Summary
The cilium is a specialized extension of the cell where many specific proteins are admitted and retained, while many others are excluded or expelled. In order to maintain the organelle, the cell must possess mechanisms for the selective gating of protein entry, as well as for the targeted transport of proteins to the cilium from their sites of synthesis within the cell. We hypothesized that the cell employs cytoplasmic vesicles as vehicles not only for the transport of proteins destined for the ciliary membrane, but also for the transport of axonemal proteins to the cilium by means of peripheral association with vesicles. To test this hypothesis we employed two different experimental strategies: 1. the isolation and biochemical characterization of cytoplasmic vesicles that carry ciliary proteins; and 2. the in situ localization of ciliary proteins on cytoplasmic vesicle surfaces using gold labeling and electron microscopy. Our findings indicate that structural proteins destined for the ciliary axoneme are attached to the outer surfaces of cytoplasmic vesicles that carry integral ciliary membrane proteins during the process of ciliary growth.
Results
Isolation of a class of cytoplasmic vesicles that carry proteins of the ciliary membrane and axoneme
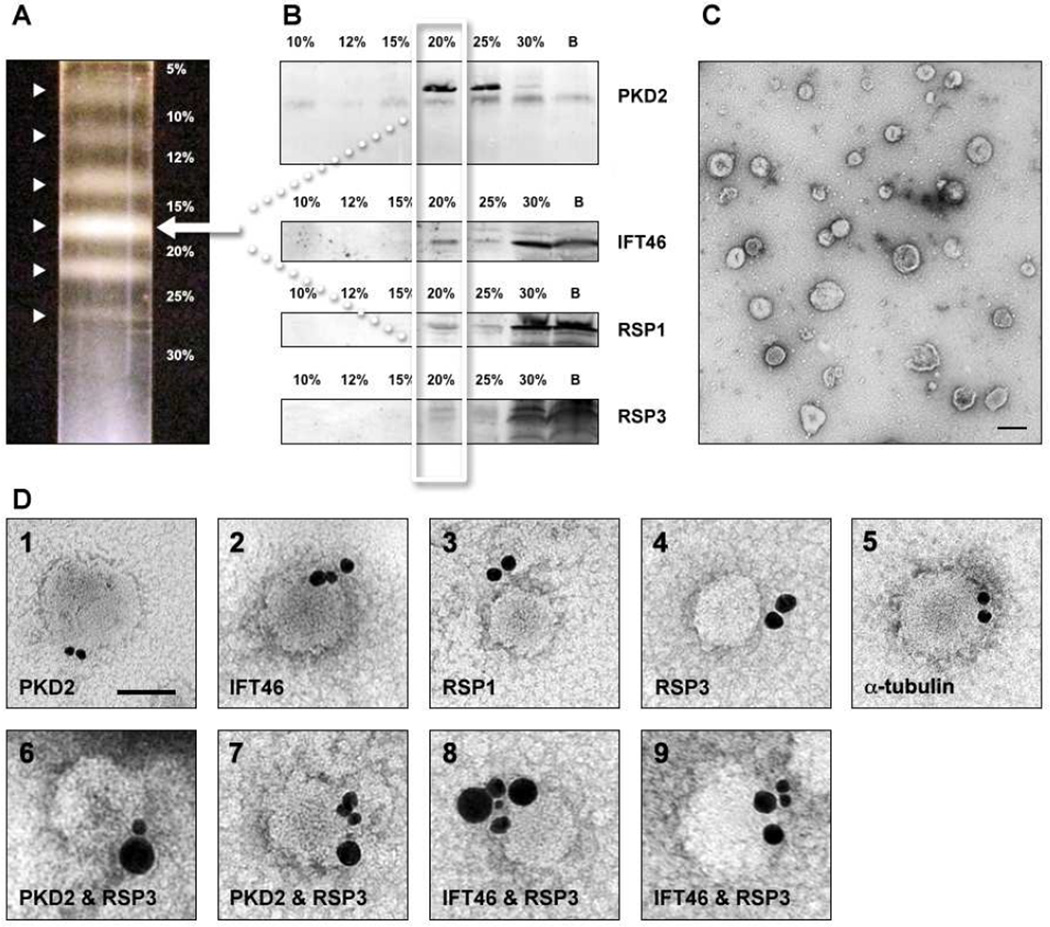
To obtain a vesicle-rich cytoplasmic extract, cell wall-less Chlamdyomonas were subjected to a brief pulse of mechanical stress using a motor-driven blade. Under these conditions, the cells released cytoplasm and vesicles, but remained largely intact - retaining most big organelles such as nuclei, chloroplasts, and mitochondria. Following the removal of cell bodies and debris by low-speed centrifugation, membrane vesicles were separated from the cytoplasmic extract by floatation up through a seven-step, discontinuous OptiPrep (iodixanol) density gradient. After floatation-centrifugation, visibly prominent membrane bands were observed at each of the gradient's six density interfaces (Figure 1, A). SDSPAGE and immunoblot analysis of each of these membrane bands revealed that the integral flagellar membrane protein, PKD2 (polycystic kidney disease 2)[1], floated to the top of the 20% gradient layer. The membrane band at this 20% layer also carried the intraflagellar transport protein, IFT46, and axonemal radial spoke proteins (RSPs) (Figure 1, B). Negative staining and electron microscopy revealed the band at the 20% layer to be composed of floated membrane vesicles, ranging in diameter from 60 nm – 100 nm (Figure 1, C).
Figure 1. Isolation of cytoplasmic vesicles with associated ciliary proteins.
A. Cytoplasmic extract, obtained by mechanical disruption of cells, was placed at the bottom of a seven-step, discontinuous OptiPrep (iodixanol) density gradient. During high-speed centrifugation, various classes of membrane vesicles in the extract float up to different interfaces of the gradient according to their density (bands indicated by white arrow heads). B. Isolated gradient fractions were subjected to SDSPAGE and analyzed on immunoblots probed with antibodies specific for ciliary membrane and axonemal proteins. The class of membrane vesicles that floats to the 20% gradient interface carries with it PKD2, IFT and radial spoke proteins (RSPs). A fraction from the bottom of the gradient (30%) was loaded to the lane labeled B. C. The fraction at the 20% gradient interface was determined by negative staining and TEM to be composed of membrane vesicles ranging in diameter from ~60 – 120 nm. The scale bar in panel C1 represents 100 nm. D. Silver-enhanced, immunogold labeling of intact, negatively-stained vesicles from the 20% gradient interface fraction showed the presence of PKD2, IFT46, RSPs, and α-tubulin on their outside surfaces (1 – 5). Double labeling performed sequentially with differential silver enhancement revealed that RSP3 (larger silver-enhanced gold particles) could be found associated with the same vesicles as PKD2 and IFT46 (smaller silver-enhanced gold particles) (6 – 9). The scale bar in panel D1 represents 50 nm.
Immunogold labeling of the membrane vesicles obtained from the 20% gradient layer showed that IFT46, RSPs, α-tubulin, and an epitope of the membrane-spanning protein PKD2 can all be found on the outside surfaces of the intact, isolated cytoplasmic vesicles (Figure 1, D1–5). With immunogold co-labeling and differential silver enhancement, RSP3 (larger silver-enhanced gold particles in Figure 1, D6 – 9) could be found on the same vesicles as PKD2 and IFT46 (smaller silver-enhanced gold particles in Figure 1, D6 – 9).
As observed in previous studies of cytoplasmic vesicle membrane protein complexes, such as the BBSome and clathrin [2], a small fraction of the total protein was floated on vesicles while much of it remained partitioned in the bottom, dense layer of the gradient (Figure 1, 30% and B). These dense, non-vesicle-associated portions of IFT and RSPs likely derive from the large accumulations of these proteins found in the cell at the bases of cilia. Indeed, it is known that greater than 80% of the total complement of IFT protein is located not as trains within the flagella, but in the cell body, accumulated in a pool where the transitional fibers contact the periciliary membrane [3, 4]. RSPs are observed to accumulate in the same location ([5–7], Figure 3, L and M). According to our model (Figure 4), these accumulated pools of ciliary proteins are a result of the exocytosis of cytoplasmic vesicles that carry ciliary proteins on their outer surface, thus positioning their ciliary protein cargo on the inner surface of the cell membrane. These post-exocytosis, periciliary pools represent the likely source of the substantial amounts of IFT protein and RSPs that are not vesicle-associated in the cytoplasmic extracts analyzed by density gradients in Figure 1. In the presence of 1% Triton X-100 detergent, all of the flagellar proteins remained at the bottom layer of the gradient (not shown) indicating that it was their association with membrane that floated some of them up to the interface at the top of the 20% OptiPrep layer.
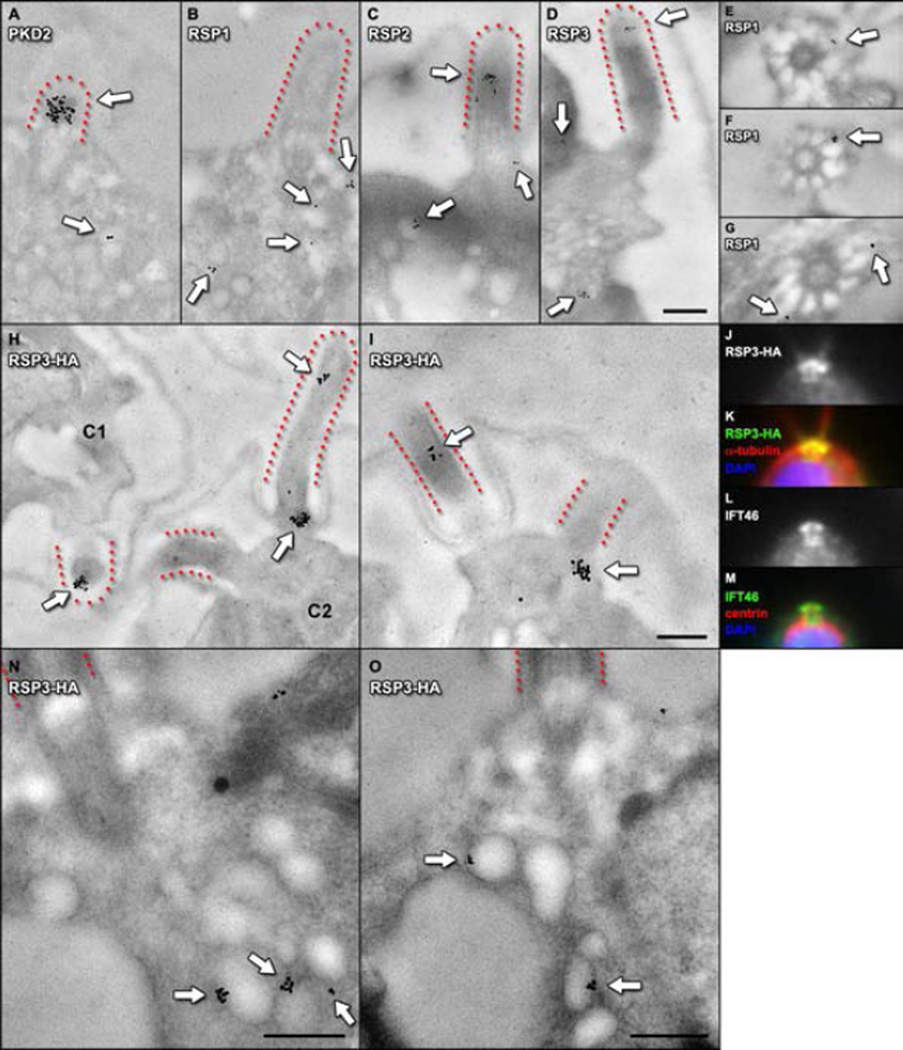
Figure 3. In situ immunogold labeling of ciliary membrane and axonemal proteins during flagellar regeneration.
A. White arrows indicate PKD2 specific gold particles clustering on the membrane of a newly forming Chlamydomonas flagellum (emphasized by dotted outline), and on membrane vesicles nearby in the cytoplasm. B – D. In addition to labeling the growing axoneme (C, D), gold particles specific for radial spoke proteins are observed on the surface of membrane vesicles in the cytoplasm beneath newly forming flagella (RSP1, RSP2 and RSP3; white arrows). E – G. Cross sections proximal to the base of newly forming flagella show transitional fibers radiating from basal bodies. RSP specific gold particles are found clustered at the cell membrane where transitional fibers terminate. H, I. An rsp3 null mutant cell line was rescued with an HA-tagged version of RSP3. Immunogold labeling with HA-specific antibodies showed labeling of newly forming flagellar axonemes (H, C1 and C2) and large clusters of RSP3-HA-specific gold particles were found at the region where transitional fibers attach to the cell membrane at the base of flagella (H, C1; I). J – M. Immunofluorescence localization with antibodies specific for RSP3-HA (J, K) and IFT46 (L, M) show similar patters of localization at the base of flagella, consistent with the immunogold localization results. N, O. Representative images of RSP3-HA-specific gold particles observed on membrane vesicles in the cytoplasm beneath growing flagella. The scale bars represent 200 nm.
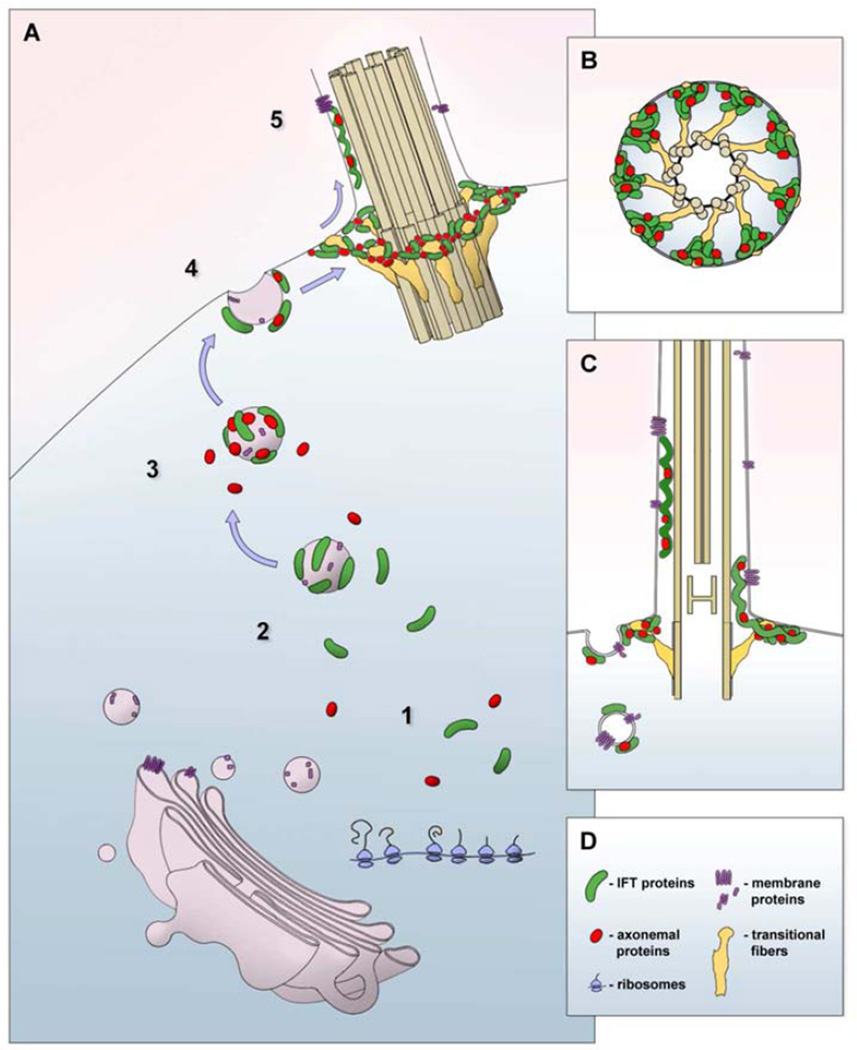
Figure 4. A model for the movement of ciliary proteins from the cytoplasm to the base of the cilium by association with cytoplasmic vesicles.
A. 1. IFT proteins (green) and axonemal proteins (red) are synthesized on free polysomes (blue) in the cytoplasm. 2. IFT proteins associate with the outer surface of cytoplasmic vesicles. 3. Axonemal proteins associate with IFT complexes on the surface of cytoplasmic vesicles. 4. IFT and axonemal protein complexes are delivered to the inner surface of the cell membrane following fusion (exocytosis) of cytoplasmic vesicles. 5. IFT trains carrying axonemal proteins emerge from a membrane-associated pool of complexes around the transitional fibers (yellow) at the ciliary base. B. A cartoon representation of a cross sectional view through the region where the transitional fibers radiate from the basal body to their points of contact with the cell membrane at the base of the cilium. A pool of IFT and axonemal proteins accumulates at the place where transitional fibers meet the membrane. C. A cartoon representation of a longitudinal sectional view through the base of the cilium. Transitional fibers (yellow) extend from the basal body to the cell membrane. IFT trains are shown originating from the membrane-associated pool of IFT and axonemal proteins observed where the transitional fibers meet the cell membrane. The locations of membrane-spanning ciliary proteins within this model are shown in purple.
The appearance of ciliary proteins on cytoplasmic vesicles is amplified during flagellar regeneration
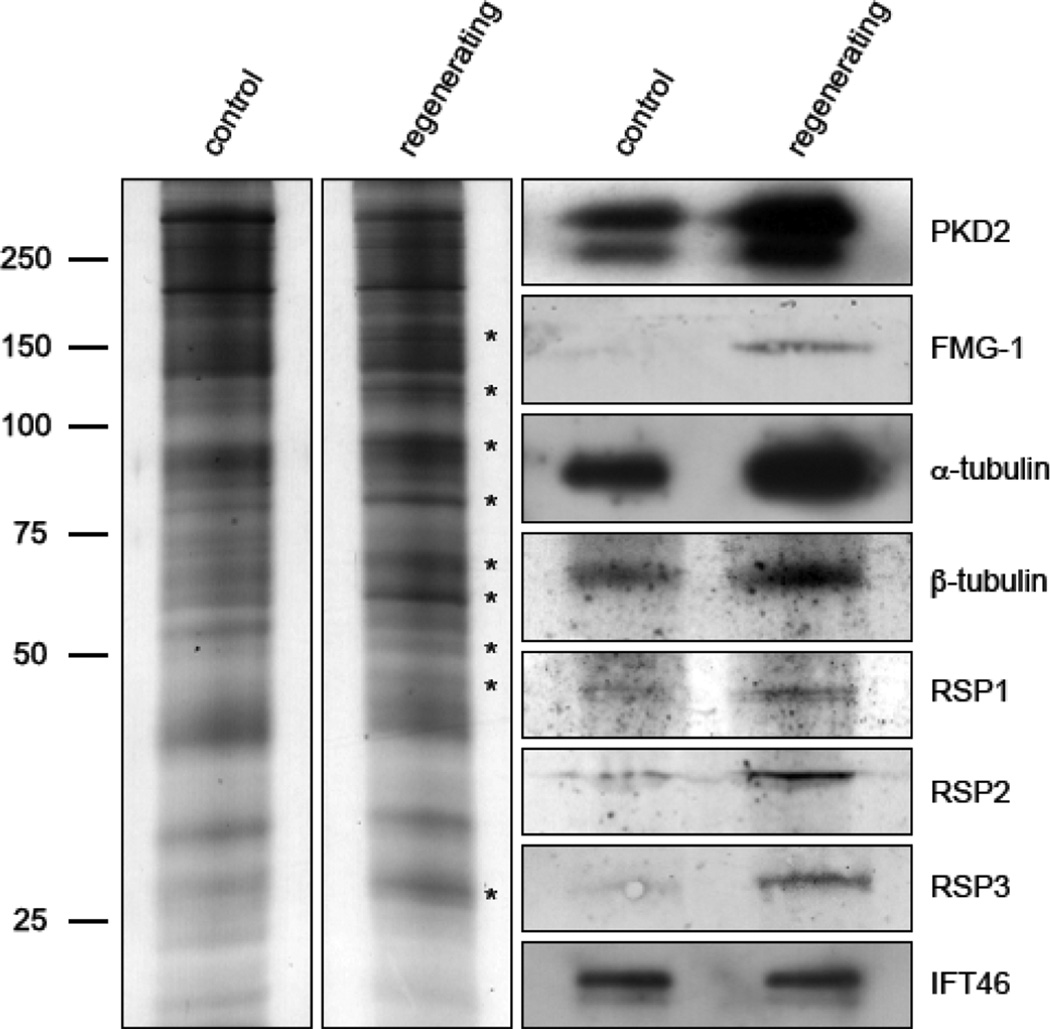
Because it has been clearly demonstrated that flagellar protein synthesis is up-regulated in cells with regenerating flagella [8, 9], cytoplasmic membrane vesicles were obtained from OptiPrep gradients, as above, from two populations of cells for comparison: one population in an early stage of synchronous flagellar growth (regenerating) and a control population with full length flagella. Equal amounts of protein from both 20% OptiPrep vesicle fractions were compared by SDSPAGE and immunoblotting (Figure 2). Silver-stained, polyacrylamide gels showed several band differences between vesicles from control cells and vesicles from cells with regenerating flagella (the most prominent differences are marked by asterisks, Figure 2). Immunoblots indicated that the amounts of several ciliary proteins were increased in the vesicle fraction from regenerating cells. These included the major flagellar membrane glycoprotein (FMG-1) and PKD2 of the flagellar membrane, as well as tubulin and RSPs of the flagellar axoneme (Figure 2). The amount of IFT46 associated with vesicles increased little during regeneration, perhaps reflecting the more general role for vesicle-associated IFT in vesicle trafficking suggested elsewhere [10–12]. Probing of these immunoblots with antibodies specific for proteins of the chloroplast membrane (photosystem II protein D1, PsbA), mitochondrial membrane (alternative oxidase, AOX1), and plasma membrane (H+ATPase) gave negative results, indicating that these membrane compartments are not significant contaminants of the vesicle preparation (Figure S1). When compared by SDSPAGE and immunoblotting, the bottom, 30% OptiPrep layers from regenerating and control cells did not show a detectable difference in their respective levels of IFT, RSP, or α-tubulin (Figure S3).
Figure 2. Proteins of the ciliary membrane and axoneme associate with cytoplasmic vesicles in increased amounts during flagellar regeneration.
The 20% interface vesicle fraction was obtained from control (full length flagella) and cells undergoing flagellar growth (regenerating) and compared by SDSPAGE and immunoblotting. Shown to the left are silver-stained SDSPAGE gels resulting from equal protein loads of control and regenerating vesicle fractions. Prominent band differences are given asterisks. Shown to the right are immunoblots probed with antibodies specific for the indicated ciliary proteins.
Localization of ciliary proteins on cytoplasmic membrane vesicles in situ during ciliary growth
To verify that the gradient co-floatation of axonemal proteins with membrane vesicles was indeed due to an association of these proteins with cytoplasmic vesicles in situ, and not the result of contamination of the isolated vesicle fraction with axonemal proteins during cell disruption, cells harvested during flagellar growth were observed by thin section TEM with gold antibody labeling. First, the in situ localization of PKD2, as a representative protein of the flagellar membrane, was examined [1]. Newly-forming flagella (Figure 3, marked with dotted outlines) were labeled with clusters of antibody-conjugated gold particles specific for PKD2. This was particularly evident in tangential sections through the budding flagellar membrane, such as the one shown in panel A of Figure 3. As expected, PKD2-specific gold particles were also found on membrane vesicles in the region of cytoplasm beneath the growing flagella (Figure 3, A). Next, the in situ localizations of RSPs, as representative proteins of the flagellar axoneme, were examined. RSP-specific gold particles labeled growing flagella (Figure 3, C, D, H), and were also routinely observed clustering at the periphery of vesicles in the cytoplasm (Figure 3, B – D, N, O).
In order to assess quantitatively for antibody specificity and to control for non-specific background labeling, the in situ gold localization experiments were repeated with an RSP3 null mutant (pf14) that had been rescued with a hemagglutinin-tagged version of RSP3 (RSP3-HA) [6]. In the RSP3-HA-expressing cells, HA-specific antibodies gave the same pattern of localization observed with RSP-specific antibodies in wildtype cells during regeneration (Figure 3, N, O). The amounts of HA gold clusters per unit area of nucleus, chloroplast, and cytoplasm were quantified in RSP3-HA cell sections and in wildtype control cell sections lacking the HA epitope (Figure S2, A). A comparison between RSP3-HA and wildtype control sections revealed a low level of non-specific, background labeling in the nucleus and chloroplast regions that remained constant whether or not the HA epitope was present. However, in the region of cytoplasm between the nucleus, chloroplast and basal bodies, where cytoplasmic vesicles are found, the number of RSP3-HA gold particle clusters per unit area was several fold higher in the RSP3-HA cell sections, with only a low level of nonspecific labeling found in wildtype controls. The distance between each RSP3-HA-specific gold particle cluster found in the cytoplasm and the nearest vesicle membrane surface was measured. The distribution of distances, displayed graphically in Figure S2 B, shows that nearly all RSP3-HA-specific labeling in the cytoplasm resided adjacent to vesicle membrane surfaces (a distance measurement of zero). These findings indicate that the cytoplasmic vesicle labeling specific for RSP3 is not the result of nonspecific, background labeling, and, more importantly, that the associations observed between axonemal RSPs and isolated vesicles could be corroborated in situ.
Localization of ciliary axonemal proteins in a pool at the periciliary membrane
Striking accumulations of both RSP3 and IFT46 were observed at the bases of flagella by immunofluorescence localization (Figure 3, J – M). The accumulation of RSPs, at the resolution afforded by widefield fluorescence microscopy, is characterized by a bright lobe of fluorescence in the region of each basal body with an arm of fluorescence extending interiorly, within the so-called “zone of exclusion” terminating near the nucleus. This matches the characteristic pattern of fluorescence localization observed for IFT46 and other IFT complex B proteins (Figure 3, L and M; [5–7]). A previous TEM immunogold labeling study identified the transitional fibers at the bases of flagella as the docking sites for IFT particles [3]. Here, in TEM cross sections through the region at the base of the flagellum where transitional fibers are visible, clusters of RSP-specific gold particles localized specifically to the point of contact between the transitional fibers and the cell membrane; the same pattern previously observed for the IFT complex B protein, IFT52 (Figure 3, E – G) [3]. In longitudinal sections through this region of the cell membrane at the bases of flagella, large clusters of RSP-specific gold particles were observed (Figure 3, H and I). These findings suggest that the RSPs associated with cytoplasmic vesicles during flagellar growth are ultimately delivered to a pool of axonemal cargo together with IFT proteins at the cell membrane where transitional fibers are attached.
Discussion
A substantial up-regulation of synthesis of flagellar proteins occurs in Chlamydomonas soon after the removal of flagella [8, 9]. With this burst of protein synthesis, cells quickly begin the regeneration of new flagella within a duration of only a few minutes. Because the ciliary membrane and its specialized set of membrane-spanning proteins ultimately derive from a post-Golgi vesicle source, a route of transit exists in which cytoplasmic vesicles carry ciliary membrane proteins from their sites of synthesis within the cell. Little is known, however, about how non-membrane-spanning proteins arrive at the cilium. How do structural proteins of the axoneme such as radial spokes and tubulin, for example, travel from their cytoplasmic site of synthesis on free polysomes to their point of entry at the base of the cilium? We hypothesized that axonemal proteins are carried peripherally on the outside surfaces of vesicles already on their way to deliver ciliary membrane proteins and lipids to the growing cilium.
Documented here is the finding that structural proteins, destined for incorporation into the assembling ciliary axoneme, are associated with cytoplasmic vesicles during the process of ciliary growth. By utilizing a unique set of advantages offered by the Chlamydomonas flagella-regenerating system, we have visualized these vesicles in isolation as well as in situ and show that they carry proteins of both the membrane and axoneme of the cilium. Our data indicate that the association of axonemal proteins with cytoplasmic vesicles is significantly increased and more readily detected during flagellar regeneration; a time when production of these proteins is up-regulated (Figure 2) [8]. Previous studies have identified an association between IFT proteins and cytoplasmic membrane vesicles in a variety of cell types [7, 10–15]. However, the detection of a transient, peripheral association between axonemal proteins, such as RSPs, and vesicles may necessitate that the system under study be in the process of actively building a new cilium. A cell that is not in the process of growing a new cilium may be engaged in little cytoplasmic trafficking of axonemal proteins to the ciliary base. Consistent with this is the observation that, like IFT proteins, RSPs accumulate in a substantial pool at the base of the flagellum (Figure 3). Given such a stockpile of RSPs, it is not surprising that one detects little association of RSPs with cytoplasmic vesicles in the non-regenerating cell (Figure 2).
Central to the model proposed in Figure 4 is the association of IFT proteins with membrane vesicles following their synthesis in the cytoplasm on free polysomes. It has been proposed, based on the homology of IFT proteins to components of the COPI and clathrin vesicle coat proteins, that IFT evolved from a specialized form of coated vesicle transport [16–18]. Since this proposal, multiple studies have provided evidence for the involvement of IFT proteins in vesicle trafficking and exocytosis. An association between IFT20 and membrane vesicles of the Golgi complex was observed in mammalian cells, and this cytoplasmic membrane localization was required for the vesicle trafficking of membrane proteins to the cilium [13–15]. Several IFT components are expressed in non-ciliated T lymphocytes where they play a role in vesicle exocytosis and are required for immune synapse formation [10]. The association of IFT proteins with cytoplasmic vesicles was further documented by immunoelectron microscopy showing IFT proteins in situ on post-Golgi vesicles in photoreceptor cells, and on vesicles in the post-synaptic terminals of non-ciliated secondary neurons in mice [11]. IFT proteins have also been found by immunoelectron microscopy on vesicles around the forming mitotic cleavage furrow of Chlamydomonas [7]. Thus, there are precedents for the association of IFT proteins with membrane vesicles in the cytoplasm as illustrated in the model proposed here.
Though the results of our immunogold co-labeling experiments indicate that RSPs can be found on the same cytoplasmic vesicles that are carrying IFT proteins (Figure 1, D6 – 9), it is possible that RSPs bind vesicle membranes directly, or via an intermediary other than IFT proteins. Evidence of a direct association between tubulin and membrane, for example, has been reported [19–22]. It is possible that the vesicle association of RSPs reported here takes place between the membrane and IFT proteins, or occurs independently of membrane associated IFT proteins. This scenario would be in contrast to the order of events proposed in Figure 4, where IFT proteins are depicted as associating with membrane vesicles first and mediating the membrane vesicle association of axonemal proteins like RSPs. Figure 2 shows that only trace amounts of RSPs are detected in association with cytoplasmic vesicles isolated from control cells that are not actively building flagella. IFT46 however is detected in appreciable amounts on cytoplasmic vesicles isolated from both control and regenerating cells (Figure 2). This finding suggests that the vesicle membrane association of IFT proteins is not dependent on axonemal cargo binding, and that RSPs, for example, are not associating with membrane vesicles first and then recruiting IFT proteins.
The prevailing idea is that IFT complexes bind axonemal cargos and mediate their transport within the cilium, and high-resolution EM tomographic studies have revealed a tight association of IFT complexes with the inner surface of the ciliary membrane [23]. Our findings show that IFT proteins and axonemal cargoes are both found on cytoplasmic vesicles during flagellar growth, and that axonemal cargoes are subsequently found accumulated together with IFT proteins at the point of contact between transitional fibers and the cell membrane (Figure 3). Taken together, this information suggests that IFT complexes assemble on membrane surfaces following IFT protein synthesis and tend to maintain an association with membrane while carrying out their cellular functions.
The cilium is a dynamic cell organelle whose formation and maintenance require a membrane flux [24]. The provision of new membrane to the cilium takes place in the form of vesicles that travel to and fuse with its adjoining cell membrane. In addition to this process, cilia also require a flux of soluble, non-membrane-spanning proteins in order to build and maintain their dynamic architecture. The data reported here showing that axonemal proteins associate with the outside surface of cytoplasmic vesicles during ciliary growth, suggest that the cell is coupling the delivery of structural components with the delivery of membrane. A cell biological process such as this would provide an efficient solution that satisfies two needs with essentially one transport modality.
Experimental Procedures
Cell Culturing and Extraction of Cytoplasm
Chlamydomonas reinhardtii strains used in these studies included cell wall-less strain, cw15 (Chlamydomonas Culture Center), and paralyzed flagella strain, pf14, that was rescued by expression of an HA-tagged RSP3 [25]. For extraction of cytoplasm, cw15 cells were cultured in 8 L of M1 medium [26] on a light-dark cycle of 12h:12h at 18 C with constant aeration. When a density of 106 cells ml−1 was reached, cells were harvested by low speed centrifugation, resuspended in M1 medium and deflagellated by pH shock as described previously [27, 28]. During the first few minutes of synchronous flagellar regeneration, cells were harvested by centrifugation and their cytoplasm was extracted by mechanical disruption with an Omnimixer (Sorvall) as described previously [6]. A clarified cytoplasmic extract was obtained by centrifugation at 16,000 g for 15 minutes to remove cells and debris.
Isolation of cytoplasmic vesicles
Separation of vesicle membranes from the cytoplasmic extract was achieved by floatation-centrifugation through a seven-step, OptiPrep (Sigma-Aldrich) gradient. A mixture of 2 ml cytoplasmic extract and 2 ml 60% OptiPrep was loaded to the bottom of a 15 ml ultracentrifuge tube. Additional 1 ml OptiPrep layers of 25%, 20%, 15%, 12%, 10%, and 5%, diluted with HMDEK buffer (10 mM Hepes, pH 7.2, 5 mM MgSO4, 1 mM DTT, 0.5 M EDTA, and 25 mM KCl), were sequentially overlaid by pipette to create the discontinuous step gradient. Gradients were centrifuged for 3 hours at 200,000 g. Following floatation-centrifugation, membrane bands were visible to the naked eye at each density interface and were extracted manually by pipette. The preparations of plasma membranes and flagellar membranes used in figure S1 were obtained using previously published methods [29, 1]
Immunolectron Microscopy
For detailed immunogold-labeling methods, please see the Supplemental Information section.
Immunofluorescence Microscopy
Widefield immunofluorescence microscopy was performed according to previously published methods [7].
Supplementary Material
Highlights.
-
▪
Axonemal proteins associate with cytoplasmic vesicles during ciliary growth
-
▪
Axonemal proteins pool together with IFT proteins at the periciliary membrane
-
▪
A novel mechanism for the transport of structural proteins to the cilium
Acknowledgements
We thank Dennis Diener for his insightful comments and Kaiyao Huang for his preparations of Chlamydomonas plasma membrane. We are grateful to Robert Bloodgood for sharing FMG-1 antibodies. This work was supported by grants from the National Institutes of Health (GM014642 to J.L.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J. Cell Biol. 2007;179:501–514. doi: 10.1083/jcb.200704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 3.Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Fan ZC, Williamson SM, Qin H. Intraflagellar transport (IFT) protein IFT25 is a phosphoprotein component of IFT complex B and physically interacts with IFT27 in Chlamydomonas. PLoS One. 2009;4:e5384. doi: 10.1371/journal.pone.0005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou Y, Qin H, Follit JA, Pazour GJ, Rosenbaum JL, Witman GB. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J. Cell Biol. 2007;176:653–665. doi: 10.1083/jcb.200608041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J. Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood CR, Wang Z, Diener D, Zones J, Rosenbaum J, Umen J. IFT proteins accumulate during cell division and localize to the cleavage furrow in Chlamydomonas. PLoS ONE. 2012;7:e30729. doi: 10.1371/journal.pone.0030729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefebvre PA, Silflow CD, Wieben ED, Rosenbaum JL. Increased levels of mRNAs for tubulin and other flagellar proteins after amputation or shortening of Chlamydomonas flagella. Cell. 1980;20:469–477. doi: 10.1016/0092-8674(80)90633-9. [DOI] [PubMed] [Google Scholar]
- 9.Baker EJ, Schloss JA, Rosenbaum JL. Rapid changes in tubulin RNA synthesis and stability induced by deflagellation in Chlamydomonas. J. Cell Biol. 1984;99:2074–2081. doi: 10.1083/jcb.99.6.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, Rosenbaum JL, Baldari CT. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat. Cell Biol. 2009;11:1332–1339. doi: 10.1038/ncb1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedmak T, Wolfrum U. Intraflagellar transport molecules in ciliary and nonciliary cells of the retina. J. Cell Biol. 2010;189:171–186. doi: 10.1083/jcb.200911095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sedmak T, Wolfrum U. Intraflagellar transport proteins in ciliogenesis of photoreceptor cells. Biol. Cell. 2011;103:449–466. doi: 10.1042/BC20110034. [DOI] [PubMed] [Google Scholar]
- 13.Follit JA, San Agustin JT, Xu F, Jonassen JA, Samtani R, Lo CW, Pazour GJ. The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet. 2008;4:e1000315. doi: 10.1371/journal.pgen.1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keady B, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, Jonassen JA, Subramanian R, Lo C, Pazour GJ. IFT25 couples movement of the hedgehog signaling components to intraflagellar transport. Molec. Biol. Cell. 2011;22(suppl) doi: 10.1016/j.devcel.2012.04.009. Abstract #128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- 17.Jekely G, Arendt D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays. 2006;28:191–198. doi: 10.1002/bies.20369. [DOI] [PubMed] [Google Scholar]
- 18.van Dam TJ, Townsend MJ, Turk M, Schlessinger A, Sali A, Field MC, Huynen MA. Evolution of modular intraflagellar transport from a coatomer-like progenitor. Proc. Natl. Acad. Sci. U.SA. 2013;110:6943–6948. doi: 10.1073/pnas.1221011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephens RE. Reconstitution of ciliary membranes containing tubulin. J. Cell Biol. 1983;96:68–75. doi: 10.1083/jcb.96.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephens RE. Tubulin in sea urchin embryonic cilia: characterization of the membrane-periaxonemal matrix. J. Cell Sci. 1991;100(Pt 3):521–531. doi: 10.1242/jcs.100.3.521. [DOI] [PubMed] [Google Scholar]
- 21.Stephens RE. Tubulin in sea urchin embryonic cilia: post-translational modifications during regeneration. J. Cell Sci. 1992;101(Pt 4):837–845. doi: 10.1242/jcs.101.4.837. [DOI] [PubMed] [Google Scholar]
- 22.Wolff J. Plasma membrane tubulin. Biochim. Biophys. Acta. 2009;1788:1415–1433. doi: 10.1016/j.bbamem.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Pigino G, Geimer S, Lanzavecchia S, Paccagnini E, Cantele F, Diener DR, Rosenbaum JL, Lupetti P. Electron-tomographic analysis of intraflagellar transport particle trains in situ. J. Cell Biol. 2009;187:135–148. doi: 10.1083/jcb.200905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dentler W. A role for the membrane in regulating Chlamydomonas flagellar length. PLoS One. 2013;8(1):e53366. doi: 10.1371/journal.pone.0053366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson KA, Rosenbaum JL. Polarity of flagellar assembly in Chlamydomonas. J. Cell Biol. 1992;119:1605–1611. doi: 10.1083/jcb.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris EH. A Comprehensive Guide to Biology and Laboratory Use. San Diego, CA: Academic Press; 1989. The Chlamydomonas Sourcebook. [DOI] [PubMed] [Google Scholar]
- 27.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witman GB, Carlson K, Berliner J, Rosenbaum JL. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J. Cell Biol. 1972;54:507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolle R, Nultsch W. Characterization of D-[3H]c/s-diltiazem binding to membrane fractions and specific binding of calcium channel blockers to isolated flagellar membranes of Chlamydomonas reinhardti. J. Cell. Sci. 1988;90:457–463. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.