Abstract
Dyskerin is a conserved, nucleolar RNA-binding protein implicated in an increasing array of fundamental cellular processes. Germline mutation in the dyskerin gene (DKC1) is the cause of X-linked dyskeratosis congenita. Conversely, wild-type dyskerin is overexpressed in sporadic cancers, and high-levels may be associated with poor prognosis. It was previously reported that acute loss of dyskerin function via siRNA-mediated depletion slowed the proliferation of transformed cell lines. However, the mechanisms remained unclear. Using human U2OS osteosarcoma cells, we show that siRNA-mediated dyskerin depletion induced cellular senescence as evidenced by proliferative arrest, senescence-associated heterochromatinization and a senescence-associated molecular profile. Senescence can render cells resistant to apoptosis. Conversely, chromatin relaxation can reverse the repressive effects of senescence-associated heterochromatinization on apoptosis. To this end, genotoxic stress-induced apoptosis was suppressed in dyskerin-depleted cells. In contrast, agents that induce chromatin relaxation, including histone deacetylase inhibitors and the DNA intercalator chloroquine, sensitized dyskerin-depleted cells to apoptosis. Dyskerin is a core component of the telomerase complex and plays an important role in telomere homeostasis. Defective telomere maintenance resulting in premature senescence is thought to primarily underlie the pathogenesis of X-linked DC. Since U2OS cells are telomerase-negative, this leads us to conclude that loss of dyskerin function can also induce cellular senescence via mechanisms independent of telomere shortening.
Keywords: Senescence-associated heterochromatinization, dyskeratosis congenita, DNA damage, U2OS cells, chloroquine, histone deacetylase inhibitor
INTRODUCTION
Dyskerin is a highly conserved nucleolar protein required for the biosynthesis, maturation, stabilization and function of ribonucleoproteins (RNPs) that incorporate non-coding H/ACA RNAs [1]. H/ACA RNAs include subsets of small nucleolar RNAs (snoRNAs) and Cajal body RNAs, respectively, telomerase RNA (TERC) (which harbors an H/ACA domain at its 3′ end) and at least 350 additional RNAs that have yet to be ascribed specific functions [1, 2]. To this end, dyskerin appears to be implicated in an array of fundamental cellular processes. In addition to well-described roles in telomere maintenance [3] and post-transcriptional processing of nascent rRNA [4], dyskerin has also been implicated in regulation of spliceosomal RNA maturation [1], internal ribosome entry site (IRES)-mediated mRNA translation [5], cell proliferation, morphology and adhesion [6-8], mitotic progression [9], and processing of a subset of H/ACA snoRNA-derived microRNAs [10, 11]. It is not yet known if dyskerin regulates all of these processes via binding to H/ACA RNAs. Nonetheless, the biologic importance of dyskerin cannot be understated.
Complete dyskerin ablation is lethal in mice, Drosophila, and yeast [12]. In humans, germline mutation in the dyskerin gene (DKC1) is the cause of X-linked dyskeratosis congenita (DC) [3]. DC is a rare, heritable disorder associated with a wide-ranging and variably severe phenotype, including aplastic anemia, pulmonary fibrosis, cancer susceptibility and signs of premature aging. Telomere dysfunction is thought to primarily underlie the pathogenesis of X-linked DC [4, 13]. Through a direct association with TERC, dyskerin plays an important role in telomere homeostasis and maintenance of genomic integrity. Disruption of this interaction impairs telomerase activity, leading to excessive telomere shortening and premature cellular senescence [4, 13].
Although dyskerin mutation may increase cancer susceptibility, wild-type dyskerin and/or its mRNA are overexpressed in various sporadic cancer types, and high-levels may be associated with poor prognosis [14-16]. We and others previously demonstrated that acute loss of dyskerin function via siRNA-mediated depletion slowed the proliferation of transformed human cell lines [6, 7]. However, the mechanism remained unclear.
Described herein, we show that targeted dyskerin depletion induced the senescence of U2OS osteosarcoma cells as evidenced by proliferative arrest, senescence-associated heterochromatinization, and a senescence-associated global gene expression profile. Chromatin compaction can promote cell survival, and this can be reversed through the use of agents that induce chromatin relaxation [17-19]. To this end, dyskerin-depleted cells were resistant to apoptosis induced by genotoxic stress, whereas agents that induce chromatin relaxation sensitized the cells to apoptosis. U2OS cells are telomerase-negative and do not express either TERC or telomerase reverse transcriptase [6]. This leads us to conclude that loss of dyskerin can induce cellular senescence via mechanisms independent of telomere dysfunction.
MATERIALS AND METHODS
Cell culture
U2OS cells (American Type Culture Collection, Manassas, VA) were grown in Dulbecco’s minimal essential medium with GlutaMAX (Invitrogen, Carlsbad, CA) and 10% fetal bovine serum with 100 IU/mL penicillin and 100 IU/mL streptomycin (Invitrogen). Quality control was sporadically performed using the Mycoplasma Plus™ PCR Primer Set (Agilent Technologies, Santa Clara, CA).
siRNA transfections
Custom-designed (#1) and pre-designed (#2) ON-TARGETplus SMARTPool siRNA duplexes targeting DKC1 and negative control, ON-TARGETplus Non-Targeting siRNA pools #1 and #2 were obtained from Dharmacon (Lafayette, CO). The siRNA sequences are listed in Table S1. Transfections were performed using Lipofectamine 2000 (Invitrogen), as previously described [6].
Chemical reagents and antibodies
Doxorubicin, neocarzinostatin, trichostatin A, suberoylanilide hydroxamic acid (SAHA), chloroquine and bafilomycin were obtained from Sigma-Aldrich (St. Louis, MO). Antibodies recognizing dyskerin and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All other antibodies, including secondary antibodies, were obtained from Cell Signaling Technology (Danvers, MA).
Protein extraction and immunoblotting
Protein extractions and Western blots were performed as previously described [6]. In most cases, the blots were stripped and re-probed with a different antibody.
Cell proliferation and apoptosis analyses
Proliferation was assessed by incubating the cells with 10 μM 5-ethynyl-2′-deoxyuridine (EdU) for 16 hours and then analyzed using the Click-iT® EdU Alexa Fluor® 647 Flow Cytometry Assay Kit (Invitrogen) as per the manufacturer’s protocol. Apoptosis was measured using the Alexa Fluor® 488 Annexin V/Dead Cell Apoptosis Kit (Invitrogen), as previously described [9]. Analyses were performed on a BD™ LSR II flow cytometer (BD Biosciences, Sparks, MD) and tabulated using FlowJo Version 10 (Tree Star, Ashland, OR). Statistical analyses were performed using Student’s t-test; p < 0.05 was considered statistically significant.
Indirect immunofluorescence and analysis
Cells were grown and transfected in 4-well Lab Tek chamber slides (Thermo Scientific, Rochester, NY). At the indicated time points, the cells were fixed, permeabilized, immunolabeled, and analyzed as previously described [9].
RNA extraction and analysis
Total RNA was isolated using the miRNeasy kit (Qiagen, Valencia, CA) as per the manufacturer’s protocol. The miScript PCR system (Qiagen) was used for quantitative RT-PCR on a 7500 Real Time PCR System (Applied Biosystems, Carlsbad, California). All Quantitect primers were obtained from Qiagen. Gene expression profiling was performed using the GeneChip Human Gene 1.0 ST array (Affymetrix, Santa Clara, CA). The associated technical work and bioinformatics analyses were conducted by the University of Pennsylvania Molecular Profiling Core.
RESULTS
Almost all of the studies described herein were performed over a 72-144 hour time frame, depending on the specific experiment. Thus we reasoned that any observed phenotype associated with dyskerin depletion would not be a result of accelerated telomere shortening. To further ensure this, we employed telomerase-negative U2OS cells which express relatively high levels of dyskerin [6, 15]. After 72 hrs, dyskerin expression was reduced more than 80-90% relative to the controls irrespective of the siRNA used (Fig. S1).
Loss of dyskerin function arrests cell proliferation
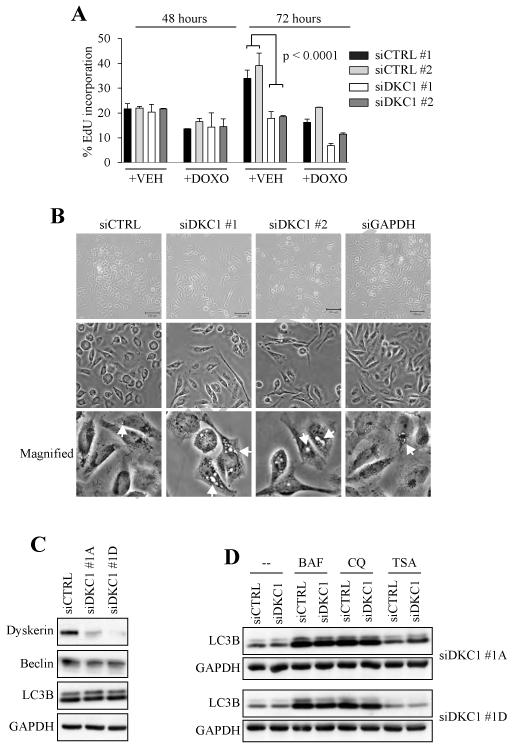
Forty-eight hrs after siRNA transfection there were no appreciable differences in EdU incorporation between the control (siCTRL) and dyskerin-depleted (siDKC1) cells (Fig. 1A). After 72 hrs siCTRL cells continued to incorporate EdU, whereas siDKC1 cells showed no additional uptake (p < 0.0001). Cells transfected with two distinct DKC1 siRNAs (siDKC1 #1 and siDKC1 #2) yielded similar results. This reaffirmed our previous findings that acute loss of dyskerin function arrested U2OS proliferation [6]. EdU incorporation was reduced in a relatively equal proportion of siCTRL and siDKC1 cells following 24 hr treatment with the genotoxic agent doxorubicin (DOXO) (Fig. 1A). In addition to no appreciable differences in apoptosis between untreated siCTRL and siDKC1 cells (see Fig. 3A), this confirmed that siDKC1 cells were still viable and responsive to genotoxic stress.
Figure 1. Loss of dyskerin triggers a proliferative arrest but not autophagy.
A, Transfected U2OS cells were pulsed for 16 hrs with 10 μM EdU in the presence and absence of DOXO (0.25 μg/mL) and then analyzed by flow cytometry. VEH = water, which was the DOXO diluent. B, Six days after transfection, siDKC1 cells exhibited thinned and elongated cellular processes compared to siCTRL and siGAPDH cells. In addition, numerous vacuoles could be seen, arranged mostly in a perinuclear pattern (arrows). Two different DKC1 siRNAs elicited similar morphologic changes as evaluated by phase contrast microscopy. C, Autophagy markers were not upregulated after dyskerin depletion. D, Chloroquine (CQ) and bafilomycin (BAF) induced autophagic flux in the presence and absence of dyskerin. Trichostatin A (TSA) had no effect on LC3B cleavage.
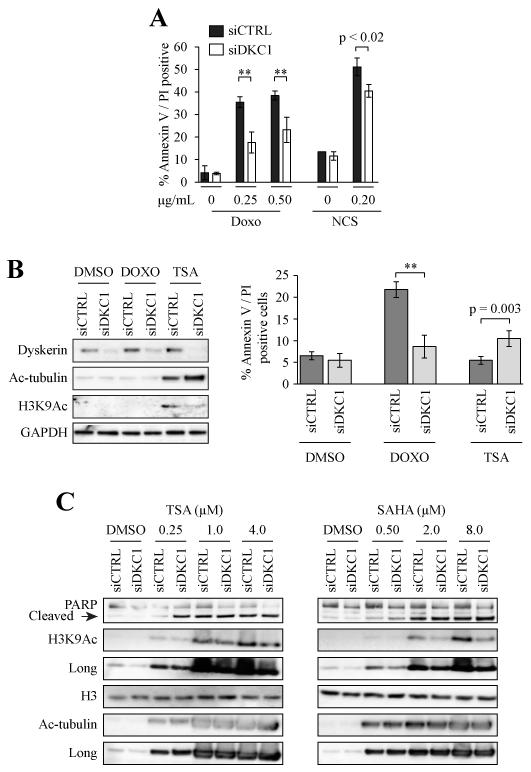
Figure 3. Apoptosis is suppressed following genotoxic stress but enhanced with histone deacetylase inhibition in dyskerin-depleted cells.
A, 48 hrs after transfection the cells were treated for 24 hrs with DOXO or neocarzinostatin (NCS). Floating and adherent cells were collected and apoptosis was assessed. Error bars denote the standard deviations derived from parallel transfections performed in triplicate wells. All experiments were performed at least in duplicate; one representative experiment is shown. B, Cells were treated with either DOXO (0.25 μg/mL) or TSA (250 nM) for 24 hrs. The cells were sensitized to TSA-induced apoptosis. Left panel, western blot, right panel flow cytometric analysis. C, Cells were treated for 16 hrs with different concentrations of TSA or SAHA, respectively. There was increased apoptosis of siDKC1 cells compared to siCTRL cells as measured by more complete PARP cleavage. As expected, H3K9Ac and Ac-tubulin levels were increased following HDAC inhibition. **p < 0.001
Autophagy is not induced by dyskerin depletion
We and others previously noted that dyskerin-depleted cells exhibited unusual morphology whereby their cytoplasmic processes were thinned and markedly elongated [6, 7]. As soon as 72 hrs but more prominently six days after transfection, there was also cytoplasmic vacuolization with most of the vacuoles arranged in a perinuclear pattern (Fig. 1B). The vacuoles were qualitatively larger than those seen in siCTRL and GAPDH knockdown cells.
The vacuolization was reminiscent of the autophagosomes that form during the course of autophagy. Autophagic flux as measured by accumulation of the LC3B cleavage product (LC3B II) is considered a reliable measure of autophagy; LC3B II is recruited to autophagosomes [20]. However, whole lysates did not reveal any appreciable increase in LC3B II or of the autophagy marker Beclin following dyskerin depletion (Fig. 1C). There was also no increase in autophagic flux following overnight treatment with chemical autophagy inhibitors chloroquine (CQ) and bafilomycin (Fig. 1D).
Dyskerin-depleted cells exhibit a senescence-associated gene expression profile
To identify factors that could help explain the proliferative arrest, we investigated the effects of dyskerin depletion on global mRNA expression using the GeneChip Human Gene 1.0 ST array. After 72 hrs of knockdown, loss of dyskerin was associated with a genetic profile in which only 96 genes were upregulated (p ≤ 0.001) and 142 genes were downregulated at least 2-fold (p < 0.02) relative to siCTRL cells. As expected, some of the downregulated genes included DKC1 and H/ACA RNAs (not shown).
No known autophagy regulatory genes were upregulated. Instead, there was an enrichment (>20%) in genes, including SERPINB5, SPINK1, SPP1, and MMP3, that encode secretory proteases and other factors that regulate extracellular matrix. Pro-inflammatory genes (IL24, CFH, DEFB103A, PROS1, CD22), and factors that regulate responses to endogenous stress (HMOX1, MGST1, TXNIP) were also enriched. A review of the literature revealed that at least 57/96 (59.4%) of the upregulated genes ( 2-fold), including six of the eight most highly upregulated genes, show increased levels during senescence and/or organismal aging in various model systems (Fig. 2A, Supp. Fig S2). Many of these genes are also known to promote cellular survival and/or confer chemoresistence.
Figure 2. Dyskerin depletion triggers genetic and epigenetic changes characteristic of cellular senescence.
A, Genes highlighted in green are either prototypic senescence markers or shown to be upregulated during senescence / aging in several different studies. Genes highlighted in yellow have been associated with senescence / aging in at least one published study. B, Three and six days after transfection, the cells were fixed and labeled with an antibody recognizing H3K9Me3 and DAPI. The punctate H3K9Me3 staining characteristic of SAH was evident at the three day time point but more readily apparent six days after transfection. C, In parallel, whole cell lysates were harvested and assessed by immunoblot 72 hrs after transfection. Total H3K9Me3 was slightly increased whereas H3K9Ac was reduced by almost 50% in siDKC1 cells. Using NIH Image J, relative expression was measured as the densitometric ratios of the marked H3K9 / native H3 in siDKC1 cells normalized to their respective ratios in siCTRL. Standard deviations were derived from analyses of at least three independent transfections.
Downregulated genes included pro-apoptotic genes such as TGFB1, TGFBR1, and notably, caspase 3 (CASP3); multiple collagens (COL4A1, COL6A3, COL8A1), matrix metallopeptidases (MMP9, MMP13, MMP14), serine proteases (PRSS1, PRSS2, TRY6), and chemokines (CCL2, CCL24, CX3CL1). We validated the expression levels of several factors by qRT-PCR and/or Western blot (Supp. Fig. S3).
Together, this suggested that loss of dyskerin triggered a pro-senescence, anti-apoptotic molecular signature in U2OS cells that was reminiscent of a senescence-associated secretory phenotype (SASP). The SASP is characterized by the dysregulated expression of soluble signaling proteins including growth factors, secretory proteases and other modulators and components of the extracellular matrix, such as collagens and laminin [21]. We note that levels of prototypical SASP biomarkers, including IL-6, IL-8, IGFBP5 and VEGF, were either unaffected or reduced (not shown). However, dyskerin depletion was recently shown to enhance VEGF protein expression in breast cancer cells [22].
Loss of dyskerin triggers heterochromatinization in U2OS cells
Senescent cells tend to become flatter and exhibit enlarged nuclei relative to proliferating cells [17]; this phenotype was not evident in dyskerin-depleted cells. As previously reported [6, 7], senescence-associated β-galactosidase levels were no different between the siDKC1 and siCTRL cells (not shown). p53 and CDKN1a (p21Cip1/Waf1) are often upregulated during senescence [17], but their levels were unaffected after dyskerin knockdown (Supp. Fig. S3 and see Fig. 3). Another senescence marker p16INK4a is inherently silenced in U2OS cells [23]. This led us and others [6, 7] to previously conclude that acute loss of dyskerin did not induce senescence. Yet, expression of the SASP prompted us to re-evaluate those conclusions. In particular, we examined the cells by epifluorescence microscopy for evidence of senescence-associated heterochromatinization (SAH) as measured by a change in immuno-localization of lysine 9-trimethylated histone H3 (H3K9Me3) [18, 24]. To this end, there was re-distribution of H3K9Me3 from a diffuse nuclear pattern in the control cells to a more punctate pattern in the siDKC1 cells (Fig. 2B). These changes in chromatin structure were evident 72 hrs after transfection but most apparent six days after knockdown.
As measured by immunoblot, total H3K9Me3 expression was modestly increased while native histone H3 levels remained unchanged (Fig. 2C). In contrast, acetylated H3K9 (H3K9Ac) levels were decreased by almost 50% relative to siCTRL cells. Histone hypoacetylation is also associated with SAH [18, 24]. Together, these findings suggested that acute loss of dyskerin function induced a senescence-like state.
Loss of dyskerin limits apoptosis induced by genotoxic stress
SAH can promote cellular survival [17-19]. Previous reports suggest that loss of dyskerin function renders cells acutely resistant to apoptosis, including that caused by DNA damage [5, 7, 25]. To assess this in our model system, we knocked down DKC1 for 48 hrs and then treated the cells with the topoisomerase II inhibitor DOXO, and the radiomimetic neocarzinostatin, respectively, for 24 hours. As measured by annexin V – propidium iodide labeling, there was a significant suppression of apoptosis in the siDKC1 cells relative to siCTRL with all treatments (Fig. 3A; p < 0.001).
Histone deacetylase inhibition sensitizes dyskerin-depleted cells to apoptosis
Histone deacetylase inhibition (HDACi) promotes chromatin relaxation thereby reversing the repressive effects of SAH on apoptosis [18, 24]. To this end, a 24 hr treatment with the histone deacetylase inhibitor (HDACi) trichostatin A (TSA) hyper-sensitized siDKC1 cells to apoptosis. There was increased Annexin V / PI-positive cells (Fig. 3B) and more complete PARP cleavage (Fig. 3C) relative to siCTRL cells. This was particularly evident with the 250 nM TSA treatment, but apoptosis of siDKC1 cells was increased with increasing HDACi concentration (Fig. 3C). Treatment with the HDACi SAHA yielded similar effects (Fig. 3C).
We note that like H3K9Ac, acetylated α-tubulin (Ac-tubulin) levels were also decreased in untreated siDKC1 cells relative to siCTRL cells (Figs. 3B, C). After HDACi treatment, Actubulin levels were at least equivalent in siCTRL and siDKC1 cells. In contrast, H3K9Ac levels in HDACi-treated siDKC1 cells typically remained lower than in siCTRL cells after HDACi treatment. The mechanisms by which loss of dyskerin reduced H3K9 and α-tubulin acetylation requires further study.
Chloroquine potentiates DOXO-induced apoptosis in dyskerin-depleted cells
CQ intercalates into DNA thereby relaxing chromatin through a mechanism that is distinct from HDACi [26, 27]. To further test our hypothesis that SAH induced the survival of dyskerin-depleted cells, transfected cells were treated with DOXO, 100 μM CQ, and CQ + DOXO, respectively, for 24 hrs. DOXO-induced apoptosis was suppressed in the absence of dyskerin. Unlike with HDACi, there was no significant increase in apoptosis following treatment with only CQ. However, the combination of DOXO and CQ significantly potentiated apoptosis in siDKC1 cells beyond the level seen in siCTRL cells (Fig. 4A, B). Moreover, total annexin V positivity (live and dead cells) was also increased (Fig. 4C). This suggested that co-treatment of CQ with DOXO relieved the suppressive effects of dyskerin depletion on DOXO-induced apoptosis.
Figure 4. Chloroquine sensitizes DOXO-treated siDKC1 cells to apoptosis.
A, Cells were treated with DOXO (0.25 μg/mL), CQ (100 μM), and DOXO+CQ for 24 hrs. Co-treatment with CQ significantly potentiated DOXO-induced cell death in the absence of dyskerin. B, Quantification of apoptosis. Error bars denote the standard deviations derived from parallel transfections performed in triplicate wells. All experiments were performed at least in duplicate; one representative experiment is shown. C, Total Annexin V positivity (live and dead cells) was also increased in the co-treated siDKC1 cells. D, CQ but not DOXO induced autophagic flux in siCTRL and siDKC1 cells. **p < 0.001
CQ is well-regarded as a chemosensitizer and an inhibitor of autophagy [28]. Autophagy has been shown to limit DOXO-induced apoptosis [20, 29]. Co-treatment with bafilomycin, another autophagy inhibitor, did not potentiate DOXO-induced apoptosis in siDKC1 cells (not shown). Moreover, there was no evidence of autophagic flux following DNA damage in siCTRL and siDKC1 cells (Fig. 4D). Unlike CQ, TSA treatment did not induce autophagic flux (Fig. 1D). This suggests that the mechanism by which CQ sensitized siDKC1 cells to DOXO was not through autophagy inhibition. This leads us to conclude that the survival of siDKC1 cells following DNA damage was attributable to induction of cellular senescence, and that CQ and HDACi potentiated apoptosis through chromatin modulation, albeit by different mechanisms. Nonetheless, we cannot exclude the possibility that loss of dyskerin may have triggered a non-canonical form of autophagy which may have partially contributed to the survival phenotype. This will require further investigation.
DISCUSSION
Patient-derived X-linked DC cells exhibit telomere dysfunction and premature senescence [3, 13]. Our findings suggest that acute loss of dyskerin function can also promote a senescent state by mechanisms that are independent of telomere shortening. The senescence phenotypes and the effects of transient and constitutive loss of normal dyskerin function may be cell-type specific [5, 13, 17, 30]. SAH is also not a universal feature of cellular senescence; it is preferentially triggered by oncogenic stress [18, 30]. RAS-induced oncogenic stress has been shown to promote cellular survival in response to genotoxic stress; HDACi treatment can reverse these effects [18, 31]. Dyskerin has been characterized as a tumor suppressor [4], and only rarely has loss of tumor suppressor protein function been linked to SAH [31-33].
It is possible that the mechanisms by which dyskerin knockdown triggered cellular senescence may be indirect. Dyskerin depletion is known to increase the turnover of its binding partners NHP2, NOP10, and NAF1, and reduce functional interactions with SHQ1 [34, 35]. Each of these proteins is implicated in H/ACA RNP biogenesis. Inactivation of SHQ1 was previously shown to confer resistance to interferon - and retinoic acid-induced apoptosis [36]. Thus, studies to examine these other H/ACA RNP regulatory proteins in the context of cellular senescence and apoptosis resistance will be worthwhile.
Loss of dyskerin function also results in decreased levels of H/ACA RNAs [6, 37]. It was recently reported that Drosophila and human chromatin-associated RNAs are enriched in snoRNAs [38]. Removal of these snoRNAs resulted in chromatin compaction and restoration of the RNAs induced chromatin relaxation. Bellodi et al [39] reported that H/ACA RNP dysfunction resulting from dyskerin mutation may contribute to the diverse phenotype associated with X-linked DC. Thus, it is conceivable that the effects we observed in our studies could be mediated by either H/ACA RNAs and/or other proteins that are dysregulated by dyskerin depletion, rather than directly by dyskerin loss alone. This will be addressed in future studies.
Supplementary Material
Highlights.
Dyskerin depletion triggers cellular senescence in U2OS osteosarcoma cells
Dyskerin-depleted cells are resistant to apoptosis induced by genotoxic stress
Chromatin relaxation sensitizes dyskerin-depleted cells to apoptosis
ACKNOWLEDGEMENTS
We thank Drs. Monica Bessler, Philip Mason and Sarah Millar for helpful discussions and/or review of the manuscript, and Dr. John Tobias of the Penn Molecular Profiling Core for the bioinformatics analyses.
This work was supported by funds from National Institutes of Health grant DE021428, the Skin Disease Research Center of the University of Pennsylvania (NIAMS P30-AR057217) and the Abramson Cancer Center Pilot Projects Program (P30-CA016520).
Abbreviations
- DC
dyskeratosis congenita
- DOXO
doxorubicin
- HDACi
histone deacetylase inhibitor
- TSA
trichostatin A
- CQ
chloroquine
- (SAH)
senescence-associated heterochromatinization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Meier UT. How a single protein complex accommodates many different H/ACA RNAs. Trends Biochem. Sci. 2006;31:311–315. doi: 10.1016/j.tibs.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jady BE, Ketele A, Kiss T. Human intron-encoded Alu RNAs are processed and packaged into Wdr79-associated nucleoplasmic box H/ACA RNPs. Genes Dev. 2012;26:1897–1910. doi: 10.1101/gad.197467.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 4.Ruggero D, Grisendi S, Piazza F, et al. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science. 2003;299:259–262. doi: 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- 5.Bellodi C, Kopmar N, Ruggero D. Deregulation of oncogene-induced senescence and p53 translational control in X-linked dyskeratosis congenita. EMBO J. 2010;29:1865–1876. doi: 10.1038/emboj.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alawi F, Lin P. Dyskerin is required for tumor cell growth through mechanisms that are independent of its role in telomerase and only partially related to its function in precursor rRNA processing. Mol. Carcinog. 2011;50:334–345. doi: 10.1002/mc.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sieron P, Hader C, Hatina J, et al. DKC1 overexpression associated with prostate cancer progression. Br. J. Cancer. 2009;101:1410–1416. doi: 10.1038/sj.bjc.6605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tortoriello G, de Celis JF, Furia M. Linking pseudouridine synthases to growth, development and cell competition. FEBS J. 2010;277:3249–3263. doi: 10.1111/j.1742-4658.2010.07731.x. [DOI] [PubMed] [Google Scholar]
- 9.Alawi F, Lin P. Dyskerin localizes to the mitotic apparatus and is required for orderly mitosis in human cells. PLoS One. 2013;8:e80805. doi: 10.1371/journal.pone.0080805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alawi F, Lin P. Loss of dyskerin reduces the accumulation of a subset of H/ACA snoRNA-derived miRNA. Cell Cycle. 2010;9:2467–2469. doi: 10.4161/cc.9.12.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott MS, Avolio F, Ono M, et al. Human miRNA precursors with box H/ACA snoRNA features. PLoS Comput. Biol. 2009;5:e1000507. doi: 10.1371/journal.pcbi.1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angrisani A, Vicidomini R, Turano M, et al. Human dyskerin: beyond telomeres. Biol. Chem. 2014 doi: 10.1515/hsz-2013-0287. doi: 10.1515/hsz-2013-0287. [DOI] [PubMed] [Google Scholar]
- 13.Kirwan M, Beswick R, Walne AJ, et al. Dyskeratosis congenita and the DNA damage response. Br. J. Haematol. 2011;153:634–643. doi: 10.1111/j.1365-2141.2011.08679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B, Zhang J, Huang C, et al. Dyskerin overexpression in human hepatocellular carcinoma is associated with advanced clinical stage and poor patient prognosis. PLoS One. 2012;7:e43147. doi: 10.1371/journal.pone.0043147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alawi F, Lin P, Ziober B, et al. Correlation of dyskerin expression with active proliferation independent of telomerase. Head Neck. 2011;33:1041–1051. doi: 10.1002/hed.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Stedingk K, Koster J, Piqueras M, et al. snoRNPs regulate telomerase activity in neuroblastomas and are associated with poor prognosis. Transl. Oncol. 2013;6:447–457. doi: 10.1593/tlo.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell. Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 18.Di Micco R, Sulli G, Dobreva M, et al. Interplay between oncogene-induced DNA damage response and heterochromatin in senescence and cancer. Nat. Cell Biol. 2011;13:292–302. doi: 10.1038/ncb2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murga M, Jaco I, Fan Y, et al. Global chromatin compaction limits the strength of the DNA damage response. J. Cell. Biol. 2007;178:1101–1108. doi: 10.1083/jcb.200704140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abedin MJ, Wang D, McDonnell MA, et al. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 2007;14:500–510. doi: 10.1038/sj.cdd.4402039. [DOI] [PubMed] [Google Scholar]
- 21.Coppe JP, Desprez PY, Krtolica A, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocchi L, Pacilli A, Sethi R, et al. Dyskerin depletion increases VEGF mRNA internal ribosome entry site-mediated translation. Nucleic Acids Res. 2103;41:8308–8318. doi: 10.1093/nar/gkt587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGarvey KM, Fahrner JA, Greene E, et al. Silenced tumor suppressor genes reactivated by DNA demethylation do not return to a fully euchromatic chromatin state. Cancer Res. 2006;66:3541–3549. doi: 10.1158/0008-5472.CAN-05-2481. [DOI] [PubMed] [Google Scholar]
- 24.Prieur A, Besnard E, Babled A, et al. p53 and p16(INK4A) independent induction of senescence by chromatin-dependent alteration of S-phase progression. Nat. Commun. 2011;2:473. doi: 10.1038/ncomms1473. [DOI] [PubMed] [Google Scholar]
- 25.Montanaro L, Calienni M, Bertoni S, et al. Novel dyskerin-mediated mechanism of p53 inactivation through defective mRNA translation. Cancer Res. 2010;70:4767–4777. doi: 10.1158/0008-5472.CAN-09-4024. [DOI] [PubMed] [Google Scholar]
- 26.Loehberg CR, Thompson T, Kastan MB, et al. Ataxia telangiectasia-mutated and p53 are potential mediators of chloroquine-induced resistance to mammary carcinogenesis. Cancer Res. 2007;67:12026–12033. doi: 10.1158/0008-5472.CAN-07-3058. [DOI] [PubMed] [Google Scholar]
- 27.Turaga RV, Massip L, Chavez A, et al. Werner syndrome protein prevents DNA breaks upon chromatin structure alteration. Aging Cell. 2007;6:471–481. doi: 10.1111/j.1474-9726.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- 28.Savarino A, Lucia MB, Giordano F, et al. Risks and benefits of chloroquine use in anticancer strategies. Lancet Oncol. 2006;7:792–793. doi: 10.1016/S1470-2045(06)70875-0. [DOI] [PubMed] [Google Scholar]
- 29.Karantza-Wadsworth V, Patel S, Kravchuk O, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosar M, Bartkova J, Hubackova S, et al. Senescence-associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type- and insult-dependent manner and follow expression of p16(ink4a) Cell Cycle. 2011;10:457–468. doi: 10.4161/cc.10.3.14707. [DOI] [PubMed] [Google Scholar]
- 31.Tu Z, Aird KM, Bitler BG, et al. Oncogenic RAS regulates BRIP1 expression to induce dissociation of BRCA1 from chromatin, inhibit DNA repair, and promote senescence. Dev. Cell. 2011;21:1077–1091. doi: 10.1016/j.devcel.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Trotman LC, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young AP, Schlisio S, Minamishima YA, et al. VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat. Cell Biol. 2008;10:361–369. doi: 10.1038/ncb1699. [DOI] [PubMed] [Google Scholar]
- 34.Grozdanov PN, Fuentes N. Fernandez, Fiser A, et al. Pathogenic NAP57 mutations decrease ribonucleoprotein assembly in dyskeratosis congenita. Hum. Mol. Genet. 2009;18:4546–4551. doi: 10.1093/hmg/ddp416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grozdanov PN, Roy S, Kittur N N, et al. SHQ1 is required prior to NAF1 for assembly of H/ACA small nucleolar and telomerase RNPs. RNA. 2009;15:1188–1197. doi: 10.1261/rna.1532109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofmann ER, Nallar SC, Lin L, et al. Identification and characterization of GRIM-1, a cell-death-associated gene product. J. Cell Sci. 2010;123:2781–2791. doi: 10.1242/jcs.070250. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Ge J, Crosby SD, Heinz ME, et al. SnoRNA microarray analysis reveals changes in H/ACA and C/D RNA levels caused by dyskerin ablation in mouse liver. Biochem. J. 2010;429:33–41. doi: 10.1042/BJ20091898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schubert T, Pusch MC, Diermeier S, et al. Df31 protein and snoRNAs maintain accessible higher-order structures of chromatin. Mol. Cell. 2012;48:434–444. doi: 10.1016/j.molcel.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Bellodi C, McMahon M, Contreras A, et al. H/ACA small RNA dysfunctions in disease reveal key roles for noncoding RNA modifications in hematopoietic stem cell differentiation. Cell Rep. 2013;3:1493–1502. doi: 10.1016/j.celrep.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.