Abstract
Objective
Carotid endarterectomy (CEA) for symptomatic carotid artery stenosis and intravenous tPA (IV-tPA) for acute ischemic stroke are proven therapies; however, the safety of CEA in stroke patients who recently received IV-tPA has not been established.
Methods
We performed a retrospective review of a consecutive series of patients who underwent CEA for symptomatic carotid artery stenosis. The primary safety endpoint was post-operative symptomatic intracerebral hemorrhage (sICH). A univariate analysis of potential risk factors for sICH including IV-tPA therapy, timing of CEA, degree of arterial stenosis, and severity of presenting ischemic stroke was performed. Factors with p<0.1 on univariate analysis were tested with multivariate logistic regression.
Results
The cohort included 142 patients. Three suffered sICH following CEA – 2 of 11 patients treated with IV-tPA (18.2%) and 1 of 131 patients not treated with IV-tPA (0.8%). Both IV-tPA patients suffering sICH underwent CEA within 3 days of tPA administration. On univariate analysis, IV-tPA (p = 0.02), female gender (p=0.09), shorter time between ischemic event and CEA (p=0.06), and lower mean arterial pressure during the first 48 hours of admission (p=0.08) were identified as potential risk factors for sICH. On multivariate analysis, IV-tPA was the only significant risk factor (p=0.002 by stepwise backward elimination logistic regression; p=0.03 by nominal logistic regression).
Conclusion
Based on this case series, IV-tPA is an independent risk factor for sICH following CEA. This suggests that CEA should be pursued cautiously in patients who recently received IV-tPA. Early surgery may be associated with an increased risk for sICH.
Keywords: Carotid Endarterectomy, Thrombolysis, Tissue Plasminogen Activator, Intracerebral Hemorrhage
INTRODUCTION
Thrombolysis with intravenous recombinant tissue plasminogen activator (IV-tPA) is the only proven therapy for patients presenting with acute ischemic stroke. For a variety of reasons including a recently extended therapeutic window of opportunity for IV-tPA (up to 4.5 hours from ictus),1 the establishment and rapid growth of primary stroke centers designed to treat appropriately selected ischemic stroke patients with IV-tPA,2 and the increasing number of states that have mandated coordinated regional stroke care,3 the number of eligible ischemic stroke patients being treated with IV-tPA therapy is expected to increase in the coming years.4
Carotid endarterectomy (CEA) is an established revascularization procedure for secondary stroke prevention in patients with symptomatic extracranial carotid artery atherosclerosis.5 Current guidelines recommend that appropriately selected patients with nondisabling acute ischemic stroke or TIA should be offered CEA if the degree of carotid artery stenosis is ≥50% on catheter angiography.5 Moreover, the maximum benefit of CEA has been shown to occur in patients who undergo surgical treatment within 2 weeks of the index ischemic event, leading many to recommend early (≤2 weeks from ictus) rather than late (>2 weeks from ictus) CEA for symptomatic patients with carotid artery stenosis.5, 6
As a result of these guidelines and ongoing efforts to promote their implementation, a subset of patients has emerged who may undergo CEA soon after IV-tPA thrombolysis. However, the safety and optimal timing of CEA in this group of patients remains unclear, with only three small case series with conflicting results having been reported to date.7–9 To further examine this important issue, we retrospectively examined whether antecedent IV-tPA administration affects the risk of symptomatic intracerebral hemorrhage (sICH) in a consecutive series of patients who underwent CEA for symptomatic carotid artery stenosis.
SUBJECTS AND METHODS
A retrospective chart review of a consecutive series of patients who underwent CEA for symptomatic carotid artery stenosis at our institution between 1995 and 2007 was performed, after obtaining Institutional Review Board approval. Data collected include demographics, comorbidities (hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, chronic heart failure), history of previous stroke or transient ischemic attack (TIA), ongoing medications at admission (especially antiplatelet or anticoagulant therapy), smoking status, clinical presentation [TIA or stroke, National Institutes of Health Stroke Scale (NIHSS) at presentation), admission laboratory values (especially platelet counts, prothrombin time and activated partial thromboplastin time), brain imaging findings (CT or MRI) at admission and during hospitalization, degree of carotid artery stenosis on digital subtraction angiography, mean and maximum mean arterial pressure (MAP) at various time points during hospitalization, and medical and surgical treatment details. Self reported history was used for identification of comorbidities and medications at admission. Clinical presentation was considered to be TIA if the patient presented with an acute focal neurological deficit(s) lasting less than 24 hours, and stroke if there was acute onset focal neurological deficit(s) lasting greater than 24 hours. NIHSS score was retrospectively assessed if absent in medical records.10 North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria were used to measure and classify the degree of carotid artery stenosis.11 The prespecified primary end point was sICH, defined as CT documented evidence of hemorrhage that temporally correlated to clinical deterioration in the patient as assessed by the treating neurologist or neurosurgeon. Patients were excluded from the study if adequate clinical data were not available in the medical records.
All patients underwent CEA under general anesthesia. Intraoperative neuromonitoring with electroencephalography (EEG) was routinely employed. Carotid artery shunting was performed selectively if EEG changes suggestive of impaired cerebral perfusion were observed. In all patients, a bolus of intravenous heparin was administered before cross clamping of the internal carotid artery. All patients received preoperative antiplatelet therapy with aspirin.
Data were analyzed using JMP version 9.0 (SAS Institute, Cary, NC). Baseline variables were compared between patients segregated by IV-tPA treatment status (treated vs. not treated) using Fisher’s exact test for categorical variables and Mann-Whitney U test for continuous variables. Variables with p < 0.05 were considered statistically significant. For univariate analysis of potential risk factors for post-CEA sICH, categorical variables were compared using Fisher’s exact test and continuous variables were compared using Mann-Whitney U test. Variables with p<0.1 on univariate analyses were selected for multivariate analysis. Two separate multivariate regression models were constructed. In the first model, a stepwise backward elimination logistic regression procedure was employed. In the second model, a nominal logistic regression procedure was utilized. A p<0.05 was considered statistically significant in the multivariate analyses.
In order to assess the safety of CEA in tPA treated patients, data from our series was pooled with data from 3 published case series,7–9 and the incidence of sICH was determined. The pooled incidence of sICH and 95% confidence interval (CI) for the pooled data was calculated as described.12
RESULTS
Patient Characteristics
One hundred forty two patients (55.6% male) underwent CEA for symptomatic carotid stenosis. The median age of presentation was 66.5 years (IQR = 58 – 74 years). The majority of patients (85%) had risk factors for stroke including hypertension (69%), diabetes mellitus (27%), hyperlipidemia (44%) and cardiac disease (35%). Of the 142 patients, 11 were treated with IV-tPA and 131 were not treated with IV-tPA. Further description of the clinical characteristics of the 11 IV-tPA treated patients is provided in Table 1.
Table 1. Clinical characteristics of IV-tPA treated patients.
NIHSS - National Institutes of Health Stroke Scale, sICH - Symptomatic intracerebral hemorrhage, CEA - Carotid Endarterectomy, NASCET - North American Symptomatic Carotid Endarterectomy Trial
| Patient | Age (years) |
Gender | Admission Medications | NIHSS at admission |
NIHSS after thrombolysis* |
Degree of Carotid Stenosis (NASCET Criteria)1 |
Symptom to CEA interval (days) |
sICH | Other Complications |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Antiplatelet Therapy |
Anticoagulant Therapy |
|||||||||
| 1 | 76 | M | None | None | 0 | 0 | 50 | 2 | No | None |
| 2 | 68 | F | None | None | 7 | 4 | 80 | 10 | No | None |
| 3 | 69 | M | None | None | 0 | 0 | 60 | 1 | No | TIAs |
| 4 | 47 | F | None | None | 10 | 3 | 99 | 2 | Yes | None |
| 5 | 70 | F | Aspirin | None | 6 | 1 | 75 | 3 | Yes (Twice) |
Ventriculitis, Death |
| 6 | 53 | M | None | None | 16 | 23+ | 75 | 49 | No | None |
| 7 | 72 | F | Aspirin | None | 2 | 0 | 85 | 85 | No | None |
| 8 | 61 | M | None | None | 15 | 12 | 80 | 30 | No | None |
| 9 | 72 | M | None | None | - | - | 80 | 21 | No | None |
| 10 | 51 | M | Aspirin | None | 24 | 16 | 85 | 181 | No | None |
| 11 | 56 | M | None | None | 22 | 14 | 95 | 38 | No | None |
NIHSS was obtained 24 hours after thrombolysis.
Patient had hemorrhagic conversion of ischemic infarct after tPA administration. However, his neurological function improved significantly over the next 6 weeks and he eventually underwent CEA without complications.
Primary Outcome – Symptomatic Intracerebral Hemorrhage (sICH)
Three patients (2.1%) had post-CEA ICH. Among these 3 patients, two had received IV-tPA (2/11, 18.2%) and one had not received IV-tPA (1/131, 0.8%). On univariate analysis of potential risk factors for post-CEA sICH, there were 4 statistically significant (p<0.1) risk factors: IV-tPA administration (p=0.02), time interval between index ischemic event and CEA (p=0.06), female gender (p=0.09), and lower mean arterial pressure (MAP) during the first 48 hours of hospital admission (p=0.08). Risk factors that were not statistically significant include age at presentation, comorbidities, smoking history, presenting symptom, NIHSS score at presentation, antiplatelet or anticoagulant therapy at admission, degree of carotid stenosis, postoperative anticoagulation, mean or maximum MAP at presentation, immediately before CEA and the first 48 hours after CEA, and maximum MAP during the first 48 hours of hospital admission. The univariate analysis has been summarized in Table 2.
Table 2. Univariate analysis of potential risk factors for symptomatic ICH following CEA.
TIA - Transient Ischemic Attack, sICH - Symptomatic intracerebral hemorrhage, NIHSS - National Institutes of Health Stroke Scale, CEA - Carotid Endarterectomy, NASCET - North American Symptomatic Carotid Endarterectomy Trial, tPA - Recombinant Tissue Plasminogen Activator
| sICH (n=3) |
No sICH (n=139) |
P | |||
|---|---|---|---|---|---|
| Age (years) | 70 (47–87)* | 66 (58 – 74)* | 0.7 | ||
| Gender | Female | 3 | 60 | 0.09 | |
| Comorbidities | Hypertension | 3 | 95 | 0.6 | |
| Hyperlipidemia | 0 | 63 | 0.3 | ||
| Diabetes Mellitus | 0 | 39 | 0.6 | ||
| Cardiac Disease | 1 | 50 | 1.0 | ||
| Smoking History | 1 | 85 | 0.6 | ||
| Clinical Presentation | Stroke | 3 | 78 | 0.3 | |
| TIA | 0 | 61 | |||
| Baseline NIHSS | 6 (0–10)* | 2 (0–8)* | 0.7 | ||
| Anticoagulant Therapy | At presentation | 1 | 27 | 0.6 | |
| Before CEA | 2 | 43 | 0.6 | ||
| After CEA | 3 | 92 | 1.0 | ||
| Antiplatelet Therapy | At presentation | 3 | 110 | 0.4 | |
| tPA Administration | 2 | 9 | 0.02 | ||
| Stenosis Grade | >70% (NASCET Criteria)1 | 3 | 118 | 1.0 | |
| Symptom to CEA Interval (days) | 3 (2–10)* | 18 (6–61)* | 0.06 | ||
| Abnormal Platelet Count | 0 | 9 | 1.0 | ||
| Mean Arterial Pressure | At admission | 95 | 104 | 0.4 | |
| First 48 hours after admission | Mean Maximum |
87 108 |
97 111 |
0.08 0.8 |
|
| Immediately before CEA | 87 | 97 | 0.3 | ||
| Intraoperative | Mean Maximum |
91 118 |
97 115 |
0.3 0.8 |
|
| First 48 hours after CEA# | Mean Maximum |
87 103 |
87 105 |
0.5 0.8 |
Median (Interquartile Range)
Until onset of hemorrhage in patients who developed sICH
On backward stepwise elimination logistic regression analysis of the four risk factors that were significant on univariate analysis, IV-tPA administration emerged as the only statistically significant independent risk factor for sICH after CEA (p=0.002). The other three variables which were significant on univariate analysis- gender (p=0.051), time interval between index ischemic event to CEA (p=0.13) and mean MAP during the first 48 hours after admission (p=0.11) were not found to be statistically significant. Similarly, on nominal logistic regression analysis, IV-tPA administration was the only statistically significant independent risk factor for sICH after CEA (OR=26.8, 95% CI 1.22 – 1437.2; p=0.03). The other three variables - gender (p=0.052), time interval between index ischemic event to CEA (p=0.41) and mean MAP during the first 48 hours after admission (p=0.52) were not statistically significant.
DISCUSSION
In the present series, antecedent IV-tPA administration was identified as a strong and independent risk factor for sICH following CEA, with 18.2% of IV-tPA patients suffering a post-operative sICH (2 of 11) vs. 0.8% of non-IV-tPA treated patients (1 of 131). Both IV-tPA patients who suffered a post-operative sICH in our series underwent CEA within 3 days of thrombolytic therapy, while no IV-tPA patient who underwent CEA > 3 days after thrombolytic therapy developed a sICH. These data question the safety of CEA for patients with symptomatic carotid artery stenosis who have recently been treated with IV-tPA.
Three retrospective single institution case series have previously examined the safety of CEA following intravenous thrombolytic therapy. McPherson et al.9 reported a series of 5 ischemic stroke patients who were treated with IV-tPA either alone (2 patients) or in combination with intra-arterial tPA (3 patients) followed by CEA for residual carotid artery stenosis. All surgeries were performed within 2 days of thrombolytic therapy (median = 1 day). They reported no peri-operative complications including no sICH. The incidence of post-operative sICH in this series was therefore 0.0%. Bartoli et al.7 reported a series of 12 ischemic stroke patients who were treated with IV-tPA followed by CEA. All surgeries were performed within 16 days of thrombolytic therapy (median = 8 days). They reported one peri-operative complication – a sICH that occurred in a patient who underwent CEA 1.5 days after thrombolytic therapy. The incidence of post-operative sICH in this series was therefore 8.3%. Crozier et al.8 reported a case series of 10 ischemic stroke patients who were treated with IV-tPA followed by CEA. All surgeries were performed within 23 days of thrombolytic therapy (median = 8 days). They reported no major cerebrovascular morbidity or death. The incidence of post-operative sICH in this series was therefore 0.0%.
When pooling data from the four available case series (including ours), the incidence of sICH following CEA in patients treated with IV-tPA was 10.5% (95% CI: 0.7% – 20.3%). This is in stark contrast to the reported incidence of post-operative sICH in patients without antecedent IV-tPA therapy. In our retrospective case series of 131 non-IV-tPA treated patients, this incidence was 0.8%. In a retrospective single institution case series of 2362 non-IV-tPA treated patients, this incidence was 0.6%.13 In the prospective North American Symptomatic Carotid Endarterectomy Trial (NASCET), of 1415 non-IV-tPA treated patients, this incidence was 0.22%. Taken together, these data strongly suggest that the risk of sICH following CEA is significantly increased in ischemic stroke patients who were recently treated with IV-tPA.
Regarding the timing of CEA in patients with symptomatic carotid artery stenosis, recent guidelines advocate that surgery be performed within 2 weeks of the presenting ischemic event.5 Importantly, this recommendation is primarily based on data obtained from NASCET and the European Carotid Surgery Trial – randomized control trials that did not include patients who were treated with IV-tPA. Therefore, the applicability of this recommendation to IV-tPA patients is unclear. In particular, CEA performed very early after thrombolytic therapy appears problematic, as all 3 reported cases of post-operative sICH in IV-tPA treated patients occurred when CEA was performed within 3 days of thrombolysis.
Regarding underlying mechanism for the observed increased risk of sICH following CEA in IV-tPA treated patients, a direct anticoagulant effect of IV-tPA seems unlikely given that tPA has a very short half-life (3–5 minutes).14 A more likely explanation is that tPA activates a variety of downstream molecular cascades, one or more of which become the downstream effector(s) that cause sICH after CEA. For example, tPA is known to induce expression of the potent proteolytic enzyme matrix metalloproteinase 9 (MMP9) following ischemic stroke,15,16 and increased MMP9 levels have been linked to hemorrhagic transformation following brain ischemia.17 In the context of CEA where heparin is administered intra-operatively and cerebral perfusion is often substantially augmented following plaque removal, it is quite plausible that tPA-induced MMP9 upregulation would create a cerebral environment that is vulnerable to sICH development.
Our study has several limitations. First, it is a retrospective series and is therefore subject to limitations inherent to this study design. Second, the sample size of IV-tPA treated patients who subsequently underwent CEA was small. Third, the incidence of the primary endpoint, sICH, was rare. The latter limitations may have led the study to be underpowered to detect risk factors other than IV-tPA therapy (e.g. timing of CEA after thrombolysis, severity of stroke by NIHSS). Due to these limitations, prospective studies examining larger patient cohorts will be needed to validate our findings.
In conclusion, data from this single institution case series indicate that antecedent IV-tPA administration likely increases the risk of sICH following CEA. This finding suggests that caution should be exercised when undertaking a decision to perform CEA in ischemic stroke patients who recently received IV-tPA. One potential strategy for reducing this increased hemorrhage risk may be to delay CEA for 3 or more days following thrombolytic therapy, given that post-operative sICH has not been reported in IV-tPA treated patients when CEA was performed in delayed fashion.
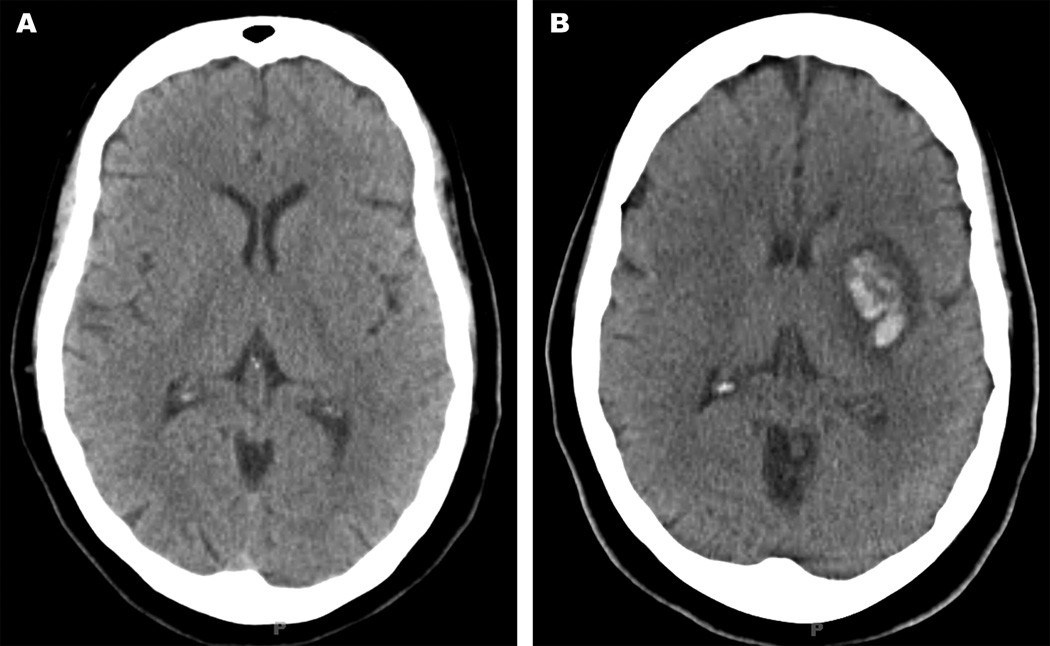
Figure 1.
A 47-year old female presented with acute ischemic stroke in the left middle cerebral artery territory and an admission NIHSS of 10. There was no evidence of ICH at presentation (Figure 1A). She was subsequently administered tPA and post-thrombolysis NIHSS was 3. Digital subtraction angiography demonstrated 99% stenosis in the proximal left internal carotid artery, and she underwent carotid endarterectomy 2 days after presentation. The patient experienced acute worsening of her pre-operative aphasia on post-operative day 2, and head CT showed an ICH (Figure 1B). The patient’s aphasia had fully recovered in late follow up.
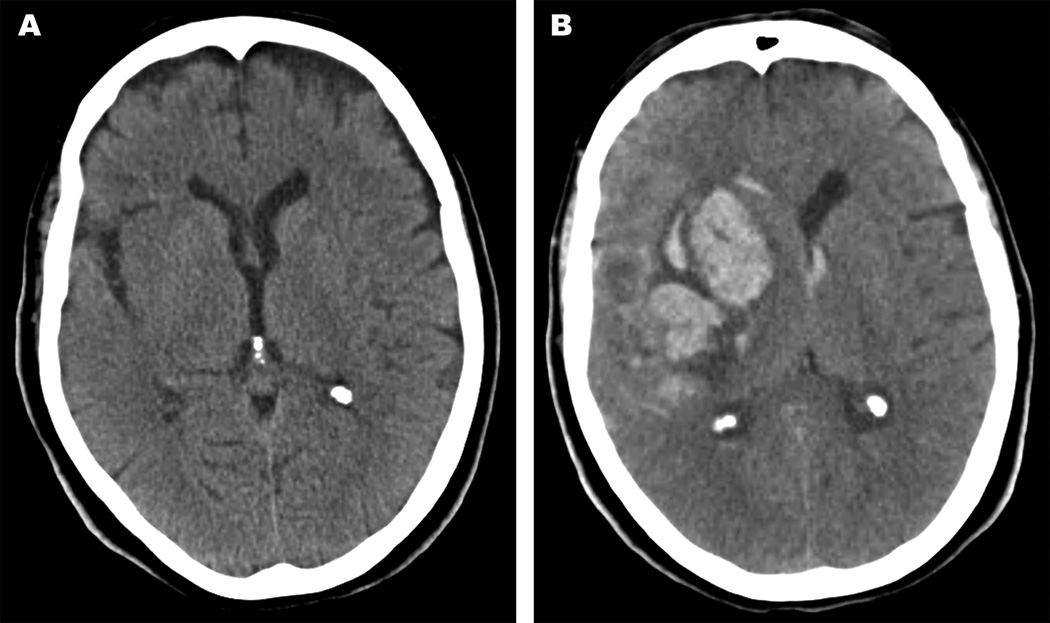
Figure 2.
A 70-year old female presented with acute ischemic stroke in the right middle cerebral artery distribution and an admission NIHSS of 6. There was no evidence of ICH at presentation (Figure 2A). She underwent thrombolysis with tPA and post-thrombolysis NIHSS was 1. Digital subtraction angiography demonstrated 75% stenosis in the proximal right internal carotid artery, and she underwent carotid endarterectomy 3 days after presentation. The patient experienced acute mental status decline and left hemiparesis on post-operative day 1, and head CT showed an ICH (Figure 2B). A craniotomy and clot evacuation was emergently performed, resulting in improved neurological status. However, the patient developed additional complications following her craniotomy including repeat sICH as well as ventriculitis resulting in further deterioration of her neurological status. Ultimately, the family withdrew care and the patient died 38 days after presentation.
Table 3. Comparison of tPA treatment groups.
tPA - Recombinant Tissue Plasminogen Activator, TIA - Transient Ischemic Attack, sICH - Symptomatic intracerebral hemorrhage, NIHSS - National Institutes of Health Stroke Scale, CEA - Carotid Endarterectomy, NASCET - North American Symptomatic Carotid Endarterectomy Trial
| Treated with tPA (n = 11) |
Not treated with tPA (n = 131) |
P | |||
|---|---|---|---|---|---|
| Age (years) | 68 (53–72) | 66 (58–75) | 0.8 | ||
| Gender | Female | 4 | 59 | 0.3 | |
| Comorbidities | Hypertension | 9 | 89 | 0.5 | |
| Hyperlipidemia | 6 | 57 | 0.5 | ||
| Diabetes Mellitus | 3 | 36 | 1.0 | ||
| Cardiac Disease | 2 | 49 | 0.3 | ||
| Smoking History | 8 | 78 | 0.3 | ||
| Clinical Presentation | Stroke TIA |
11 0 |
70 61 |
0.002 | |
| Baseline NIHSS+ | 9 (2–17)* | 4 (1–9)* | 0.2 | ||
| Anticoagulant Therapy | At presentation | 3 | 25 | 0.7 | |
| Before CEA | 7 | 38 | 0.2 | ||
| After CEA | 9 | 86 | 0.5 | ||
| Antiplatelet Therapy | At presentation | 10 | 90 | 1.0 | |
| sICH after CEA | 2 | 1 | 0.02 | ||
| Stenosis Grade (%) | >70% | 9 | 112 | 0.6 | |
| Symptom to CEA Interval (days) | 22 (2–49)* | 14 (6–61)* | 0.46 | ||
| Abnormal Platelet Count | 1 | 8 | 0.5 | ||
| Mean Arterial Pressure | At admission | 101 | 104 | 0.5 | |
| First 48 hours after admission |
Mean Maximum |
93 114 |
97 110 |
0.3 0.3 |
|
| Immediately before CEA | 95 | 97 | 0.7 | ||
| Intraoperative | Mean Maximum |
93 116 |
97 115 |
0.2 0.8 |
|
| First 48 hours after CEA# | Mean Maximum |
88 110 |
87 105 |
0.7 0.06 |
Median (Interquartile Range)
Patients with ischemic stroke
Until onset of hemorrhage in patients who developed sICH
ACKNOWLEDGMENTS
Source of Funding
This study was supported by NIH grants - NINDS P50 NS055977 (C.P.D., G.J.Z.), NINDS U01 NS58728 (C.P.D.), and NINDS R01 NS051631 (C.P.D).
Footnotes
Presentation Information: Portions of this work were presented in abstract form at the International Stroke Conference 2011, Los Angeles, USA, February 9, 2011.
DISCLOSURE
None
REFERENCES
- 1.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: A science advisory from the american heart association/american stroke association. Stroke. 2009;40:2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Govan L, Weir CJ, Langhorne P. Organized inpatient (stroke unit) care for stroke. Stroke. 2008 doi: 10.1161/STROKEAHA.106.478842. [DOI] [PubMed] [Google Scholar]
- 3.Derdeyn CP, Panagos PD. Stroke center certification: Where are we in 2010? Journal of NeuroInterventional Surgery. 2010;2:41–43. doi: 10.1136/jnis.2009.001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dirks M, Niessen LW, van Wijngaarden JD, Koudstaal PJ, Franke CL, van Oostenbrugge RJ, Huijsman R, Lingsma HF, Minkman MM, Dippel DW. Promoting thrombolysis in acute ischemic stroke. Stroke. 2011;42:1325–1330. doi: 10.1161/STROKEAHA.110.596940. [DOI] [PubMed] [Google Scholar]
- 5.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, Hertzberg VS, McIff EB, Moore WS, Panagos PD, Riles TS, Rosenwasser RH, Taylor AJ. 2011 asa/accf/aha/aann/aans/acr/asnr/cns/saip/scai/sir/snis/svm/svs guideline on the management of patients with extracranial carotid and vertebral artery disease: A report of the american college of cardiology foundation/american heart association task force on practice guidelines, and the american stroke association, american association of neuroscience nurses, american association of neurological surgeons, american college of radiology, american society of neuroradiology, congress of neurological surgeons, society of atherosclerosis imaging and prevention, society for cardiovascular angiography and interventions, society of interventional radiology, society of neurointerventional surgery, society for vascular medicine, and society for vascular surgery. Stroke. 2011 doi: 10.1016/j.jacc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–924. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 7.Bartoli MA, Squarcioni C, Nicoli F, Magnan PE, Malikov S, Berger L, Lerussi GB, Branchereau A. Early carotid endarterectomy after intravenous thrombolysis for acute ischaemic stroke. Eur J Vasc Endovasc Surg. 2009;37:512–518. doi: 10.1016/j.ejvs.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Crozier JE, Reid J, Welch GH, Muir KW, Stuart WP. Early carotid endarterectomy following thrombolysis in the hyperacute treatment of stroke. Br J Surg. 2011;98:235–238. doi: 10.1002/bjs.7306. [DOI] [PubMed] [Google Scholar]
- 9.McPherson CM, Woo D, Cohen PL, Pancioli AM, Kissela BM, Carrozzella JA, Tomsick TA, Zuccarello M. Early carotid endarterectomy for critical carotid artery stenosis after thrombolysis therapy in acute ischemic stroke in the middle cerebral artery. Stroke. 2001;32:2075–2080. doi: 10.1161/hs0901.095679. [DOI] [PubMed] [Google Scholar]
- 10.Kasner SE, Chalela JA, Luciano JM, Cucchiara BL, Raps EC, McGarvey ML, Conroy MB, Localio AR. Reliability and validity of estimating the nih stroke scale score from medical records. Stroke. 1999;30:1534–1537. doi: 10.1161/01.str.30.8.1534. [DOI] [PubMed] [Google Scholar]
- 11.North american symptomatic carotid endarterectomy trial. Methods, patient characteristics, and progress. Stroke. 1991;22:711–720. doi: 10.1161/01.str.22.6.711. [DOI] [PubMed] [Google Scholar]
- 12.Young-Xu Y, Chan KA. Pooling overdispersed binomial data to estimate event rate. BMC Med Res Methodol. 2008;8:58. doi: 10.1186/1471-2288-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piepgras DG, Morgan MK, Sundt TM, Jr, Yanagihara T, Mussman LM. Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg. 1988;68:532–536. doi: 10.3171/jns.1988.68.4.0532. [DOI] [PubMed] [Google Scholar]
- 14.Chandler WL, Alessi MC, Aillaud MF, Henderson P, Vague P, Juhan-Vague I. Clearance of tissue plasminogen activator (tpa) and tpa/plasminogen activator inhibitor type 1 (pai-1) complex: Relationship to elevated tpa antigen in patients with high pai-1 activity levels. Circulation. 1997;96:761–768. doi: 10.1161/01.cir.96.3.761. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, Huang PL, Wang X, Montaner J, Lo EH. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36:1954–1959. doi: 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- 16.Ning M, Furie KL, Koroshetz WJ, Lee H, Barron M, Lederer M, Wang X, Zhu M, Sorensen AG, Lo EH, Kelly PJ. Association between tpa therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006;66:1550–1555. doi: 10.1212/01.wnl.0000216133.98416.b4. [DOI] [PubMed] [Google Scholar]
- 17.Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, Quintana M, Alvarez-Sabin J. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]