Summary
Background
Cell migration, angiogenesis, inflammation, and extracellular matrix remodeling are key events in wound healing. Natural products, including fatty acids (FAs), can accelerate wound healing by modulating the aforementioned events.
Aims
This study aims to evaluate the effect of lucuma (Pouteria lucuma O Kezte) nut oil (LNO) on fibroblasts migration, angiogenesis, inflammation, bacterial and fungal growth, and wound healing.
Methods
GC–MS analysis of FAs methyl esters (FAMES) was used for chemical characterization of LNO. In vitro studies were carried out with LNO investigating the induction of cell migration, cytoskeleton remodeling of human fibroblasts, inhibition of LPS-induced nitric oxide production in macrophages, and antibacterial and antifungal effects. Two in vivo studies were carried out to study LNO’s effect on angiogenesis and wound healing: (i) tail fin regeneration in transgenic zebrafish larvae expressing enhanced green fluorescent protein (EGFP) in vascular endothelial cells was used to study vessel sprouting and wound healing and (ii) the closure of wounds was evaluated in CD-1 mice after topical applications of LNO-containing formulations.
Results
Lucuma nut oil is a mixture of FAs, 99.7% of which were characterized. Major components of LNO (w/w) are linoleic acid (38.9%), oleic acid (27.9%), palmitic acid (18.6%), stearic acid (8.9%), and γ linolenic acid (2.9%). In vitro studies showed that LNO significantly promoted migration and vinculin expression in human fibroblasts. LNO decreased LPS-induced nitric oxide production and did not display significant antibacterial or antifungal effects. LNO induced tail fin regeneration in transgenic zebrafish larvae 48 h after tail fin amputation and significantly accelerated cutaneous wound closure in CD-1 mice.
Conclusions
Natural FAs from P. lucuma nut promote skin regeneration and, thus, may have applications in medicine and skin care.
Keywords: fatty acids, lucuma, Pouteria lucuma, transgenic zebrafish, wound healing
Introduction
Fatty acids (FAs) from natural sources play important roles in human health and have diverse acute or chronic anti-inflammatory effects.1–4 Linoleic acid (LA) and oleic acid (OA) are frequently found in natural oils. Experimental5,6 and clinical studies2,7 have reported LA and OA as potential modulators of skin inflammation and regeneration but with low clinical efficacy.5,6,8 Another anti-inflammatory natural fatty acid is γ-linolenic acid, reported to have potential therapeutic use in treating psoriasis and inflammatory skin disorders including itching, eczema, and dryness.2,3,5,9 These experimental and clinical findings have opened interesting avenues of research for synergistic combinations of natural FAs in skin inflammation and regeneration. The mechanisms of pharmacologic action of FAs in skin physiology are not yet well understood.10 Several reports suggest that FAs can be metabolized by cyclooxygenases and lipoxygenases to produce anti-inflammatory eicosanoids.3,4 It is also known that free and conjugated FAs contribute to the cell membrane architecture of fibroblasts, keratinocytes, leukocytes, and other relevant cells of skin layers.6,7,11 Besides their structural function in biologic membranes, FAs are important modulators of the cell–cell interactions and intracellular signaling pathways leading to cell migration.12 Thus, the alteration of the composition of FAs and phospholipids in the cell membrane can modify its fluidity and change the binding of cytokines to their receptors affecting inflammatory responses to injuries and the wound-healing process.1 To date, the role of FAs in inflammation and tissue responses to injuries is not completely elucidated, but it seems likely that the pharmacologic effects of FAs involve changes in the expression of genes implicated in cell migration, inflammatory, and immune response to pathogenic bacteria and fungus.3,9 FAs from natural sources, such as soybean, corn, and canola oils, are among the most consumed worldwide for their positive effect on human health.4 However, natural oils rich in bioactive FAs, with unique biochemical characteristics and pharmacologic effects, can be found in many lesser known crops, making them valuable products for pharmaceutical and cosmeceutical applications.4,8,11
The edible fruit of Pouteria lucuma O. Ktze, commonly named “lucuma” in Chile and Peru, is widely consumed in these countries.13 However, there is virtually no information on the effects of lucuma fruit on human health. Only one report suggests in vitro effects of aqueous extracts of lucuma fruit on models of diabetes and hypertension.14 Lucuma fruit consists of the edible fleshy outer part and a hard inner seed (nut) that is inedible and is usually discarded when the fruit is consumed or processed. However, lucuma nut is an important source of natural FAs. In this work, we report for the first time the biochemical composition, and anti-inflammatory and wound-healing effect of FAs from lucuma nut oil (LNO). In addition, we described the effects of LNO on inflammation, cell migration, angiogenesis, bacterial and fungal growth, and wound healing using in vivo and in vitro models.
Materials and methods
General
Murine RAW264.7 macrophages were obtained from the American Type Culture Collection (ATCC). Human neonatal fibroblasts were obtained from Cascade Biologics Catalog Number: C-004-5C. LNO was obtained by extracting 150 g of ground lucuma nut with 2.25 L of heptane for 12 h. The extraction was done under gentle agitation, protected from light and at room temperature. After extraction, heptane was removed from the extract under vacuum, creating yellow oil that was stored at −20 °C protected from light.
Chemical characterization of LNO
A 100 µL aliquot of LNO was added to 1 mL methanolic HCl and heated for 1 h at 100 °C, gently shaken every 15 min for 1 min. Vials were cooled in an ice bath and 2 mL of hexane was added. Samples were gently stirred in a tabletop incubator for 5 min at 120 rpm and then centrifuged for 3 min at 2100 g. The organic phase was transferred to a new vial, dried under a stream of nitrogen and reconstituted in 100 µL of methylene chloride. This extract was diluted 1:10 twice for further analysis. A 1 µL aliquot of the diluted sample was injected to a heated (250 °C) split (50:1) injector and separated on an SGE BPX70 (highly polar cyanopropyl polysilphenylsiloxane) capillary column (60 m × 0.25 mm ID × 0.25 µm film). Oven conditions were 100 °C hold 2 min, ramp 5 °C/min to 150 °C hold 10 min; ramp 2 °C/min to 200 °C hold 8 min, ramp 5 °C/min to 240 hold 1 min. The carrier gas was helium at 1 mL/min. Mass spectra from 40 to 550 m/z were acquired using a dual-stage quadrupole with source at 250 °C and +EI ionization of 70 eV. A 37-component food industry fatty acid mixture at 100 µg /mL each was injected for verification.
In vitro studies
Determination of cell migration
Human neonatal fibroblasts were maintained in Dulbecco’s modified Eagle medium (DMEM) + 10% fetal bovine serum (FBS) and were kept in a humidified 37 °C incubator with 5% CO2. Cells were plated at a density of 1 × 104 cells per well in a 24-well plate and incubated for 24 h, or until 100% confluency was reached. Each monolayer was wounded by dragging a pipette tip across the bottom of the well, using a straight edge as a guide (approximately 1.3 mm in width). The media was removed and cells were washed with 500 µL PBS twice to remove detached cells or cell debris. The cells were treated as follows: DMEM + 10% FBS + vehicle (0.06% glycerol, positive control), DMEM + vehicle (0.06% glycerol, negative control), DMEM + vehicle (0.06% glycerol) + LNO (60 µg/mL), DMEM + vehicle (0.06% glycerol) + LA (24 µg/mL) and DMEM + vehicle (0.06% glycerol) + oleic acid (18 µg/mL). Photographs were taken of each well to measure the initial area of the wound. After 24 h incubation, the plate was photographed again. The scratched area was measured using Acrobat Photoshop CS software, and percentage of wound closure at each time point was derived by following formula: (1 – [current wound size/initial wound size]) × 100. The effect of LNO on fibroblast migration was also assessed by Oris° Cell Migration Assay from Platypus Technologies according to manufacturer’s instructions. Briefly, human neonatal fibroblasts were maintained in DMEM + 10% FBS and kept in a humidified 37 °C incubator with 5% CO2. Then, 25 000 cells/well were seeded and allowed to adhere to the collagen I-coated plate for 24 h, serum-starved for 24 h, and treated with LNO or vehicle for 72 h. The stoppers were then removed from the 96-well plate, cells were labeled with Calcein AM 4.0 µm, and migration to the exclusion zone was measured by fluorescent microscopy.
Studies on cytoskeleton remodeling of dermal fibroblasts
Human fibroblasts were seeded onto 24-well plates containing glass cover slips, serum-starved for 24 h, and treated with LNO 60 µg/mL of vehicle for 24 h. For staining of stress fibers and vinculin, cells were fixed with ice cold 4% paraformaldehyde in PBS for 15 min and permeabilized with ice cold 0.5% (vol/vol) Triton X-100 in PBS for 5 min at room temperature. Cells were washed with PBS and then blocked with 3% (wt/vol) Bovine Serum Albumin (BSA) in PBS for 1 h. Vinculin mAb FITC-Conjugate (Sigma, Cat F7053 Sigma Aldrich, St Luis, Palo Alto, CA, USA) 1/200 or Rodamine Phallodin 140 nm (Cytoskeleton Inc., 1830 S Acoma St, Denver CO 80223, USA), diluted in 3% BSA (wt/vol) in PBS, was applied, and the cells were incubated overnight at 4 °C. Cells were washed again for at least 20 min with PBS then mounted in 80% glycerol PBS and analyzed by fluorescent microscopy.
Determination of anti-inflammatory effect of LNO
Anti-inflammatory effect of LNO was assessed by measuring the inhibition of nitric oxide production in LPS-induced macrophages. Nitrite in the culture medium was used as an indirect measurement of nitric oxide according to the protocol described elsewhere15 with minor modifications. Briefly, RAW macrophages were plated at a minimum density of 0.4 105 cells/well in a 24-well plate and grown for 24 h. Stock solutions of LNO were added to the medium 2 h before the LPS stimulation. Cells were stimulated with 1 µg/mL LPS (Sigma-Aldrich, Inc., St. Louis, MO, USA). After 8 h, conditioned media (50 µL) were removed and immediately mixed with 50 µL of Griess reagent (10% sulfanilamide, 1% naphthaleneethylenediamine dihydrochloride in 5% H3PO4). After incubation for 15 min at room temperature in darkness, the samples were read at 540 nm using a BIOTEK micro-plate spectrophotometer. LNO did not show any cytotoxic effect at the concentrations used in our studies as evaluated by MTT viability assay.
Antibacterial and antifungal assays
The bacteria and fungi studied were Escherichia coli DH5α (ATCC # 47093), Staphylococcus aureus (ATCC # 12600), Enterobacter aerogenes (ATCC # 13048), Mycobacterium rodococcus DK17 (kindly provided by Dr. Gerben Zylstra, Biotech Center, Rutgers University), and Saccharomyces cerevisiae (ATCC # 201459). The medium for the growth of all bacteria was Luria-Bertani Media (LB) agar at 37 °C. The medium for the growth of S. cerevisiae was potato dextrose agar (PDA). E. coli and S. aureus were grown on LB agar plates at 37 °C. E. aerogenes and M. rodococcus Dκ17 were grown on LB agar plates at 30 °C. S. cerevisiae was grown on PDA plates at 30 °C. For inhibition experiments, LB broth was used for all bacteria and potato dextrose broth was used for testing S. cerevisiae. The minimal inhibitory concentration values were determined by broth dilution assay, using a method modified from Jones et al.,16 and Ferraro.17
In vivo studies
Transgenic zebrafish larvae as a model of wound healing
Transgenic zebrafish (Danio rerio), genotype Tg(fli1a: EGFP)y1/+, with the fli1 promoter driving the expression of enhanced green fluorescent protein (EGFP) in the vascular endothelial cells was used to track wound healing in real time by direct observation of angiogenesis.18 This transgenic zebrafish model was obtained from Zebrafish International Resource Center, ZFIN ID ZDB-GENO-070209-102. Zebrafish larvae (48 h post-fertilization) were sedated and the tail primordia were cut posterior to the notochord. For this assay, the larvae were placed in a 24-well plate and treated with LNO 10–100 µg/mL dissolved in water. Primordia re-growth was measured using fluorescence microscopy and image analysis of fluorescent endothelial cells. For this assay, the fin fluorescent area was normalized against the total fin area. The effects of LNO were analyzed and compared to the positive control CGS-21680 (2-p-(2-Carboxyethyl) phenethylamino-5′-N-ethylcarboxamidoadenosine).
Murine model of wound healing
This in vivo wound-healing assay was performed by MDS Pharma Services, Taiwan, MDSPS Testing Codes 1115924, 1079329, according to the protocol described elsewhere.10,19 Briefly, groups of five male CD-1 mice weighing 22 ± 2g were used for this assay. Under ether anesthesia, the shoulder and back region of each animal was shaved and a sharp punch (ID 12 mm) was used to remove the skin including panniculus carnosus and adherent tissues. The wounded area was traced onto clear plastic sheets on days 3, 5, 7, 9, and 11 and quantified by using Image-Pro Plus Software (Media Cybernetics, Inc., 4340 East-West HWY, Suite 400 Bethesda, MD, USA). Adenosine agonist CGS, LNO or vehicle were topically applied immediately after injury and once daily thereafter for a total of 10 consecutive days.
Results
Chemical characterization of LNO
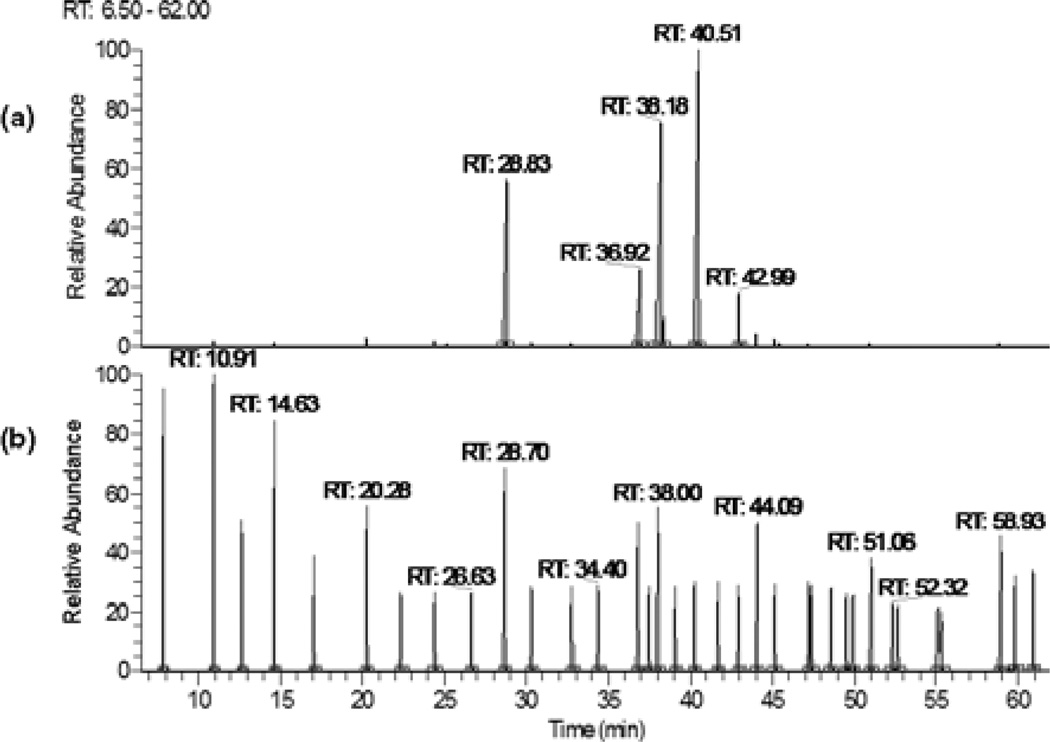
Lucuma nut oil was obtained by heptane extraction of ground lucuma nut yielding 2.6% (w/w) of the nut powder. The composition of LNO was identified by GC/MS analyses as LA (38.9%), oleic acid (27.9%), palmitic acid (18.6%), stearic acid (8.9%), and γ linolenic acid (2.9%). An additional 16 FAs were found in minor concentrations (Table 1, Fig. 1). Five minor FAs were not identified.
Table 1.
Chemical composition of lucuma nut oil
| %Area* | Fatty acid | Fatty acid methyl ester (FAME) | Carbon† | RT‡ |
|---|---|---|---|---|
| 38.85 | Linoleic acid | Methyl linoleate (cis-9,12) | 18:2 | 40.51 |
| 27.85 | Oleic acid | Methyl oleate (cis-9) | 18:1 | 38.18 |
| 18.63 | Palmitic acid | Methyl palmitate | 16:0 | 28.83 |
| 8.94 | Stearic acid | Methyl stearate | 18:0 | 36.92 |
| 2.93 | γ linolenic acid | Methyl γ-linolenate (cis-6,9,12) | 18:3 | 42.99 |
| 0.61 | Arachidic acid | Methyl arachidate | 20:0 | 44.01 |
| 0.42 | Myristic acid | Methyl myristate | 14:0 | 20.24 |
| 0.34 | Eicosenoic acid | Methyl eicosenoate (cis-11) | 20:1 | 45.09 |
| 0.24 | Pentadecanoic acid | Methyl pentadecanoate | 15:0 | 24.34 |
| 0.17 | Palmitoleic acid | Methyl palmitoleate (cis-9) | 16:1 | 30.29 |
| 0.14 | Decanoic acid | Methyl decanoate | 10:0 | 10.90 |
| 0.11 | Heptadecanoic acid | Methyl heptadecanoate | 17:0 | 32.76 |
| 0.1 | Lauric acid | Methyl laurate | 12:0 | 14.60 |
| 0.1 | Behenic acid | Methyl behenate | 22:0 | 50.90 |
| 0.08 | ND | ND | – | 45.42 |
| 0.07 | ND | ND | – | 25.16 |
| 0.07 | Eicosadienoic acid | Methyl eicosadienoate (cis-11,14) | 20:2 | 47.17 |
| 0.05 | Erucic acid | Methyl erucate (cis-13) | 22:1 | 52.21 |
| 0.04 | ND | ND | – | 46.75 |
| 0.04 | Heneicosanoic acid | Methyl heneicosanoate | 21:0 | 47.33 |
| 0.04 | Docosadienoic acid | Methyl docosadienoate (cis-13,16) | 22:2 | 52.65 |
| 0.04 | Lignoceric acid | Methyl lignocerate | 24:0 | 58.77 |
| 0.03 | ND | ND | – | 55.58 |
| 0.02 | Octanoic acid | Methyl octanoate | 8:0 | 7.85 |
| 0.02 | ND | ND | – | 60.02 |
| 0.01 | ND | ND | – | 47.50 |
| 0.01 | ND | ND | – | 47.88 |
| 0.01 | Eicosapentoic acid | Methyl eicosapentanoate (cis,5,8,11,14,17) | 20:5 | 55.17 |
| 0.01 | ND | ND | – | 63.43 |
All peak areas integrated and summed. Area of each FAME was divided by summed peak area of all FAMEs to calculate % area.
Total carbon number and unsaturation (if any), e.g., 16:0, represents the completely saturated palmitic acid.
RT, retention time in minutes (see Fig. 1).
ND, Not determined.
Figure 1.
Chromatographic profile of FAMEs from lucuma nut oil (LNO) analyzed by GC–MS. (a) Chromatograms of LNO 10 dilution and (b) food industry standard mixture of 37 FAMEs; both LNO and standards were injected at 100 µg/mL.
Determination of cell migration
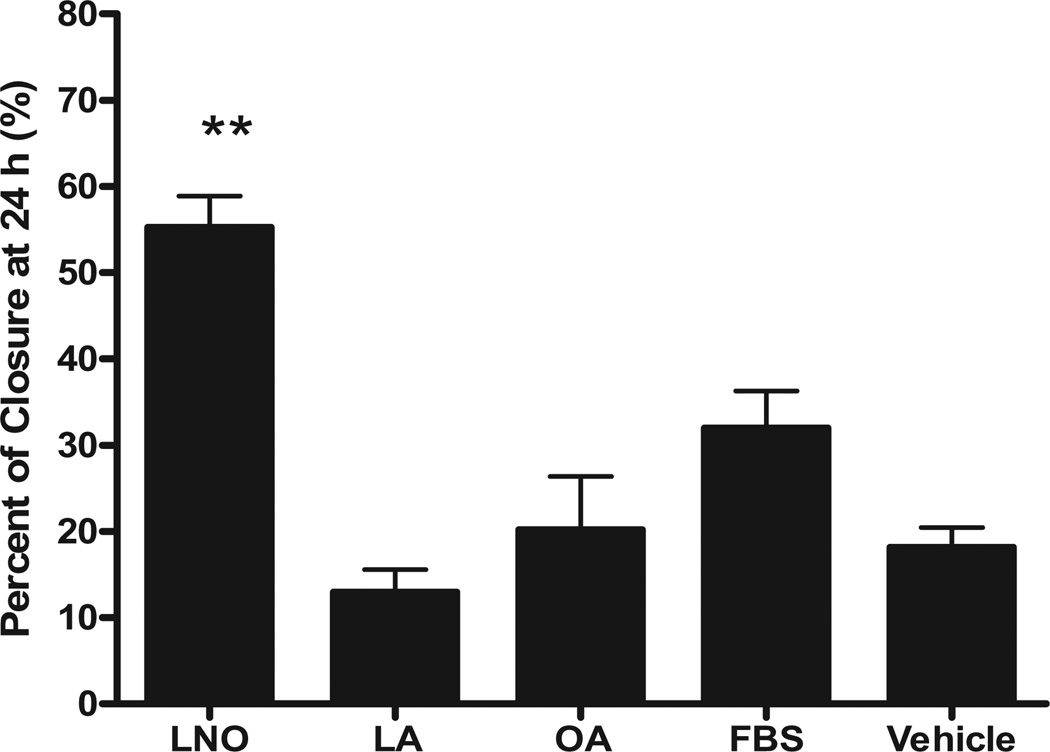
Microscopic observation of fluorescently labeled fibroblasts demonstrated that LNO promotes migration of human fibroblast into a 2- mm exclusion zone (Fig. 2). As pure FAs have been reported as potential wound-healing agents,5 we compared the effect of LNO with pure LA and OA on scratched fibroblasts. As shown in Table 1, LA and OA are the major FAs of LNO. Our results demonstrated that the wound-healing effect of LNO cannot be explained by the sole presence of LA and OA acids, as equivalent concentrations of these pure FAs were not able to reproduce the effect of LNO on fibroblasts migration (Fig. 3).
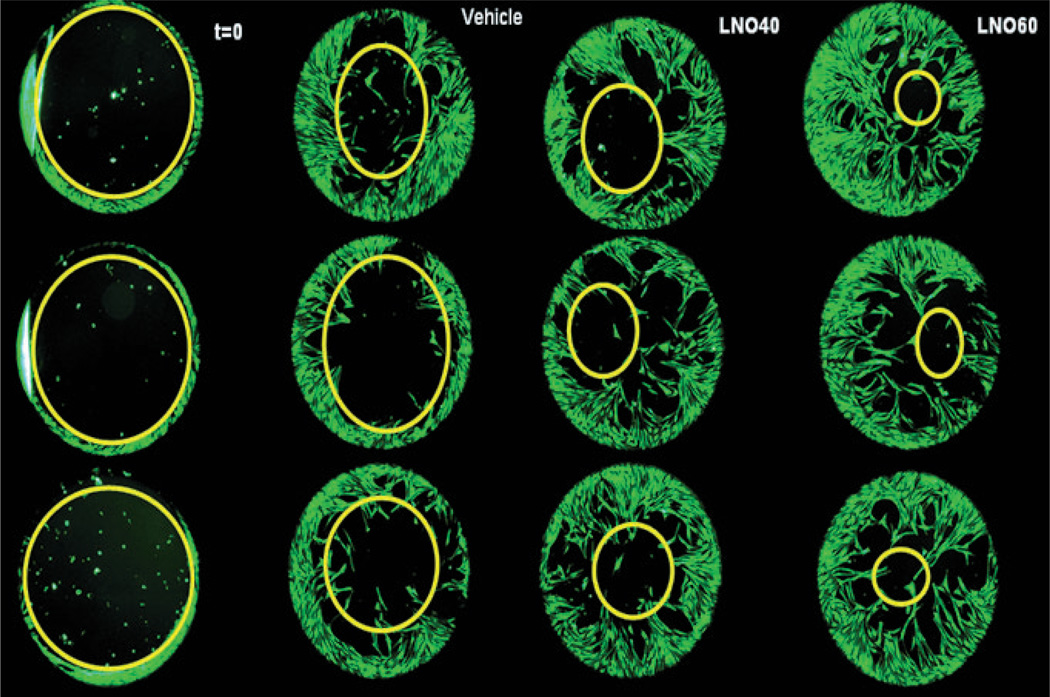
Figure 2.
Effect of lucuma nut oil (LNO) on human fibroblasts migration. Human neonatal fibroblasts were labeled with the green fluorescent probe Calcein-AM. The effect of LNO on fibroblast migration was evaluated using the ORIS° cell migration assay as described in the experimental section. Digital images were taken 72 h after starting the experiment. From left to right: time zero (t = 0), Vehicle, LNO 40 µg/mL (LNO 40) and LNO 60 µg/mL (LNO 60).
Figure 3.
Effect of lucuma nut oil (LNO) on scratched monolayer of human fibroblasts. Monolayers of fibroblasts were scratched and treated with LNO 60 µg/mL (n = 3) or with equivalent concentrations of pure fatty acids (FAs), oleic acid (OA, n = 4), linoleic acid (LA, n = 3) or 10% fetal bovine serum (FBS, n = 4) as positive control. The effect of LNO and FAs was evaluated as described in the experimental section. Values are the mean ± standard error (**P < 0.01 vs. FBS, one-way anova, Newman–Keuls multiple comparison test).
Studies on cytoskeleton remodeling of dermal fibroblasts
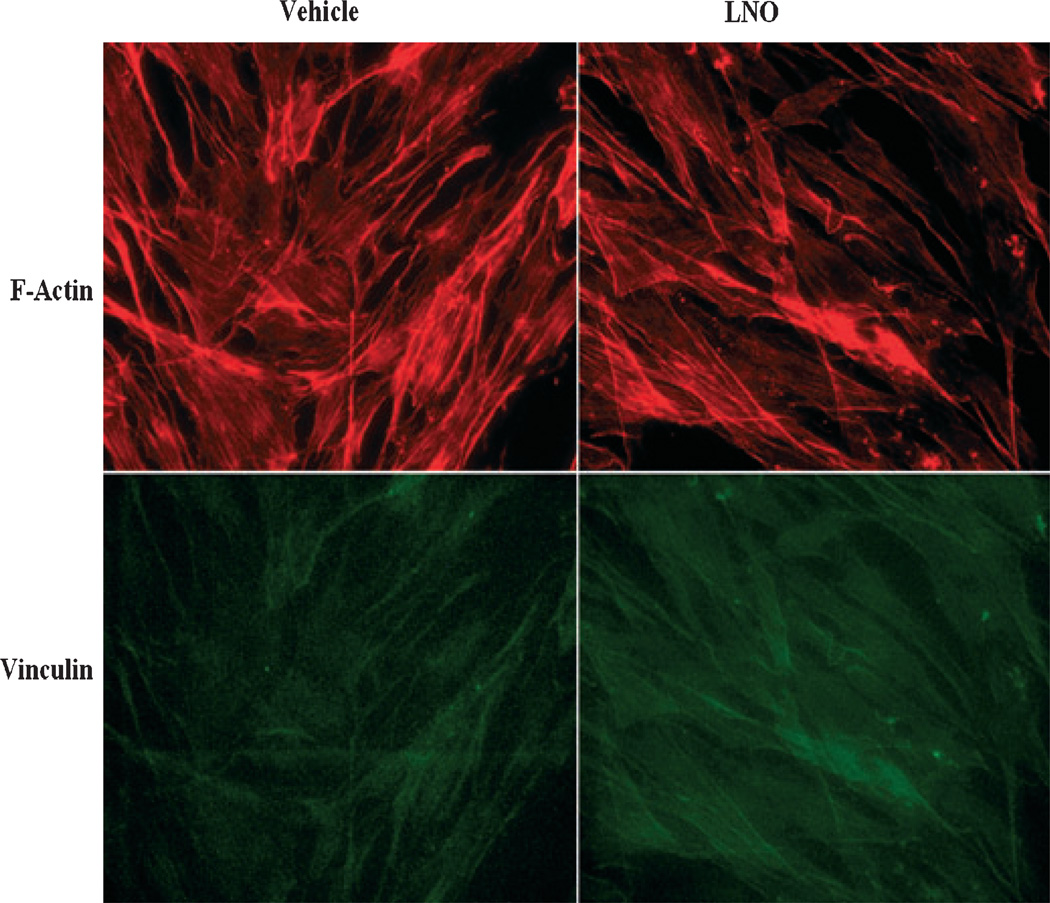
The results from immunocytochemical analyses showed that LNO at 60 µg/mL induced a reorganization of actin microfilaments in fibroblasts, as it was observable by less stress fiber formation and increased the expression of vinculin with respect to nontreated cells (Fig. 4). This is in accordance with other reports in which increased vinculin expression and reorganization of actin filaments in fibroblasts favor cell motility and wound healing.20
Figure 4.
Effect of lucuma nut oil (LNO) on vinculin expression and actin fibers organization in fibroblasts. Human dermal fibroblasts were treated with LNO 60 µg/mL or vehicle and doubly stained with rhodamine phalloidin (red) and mAb anti-vinculin (green). LNO caused mild decrease in stress fibers (F-actin) and increased the immunoreactivity to vinculin.
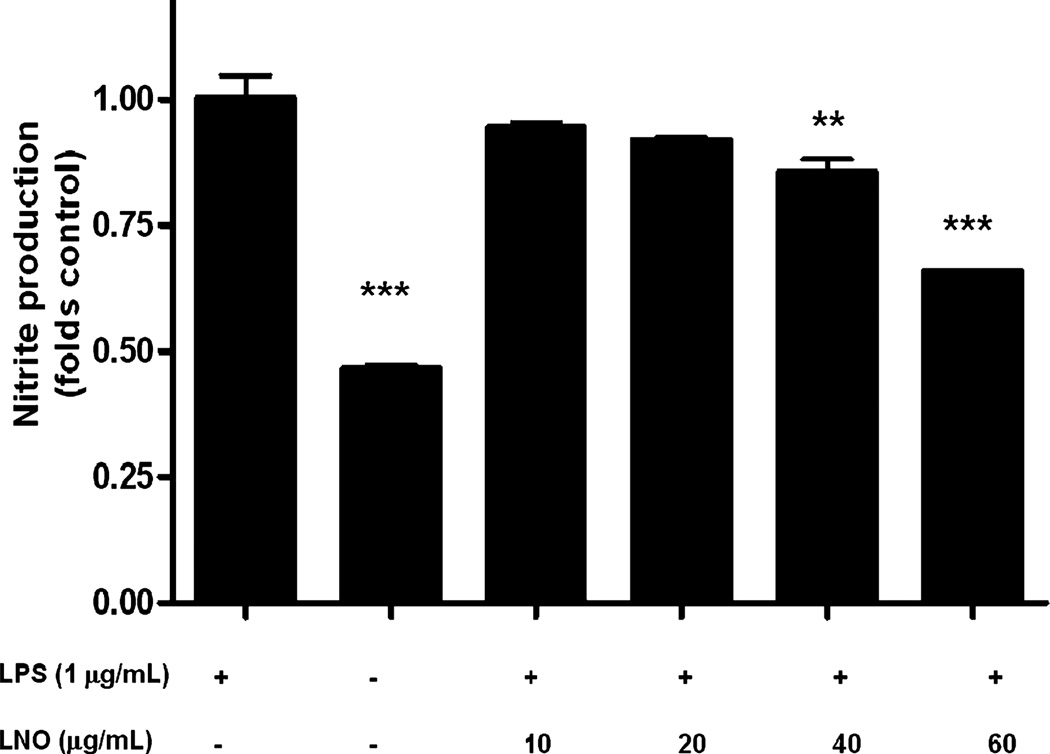
Effect of LNO in NO production
Lucuma nut oil promoted a moderate but significant decrease in the concentration of nitric oxide relative to nontreated controls in LPS-stimulated macrophages (Fig. 5).
Figure 5.
Effect of lucuma nut oil (LNO) on LPS-induced nitric oxide production in RAW 264.7 cells. RAW cells were pretreated with either vehicle alone or LNO for 2 h. Subsequently, LPS (1 µg/mL) was added to each well and incubated for 8 h. LPS significantly increased NO production with respect to the nonstimulated cells (control). LNO decreased LPS-induced NO production in a concentration-dependent manner. Results are expressed as mean ± standard error (***P < 0.001 vs. control **P < 0.01 vs. control, one-way anova, Newman–Keuls multiple comparison test).
Antimicrobial activity of LNO
Bacteria E. coli DH5α (ATCC # 47093), S. aureus (ATCC # 12600), E. aerogenes (ATCC # 13048), M. rodococcus DK17 and a fungus S. cerevisiae (ATCC # 201459) were tested over the range of 10–1000 µg/mL LNO concentrations. No significant inhibitory effect was observed up to the concentration of 1000 µg/mL of LNO (data not shown).
In vivo studies
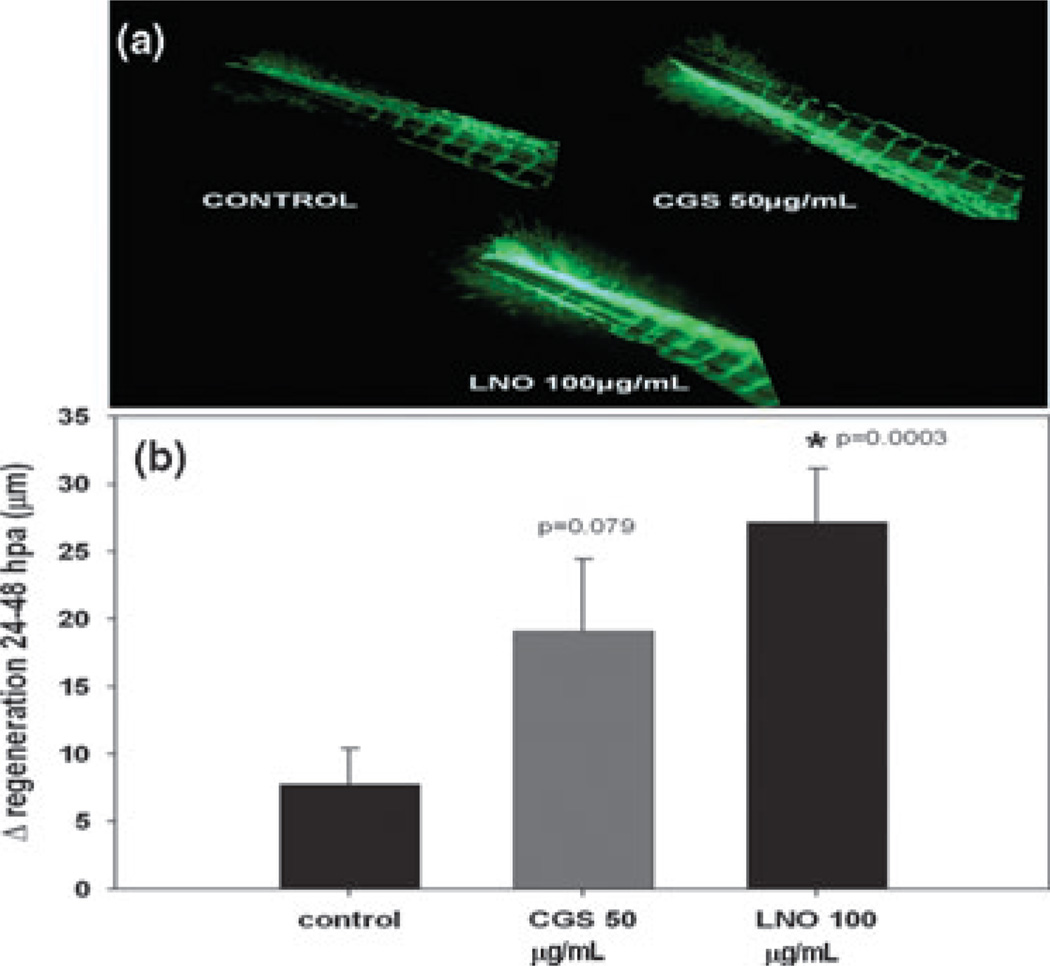
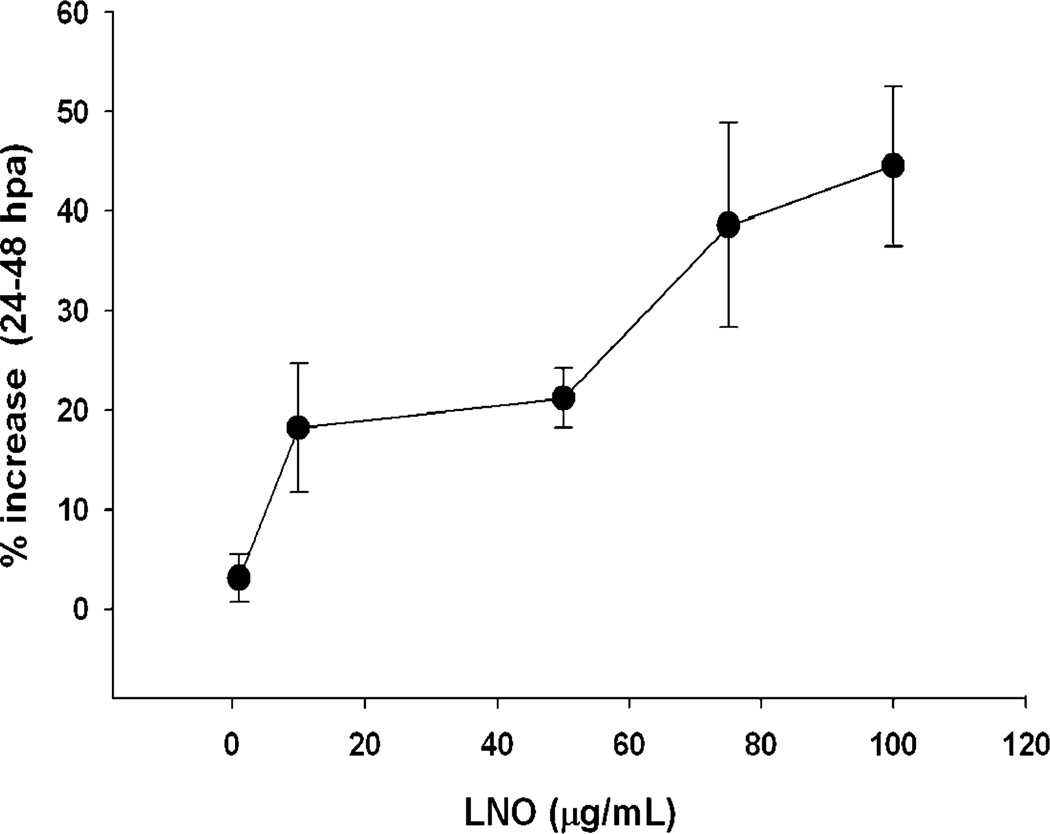
Transgenic zebrafish larvae as a model of wound healing As described in materials and methods, we amputated the tail fin of Tg(fli1a:EGFP)y1/+ transgenic zebrafish larvae and evaluated the regeneration and revascularization 48 h after amputation. LNO not only promoted faster regeneration of the amputated tail relative to negative control but also significantly promoted angiogenesis in the amputated larvae (Fig. 6). This proangiogenic effect of LNO was dose dependent over the range dose of 20–100 µg/mL (Fig. 7).
Figure 6.
Effect of lucuma nut oil (LNO) on tail fin regeneration and angiogenesis in transgenic zebrafish larvae. (a) Representative fluorescent micrographs of GFP-positive endothelial cells during tail fin regeneration induced by 100 µg/mL of LNO (LNO 100). (b) Quantitation of tail endothelial cells regeneration 48 h after amputation (hpa) of tail fin. Results were expressed as mean ± standard error of nine replicates (*P = 0.003, P = 0.079 vs. control).
Figure 7.
Dose-dependent effect of lucuma nut oil on tail fin endothelial repopulation of transgenic zebrafish larvae. The increase in fluorescent GFP-positive-endothelial cells 48 h after amputation (hpa) of tail fin was dose dependent. Results were expressed as mean ± standard error of nine replicates.
Murine wound-healing model
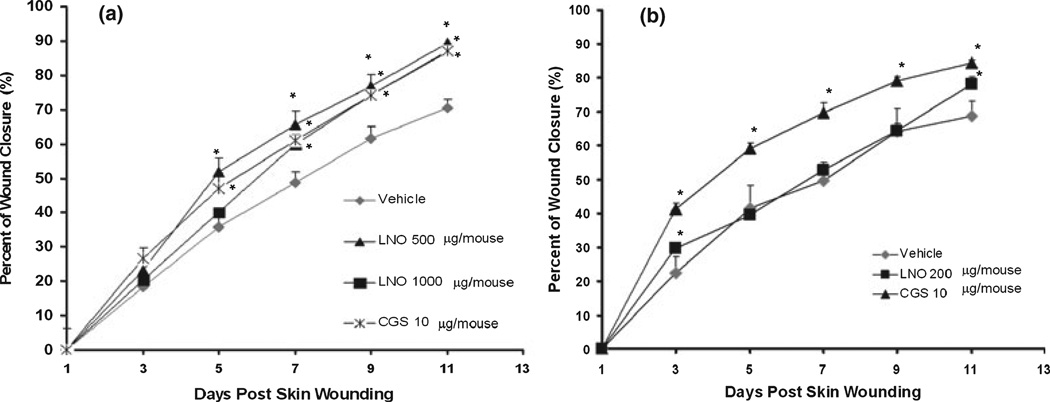
To determine the effect of LNO in cutaneous wounds, we applied formulations containing 1.0, 2.5, or 5.0% of LNO to wounded mice. Each animal received 20 µL of the formulation on the wounded area per day. Thus, the amounts of LNO daily administered to the wounded area were 200, 500, or 1000 µg. Topical daily application of LNO-containing formulations at doses of 500 and 1000 µg/wound significantly accelerated wound healing compared to nontreated animals (Figs 8 and 9, Table 2). The time at which 50% of the cutaneous wound is closed (CT50) was significantly shorter with LNO at 500 and 1000 µg/wound (Table 2). We hypothesize that this wound-healing effect of LNO might be explained by the synergistic effects of its anti-inflammatory FAs (Table 1). These results from CD-1 mice are in agreement with the data from transgenic zebrafish in which LNO not only promoted wound healing but also increased the formation of new blood vessels in wounded areas.
Figure 8.
Time course of in vivo wound-healing effect of lucuma nut oil (LNO). The percent of wound closure was evaluated during 11 days of topical application of LNO 200, 500, and 1000 µg/wound. Values are presented as mean ± standard error of five replicates (*P < 0.05 vs. vehicle). One-way anova followed by Dunnett’s test was applied for comparisons between the treated and vehicle.
Figure 9.
Progression of the dermal wound healing induced by lucuma nut oil (LNO). Representative images of the wound-healing progression in mice after daily application of LNO (500 and 1000 µg/wound), vehicle (CMC 50.5%, PBS pH7.4) and CGS (10 µg/mouse). Photographs of the wounds were taken every other day during 11 days postskin wounding (DPSW).
Table 2.
Dermal wound closure expressed as half-closure time (CT50)
| Treatment | CT50† ± SE‡ (days) |
|---|---|
| Vehicle | 7.58 ± 0.4 |
| CGS 10 µg/mouse | 6.04 ± 0.2* |
| LNO 200 µg/mouse | 6.84 ± 0.2 |
| LNO 500 µg/mouse | 5.86 ± 0.3* |
| LNO 1000 µg/mouse | 6.34 ± 0.1* |
P < 0.05, vs. vehicle (one-way anova followed by Dunnett’s test).
Time at which 50% of the skin wound is closed.
Standard error (n = 5).
LNO, lucuma nut oil.
Discussion
Fatty acids are metabolic precursors of several key mediators of inflammation and tissue regeneration, such as eicosanoids, lipoxins, and resolvins.21 However, the current evidence regarding the role of natural FAs in cutaneous wound healing continues to be inconclusive, consequently limiting their clinical use.2 Recent clinical studies have reported that topical formulations containing LA and other FAs have failed to accelerate wound healing.7 Conversely, other reports have shown that LA and OA could promote wound healing in a dose-dependent manner, increasing secretion of vascular endothelial growth factor (VEGF-α) and interleukin-1 beta (IL-1β), and decreasing TNF-α,6 consistent with proposed pro-angiogenic and anti-inflammatory effects of LA and OA.6 According to Pereira et al.,6 the anti-inflammatory effect of LA and OA in intraperitoneal neutrophils was only seen at concentrations as high as 200 µm and in the absence of any pro-inflammatory stimulus. The authors demonstrated that topical application of FAs either decreases or does not affect wound closure. Conversely, Cardoso et al.5 observed accelerated wound closure by LA and OA, but saw no increase in the formation of new blood vessels in wounded areas. A literature review reported that topical application of essential FAs in pressure ulcers, surgical wounds and skin burns could promote wound healing, but also found that the reviewed studies lacked proper methodologies.2 Thus, different pure FAs may either promote or impair wound healing.2,7 Our data suggest that the unique composition of FAs in LNO promotes wound-healing effect.
Wound healing is a highly coordinated sequence of molecular events involving inflammation, fibroblast migration, re-epithelialization, extracellular matrix (ECM) remodeling, and angiogenesis.9,22–24 Several cytokines and nitric oxide are important mediators of this process.21,25 Angiogenesis is important in the formation of granulation tissues and in supplying of oxygen to wounded tissues.26 Another important aspect in the treatments of cutaneous wounds is the prevention of bacterial and fungal infections,24 especially in immunocompromised individuals. Infected skin wounds are frequently associated with delayed wound closure, prolonged inflammation, and severe scar formation.9 Colonized or infected wounds tend to develop an abnormally prolonged inflammatory state that generates large amounts of nitric oxide.9 In this study, we tested the hypothesis that LNO, a mixture of natural FAs, promotes wound healing by modulating inflammation, cell migration, and blood vessels sprouting. We studied LNO’s effect on fibroblast migration, revascularization, bacterial and fungal growth and inflammation. Although inflammation is a normal and necessary step in the wound-healing process, uncontrolled inflammation of wounded tissues can severely damage healthy cells and impair wound healing. Taken together, our results from mice (Figs 8 and 9, Table 2), transgenic zebrafish larvae (Figs 6 and 7), and LPS-stimulated macrophages (Fig. 5) demonstrated that LNO can modulate the inflammatory process to accelerate the wound-healing process. In the transgenic zebrafish model, LNO not only accelerated wound healing but also increased new GFP-positive endothelial cells. This finding demonstrates that LNO increases vessel sprouting, thus improving perfusion of wounded areas. A previous report has shown that pure LA and OA, the major components of LNO (Table 1), accelerate cutaneous wound healing in mice but did not increase vessel sprouting in wounded areas.5 Our data from cell migration experiments led us to conclude that the in vivo wound-healing effect of LNO could relate to an increase in the migration capacity of fibroblasts.
As focal adhesions are important for fibroblast migration and adhesion,20 we expected to find changes in the expression of focal adhesion proteins, such as vinculin in fibroblasts treated with LNO. In fact, it has been described that, in fibroblasts from wounded areas, the increased expression of vinculin is correlated with a higher migratory capacity.20,27 It has been reported that during cell migration, vinculin and stress fibers play a key role in the integrin-mediated interaction of fibroblasts with the ECM.28 During the wound-healing process, integrins nucleate three distinct matrix adhesions in fibroblastic cells: focal complexes, focal adhesions and fibrillar adhesions. Focal complexes containing paxillin and vinculin, interact with β1 integrins. During cell migration, β1 integrins translocate from focal complexes to focal adhesions and ultimately to fibrillar adhesions, suggesting that a precursor–product relationship between the three types of matrix adhesion is necessary for optimum cell migration.29 Besides the increased expression of vinculin, the re-organization of the actin cytoskeleton has been associated with a higher migration capacity in fibroblasts,28,29 which was also observed in fibroblasts treated with LNO (Fig. 4). Thus, we hypothesize that LNO’s wound-healing effect observed in vivo is produced by increasing fibroblast migration capacity, promoting angiogenesis and modulating local nitric oxide production. In this study, we have compared the wound-healing efficacy of LNO with the adenosine receptor ligand CGS-21680, which is an accepted positive control in preclinical evaluation of wound-healing agents.19,30–32 Adenosine A2 receptor pathways are known to modulate wound healing19,33 and inflammatory processes.33
In conclusion, our data suggest that LNO possess a combination of natural pharmacologically active FAs, offering a potential new treatment for cutaneous wounds, skin inflammatory conditions, and general skin care. Thus, the oil from P. lucuma seems to be a good candidate for comparative studies with currently used wound-healing agents, such as petrolatum, antibiotics, and drug-eluting devices.34 These comparative studies are not included in this work, as we focused on LNO’s efficacy and potential mechanisms of action. Emollients, such as petrolatum favor granulation tissue formation, improve wound clinical appearance and accelerate wound closure.35 A similar clinical effect could be expected from LNO, as it led to a better clinical appearance and faster wound closure in wounded mice (Figs 7–9). Also, LNO produced faster revascularization in our transgenic zebrafish model of wound healing. With respect to the antibiotic formulations, LNO did not show strong antibacterial effect on planktonic bacterial forms according to our data. However, this does not preclude a potential effect of LNO on disrupting biofilm bacterial populations, which frequently colonize wounds and show very different clinical behavior and genomic profile than does the planktonic form of the same species.
Thus, this work may add a new bioactive botanical to the field of wound care.2,36,37 In addition, we report innovative approaches in the evaluation of bioactive botanicals, using sensitive analytical technologies and a transgenic zebrafish model of wound healing and revascularization.
Acknowledgments
Authors thank Ruth Dorn and Reneta Pouleva for technical assistance and Barbara Halpern for reviewing the manuscript. Research supported by a grant from Phytomedics Chile (Santiago, Chile) and NIH Center for Dietary Supplements Research on Botanicals and Metabolic Syndrome, grant # 1-P50 AT002776-01.
References
- 1.Vicanova J, Ponec M, Weerheim A, et al. Epidermal lipid metabolism of cultured skin substitutes during healing of full-thickness wounds in athymic mice. Wound Repair Regen. 1997;5:329–338. doi: 10.1046/j.1524-475X.1997.50407.x. [DOI] [PubMed] [Google Scholar]
- 2.Pieper B, Caliri MH. Nontraditional wound care: a review of the evidence for the use of sugar, papaya/papain, and fatty acids. J Wound Ostomy Continence Nurs. 2003;30:175–183. doi: 10.1067/mjw.2003.131. [DOI] [PubMed] [Google Scholar]
- 3.Simopoulos AP. Essential fatty acids in health and chronic diseases. Forum Nutr. 2003;56:67–70. [PubMed] [Google Scholar]
- 4.Simopoulos AP. Omega-3 fatty acids and antioxidants in edible wild plants. Biol Res. 2004;37:263–277. doi: 10.4067/s0716-97602004000200013. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso CR, Souza MA, Ferro EA, et al. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004;12:235–243. doi: 10.1111/j.1067-1927.2004.012216.x. [DOI] [PubMed] [Google Scholar]
- 6.Pereira LM, Hatanaka E, Martins EF, et al. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem Funct. 2008;26:197–204. doi: 10.1002/cbf.1432. [DOI] [PubMed] [Google Scholar]
- 7.Magalhaes MS, Fechine FV, Macedo RN, et al. Effect of a combination of medium chain triglycerides, linoleic acid, soy lecithin and vitamins A and E on wound healing in rats. Acta Cir Bras. 2008;23:262–269. doi: 10.1590/s0102-86502008000300009. [DOI] [PubMed] [Google Scholar]
- 8.Park KS, Lim JW, Kim H. Inhibitory mechanism of omega-3 fatty acids in pancreatic inflammation and apoptosis. Ann N Y Acad Sci. 2009;1171:421–427. doi: 10.1111/j.1749-6632.2009.04887.x. [DOI] [PubMed] [Google Scholar]
- 9.Broughton G, II, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor M, Liu S, Shi-wen X, et al. GSK-3beta in mouse fibroblasts controls wound healing and fibrosis through an endothelin-1-dependent mechanism. J Clin Invest. 2008;118:3279–3290. doi: 10.1172/JCI35381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Tull SP, Yates CM, Maskrey BH, et al. Omega-3 Fatty acids and inflammation: novel interactions reveal a new step in neutrophil recruitment. PLoS Biol. 2009;7:e1000177. doi: 10.1371/journal.pbio.1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruthig DJ, Meckling-Gill KA. Both (n-3) and (n-6) fatty acids stimulate wound healing in the rat intestinal epithelial cell line, IEC-6. J Nutr. 1999;129:1791–1798. doi: 10.1093/jn/129.10.1791. [DOI] [PubMed] [Google Scholar]
- 13.Quilter J, Ojeda EB, Pearsall DM, et al. Subsistence economy of El Paraiso, an early Peruvian site. Science. 1991;251:277–283. doi: 10.1126/science.251.4991.277. [DOI] [PubMed] [Google Scholar]
- 14.Pinto Mda S, Ranilla LG, Apostolidis E, et al. Evaluation of antihyperglycemia and antihypertension potential of native Peruvian fruits using in vitro models. J Med Food. 2009;12:278–291. doi: 10.1089/jmf.2008.0113. [DOI] [PubMed] [Google Scholar]
- 15.Park E, Quinn MR, Wright CE, et al. Taurine chloramine inhibits the synthesis of nitric oxide and the release of tumor necrosis factor in activated RAW 264.7 cells. J Leukoc Biol. 1993;54:119–124. doi: 10.1002/jlb.54.2.119. [DOI] [PubMed] [Google Scholar]
- 16.Jones RN, Barry AL, Gavin TL, et al. Susceptibility tests: microdilution and macrodilution broth procedures. In: Lennette EH, Balows A, Hausler WJ Jr, et al., editors. Manual of Clinical Microbiology. 4th edn. Washington, D.C.: American Society for Microbiology Washington, American Society for Microbiology; 1985. [Google Scholar]
- 17.Ferraro MJ. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Wayne: Natl. Comm. Clin. Lab. Stds; 1997. [Google Scholar]
- 18.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 19.Montesinos MC, Gadangi P, Longaker M, et al. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beurden HE, Snoek PA, Hoff JW, et al. In vitro migration and adhesion of fibroblasts from different phases of palatal wound healing. Wound Repair Regen. 2006;14:66–71. doi: 10.1111/j.1743-6109.2005.00090.x. [DOI] [PubMed] [Google Scholar]
- 21.Schwab JM, Serhan CN. Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol. 2006;6:414–420. doi: 10.1016/j.coph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Icre G, Wahli W, Michalik L. Functions of the peroxisome proliferator-activated receptor (PPAR) alpha and beta in skin homeostasis, epithelial repair, and morphogenesis. J Investig Dermatol Symp Proc. 2006;11:30–35. doi: 10.1038/sj.jidsymp.5650007. [DOI] [PubMed] [Google Scholar]
- 23.Nishioka T, Yasuda M, Tsutsumi K, et al. Matrixmetalloproteinases: up-regulated in subclones that survived 10-Gy irradiation. Radiat Med. 2007;25:430–431. doi: 10.1007/s11604-007-0168-9. [DOI] [PubMed] [Google Scholar]
- 24.Hampton S. Malodorous fungating wounds: how dressings alleviate symptoms. Br J Community Nurs. 2008;13:S31–S32. doi: 10.12968/bjcn.2008.13.Sup3.29470. S34, S36 passim. [DOI] [PubMed] [Google Scholar]
- 25.Yates CC, Whaley D, Hooda S, et al. Delayed reepithelialization and basement membrane regeneration after wounding in mice lacking CXCR3. Wound Repair Regen. 2009;17:34–41. doi: 10.1111/j.1524-475X.2008.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia Y, Turek JJ. Inducible nitric oxide synthase links NF-kappaB to PGE2 in polyunsaturated fatty acid altered fibroblast in-vitro wound healing. Lipids Health Dis. 2005;4:14. doi: 10.1186/1476-511X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Beurden HE, Snoek PA, Von den Hoff JW, et al. Dynamic protein expression patterns during intraoral wound healing in the rat. Eur J Oral Sci. 2005;113:153–158. doi: 10.1111/j.1600-0722.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Xu SW, Kennedy L, et al. FAK is required for TGF-beta-induced JNK phosphorylation in fibroblasts: implications for acquisition of a matrix-remodeling phenotype. Mol Biol Cell. 2007;18:2169–2178. doi: 10.1091/mbc.E06-12-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pala D, Kapoor M, Woods A, et al. Focal adhesion kinase/Src suppresses early chondrogenesis: central role of CCN2. J Biol Chem. 2008;283:9239–9247. doi: 10.1074/jbc.M705175200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montesinos MC, Desai A, Chen JF, et al. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol. 2002;160:2009–2018. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Victor-Vega C, Desai A, Montesinos MC, et al. Adenosine A2A receptor agonists promote more rapid wound healing than recombinant human platelet-derived growth factor (Becaplermin gel) Inflammation. 2002;26:19–24. doi: 10.1023/a:1014417728325. [DOI] [PubMed] [Google Scholar]
- 32.Valls MD, Cronstein BN, Montesinos MC. Adenosine receptor agonists for promotion of dermal wound healing. Biochem Pharmacol. 2009;77:1117–1124. doi: 10.1016/j.bcp.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schon MP, Schon M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J Invest Dermatol. 2006;126:1338–1347. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 34.Zilberman M, Kraitzer A, Grinberg O, et al. Drug-eluting medical implants. Handb Exp Pharmacol. 2010;197:299–341. doi: 10.1007/978-3-642-00477-3_11. [DOI] [PubMed] [Google Scholar]
- 35.Rossio-Pasquier P, Casanova D, Jomard A, et al. Wound healing of human skin transplanted onto the nude mouse after a superficial excisional injury: human dermal reconstruction is achieved in several steps by two different fibroblast subpopulations. Arch Dermatol Res. 1999;291:591–599. doi: 10.1007/s004030050460. [DOI] [PubMed] [Google Scholar]
- 36.Draelos ZD. The ability of onion extract gel to improve the cosmetic appearance of postsurgical scars. J Cosmet Dermatol. 2008;7:101–104. doi: 10.1111/j.1473-2165.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- 37.Draelos ZD, Yatskayer M, Raab S, et al. An evaluation of the effect of a topical product containing C-xyloside and blueberry extract on the appearance of type II diabetic skin. J Cosmet Dermatol. 2009;8:147–151. doi: 10.1111/j.1473-2165.2009.00428.x. [DOI] [PubMed] [Google Scholar]