Abstract
Aim
To assess medical and neurodevelopmental effects of Newborn Individualized Developmental Care and Assessment Program (NIDCAP) for a large sample of very early-born infants.
Methods
One hundred and seven singleton inborn preterm infants, <29 weeks gestational age (GA), <1250 g birth weight, enrolled in three consecutive phases, were randomized within phase to NIDCAP (treatment, E) or standard care (C). Treatment extended from admission to the Newborn Intensive Care Unit to 2 weeks corrected age (wCA). Outcome included medical, neurobehavioural and neurophysiological status at 2 wCA, and growth and neurobehavioural status at 9 months (m) CA.
Results
The C- and E-group within each of the three consecutive phases and across the three phases were comparable in terms of all background measures; they therefore were treated as one sample. The results indicated for the E-group significant reduction in major medical morbidities of prematurity as well as significantly improved neurodevelopmental (behaviour and electrophysiology) functioning at 2 wCA; significantly better neurobehavioural functioning was also found at 9 mCA.
Conclusion
The NIDCAP is an effective treatment for very early-born infants. It reduces health morbidities and enhances neurodevelopment, functional competence and life quality for preterm infants at 2 w and 9 mCA.
Keywords: Behaviour, EEG spectral coherence, Neurodevelopment, NIDCAP, Prematurity
INTRODUCTION
Preterm birth is a global obstetric challenge. About 13 million preterm deliveries occur per year world-wide, an overall incidence of about 9% (1). Medical advances have led to dramatic survival increases of very low birth weight infants (<1250 g), yet they continue to experience a range of health and neurodevelopmental challenges that extend into adolescence and young adulthood (2,3). The costs associated with prematurity, including those of a Newborn Intensive Care Unit (NICU), and the medical, educational and life-long social resources required, are considerable (2). The Newborn Individualized Developmental Care and Assessment Program (NIDCAP) (4) builds a system of care and environmental structure in the NICU specifically supportive of early brain development. Randomized controlled NIDCAP trials (RCTs) have shown reduction in medical morbidities including, reduction in length of time to independent feeding, better weight gain, shorter stays in intensive care, shorter overall hospitalization, reduction in hospital charges (5–8) and improvement in neurobehavioural and electrophysiological functioning of such infants, with significant results at 2 weeks corrected age (wCA) and 9 months corrected age (mCA). A meta-analyses acknowledged NIDCAP-based improvements in chronic lung disease, necrotizing enterocolitis, improved family outcome and behavioural advantages up to 5 years (y) CA (9). Only two studies reported no NIDCAP impact on growth or development at 2 yCA (10,11). Methodological difficulties prompt well-designed RCTs with large homogeneous samples.
DESIGN, SUBJECTS AND METHODS
Study design
A two-group [control (C) and experimental (E)] RCT design was employed. Blocking by gender (male/female) and ethnicity (Caucasian/other) was imposed prior to recruitment. Following parental consent subjects were randomly assigned to the C- or E-group. Purposefully, blinded assessors evaluated outcome at 2 wCA and 9 mCA. The study protocol was approved by the institutional review boards for human research at the birth and the outcome assessment sites.
Subjects
One hundred and fifty-four infants were eligible; 107 (51C, 56E) agreed to participate. All were delivered at a large urban regional high-risk perinatal tertiary care centre and admitted to that hospital’s NICU, a 48-bed, level III nursery.
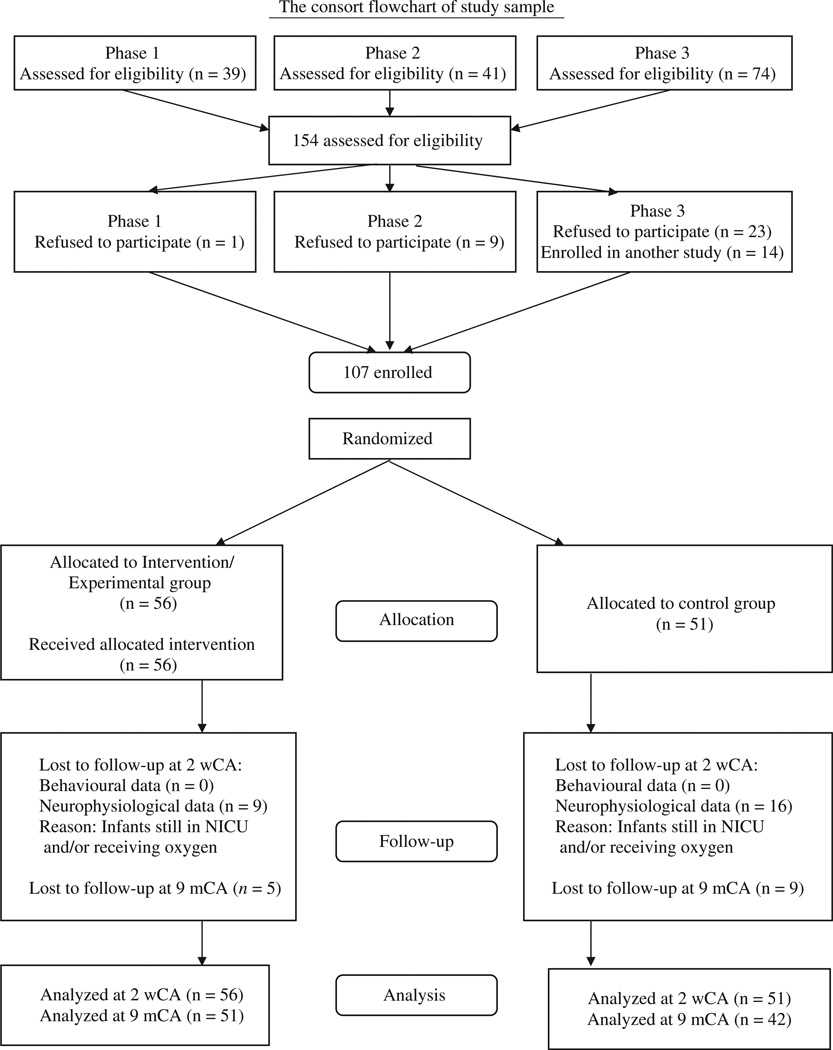
Recruitment extended across 8 years in three distinct funding phases: phase 1: October 1984–June 1986, phase 2: December 1986–March 1988, phase 3: April 1990–May 1992. Eligibility criteria included: gestational age (GA) at birth <29 weeks (12), birth weight <1250 g, conceived spontaneously, singleton, mechanical ventilation within the first 3 h and for >24 h in the first 48 h, alive at 48 h, deemed viable by the attending neonatologist, absence of chromosomal or major genetic anomalies and congenital infections, family with some knowledge of English language, living ≤25 miles of hospital. Recruitment was completed within ≤3 days after delivery. Partial results have been reported elsewhere (5,8). For subject distribution by phase, see Fig. 1. There was no difference in background criteria between refusing and participating families, and the study cohort is representative of the eligible subjects.
Figure 1.
Consort Flowchart of study sample.
Analysis of variance (ANOVA) and chi-squared test statistics showed that randomized C- and E-group infants and families were comparable on all demographical and medical background variables within and across phases. The three samples thus were combined into one. Significant societal phase effects identified included increased maternal age, decreased family size, increased number of first-born study infants and more use of breast milk over time. Significant medical care changes over time identified that more mothers received Terbutaline and prenatal steroids, infant GA at birth was lower, more infants had respiratory distress syndrome, received surfactant replacement therapy, had lower levels of inspired oxygen in the first 48 h, were diagnosed with patent ductus arteriosus, received more medications and were discharged at younger ages. Nevertheless, there were no significant group background differences per phase or for the combined phases.
Control and experimental group treatments
Control-group infants received NICU care as standard at the time of study without attempts to influence staffing or prevent spillover from E-group to C-group care. Any significant experimental effects identified are conservative, as they must exceed all contamination effects. Experimental treatment effects across phases did not decrease from phases 1 to 2, yet decreased from phases 1 and 2 to phase 3 as evaluated by neurobehaviour at 2 wCA and 9 mCA. By phase 3, the differences between the C- and E-group were the smallest. Incorporation of NIDCAP concepts into daily NICU care may be assumed to have generalized to some extent by phase 3. Nonetheless, the contamination did not eliminate significant treatment effects for all three phases.
The experimental treatment was NIDCAP (4). Two certified NIDCAP Professionals, a NICU nurse and a psychologist, worked with the E-group infants’ care teams and families to jointly plan and implement care and to structure individualized environments supportive of each infant. Weekly neurobehavioural observations were performed through hospital stay to 2 wCA. The NIDCAP Professionals provided daily support and guidance and weekly reports to the families and medical staff in adjusting care to the understanding the E-group infants’ stress and comfort signals, as well as in conceptualizing the infants as active participants in the care delivered. Formal explanation of the NIDCAP approach may be found elsewhere (4,13).
Medical and demographical background and outcome assessment
Medical information was obtained from NICU and outlying hospital medical records, abstracted double-blind by the study’s research nurse, supervised by the study neonatologists. Demographical and parent/infant medical history information additionally was obtained from parent interview by the study’s senior psychologist, blinded to infant group membership. Medical outcome was assessed from birth to 2 wCA in terms of the major medical morbidities associated with prematurity, and at 9 mCA for the interval from 2 wCA to 9 mCA. At 9 mCA, 93 infants (42C, 51E) were studied. They were comparable in demographical and medical background to the 14 infants not available for study. All infants had complete medical outcome data sets.
Neurobehavioural and neurophysiological outcome assessment
At 2 wCA, all 107 infants (51C, 56E) had complete neurobehavioural data, and 82 (35C, 47E) had complete neurophysiological data. More C-infants (16) than E-infants (9) were still hospitalized and deemed too fragile to undergo electroencephalography (EEG) study. At 9 mCA, 93 infants (42C, 51E) had growth and neurobehavioural measurements.
Assessments were performed by two independent examiners, purposefully blinded to infants’ background and group status, and videotaped for reliability checks. Parent(s) were present throughout all examinations.
Neurobehavioural outcome measures at 2 wCA
The Assessment of Preterm Infants’ Behaviour (APIB) (14,15), and the Prechtl Neurological Examination of the Full-Term Newborn Infant (Prechtl) were employed at 2 wCA (16). Six APIB system scores (autonomic, motor, state, attention, self-regulation systems and examiner facilitation), which range from 1 (well-organized behavioural regulation) to 9 (poor behavioural organization) were used for analysis. The Prechtl was reduced to 12 summary variables (17), which assess syndromes of reactivity and thresholds of functioning.
Neurophysiological outcome measures at 2 wCA
All infants were assessed neuro-EEG following the neurobehavioural assessment. Data were obtained from 20 scalp electrodes with linked ear reference. Following amplification (Neuroscan Synamps), data were digitized at 250 Hz and band pass filtered from 1 to 100 Hz with 60 Hz mains rejection filter. Subsequent analyses were limited to artifact free segments of quiet sleep, identified by the senior electro-encephalographer, blinded to subject identity.
Electro-encephalographic spectral coherence data from quiet sleep were evaluated for all subjects. Coherence between two EEG electrodes using the Laplacian reference technique is a measure of cortical coupling between the brain areas underlying the electrodes (18). The EEG spectral coherence data were represented by coherence factors, derived from an independent normative sample (N = 312) also studied at 2 wCA. Principal components analysis with Varimax rotation using a specially constructed algorithm based on singular value decomposition (18) generated 40 factors, which accounted for 65% of the variance. These factors predict GA at birth, degree of medical compromise and newborn behavioural competence (19). EEG coherence factors were generated for all subjects of this study and used in subsequent analyses.
Neurobehavioural outcome measures at 9 mCA
At 9 mCA, the infants were assessed in terms of medical diagnoses, their Pediatric Complication Scale scores (20) and their growth (weight, height, head circumference). Development was assessed with the Bayley Scales of Infant Development (Bayley) (21), which yields a Mental (MDI) and a Psychomotor Developmental Index (PDI), both with a mean (X̅) of 100 and a standard deviation (SD) of 16 and the Bayley Infant Behaviour Record (IBR), which includes eleven 9-point and three 5-point rating scales.
Data analysis
Statistical analyses used BMDP2007™ software (22). Variables were submitted to multivariate analysis of variance (MANOVA) by domain, with subsequent exploratory univariate ANOVA. In cases of unequal variances, the Browne-Forsythe test of variance (F*) was used. Categorical variables were submitted to Pearson’s chi-squared test (22,23). An a priori two-tailed probability level of p < 0.05 was used as criterion for statistical significance. Sample sizes of 51 C-group and 56 E-group infants provide 89% power for detecting mean differences of 10 points in the Bayley MDI and PDI between the groups assuming a pooled SD of 16 points (moderate effect size of 0.625) using a two-sample Student’s t-test. Based on 42 C and 51 Einfants power was 84% for detecting the 10 point mean difference (nQuery Advisor, version 7; Statistical Solutions, Saugus, MA, USA) (24). Stepwise discriminate analysis (DSC) was employed for the behavioural and electrophysiological domains at 2 wCA followed by Wilks’ lambda and jackknifed classification (25) to ascertain two-group classification success per domain. Canonical correlation analysis was employed to explore the relationships among domains.
RESULTS
Ages were comparable for the C- and E-group at 2 wCA [C: X̅ = 18.14 days (SD = 6.58); E: X̅ = 18.11 (SD = 7.16); p = 0.98] as well as at 9 mCA [C: X̅ = 9.35 m (SD = 0.48); E: X̅ = 9.40 m (SD = 0.61); p = 0.63].
Medical and demographical background
Table S1 describes the sample in terms of medical and demographical background data. There were no differences between groups except for a slight trend for the C-group to have somewhat better Obstetric Complication Scale Scores (20).
Medical outcome at 2 wCA
Group effects at 2 wCA indicated significantly improved medical status for E-group compared to C-group infants (p = 0.05), as shown in Table S2. Post hoc analysis of individual variables showed significant differences in terms of reduction in days of ventilator support and of supplemental oxygen, lower incidence of pneumothorax and reduced severity of bronchopulmonary dysplasia (BPD) (doubleblind, independent lung X-ray review). Mean postmenstrual age (PMA) at discontinuation of supplemental oxygen significantly favoured the E-group [C: X̅ = 41 weeks PMA (SD = 19 weeks); E: X̅ = 35 weeks PMA (SD = 7 weeks); p = 0.03], concordant with the lung X-ray staging results. Additional significant group effects included lower incidence and severity of intraventricular haemorrhage (IVH), lower degree of ventricular dilation, fewer days of hyperalimentation and of gavage feeding, shorter stays in the hospital and lower PMA at discharge. In addition, the E-group demonstrated significantly higher mean daily weight gains to 2 wCA and higher body weights at 2 wCA.
As Table S3 shows there was a trend for the E-group infants to show somewhat better growth than the C-group infants at 2 wCA (p < 0.097). In terms of growth percentiles for PMA, this was not the case (p = 0.21).
Neurobehavioural outcome at 2 wCA
Neurobehavioural outcome at 2 wCA showed highly significant group effects in favour of the E-group on the APIB (p = 0.0003) and a trend towards better performance on the Prechtl (p = 0.07). On the APIB (14,26), as Table S4 shows post hoc analysis showed highly improved autonomic regulation, motor system organization, state organization and self-regulation, as well as significantly reduced the need for examiner facilitation for the E-group, again many more differences than would be expected by chance.
The Prechtl (16) post hoc analysis, as Table S5 shows, demonstrated greatly improved trunk and limb posture, improved motility, higher threshold of response, more well-modulated Moro response, more stable state maintenance, a more modulated and robust cry, a lower degree of hemi-syndromes and less hyper-reactivity and/or hyporeactivity. The Total Prechtl Score also reflected significantly better performance for the E-group than C-group.
Neurophysiological outcome at 2 wCA
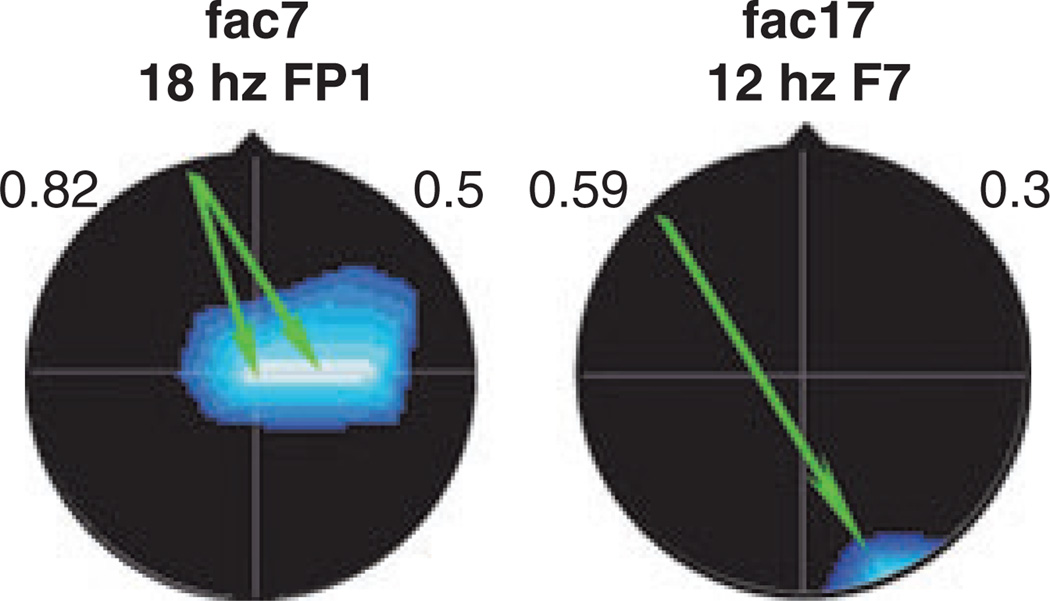
The EEG coherence results at 2 wCA showed significant group differences (p < 0.02) in favour of the E-group on Coherence Factors 7 and 17 (19), as depicted in Fig. 2.
Figure 2.
EEG spectral coherence factors at 2 wCA [C-group (n = 35), E-group (n = 47)]. Images show significant group differences: Factor 7 – connectivity between mid-central and frontal brain regions in the beta frequency band; Factor 17 – connectivity between right occipital and left anterior temporal regions in the alpha frequency band. Coherences are higher for the E-group. Head shown in vertex view, nose above, left ear to left. EEG frequency and index electrode shown above head. Background colour indicates loading on original principal components analysis (blue = negative). Arrow colour illustrates E-group coherence; green = decreased. Decreased coherence on background of negative loading indicates higher connectivity.
Factor 7 involves significantly higher E-group connectivity, i.e. greater neural pathway engagement, between midcentral to frontal brain regions in the beta frequency band, at later ages important for attention allocation, verbal memory, temporal thought and action organization, emotional states activation, social executive control, decision making and judgement (27,28). Factor 17 involved higher E-group connectivity between right occipital and left anterior temporal regions in the alpha frequency band. At later ages, this involves storage and processing of semantic information and general verbal memory functions (29).
Group classification and relationships among medical, neurobehavioural and neurophysiological domains at 2 wCA
Discriminate analysis with jackknifed classification for the medical outcome variables resulted in a 65% classification success rate. This indicates that medical outcome variables predicted C- and E-group membership at just beyond the chance level. However, DSC performed on the neurobehavioural (APIB/Prechtl) and spectral coherence outcome variables combined (Wilks’ lambda = 0.413; F = 5.97; df = 15, 63), yielded an 82.3% jackknifed classification success, and five APIB/Prechtl and 10 coherence variables formed the discriminant. Thus, brain-based behavioural and neurophysiological assessments were considerably more successful than medical outcome variables in predicting NIDCAP intervention success and later functioning than medical information.
Canonical correlation was employed to explore the relationship of the APIB/Prechtl and the EEG spectral coherence data. Bartlett’s test (χ2 = 688.44; df = 320; p < 0.0001) identified a significantly strong relationship between the behavioural and neurophysiological measures during the newborn period. DSC results indicate that the two sets nevertheless were not redundant as each variable set made a significant contribution to the successful discriminant.
Medical and behavioural outcome at 9 mCA
For the 93 infants (42C/51E; 13% attrition rate) studied at 9 mCA, medical outcome data indicated (Table S6) that E-group infants were significantly healthier than the C-group infants (p = 0.02). Post hoc exploration showed that the E-group infants had fewer medical diagnoses at 9 mCA and better scores on the Pediatric Complication Scale (20).
At 9 mCA, the E-group when compared with the C-group again showed a trend towards better absolute growth (F = 2.57; df = 3,88; p = 0.06). Post hoc exploration revealed higher E-group body weights in kilograms [C: 7.59(1.21); E: 8.12(1.14); p = 0.03] as well as higher weight percentiles for CA [C: 16.83(20.85); E: 26.42(25.51); p = 0.05]. These results indicate significantly better health and a trend toward better body weights of the E-group.
The Bayley (21) showed significantly better MDI and PDI scores for the E-group over the C-group (p = 0.0005) (Table S7). Moreover, 52% (22/42) of C-group infants scored below the mean (X̅ = 100, SD = 16) on the MDI, compared with only 18% (nine of 51) of E-group infants. On the PDI, 79% (33/42) of C-group infants scored below the mean compared with only 58% (29/51) of E-group infants. Analysis of the IBR scores (Table S8) shows significantly better scores for the E-group (p = 0.05). Four of the eleven IBR measures, namely goal directedness, attention span, as well as gross and fine muscle coordination, favour the E-group infants. This speaks strongly for the continued effectiveness of NIDCAP to 9 mCA.
DISCUSSION
Results consistently support the primary hypothesis that the NIDCAP intervention in the NICU significantly enhances medical outcome, behavioural and electrophysiological function at 2 wCA and medical and behavioural function at 9 mCA.
The E-group’s early medical improvement included a number of clinically meaningful findings, which have significant implications for the infants’ and families’ future, such as reduced incidence in IVH and decreased severity of BPD. While the same number of children in both groups still required supplemental oxygen at 36 weeks, PMA at discontinuation of supplemental oxygen was significantly higher for the C-group infants. Thus, it indicates that the experimental treatment may decrease the degree of severity of lung disease rather than prevent the occurrence of lung disease in this high-risk population.
At 2 wCA, the E-group infants showed better behavioural functioning, in autonomic, motor system, state organization and self-regulation competence. They also showed significantly less hyper-reactivity, better posture, greater reflex intactness and better overall motor regulation. EEG coherence results showed E-group infants’ enhanced cortical function in areas related to later attention, verbal memory, semantic processing, executive control and organization of thought and action. Results identified are internally consistent and provide compelling evidence for the effectiveness of NIDCAP in its continuous support to the individual infant’s behavioural integration, along with improved medical outcomes.
At 9 mCA, E-group infants continued to show better health with fewer medical problems, better weight gain and superior development in behaviour, cognitive and motor skills. These improvements predict better academic achievement and developmental progress at school age (30).
The study results validate that the foetal brain is differentially vulnerable to the repeated stressful events experienced in the traditional NICU. While the specific pathways are not fully understood, changes in white matter architecture, programmed cell death, altered inter-cortical connectivity and myelination are all implicated in compromised resultant neurodevelopment (31,32). Prevention of repeated stress is the focus of NIDCAP. NIDCAP allows for a calmer and better regulated infant, and this in turn appears to facilitate brain development, evidenced in better electrophysiological and psychological development.
This large study validates the results of previous smaller studies regarding the benefits of NIDCAP and it is in contrast with the lack of effects reported recently elsewhere (10,11). This study broadens the generalizability of the effectiveness of NIDCAP to very early-born, high-risk infants. Furthermore, the EEG coherence findings for this very high-risk, very early-born preterm population corroborate the findings from two low-risk preterm infant studies that showed NIDCAP effectiveness in differential protection of frontal lobe functions (17,33).
While medical care changed over the 8-year interval, the study involved time-matched controls, which assured that the changes did not alter the impact of the intervention. Treatment contamination of standard care by NIDCAP appeared to become evident only by the third phase of the study. However, despite contamination, the overall impact of the intervention was still highly significant.
While the results of this study are encouraging, their interpretation nevertheless requires caution. Further substantiation by a more current large sample is necessary and with follow-up to older ages. Along with the medical advances over the last years, even younger and more vulnerable infants survive. Care practice changes such as enforcement of ‘back to sleep’ recommendations to reduce Sudden Infant Death Syndrome, and the mounting life stressors for young families, warrant future investigation and follow-up.
A further question arises regarding the optimal starting point of NIDCAP. In this study, once the criteria were met and consent obtained, treatment ensued. Forty-eight hours after birth was the formal earliest starting point of treatment. It is possible that this is the most vulnerable period for a high-risk preterm infant, when blood flow velocity changes likely are at their most extreme and intubation takes place. Further research is needed of the enhanced effectiveness of NIDCAP in the critical early stabilization period immediately during, at and from birth onward.
While the exact processes underlying the effectiveness of NIDCAP remain to be determined, the study’s results demonstrate that NIDCAP improves health, brain development, functional competence and life quality in very early-born preterm infants. NIDCAP is compelling, humane and ethical, and promises to become the standard for all NICU care.
ACKNOWLEDGEMENTS
This study was supported by U.S. Department of Education grants HO24S90003, HO23C970032, HI33G20203 and R305T990294, National Institutes of Health grant RO1HD3826, and I. B. Harris Foundation grant to H. ALS, as well as National Institute of Health grant P30HD18655 to J.J. Volpe and M. Greenberg. The authors foremost thank Ms Sandra Kosta, BA for her expert support throughout all aspects of the study including design, data acquisition, analysis and manuscript preparation; Mr John Connolly, REEGT for expert EEG data acquisition; NIDCAP Professionals gretchen Lawhon, RN PhD and Deborah Buehler, PhD for their valuable contributions to the study and Johann G. Blickman, MD PhD for independent expert cranial ultrasound and lung radiograph review. The authors furthermore thank all the nurses and neonatologists for their support in the NICU, and foremost the families and infants for their participation in and commitment to the study.
Abbreviations
- ANOVA
analysis of variance
- APIB
Assessment of Preterm Infants’ Behaviour
- BPD
bronchopulmonary dysplasia
- CA
corrected age
- DSC
discriminate analysis
- EEG
electroencephalography
- GA
gestational age
- IBR
Infant Behaviour Record
- IVH
intraventricular haemorrhage
- MANOVA
multivariate analysis of variance (MANOVA)
- MDI
Mental Developmental Index
- NICU
Newborn Intensive Care Unit
- NIDCAP
Newborn Individualized Developmental Care and Assessment Program
- PDI
Psychomotor Developmental Index
- PMA
postmenstrual age
- RCT
randomized controlled trial
- SVD
singular value decomposition
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Table S1 Medical and demographical background variables.
Table S2 Medical outcome variables at 2 wCA.
Table S3 Growth measures at 2 wCA.
Table S4 Assessment of Preterm Infants Behavior (APIB) System Scores and Prechtl Neurological Examination Scores at 2 wCA.
Table S5 Prechtl Neurological Examination of the Full-Term Infant, Scores at 2 wCA.
Table S6 Medical outcome variables at 9 mCA.
Table S7 Bayley Scales of Infant Development, MDI and PDI at 9 mCA.
Table S8 Bayley Scales of Infant Development, Infant Behavior Record Scores at 9 mCA.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Villar J, Merialdi M, Gulmezoglu AM, Abalos E, Carroli G, Kulier R, et al. Characteristics of randomized controlled trials included in systematic reviews of nutritional interventions reporting maternal morbidity, mortality, preterm delivery, intrauterine growth restriction and small for gestational age and birth weight outcomes. J Nutr. 2003;133:1632–1639. doi: 10.1093/jn/133.5.1632S. [DOI] [PubMed] [Google Scholar]
- 2.Behrman RE, Stith Butler A. Preterm birth: causes, consequences, and prevention. Washington, DC: The National Academies Press; 2007. [PubMed] [Google Scholar]
- 3.Saigal S, Stoskopf B, Streiner D, Boyle M, Pinelli J, Paneth N, et al. Transition of extremely low-birth-weight infants from adolescence to young adulthood: comparison with normal birth-weight controls. J Am Med Assoc. 2006;295:667–675. doi: 10.1001/jama.295.6.667. [DOI] [PubMed] [Google Scholar]
- 4.Als H. 1986 rev 2008, ©. MA: NIDCAP Federation International Boston; Program Guide – Newborn Individualized Developmental Care and Assessment Program (NIDCAP): an Education and Training Program for Health Care Professionals. Available from http://nidcap.org. [Google Scholar]
- 5.Als H, Lawhon g, Duffy FH, McAnulty GB, Gibes-Grossman R, Blickman JG. Individualized developmental care for the very low birthweight preterm infant: medical and neurofunctional effects. JAMA. 1994;272:853–858. [PubMed] [Google Scholar]
- 6.Fleisher BF, VandenBerg KA, Constantinou J, Heller C, Benitz WE, Johnson A, et al. Individualized developmental care for very-low-birth-weight premature infants. Clin Pediatr. 1995;34:523–529. doi: 10.1177/000992289503401003. [DOI] [PubMed] [Google Scholar]
- 7.Westrup B, Kleberg A, von Eichwald K, Stjernqvist K, Lagercrantz H. A randomized controlled trial to evaluate the effects of the Newborn Individualized Developmental Care and Assessment Program in a Swedish setting. Pediatrics. 2000;105:66–72. doi: 10.1542/peds.105.1.66. [DOI] [PubMed] [Google Scholar]
- 8.Als H, Gilkerson L, Duffy FH, McAnulty GB, Buehler DM, VandenBerg KA, et al. A three-center randomized controlled trial of individualized developmental care for very low birth weight preterm infants: medical, neurodevelopmental, parenting and caregiving effects. J Dev Behav Pediatrics. 2003;24:399–408. doi: 10.1097/00004703-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochr Database Syst Rev. 2006;(Issue 2) doi: 10.1002/14651858.CD001814.pub2. Art. No: CD001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maguire CM, Veen S, Sprij AJ, Le Cessie S, Wit JM, Walther FJ, et al. Follow-up outcomes at 1 and 2 years of infants born less than 32 weeks after Newborn Individualized Developmental Care and Assessment Program. Pediatrics. 2009;123:1081–1087. doi: 10.1542/peds.2008-1950. [DOI] [PubMed] [Google Scholar]
- 11.Wielenga JM, Smit BJ, Merkus MP, Wolf MJ, van Sonderen L, Kok JH. Development and growth in very preterm infants in relation to NIDCAP in Dutch NICU: two years of follow-up. Acta Paediatr. 2009;98:291–297. doi: 10.1111/j.1651-2227.2008.01038.x. [DOI] [PubMed] [Google Scholar]
- 12.Ballard JL. A simplified assessment of gestational age. Pediatr Res. 1977;11:374. [Google Scholar]
- 13.Als H, Butler S. Newborn Individualized Developmental Care and Assessment Program (NIDCAP): changing the future for infants and families in intensive and special care nurseries. Early Child Serv. 2008;2:1–19. [Google Scholar]
- 14.Als H, Lester BM, Tronick EZ, Brazelton TB. Manual for the assessment of preterm infants’ behavior (APIB) In: Fitzgerald HE, Lester BM, Yogman MW, editors. Theory and research in behavioral pediatrics. New York: Plenum Press; 1982. pp. 65–132. [Google Scholar]
- 15.Als H. Toward a synactive theory of development: promise for the assessment of infant individuality. Inf Mental Health J. 1982;3:229–243. [Google Scholar]
- 16.Prechtl HFR. The neurological examination of the full-term infant: a manual for clinical use. 2nd ed. Philadelphia, PA: Lippincott; 1977. p. 68. Clinics in Developmental Medicine, No. 63. [Google Scholar]
- 17.Buehler DM, Als H, Duffy FH, McAnulty GB, Liederman J. Effectiveness of individualized developmental care for low-risk preterm infants: behavioral and electrophysiological evidence. Pediatrics. 1995;96:923–932. [PubMed] [Google Scholar]
- 18.Duffy FH, Jones KH, McAnulty GB, Albert MS. Spectral coherence in normal adults: unrestricted principal components analysis – relation of factors to age, gender, and neuropsychologic data. Clin Electroencephalogr. 1995;26:30–46. doi: 10.1177/155005949502600106. [DOI] [PubMed] [Google Scholar]
- 19.Duffy FH, Als H, McAnulty GB. Infant EEG spectral coherence data during quiet sleep: unrestricted principal components analysis – relation of factors to gestational age, medical risk, and neurobehavioral status. Clin Electroencephalogr. 2003;34:54–69. doi: 10.1177/155005940303400204. [DOI] [PubMed] [Google Scholar]
- 20.Littman B, Parmelee AH. Manual for pediatric complications. Los Angeles, CA: Infant Studies Project, Department of Pediatrics, School of Medicine, University of California; 1974. [Google Scholar]
- 21.Bayley N. Bayley scales of infant development. New York: The Psychological Corporation; 1969. [Google Scholar]
- 22.Dixon WJ. BMDP statistical software manual. Berkeley, CA: University of California Press; 1988. [Google Scholar]
- 23.Yates F. The analysis of multiple classifications with unequal numbers in the different classes. J Am Stat. 1934;29:51–66. [Google Scholar]
- 24.Cohen J. Statistical power for analysis for the behavioral sciences. New York: Academic Press; 1969. [Google Scholar]
- 25.Lachenbruch PA. Discriminant analysis. New York: Hafner Press; 1975. [Google Scholar]
- 26.Als H, Lester BM, Tronick EZ, Brazelton TB. Towards a research instrument for the assessment of preterm infants’ behavior. In: Fitzgerald HE, Lester BM, Yogman MW, editors. Theory and research in behavioral pediatrics. New York: Plenum Press; 1982. pp. 35–63. [Google Scholar]
- 27.D’Esposito M, Postle BR. The organization of working memory function in lateral prefrontal cortex: evidence from event-related functional MRI. In: Donald ST, Robert KT, editors. Principles of frontal lobe function. London: Oxford University Press; 2002. pp. 168–187. [Google Scholar]
- 28.Gevins AS, Gevins AS, Bressler SL, Cutillo BA, Illes J, Miller JC, et al. Effects of prolonged mental work on functional brain topography. Electroencephalogr Clin Neurophysiol. 1990;76:339–350. doi: 10.1016/0013-4694(90)90035-i. [DOI] [PubMed] [Google Scholar]
- 29.Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- 30.Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Wilson-Costello D, et al. Poor predictive validity of the Bayley scales of infant development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116:333–341. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 31.Anand KJS, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonat. 2000;77:69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- 32.Limperopoulos C, Gauvreau K, O’Leary H, Moore M, Bassan H, Eichenwald E, et al. Cerebral hemodynamic changes during intensive care of preterm infants. Pediatrics. 2008;122:e1006–e1013. doi: 10.1542/peds.2008-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Als H, Duffy F, McAnulty GB, Rivkin M, Vajapeyam S, Mulkern RV, et al. Early experience alters brain function and structure. Pediatrics. 2004;113:846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]