Abstract
Vertebrate limbs first emerge as small buds at specific locations along the trunk. Although a fair amount is known about the molecular regulation of limb initiation and outgrowth, the cellular events underlying these processes have remained less clear. We show that the mesenchymal limb progenitors arise through localized Epithelial-to-Mesenchymal Transition (EMT) of the coelomic epithelium specifically within the presumptive limb fields. This EMT is regulated at least in part by Tbx5 and Fgf10, two genes known to control limb initiation. This work shows that limb buds initiate earlier than previously thought, as a result of localized EMT rather than differential proliferation rates.
In 1971, Searls and Janners found that, at early limb stages (Hamburger-Hamilton Stage 17–18 in the chick) there is a substantial decrease in proliferation of the flank mesoderm whereas higher rates are maintained within the emerging vertebrate limb buds. Accordingly, they proposed that localized regulation of proliferation at specific levels along the body axis is responsible for limb initiation (1). However, the cellular properties of the somatopleural lateral plate cells that give rise to the limb bud have not been identified.
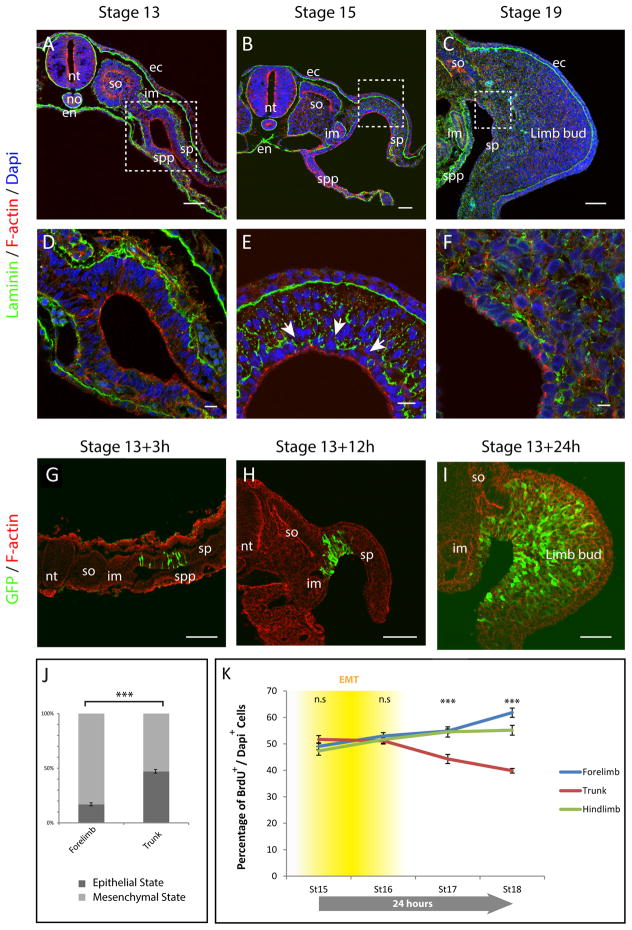
During gastrulation, the mesodermal germ layer is formed through the generation of mesenchymal cells from the epithelial epiblast. However, shortly after gastrulation a re-epithelization occurs such that essentially the entire embryo is epithelial, as defined by apical (F-actin) and basal (laminin) epithelial markers: not only are the ectoderm, neural tube and endoderm epithelial, but also the notochord, the somites, the intermediate mesoderm and the lateral plate mesoderm (i.e. splanchnopleural and somatopleural mesoderm, Fig. 1, A, D). At stage 13 in the chick, before any signs of limb bud formation, the somatopleure displays epithelial rather than mesenchymal characteristics. Molecular characterization revealed that, at this stage, F-actin, N-cadherin as well as β-catenin and aPkc localize at the apical end of somatopleure cells (Fig. 1, A, D and Fig. S1 A, D). On the other hand, Vimentin is localized at the basal end of somatopleural cells and laminin is deposited only on the basal side (Fig. 1, A, D and Fig. S1, A); demonstrating that at early stages the somatopleure is a single cell layer highly polarized pseudo-stratified columnar epithelium. These observations differ from the previous assumption that limbs originate from a preexisting mesenchymal population. Forelimb bud mesenchyme first becomes apparent at stage 14–15 whereas the more posterior hindlimb mesenchyme can be first observed only at stage 15–16 as revealed by enrichment of Vimentin expression and a concomitant a loss polarized localization of N-cadherin, β-catenin, F-actin and aPKC within somatopleural cells and basement membrane of laminin break-down(Fig. 1, B, E, and Fig. S1, B, C, E, F). Importantly, mesenchyme in the trunk region is only seen at stage 17 long after forelimb and hindlimb mesenchymes have emerged (Fig. 1, C, F and Fig. S2), thus out of order relative to the general rostral-caudal wave of development along the body axis, indicating specific regulation is involved in this timing. Similar results were found for the mouse (Fig. S3).
Fig. 1. Limb progenitors arise from EMT of the epithelial somatopleure.
(A to C) Transverse sections of stage 13 (A), 15 (B) and 19 (C) chick embryos, at the forelimb level. Sections were stained with Dapi (blue), F-actin (red) and with anti-laminin antibody (green). (D to F) are higher magnifications (A–C), white arrows point at laminin basement membrane breakdown.
(G to I) Transverse sections of chick embryos electroporated at stage 13 and harvested after 3h, 12h and 24h. Sections were stained with Phalloidin (red) and with anti GFP antibody (Green). (J) Proportion of cells in epithelial or mesenchymal state at the forelimb and trunk level. (n=3723 cells, 5 embryos; Mann-Whitney U test ***p-value=2.4×10−9. (K) Percentage of proliferating cells (nBrdU+/nDapi+) in the somatopleure of chick embryos at stage 15, 16, 17 and 18 at the level of the forelimb (blue), Trunk (red) and hindlimb (green); (n=5 embryos for each stage, over 250,000 cells were counted in total; Mann-Whitney U test, n.s: non-significant p-value>0.05; ***p-value <10−7. The timing of EMT in relation to proliferation is represented in yellow. Errors bars indicate the SEM. Scale bars represent 50μm in (A–C, G–I,) and 10μm in (D–F). nt: neural tube; no: notochord; en: endoderm; so: somite; im: intermediate mesoderm; ec: ectoderm; spp: splanchnopleure; sp: somatopleure.
Since the somatopleural lateral plate mesoderm of the limb field starts out as an epithelium and ultimately generates limb bud mesenchyme, one would expect that this occurs through an epithelial-mesenchymal transition (EMT) process. To directly demonstrate this, we performed lineage analysis with an electroporated GFP reporter in chick embryos. We found that three hours after electroporation each GFP electroporated cell exhibited a bottle-like epithelial shape (Fig. 1, G and S4, A), and localized expression of apical Ncadherin, β-catenin, aPKC, and basal Vimentin (Fig. S4, D, E, H, I, L, M, P,Q). After 12 and 24 hours, although some GFP labeled cells could still be seen in an epithelial state, the vast majority of GFP labeled cells had left the epithelium and displayed mesenchymal characteristics (Fig. 1, H, I and Fig. S4, B, C, F, G, J, K, N, O, R, S), demonstrating that virtually all mesenchymal cells of the limb bud originate from the epithelial somatopleure (Fig. 1, I).
Although EMT is delayed in the trunk relative to the limb forming regions, ultimately it is necessary to generate the ventral dermis of the body wall. We, therefore, quantified the extent of EMT process occurring in each of these regions. To this end, we electroporated the somatopleure of both the forelimb and trunk level of stage 13 chick embryos and quantified the number of GFP positive cells in the epithelial and mesenchymal state. We found that the somatopleure at the forelimb level generates 5.5 times more mesenchymal than epithelial cells; whereas at the trunk level, the number of mesenchymal cells that emerge from the somatopleure is nearly equal to the number of epithelial cells (Fig. 1J). These quantifications show that a precocious and sustained EMT process generates the limb primordium as opposed to the trunk region in which a delayed and less efficient EMT generates only the minimal amount of mesenchymal cells that will eventually contribute to the dermis of the trunk (2).
Limb bud initiation has previously been described to be the consequence of differential proliferation between limb and trunk regions starting at stage 17 (1). However, our data indicated that EMT, resulting in localized production of limb mesenchyme, begins before proliferation starts. We, therefore, decided to revisit the timing of proliferative changes in the lateral plate mesoderm using more modern approaches--automated cell counting of proliferative cells labeled using BrdU as opposed to manual counting of tritiated thymidine labeled cells in Searls and Janners study. Our study confirmed that proliferation starts to decrease at about stage 17–18 in the trunk; however, it remains sustained at a high level in the limb region (Fig. 1K). However, at stage 15–16, as mesenchyme is being generated, proliferation is uniform throughout the lateral plate mesoderm including both the trunk and limb-forming fields (Fig. 1K). Together with the lineage analysis, these observations strongly suggest that the limb bud initiates earlier than stage 17–18, through EMT and independent of proliferation rate changes. It is worth noting that when the limb bud mesenchyme is generated, it induces a source of Fgf activity in the overlying ectoderm, the AER, which serves to maintain the limb’s high level of proliferation(3). This does not occur in the trunk region, which we hypothesize is the reason for its relative decrease in mitotic activity as mesenchyme is generated. We suggest that the difference in proliferation of trunk versus limb bud mesenchyme is a result of limb bud initiation, as opposed to being a cause of it.
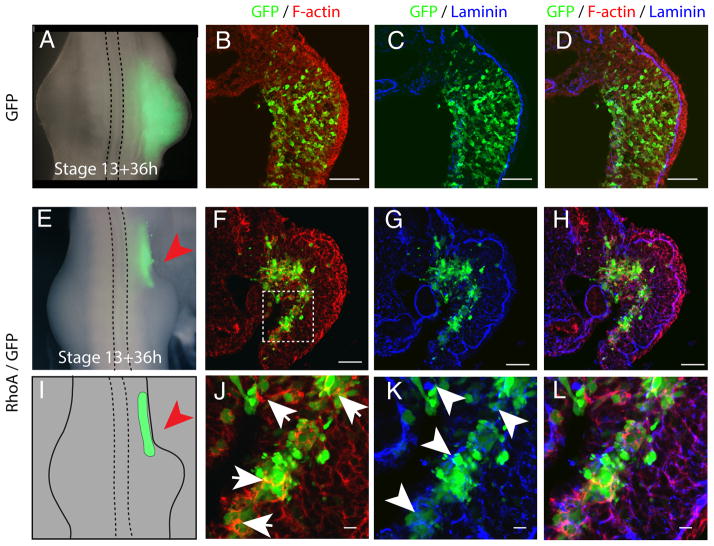
To verify that EMT of the somatopleure represents a necessary step of limb initiation, we blocked EMT in the presumptive limb region by RhoA overexpression, which abrogates EMT by inducing strong interaction of cells with extracellular matrix components (ECM) (4). We electroporated RhoA with GFP within the epithelial somatopleure. In control embryos electroporated with GFP alone, limb buds form normally (Fig. 2, A), the basement membrane of laminin breaks down and cells undergo EMT (Fig. 2, B–D). In contrast, co-electroporation of RhoA with GFP completely abrogated limb formation (Fig. 2, E, I). Sections revealed that RhoA electroporated cells were stuck in the epithelial somatopleure and failed to undergo proper EMT (Fig. 2, F–H). These cells were attached to aggregates of laminin (Fig. 2, K, L) and did not exhibit enrichment in Vimentin staining (Fig. S5, G–L). We found that RhoA overexpressing cells overexpressed F-actin, maintained adherens junction as revealed by Ncadherin and β-catenin stainings (Fig. S5, A–L), and also failed to relocalize aPKC from their apical cortex to the cytoplasm (Fig. S5, A–F) as observed in control GFP electroporated cells (Fig. S4, N, O, R, S). RhoA overexpression did not lead to dramatic reduction of proliferation (Fig. S6 A–C) or increase in apoptosis in the electroporated cells (Fig. S6 D–G), confirming that RhoA acts to prevent epithelial to mesenchymal cell state change. Thus as a consequence of this failure to undergo EMT, the electroporated cells did not participate in the initiation and formation of the limb primordium.
Fig. 2. Epithelial-to-Mesenchymal Transition of the somatopleure is a necessary step in limb bud initiation.
(A, E) Dorsal view of stage 21 chick embryos 36 h after electroporation of GFP or GFP/RhoA. (B to D and F to H) Transverse section of chick embryo electroporated with GFP or GFP/RhoA at the forelimb level and stained with Phalloidin (red), GFP (green) and Laminin antibody (blue). (J to L) higher magnification of (F to L); Note the over-stabilization of F-actin (arrows) and the presence of large aggregates of laminin (arrowheads). (I) Cartoon representing the extent of RhoA/GFP electroporation as shown in (E) and highlighting the absence of limb formation in the RhoA electroporated region, red arrow.
Scale bars represent 50μm in (B to D, and F to H) and 10μm in (J to L)
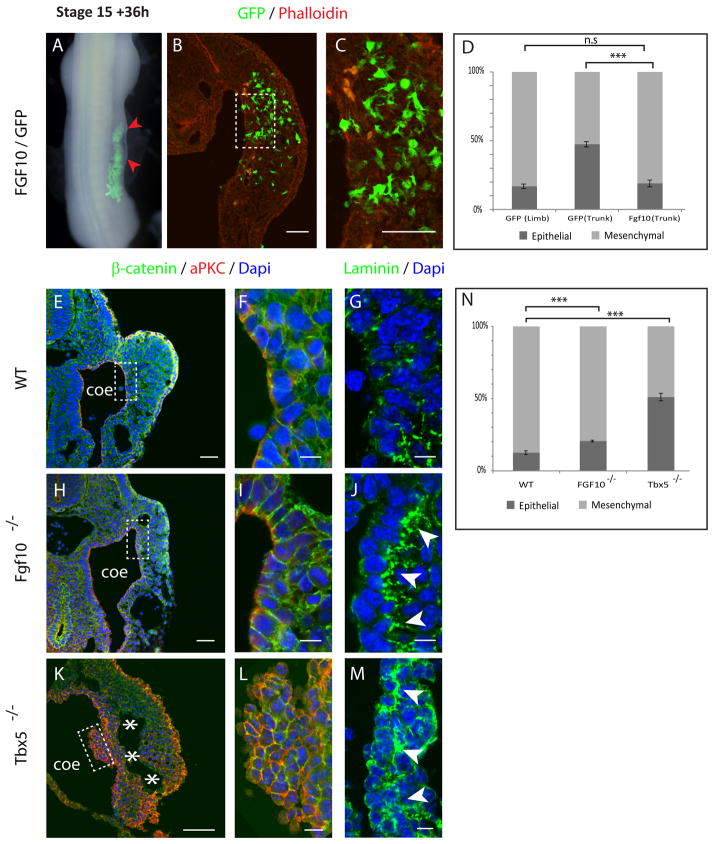
To understand how EMT is regulated in this context we explored the possibility that Snail1, a transcription factor upstream of EMT in other contexts (5), plays a similar role here, but (perhaps due to redundancy) we failed to find such a connection. We therefore turned to factors known to be involved in establishing the formation of a limb bud. Ectopic Fgf protein, applied up to stage 17, is sufficient to induce the formation of an entire additional limb from trunk tissue(6). To test whether Fgf10 promotes limb formation by inducing EMT, Fgf10 and GFP were co-electroporated into the trunk somatopleure of stage 13–14 chick embryos. As expected, 36 h after electroporation Fgf10 induced swelling of the trunk, indicating ectopic limb initiation (Fig. 3, A, red arrowheads). Sectioning through the electroporated region showed that most of the GFP positive cells had left the epithelium and had acquired a mesenchymal phenotype (81%, Fig. 3, B–D), showing that indeed EMT had taken place. The trunk is only competent to form an ectopic limb up to stage 16–17 (7, 8). This was previously interpreted as the time at which the trunk mesenchyme becomes determined and is no longer capable of being redirected to a limb fate. However, our data show that this is precisely the time at which the trunk mesenchyme is first generated. Thus we would reinterpret those results as indicating that ectopic Fgf activity can induce limb bud formation from epithelial trunk somatopleure cells, but not from mesenchymal cells of the same rostro-caudal level.
Fig. 3. Tbx5/FGF10 participates in the regulation of the EMT of the somatopleure.
(A) FGF10/GFP electroporated chick embryos showing ectopic swellings (red arrowheads). (B, C) transverse section of FGF10/GFP electroporated embryo, (C) is a higher magnification of (B). (D) Proportion of cells in mesenchymal or epithelial state (n= 1912 (FGF10/GFP), 3 embryos; Mann-Whitney U test ***p-value <0.0001; n.s: non-significant, p-value=0.38). (E, H, K) Transverse sections of mouse embryos at the forelimb level stained with Dapi (Blue), β–catenin (Green) and aPkc (red) antibody. (F, I, L) are Higher magnification of (E, H, K). (G, J, M) Transverse sections stained with Dapi (blue) and Laminin antibody (green) focusing on the somatopleure epithelium. (N) Proportion of cells in mesenchymal or epithelial state (n= 2962 (WT), 10894 (Tbx5−/−) and 3115 (FGF10−/−) cells, 3 embryos for each genotype; Mann-Whitney U test ***p-value <0.0001). Scale bars represent 50 μm in (B, C, E, H, K) and 10 μm in (F, I, L, G, J, M). Coe: coelomic cavity; asterisks: separation between epithelium and mesenchyme.
Targeted mutation of Fgf10 and Tbx5 in mice have demonstrated that these genes are necessary to initiate limb bud formation(9–12). Transverse sections of E9.5 Fgf10 −/− and Tbx5 −/− embryos confirmed that neither Tbx5 nor Fgf10 mutant embryos exhibit swellings characteristic of limb bud initiation (Fig. 3, E, H and K). Both FGF10 and Tbx5 mutant embryos showed the presence of mesenchyme in the forelimb region, however, the proportion of mesenchymal cells compared to the proportion of epithelial cells was significantly lower than that of a wild type sibling embryo (Fig. 3, N) with a stronger phenotype observed in Tbx5 −/− embryos (only 12% of cells were epithelial in wild type embryos, whereas 21% and 51% were epithelial in Fgf10 and Tbx5 deficient embryos, respectively). In Tbx5 mutant embryos, the epithelium appeared separated from the mesenchyme (Fig. 3, K, asterisks). aPkc and β–catenin staining revealed a clear hyperplasia of the somatopleure epithelium, in strong support of failure of these cells undergo EMT (Fig. 3, K, L). Finally, in Fgf10 −/− as well as in Tbx5 −/− embryos, the basement membrane of laminin does not properly break down and appears over-stabilized as opposed to WT embryos (Fig. 3, F, G, I, J, L, M). Taken together, these data show that Tbx5/Fgf10 acts on the somatopleure epithelium to regulate, at least partially, the early induction of EMT in the limb fields, the process that is at the heart of limb bud initiation.
Supplementary Material
Acknowledgments
We want to thank F. Constantini for help re-deriving the Fgf10 mutant mouse line; B. Bruneau for kindly providing Tbx5 mutant embryos; J. deMelo and P. Tschopp for help with mouse work. J.G. was a fellow of the Human Frontier Science Program. This work was supported by NIH grant R01-HD045499 to C.J.T.
References and notes
- 1.Searls RL, Janners MY. The initiation of limb bud outgrowth in the embryonic chick. Dev Biol. 1971;24:198–213. doi: 10.1016/0012-1606(71)90095-9. [DOI] [PubMed] [Google Scholar]
- 2.Mauger A. The role of somitic mesoderm in the development of dorsal plumage in chick embryos. I. Origin, regulative capacity and determination of the plumage-forming mesoderm. J Embryol Exp Morphol. 1972;28:313–341. [PubMed] [Google Scholar]
- 3.Ohuchi H, et al. The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development. 1997;124:2235–2244. doi: 10.1242/dev.124.11.2235. [DOI] [PubMed] [Google Scholar]
- 4.Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol. 2008;10:765–775. doi: 10.1038/ncb1739. [DOI] [PubMed] [Google Scholar]
- 5.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Cohn MJ, Izpisúa-Belmonte JC, Abud H, Heath JK, Tickle C. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell. 1995;80:739–746. doi: 10.1016/0092-8674(95)90352-6. [DOI] [PubMed] [Google Scholar]
- 7.Ohuchi H, et al. An Additional Limb Can Be Induced from the Flank of the Chick Embryo by FGF4. Biochemical and Biophysical Research Communications. 1995;209:809–816. doi: 10.1006/bbrc.1995.1572. [DOI] [PubMed] [Google Scholar]
- 8.Vogel A, Rodriguez C, Izpisua-Belmonte JC. Involvement of FGF-8 in Initiation, Outgrowth and Patterning of the Vertebrate Limb. Development. 1996;122:1737–1750. doi: 10.1242/dev.122.6.1737. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal P. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. doi: 10.1242/dev.00191. [DOI] [PubMed] [Google Scholar]
- 10.Rallis C, et al. Tbx5 is required for forelimb bud formation and continued outgrowth. Development. 2003;130:2741–2751. doi: 10.1242/dev.00473. [DOI] [PubMed] [Google Scholar]
- 11.Min H, et al. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekine K, et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.