Abstract
Reductions in brain volumes represent a neurobiological signature of fetal alcohol spectrum disorders (FASD). Less clear is how regional brain tissue reductions differ after normalizing for brain size differences linked with FASD and whether these profiles can predict the degree of prenatal exposure to alcohol. To examine associations of regional brain tissue excesses/deficits with degree of prenatal alcohol exposure and diagnosis with and without correction for overall brain volume, tensor-based morphometry (TBM) methods were applied to structural imaging data from a well-characterized, demographically homogeneous sample of children diagnosed with FASD (n = 39, 9.6–11.0 years) and controls (n = 16, 9.5–11.0 years). Degree of prenatal alcohol exposure was significantly associated with regionally pervasive brain tissue reductions in: (1) the thalamus, midbrain, and ventromedial frontal lobe, (2) the superior cerebellum and inferior occipital lobe, (3) the dorsolateral frontal cortex, and (4) the precuneus and superior parietal lobule. When overall brain size was factored out of the analysis on a subject-by-subject basis, no regions showed significant associations with alcohol exposure. FASD diagnosis was associated with a similar deformation pattern, but few of the regions survived FDR correction. In data-driven independent component analyses (ICA) regional brain tissue deformations successfully distinguished individuals based on extent of prenatal alcohol exposure and to a lesser degree, diagnosis. The greater sensitivity of the continuous measure of alcohol exposure compared with the categorical diagnosis across diverse brain regions underscores the dose dependence of these effects. The ICA results illustrate that profiles of brain tissue alterations may be a useful indicator of prenatal alcohol exposure when reliable historical data are not available and facial features are not apparent.
Keywords: Tensor-based morphometry, Fetal alcohol spectrum disorders, Prenatal alcohol exposure, Structural MRI, Neurodevelopment, Morphology, Brain structure
Abbreviations: AA, absolute alcohol; CSF, cerebrospinal fluid; FAS, fetal alcohol syndrome; FASD, fetal alcohol spectrum disorders; ICA, independent component analyses; MDT, minimal deformation target; MEMPRAGE, multiecho magnetization prepared rapid gradient echo; TBM, tensor-based morphometry; WISC-IV, Wechsler Intelligence Scale for Children
Highlights
-
•
Tensor-based morphometry predicts brain volume reductions in fetal alcohol syndrome.
-
•
Normalizing for brain size in FASD may mask regional differences in tissue volume.
-
•
Patterns of volumetric change are pervasive, particularly in midline structures.
-
•
Degree of prenatal alcohol exposure predicts pervasiveness of volumetric deficits.
-
•
Pattern of volumetric change may be useful in identifying individuals with FASD.
1. Introduction
Prenatal alcohol exposure causes physical and behavioral impairments (Kodituwakku, 2009; Mattson et al., 2011) that range in severity and occur through the disruption of normal neurodevelopmental processes (Ismail et al., 2010; Jacobson et al., 2011; Thompson et al., 2009), impacting both the size and the structural organization of the brain (Guerri et al., 2009; Norman et al., 2009; Roebuck et al., 1998).
While the severity of physical and behavioral symptoms is related to exposure dose and frequency (e.g., Streissguth et al., 1989, 1994; Jacobson et al., 1998; Jacobson et al., 2008), these associations are difficult to characterize as the amount of alcohol use during pregnancy is often poorly recalled in retrospective case–control studies (Jacobson et al., 2002). Instead, growth deficiencies, facial dysmorphology, and central nervous system dysfunctions are typically used to diagnose and categorize severity of exposure. Diagnosis is challenging as facial anomalies may be subtle or absent (Suttie et al., 2013). Understanding the links between extent of fetal alcohol exposure and disruptions in the structural development of the brain may thus be helpful for distinguishing individuals with fetal alcohol spectrum disorders (FASD) and elucidating vulnerable functional systems.
Few studies examining the impact of fetal alcohol exposure on brain development (for review see Lebel et al., 2011; Norman et al., 2009) have employed advanced computational image analysis methods to simultaneously determine global and local changes in brain tissue architecture. Tensor-based morphometry (TBM) uses Jacobian determinant values obtained from the linear and non-linear deformation fields required to match structures with similar intensity patterns of individual subjects to a population specific atlas. Since TBM matches structures with similar intensity patterns, it can detect local volumetric excesses and deficits in brain tissue at the voxel level (e.g., Chiang et al., 2007; Gogtay et al., 2008; Ho et al., 2010; Hua et al., 2008; Leow et al., 2006; Lepore et al., 2010; Yanovsky et al., 2009).
Only one prior investigation has applied TBM to compare children with heavy prenatal exposure to alcohol or methamphetamine and controls (Sowell et al., 2010). Although this study, which examined local changes after removing global scaling differences from the imaging data, found volumetric deficits in several regions, prenatal alcohol exposure was also associated with expansions in several regions. The latter effects, which are not consistent with findings from other neuroimaging and autopsy studies, may be due to over-compensatory expansions in local volumes occurring as a function of normalization for brain size.
Here we characterize the nature and extent of cerebral abnormalities in FASD in a prospectively recruited, demographically homogeneous sample of FASD subjects and controls using TBM methods to quantitatively map volumetric differences throughout the brain. We compared voxel-level variations in brain tissue volume in relation to both a continuous measure of oz of absolute alcohol consumed per day during pregnancy and diagnosis based on discrete classifications of FASD severity, both with and without taking global brain size differences into account. In addition, using objective, data-driven independent component analyses (ICA), we addressed whether patterns of volumetric deviation can separately predict the presence and extent of prenatal alcohol exposure.
2. Materials and methods
2.1. Participants
Participants were 55 9- to 11-year-old children (28 males, mean age 10.4 ± 0.4 years) from Cape Town, South Africa, who are enrolled in a prospective, longitudinal study of FASD (Jacobson et al., 2008, 2011). Of these, 39 were heavily exposed to alcohol and 16 were demographically similar controls. All children were from the Cape Coloured (mixed ancestry) community, which is composed mainly of descendants of white European settlers, Malaysian slaves, Khoi-San aboriginals, and black African ancestors. The incidence of fetal alcohol syndrome (FAS) in this community, situated in a geographical region supporting a wine-producing industry, is estimated to be 18–141 times greater than that in the United States (May et al., 2000, 2007). Poor socioeconomic circumstances and historical practices of compensating farm labor with wine have contributed to a tradition of heavy recreational weekend binge drinking in a portion of this population, leading to the increased incidence of FAS.
During 1998–2002, mothers initiating antenatal care at a clinic serving a predominantly Cape Coloured community were interviewed regarding their alcohol consumption using a timeline follow-back approach (Jacobson et al., 2002). At recruitment the mother was interviewed regarding incidence and amount of her drinking on a day-by-day basis during a typical 2-week period at time of conception. Volume was recorded for each type of beverage consumed each day and converted to oz absolute alcohol (AA) (Jacobson et al., 2008). The mother was then asked whether her drinking had changed since conception; if so, when the change occurred and how much she drank on a day-by-day basis during the last 2 weeks. Two groups of women were recruited: (1) heavy drinkers, who consumed 14 or more standard drinks/week (≈1.0 oz AA/day) and/or engaged in binge drinking (5 or more drinks/occasion) and (2) controls, 14 of whom abstained from drinking and 2 who drank only minimally during pregnancy (one averaged 3 drinks/occasion twice monthly, and the other drank 2 drinks on 4 occasions). The timeline follow-back interview was repeated in mid-pregnancy and again at 1 month postpartum to provide information about drinking during the latter part of pregnancy. Data from the three alcohol consumption interviews were tabulated to provide three continuous measures of drinking during pregnancy: average oz AA consumed/day (AA/day), AA/drinking day (dose/occasion), and frequency (days/week). Smoking during pregnancy was reported in terms of cigarettes smoked per day; one outlier with cigarettes/day > 3SD above the mean for this sample was recoded to 1 unit above the next highest observed value as recommended by Winer (1971). Exclusionary criteria included age < 18 years, diabetes, epilepsy, cardiac problems requiring treatment, and observant Muslims whose religious practice prohibits alcohol consumption.
In 2005 we organized a clinic, in which each child was examined for growth and FAS anomalies by two expert dysmorphologists using a standard protocol (see Jacobson et al., 2008). Based on the revised Institute of Medicine guidelines, FAS is characterized by microcephaly, growth retardation, and a distinctive craniofacial dysmorphology, including short palpebral fissures, a flat philtrum, and a thin vermilion (upper lip) (Hoyme et al., 2005). A partial FAS (PFAS) diagnosis requires the presence of at least two of these facial features, as well as microcephaly, retarded growth, or neurobehavioral deficits. Children with alcohol-related neurodevelopmental disorder exhibit neurobehavioral deficits without the characteristic facial features. Of the 39 children born to heavy drinking mothers, 7 met the criteria for full FAS, 18 for PFAS, and 14 for neither syndrome. Approval for human research was obtained from the Wayne State University Institutional Review Board and University of Cape Town Faculty of Health Sciences Human Research Ethics Committee. All mothers provided informed written consent; the children provided oral assent.
2.2. Neuropsychological assessment
Intellectual ability (IQ) for each child was assessed at our University of Cape Town Child Development Research Laboratory using the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) in English or Afrikaans, depending on the language used in the child's elementary school classroom (see Diwadkar et al., 2013).
2.3. Image acquisition
High-resolution T1-weighted structural MR images were acquired on a 3 T Allegra MRI scanner (Siemens, Erlangen, Germany) using a 3D EPI-navigated (Tisdall et al., 2009) multiecho magnetization prepared rapid gradient echo (MEMPRAGE) (van der Kouwe et al., 2008) sequence optimized for morphometric analyses using FreeSurfer software. Imaging parameters were as follows: FOV: 256 × 256 mm; 128 sagittal slices; TR: 2530 ms; TE: 1.53/3.21/4.89/6.57 ms; TI: 1100 ms; flip angle: 7°; voxel size: 1.3 × 1.0 × 1.3 mm3; and acquisition time: 8:07 min. The 3D EPI navigator provided real-time motion tracking and correction, substantially reducing motion artifacts in the images, even in the presence of frequent subject motion.
2.4. Image processing
TBM is an advanced, relatively unbiased and mostly automated image analysis approach that allows variations in brain tissue structure (gray and white matter and cerebrospinal fluid (CSF)) to be determined and compared between subjects throughout the entire brain. In brief, TBM identifies brain structural differences from the gradients of linear and/or non-linear deformation fields required to align or warp individual target brains to an anatomical reference (Ashburner et al., 2003). Comparisons of three-dimensional Jacobian determinant maps derived from the deformation fields made at the voxel level then determine both local and global changes in brain tissue volume in association with individual differences such as the extent/severity of prenatal alcohol exposure investigated here.
Prior to applying TBM, non-brain tissue (scalp and meninges) was removed from each image volume using brain masks created with FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/). Any brain segmentation errors were corrected on a slice-by-slice basis. Image volumes were corrected for signal intensity and magnetic field inhomogeneity artifacts (Sled and Pike, 1998). TBM workflows including procedures similar to those detailed in prior studies (Chiang et al., 2007; Gogtay et al., 2008; Ho et al., 2010; Hua et al., 2008; Leow et al., 2006; Lepore et al., 2010) were implemented in the LONI Pipeline environment (Dinov et al., 2009; Dinov et al., 2010). First, each preprocessed image volume was registered to a single template image using an affine 9-parameter registration to adjust for global brain scale and alignment. Transformation matrices were retained for subsequent processing steps allowing the data to be assessed without global scaling. Next, images from the control group were used to create a minimal deformation target (MDT) or average anatomical template. For this processing step, each individual volume was aligned to all other volumes using a mutual information (MI)-based inverse-consistent algorithm, followed by applying the inverse of the mean displacement field from all subjects to the MDT. Finally, image volumes from all subjects were each separately and similarly aligned to the MDT by nonlinearly deforming the anatomy of each individual image to the anatomical template while optimizing the regularity of the deformation by quantifying the inverse consistent symmetric Kullback–Leibler (KL)-distance between the MDT and the resulting deformation to allow for unbiased registration (Hua et al., 2011).
The Jacobian operator was then applied to the deformation fields required to non-linearly match each subject's anatomy to the MDT to produce univariate Jacobian determinants (i.e., Jacobian maps). These 3D Jacobian maps represent relative tissue volume differences between each individual and the MDT after factoring out global scaling differences and may be compared voxel-wise across the entire brain to reveal local changes in tissue structure between subjects. To determine tissue-specific differences without factoring out global scaling differences, the scaling factors obtained from the affine transformations in earlier processing steps were applied to the Jacobians at each voxel. Thus, we could map changes in brain tissue structure both with and without normalizing for overall brain size differences. This is beneficial, as children with prenatal alcohol exposure tend to have smaller brain volume and small head circumference is one of the diagnostic criteria for FAS and PFAS.
In addition, to provide independent verification of TBM findings, FreeSurfer (Fischl et al., 2002, 2004), an automated brain segmentation program, was used to measure the volumes of 22 discrete subcortical structures and 34 cortical regions.
2.5. Statistical analyses
Regression analyses, employing computational tools provided by the Statistics Online Computational Resource (http://www.SOCR.ucla.edu) (Chu et al., 2009), were used to relate local variations in brain structure to the continuous measure of prenatal alcohol exposure (AA/day) for all subjects both with and without normalizing for brain size. The AA/day measure was log transformed to reduce skewness. Jacobian determinants indexing both affine and non-linear deformations (i.e., representing changes in unscaled/native space) and Jacobians indexing non-linear deformations only (i.e., representing changes in scaled/normalized space) were each assessed in relation to AA/day. The General Linear Model was used to compare groups based on discrete categorizations of FASD (FAS and PFAS compared to controls), again with and without normalizing for brain size; that is, in scaled and unscaled data. Children with heavy prenatal exposure not meeting the criteria for FAS or PFAS were excluded from the diagnostic group comparisons.
Probability values for regions surviving an uncorrected threshold of p < 0.05 were color coded in red and mapped onto the MDT. Since comparisons were made at thousands of voxels, uncorrected probability maps were then thresholded using a False Discovery Rate (FDR) of 5% (q-value = 0.05) according to the implementation by Storey et al. (2002, 2004). Regional effects surviving FDR thresholding were color coded in yellow and superimposed onto the uncorrected probability maps. Color-coded r- and beta maps were used to indicate the direction and magnitude of effects for AA/day and diagnosis, respectively.
Log transformed Jacobian maps of the unscaled data were used in ICA to determine whether the pattern of volumetric variations throughout the brain predicted the degree of prenatal alcohol exposure and/or diagnostic status. The dimensionality of the data was reduced using a principal components analysis, planned a priori to retain 10 dimensions, which were deemed to be sufficient to cover the candidate sources (e.g., sex, age, alcohol exposure, smoking), while also allowing for the possibility of some unquantified sources (e.g., genetic variation, infectious disease exposure).
An ICA component that showed significant correlations with both degree of prenatal alcohol exposure and diagnosis was mapped to show FDR-thresholded results superimposed on the uncorrected probability maps in yellow and red, respectively.
3. Results
3.1. Sample characteristics
Sample demographics are summarized in Table 1. All children were scanned within a narrow 1.5-year age range (9.5–11.0 years). There were no significant differences in sex distribution across the four diagnostic groups (χ2(3) = 2.69, p > 0.20). However, sex was included as a covariate in the analyses of the effects of degree of prenatal alcohol exposure and diagnosis because males have larger brain volumes than females and the groups were not matched on a per subject basis for this variable. Alcohol consumption among the drinking mothers was very heavy. Mothers of children with FAS averaged 8.4 drinks/occasion during pregnancy; PFAS, 7.6; heavily exposed nonsyndromal, 5.6. Reflecting the high degree of socioeconomic disadvantage in this community, IQ scores were very low even among the controls. IQ in the alcohol-exposed children was even lower than that in the controls, F(1,53) = 7.32, p = 0.009, and inversely correlated with prenatal alcohol exposure, r = −0.50, p < 0.01. However, IQ was not modeled as a confounder in the TBM analyses because effects of prenatal alcohol exposure on IQ are presumably mediated by alcohol effects on regional brain volumes. As expected, total intracranial volume as measured by FreeSurfer also decreased in relation to degree of prenatal alcohol exposure, r = −0.31, p = 0.02.
Table 1.
Sample characteristics by diagnostic group (N = 55).
| FAS | PFAS | HE | Control | F or χ2 | |
|---|---|---|---|---|---|
| (7) | (18) | (14) | (16) | ||
| Maternal/primary caregiver characteristics | |||||
| Age at delivery | 30.6 (9.1) | 26.5 (6.9) | 24.7 (5.4) | 27.1 (4.2) | 1.70 |
| Years of educationa |
9.3 (2.4) | 6.5 (2.4) | 9.2 (2.2) | 10.1 (1.4) | 9.41⁎⁎⁎ |
| Married (%)a |
42.9 | 27.8 | 42.9 | 62.5 | 4.16 |
| Parity | 3.1 (1.4) | 2.6 (1.9) | 1.9 (0.8) | 1.8 (0.9) | 2.50† |
| Alcohol during pregnancy | |||||
| oz AA/day | 1.9 (2.6) | 1.0 (0.7) | 0.5 (0.5) | 0.01 (0.03) | 6.38⁎⁎⁎ |
| oz AA/occasion | 4.2 (2.6) | 3.8 (1.8) | 2.8 (1.6) | 0.2 (0.5) | 17.82⁎⁎⁎ |
| Frequency (days/week) | 2.1 (2.2) | 2.0 (1.0) | 1.1 (0.9) | 0.04 (0.1) | 11.12⁎⁎⁎ |
| Cigarettes/day during pregnancy | |||||
| 9.5 (5.1) | 8.2 (5.9) | 8.4 (7.3) | 3.7 (9.9) | 1.57 | |
| Child characteristics | |||||
| Child gender (% male) | 28.6 | 61.1 | 57.1 | 43.8 | 2.69 |
| Age | |||||
| Neurobehavioral assessment (years) | 9.1 (0.3) | 9.4 (0.4) | 9.6 (0.5) | 9.3 (0.4) | 2.85⁎ |
| Neuroimaging scan (years) | 10.0 (0.5) | 10.6 (0.4) | 10.6 (0.2) | 10.3 (0.4) | 4.84⁎⁎ |
| Weight (kg) | 21.3 (2.7) | 25.9 (2.8) | 37.6 (16.1) | 29.5 (6.8) | 6.43⁎⁎⁎ |
| Height (cm) | 120.8 (3.9) | 128.4 (5.0) | 136.4 (10.0) | 129.7 (5.8) | 8.89⁎⁎⁎ |
| Head circumference (cm) | 49.6 (2.0) | 50.7 (1.6) | 52.6 (1.7) | 52.6 (1.0) | 9.89⁎⁎⁎ |
| WISC-IV IQ | 65.0 (8.7) | 63.6 (10.3) | 72.8 (8.2) | 74.8 (8.1) | 5.65⁎⁎ |
| Volumetric measures (cm3) | |||||
| Total intracranial volume | 1223 (127) | 1340 (122) | 1435 (126) | 1359 (86) | 5.50⁎⁎ |
Values are mean (SD) or %. FAS = fetal alcohol syndrome, PFAS = partial FAS, HE = heavily exposed nonsyndromal.
Obtained from mother or primary caregiver at 9-year follow-up assessment visits.
p < 0.10.
p < 0.05.
p < 0.01.
p < 0.001.
3.2. Effects of brain normalization
Regression analysis was used to examine the effects of brain size normalization on scaled (i.e., Jacobians representing non-linear deformations only) as compared to unscaled (i.e., Jacobians representing both affine and non-linear deformations) data across subjects. As expected, in the unscaled data, total brain volume was highly correlated with Jacobian determinants averaged throughout the brain, r = 0.98, p < 0.0001 (Fig. 1). By contrast, total brain volume was not significantly related to Jacobian determinants after global scaling differences were removed from the data by linear spatial normalization, r = −0.23, p = 0.08. Kolmogorov–Smirnov statistics showed that mean Jacobian values were normally distributed in both the normalized and unscaled data, both ps > 0.20.
Fig. 1.
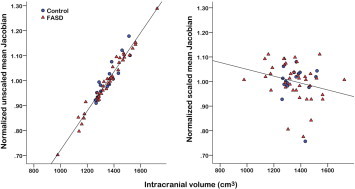
Relation of total brain volume to Jacobian values averaged across the entire brain in unscaled / native (left) and normalized / scaled (right) space for each subject. FASD = fetal alcohol spectrum disorders.
3.3. TBM effects of the extent of prenatal alcohol exposure
Statistical maps showing relations between prenatal exposure and variations in regional brain tissue volume in unscaled data (without normalizing for brain volume) are shown in Fig. 2. Probability values are mapped in the lower panel of the figure where p-values surviving FDR thresholding at 5% are encoded in yellow and superimposed on the uncorrected probability values (p < .05) represented in red. Color-coded r-maps at the top of Fig. 2 indicate the strength and direction of the relations at each voxel. For descriptive purposes, the relation between AA/day and Jacobian values averaged within regions exhibiting significant effects (p < .05) is plotted on the right. Slice views showing r-values across the entire brain are shown in Supplementary Fig. 1. As can be seen from the statistical maps in Fig. 2, greater AA/day was associated with significant reductions in brain tissue volume in four regions: (1) a bilateral region encompassing the thalamus, midbrain, and the ventromedial portion of the frontal lobe, (2) a medial bilateral region encompassing the upper surface of the cerebellum and inferior surfaces of the occipital lobe, (3) the bilateral dorsolateral frontal lobe, and (4) the precuneus extending bilaterally into superior parietal lobule and parietal central white matter. No regions showed significant positive relations (expansions) between degree of exposure and local brain tissue changes.
Fig. 2.
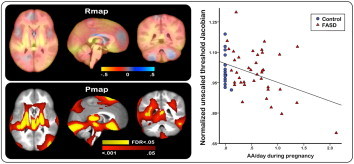
Statistical maps relating the degree of prenatal alcohol exposure to local brain tissue differences in unscaled data, adjusted for child sex. Top: color encoded r -values showing the relations between oz absolute alcohol (AA) / day and regional brain tis- sue reductions (hot colors) and expansions (cool colors). 1 oz AA ≈2 standard drinks. Bottom: significant effects of oz AA / day shown in corresponding slice views in red (uncorrected p < .05), and effects surviving an FDR threshold of q = .05 super- imposed in yellow. Right: mean Jacobian values from each subject, masked to include only regions where the uncorrected p value for AA / day was < .05, plotted as a function of oz AA / day during pregnancy.
Statistical analysis of relations between AA/day and regional brain volume in scaled data, where the Jacobians represent only local non-linear deformations, did not survive FDR thresholding at any brain location. Uncorrected probability maps and corresponding r-maps for these results, as well as slice views showing r-values across the entire brain, are shown in Fig. 3 and Supplementary Fig. 2, respectively. Although the results did not survive FDR correction, the pattern of regional effects differed markedly from that seen in the unscaled data, with alcohol exposure relating to localized volumetric expansions in spatially distinct mesial prefrontal, insula, lingual and cerebellar regions, which are all located adjacent to extra-cortical CSF and showed minimal effects prior to normalizing for brain size. The relation between AA/day and Jacobian values averaged in areas showing significant effects (p < .05) is plotted in Fig. 3 on the right.
Fig. 3.

Statistical maps relating the degree of prenatal alcohol exposure to local brain tissue differences in scaled data, adjusted for child sex. Top: color encoded r -values showing the relation between oz absolute alcohol (AA) / day and regional brain tissue reductions (hot colors) and expansions (cool colors). Bottom: significant effects of oz AA / day shown in corresponding slice views in red (uncorrected p < .05). Effects did not survive FDR thresholding at q = .05 at any brain location. Right: mean Jacobian values from each subject, masked to include only regions where the uncorrected p value for AA / day was < .05, plotted as a function of oz AA / day during pregnancy.
3.4. TBM effects of diagnosis
Statistical maps of the effects of diagnosis (FAS and PFAS compared to controls) on regional brain tissue volumes in unscaled data are shown in Fig. 4. In contrast to the effects observed for the continuous measure of prenatal alcohol exposure in Fig. 2, only a few regions survived FDR correction at 5%, although sub-threshold effects were observed in similar regions and in the same direction. Fig. 4 shows p-values surviving FDR-thresholding in yellow superimposed onto the uncorrected p-values indicated in red (lower panel) and corresponding beta-values encoded in color in the top panel. For descriptive purposes, Jacobians averaged in brain areas showing significant effects have been plotted by diagnosis on the right of Fig. 4 (p < .05). Slice views showing beta-values across the entire brain are provided in Supplementary Fig. 3.
Fig. 4.
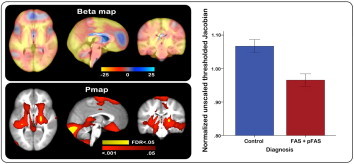
Statistical maps showing the effects of diagnosis (fetal alcohol syndrome (FAS) and partial FAS (pFAS) compared to controls) on regional brain tissue volumes in un- scaled data, adjusted for child sex. Top: color encoded beta -values showing the effects of diagnosis on FASD-related regional brain tissue reductions (hot colors) and expan- sions (cool colors). Bottom: significant effects of diagnosis are shown in corresponding slice views in red (uncorrected p < .05), and p -values surviving FDR correction ( q = .05) are superimposed in yellow. Right: mean Jacobian values from each subject, masked to include only regions where the uncorrected p value for an effect of diagnosis was < .05, plotted by diagnostic status.
Statistical analysis of the effects of diagnosis in scaled data did not survive FDR thresholding at any brain location. Uncorrected probability and beta-maps, as well as slice views showing beta-values across the entire brain, are shown in Supplementary Figs. 4 and 5, respectively. Jacobians averaged within regions showing significant effects are plotted by diagnosis on the right in Supplementary Fig. 4 (p < .05). Notably, as for the analysis of the continuous measures of alcohol exposure in relation to the scaled data, regional expansions in brain tissue volume were observed in the FAS/PFAS group in localized mesial prefrontal, perisylvian and cerebellar regions adjacent to CSF.
3.5. ICA
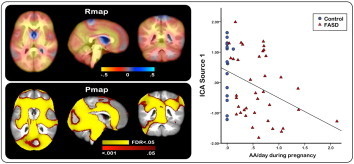
To determine whether regional variations in brain tissue volume might independently predict the degree of prenatal exposure to alcohol and/or diagnostic group membership, the log transformed Jacobian maps obtained from each child were subjected to ICA. Since the regional deformations did not relate significantly to FASD after normalization for total brain volume, ICA were performed only on the TBM analyses incorporating both linear and non-linear deformations (i.e., the unscaled data). The dimensionality of the data was reduced using principal components analysis, retaining the 10 dimensions explaining the greatest variance. These sources were examined to determine which most strongly related to AA/day or diagnostic group. A subgaussian source explaining 11.7% of the variance significantly predicted the degree of alcohol exposure, r = −0.366, p < 0.006 and, to a lesser extent, the diagnostic group, F(1,54) = 4.11, p = 0.048.
Fig. 5 shows significant relations of the ICA source associated with degree of alcohol exposure and diagnosis to regional changes in brain tissue structure. p-Values surviving FDR-thresholding shown in yellow are superimposed onto the uncorrected p-values indicated in red (lower panel), and the corresponding r-values in the top panel indicate the magnitude and direction of the effects. Slice views showing the correlations across the entire brain are provided in Supplementary Fig. 6. The graph shown in the right panel of Fig. 5 indicates the relation between AA/day and the ICA source averaged over voxels where the uncorrected p value for an effect of the ICA source was p < .05. Three of the four regions weighted most heavily in this ICA source – the thalamus/midbrain, the supero-medial cerebellum/inferior surface of the occipital lobe, and the precuneus – were regions that also showed significant fetal alcohol-related reductions in brain tissue volume in the TBM analysis (see Fig. 2). The fourth TBM region, the dorsolateral frontal lobe, was not heavily weighted in the ICA source, which instead included a frontal region extending bilaterally through the anterior cingulate gyrus and anterior portions of the corpus callosum. None of the other ICA sources showed significant associations with degree of exposure or diagnosis.
Fig. 5.
Group differences on corrected putamen (p = .005) and hippocampus (p < .001) volumes between participants with spina bifida myelomeningocele (SBM; n = 48) and typically developing controls (TD; n = 18). Values reflect averages between left and right hemispheres. Note: bars represent standard error.
3.6. TBM effects of prenatal exposure to maternal smoking
Regression analysis performed to examine the relation of number of cigarettes smoked/day by mothers during pregnancy to regional brain volumes did not survive FDR thresholding (q = .05) at any brain location in either the scaled or unscaled data.
3.7. FreeSurfer segmentation results
For comparative purposes, volumes of the discrete structures and regions that correspond most closely to the regions where we saw significant deformations in the TBM analysis were examined in relation to degree of alcohol exposure (Supplementary Table 1). Correlations of these regions with total intracranial volume ranged from r = 0.14–0.75, median = 0.55, with particularly strong correlations for the midline structures, such as the thalamus, midbrain, and precuneus. Moderate negative correlations with prenatal alcohol exposure were seen for the thalamus, midbrain, lingual gyrus, precuneus, and superior parietal lobule, many of which continued to be significant even after controlling for total intracranial volume; correlations were somewhat lower for the cerebellum, which FreeSurfer does not segment into subregions, and the frontal lobe. By contrast, prenatal alcohol exposure was not associated with volumetric increases in any of the 34 cortical and 22 subcortical regions measured by FreeSurfer.
4. Discussion
TBM analysis of regional differences in brain tissue volume in children with FASD demonstrated that (i) regional reductions in brain volume are more strongly predicted by a continuous measure of prenatal alcohol exposure obtained during pregnancy than by FASD diagnosis; (ii) normalizing for overall brain size may not be appropriate for disorders in which smaller head circumference forms part of the diagnostic criteria and may generate spurious expansions in regions that are relatively unaffected by prenatal alcohol exposure; (iii) volumetric reductions are not seen in discrete brain structures but rather in several broad regions, most of which involve medial structures; and (iv) identification of the patterns of local changes in brain tissue volume using a data driven ICA approach can identify a latent source in anatomical data that produces a spatial pattern of regional volume changes similar to that associated with prenatal alcohol exposure. The latter finding suggests that ICA may be useful in identifying children exposed to alcohol prenatally and degree of prenatal exposure if no other diagnostic information is available.
A growing number of in vivo neuroimaging studies have reported FASD-related changes in brain structure that extend beyond early reports of microencephaly, callosal agenesis, and ventriculomegaly seen in severe cases at autopsy (Roebuck et al., 1998). These reports indicate that reductions in cerebral volume in FASD are highly reproducible (Lebel et al., 2011; Norman et al., 2009) even at low- to moderate exposure levels (Eckstrand et al., 2012). Smaller than normal gray and white matter tissue compartments and cerebellar volumes are also widely reported, but are often not dissociable from overall brain volume reductions.
Reports of more focal changes in cerebral structure have been less consistent. Brain tissue reductions have been reported in almost every brain region and structure measured (Lebel et al., 2011), but findings are less regionally reproducible across groups, and paradoxical increases in cortical thickness and density have also been reported (Sowell et al., 2002; Sowell et al., 2008). Some structures have been reported to be disproportionately smaller after statistical adjustment for total brain volume; for example, the caudate nucleus (Archibald et al., 2001; Astley et al., 2009; Chen et al., 2012) and the hippocampus (Nardelli et al., 2011; Willoughby et al., 2008). However, other studies find that these same structures are not disproportionately smaller after adjusting for total brain size (for caudate nucleus—Roussotte et al., 2012; Riikonen et al., 2005; for hippocampus—Astley et al., 2009; Coles et al., 2011).
Because severity of impact depends on the degree of exposure (Astley et al., 2009; Guerri et al., 2009), exposure differences within and across study samples may contribute to inconsistencies, particularly if group comparisons are based solely on diagnosis. Additional factors that may contribute to discrepancies regarding regional susceptibility to the teratogenic effects of alcohol include developmental stage, sex, race/ethnicity, and other sample-specific characteristics. For example, in a recent longitudinal study investigating change in cortical volumes over time, Lebel et al. (2012) found different developmental trajectories in alcohol-exposed children than control subjects, particularly in posterior parietal association regions of the brain. That study also found that maturational changes in some cortical regions were related to the amount of alcohol exposure measured by trimester, indicating that patterns of regional abnormalities may be influenced by timing of exposure. Since effects of prenatal alcohol exposure continue to manifest during brain maturation, genetic factors and additional environmental risks may also act as moderating factors (Jacobson et al., 2004; Jacobson et al., 2006; Jones, 2011; May et al., 2008; Thompson et al., 2009; Warren and Li, 2005).
To date, most prior studies have focused on discrete neuroanatomical regions; few examined regional volumetric variation in a single, comprehensive analysis. Our study is among the first to use TBM to simultaneously determine global and local differences in brain tissue architecture. Further, our homogeneous sample assessed across a narrow age range ensures that observed effects are not confounded by interactions with age.
What emerged from our TBM analysis was not evidence of deformation in certain discrete brain structures but rather volumetric reductions in several broad regions, including the parietal lobe (precuneus extending into the superior parietal lobule), the supero-medial cerebellum extending to the inferior surfaces of the occipital lobe, and a large subcortical region encompassing the thalamus and midbrain, extending into the ventromedial portion of the frontal lobe. One notable feature of these deformation patterns is that most involve medial brain regions. This finding of volumetric reductions in medial brain regions is consistent with extensive animal model evidence that neural tube midline tissue is particularly vulnerable to alcohol exposure during embryogenesis (Zhou et al., 2003). Heavy alcohol exposure during gestational day 7 in mice leads to a spectrum of dysmorphology affecting the median aspects of the developing face (e.g., palpebral fissures, philtrum, and vermilion) and brain (including thalamic nuclei and corpus callosum) (Sulik et al., 1981). Even moderate levels of embryonic alcohol exposure cause subtle neural tube midline defects that can influence later brain development (Zhou et al., 2003).
Although the pattern of regional effects associated with AA/day and diagnosis was similar in the uncorrected data (color-coded in red in Figs. 2 and 4, respectively), after FDR adjustment, degree of alcohol exposure was a better predictor of regional brain structural deformations than diagnosis. The greater sensitivity of the quantitative measure of prenatal exposure in detecting regional brain contractions is impressive given that diagnosis was performed by internationally-respected dysmorphologists and that reduced head circumference is a critical element in that diagnosis. The predictive validity of our prospectively ascertained maternal self-reports of alcohol consumption during pregnancy for subtle brain deformations is particularly encouraging since self-reports can be problematic due to difficulties in recalling specific quantities of alcohol consumed and the frequency of binge episodes. By contrast to cigarette smoking, which tends to be relatively stable over time (Jacobson and Jacobson, 1992) and that was not shown to associate with regional variations in volume after FDR correction, alcohol consumption is typically episodic. That is, heavy binging may occur on some weekends, abstention on others, particularly in an economically-disadvantaged population in which funds for alcohol are not always available. A major advantage of the timeline follow-back approach used in this study is that it aids recall by encouraging the respondent to focus on what activities she engaged in on a daily basis during a recent 2-week period, where she spent each evening and weekend, and with whom. We have previously shown that maternal reports of alcohol consumption using this approach obtained contemporaneously during pregnancy are more predictive of alcohol-related infant growth and neurobehavioral deficits when compared with retrospective reports obtained at 1-year postpartum (Jacobson et al., 2002).
Studies that have examined the effects of prenatal alcohol exposure on discrete structures or regions have typically adjusted for total brain volume to determine whether a given region is smaller relative to total brain size. An important advantage of TBM is that it examines deformations simultaneously in every voxel of the brain, making clear which regions are most affected without having to control for total brain volume. The finding by Sowell et al. (2010) that FASD was associated with expansions in certain regions after adjustment for total brain volume, which we also observed in our own scaled data, suggests that removal of the variance associated with overall brain volume can cause the volume of some regions that are less severely affected by prenatal alcohol to become artificially inflated. The TBM findings in the Sowell et al. study were supported by a finding that the pattern of structural deformation seen in that study predicted group membership with 72% accuracy in follow-up jackknife analyses. However, the discriminant function analysis performed was conducted using Jacobian values only from regions already shown to differ significantly between groups, thus biasing results towards positive outcomes.
Prenatal alcohol exposure was associated with reductions in the volumes of most of the FreeSurfer regions corresponding to those identified in the unscaled TBM analysis. Conversely, prenatal alcohol was not related to volumetric increases in any of the FreeSurfer regions, providing additional support for the inference that the volumetric increases seen in the scaled data are spurious. The moderate-to-strong correlations of the FreeSurfer regional volumes with total brain volume are compatible with the suggestion that statistical adjustment for total brain volume is likely to remove much of the variance shared by AA/day and a given region, thereby potentially obscuring the associations revealed in the unscaled TBM analysis.
Notably, ICA provided independent confirmation of the regions of structural deformation linked to prenatal alcohol exposure in the TBM analysis. Among the 10 ICA generated sets of deformation patterns, one was significantly correlated with both maternal report of alcohol consumption during pregnancy and FASD diagnosis. Brain regions receiving the greatest weights in that set were strikingly similar to the brain regions found to be most strongly affected by prenatal alcohol exposure in the TBM analysis, providing independent evidence for the FASD-related deformation patterns that emerged in the TBM analysis. These findings suggest that a data-driven ICA approach to analyze MRI scans might provide an indicator of prenatal alcohol exposure in cases for which the facial features are not apparent.
5. Conclusions
This study applied sensitive TBM methods to quantitatively map relations between a continuous measure of prenatal alcohol exposure and morphometric change throughout the brain in a sample of children for whom detailed prenatal exposure information was available. This new evidence supports a dose-dependent relation between prenatal alcohol exposure and the structural organization of the brain in childhood. Our continuous measure of alcohol exposure during pregnancy was markedly more sensitive for detecting alcohol-related changes in brain tissue structure than FASD diagnosis. The data suggest that adjusting for brain size is not ideal and can hide important regional volumetric differences in disorders such as FASD, where reduced overall brain size is among the diagnostic criteria. ICA showed that the pattern of local brain tissue reductions found in this TBM analysis predicted extent of prenatal alcohol exposure as well as diagnosis, providing confirmation of the alcohol exposure-related deformation patterns reported here.
Acknowledgments
This study was supported byNIH grants R01AA016781, R21AA017410, and U01-AA014790; South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa; Medical Research Council of South Africa; Fulbright South Africa Research/Scholar Award; Joseph Young Sr., Fund, State of Michigan; and Ellison Medical Foundation. We thank Bruce Spottiswoode, PhD, the CUBIC radiographers Marie-Louise de Villiers and Nailah Maroof, and our UCT and WSU research staff Nicolette Hamman, Mariska Pienaar, Maggie September, Emma Makin, Renee Sun, and Neil Dodge. We also thank the parents and children for their long-term participation in and contribution to the study. The authors declare no competing financial interests.
Appendix A. Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2014.04.001.
Appendix A. Supplementary material
The following are the supplementary data to this article:
Supplementary material for A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure
References
- Archibald S.L., Fennema-Notestine C., Gamst A., Riley E.P., Mattson S.N., Jernigan T.L. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Developmental Medicine and Child Neurology. 2001;43:148–154. 11263683 [PubMed] [Google Scholar]
- Ashburner J., Csernansky J.G., Davatzikos C., Fox N.C., Frisoni G.B., Thompson P.M. Computer-assisted imaging to assess brain structure in healthy and diseased brains. Lancet Neurology. 2003;2:79–88. doi: 10.1016/s1474-4422(03)00304-1. 12849264 [DOI] [PubMed] [Google Scholar]
- Astley S.J., Aylward E.H., Olson H.C., Kerns K., Brooks A., Coggins T.E., Davies J., Dorn S., Gendler B., Jirikowic T., Kraegel P., Maravilla K., Richards T. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcoholism, Clinical and Experimental Research. 2009;33:1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. 19572986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Coles C.D., Lynch M.E., Hu X. Understanding specific effects of prenatal alcohol exposure on brain structure in young adults. Human Brain Mapping. 2012;33:1663–1676. doi: 10.1002/hbm.21313. 21692145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M.C., Dutton R.A., Hayashi K.M., Lopez O.L., Aizenstein H.J., Toga A.W., Becker J.T., Thompson P.M. 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. NeuroImage. 2007;34:44–60. doi: 10.1016/j.neuroimage.2006.08.030. 17035049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu A., Cui J., Dinov I.D. SOCR analyses—an instructional java web-based statistical analysis toolkit. Journal of Online Learning and Teaching / MERLOT. 2009;5:1–18. 21546994 [PMC free article] [PubMed] [Google Scholar]
- Coles C.D., Goldstein F.C., Lynch M.E., Chen X., Kable J.A., Johnson K.C., Hu X. Memory and brain volume in adults prenatally exposed to alcohol. Brain and Cognition. 2011;75:67–77. doi: 10.1016/j.bandc.2010.08.013. 21067853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinov I., Lozev K., Petrosyan P., Liu Z., Eggert P., Pierce J., Zamanyan A., Chakrapani S., Van Horn J., Parker D.S., Magsipoc R., Leung K., Gutman B., Woods R., Toga A. Neuroimaging study designs, computational analyses and data provenance using the LONI pipeline. PLOS One. 2010;5 doi: 10.1371/journal.pone.0013070. 20927408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinov I.D., Van Horn J.D., Lozev K.M., Magsipoc R., Petrosyan P., Liu Z., Mackenzie-Graham A., Eggert P., Parker D.S., Toga A.W. Efficient, distributed and interactive neuroimaging data analysis using the LONI pipeline. Frontiers in neuroinformatics. 2009;3:22. doi: 10.3389/neuro.11.022.2009. 19649168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar V., Meintjes E.M., Goradia D., Dodge N.C., Molteno C.D., Jacobson J.L., Jacobson S.W. Differences in cortico-striatal-cerebellar activations during working memory in syndromal and non-syndromal children with prenatal alcohol exposure. Human Brain Mapping. 2013;34:1931–1945. doi: 10.1002/hbm.22042. 22451272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstrand K.L., Ding Z., Dodge N.C., Cowan R.L., Jacobson J.L., Jacobson S.W., Avison M.J. Persistent dose-dependent changes in brain structure in young adults with low-to-moderate alcohol exposure in utero. Alcoholism, Clinical and Experimental Research. 2012;36:1892–1902. doi: 10.1111/j.1530-0277.2012.01819.x. 22594302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., Van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. 11832223 [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Segonne F., Salat D.H., Busa E., Seidman L.J., Goldstein J., Kennedy D., Caviness V., Makris N., Rosen B., Dale A.M. Automatically parcellating the human cerebral cortex. Cerebral Cortex (New York, N.Y.: 1991) 2004;14:11–22. doi: 10.1093/cercor/bhg087. 14654453 [DOI] [PubMed] [Google Scholar]
- Gogtay N., Lu A., Leow A.D., Klunder A.D., Lee A.D., Chavez A., Greenstein D., Giedd J.N., Toga A.W., Rapoport J.L., Thompson P.M. Three-dimensional brain growth abnormalities in childhood-onset schizophrenia visualized by using tensor-based morphometry. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15979–15984. doi: 10.1073/pnas.0806485105. 18852461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C., Bazinet A., Riley E.P. Foetal alcohol spectrum disorders and alterations in brain and behaviour. Alcohol and Alcoholism. 2009;44:108–114. doi: 10.1093/alcalc/agn105. 19147799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A.J., Raji C.A., Becker J.T., Lopez O.L., Kuller L.H., Hua X., Lee S., Hibar D., Dinov I.D., Stein J.L., ADNI Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiology of Aging. 2010;31:1326–1339. doi: 10.1016/j.neurobiolaging.2010.04.006. 20570405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme H.E., May P.A., Kalberg W.O., Kodituwakku P., Gossage J.P., Trujillo P.M., Buckley D.G., Miller J.H., Aragon A.S., Khaole N., Viljoen D.L., Jones K.L., Robinson L.K. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. 15629980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X., Gutman B., Boyle C.P., Rajagopalan P., Leow A.D., Yanovsky I., Kumar A.R., Toga A.W., Jack C.R., Jr., Schuff N., Alexander G.E., Chen K., Reiman E.M., Weiner M.W., Thompson P.M. Accurate measurement of brain changes in longitudinal MRI scans using tensor-based morphometry. Neuroimage. 2011;57:5–14. doi: 10.1016/j.neuroimage.2011.01.079. 21320612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X., Leow A.D., Lee S., Klunder A.D., Toga A.W., Lepore N., Chou Y.Y., Brun C., Chiang M.C., Barysheva M., Jack C.R., Jr., Bernstein M.A., Britson P.J., Ward C.P., Whitwell J.L., Borowski B., Fleisher A.S., Fox N.C., Boyes R.G., Barnes J., Harvey D., Kornak J., Schuff N., Boreta L., Alexander G.E., Weiner M.W., Thompson P.M., Alzheimer's Disease Neuroimaging Institute 3D characterization of brain atrophy in Alzheimer's disease and mild cognitive impairment using tensor-based morphometry. Neuroimage. 2008;41:19–34. doi: 10.1016/j.neuroimage.2008.02.010. 18378167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail S., Buckley S., Budacki R., Jabbar A., Gallicano G.I. Screening, diagnosing and prevention of fetal alcohol syndrome: is this syndrome treatable? Developmental Neuroscience. 2010;32:91–100. doi: 10.1159/000313339. 20551645 [DOI] [PubMed] [Google Scholar]
- Jacobson J.L., Jacobson S.W., Sokol R.J., Ager J.W. Relation of maternal age and pattern of pregnancy drinking to functionally significant cognitive deficit in infancy. Alcoholism, Clinical and Experimental Research. 1998;22:345–351. doi: 10.1111/j.1530-0277.1998.tb03659.x. 9581639 [DOI] [PubMed] [Google Scholar]
- Jacobson S.W., Jacobson J.L. Early exposure to PCBs and other suspected teratogens: assessment of confounding. In: Greenbaum C., Auerbach J., editors. Longitudinal Studies of Infants Born at Psychological Risk. Ablex; Norwood, NJ: 1992. pp. 135–154. [Google Scholar]
- Jacobson S.W., Chiodo L.M., Jacobson J.L., Sokol R.J. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. 11986441 [DOI] [PubMed] [Google Scholar]
- Jacobson S.W., Jacobson J.L., Sokol R.J., Chiodo L.M., Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcoholism: Clinical & Experimental Research. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. 15547461 [DOI] [PubMed] [Google Scholar]
- Jacobson S.W., Carr L.G., Croxford J., Sokol R.J., Li T.-K., Jacobson J.L. Protective effects of the alcohol dehydrogenase-ADH1B allele in children exposed to alcohol during pregnancy. Journal of Pediatrics. 2006;148:30–37. doi: 10.1016/j.jpeds.2005.08.023. 16423594 [DOI] [PubMed] [Google Scholar]
- Jacobson S.W., Stanton M.E., Molteno C.D., Burden M.J., Fuller D.S., Hoyme H.E., Robinson L.K., Khaole N., Jacobson J.L. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jacobson S.W., Jacobson J.L., Stanton M.E., Meintjes E.M., Molteno C.D. Biobehavioral markers of adverse effect in fetal alcohol spectrum disorders. Neuropsychology Review. 2011;21:148–166. doi: 10.1007/s11065-011-9169-7. 21541763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.L. The effects of alcohol on fetal development. Birth Defects Research. Part C, Embryo Today: Reviews. 2011;93:3–11. doi: 10.1002/bdrc.20200. 21425437 [DOI] [PubMed] [Google Scholar]
- Kodituwakku P.W. Neurocognitive profile in children with fetal alcohol spectrum disorders. Developmental Disabilities Research Reviews. 2009;15:218–224. doi: 10.1002/ddrr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Roussotte F., Sowell E.R. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychology Review. 2011;21:102–118. doi: 10.1007/s11065-011-9163-0. 21369875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Mattson S.N., Riley E.P., Jones K.L., Adnams C.M., May P.A., Bookheimer S.Y., O'Connor M.J., Narr K.L., Kan E., Abaryan Z., Sowell E.R. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2012;32:15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. 23115162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow A.D., Klunder A.D., Jack C.R., Jr., Toga A.W., Dale A.M., Bernstein M.A., Britson P.J., Gunter J.L., Ward C.P., Whitwell J.L., Borowski B.J., Fleisher A.S., Fox N.C., Harvey D., Kornak J., Schuff N., Studholme C., Alexander G.E., Weiner M.W., Thompson P.M. Longitudinal stability of MRI for mapping brain change using tensor-based morphometry. Neuroimage. 2006;31:627–640. doi: 10.1016/j.neuroimage.2005.12.013. 16480900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore N., Voss P., Lepore F., Chou Y.Y., Fortin M., Gougoux F., Lee A.D., Brun C., Lassonde M., Madsen S.K., Toga A.W., Thompson P.M. Brain structure changes visualized in early- and late-onset blind subjects. NeuroImage. 2010;49:134–140. doi: 10.1016/j.neuroimage.2009.07.048. 19643183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson S.N., Crocker N., Nguyen T.T. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychology Review. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. 21503685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P.A., Brooke L., Gossage J.P., Croxford J., Adnams C., Jones K.L., Robinson L., Viljoen D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. American Journal of Public Health. 2000;90:1905–1912. doi: 10.2105/ajph.90.12.1905. 11111264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P.A., Gossage J.P., Marais A.S., Adnams C.M., Hoyme H.E., Jones K.L., Robinson L.K., Khaole N.C., Snell C., Kalberg W.O., Hendricks L., Brooke L., Stellavato C., Viljoen D.L. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug and Alcohol Dependence. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. 17127017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P.A., Gossage J.P., Marais A.S., Hendricks L.S., Snell C.L., Tabachnick B.G., Stellavato C., Buckley D.G., Brooke L.E., Viljoen D.L. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcoholism, Clinical and Experimental Research. 2008;32:738–753. doi: 10.1111/j.1530-0277.2008.00634.x. 18336634 [DOI] [PubMed] [Google Scholar]
- Nardelli A., Lebel C., Rasmussen C., Andrew G., Beulieu C. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcoholism, Clinical and Experimental Research. 2011;35:1404–1417. doi: 10.1111/j.1530-0277.2011.01476.x. 21575012 [DOI] [PubMed] [Google Scholar]
- Norman A.L., Crocker N., Mattson S.N., Riley E.P. Neuroimaging and fetal alcohol spectrum disorders. Developmental Disabilities Research Reviews. 2009;15:209–217. doi: 10.1002/ddrr.72. 19731391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen R.S., Nokelainen P., Valkonen K., Kolehmainen A.I., Kumpulainen K.I., Kononen M., Vanninen R.S., Kuikka J.T. Deep serotonergic and dopaminergic structures in fetal alcohol syndrome: a study with nor-β-CIT-single-photon emission computed tomography and magnetic resonance imaging volumetry. Biological Psychiatry. 2005;57:1565–1572. doi: 10.1016/j.biopsych.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Roebuck T.M., Mattson S.N., Riley E.P. A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism, Clinical and Experimental Research. 1998;22:339–344. doi: 10.1111/j.1530-0277.1998.tb03658.x. 9581638 [DOI] [PubMed] [Google Scholar]
- Roussotte F.F., Sulik K.K., Mattson S.N., Riley E.P., Jones K.L., Adnams C.M., May P.A., O'Connor M.J., Narr K.L., Sowell E.R. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Human Brain Mapping. 2012;33:920–937. doi: 10.1002/hbm.21260. 21416562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled J.G., Pike G.B. Standing-wave and RF penetration artifacts caused by elliptic geometry: an electrodynamic analysis of MRI. IEEE Transactions on Medical Imaging. 1998;17:653–662. doi: 10.1109/42.730409. 9845320 [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Leow A.D., Bookheimer S.Y., Smith L.M., O'Connor M.J., Kan E., Rosso C., Houston S., Dinov I.D., Thompson P.M. Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2010;30:3876–3885. doi: 10.1523/JNEUROSCI.4967-09.2010. 20237258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Mattson S.N., Kan E., Thompson P.M., Riley E.P., Toga A.W. Abnormal cortical thickness and brain–behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cerebral Cortex (New York, N.Y.: 1991) 2008;18:136–144. doi: 10.1093/cercor/bhm039. 17443018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Mattson S.N., Tessner K.D., Jernigan T.L., Riley E.P., Toga A.W. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cerebral Cortex (New York, N.Y.: 1991) 2002;12:856–865. doi: 10.1093/cercor/12.8.856. 12122034 [DOI] [PubMed] [Google Scholar]
- Storey J.D. A direct approach to false discovery rates. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2002;64:479–496. [Google Scholar]
- Storey J.D., Taylor J.E., Siegmund D. Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: a unified approach. Journal of the Royal Statistical Society B. 2004;66:187–205. [Google Scholar]
- Streissguth A.P., Sampson P.D., Carmichael Olson H., Bookstein F.L., Barr H.M., Scott M., Feldman J., Mirsky A.F. Maternal drinking during pregnancy: attention and short-term memory in 14-year-old offspring—a longitudinal prospective study. Alcoholism, Clinical and Experimental Research. 1994;18:202–218. doi: 10.1111/j.1530-0277.1994.tb00904.x. 8198221 [DOI] [PubMed] [Google Scholar]
- Streissguth A.P., Barr H.M., Sampson P.D., Darby B.L., Martin D.C. IQ at age 4 in relation to maternal alcohol use and smoking during pregnancy. Developmental Psychology. 1989;25:3–11. [Google Scholar]
- Sulik K.K., Johnston M.C., Webb M.A. Fetal alcohol syndrome embryogenesis in a mouse model. Science. 1981;214:936–938. doi: 10.1126/science.6795717. 6795717 [DOI] [PubMed] [Google Scholar]
- Suttie M., Foroud T., Wetherill L., Jacobson J.L., Molteno C.D., Meintjes E.M., Hoyme H.E., Khaole N., Robinson L., Riley E., Jacobson S.W., Hammond P. Facial dysmorphism across the fetal alcohol spectrum. Pediatriia. 2013;131:e779–e788. doi: 10.1542/peds.2012-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B.L., Levitt P., Stanwood G.D. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nature Reviews Neuroscience. 2009;10:303–312. doi: 10.1038/nrn2598. 19277053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdall M.D., Hess A.T., Van der Kouwe A.J.W. MPRAGE using EPI navigators for prospective motion correction. Proceedings of the International Society for Magnetic Resonance in Medicine. 2009;17:4656. [Google Scholar]
- van der Kouwe A.J.W., Benner T., Salat D.H., Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40:559–569. doi: 10.1016/j.neuroimage.2007.12.025. 18242102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren K.R., Li T.-K. Genetic polymorphisms: impact on the risk of fetal alcohol spectrum disorders. Birth Defects Research. Part A, Clinical and Molecular Teratology. 2005;73:195–203. doi: 10.1002/bdra.20125. 15786496 [DOI] [PubMed] [Google Scholar]
- Willoughby K.A., Sheard E.D., Nash K., Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. Journal of the International Neuropsychological Society. 2008;14:1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Winer B.J. Statistical Principles in Experimental Design. second edition. McGraw-Hill; New York: 1971. [Google Scholar]
- Yanovsky I., Leow A.D., Lee S., Osher S.J., Thompson P.M. Comparing registration methods for mapping brain change using tensor-based morphometry. Medical Image Analysis. 2009;13:679–700. doi: 10.1016/j.media.2009.06.002. 19631572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F.C., Sari Y., Powrozek T., Goodlett C.R., Li T.-K. Moderate alcohol exposure compromises neural tube midline development in prenatal brain. Developmental Brain Research. 2003;144:43–55. doi: 10.1016/s0165-3806(03)00158-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for A tensor-based morphometry analysis of regional differences in brain volume in relation to prenatal alcohol exposure


