Abstract
Despite our decades of experience with Kaposi Sarcoma its true nature remains elusive. This angioproliferative disease of the vascular endothelium has a propensity to involve visceral organs in the immunocompromised population. There are four variants of the disease and each has its own pathogenesis and evolution. While the common sources of upper gastrointestinal bleeding are familiar to surgeons and critical care physicians, here we present the exceedingly rare report of upper gastrointestinal bleeding attributable to this malady, explore its successful management, and review the various forms of Kaposi Sarcoma including the strategies in regard to their management.
Keywords: Kaposi Sarcoma, Upper gastrointestinal bleed, Endoscopy, HAART, Clinical management
Introduction
The incidence of hospitalization due to upper gastrointestinal bleeding (UGIB) ranges between 50 and 150 cases per 100,000 [1]. High volume intensive care units (ICUs) have become proficient at resuscitating and managing the common causes of UGIB (Table 1). Peptic ulcers are the most common etiology of UGIB, accounting for 30-50% of cases [2]; Mallory-Weiss tears, esophageal and gastric varices, angiodysplasias and arteriovenous malformations, hemobilia, hemorrhagic/erosive gastropathy (e.g., due to NSAIDs or alcohol consumption), and erosive esophagitis are additional etiologies of UGIB [2]. Upper gastrointestinal hemorrhage associated with neoplasms, however, is less common and within that subset is the exceedingly rare occurrence of bleeding attributable to Kaposi Sarcoma (KS) [4].
Table 1. Common Sources and Prevalence of Upper Gastrointestinal Bleeding*.
| Source | Prevalence (%) |
|---|---|
| Duodenal Ulcer | 24.3 |
| Gastric Erosions | 23.4 |
| Gastric Ulcer | 21.3 |
| Esophagogastric Varices | 10.3 |
| Mallory-Weiss Tear | 7.2 |
| Esophagitis | 6.3 |
| Erosive Duodenitis | 5.8 |
Defined as >5% in American Society for Gastrointestinal Endoscopy bleeding survey of 2225 patients.
KS is a multicentric neoplasm consisting of multiple vascular nodules appearing on cutaneous surfaces, mucous membranes, and less commonly viscera [5]. Massive GI bleeding from visceral KS is rare, with few cases reported (Table 2) [6, 7]. In the United States, KS is most commonly associated with immunosuppression from the Human Immunodeficiency Virus (HIV) or chronic use of transplant anti-rejection medications [8]. Early in the AIDS epidemic the incidence of KS reached >50%, but decreased to 15% by 1989 [9]. A further drop in incidence to <1% occurred in the early 2000s with widespread use of the highly active anti-retroviral therapy (HAART) [10].
Table 2. Literature Review - Gastrointestinal Bleeding as a Result of Kaposi Sarcoma - Presentations, Treatments and Outcomes.
| Author | Year | Age/ Gender |
Presentation | History | GI Location | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Salako Abdulkadir14 | 2005 | 56 yo / M | melena | h.pylori s/p treatment | jejunum, ileum | small bowel resection | death from rupture of hepatic kaposi, HIV testing negative |
| Ablin41 | 1998 | 73 yo / M | melena, fever | vasculitis on immunosuppressants | gastric, LI, rectum | vinblastine, cessation of immunosuppressants | continued hemorrhage, death |
| Balachandra42 | 2006 | 61 yo / M | GI bleeding | Inuit, NSAID use | stomach, ileocecal valve | doxorubicin | alive at 2.5 yrs HIV testing negative |
| Bryk43 | 1978 | 78 yo / M | weakness, malaise, melena | Known Cutaneous Kaposi | duodenum, jejunum | local radiotherapy, vinblastine, cyclophosphamide | death from continued bleeding |
| Calzona44 | 2002 | 15 yo / F | melena, lymphadenopathy, fever | Renal Txp | stomach | cessation of immunosuppressive therapy | bleeding stopped at 16 days, remission at 6 months |
| Cairncross45 | 2009 | 5 yo / F | “massive GI bleed” | HIV, CD4 14 | unknown | none | Death |
| De Blasio46 | 2005 | 42 yo / M | anemia, lymphadenopathy | renal txp | gastric, liver, inguinal lymph nodes | sub-total gastrectomy | death few days following surgery |
| Lin47 | 2002 | 41 yo / F | lymphadenopathy, tarry stools | Renal Txp | Esophagus, stomach | none listed | Unknown |
| Kahl48 | 2007 | 39 yo | hematochezia | none | stomach, SI, LI | none listed | New HIV Dx |
| Neville12 | 1996 | 53 yo / M | melena, anemia | HIV, known cutaneous KS | SI | chemo, radiation | resolution of symptoms |
| Potter49 | 1984 | 35 yo / M | hematochezia, abdominal distention | Known HIV / AIDS | esophagus, duodenum, SI, appendix, LI, Liver, GB, pancreas | TPN, bowel rest, vinblastine, adriamycin, bleomycin, vincristine, actinomycin, DTIC | progression of kaposi, death due to respiratory failure (PCP) |
| Rodriguez10 | 2012 | 30 yo / M | anal pain, hematemesis | HIV, known cutaneous KS | stomach, duodenum, rectum | HAART, doxorubicin, radiation | unknown |
| Stribling51 | 1978 | 59 yo / M | skin lesions, GI bleed | Renal Txp | Esophagus, stomach, SI, LI, liver, adrenals, retroperitoneum | Laparotomy, bowel resection | Death |
| Wien52 | 1991 | 66 yo / M | upper GI bleed | alcoholic liver disease | stomach | radiation | resolution of gastric lesions |
| Yildiz53 | 2010 | 52 yo / M | growing palate mass, melena, hematemesis | known cutaneous classic KS, HIV negative | palate, esophagus, stomach, lung, liver | Adriablastine, vincristine, bleomycin | partial remission |
Pub Med and Google Scholar searches for “gastrointestinal bleeding AND Kaposi”.
Location Abbreviations: SI = Small Intestine, LI = Large Intestine, GB = gall bladder
In the context of the above observations, it is important to note that while approximately 50,000 new HIV infections are diagnosed in the Unites States yearly, more than one million individuals in the United States do not know they are infected [11]. Despite the overall decreasing morbidity and mortality trend in the HIV-infected population, the potential number of patients with advanced opportunistic infections and malignancies continues to be significant, and is further amplified by patients who are non-compliant with HAART or whose virus is resistant to current anti-retroviral regimens [12].
Here we report an unusual case of massive UGIB directly attributable to HIV-associated KS complicated by thrombocytopenia of advanced, undiagnosed human immunodeficiency virus infection. We also present a review of the literature for KS-induced UGIB and important aspects specific to the management of the patient with newly-diagnosed, advanced HIV infection.
Case Report
A middle-aged female presented to the Emergency Department (ED) with a chief complaint of facial edema following a visit to a dental clinic three days prior to admission. During that visit, the patient underwent extraction of an infected tooth and was started on penicillin. She continued to have swelling and pain in the left buccal area, and on the morning of hospital admission she noted increased purulent drainage from this area. While en route to the ED she had one episode of hematemesis, approximately 200 mL in volume. Upon further questioning, the patient stated that since the dental extraction, she has also been taking high-dose ibuprofen frequently to help with her severe pain. She denied any past medical or surgical history, although admittedly she rarely sought medical care and felt herself to be generally healthy. She denied tobacco, alcohol or drug abuse.
On initial evaluation, she was a thin-appearing female with notable erythema and edema in the left buccal and periorbital area. On examination her blood pressure was 123/74 mmHg, heart rate 127 beats/minute, , respiratory rate 33 breaths/min, temperature 97.3 °F, and an oxygen saturation of 100% on room air. Within the middle of the buccal area of erythema was a spontaneously draining abscess containing thick, purulent material. There was no obvious blood in the oropharynx. The remainder of her physical exam was unremarkable. Microbiology culture specimens were sent broad spectrum antibiotics were started.
Shortly after her initial evaluation the patient experienced a second episode of hematemesis measuring approximately 250 mL. A nasogastric tube was placed, but it was not possible to clear the lavage output. Large-bore, central venous access was established and fluid resuscitation began. Her initial laboratory evaluation demonstrated hemoglobin of 7.3 g/dl, international normalized ratio (INR) of 1.1, and white blood cell count of 18,800/μL. Concurrent to this most recent episode of hematemesis, her clinical course began to rapidly deteriorate and the patient quickly became hypotensive and increasingly tachycardic. After a third, larger episode of hematemesis occurred (approximately 800 mL), the patient's trachea was intubated for airway protection and gastroenterology was consulted for emergent esophagogastroduodenoscopy (EGD). Upon examination bright red blood was found throughout the esophagus and stomach. There were numerous (>8) 1-2 cm cratered, nodular gastric ulcers within the gastric body. Some of the ulcers in the distal body of the stomach had a visible vessel with stigmata of recent bleeding. The largest lesion seen in the proximal gastric body was 20 mm in largest dimension with actively blood extravasation visible vessel within the ulcer bed. Multimodality therapy consisting of electrocoagulation, endoscopic clip application, and local epinephrine injections were utilized to slow the bleeding. The proximal duodenum and antrum appeared to be grossly normal. Biopsies of the lesions were obtained.
Following the upper endoscopy, the patient continued to have sanguineous nasogastric tube output. Given these findings, an arteriogram was performed with intent to perform embolization of any active intragastric bleeding. However, no active bleeding was identified on the study. A repeat upper endoscopy performed approximately 24 hours after the initial EGD demonstrated no active bleeding and re-visualization of the ulcerative lesions (Figure 1).
Figure 1.
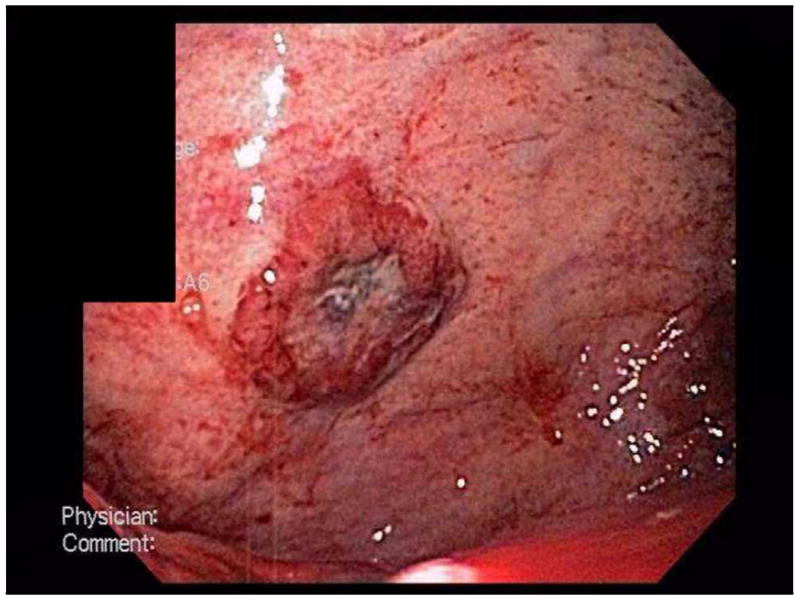
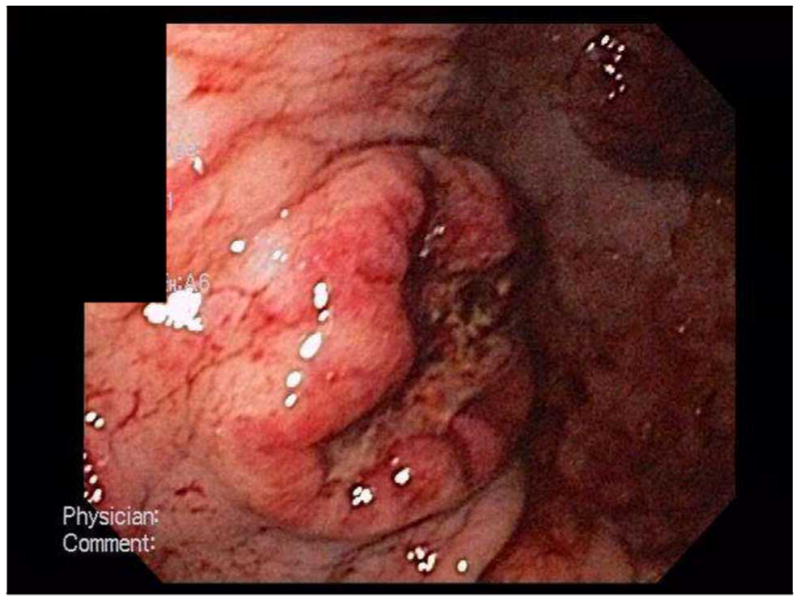
EGD photos of gastric fundus demonstrating ulcers.
The patient was transfused 8 units of packed red blood cells during her first 48 hours in the ICU. The patient gradually stabilized and began to improve. On hospital day four, her pathology results were finalized, showing focal proliferating spindle cells at the edge of the ulcer, granulation tissue positive for CD31, HHV-8 and CD117, and stains negative for S100 and SMA. Overall, these findings were consistent with Kaposi's Sarcoma. Upon subsequent testing, the patient's HIV results came back positive, with a viral load of 2,567,006 copies/mL. The patient's CD4 count was 5 cells/mm3.
Over the next several days, she continued to require intermittent packed red blood cell transfusions. In addition, she was diagnosed with HIV-Related Immune Thrombocytopenia Purpura which responded to treatment with intravenous immunoglobulin. The bleeding gradually subsided to the point where she was able to take oral medications, and HAART therapy was initiated consisting of Darunavir, Ritonavir and Emtricitabine/Tenofovir. Her clinical status rapidly improved. Positron emission tomography (PET) revealed multifocal hypermetabolic lesions within the stomach and small bowel (Figure 2). She received one dose of paclitaxel prior to discharge in order to induce regression of her sarcomas. On hospital day 28 she was discharged to home in stable condition.
Figure 2.
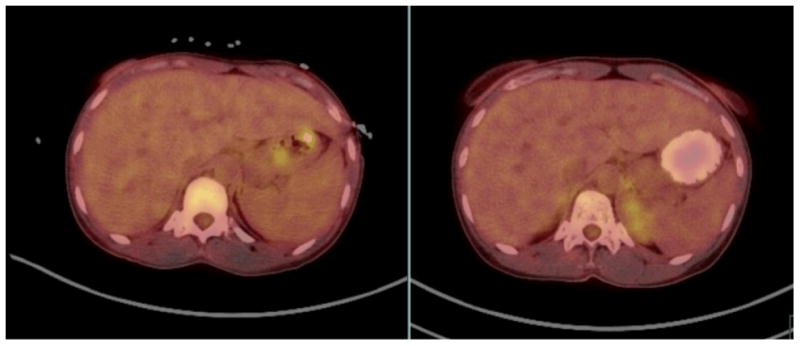
PET/CT of abdomen: [Left] Imaging performed prior to treatment (without oral contrast); [Right] Imaging obtained three months after initiation of medical treatment on the right (with oral contrast).
The original mouth lesion failed to heal completely, and was thus biopsied. This revealed Kaposi sarcoma as well. She continued outpatient chemotherapy and HAART, and at her six-month follow-up appointment her viral load was reduced to <40 copies/mL and the CD4 count increased to 46 cells/mm3. Repeat PET scan after five cycles of Taxol demonstrated significant improvement in hypermetabolic activity in the stomach with mild residual activity (Figure 2). She completed six doses of paclitaxel and has experienced no additional episodes gastrointestinal bleeding. Twenty months after her initial diagnosis, her CD4 count was stable between 190-250 Cells/mm3.
Discussion
Kaposi sarcoma is a low-grade vascular tumor first described in 1872 as a cutaneous “pigmented sarcoma” [5]. Since then the understanding of the different subtypes of KS has advanced greatly with the common theme being the involvement of the human herpesvirus-8 (HHV-8) infection [13]. The classic variant of KS is found predominantly in older men of Eastern European and Mediterranean origin, presenting with multiple red-to-purple nodules on lower extremities and rare involvement of the GI tract [14]. The second variant is the lymphadenopathy-associated form of KS, also called the endemic or African form. This variant is very aggressive and is often found in South Africa in young Bantu children with local or generalized lymphadenopathy. Skin lesions are rare in this variant [16]. The third variant of KS is the iatrogenic, transplant- or immunosuppression-associated form, which develops in patients on immunosuppressive therapy. Lesions appear on the skin, but in approximately half of the cases they are also found in internal organs and lymph nodes [8]. The fourth variant of Kaposi sarcoma is the AIDS-associated (epidemic) form. This variant is the most common AIDS-associated tumor in the United States. AIDS-associated Kaposi sarcoma has no preferred locations and tends to be more scattered, with involvement of the lymph nodes and the gastrointestinal tract occurring relatively early [13, 17].
In the United States, KS is most commonly associated with immunosuppression from the Human Immunodeficiency Virus (HIV) or chronic use of transplant anti-rejection medications [8]. During the early years of the HIV epidemic, KS was one of the most common clinical manifestations of AIDS. The incidence of KS associated with the early period of the AIDS epidemic (>50%) declined markedly by the late 1980s (15%) [9], and then further dropped to <1% by the early 2000s with the use of the highly active anti-retroviral therapy (HAART) [10]. Approximately 80-90% of HIV-associated KS cases are identified in homosexual males, suggesting the HHV-8 as a transmitting agent [18].
Visceral involvement is common in AIDS-related KS but is often asymptomatic. Post-mortem studies suggest that >25% of patients with AIDS-related KS have visceral lesions commonly involving of the stomach, bowel, liver, spleen, and lungs [5]. Most of the clinical data regarding the visceral presentation of KS is limited to case reports and series. Rare reports of intussusception, appendicitis, bowel obstruction, and perforation have been reported [19-21]. Table 2 reviews the reports of Kaposi presenting in the form of gastrointestinal hemorrhage.
KS is usually indolent, and requires no specific treatment with fewer than 10% of patients with KS dying of their malignancy. Many cases of KS regress following the initiation of HAART. Therefore aggressive treatment is reserved for cases with severe cosmetic problems or functional limitations with joint involvement. Recommendations for symptomatic visceral lesions are limited due to the rare nature of the disease. However there are treatment options, and management of AIDS-Associated Kaposi's Sarcoma is based on the extent of disease (Table 3). Paclitaxel is the most attractive antineoplastic agent with acceptable tolerance. This is also used as rescue therapy for those that fail classical regimens. Neutropenia is the major side effect, occurring in 60% [22].
Table 3. Management of AIDS-Associated Kaposi's Sarcoma.
| 1. Observation and optimization of antiretroviral therapy |
2. Single or limited number of lesions:
|
3. Antineoplastic Agents:
|
Fatahzadeh40
Essentially, KS therapy is palliative in that it seeks to eliminate tumor-related edema, improve cosmetic results and the functioning of limbs. The management approaches to classic KS, endemic KS, iatrogenic KS, and epidemic will differ as indicated above. Systemic therapy is applicable to the four types of KS. However, side effects, duration of response, and how efficacious the therapy will vary among the different forms of KS. New therapies target prevention of KSHV prevention in conjunction with treatment of KS. Gancyclovir, foscarnet, and valgancyclovir have all demonstrated promise. However, anti-herpes drugs alone are inadequate in that their administration with HAART offers the best hope to patients. Other innovative approaches are being brought to bear on KS, such as tyrosine kinase, VEGF, angiogenesis, and matrix metalloproteinases [15].
Conclusions
Acute upper gastrointestinal bleeding associated with KS is very uncommon. Successful management involves multi-modality approach, including endoscopy and HAART. In some cases, KS lesions have regressed following adequate HIV-targeted therapy. A variety of other therapeutic agents have been described in the management of KS, but there is no universal agreement or standardized approach to this challenging clinical problem.
Footnotes
Conflict of Interest: The authors report that they have no conflicts of interest to report.
References
- 1.Button LA, Roberts SE, Evans PA, et al. Hospitalized incidence and case fatality for upper gastrointestinal bleeding from 1999 to 2007: a record linkage study. Aliment Pharmacol Ther. 2011;33(1):64–76. doi: 10.1111/j.1365-2036.2010.04495.x. [DOI] [PubMed] [Google Scholar]
- 2.Fleischer D. Etiology and prevalence of severe persistent upper gastrointestinal bleeding. Gastroenterology. 1983;84(3):538–43. [PubMed] [Google Scholar]
- 3.Katz PO, Salas L. Less frequent causes of upper gastrointestinal bleeding. Gastroenterol Clin North Am. 1993;22(4):875–89. [PubMed] [Google Scholar]
- 4.Bello Rodriguez L, Pardeiro Pértega R, Couto Wörner I, et al. Upper gastrointestinal bleeding due to gastric and duodenal Kaposi's sarcoma. Rev Esp Enferm Dig. 2012;104(1):33–4. doi: 10.4321/s1130-01082012000100007. [DOI] [PubMed] [Google Scholar]
- 5.Hengge UR, Ruzicka T, Tyring SK, et al. Update on Kaposi's sarcoma and other HHV8 associated diseases. Part 1: epidemiology, environmental predispositions, clinical manifestations, and therapy. Lancet Infect Dis. 2002;2(5):281–92. doi: 10.1016/s1473-3099(02)00263-3. [DOI] [PubMed] [Google Scholar]
- 6.Neville CR, Peddada AV, Smith D, et al. Massive gastrointestinal hemorrhage from AIDS-related Kaposi's sarcoma confined to the small bowel managed with radiation. Med Pediatr Oncol. 1996;26(2):135–8. doi: 10.1002/(SICI)1096-911X(199602)26:2<135::AID-MPO12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Salako AA, Adisa AO, Ojo OS, et al. Severe gastrointestinal haemorrhage due to primary intestinal Kaposi's sarcoma - a case report. Niger Postgrad Med J. 2007;14(4):352–4. [PubMed] [Google Scholar]
- 8.Sampaio MS, Cho YW, Qazi Y, et al. Posttransplant malignancies in solid organ adult recipients: an analysis of the U.S. National Transplant Database. Transplantation. 2012;94(10):990–8. doi: 10.1097/TP.0b013e318270bc7b. [DOI] [PubMed] [Google Scholar]
- 9.Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi's sarcoma. J Am Acad Dermatol. 1990;22(6 Pt 2):1237–50. doi: 10.1016/0190-9622(90)70169-i. [DOI] [PubMed] [Google Scholar]
- 10.Rabkin CS. AIDS and cancer in the era of highly active antiretroviral therapy (HAART) Eur J Cancer. 2001;37(10):1316–9. doi: 10.1016/s0959-8049(01)00104-6. [DOI] [PubMed] [Google Scholar]
- 11.Huppmann AR, Orenstein JM. Opportunistic disorders of the gastrointestinal tract in the age of highly active antiretroviral therapy. Hum Pathol. 2010;41(12):1777–87. doi: 10.1016/j.humpath.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Hughes SC. Human immunodeficiency virus and obstetric anesthesia. Anesthesiol Clin. 1998;16(2):397–418. [Google Scholar]
- 13.Mwakigonja AR, Pyakurel P, Kokhaei P, et al. Human herpesvirus-8 (HHV-8) sero-detection and HIV association in Kaposi's sarcoma (KS), non-KS tumors and non-neoplastic conditions. Infect Agent Cancer. 2008;3:10. doi: 10.1186/1750-9378-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laor Y, Schwartz RA. Epidemiologic aspects of american Kaposi's sarcoma. J Surg Oncol. 1979;12(4):299–303. doi: 10.1002/jso.2930120403. [DOI] [PubMed] [Google Scholar]
- 15.Fatahzadeh M. Kaposi sarcoma: review and medical management update. Oral Surg Oral Med Oral Path Oral Radiol. 2012;113(1):2–16. doi: 10.1016/j.tripleo.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Friedman SL, Wright TL, Altman DF. Gastrointestinal Kaposi's sarcoma in patients with acquired immunodeficiency syndrome. Endoscopic and autopsy findings. Gastroenterology. 1985;89(1):102–8. doi: 10.1016/0016-5085(85)90750-4. [DOI] [PubMed] [Google Scholar]
- 17.Beral V, Peterman TA, Berkelman RL, et al. Kaposi's sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335(8682):123–8. doi: 10.1016/0140-6736(90)90001-l. [DOI] [PubMed] [Google Scholar]
- 18.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20(12):1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 19.Zebrowska G, Walsh NM. Human immunodeficiency virus-related Kaposi's sarcoma of the appendix and acute appendicitis. Report of a case and review of the literature. Arch Pathol Lab Med. 1991;115(11):1157–60. [PubMed] [Google Scholar]
- 20.Wang NC, Chang FY, Chou YY, et al. Intussusception as the initial manifestation of AIDS associated with primary Kaposi's sarcoma: a case report. J Formos Med Assoc. 2002;101(8):585–7. [PubMed] [Google Scholar]
- 21.Coetzee T, Le Roux CG. Kaposi sarcoma--presentation with intestinal obstruction. S Afr Med J. 1967;41(17):442–5. [PubMed] [Google Scholar]
- 22.Hermans P. Kaposi's sarcoma in HIV-infected patients: treatment options. HIV Med. 2000;1(3):137–42. doi: 10.1046/j.1468-1293.2000.00027.x. [DOI] [PubMed] [Google Scholar]
- 23.Ablin J, Ackerman Z, Eliakim R, et al. Diffuse gastrointestinal hemorrhage as a presentation of systemic Kaposi sarcoma. Am J Gastroenterol. 1998;93(8):1390–1. doi: 10.1111/j.1572-0241.1998.01390.x. [DOI] [PubMed] [Google Scholar]
- 24.Balachandra B, Tunitsky E, Dawood S, et al. Classic Kaposi's sarcoma presenting first with gastrointestinal tract involvement in a HIV-negative Inuit male--a case report and review of the literature. Pathol Res Pract. 2006;202(8):623–6. doi: 10.1016/j.prp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Bryk D, Farman J, Dallemand S, et al. Kaposi's sarcoma of the intestinal tract: roentgen manifestations. Gastrointest Radiol. 1978;3(4):425–30. doi: 10.1007/BF01887107. [DOI] [PubMed] [Google Scholar]
- 26.Calzona A, Naso P, Puliatti C, et al. Massive gastrointestinal hemorrhage in a renal transpant recipient due to visceral Kaposi's sarcoma. Endoscopy. 2002;34(2):179. doi: 10.1055/s-2002-19842. [DOI] [PubMed] [Google Scholar]
- 27.Cairncross LL, Davidson A, Millar AJ, et al. Kaposi Sarcoma in children with HIV: a clinical series from Red Cross Children's Hospital. J Pediatr Surg. 2009;44(2):373–6. doi: 10.1016/j.jpedsurg.2008.10.087. [DOI] [PubMed] [Google Scholar]
- 28.De Blasio A, Palmiero G, Russo D. Kaposi's sarcoma occuring in a young black man after kidney transplantation. Nephrol Dial Transplant. 2005;20(12):2839–41. doi: 10.1093/ndt/gfi182. [DOI] [PubMed] [Google Scholar]
- 29.Lin CH, Hsu CW, Chiang YJ, et al. Esophageal and gastric Kaposi's sarcomas presenting as upper gastrointestinal bleeding. Chang Gung Med J. 2002;25(5):329–33. [PubMed] [Google Scholar]
- 30.Kahl P, Buettner R, Friedrichs N, et al. Kaposi's sarcoma of the gastrointestinal tract: report of two cases and review of the literature. Pathol Res Pract. 2007;203(4):227–31. doi: 10.1016/j.prp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Potter DA, Danforth DN, Jr, Macher AM, et al. Evaluation of abdominal pain in the AIDS patient. Ann Surg. 1984;199(3):332–9. doi: 10.1097/00000658-198403000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stibling J, Weitzner S, Smith GV. Kaposi's sarcoma in renal allograft recipients. Cancer. 1978;42(2):442–6. doi: 10.1002/1097-0142(197808)42:2<442::aid-cncr2820420210>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Wien FE, Samanta A, Venkataseshan VS, et al. Gastric hemorrhage and Kaposi's sarcoma treated with radiotherapy. N J Med. 1991;88(1):42–5. [PubMed] [Google Scholar]
- 34.Yildiz B, Ersoz S, Fidan E, et al. Classic Kaposi's sarcoma with multiple visceral organ involvement presenting with gastrointestinal bleeding. HealthMED. 2010;4(4):1103–6. [Google Scholar]


