma membrane, respectively (Csoka et al., 2001; Tammi et al., 2001). Thus, inhibition of pH-sensitive hyaluronan degradation by pre-treatment with chloroquine allowed us to demonstrate increased hyaluronan synthesis by macrophages in response to LPS. However, hyaluronan turnover and degradation is complex. While the hyaluronidases are responsible for much of hyaluronan catabolism, other enzymatic and non-enzymatic mechanisms that are not inhibitable with chloroquine also contribute. Therefore, it is likely that our results still underestimate total hyaluronan synthesis by macrophages. These results indicate that macrophages rapidly synthesize and degrade hyaluronan, consistent with the highly metabolic and motile phenotype of these cells. This rapid turnover is likely central to the acute inflammatory response to bacterial infection. Several other studies have shown that low molecular weight hyaluronan degradation products may be important regulators of macrophage function during inflammatory responses and that subsequent clearance of these degradation products is essential for resolution of the inflammatory response (Jiang et al., 2011).
We have shown that the versican and Has1 genes are upregulated during differentiation of the human monocytic leukemia cell line, THP1, from monocytes into macrophages (Chang et al., 2012). Transcription profiling of human monocyte-to-macrophage polarization indicates that versican (i.e., chondroitin sulfate proteoglycan 2) is one of the genes that is induced by M1 activation using the combination of LPS and interferon gamma in vitro (Martinez et al., 2006). Similarly, the present in vitro studies indicate that versican and Has1 are induced by M1 macrophage activation with LPS. Hyaluronan interactions with its cell surface receptors, CD44 and RHAMM, are important to inflammatory rolling, adhesion, and cell activation (Hall et al., 1994; DeGrendele et al., 1996) and hyaluronan-containing matrices have been shown to possess immunomodulating properties that dampen M1 inflammatory macrophage functions (Franz et al., 2013). Our in vivo studies indicate positive versican staining in the majority of, but not all, alveolar macrophages in mice exposed to LPS, suggesting that these alveolar macrophages are heterogeneous and differentially activated. This is in keeping with the understanding that alveolar macrophages have diverse roles in maintaining homeostasis and responding to inflammatory crises (Duan et al., 2012) and are heterogeneous in a number of ex vivo functional assays indicative of differential macrophage activation, e.g., release of soluble mediators and migration towards a chemotactic peptide (Brannen and Chandler, 1988). Thus, versican and hyaluronan appear to be markers of pro-inflammatory conditions.
The current data show that LPS exerts differential effects on macrophages and epithelial cells with respect to versican and hyaluronan synthase expression. Thus, it is likely that the selective acute increases in versican and Has1 seen in the whole lung after IT administration of LPS reflect, at least in part, the macrophage response, whereas the whole lung increase in Has2 reflects, at least in part, the epithelial response. The differential regulation of the hyaluronan synthases in macrophages and epithelial cells treated with LPS suggests that the regulation of the genes for these three membrane-bound enzymes differs. The relative specific activities of these Has isoforms and the size distributions of their hyaluronan products are different (Itano et al., 1999; Jiang et al., 2007). We find that LPS induces Has1 mRNA expression by macrophages. Has1 synthesizes a relatively high molecular weight product (Itano et al., 1999). The anti-inflammatory properties of high molecular weight hyaluronan are evidenced by its ability to decrease LPS-induced inflammation in microglial cells (Austin et al., 2012), to promote immune tolerance by augmenting regulatory T cell function cells (Bollyky et al., 2009), and to protect against lung injury in a variety of models (Lennon and Singleton, 2011). We also find that LPS induces Has2 mRNA expression by airway epithelial cells. Lung epithelial cell-specific overexpression of Has2 and its high molecular weight hyaluronan product has been shown to be protective against acute lung injury, in part through TLR-dependent interactions (Jiang et al., 2005).
While macrophages and epithelial cells are the first airway cells to encounter and respond to airborne pathogens, endothelial cells and fibroblasts in the underlying tissue are also likely to respond to infection. The studies reported here, and work of others, indicate that these underlying cells also are involved in the expression and accumulation of versican and hyaluronan. Our immunohistochemical studies of whole lung demonstrate versican in the cytoplasm of endothelial cells within pulmonary veins, and in vitro studies have shown that activation of primary human pulmonary endothelial cells with tumor necrosis factor-α or vascular endothelial growth factor increases the expression of the versican isoforms, V1, V2, and V3 (Cattaruzza et al., 2002). Analyses of bronchoalveolar lavage fluid show that LPS stimulates the production of both tumor necrosis factor-α and vascular endothelial growth factor (data not shown), suggesting that these two inflammatory mediators may contribute to the increase in versican expression by pulmonary endothelial cells. In addition, lung fibroblasts treated with the viral mimetic, poly I:C, secrete a matrix rich in versican and hyaluronan to which T cells bind avidly (Evanko et al., 2012). Activation of fibroblasts by poly I:C requires the adaptor molecule Trif, a signaling molecule that becomes activated when LPS binds to TLR4 (Yamamoto et al., 2003). These findings suggest that activation of TLR4 signaling pathways in lung fibroblasts results in a pericellular matrix rich in versican and hyaluronan. Thus, multiple cell types within the lung have the capacity for increased production of versican and hyaluronan in response to inflammatory conditions.
In summary, these findings suggest that versican and hyaluronan synthesis play an important role in the innate immune response to gram-negative lung infection. Whether versican and hyaluronan are themselves pro-inflammatory molecules or simply a response to inflammation is not yet clear. Understanding the functional roles that these molecules have in the innate immune response to lung infection is the focus of ongoing studies.
4. Materials and methods
4.1. Reagents
E. coli 91–100696 serotype K1 was a clinical isolate obtained from a patient with bacteremia due to biliary sepsis. LPS from E. coli serotype 0111:B4 was purchased from List Biological Laboratories (Campbell, CA). Ultrapure LPS-EB from E. coli serotype 0111:B4 was purchased from Invivogen (San Diego, CA). Colloidal carbon was prepared as previously described (Frevert et al., 2000). The polyclonal rabbit anti-versican antibody (β-GAG) was from Chemicon International (Temecula, CA). Gene-specific TaqMan primer-probe mixes used for quantitative real time PCR of versican, decorin, biglycan, Has1, Has2, Has3, hyaluronidase 1 and 2, and 18S mRNA were purchased from Applied Biosystems (Foster City, CA). IL-4, IL-13, and IL-10were purchased from Peprotech (Rocky Hill, NJ).
4.2. Preparation of bacteria
The use of E. coli serotype K-1 in mice was previously described (Matute-Bello et al., 2001). Methods used to pass and store the bacteria have been detailed elsewhere (Frevert et al., 2000). On the day before an experiment, a frozen aliquot of E. coli was thawed, inoculated into 50 ml Lennox-B broth, and incubated overnight at 37 °C in a shaking incubator. The bacteria were recovered by centrifugation, washed once in PBS, and resuspended in sterile water to 2 × 1010 CFU/ml. Bacterial concentrations were confirmed by quantitative culture using the pour plate method.
4.3. Animal protocols
C57BL/6 and TLR-4−/− mice (7–8 weeks age) (Jackson Laboratories) were housed in an SPF animal facility at the University of Washington and the UW Institutional Animal Care and Use Committee approved all studies performed. Using methods that were previously described, the intratracheal (IT) instillation of live E. coli, E. coli 0111:B4 LPS (1 µg/g), or PBS was performed in mice anesthetized with 3–4% isoflurane (Matute-Bello et al., 2001). To identify the location where PBS, bacteria, or LPS or ultrapure LPS-EB were instilled in lungs at necropsy and in tissues sections, 1% colloidal carbon was mixed into these solutions as previously described (Frevert et al., 2000). Following the IT instillation, mice were recovered and returned to a cage where they were allowed access to food and water for the remainder of the study. At 2, 6, and 24 h after the IT instillation, mice were euthanized with 120 mg/kg pentobarbital. Whole blood was obtained by direct puncture and the plasma was collected and stored at −70 °C until use. The thoracic cavity was opened by a midline incision and the lungs were removed and placed into RNAlater (Ambion, Austin, TX) and stored at 4 °C overnight for the extraction of RNA. For animal studies where tissues were obtained for immunohistochemistry (IHC), C57BL/6 mice were instilled with LPS, euthanized at specified times, then the lungs were cannulated with a 24-gage catheter and perfusion fixed using 10% neutral buffered formalin and a perfusion pressure of 21 cm H2O. Lungs were then processed for light microscopy as previously described (Jiang et al., 2005).
4.4. Isolation of RNA from lung tissue
Total RNA was isolated from lung tissue using the Absolute RNA Miniprep Kit (Stratagene, La Jolla, CA). Genomic DNA was digested by incubation with DNAse I (Ambion) and RNA was reverse transcribed using the High Capacity cDNA Archive Kit (Applied Biosystems) at 25 °C for 10 min, at 37 °C for 2 h, and at 90 °C for 5 min. The resulting cDNAwas used for standard polymerase chain reaction (PCR) and quantitative real-time PCR.
4.5. Measurement of mRNA using quantitative real time PCR
Quantitative real-time PCR was performed using an ABI PRISM7000 Sequence Detector and TaqMan gene expression assays for versican, biglycan, decorin, the hyaluronan synthases, Has1, Has2, and Has3, and 18S (Life Technologies, Grand Island, NY). Real-time PCR was carried out in a total volume of 25 µl with a master mixture including all reagents required for PCR and gene-specific TaqMan primer-probe mixes. Cycle parameters were 50 °C for 2 min, 95 °C for 10min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1min. Delta Ct (ΔCt) was calculated as the difference in threshold cycles (Ct) for the target genes compared to 18S. Relative mRNA levels were calculated by the 2−ΔΔCt method (Livak and Schmittgen, 2001) to express the fold increase over values obtained from RNA collected from untreated mice (i.e., normal lungs) or from untreated cells. No template controls and no reverse transcriptase controls were performed simultaneously.
4.6. Preparation of tissue for immunohistochemistry
Lung tissue was fixed by IT instillation of 4% paraformaldehyde at 21 cm H2O pressure. After inflation, the lungs were immersed in 4% paraformaldehyde, then embedded in paraffin and sectioned using standard methods and used for the immunohistochemical analysis.
4.7. Immunohistochemistry
IHC for versican and affinity histochemistry for hyaluronan were performed with paraffin-embedded tissue. After deparaffinization, endogenous peroxidases were blocked using H2O2 in methanol and tissue sections were rehydrated in a series of graded ethanol. For versican IHC, sections were digested with 0.2 U/ml of chondroitinase ABC to remove chondroitin sulfate side chains and expose versican epitopes. Tissues were then incubated with 5% Carnation non-fat milk in PBS for tissue blocking, followed by overnight incubation with either rabbit anti-versican polyclonal antibody or isotype-matched control IgG. This was followed by biotinylated goat anti-rabbit IgG antibody for 2 h at room temperature. The tissue sections were rinsed twice with PBS and then incubated with the Vector “Elite” ABC-HP kit (Burlingame, CA) in a moist chamber for 30 min at room temperature. Detection was performed using the Vector NovaRed substrate (Burlingame, CA) for 10 min at room temperature. Slides were counterstained with Gills #3 hematoxylin. The same protocol was used for hyaluronan affinity histochemistry, except that the tissue was not treated with chondroitinase ABC and biotinylated hyaluronan binding protein (b-HABP, the N-terminal hyaluronan binding region of aggrecan which has been biotinylated) was used instead of the primary and secondary antibodies.
4.8. Assessment of positive staining for versican and hyaluronan by IHC
The anatomical location of the positive staining for versican and hyaluronan in lungs was identified using tissues collected from animals treated with PBS or LPS for 6 h. A comparative pathologist (CWF) evaluated tissues collected from all animals and the positive staining for versican and hyaluronan was identified in six anatomical locations, bronchioles, bronchial vessels, pulmonary veins, alveolar septa, alveolar macrophages and white blood cells. Colloidal carbon was co-instilled with the PBS and LPS to identify the gross and microscope location of LPS deposition in the lungs. Alveolar macrophages exposed to LPS were identified by cellular morphology and the presence of colloidal carbon in their cytoplasm. Each animal (n = 4 for PBS and n = 6 for LPS treated mice) were individually scored as having positive staining for versican or HA in the six anatomical locations. This analysis was performed in a blinded manner.
4.9. In vitro cell culture studies
Bone marrow-derived macrophages (BMDMs) were isolated from femurs and tibia of mice and cultured in “macrophage medium” (RPMI 1640, 10% FBS, 30% L929 cell supernatant, 2 mM l-glutamine, 100 IU/mL penicillin, and 100 µg/mL streptomycin) (Tanino et al., 2012). After 24 h, adherent cells were placed in macrophage medium, and cultured for 6 days. Macrophages were then re-plated in macrophage medium in a 6-well tissue culture dish at a density of 2 × 106 cells/well for 24 h and then stimulated in the presence or absence of LPS (10 or 100 ng/mL), ultrapure LPS-EB (10 or 100 ng/ml), a combination of IL-4 (10 ng/ml) and IL-13 (10 ng/ml), or IL-10 (10 ng/ml) in RPMI with 10% FBS for 4, 24, or 48 h. Murine alveolar macrophages were isolated from BAL fluid, cultured for 24 h in macrophage medium, and then stimulated with 100 ng/ml LPS for 4 h. Primary mouse tracheal epithelial cells were cultured at an air–liquid interface, as previously described, and stimulated with LPS (10 or 100 ng/mL) for 4 and 24 h (Kassim et al., 2007).
4.10. Isolation of RNA from cell cultures and quantitative real time PCR
Total RNA was obtained from cell culture monolayers using the RNeasy Mini Kit from Qiagen (Valencia, CA). Complementary DNA was prepared by reverse transcription using the High Capacity cDNA Reverse Transcription Kit from Applied Biosciences (Carlsbad, CA). Quantitative real-time PCR was performed using an ABI PRISM 7000 Sequence Detector (Applied Biosystems) to quantify the mRNA expression of versican, the hyaluronan synthases (Has1, Has2, and Has3), the hyaluronidases (Hyal1 and Hyal2), iNOS, Arg1, and 18S as described above.
4.11. Isolation of proteoglycans
Cell medium was collected and combined with protease inhibitors (5 mM benzamidine, 100 mM 6-aminohexanoic acid, and 1 mM phenylmethylsulfonyl fluoride). The cell layer was rinsed with phosphate-buffered saline and solubilized in 8 M urea buffer (8 M urea, 2 mM EDTA, 0 or 0.25 M NaCl, 50 mM Tris–HCl, and 0.5% Triton X-100 detergent, pH 7.4) containing protease inhibitors (Schonherr et al., 1991, 1993). The media contained secreted proteoglycans,whereas cell layers contained cell membrane-associated, intracellular and extracellular matrix proteoglycans. Media and cell layer extracts were concentrated and purified by ion-exchange chromatography on diethylaminoethyl-Sephacel in 8 M urea buffer with 0.25 M NaCl and eluted with 8 M urea buffer containing 3 M NaCl (Schonherr et al., 1991, 1993).
4.12. Versican Western
For Western immunoblotting, proteoglycans isolated by ion-exchange chromatography were digested with 0.05 units chondroitin ABC lyase (North Star BioProducts, East Falmouth, MA) in 0.3 M Tris–HCl, pH 8.0, 0.6 mg/ml bovine serum albumin and 18 mM sodium acetate with protease inhibitors for 3 h at 37 °C) and run on SDS-PAGE (4–12% with 3.5% stacking gel) under reducing conditions (Laemmli, 1970). Separated proteins were electrophoretically transferred to 0.2 µm nitrocellulose membranes (GE Healthcare, Piscataway, NJ) using a BioRad Transblot SD Semi-Dry Transfer Cell (BioRad, Hercules, CA) (Olin et al., 1999). The transferred proteins were then detected with the primary antibody to versican (Millipore), and enhanced chemiluminescence (Western-Light Chemiluminescent Detection System) with proprietary luminescent substrate (Applied Biosystems, Foster City, CA) (Lemire et al., 2007).
4.13. Quantification of [3H]-hyaluronan synthesis
Macrophage cultures were metabolically labeled with [3H]-glucosamine (50 µCi/ml) in the presence or absence of 100 ng/ml LPS and/or 0.5mMchloroquine for 2, 4, 6 and 24 h. Media and cell layer fractions were harvested separately and digested with pronase (100 µg/ml) in 0.5 M Tris pH 6.5 overnight at 37 °C. Following digestion, the pronase was inactivated by heating to 100 °C for 20 min. Radiolabeled macromolecules were then recovered and separated from unincorporated precursor by precipitation on nitrocellulose membranes using slot blot analysis (Agren et al., 1994). Briefly, 200 µl of sample was added to an equal volume of 2% cetylpyridinium chloride, 50 mM NaCl (CPC wash solution) and the solution blotted onto 0.45 µm nitrocellulose membrane (Schleicher and Schuell, Keene, NH). The membrane was washed six times in CPC wash solution and once in deionized water before air-drying at room temperature overnight. Incorporation of [3H]-glucosamine into hyaluronan was measured by digesting an equivalent radiolabeled aliquot with Streptomyces hyaluronidase (2 U/ml) for 24 h at 37 °C before slot blotting. Hyaluronan was measured as the amount of hyaluronidase-sensitive material precipitated to the nitrocellulose membrane. All scintillation counting was done on Beckman LS 6500 (Beckman Instruments, Fullerton, CA).
4.14. Hyaluronan Enzyme-linked Sorbent Assay (ELSA)
Media was digested with 300 µg/ml pronase for 18 h at 37 °C. Following digestion, the pronase was inactivated by heating to 100 °C for 20 min. The hyaluronan ELSA is a modification (Wilkinson et al., 2004) of a previously described (Underhill et al., 1993) competitive ELSA in which the samples to be assayed are first mixed with biotinylated hyaluronan binding protein and then added to hyaluronan-coated microtiter plates; therefore the final signal is inversely proportional to the amount of hyaluronan in the sample. Specifically, Nunc Maxisorp 96-well plates were coated with an excess of hyaluronan (Sigma), which was covalently cross-linked to bovine serum album to enhance its retention by the plastic, and blocked with PBS containing serum. Hyaluronan standards and media samples were pre-incubated with b-HABP. After incubation, the mixtures were added to the wells and excess b-HABP bound to the hyaluronan in the wells, while b-HABP already bound to hyaluronan was washed away. Thus, increasing amounts of hyaluronan resulted in decreasing amounts of b-HABP available to be retained in the wells. Bound b-HABP was detected colorimetrically with peroxidase-labeled streptavidin, H2O2, and the peroxidase substrate 2,2 azinobis (3-ethylbenzthiazoline sulfonic acid) in sodium citrate buffer, pH 4.2. The reaction product was monitored spectrophotometrically at OD405.
4.15. Statistical Analysis
A t-test and p < 0.05was used for statistical analysis of data collected from studies where comparisons were made between PBS- and LPS-treated animals. Statistical analysis of multiple groups was performed using log-transformed data and two-way ANOVA with Bonferroni's Multiple Comparison Test and p < 0.05. Values are reported as means ± standard error of the means (SEM) unless otherwise specified.
Acknowledgements
This work was supported by National Institutes of Health grants HL098067 (to C.W.F. and T.N.W.), RR030249-02 (to C.W.F.) and by the VA Medical Research Service (to C.W.F.).
The authors would like to thank Drs. Adeline Hajjar and Susan Perigo for helpful discussions. The authors also thank Dowon An, Gina Kiske, Vivian Lee, Steven Mongovin, and TaraWigmosta for technical assistance.
Abbreviations
- TLRs
toll-like receptors
- LPS
lipopolysaccharide
- IT
intratracheal
- PCR
polymerase chain reaction
- IHC
immunohistochemistry
- Ct
threshold cycle
- b-HABP
biotinylated hyaluronan binding protein
- BMDMs
bone marrow derived macrophages
- Has
hyaluronan synthase
- Hyal
hyaluronidase
- ELSA
enzyme-linked sorbent assay
- SEM
standard error of the means.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agren UM, Tammi R, Tammi M. A dot-blot assay of metabolically radiolabeled hyaluronan. Anal. Biochem. 1994;217:311–315. doi: 10.1006/abio.1994.1124. [DOI] [PubMed] [Google Scholar]
- Austin JW, Gilchrist C, Fehlings MG. High molecular weight hyaluronan reduces lipopolysaccharide mediated microglial activation. J. Neurochem. 2012;122:344–355. doi: 10.1111/j.1471-4159.2012.07789.x. [DOI] [PubMed] [Google Scholar]
- Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycan deposition in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1996;154:1819–1828. doi: 10.1164/ajrccm.154.6.8970376. [DOI] [PubMed] [Google Scholar]
- Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycans in granulomatous lung diseases. Eur. Respir. J. 1997;10:2731–2737. doi: 10.1183/09031936.97.10122731. [DOI] [PubMed] [Google Scholar]
- Berg JT, Lee ST, Thepen T, Lee CY, Tsan MF. Depletion of alveolar macrophages by liposome-encapsulated dichloromethylene diphosphonate. J. Appl. Physiol. 1993;74:2812–2819. doi: 10.1152/jappl.1993.74.6.2812. [DOI] [PubMed] [Google Scholar]
- Blackwood RA, Cantor JO, Moret J, Mandl I, Turino GM. Glycosaminoglycan synthesis in endotoxin-induced lung injury. Proc. Soc. Exp. Biol. Med. 1983;174:343–349. doi: 10.3181/00379727-174-41746. [DOI] [PubMed] [Google Scholar]
- Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4 + CD25+ regulatory T cells. J. Leukoc. Biol. 2009;86:567–572. doi: 10.1189/jlb.0109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollyky PL, Bogdani M, Bollyky JB, Hull RL, Wight TN. The role of hyaluronan and the extracellularmatrix in islet inflammation and immune regulation. Curr. Diab. Rep. 2012;12:471–480. doi: 10.1007/s11892-012-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannen AL, Chandler DB. Alveolar macrophage subpopulations' responsiveness to chemotactic stimuli. Am. J. Pathol. 1988;132:161–166. [PMC free article] [PubMed] [Google Scholar]
- Cattaruzza S, Schiappacassi M, Ljungberg-Rose A, Spessotto P, Perissinotto D, Morgelin M, Mucignat MT, Colombatti A, Perris R. Distribution of PG-M/versican variants in human tissues and de novo expression of isoform V3 upon endothelial cell activation, migration, and neoangiogenesis in vitro. J. Biol. Chem. 2002;277:47626–47635. doi: 10.1074/jbc.M206521200. [DOI] [PubMed] [Google Scholar]
- Chang MY, Chan CK, Braun KR, Green PS, O'Brien KD, Chait A, Day AJ, Wight TN. Monocyte-to-macrophage differentiation: synthesis and secretion of a complex extracellular matrix. J. Biol. Chem. 2012;287:14122–14135. doi: 10.1074/jbc.M111.324988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Correlation of Hyaluronan Deposition with Infiltration of Eosinophils and Lymphocytes in a Cockroach-Induced Murine Model of Asthma. Glycobiology. 2013;23:43–58. doi: 10.1093/glycob/cws122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20:499–508. doi: 10.1016/s0945-053x(01)00172-x. [DOI] [PubMed] [Google Scholar]
- Day AJ, de la Motte CA. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 2005;26:637–643. doi: 10.1016/j.it.2005.09.009. [DOI] [PubMed] [Google Scholar]
- de Medeiros Matsushita M, da Silva LF, dos Santos MA, Fernezlian S, Schrumpf JA, Roughley P, Hiemstra PS, Saldiva PH, Mauad T, Dolhnikoff M. Airway proteoglycans are differentially altered in fatal asthma. J. Pathol. 2005;207:102–110. doi: 10.1002/path.1818. [DOI] [PubMed] [Google Scholar]
- DeGrendele HC, Estess P, Picker LJ, Siegelman MH. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: a novel lymphocyte-endothelial cell primary adhesion pathway. J. Exp. Med. 1996;183:1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan M, Li WC, Vlahos R, Maxwell MJ, Anderson GP, Hibbs ML. Distinct macrophage subpopulations characterize acute infection and chronic inflammatory lung disease. J. Immunol. 2012;189:946–955. doi: 10.4049/jimmunol.1200660. [DOI] [PubMed] [Google Scholar]
- Edelstam GA, Laurent UB, Lundkvist OE, Fraser JR, Laurent TC. Concentration and turnover of intraperitoneal hyaluronan during inflammation. Inflammation. 1992;16:459–469. doi: 10.1007/BF00918972. [DOI] [PubMed] [Google Scholar]
- Esko JD, Lindahl U. Molecular diversity of heparan sulfate. J. Clin. Invest. 2001;108:169–173. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Potter-Perigo S, Bollyky PL, Nepom GT, Wight TN. Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol. 2012;31:90–100. doi: 10.1016/j.matbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggian J, Fosang AJ, Zieba M, Wallace MJ, Hooper SB. Changes in versican and chondroitin sulfate proteoglycans during structural development of the lung. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R784–R792. doi: 10.1152/ajpregu.00801.2006. [DOI] [PubMed] [Google Scholar]
- Forteza R, Lieb T, Aoki T, Savani RC, Conner GE, Salathe M. Hyaluronan serves a novel role in airway mucosal host defense. FASEB J. 2001;15:2179–2186. doi: 10.1096/fj.01-0036com. [DOI] [PubMed] [Google Scholar]
- Franz S, Allenstein F, Kajahn J, Forstreuter I, Hintze V, Moller S, Simon JC. Artificial extracellular matrices composed of collagen 1 and high-sulfated hyaluronan promote phenotypic and functional modulation of human pro-inflammatory M1 macrophages. Acta Biomater. 2013;9:5621–5629. doi: 10.1016/j.actbio.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Frevert CW, Matute-Bello G, Skerrett SJ, Goodman RB, Kajikawa O, Sittipunt C, Martin TR. Effect of CD14 blockade in rabbits with Escherichia coli pneumonia and sepsis. J. Immunol. 2000;164:5439–5445. doi: 10.4049/jimmunol.164.10.5439. [DOI] [PubMed] [Google Scholar]
- Frey H, Schroeder N, Manon-Jensen T, Iozzo RV, Schaefer L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 2013;280:2165–2179. doi: 10.1111/febs.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzumi M, Shinomiya H, Shimizu Y, Ohishi K, Utsumi S. Endotoxin-induced enhancement of glucose influx into murine peritoneal macrophages via GLUT1. Infect. Immun. 1996;64:108–112. doi: 10.1128/iai.64.1.108-112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Wight TN, Frevert CW. Proteoglycans: key regulators of pulmonary inflammation and the innate immune response to lung infection. Anat. Rec. (Hoboken) 2010;293:968–981. doi: 10.1002/ar.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar AM, Ernst RK, Fortuno ES, III, Brasfield AS, Yam CS, Newlon LA, Kollmann TR, Miller SI, Wilson CB. Humanized TLR4/MD-2 mice reveal LPS recognition differentially impacts susceptibility to Yersinia pestis and Salmonella enterica. PLoS Pathog. 2012;8:e1002963. doi: 10.1371/journal.ppat.1002963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CL, Wang C, Lange LA, Turley EA. Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase activity. J. Cell Biol. 1994;126:575–588. doi: 10.1083/jcb.126.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren R, Samuelsson T, Laurent TC, Modig J. Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1989;139:682–687. doi: 10.1164/ajrccm/139.3.682. [DOI] [PubMed] [Google Scholar]
- Hattori N, Carrino DA, Lauer ME, Vasanji A, Wylie JD, Nelson CM, Apte SS. Pericellular versican regulates the fibroblast–myofibroblast transition: a role for ADAMTS5 protease-mediated proteolysis. J. Biol. Chem. 2011;286:34298–34310. doi: 10.1074/jbc.M111.254938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu. Rev. Cell Dev. Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela TA, Jauhiainen M, Auriola S, Kauhanen M, Tiihonen R, Tammi MI, Tammi RH. Mannose inhibits hyaluronan synthesis by down-regulation of the cellular pool of UDP-N-acetylhexosamines. J. Biol. Chem. 2008;283:7666–7673. doi: 10.1074/jbc.M706001200. [DOI] [PubMed] [Google Scholar]
- Karlinsky JB. Glycosaminoglycans in emphysematous and fibrotic hamster lungs. Am. Rev. Respir. Dis. 1982;125:85–88. doi: 10.1164/arrd.1982.125.1.85. [DOI] [PubMed] [Google Scholar]
- Kassim SY, Gharib SA, Mecham BH, Birkland TP, Parks WC, McGuire JK. Individual matrix metalloproteinases control distinct transcriptional responses in airway epithelial cells infected with Pseudomonas aeruginosa. Infect. Immun. 2007;75:5640–5650. doi: 10.1128/IAI.00799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay MA, Gao X, Washington MK, Parman KS, Sadikot RT, Blackwell TS, Christman JW. Macrophages are necessary for maximal nuclear factor-kappa B activation in response to endotoxin. Am. J. Respir. CellMol. Biol. 2002;26:572–578. doi: 10.1165/ajrcmb.26.5.4748. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemire JM, Chan CK, Bressler S, Miller J, LeBaron RG, Wight TN. Interleukin-1beta selectively decreases the synthesis of versican by arterial smooth muscle cells. J. Cell. Biochem. 2007;101:753–766. doi: 10.1002/jcb.21235. [DOI] [PubMed] [Google Scholar]
- Lennon FE, Singleton PA. Role of hyaluronan and hyaluronan-binding proteins in lung pathobiology. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;301:L137–L147. doi: 10.1152/ajplung.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Malmstrom J, Larsen K, Hansson L, Lofdahl CG, Norregard-Jensen O, Marko-Varga G, Westergren-Thorsson G. Proteoglycan and proteome profiling of central human pulmonary fibrotic tissue utilizing miniaturized sample preparation: a feasibility study. Proteomics. 2002;2:394–404. doi: 10.1002/1615-9861(200204)2:4<394::AID-PROT394>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Mapleson JL, Buchwald M. Effect of cycloheximide and dexamethasone phosphate on hyaluronic acid synthesis and secretion in cultured human skin fibroblasts. J. Cell. Physiol. 1981;109:215–222. doi: 10.1002/jcp.1041090204. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Frevert CW, Liles WC, Nakamura M, Ruzinski JT, Ballman K, Wong VA, Vathanaprida C, Martin TR. Fas/Fas ligand system mediates epithelial injury, but not pulmonary host defenses, in response to inhaled bacteria. Infect. Immun. 2001;69:5768–5776. doi: 10.1128/IAI.69.9.5768-5776.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meran S, Thomas D, Stephens P, Martin J, Bowen T, Phillips A, Steadman R. Involvement of hyaluronan in regulation of fibroblast phenotype. J. Biol. Chem. 2007;282:25687–25697. doi: 10.1074/jbc.M700773200. [DOI] [PubMed] [Google Scholar]
- Merrilees MJ, Ching PS, Beaumont B, Hinek A, Wight TN, Black PN. Changes in elastin, elastin binding protein and versican in alveoli in chronic obstructive pulmonary disease. Respir. Res. 2008;9:41. doi: 10.1186/1465-9921-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzon ME, Fregien N, Schmid N, Falcon NS, Campos M, Casalino-Matsuda SM, Forteza RM. Reactive oxygen species and hyaluronidase 2 regulate airway epithelial hyaluronan fragmentation. J. Biol. Chem. 2010;285:26126–26134. doi: 10.1074/jbc.M110.135194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettelbladt O, Hallgren R. Hyaluronan (hyaluronic acid) in bronchoalveolar lavage fluid during the development of bleomycin-induced alveolitis in the rat. Am. Rev. Respir. Dis. 1989;140:1028–1032. doi: 10.1164/ajrccm/140.4.1028. [DOI] [PubMed] [Google Scholar]
- Nettelbladt O, Tengblad A, Hallgren R. Lung accumulation of hyaluronan parallels pulmonary edema in experimental alveolitis. Am. J. Physiol. 1989;257:L379–L384. doi: 10.1152/ajplung.1989.257.6.L379. [DOI] [PubMed] [Google Scholar]
- Nielsen MJ, Madsen M, Moller HJ, Moestrup SK. The macrophage scavenger receptor CD163: endocytic properties of cytoplasmic tail variants. J. Leukoc. Biol. 2006;79:837–845. doi: 10.1189/jlb.1005602. [DOI] [PubMed] [Google Scholar]
- Olin KL, Potter-Perigo S, Barrett PH, Wight TN, Chait A. Lipoprotein lipase enhances the binding of native and oxidized low density lipoproteins to versican and biglycan synthesized by cultured arterial smooth muscle cells. J. Biol. Chem. 1999;274:34629–34636. doi: 10.1074/jbc.274.49.34629. [DOI] [PubMed] [Google Scholar]
- Parish CR. The role of heparan sulphate in inflammation. Nat. Rev. Immunol. 2006;6:633–643. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Potter-Perigo S, Johnson PY, Evanko SP, Chan CK, Braun KR, Wilkinson TS, Altman LC, Wight TN. Polyinosine-polycytidylic acid stimulates versican accumulation in the extracellular matrix promoting monocyte adhesion. Am. J. Respir. Cell Mol. Biol. 2010;43:109–120. doi: 10.1165/rcmb.2009-0081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonherr E, Jarvelainen HT, Sandell LJ, Wight TN. Effects of platelet-derived growth factor and transforming growth factor-beta 1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J. Biol. Chem. 1991;266:17640–17647. [PubMed] [Google Scholar]
- Schonherr E, Jarvelainen HT, Kinsella MG, Sandell LJ, Wight TN. Platelet-derived growth factor and transforming growth factor-beta 1 differentially affect the synthesis of biglycan and decorin by monkey arterial smooth muscle cells. Arterioscler. Thromb. 1993;13:1026–1036. doi: 10.1161/01.atv.13.7.1026. [DOI] [PubMed] [Google Scholar]
- Sheng W, Wang G, Wang Y, Liang J, Wen J, Zheng PS, Wu Y, Lee V, Slingerland J, Dumont D, Yang BB. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol. Biol. Cell. 2005;16:1330–1340. doi: 10.1091/mbc.E04-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L143–L152. doi: 10.1152/ajplung.00030.2004. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Belperio JA, Keane MP. Cytokines in innate host defense in the lung. J. Clin. Invest. 2002;109:699–705. doi: 10.1172/JCI15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammi R, Rilla K, Pienimaki JP, MacCallum DK, Hogg M, Luukkonen M, Hascall VC, Tammi M. Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J. Biol. Chem. 2001;276:35111–35122. doi: 10.1074/jbc.M103481200. [DOI] [PubMed] [Google Scholar]
- Tammi RH, Passi AG, Rilla K, Karousou E, Vigetti D, Makkonen K, Tammi MI. Transcriptional and post-translational regulation of hyaluronan synthesis. FEBS J. 2011;278:1419–1428. doi: 10.1111/j.1742-4658.2011.08070.x. [DOI] [PubMed] [Google Scholar]
- Tanino Y, Chang MY, Wang X, Gill SE, Skerrett S, McGuire JK, Sato S, Nikaido T, Kojima T, Munakata M, Mongovin S, Parks WC, Martin TR, Wight TN, Frevert CW. Syndecan-4 regulates early neutrophil migration and pulmonary inflammation in response to lipopolysaccharide. Am. J. Respir. Cell Mol. Biol. 2012;47:196–202. doi: 10.1165/rcmb.2011-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- Underhill CB, Nguyen HA, Shizari M, Culty M. CD44 positive macrophages take up hyaluronan during lung development. Dev. Biol. 1993;155:324–336. doi: 10.1006/dbio.1993.1032. [DOI] [PubMed] [Google Scholar]
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr. Opin. Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- Wight TN. Arterial remodeling in vascular disease: a key role for hyaluronan and versican. Front. Biosci. 2008;13:4933–4937. doi: 10.2741/3052. [DOI] [PubMed] [Google Scholar]
- Wilkinson TS, Potter-Perigo S, Tsoi C, Altman LC, Wight TN. Pro- and anti-inflammatory factors cooperate to control hyaluronan synthesis in lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2004;31:92–99. doi: 10.1165/rcmb.2003-0380OC. [DOI] [PubMed] [Google Scholar]
- Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Kimata K. Repression of a malignant cell-substratum adhesion phenotype by inhibiting the production of the anti-adhesive proteoglycan, PG-M/versican. J. Cell Sci. 1994;107(Pt 9):2581–2590. doi: 10.1242/jcs.107.9.2581. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Suzuki S, Akiyama SK, Yamada KM, Kimata K. Regulation of cell-substrate adhesion by proteoglycans immobilized on extracellular substrates. J. Biol. Chem. 1989;264:8012–8018. [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Yuan H, Tank M, Alsofyani A, Shah N, Talati N, LoBello JC, Kim JR, Oonuki Y, de la Motte CA, Cowman MK. Molecular mass dependence of hyaluronan detection by sandwich ELISA-like assay and membrane blotting using biotinylated hyaluronan binding protein. Glycobiology. 2013;23:1270–1280. doi: 10.1093/glycob/cwt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng PS, Vais D, Lapierre D, Liang YY, Lee V, Yang BL, Yang BB. PG-M/versican binds to P-selectin glycoprotein ligand-1 and mediates leukocyte aggregation. J. Cell Sci. 2004;117:5887–5895. doi: 10.1242/jcs.01516. [DOI] [PubMed] [Google Scholar]