Abstract
The acute response of the rodent subventricular zone (SVZ) to traumatic brain injury (TBI) involves a physical expansion through increased cell proliferation. However, the cellular underpinnings of these changes are not well understood. Our analyses have revealed that there are two distinct transit-amplifying cell populations that respond in opposite ways to injury. Mash1+ transit-amplifying cells are the primary SVZ cell type that is stimulated to divide following TBI. In contrast, the EGFR+ population, which has been considered to be a functionally equivalent progenitor population to Mash1+ cells in the uninjured brain, becomes significantly less proliferative after injury. Although normally quiescent GFAP+ stem cells are stimulated to divide in SVZ ablation models, we found that the GFAP+ stem cells do not divide more after TBI. We found, instead, that TBI results in increased numbers of GFAP+/EGFR+ stem cells via non-proliferative means—potentially through the dedifferentiation of progenitor cells. EGFR+ progenitors from injured brains only were competent to revert to a stem cell state following brief exposure to growth factors. Thus, our results demonstrate previously unknown changes in lineage relationships that differ from conventional models and likely reflect an adaptive response of the SVZ to maintain endogenous brain repair after TBI.
Keywords: subventricular zone, stem cells, GFAP, proliferation, neurogenesis, brain injury, CldU, TBI
1. INTRODUCTION
While increased proliferation and an expansion in the size of the SVZ are well known phenomena after brain injury, the cellular underpinnings of this effect are not well understood. In addition, although injury-induced neurogenesis has been detected in the adult brain from the subventricular zone (SVZ) and hippocampus (Gould et al., 1997; Yagita et al., 2001; Parent et al., 2002; Thored et al., 2006) and in non-neurogenic regions (Tonchev et al., 2003; Yamamoto, et al., 2001; Magavi et al., 2000) the regenerative capacity of the brain remains low (Arvidsson et al., 2002). Thus, therapeutic intervention aimed at certain cell populations or within specific time-frames post-injury are needed to enhance and support the endogenous neurogenic response.
Under uninjured conditions, stem cells in the SVZ are a relatively quiescent population of cells (Morshead et al., 1994; Doetsch et al., 1999; Garcia et al., 2004; Imura et al., 2003), while transit-amplifying progenitors (Doetsch et al., 2002; Cesetti et al., 2009; Kim et al., 2009) and some neuroblasts (Brown et al., 2003) are populations of actively proliferating cells. According to the current models of SVZ lineage progression, the development from slowly-dividing GFAP+ stem cell to migrating neuroblast occurs following activation and co-expression of epidermal growth factor receptor (EGFR). These GFAP+/EGFR+ stem cells give rise to GFAP−/EGFR+ transit amplifying cells, which rapidly divide to generate DCX+ neuroblasts and continue to divide as they migrate toward the olfactory bulb where they become functional interneurons (Pastrana et al., 2009). Although studies utilizing the deletion of specific SVZ cell populations demonstrate this specific pattern of cellular hierarchy in the uninjured brain, it is unknown whether injury-induced SVZ cell proliferation involves changes to this normal lineage progression, and which specific cell phenotypes are most affected. Resolution of this post-injury biology is important for understanding the ability of SVZ-derived stem and progenitor cells to contribute to the brain’s natural repair process.
To address this gap in knowledge we examined the cellular changes within the SVZ after traumatic brain injury (TBI) in a murine model. We identified a significant non-proliferative increase in neural stem cells and a divergent response to injury by different transit amplifying progenitor populations. Our data also suggest that injury-induced signaling through the EGF receptor may result in the dedifferentiation of a progenitor population back into a stem cell state. Thus, these alterations to cell lineage relationships in the SVZ are likely to be important regulators of the enhanced proliferation and neurogenesis known to be induced by brain injury. Therefore, EGFR-signaling in particular may be an important therapeutic target for optimizing the post-injury cellular response to promote functional recovery.
2. MATERIALS AND METHODS
Animals
C57Bl/6 mice purchased from Charles River Laboratory were housed under NIH guidelines and all experiments were conducted in accordance with the University of California, Los Angeles, (UCLA) Chancellor’s Animal Research Committee and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. Transgenic mice expressing the herpes simplex virus-thymidine kinase from the mouse glial fibrillary acid protein promoter (GFAP-TK mice) were supplied by the Sofroniew Lab at UCLA. The pattern and regulation of transgene-derived HSV-TK expression is similar to that of endogenous GFAP, to the extent that 100% of TK cells co-localize with GFAP in both uninjured mice (Garcia et al., 2004) or in stab wound-injured mice (Bush et al., 1998). Adult male mice at least 3 months of age were used in all experiments.
5-Chloro-2′-deoxyuridine (CldU) labeling
To label cells that were actively proliferating on the day of euthanasia (1, 3, or 7 days post-injury), 42.5 mg/kg 5-chloro-2′-deoxyuridine (CldU) was administered intraperitoneally every 2 hours over the course of 8 hours (4 injections total) and mice were euthanized 2 hours after the last injection. To identify GFAP-TK+ cells that arise from actively dividing cells after injury, animals were injected with CldU immediately after injury and every 2 hours thereafter for a total of 4 injections and animals were euthanized 3 days following injury.
5-Iodo-2′-deoxyuridine (IdU) labeling
For label-retaining experiments, intraperitoneal (IP) injections of IdU (Sigma I7125; 57.5 mg/kg) were administered to adult mice, once daily for three weeks to label all dividing cells, even the slowly dividing stem cells. Naïve animals were euthanized immediately or after a label washout period of 10 days in which no injections were given. Over this 10 day wash out period the IdU label intensity within proliferating cells will diminish by half with every division, so that fast dividing cells become dim or undetectable and quiescent cells remain brightly labeled (see results). Animals in the injury group received a TBI 7 days after the last IdU injection and were euthanized 3 days post-injury (corresponding to a total 10- day washout). IdU was prepared at a concentration of 3.2 mg/ml in .08N NaOH in sterile saline and pH was neutralized using concentrated HCl.
Controlled Cortical Impact (CCI) Injury
Mice were anesthetized with isofluorane and positioned within a mouse stereotaxic frame. Following a longitudinal skin incision, a 6mm diameter craniotomy was made centered at 3mm posterior to Bregma and 3mm lateral to the midline. Cortical injury was performed with a flat, 3mm diameter metal tip attached to the CCI device, at 15psi and to a depth of 0.6 mm below the dura. The skull flap was replaced and glued in place with Loctite Ultra Gel-Control super glue before suturing the wound closed. (See Myer et al., 2006)
Tissue Fixation, Brain Sectioning & Immunohistochemistry
Mice were deeply anesthetized with pentobarbital (50mg/kg) and perfused/fixed with .9% PBS followed by 4% paraformaldehyde. 40 μm coronal sections were cut and standard, multi-label fluorescent immunohistochemistry was performed using the following antibodies: rat anti-BrdU/CldU (1:250, Accurate Chemicals, Westbury, NY, Clone BU 1/75), mouse anti-BrdU/IdU (1:250, BD, Franklin Lakes, NJ, Clone B44), rabbit anti-GFAP (1:2000, Dako, Carpinteria, CA), rabbit anti-TK (from Dr. Michael Sofroniew), sheep anti-EGFR (1:5000, Capralogics, Hardwick, MA), mouse anti-Mash1 (1:250, BD, Franklin Lakes, NJ), rabbit anti-DCX (1:500, Abcam, Cambridge UK), rabbit anti-Iba1 (1:250, Wako, Richmond, VA). Alexafluorophore-conjugated secondary probes (Molecular Probes, Carlsbad, CA) were used. For CldU and IdU staining, antigen-retrieval was performed by first incubating sections in 10mM sodium citrate at >95°C for 15 minutes followed by incubation in 2N HCl at 37°C for 25 minutes.
Contusion Analysis
Six sections (480μm apart) were Nissl-stained, digitally scanned and analyzed for contusion volume analysis using previous published methods (Chen et al., 2003). Single, representative sections at Bregma +0.48 from 12 mice at 7 days after injury were scanned on a flatbed scanner and digitally co-registered by affine transformations (Jenkinson & Smith, 2001). Lesioned areas were then manually outlined before images were merged by summation, rescaled and colorized to represent the degree of lesion overlap.
Microscopy
Stereological analysis was performed to quantify the following populations of cells: CldU, GFAP-TK, EGFR, CldU/MASH1, CldU/GFAP-TK and CldU/EGFR. The estimated total number of single- and double-labeled cells was determined by epifluorescence microscopy using unbiased stereology cell counts with the optical fractionator method, as implemented by StereoInvestigator software (MicroBrightfield, Williston, VT, USA) in four 40 μm sections that were 240 um apart within the SVZ region beginning rostrally at the genu of the corpus callosum. Counting regions were grossly defined under DAPI nuclear stain immunofluorescence by contouring an area extending from the junction of the lateral ventricle and dorsolateral SVZ to 100 μm medially, 100 μm ventrally and 175 μm laterally. Labeled cells were counted at 100x total magnification using a grid size of 35 x 35 μm with a sampling box of 15 x 15 μm and were reported as estimated total cell populations.
Accurate stereological quantification was not possible for all cell populations: the abundance of DCX+ cells throughout the SVZ and staining within their processes did not allow for unambiguous identification of double-labeled cells. Additionally, limitations in available filter sets did not allow for stereological analysis of triple-labeled populations (CldU/GFAP-TK/EGFR). Therefore, confocal microscopy was used for identification and semi-quantification of the CldU/DCX population and of cells triple-labeled for CldU/GFAP-TK/EGFR. Three 75 um2 fields-of-view (FOVs) were analyzed for cell numbers within four sections (12 FOVs total) as described for use with stereology. FOVs were chosen to include the entire cell-dense areas directly lateral to, and continuing dorsolaterally from the lateral ventricle/dorsolateral SVZ junction. Cell numbers were expressed as percentages of total cells analyzed for each brain. Semi-quantitative data for total cell numbers of double- and triple- labeled populations for comparative purposes in Supplemental Table 1 were derived from the percentages of total cell populations obtained with confocal microscopy Z-stack analysis and the corresponding total stereological counts of CldU+, EGFR+ and GFAP+ cells that were obtained from the same brains. Statistical analysis was performed on the original percent-calculated data.
The same tissue sections (n=4/brain) were used to measure dorsolateral SVZ thickness, which was accomplished using DAPI-illuminated sections by measuring the distance from the ventricle to the edge of the cell dense region directly lateral to the ventricle/dorsolateral SVZ junction at 40x magnification. Each measurement was repeated 3 times within each section and for 4 sections/brain.
Flow Cytometry
Dissected SVZ tissue was enzymatically digested using Accumax (Sigma-Aldrich, USA) and mechanically triturated using a fire-polished Pasteur pipette into a single cell suspension. The cells were immunostained with an EGFR antibody conjugated with FITC secondary antibody (Cell Signaling Technology, Danvers, MA) in Hibernate media (BrainBits, Springfield, IL) with 4% normal goat serum. The cells were analyzed using a FACSDiVa cell sorter (BD Biosciences, San Jose, CA) flow cytometer and FlowJo analysis software (Treestar, Ashland, OR) for the collection of live EGFR+ cells. Sort gates were set by side and forward scatter to eliminate dead and aggregated cells and with negative control cell samples. For assessing intracellular GFAP expression the EGFR+ cells were placed in culture media with or without 20ng/ml EGF for 4 hours. After the incubation period the cells were fixed and permeabilized using the Fix & PERM kit (Invitrogen, Grand Island, NY) and immunostaining with polyclonal GFAP (Invitrogen) and anti-rabbit PerCP secondary (Invitrogen). Cells were then analyzed using the cell sorter as described above.
Clonal Neurosphere-forming Assay
The EGFR+ sorted cells were cultured at clonal density as described previously (Le Belle et al., 2011). Briefly, the cells were seeded into 96-well plates at 100 cells per ml of media. Clonally-derived neurospheres were counted and their diameters measured using a brightfield illumination and image analysis software (MCID, Imaging Research, St. Catherines, ON, Canada). A minimum diameter cutoff of 40 μm was used in defining a neurosphere. Intact clonal neurospheres were also plated down on poly-D-lysine- (100 μg/ml; Invitrogen) and laminin-coated (10 μg/ml; Invitrogen) glass coverslips in mitogen-free media for 7 days to differentiate for multipotency analysis.
Immunocytochemistry
Differentiated cells were fixed in 4% paraformaldehyde in PBS for 20 min. Immunostaining was carried out using standard protocols. Primary antibodies and dilutions were as follows: β-tubulin type III monoclonal (TuJ1, 1:500; Sigma, St. Louis, MO), GFAP polyclonal (1:1,000; DAKO, Carpenteria, CA), O4 (1:50 hybridoma supernatant; gift from De Vellis lab). Cells were then reacted with appropriate secondary antibodies (Alexa 488 or 568, Invitrogen); 1:2000) and Hoescht nuclear stain (1:5000).
Label-Retaining Cell Intensity Analysis
Animals were given daily IdU injections for 3 weeks to label all dividing SVZ cells, including the slowly dividing stem cell population. This was followed by a 10-day washout period during which time all dividing cells would dilute the IdU label and quiescent cells can be identified. TBI was performed on day 3 of the washout period. Perfusion fixation was carried out on day 10 of the washout period. All brain sections for analysis were immnuostained for IdU and GFAP at the same time using the same antibody solutions and sections were photographed at the same exposure time using epi-fluorescence at 40x magnification. Raw images were not adjusted for brightness or contrast. All images were photographed with an exposure time of 800 milliseconds, using an RS Photometrics CoolSNAP camera and Image Pro Plus software (Media Cybernetics, Inc. Bethesda, MD, USA). After images were collected, cells were visualized in Image J (Rasband, 2008) and regions of interest were drawn manually around only whole cells that were completely in focus. Cell intensity values were measured for each cell of interest (found within 2 fields of view (FOV)/section over 4 sections). FOVs were chosen to include the entire cell-dense areas directly lateral to, and continuing dorsolaterally from the ventricle/dorsolateral SVZ junction. Intensity values for cells within an individual image were corrected by subtracting the average intensity value of the background. IdU+ cells analyzed within the SVZ of an individual animal were segregated into groups based on label intensity where the most intense label retention represents the cells that did not divide during the 10-day washout period. The non-dividing group of Label Retaining Cells (LRCs) corresponded to the top 40% intensity of IdU label (~6% of the total LRC cell population). The quiescence of this high intensity LRC population was confirmed by lack of the proliferation marker CldU, given immediately before perfusion fixation. The remaining, dimmer IdU cell populations that were not true LRCs were indeed shown to be proliferative by the CldU marker and were therefore excluded from the LRC analysis. The population of high-intensity label-retaining cells was then evaluated for co-expression of GFAP and the number of GFAP+ LRCS was quantified stereologically.
Statistical Analysis
For each quantitative analysis including thickness measurement, cell counting, clonal neurosphere analysis and contusion volume analysis, the mean ± standard errors of at least three independent experiments were calculated and statistical significance tests (t-test or one-way ANOVA with Tukey’s HSD post hoc) were performed using the “R” statistical package (R Development Core Team, 2008). Statistical significance (α) was set at p < 0.05 for all comparisons.
3. RESULTS
3.1 TBI increases the size of the SVZ and the number of proliferating SVZ cells
We confirmed that SVZ proliferation and expansion occurs in the moderate controlled cortical impact injury model of traumatic brain injury (TBI) used in these studies and that it did not directly involve injury to the SVZ itself (Fig. 1A). Using an 8-hour exposure to the thymidine analogue 5-chloro-2′-deoxyuridine (CldU) on the day of euthanasia post-injury, we found that the number of actively dividing SVZ cells was significantly increased relative to uninjured (naïve) controls in the dorsolateral SVZ at 1, 3 and 7 days following TBI (p<0.05, Fig. 1B–E). Accordingly, we observed an approximately 25% expansion in the thickness of the SVZ by three days post-injury (p<0.05, compared to controls Fig. 1F). While it is known that there is a substantial inflammatory response within the injured cortex after TBI, consisting of dividing glial and inflammatory cells (Chen et al., 2003), it was not known whether this would occur within the SVZ and contribute to the SVZ expansion after injury. We found almost no change in the proliferation of IBA1+ microglia in the SVZ after injury compared to naïve (Fig. 1G–I).
Figure 1. Brain injury increases the size of the SVZ and the number of proliferating SVZ cells.
(A) Contusion injury overlap map at one representative antero-posterior level of the SVZ computed by co-registering thymine-stained sections from 12 injured mice at 3 days post-injury, demonstrating both the low variation in injury size within the group and the absence of direct injury to the SVZ (yellow red= injury overlap from 12–1 mouse, respectively. (B) Schematic of the labeling paradigm used to detect dividing cells at 3 different time points after injury. (C) Total numbers of actively dividing cells in the dorsolateral SVZ were significantly increased relative to naïve at all time-points after injury (n= 5/group, P <0.05), as indicated by immunostaining for CldU (D) and inset (E) (scale bar D; 100 um, E; 20 um). (F) By 3-days post-injury, SVZ thickness was increased by ~25% relative to naïve (n= 3/group, P <0.05). The observed proliferative effect of injury was not due to a local inflammatory response within the SVZ as observed by immunostaining for CldU (green) and microglia marker, Iba1 (blue) at low power (G) and at high power (H) in which the percent of dividing cells that were microglia (I) was less than 8% in both naïve and in injured (3-day) SVZ (n= 3/group, scale bar G; 100 um, H; 10 um).
3.2 Injury does not induce proliferation of DCX+ neuroblasts within the SVZ
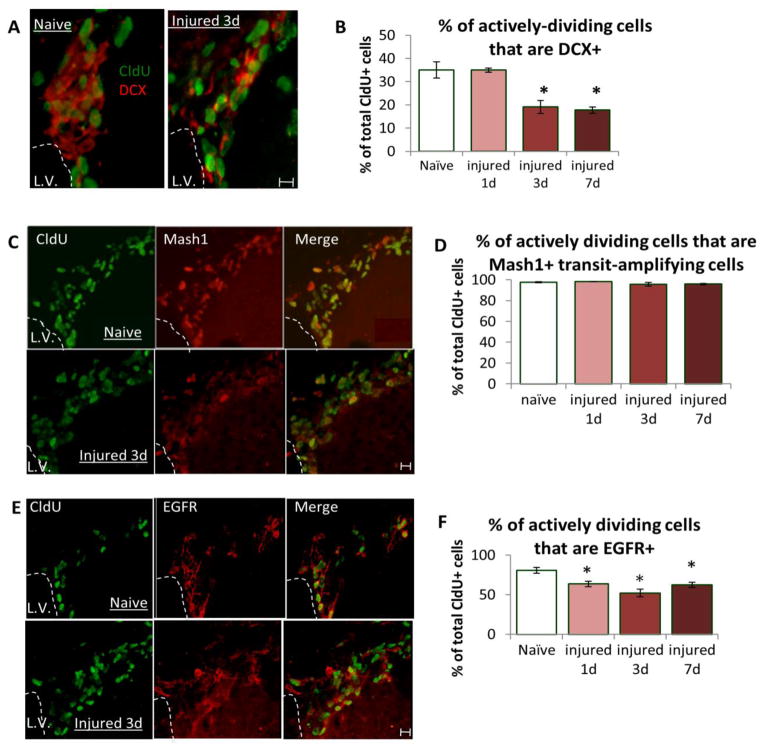
In order to determine which cells are directly responsible for the increased numbers of actively dividing cells in the SVZ after injury, we quantified the amount of cell division in a number of different cell phenotypes at 1, 3, and 7 days after injury (Fig. 1B). We first looked at DCX+ neuroblasts for their potential contribution to the post-injury increases in SVZ proliferation. We found that 35% of the actively dividing (CldU+) cells within the uninjured SVZ expressed DCX and this percentage was unchanged at 1-day post-injury (Fig. 2A, B). However, the proliferation of the DCX population significantly decreased to 19% and 17% by 3 and 7 days post-injury, respectively (P<0.05, Fig. 2B). This decrease could result from less DCX+ cell proliferation or from an increase in the migration of these cells away from the SVZ. In fact, increased total numbers of DCX+ cells were detected in the corpus callosum underlying the cortical injury and in the cortex itself (data not shown). Regardless of the cause of the decrease in dividing DCX+ cells in the SVZ, this data demonstrates that DCX+ cells do not significantly contribute to the proliferative expansion of the SVZ after injury.
Figure 2. Injury alters DCX+ neuroblast proliferation and reveals two different populations of SVZ transit-amplifying cells.
(A) Immunostaining for CldU (green) and DCX (red) in the naïve and injured dorsolateral SVZ revealed that (B) 35% of actively dividing SVZ cells in the naïve mouse are DCX+ neuroblasts (open bar) and that this percentage was significantly decreased starting at day 3 post-injury (red filled bar) and remained decreased on day 7 (dark red, filled bar). (C) Immunostaining for CldU (green) and Mash1 (red) in the dorsolateral SVZ revealed that (D) >96% of actively dividing SVZ cells in naïve and at all time points after injury were Mash1+ suggesting that, since total numbers of CldU+ cells are increased after injury, it is the Mash1+ transit-amplifying cell population that is stimulated by injury to divide. (E) Immunostaining for CldU (green) and EGFR (red) in the naïve and injured SVZ revealed that (F) 81% of actively dividing cells are EGFR+ in naïve mice SVZ (open bar) confirming that the majority of EGFR+ cells are a transit-amplifying population. However, unlike Mash1+ cells that are stimulated to divide following injury, a significantly smaller percentage of dividing cells are EGFR+ at all time points after injury (filled bars). All experiments, n= 3/group, P <0.05, each scale bar = 10um.
3.3 Mash1+ but not EGFR+ transit-amplifying cells contribute significantly to injury-induced SVZ proliferation
Although Mash1+ and EGFR+ cells are both transit amplifying cell populations, which overlap significantly in the uninjured SVZ (Kim et al., 2009; Pastrana et al., 2009; Ciccolini et al., 2005), we have found that these two populations respond very differently to TBI. Nearly all actively dividing (CldU+) cells in the SVZ (>96%) of both naïve and injured mice were Mash1+ transit amplifying cells (Fig. 2C, D), and conversely >90% of Mash1+ SVZ cells were CldU+ in both naïve and injured mice (data not shown). This shows that the actively dividing cell population within the SVZ after injury consists mainly of the Mash1+ transit-amplifying cells, and it is these cells that underlie the injury-induced expansion of the SVZ.
Among the population of actively dividing SVZ cells in the uninjured mouse, 81% expressed EGFR (Fig. 2E, F), in agreement with previous reports that EGFR is expressed largely by transit amplifying cells and overlaps to some degree with the Mash1 population (Doetsch et al., 2002; Pastrana et al., 2009; Kim et al, 2009; Cesetti, et al., 2009). However in contrast to the Mash1+ transit amplifying cells, the percent of dividing (CldU+) cells that were EGFR+ was significantly reduced to 64% on day 1 post-injury and remained significantly lower than naïve on days 3 and 7 (P<0.05, Fig. 2E, F). This reveals that it is the non-overlapping Mash1+/EGFR− population that was primarily responsible for the increased proliferative response. Because almost all of the CldU+ cells are Mash1+ and vice versa in both injured and naïve brain, CldU can be used as a proxy for Mash1 in these studies in order to assess the extent of overlap between Mash1 and other cellular markers. In doing this we can determine that prior to injury ~81% of Mash1+ transit amplifying cells co-expressed EGFR but this is reduced to less than 64% after injury. Thus, the cells that continue to co-express both EGFR and Mash1 remain proliferative post-injury but the EGFR+ cells that no longer co-express Mash1 are quiescent (see Supplemental Table 1).
3.4 Brain injury increases the total number of GFAP+ SVZ cells, but not through increased proliferation
In published experiments, ablation of the fast dividing progenitor cells using an anti-mitotic drug stimulates proliferation of the previously quiescent GFAP+ stem cell population, resulting in the repopulation of the entire SVZ niche following drug removal (Doetsch et al., 1999b, 2002). This understanding of lineage progression suggests that increases in highly proliferative Mash1+ cells that we observe after injury would result from increases in the number of dividing GFAP+ stem cells from which the transit amplifying cell populations arise. Therefore, we tested this by examining the population of actively dividing (CldU+) cells that were GFAP+ following injury. However, unlike the ablation models, we found the SVZ GFAP+ population were not stimulated to divide more and, in fact, initially decreased proliferation at day 1 post-injury (Fig. 3A, B).
Figure 3. The total numbers of GFAP+ cells increase without proliferation.
(A) Immunostaining for CldU, EGFR and GFAP-TK (green/red/blue, scale bar = 10um) revealed that (B) actively dividing cells were rarely GFAP+ SVZ astrocytes under naïve conditions (12%, open bar) and this did not change with cortical injury (closed bars). (C) Starting at 3-days post-injury, the total number of GFAP+ SVZ astrocytes and therefore the pool of potential stem cells was increased. This ~3300 cell increase in GFAP+ cells was not due to proliferation of GFAP+ cells themselves as (D), there was no significant change in GFAP+ cells that were actively dividing in the SVZ after injury. (E) A paradigm of Idu labeling of all cells before injury followed by a washout period (F) allowed the identification of the brightest label-retaining cells compared to no-washout. (G) Additional labeling with CldU prior to perfusion confirmed that these high-intensity, label-retaining IdU+ cells were indeed non-proliferative 3 days after injury and similar to naive. (H) Double-labeling with GFAP demonstrated that this quiescent population of high-intensity IdU+/GFAP+ cells increased 3-days post-injury. All experiments, n= 3/group, P <0.05, each scale bar = 10um.
In apparent contradiction to this finding of no significant change in proliferation by the GFAP+ cells in the SVZ, we observed that the total number of SVZ GFAP+ cells was significantly increased by day 3 after injury. Beginning on day 3 post-injury the total number of GFAP+ cells was increased by 36% relative to naïve, and remained at this elevated level on day 7 (P<0.05, Fig. 3C). Thus, because there was no significant change in the very small percentage of GFAP+ cells that were actively dividing (and no significant difference in total numbers of GFAP+/CldU+, Supplemental Table 1) over this 3-day period post-injury (Fig. 3D), this suggests the expansion of the pool of GFAP+ cells after injury occurred without proliferation of GFAP+ cells.
To confirm that the GFAP+ SVZ population was increasing without proliferation after injury, we used label-retention of the thymidine analogue IdU to directly determine the relative quiescence of the GFAP+ population after injury. In this labeling paradigm IdU was given daily for 3 weeks in order to label even the very slow-dividing, relatively quiescent GFAP+ cell population in the SVZ before injury. This was followed by a 10-day washout period during which time any cells that divide would dilute the IdU label with each division (Fig. 3E). Label intensity measurements were then used to identify label-retaining cells (LRCs), which reflect cells that have not undergone division over the 10-day washout period in the injured and naïve SVZ (Fig. 3F). We confirmed that the highest intensity LRCs after the 10-day washout were indeed not dividing in both the injured and naïve SVZ by looking for any double-labeling with a second thymidine analog, CldU, given on the day of perfusion/fixation (Fig. 3G). Thus, our quantification of GFAP+ LRCs demonstrated that there was a significant increase in the number of non-dividing GFAP+ cells in the SVZ after injury (Fig. 3H), which is in agreement with our previous data which also indicate that there is a non-proliferative increase in the total number of GFAP+ stem cells by day 3 after injury (Fig 3C–D).
3.5 Mash1+ transit-amplifying cells do not re-acquire GFAP expression in response to injury
There is evidence that SVZ cell lineage progression is not necessarily unidirectional and can be affected by specific extracellular cues (Doetsch et al., 2002). Therefore, in order to test the possibility that the non-proliferative increase in the SVZ GFAP+ cell population that we observed after injury could result from a proliferating progenitor re-acquiring GFAP expression and becoming quiescent, we administered CldU label once every two hours for eight hours immediately after cortical injury. Mice were euthanized three days later and the number of CldU+ cells that expressed GFAP was quantified (Fig. 4A). If actively proliferating cells were capable of reverting back to a GFAP+ phenotype, then we would detect a significant increase in CldU+/GFAP+ cells at 3 days post-injury/post-labeling. However, we found that the number of SVZ cells that were CldU+/GFAP+ 3 days after labeling was not significantly increased after injury (P >0.05; Fig. 4B). Therefore, injury-induced increases in total GFAP+ cells (by ~3300 cells, Fig. 3C) cannot be accounted for by the reversion of a population of proliferating cells back to a quiescent GFAP-expressing phenotype. This is in agreement with our finding that the number of GFAP+ SVZ cells which divide is a very small population that is essentially unchanged after injury (Fig. 3D).
Figure 4. SVZ stem cells increase in number after brain injury without proliferation and they are not derived from a transit amplifying population.
(A) Schematic of labeling paradigm to determine if the rapidly dividing cell population, which is synonymous with the MASH1+ population can contribute to the increased GFAP+ population. (B) The small, non-significant increase (250 cells) in CldU+/GFAP+ cells could not account for the significantly larger, 3300 total GFAP+ cell increase accumulated by day 3 post-injury in Fig 3C. (C) Although the total numbers of EGFR+ SVZ cells were decreased initially, they were significantly increased by day 7, which also occurred without increased proliferation in the EGFR+ population in Fig. 2F. (D) The number of co-labeled EGFR+/GFAP+ SVZ stem cells significantly increased at all time points after injury. (E) However, the number of these cells that were actively proliferating post-injury was significantly decreased at all time points, suggesting a non-proliferative increase in their number. (F) In vitro serial clonal neurosphere assays confirm that the EGFR+ SVZ cells from the injured brain had significantly increased self-renewal at all clonal passages (neurosphere number; P<0.01) but no significant increases in overall cell proliferation (sphere diameter) under the stimulation of growth factor or in the percentage of multipotent clonal spheres. (G) The percentage of EGFR+ cells that were dividing was significantly lower at all time points after injury while (H) the percentage of EGFR+ cells that co-labeled for GFAP was increased at all time points, further suggesting an altered SVZ lineage progression after injury. All experiments, n= 3/group, P <0.05.
3.6 There is a non-proliferative rise in EGFR+/GFAP+ neural stem cell population after injury
Similar to the GFAP+ cells in the SVZ, the total number of EGFR+ cells was significantly increased by day 7 in the injured brains (Fig. 4C). This occurred despite the fact that the number of actively dividing cells that were EGFR+ was significantly lower at all post-injury time points (P<0.05) as shown above (Fig. 2F). Thus, both GFAP+ and EGFR+ cells increase in number without proliferation after TBI, suggesting a change in normal lineage progression within the SVZ.
Co-expression of EGFR and GFAP has been shown to identify activated stem cells in the uninjured brain (Pastrana et al., 2009). Therefore we examined whether the non-proliferative increase in total numbers of GFAP+ cells that we measured (Fig. 3C) was related to increase in the number of GFAP+/EGFR+ stem cells. We found that there was an immediate and sustained increase in total numbers of GFAP+/EGFR+ cells by 65% at day 1 post-injury compared to naive, followed by increases of 132 and 265% at 3 and 7 days after injury, respectively (P<0.05 Fig. 4D). This increase in the number of double-labeled stem cells occurred despite a significant and sustained decrease in their proliferation after injury (P<0.05 Fig 4E). Furthermore, this increase in EGFR+/GFAP+ cells accounted for nearly 80% of the increase in total GFAP+ cells that occurred on days 3 and 7 after injury (Fig. 3C), suggesting that EGFR+ cells play an important role in expanding the pool of potential stem cells after injury. In this case, the injured SVZ does not appear to follow the conventional lineage progression model where stem cells divide to give rise to transit amplifying progeny.
Although EGFR+/GFAP+ SVZ cells have already been determined to represent a stem cell population in the uninjured brain (Pastrana et al., 2009), we sought to confirm that the non-proliferative increase in GFAP+/EGFR+ cells also reflects an increase in the number of bona fide stem cells within the injured SVZ. To do this we used in vitro serial clonal neurosphere formation to establish that the injured SVZ contained a larger number of self-renewing multipotent stem cells via the functional assay. Because there is an increase in the total number of EGFR+ cells in the SVZ after injury and there is evidence that EGFR+ progenitors can have similar self-renewal and multipotent properties as neural stem cells (Doetsch et al., 2002), we first normalized the number of EGFR+ cells from naïve and injured mice by Fluorescence Activated Cell (FAC) sorting and then plated the cells at clonal density for three consecutive clonal passages. The cells from the injured mice consistently demonstrated a greater self-renewal capacity, and maintained multipotent differentiation potential in keeping with the properties of increased EGFR+/GFAP+ stem cell numbers, despite there being no evidence for an increase in their proliferation in vivo after injury (Fig. 4F). Furthermore, these functional data also demonstrate that increased EGFR+/GFAP+ cells are not representative of infiltrative gliosis in the SVZ.
3.7 Increased stem cell numbers are consistent with an altered SVZ cell lineage progression after injury
SVZ transit-amplifying cells have been shown to be capable of reverting back to a more immature progenitor cell phenotype under special circumstances (Doetsch et al., 2002). Our in vivo data suggests that the effect of injury might be to slow, prevent or even to reverse the normal lineage progression of GFAP+/EGFR+ stem cells to actively dividing EGFR+/CldU+ transit-amplifying cells. Although we found an increase in the total numbers of EGFR+ cells by day 7 after injury (Fig. 4C), the percentage that were actively dividing (EGFR+/CldU+) was significantly reduced (Fig. 4G). Despite fewer EGFR+ cells undergoing division after injury, more EGFR+ cells co-expressed GFAP, increasing significantly from ~15% in naïve SVZ to 32% by 7 days after injury (p<0.05, Fig. 4H). This indicates that the injury-induced increase in the SVZ stem cell (GFAP+/EGFR+) population does not promote the normal progression of these cells into actively dividing (EGFR+/GFAP−) transit-amplifying cells.
We next tested the hypothesis that the EGFR+ transit amplifying cells from the injured brain have the competence to revert back into a stem cell state by re-acquiring GFAP expression in agreement with others (Doetsch et al., 2002). To do this we first FAC-sorted EGFR+ cells from the injured (3d) SVZ, divided the samples in half, and then incubated them with either epidermal growth factor-supplemented media or non-growth factor supplemented media for 4 hours, which is significantly shorter than typical in vitro cell cycle times of 12–24 hours. After the short incubation with growth factor we determined by flow-cytometry acquisition that the number of EGFR+/GFAP+ cells had increased compared to the same cells that were not exposed to EGF (P=0.04; Fig. 5A, B). However, the same EGF exposure did not change the number of double-labeled cells from the uninjured SVZ (Fig. 5C), which would suggest that injury-induced signals in vivo prime some EGFR+ cells to up-regulate GFAP expression but not proliferation in response to receptor activation. Thus, EGF receptor activation in vivo after injury may underlie the non-proliferative, de-differentiation expansion of the SVZ stem cell population (Fig. 5D).
Figure 5. Increased stem cell numbers from the non-proliferative population after injury may result from a reversion/conversion of the EGFR+ transit amplifying cells.
(A) Following injury, in vitro exposure to EGF for 4 hours resulted in a significant increase in the number of EGFR+ cells that co-expressed GFAP (relative to no-EGF exposure), demonstrating the competence of the cells from the SVZ of the injured brain to re-acquire GFAP expression. (B) Scatter plots of FACS acquisition for GFAP. (C) SVZ cells from naïve mice did not re-acquire GFAP expression, indicating that some injury-induced signaling must influence cellular response to growth factor stimulation. (D) Representation of a change in lineage progression after injury whereby a population of non-proliferative EGFR+/CldU− cells (gray circle/no border) becomes GFAP+ (gray astrocyte/black border) at the expense of becoming proliferative transit-amplifying cells (gray circle/black border). All experiments, n= 3/group, P <0.05.
4. DISCUSSION
Post-injury hippocampal neurogenesis has been demonstrated to have positive effects on functional recovery (Kernie & Parent, 2010; Tsai et al., 2013). However, the neurogenic progenitors are not known to migrate outside of the hippocampus itself to effect repair elsewhere in the brain. SVZ-derived progenitors, on the other hand, have been shown to migrate to distal sites of injury throughout the brain where they contribute new neurons for many months following injury. The long-term survival and functional recovery provided by these cells however, is quite limited (Parent et al., 2002, 2003; Ramaswamy et al., 2005; Blizzard et al., 2011; Ohab et al., 2006; Gregorian et al., 2009; Kreuzberg et al., 2010; Jin et al., 2001). Therefore, given the encouraging example of neurogenic recovery of function in the hippocampus, it is hoped that therapeutic approaches aimed at specific SVZ cell populations or within critical time periods post-injury may be used to enhance repair throughout the brain.
Although we know that post-injury cortical neurogenesis primarily originates from the SVZ, the early response of specific cell phenotypes within the SVZ and the cell types that are directly responsible for the injury-induced expansion of SVZ is not well understood. A subpopulation of GFAP+ cells in the adult mammalian SVZ has been identified as endogenous neural stem cells, and is thought to be relatively quiescent under normal conditions (Doetsch et al., 1999a; Garcia et al., 2004; Imura et al., 2003). GFAP+ stem cells (type B cells) are thought to generate rapidly dividing EGFR+, Mash1+, and Dlx2+ transit amplifying progenitors (type C cells) which in turn give rise to CD24+ and DCX+ neuroblasts (type A cells) which migrate to the olfactory bulb in the uninjured brain (Doetsch et al., 1999b, 2002). This knowledge, based partly on ablation experiments that used the anti-mitotic agent, Ara-C, to eliminate the faster dividing progenitors in the SVZ showed that a slowly-dividing population of cells was able to repopulate all of the different cell types in the SVZ, and to rescue endogenous neurogenesis (Doetsch et al., 1999a). From this work it has been extrapolated that a similar paradigm occurs in response to brain injury, where the normally quiescent stem cells are stimulated to divide, resulting in the increases in fast-dividing transit amplifying cells and neuroblast production. However, contrary to this conventional wisdom, we have shown that although the neural stem cell population does expand in response to traumatic brain injury, this does not result from more proliferation after injury.
We find that the Mash1-expressing transit-amplifying cells are the primary cell type directly responsible for injury-induced increases in SVZ size and cell proliferation. No other cell type examined, including GFAP+, EGFR+ or DCX+ cells increased division in response to injury. In fact, the EGFR+ cells, which under normal conditions have been shown to also be largely a transit-amplifying population (Doetsch et al., 2002), divide less after injury. Although both EGFR+ and Mash1+ SVZ cells are both transit amplifying progenitor populations, we observed that even in naïve brains the EGFR+ cells were less proliferative than the Mash1+ cells (98% of Mash1+ cells actively proliferate versus 46% of EGFR+ cells) and are even less proliferative after injury. Thus, there is a non-overlapping, EGFR+/Mash1− population that is revealed by injury to be a non-transit amplifying progenitor population within the SVZ that has a divergent response to TBI from the Mash1+ population. Since the Mash1+ cells are the primary proliferative population in the SVZ both before and after injury, they clearly would not directly contribute to the non-proliferative increases we observed in EGFR+/GFAP+ stem cells. Our data suggest that it is the quiescent EGFR+/Mash1-negative population, which reverts to a stem cell state and up-regulates GFAP expression in response to injury-induced signaling. Consequently, it is the decreased proliferation of the EGFR+/Mash1− population and altered lineage progression, which underlies the increase in label-retaining SVZ stem cells between naïve and injured brain.
Unlike ablation models which suggest that SVZ cell lineage progression involves an activation of stem cell proliferation that give rise to highly proliferative progenitors, brain injury results in an increase in GFAP+ stem cells and EGFR+ progenitors without any corresponding increase in their proliferation. In vitro serial clonal density assays performed with EGFR+ cells isolated from the SVZ confirmed that the non-proliferative increase in EGFR+/GFAP+ cell number that we observed does indeed correspond to an increase in a self-renewing, multipotent stem cell population within the SVZ of the injured brain. Although it has been shown that EGF-responsive SVZ transit amplifying cells can also have multipotent, self-renewing capacities indistinguishable from neural stem cells in the presence of growth factor (Doetsch et al., 2002), we observed that injury-induced increases in self-renewal are still reflected in vitro when the number of EGFR+ cells have been normalized from the injured and naïve brains. Even though the number of MASH1+ transit amplifying cells was also increased in the SVZ after injury, MASH1+ cells were shown to not make any contribution to self-renewal in functional clonal neurosphere assays (Parras et al., 2004). Thus, despite equal exposure to growth factor and equal numbers of EGFR+ SVZ cells being plated in our clonal assays, the increased capacity for self-renewal could be detected in cells from the SVZ of the injured brain, indicating an increase in neural stem cell number in agreement with our in vivo observations.
The increase in neural stem cells without proliferation after injury supports the idea that SVZ lineage progression is not necessarily unidirectional. For example, increased exposure to EGF in the SVZ of uninjured mice has been shown to induce a reversion of the EGFR+ transit amplifying progenitor population back to a stem cell state (Doetsch et al., 2002). In agreement with this explanation, our data show that a purified population of EFGR+ cells isolated by FACS from the SVZ of injured animals are capable of “turning on” GFAP expression consistent with a reversion to an EGFR+/GFAP+ activated stem cell state following a brief exposure to EGF whereas the same cells from the uninjured brain were not competent to do this. Therefore, these findings show that the increase in the number of EGFR+/GFAP+ stem cells can be derived from an EGFR+ transit amplifying population and suggests that injury-induced signals may alter the direction of normal cell lineage relationships. Correlative evidence supports the idea that EGFR ligands influence the cellular response in the SVZ after injury: EGF and TGF-α have been shown to promote the expansion of transit-amplifying cells in the SVZ at the expense of neuroblast production, similar to what we observed after TBI (Doetsch et al., 2002; Kim et al., 2009; Craig et al 1996; Ninomiya et al 2006; Alagappan et al., 2009). Furthermore, both EGF and TGF-α are substantially upregulated following brain injury (Sundholm et al., 2005; Helmy et al., 2010).
Finally, although we show that the increase in SVZ stem cells does not occur via proliferation in vivo post-injury, this does not rule out the possibility that they leave this quiescent state at times after the 7 day time point of the current data. After stroke, new neurons migrate from the SVZ to the site of injury for up to 4 months following injury, indicating a sustained increase in neurogenesis (Thored et al., 2006; Leker et al., 2007). This long-term supply of neuroblasts would likely require stem cell proliferation to replenish and maintain the fast dividing progenitor pool from which the neuroblasts are derived in the SVZ. It is therefore conceivable that this acute 7-day period, which we have studied, represents a “priming” or activating period for the stem cell pool during which time the number of stem cells increases without proliferation. Maintaining the balance between the different populations of stem and progenitor cells within the SVZ niche may be critical for promoting and manipulating neurogenesis under both normal and injured conditions.
Few studies have closely examined the specific cell populations within the SVZ that respond to injury after different insults. Therefore, it is uncertain if our findings are unique to traumatic brain injury or if there is a comparable response in the SVZ after other types of injuries. Certainly, we have established that the TBI response in the intact SVZ is significantly altered from the response after ablation of different cell populations in the SVZ. Thus, detailed knowledge of the diverse cellular response to brain injuries would significantly aid in the development of appropriate therapeutic approaches aimed at specific cell populations, signaling pathways, or within specific time periods post-injury in order to enhance endogenous neurogenesis and brain repair.
Supplementary Material
Highlights.
Expansion of the SVZ niche after TBI is mainly due to Mash1 transit amplifying cells.
EGFR+ progenitors decrease proliferation and can become GFAP+ after injury.
TBI increases the number of EGFR+/GFAP+ SVZ stem cells without proliferation.
These data indicate the likelihood of an altered lineage progression after TBI.
Acknowledgments
Support: NIH NINDS grant #’s R01 NS055910, NRSA 5F31NS064697-02 & UCLA Brain Injury Research Center
We wish to thank Dr. David McArthur for helpful discussion about the statistics. This work was supported by the UCLA Brain Injury Research Center, and Award Number R01 NS055910 and NRSA 5F31NS064697-02 from the National Institute of Neurological Disorders and Stroke (NINDS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alagappan D, Lazzarino Da, Felling RJ, et al. Brain injury expands the numbers of neural stem cells and progenitors in the SVZ by enhancing their responsiveness to EGF. ASN Neuro. 2009;1(2):95–111. doi: 10.1042/AN20090002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Blizzard CA, Chuckowree JA, King AE, et al. Focal damage to the adult rat neocortex induces wound healing accompanied by axonal sprouting and dendritic structural plasticity. Cereb Cortex. 2011;21(2):281–91. doi: 10.1093/cercor/bhq091. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;10(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, et al. Fulminant Jejuno-Ileitis following Ablation of Enteric Glia in Adult Transgenic Mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- Cesetti T, Obernier K, Bengtson CP, et al. Analysis of stem cell lineage progression in the neonatal subventricular zone identifies EGFR+/NG2- cells as transit-amplifying precursors. Stem Cells. 2009;27(6):1443–54. doi: 10.1002/stem.74. [DOI] [PubMed] [Google Scholar]
- Chen X-H, Iwata A, Nonaka M, et al. Neurogenesis and glial proliferation persist for at least one year in the subventricular zone following brain trauma in rats. J Neurotraum. 2003;20(7):623–31. doi: 10.1089/089771503322144545. [DOI] [PubMed] [Google Scholar]
- Ciccolini F, Mandl C, Hölzl-Wenig G, et al. Prospective isolation of late development multipotent precursors whose migration is promoted by EGFR. Dev Biol. 2005;284(1):112–25. doi: 10.1016/j.ydbio.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Craig G, Der Kooyl V, Reynolds A, et al. In Vivo Growth Factor Expansion Neural Precursor Cell Populations. J Neurosci. 1996;16(8):2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999a;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. P Natl Acad Sci USA. 1999b;96(20):11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, et al. EGF Converts Transit-Amplifying Neurogenic Precursors in the Adult Brain into Multipotent Stem Cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Garcia a DR, Doan NB, Imura T, et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7(11):1233–41. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Gregorian C, Nakashima J, Le Belle J, et al. Deletion in Adult Neural Stem/Progenitor Cells Enhances Constitutive Neurogenesis. Stem Cells. 2009;29(6):1874–1886. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience. 1997;80(2):427–36. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Helmy A, Carpenter KLH, Menon DK, et al. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cerebr Blood F Met. 2010;31(2):658–670. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Kornblum HI, Sofroniew MV. The Predominant Neural Stem Cell Isolated from Postnatal and Adult Forebrain But Not Early Embryonic Forebrain Expresses GFAP. Stem Cells. 2003;23(7):2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. P Natl Acad Sci USA. 2001;98(8):4710–5. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 2010;37(2):267–74. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Comte I, Szabo G, et al. Adult mouse subventricular zone stem and progenitor cells are sessile and epidermal growth factor receptor negatively regulates neuroblast migration. Plos One. 2009;4(12):e8122. doi: 10.1371/journal.pone.0008122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzberg M, Kanov E, Timofeev O, et al. Increased subventricular zone-derived cortical neurogenesis after ischemic lesion. Exp Neurol. 2010;226(1):90–99. doi: 10.1016/j.expneurol.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8(1):59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leker RR, Soldner F, Velasco I, et al. Long lasting regeneration after ischemia in the cerebral cortex. Stroke. 2007;38(1):153. doi: 10.1161/01.STR.0000252156.65953.a9. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405(6789):951–5. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, et al. Neural stem cells in the adult mammalian forebrain: A relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, et al. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Ninomiya M, Yamashita T, Araki N, et al. Enhanced neurogenesis in the ischemic striatum following EGF-induced expansion of transit-amplifying cells in the subventricular zone. Neurosci Lett. 2006;403(1–2):63–7. doi: 10.1016/j.neulet.2006.04.039. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, et al. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–130016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, et al. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52(6):802–13. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Parent JM. Injury-Induced Neurogenesis in the Adult Mammalian Brain. Neuroscientist. 2003;9(4):261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Parras CM, Galli R, Britz O, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. Embo J. 2004;23(22):4495–505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana E, Cheng L-C, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. P Natl Acad Sci USA. 2009;106(15):6387–92. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- Ramaswamy S, Goings G, Soderstrom K. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053:38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Sundholm-Peters NL, Yang HKC, Goings GE, et al. Subventricular zone neuroblasts emigrate toward cortical lesions. J Neuropath Exp Neur. 2005;64(12):1089–100. doi: 10.1097/01.jnen.0000190066.13312.8f. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24(3):739–47. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Tonchev A, Yamashima T, Zhao L, et al. Proliferation of neural and neuronal progenitors after global brain ischemia in young adult macaque monkeys. Mol Cell Biol. 2003;23(2):292–301. doi: 10.1016/s1044-7431(03)00058-7. [DOI] [PubMed] [Google Scholar]
- Tsai Y-W, Yang Y-R, Sun SH, et al. Post ischemia intermittent hypoxia induces hippocampal neurogenesis and synaptic alterations and alleviates long-term memory impairment. J Cerebr Blood F Met. 2013;33(5):764–73. doi: 10.1038/jcbfm.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita Y, Kitagawa K, Ohtsuki T, et al. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke. 2001;32(8):1890–6. doi: 10.1161/01.str.32.8.1890. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Yamamoto N, Kitamura T, et al. Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp Neurol. 2001;172(1):115–27. doi: 10.1006/exnr.2001.7798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.