Abstract
One of the best studied hormone-behavior interactions is the transient rise in testosterone (T) associated with male-male aggression. However, recent research on songbirds has demonstrated numerous exceptions to this pattern. One species previously thought to elevate T in response to a simulated territorial intrusion is the dark-eyed junco (Junco hyemalis). Here, we show that under most circumstances male juncos do not elevate circulating T or CORT levels in response to social stimuli, despite being physiologically capable of elevating T as indicated by their response to GnRH. The lack of hormonal response was found regardless of the sex of the social stimulus (singing male vs. soliciting female), its sensory modality (song only, song + live lure, song + taxidermic mount), or the timecourse of sampling. Notably, males did elevate T levels when exposed to a simulated territorial intrusion in the days following simulated predation of their chicks. Whether the high T seen in these narrow circumstances represents stage-dependent social modulation of T or re-activation of male reproductive physiology in preparation for re-nesting (i.e. socially independent T modulation) remains to be determined. It is clear, however, that activation of the HPG axis is highly context-specific for male juncos. These results highlight important and unresolved issues regarding the socially mediated component of the challenge hypothesis and how it relates to the evolution of hormone-mediated traits.
Keywords: challenge hypothesis, testosterone, social challenge, corticosterone, androgen responsiveness, simulated territorial intrusion, simulated courtship interaction
1. INTRODUCTION
Circulating testosterone (T) in male vertebrates is often elevated during periods of social instability, a pattern that has been interpreted as an adaptive physiological mechanism enabling greater investment in activity, energy mobilization, and resource or mate defense (Wingfield et al., 1990). This pattern, known as the ‘challenge hypothesis,’ has been observed in three interconnected contexts. The first context is that of an acute social challenge, such as a male-male aggressive encounter, that leads to activation of the hypothalamo-pituitary-gonadal (HPG) axis, causing T secretion from the testes and elevation of T in circulation shortly thereafter (Marler et al., 2005; Wingfield, 1984) (referred to as male-male androgen responsiveness, or Rmale-male, in Goymann et al. (2007)). The second context is seasonal variation in circulating T, which often tracks social instability, such that circulating T in males is higher during periods of territory establishment or female fertility, lower during parental phases, and even lower outside of the breeding season (Wingfield et al., 1987) (referred to as seasonal androgen responsiveness, or Rseasonal in Goymann et al. (2007)). The third context relates to among-species variation in T. For instance, males in species with polygynous mating systems exhibit high levels of aggression and little parental behavior, and they usually sustain comparatively higher levels of T across the season compared to males in monogamous species that have elevate T only during territory and lower T during the parental phase (Wingfield et al., 1987; Wingfield et al., 1990; Wingfield et al., 1982).
Because prolonged elevation of T can be costly (Wingfield et al., 2001), particularly with regard to reduced fecundity owing to reduced parental care (Hegner and Wingfield, 1987; Ketterson et al., 1992), each of these patterns is thought to reflect an adaptive solution to the trade-off between mating and parental effort. For example, elevation of T is less common in single-brooded species in which extended mating opportunities are few and the benefits of paternal behavior are ongoing (Wingfield and Hunt, 2002), or in species in which paternal care is essential (Goymann et al., 2007; Lynn, 2008). Elevation of T may also be temporally restricted only to discrete periods of intense social instability, such as territory establishment (Wingfield et al., 1990).
While seasonal and interspecific components of the challenge hypothesis have been well supported in several vertebrate lineages (Hirschenhauser and Oliveira, 2006; Oliveira et al., 2002; Wingfield et al., 1990), including humans (Archer, 2006), a growing number of studies on songbird species have led to questions regarding the general applicability of the social component of the challenge hypothesis and how it relates to seasonal and interspecific components (Goymann, 2009; Goymann et al., 2007). For instance, males in some species exhibit a robust seasonal pattern of elevated T that co-occurs with territory establishment, but they do not elevate T in response to a simulated territorial intrusion (STI) (e.g. Landys et al., 2007; Moore et al., 2004a; Moore et al., 2004b). Indeed a review of the literature in songbirds reveals that Rseasonal maps onto species-specific indices of aggression and parental care, but Rmale-male does not (Goymann, 2009; Goymann et al., 2007), and the effect sizes for Rmale-male and Rseasonal are largely uncorrelated among species (Goymann et al., 2007). These discrepancies not only suggest that Rmale-male and Rseasonal may represent fundamentally different components of androgen responsiveness, but they also indicate that our understanding of when the challenge hypothesis applies and when it does is incomplete.
Variation in methods used to assess Rmale-male might explain its absence in some previous studies. For example, several studies have suggested that playback alone is insufficient to bring about T elevation in circulation (Cramer, 2012; Dufty and Wingfield, 1990; Fokidis et al., 2011; Wingfield and Wada, 1989), and other studies have reported that the use of taxidermic mounts versus live decoys can influence the strength of hormonal responses to STI (Scriba and Goymann, 2008; Scriba and Goymann, 2010). The timecourse of STIs has also varied among studies: Most studies analyze hormonal responses within approximately 30 min of the beginning of the intrusion, but latencies from STI initiation to hormonal sampling have varied from roughly ten minutes to over two hours (Addis et al., 2013; Landys et al., 2007; McGlothlin et al., 2008; Moore et al., 2004a; Van Duyse et al., 2004; Wikelski et al., 1999; Wingfield and Wada, 1989), leaving null results open to the criticism that hormone levels may not have been assessed at the appropriate time.
Our primary goal in this study was to determine the circumstances under which social instability might lead to elevated testosterone. The dark-eyed junco (Junco hyemalis) is an excellent subject for this experiment because both proximate and evolutionary mechanisms shaping T-mediated phenotypes are well characterized in this species (Ketterson et al., 2009; Ketterson et al., 1992; McGlothlin and Ketterson, 2008; McGlothlin et al., 2005), and long-term studies have shown strong connections between a number of fitness-related traits and both experimentally elevated T and natural variation in T (Greives et al., 2006; Ketterson et al., 1992; McGlothlin et al., 2008; McGlothlin et al., 2007; Reed et al., 2006). Among-male variation in the physiological capacity to elevate T (i.e. the potential androgen responsiveness, or Rpotential, following Goymann et al. (2007)), which is assessed by comparing baseline T levels to those after stimulation of the HPG axis with exogenous GnRH, is correlated with reproductive success measured in the wild, suggesting evolutionary significance to variation among individuals in their ability to elevate T (McGlothlin et al., 2010). Dark-eyed juncos also have exactly the sort of life history for which social modulation of T is thought to be selectively advantageous (Goymann, 2009; Goymann et al., 2007). They are socially monogamous, temperate-breeding songbirds with biparental care and multiple broods per season (Nolan et al., 2002). Seasonal variation in circulating T in male juncos closely matches the predictions of the seasonal component of the challenge hypothesis, i.e., T peaks as breeding begins, is lower during parental care, and declines at the end of the season (Ketterson et al., 2005).
Importantly, though, there are conflicting findings as to whether male juncos elevate T in response to a social challenge, making it difficult to connect the wealth of knowledge on the evolution of T-mediated traits in this system with the theoretical framework of the challenge hypothesis. On the one hand, males exposed to song playback and a live male lure for an average of 37 min in the days immediately following a nest predation event had significantly higher T than they did 2–3 days earlier while they were rearing chicks (McGlothlin et al., 2008). Further, males that were more aggressive in response to an STI also elevated T to a greater degree in response to a GnRH challenge, suggesting that an STI acted on the HPG axis in the same way as GnRH (McGlothlin et al., 2007). On the other hand, in another study when male juncos were presented with 10 to 12.5 min of playback without a visual stimulus and captured approximately 30 min after the initiation of the playback, they did not show elevated T or CORT as compared to control males (Rosvall et al., 2012b). These two studies might be taken to indicate that a long social challenge elicited a hormonal response while a brief one did not. However the studies differed in several important ways. For example the longer challenge was accompanied by a live lure, while the shorter playback lacked a visual stimulus (i.e. no live lure or taxidermic mount). More importantly, the longer playback was performed on males whose nests had been experimentally failed just a few days earlier and whose mates were presumably fertile. Thus, the hormonal response may have reflected female fertility as opposed to the presence of a male lure. More generally, it is unclear whether variation in hormone elevation across studies in this and other species reflects the duration of the trial, the nature of the visual stimulus presented, or the reproductive state of the subject.
Here, we addressed these issues by presenting free-living male juncos with social treatments that varied in the duration of exposure to the simulated intruder (≥ 12.5 min, ≥ 25 min, ≥ 60 min), the sex of the intruder (male accompanied by male song; female accompanied by pre-copulatory trill), and the modality of social interaction (auditory only; auditory + taxidermic mount; auditory + live lure). We did not execute all permutations of these parameters, instead prioritizing certain comparisons that would shed light upon the specific circumstances (if any) that lead to socially mediated changes in circulating hormone levels (see Figure 1 for schematic of treatments). We also replicated McGlothlin et al. (2008) to compare socially challenged males that were experimentally induced to re-nest vs. those that were not re-nesting. To assess whether any magnitude of social elevation was on par with Rpotential for this species, we contrasted T levels collected from socially challenged and control males with those of date-matched samples collected from others males given a GnRH challenge. While our primary focus was T, we opportunistically measured CORT levels in these plasma samples, to test whether social challenges alter CORT signaling in the periphery, as demonstrated in other species (Landys et al., 2007).
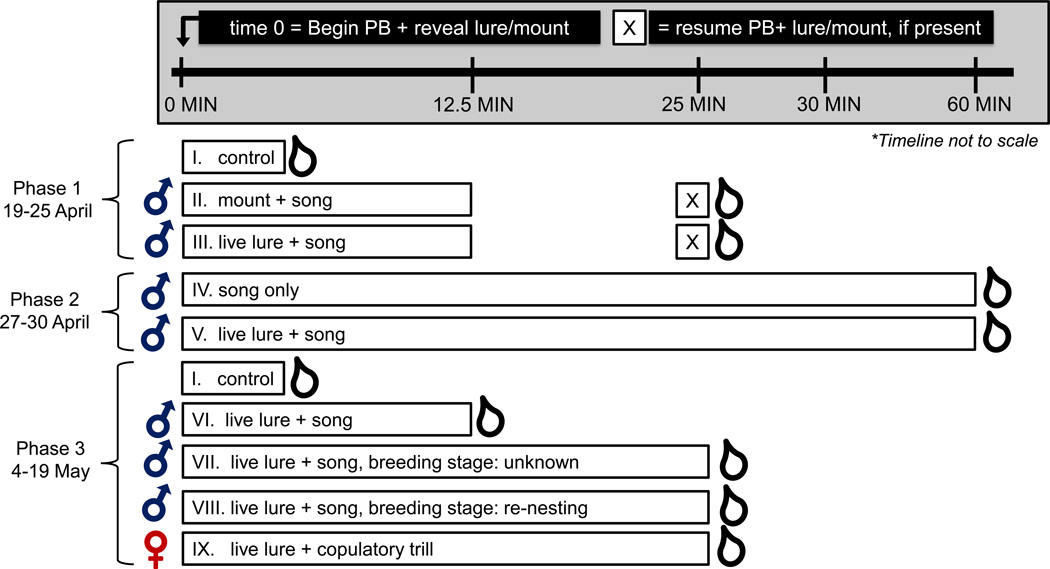
Figure 1. Schematic of social treatments.
Open boxes denote the duration of exposure to playback (PB) and/or a live lure or mount, as indicated. Male and female symbols denote the sex of the social stimulus. All trials simulating male intrusion were accompanied by male song, whereas trials simulating courtship interactions with females were accompanied by copulatory trills. “0 MIN” indicates the beginning of the trial, X indicates re-started playback (if applicable), and droplets indicate the approximate timing of blood sampling. Roman numerals are used as short-hand for these treatments in subsequent figures.
2. MATERIALS AND METHODS
2.1 Study area and focal animals
All procedures were approved by the Bloomington Institutional Animal Care and Use Committee (BIACUC). For this study, we captured and collected blood from 90 male juncos in the area surrounding the University of Virginia’s Mountain Lake Biological Station, USA (37°22’N, 80°32’W) between 17 April and 19 May 2012. Twenty-two males were sampled before and after a GnRH challenge. Sixty-eight additional males were sampled after social challenges or control captures, and nine of these social challenge birds were sampled twice in response to different social treatments, separated by ≥5 days (range: 5 to 24 d, 11.3 ± 2.1 d). We did not test neighbors on the same day, and we aborted trials in which more than one male appeared, so as to avoid inadvertently capturing males on territory boundaries. Subjects were randomly assigned to a control or treatment group prior to each trial.
All subjects were in the early- to mid-breeding period, as evidenced by date, enlarged cloacal protuberances, and incidental capture of females showing brood-patches. Demographic data from the long-term study population nearby shows that the median egg one date for first nests in 2012 was 13 May (first quartile: 28 April; third quartile: 26 May). These data suggest that most males in this study were likely to have mates that were building, laying, or incubating their first nest of the season, with few males attending to offspring.
2.2 Hormonal sampling in response to social stimuli and controls
We collected hormone samples from control males and males exposed to one of 8 different social stimuli (summarized in Figure 1). This portion of the study was conducted in 3 phases. Phase 1 (19 April to 25 April) focused on the contrasts among controls and STIs with live lures or taxidermic mounts. Each male experienced a 12.5 min STI with either a live lure or taxidermic mount. The visual stimulus was then covered, and we waited an additional 12.5 min before attempting to capture the bird. This delay was intended to allow approximately 30 min from the initial challenge to blood sampling because T levels in circulation peak approximately 30 min after GnRH challenge (Jawor et al., 2006), and average latencies to blood sampling in prior STIs in this system were comparable (36.9, 34.9 and 27.0 min in the protocols described in McGlothlin et al. (2008) and Rosvall et al. (2012b)) Phase 2 (27 to 30 April) focused on longer latencies to elevate hormones (60 min song only, 60 min live lure + song). Phase 3 (4 to 19 May) employed shorter latencies (12.5 min STI with live lure) and simulated courtship interactions that included a live female lure. Phase 3 also included STIs in which live lures were visible the entire 25 min. This modification of the STIs from Phase 1 was included in case prolonged contact with the intruder was necessary to establish a hormonal response. These 25-min STIs were performed on either the 2nd day after a simulated predation event (replicating McGlothlin et al. (2008)), or without any such manipulation, such that the breeding stage was likely to be building, laying, or incubating, as summarized above. Simulated predation of nestlings involved removal of nestlings to induce re-nesting; all pairs immediately began re-nesting, as evidenced by females observed with nesting material.
While the timing, visual stimuli and audio stimuli varied among different treatments, all audio stimuli were standardized to play at 85–90 dB measured at 1m with a sound pressure level meter, which is a natural amplitude in this species (Nolan et al., 2002). For each trial, we placed a speaker (Altec Lansing iM237) and iPod on the ground adjacent to a cloaked lure or mount (if present). For all trials involving a mount, we used one taxidermic mount of a male junco perched in a natural position. For live lures, we used one randomly selected sex-appropriate individual from a population of juncos maintained in captivity (n = 5 males, 5 females). We also set up and furled a mist-net out of the way of the speaker and/or lure, and we retreated at least 15 m before beginning the specific protocol summarized in Figure 1. At the pre-designated time, we unfurled the mist-net, re-started playback, and uncovered the lure (if present). Capture playback included a mix of junco vocalizations that prior work has shown to have no detectable effect on T or CORT signaling (Rosvall et al., 2012b).
For all simulated territorial intrusions, we broadcast recordings of male long-range songs that were recorded ≥1 km away in a previous year (see Reichard et al. (2011) for further details). Each song playback consisted of one song type repeated every 10 sec for the duration of the trial (12.5 min, 25 min, or 60 min). We used 8 song types recorded from 8 different males, and we balanced use of each song type by treatment.
For simulated courtship interactions (hereafter, SCI), female lures were accompanied by audio playback of a pre-copulatory trill, a vocalization produced by females during a copulation solicitation display (Nolan et al., 2002). At the time of the study, we were limited to a single high quality recording of this trill, and consequently, the audio stimulus was pseudo-replicated, though the live lure was not. Subsequent work has shown that these pre-copulatory trills are highly stereotyped among females (i.e. identical note type), making it unlikely that our stimulus was atypical (see Reichard et al., 2013). Furthermore, we noted that all males responded to SCIs with typical courtship behaviors (e.g. ptiloerection of the body feathers, tail spreading, short-range song, lack of dives or aggression), which are the same behaviors males perform in the presence of soliciting females (Enstrom et al., 1997).
Control hormone samples were collected from males that were captured in mist nets using targeted playback of a mix of junco vocalizations (n = 18 males; 8 in Phase 1, 10 in Phase 3). All males were captured and bled very rapidly (latency to capture: 103 ± 18 sec after initiation of playback; latency to completion of blood sampling: 290 ± 26 sec), suggesting that hormone levels from these males represent unstimulated levels of T and CORT.
Upon capture, we collected up to 150uL of blood from the wing vein from each male into microhematocrit tubes. Males were then banded and immediately released. Blood was stored on ice in the field and centrifuged later the same day. Plasma was drawn off the top and stored at −20°C until later enzyme immunoassays.
2.3 Hormone sampling to assess response to GnRH challenge
We compared the above described control and socially challenged males to another set of males whose Rpotential was assessed, so that we could ask whether any T elevation seen in response to social stimuli was similar in magnitude to what can be achieved by exogenous activation of the HPG axis (Rmale-male vs. Rmale-female vs. Rpotential). Fifty-six male juncos were given GnRH challenges in 2012. We randomly selected 22 of these males for our comparison, balancing for date against the control and socially challenged males described above. The methods used to assess Rpotential have been described in detail elsewhere (Jawor et al., 2006; McGlothlin et al., 2010). Briefly, males were captured with seed-baited potter traps or mistnets, and we noted the time at which each bird was retrieved from the net or trap (“capture time”). Birds were transported to the nearby laboratory, where an initial blood sample was taken (“pre-GnRH”), and we noted the time elapsed since capture. Immediately thereafter, males were injected intramuscularly with 1.25 µg of chicken GnRH (American Peptide, #54-8-23) dissolved in 50 µL of PBS. Thirty minutes later, a second blood sample was taken (“post-GnRH”). Blood samples were stored at −4°C and processed later the same day as described above (centrifugation, plasma isolation and storage).
2.4 Hormone assays
Commercially available enzyme immunoassays (EIA) were employed to analyze T (Enzo #901–065) and CORT (Cayman Chemical #500655), using 20µL and 10µL of plasma respectively and protocols optimized for use in the junco (Clotfelter et al., 2004; Rosvall et al., 2012b). Each EIA followed the same overall procedure: addition of a trace amount of tritiated hormone (~2000 CPM), extraction with diethyl ether (2× for T EIAs; 3× for CORT EIAs), evaporation with N2, reconstitution in ethanol (50µL) and assay buffer (300µL for T; 600µL for CORT), and plating in duplicate along with a standard curve according to the manufacturers guidelines. We used extraction efficiencies to correct for incomplete recoveries (for T: 79.8 ± 0.6%; for CORT: 91.8 ± 0.3%). We used samples repeated within and among plates to calculate intra- and inter-plate variability. For CORT, average intraplate variation was 12.2% and interplate variation was 18.4%. For T, intraplate variation averaged 4.7%. The final two plates, which included exactly half of the GnRH challenge samples in this study, inadvertently used a different interplate standard than the other four plates, and so we tested for an effect of plate number on pre- and post-GnRH challenge T levels. We found no effect of plate number (ANOVA: F = 0.89, p = 0.45), and we note that interplate variation for these two plates was 10.9%, and it was 15.6% for the other four T plates.
2.5 Statistical analyses
All statistical analyses used JMP v. 10.0.0 (SAS Institute, Cary, NC, USA). Results are reported as mean ± one standard error unless otherwise noted, and all tests are two-tailed. All hormone data were natural log transformed to achieve normality.
In preliminary analyses on the control and socially stimulated males, we tested for an effect of individual bird identity on T and CORT because we sampled 9 males twice in response to different treatments. We found no significant effect of bird identity on T or CORT levels (ANOVA, F < 1.44, p > 0.30). We also tested for and found no effect of song type on hormone levels (ANOVA, F <1.53, p > 0.19). Therefore, we did not consider individual identify or song type in further analyses of social treatments and controls.
Because we balanced social treatments by date within each phase of our experiment, but we did not balance among phases, we first separately analyzed hormone data from each of the three phases of our experiment. For T, we used ANOVA with treatment as an independent variable; for CORT, we used ANCOVA with treatment and handling time as independent variables. In cases with 2 or more treatment groups per phase, we used Tukey HSD tests to contrast specific social treatments within each phase. We included handling time in CORT analyses but not in T analyses because handling times were shorter than the time frame on which T is thought to change, but long enough that CORT might be affected (3.57 ± 0.19 min from net to bleed end, n = 77, range: 1.65 to 10.97 min). To further minimize handling time effects, we did not include the few samples with > 5 min handling time in our CORT analyses, thus reducing the sample size for CORT to n = 69. As a further test of these handling time effects on CORT, we followed this analysis with an ANOVA of only those Phase 3 STIs in which males were bled within 3.5 min of capture (i.e. a time frame short enough that handling induced CORT changes ought to be negligible), asking whether male CORT levels were significantly different among males captured after ~1 min, ~15 min, and ~30 min of STI (i.e. control vs. 12.5 min STI vs. 25 min STI).
As a secondary tool to contrast results from all three phases, we used linear regression to analyze all treatment groups collectively, while controlling for date (in both T and CORT models) and handling time (CORT model only), followed by post-hoc Tukey HSD tests to contrast the specific social stimuli and controls.
Handling times for birds receiving GnRH challenges vs. controls/social stimuli were sufficiently different so as to preclude direct comparison in these models (average latency for capture to pre-GnRH bleed = 38.4 ± 4.3 min), so we used a paired t-test to assess the effect of GnRH administration on T levels. We used Pearson correlations to examine relationships between pre-challenge T, pre-challenge CORT, and post-challenge T. Finally, we used unpaired t-tests to contrast pre-GnRH T and CORT levels with those of control males from the social challenge component of the experiment, and to contrast post-GnRH T levels with those of any social treatment that led to elevated T.
3. RESULTS
None of the social treatments had significant effects on T levels, except for exposure to a 25-min STI after a simulated predation event, i.e. while females were re-nesting (Table 1, Figure 2A–C). None of the social stimuli had significant effects on circulating CORT levels (Table 2, Figure 3A–C). Post-hoc analyses on only those Phase 3 males that were bled very rapidly also show no differences in CORT (ANOVA: F2,19 = 0.36, p = 0.70), and they reveal that males had typical baseline levels of CORT (back transformed mean ± se for controls: 13.9 ± 3.2 ng/mL, for 12.5 min STI: 10.7 ± 1.7 ng/mL, for 25 min STI: 12.1 ± 4.1 ng/mL).
Table 1.
ANOVA results for testosterone, with each phase of the experiment analyzed separately. Post-hoc Tukey tests are reported for analyses with ≥3 treatment groups. See Figure 1 for summary of treatments and phases.
| F | df | P | Post-hoc Tukey | |
|---|---|---|---|---|
| Phase 1 (n = 24) | 1.15 | 2, 21 | 0.34 | all p > 0.31 |
| Phase 2 (n = 13) | 0.38 | 1, 11 | 0.55 | |
| Phase 3 (n = 40) | 7.9 | 4, 35 | <0.0001 | STI during re-nest vs. others: p < 0.0030 all other pairwise comparisons: p > 0.51 |
Figure 2. Testosterone.
Plasma T did not differ significantly between controls and experimentals in any social treatment (A–C) except for males that experienced a 25-min STI in the days immediately following a simulated nest predation event. (D) T levels post-GnRH challenge were significantly higher than T-levels pre-GnRH challenge. Bars represent back-transformed means, and error bars show one standard error from the mean.
Table 2.
ANCOVA results for corticosterone, with each phase of the experiment analyzed separately. Whole model results are followed by results for each fixed effect (treatment, handling time). Post-hoc Tukey tests are reported for analyses with ≥ 3 treatment groups. See Figure 1 for summary of treatments and phases.
| F | df | p | Post-hoc Tukey | ||
|---|---|---|---|---|---|
| Phase 1 (n = 19) | 2.60 | 3, 15 | 0.090 | ||
| Treatment | 0.66 | 2 | 0.53 | all p > 0.51 | |
| Handling time | 1.07 | 1 | 0.020 | ||
| Phase 2 (n = 12) | 2.33 | 2, 9 | 0.15 | ||
| Treatment | 1.92 | 1 | 0.20 | ||
| Handling time | 3.98 | 1 | 0.077 | ||
| Phase 3 (n = 38) | 0.93 | 5, 32 | 0.47 | ||
| Treatment | 0.54 | 4 | 0.71 | All p > 0.73 | |
| Handling time | 1.52 | 1 | 0.23 | ||
Figure 3. Corticosterone.
Plasma CORT did not differ significantly among any social treatment (A–C), but CORT levels prior to GnRH challenge (D) were significantly higher than those of all other males (A–C). Bars represent back-transformed means, and error bars show one standard error from the mean. Note that the scale of the y-axis for D differs from A–C.
Regression analyses that collectively analyzed all social treatments and controls supported these same results. For testosterone: R2adj = 0.29, F9,67 = 4.46, p = 0.0001; Date: F = 12.0, p = 0.0009; Treatment: F = 4.20, p = 0.0004; Tukey HSD: STI during re-nest vs. other groups: p < 0.0023, All other pairwise comparisons: p > 0.71. For corticosterone: R2adj = 0.21, F10,58 = 2.78, p = 0.0071; Date: F = 0.41, p = 0.52; Handling time: F = 7.48, p = 0.0083; Treatment: F = 1.33, p = 0.24; Tukey HSD: p > 0.38 for all comparisons. Plasma T and CORT levels were uncorrelated among control and socially stimulated males (r = 0.038, n = 69, p = 0.75).
GnRH challenges significantly increased T levels (paired t-test: t1,21 = 6.90, n = 22, p < 0.0001), on average by 3.84 ± 0.54 ng/mL (range: −0.50 to 9.61 ng/mL, first quartile: 2.18 ng/mL, 3rd quartile: 5.75 ng/mL, Figure 2D). Pre-challenge and post-challenge T were not correlated (r = 0.27, n = 22, p = 0.23). Males with lower pre-challenge T had significantly higher CORT (r = −0.50, n = 20, p = 0.025, Figure 4), suggesting a stress-induced reduction in plasma T. However, circulating CORT levels prior to GnRH challenge had no detectable relationship with T levels post-GnRH challenge (r = 0.021, n = 20, p = 0.93). Consistent with the interpretation that the lower T in males given GnRH challenges was stress-related, control males that were captured and bled immediately on their territories had significantly higher T levels and significantly lower CORT levels compared to males that were transported to a central laboratory prior to GnRH challenge (For T, unpaired t-test: t = 2.88, p = 0.0048; for CORT: unpaired t-test: t = 6.94, p < 0.0001, Figure 3D). GnRH-induced T levels were significantly lower than T levels seen in males sampled after STIs while their females were re-nesting (unpaired t-test: t = 2.03, p = 0.045).
Figure 4.
Prior to GnRH challenge, plasma T and CORT were negatively correlated.
4. DISCUSSION
Socially mediated shifts in hormones are thought to facilitate behavioral and physiological responses to the social environment; however, emerging evidence suggests that social interactions may not always affect circulating hormones as initially hypothesized, at least not in many songbirds (Goymann, 2009; Goymann et al., 2007). Here, we staged a series of social interactions with free-living male juncos to ask which social conditions, if any, bring about changes in T or CORT. Our findings reveal significantly elevated T only in males that experienced a stimulated territorial intrusion while females were re-nesting following removal of their chicks (simulated predation). Males did not elevate T after identical STIs at other stages of breeding, nor after simulated courtship interactions. We also found that neither the duration of STIs nor the sensory modality was related to whether or not males elevated T in response to an STI. These failures to elevate T occurred despite clear evidence that males can elevate T in response to exogenous GnRH. While some species appear to socially modulate CORT instead of T (Landys et al., 2007), we found no evidence of such a CORT response. Thus, under most circumstances, male dark-eyed juncos do not appear to socially modulate T or CORT.
These findings resolve methodological concerns from prior examinations of the socially mediated component of the challenge hypothesis (Rmale-male) in juncos (McGlothlin et al., 2008; Rosvall et al., 2012b), and they therefore make it clear that modulation of T induced by social challenges is either absent or highly context specific (i.e. it occurs only while re-nesting). We further extend these conclusions from the realm of social challenges and into that of social opportunities by showing that males also do not elevate T in response to simulated courtship interactions. Like other recent additions to the growing list of exceptions to the challenge hypothesis (e.g. Apfelbeck and Goymann, 2011), the life history of the junco is similar to species that do elevate T in response to social challenges. Further, there are well-established linkages between T and phenotypic evolution in this species (Ketterson et al., 2009; Ketterson et al., 1992; McGlothlin and Ketterson, 2008; McGlothlin et al., 2005; McGlothlin et al., 2010), highlighting important and unresolved issues regarding the socially mediated component of the challenge hypothesis (Rmale-male) and how it relates to the evolution of hormone-mediated traits.
4.1 Methodology does not affect social modulation of hormones
By using a variety of social treatments, we were able to discount the role of several methodological variants that have made some prior tests of the challenge hypothesis difficult to interpret, and in doing so, our results demonstrate that presence or absence of modulation of T in male juncos relates more to a biological issue (re-nesting) than a methodological one. For example, our use of live lures and taxidermic mounts showed that the sensory modality of the simulated intruder did not affect hormone signaling. Paired with prior work that measured hormone responses to auditory playbacks alone (Rosvall et al., 2012b), these data show that neither playback, nor playback accompanied by a visual stimulus alters T or CORT signaling in this system.
In addition, we varied the length of exposure to the stimulated territorial intrusion, from approximately 1 min in controls to approximately 12.5, 25, and 60 minutes in other treatments. Most other studies that report a T rise in response to a social challenge find it within this timeframe (Gleason et al., 2009; Goymann, 2009; Goymann et al., 2007; Hirschenhauser et al., 2003; Oliveira et al., 2002; but see Wikelski et al., 1999), and exogenous treatment with LH or GnRH also leads to a sharp T rise within this timeframe (Deviche et al., 2012; Jawor et al., 2006), suggesting that that the lack of T response seen here is unlikely to be a consequence of sampling before T has elevated, or after return to baseline.
Some studies suggest that a T response to an aggressive challenge may require that a focal male wins the interaction (Apfelbeck et al., 2011; Gleason et al., 2009; Oliveira, 2009), and the nature of STIs (protracted singing from an intruder without retreat) might not sufficiently mimic natural contests that yield a winner and loser. Among males sampled ~25 min after the intrusion began, we varied whether the live male lure was visible and whether song playback continued during the entire trial or not, providing an opportunity for an apparent retreat on the part of the simulated intruder, though we did not detect T elevation in any of these treatments.
By comparing control and socially challenged males to males treated with GnRH challenges, our findings lend support to the hypothesis that a lack of social T elevation was also unrelated to physiological inability to elevate T (Apfelbeck and Goymann, 2011). Furthermore, T levels seen in males that were re-nesting were roughly an order of magnitude higher than date-matched control samples, suggesting that males may be capable of elevating T, but they do not do so in responses to most STIs.
A final methodological consideration in tests of the challenge hypothesis is whether controls accurately represent unstimulated males, given that free-living territorial songbirds hardly live in social isolation. In this study, T levels measured in control males are comparable to established seasonal profiles of baseline T in this species from other research. Specifically, baseline T levels were ~3–4 ng/mL at the beginning of the study, and they declined to ~1 ng/mL near the end of the study (Ketterson and Nolan, 1992). We note that the trend towards slightly higher T in the Phase 1 control males is largely driven by one male whose T was nearly two-fold higher than any other Phase 1 male (18.4 ng/mL). Without this point, the back-transformed mean for this group is only 3.3 ng/mL.
4.2 Under what circumstances do males elevate T and why?
The challenge hypothesis predicts that males elevate T in response to a social challenge because elevated T levels are adaptive in the face of social instability (Wingfield et al., 1990). Hypotheses posed to explain the adaptive advantage of a socially insensitive HPG axis have pointed to single-broodedness, short breeding seasons, or the essential nature of paternal care (Goymann et al., 2007; Wingfield and Hunt, 2002) because, in each of these circumstances, there is a greater premium for males to focus on caring for young instead of vying for females or territorial position, and elevated T can interfere with parental care. Juncos from our population, however, are not single-brooded, nor do they have essential paternal care. Pairs regularly raise 2–3 broods per season, and the breeding season lasts approximately 4 months (Nolan et al., 2002). In addition, although male juncos provision nestlings and defend them against predators (Nolan et al., 2002), experimental male removals have shown that females can almost fully compensate for reduced paternal care (Wolf et al., 1990), suggesting that juncos do not have ‘essential’ paternal care. Thus, based upon the life history of the junco, we should expect males to elevate T in response to social challenges, but under most circumstances they did not.
In the one situation in which males did elevate T, we cannot yet distinguish between two options: (1) that systemic T elevation in response to STI is highly stage-specific, such that males socially elevate T only while their mates are re-nesting, or (2) that social challenges in the form of STIs have no direct effect on T signaling, and the elevated T seen in these males was entirely a consequence of the loss of their brood and subsequent fertility of their mates. Full resolution of this issue will require future work to compare hormonal response to an STI in males whose mates are re-nesting with unchallenged controls in this same breeding stage. However, certain results from this study and others shed light on the relative likelihood of stage-dependent social modulation of T vs. stage-dependent – but socially independent – modulation of T.
On the one hand, stage-dependent modulation of T is quite common. For example, males often show seasonal changes in T, with peaks reported while males are establishing territories and attracting mates, and again as males begin their second broods (Wingfield et al., 1990). Male juncos indeed have an early season peak of T that is on par with the level of T we found in the re-nesting males (Deviche et al., 2000; Ketterson et al., 2005), though we are not aware of data that address whether male juncos elevate T after a failed nesting attempt without a concomitant social challenge. Data from other North American sparrows also demonstrate an elevation of T when re-nesting after a failed attempt (Wingfield and Farner, 1979; Wingfield and Goldsmith, 1990), even though such an elevation does not occur when re-nesting is after a successful brood (Wingfield and Moore, 1987). These data suggest that the presence of chicks induces a state of quiescence for the HPG axis (Apfelbeck and Goymann, 2011; Calisi et al., 2011), and removal of chicks in the form of predation is sufficient to release males from this dampened HPG axis, resulting in stage-dependent modulation of T.
To what degree, then, might this stage-dependent modulation of T be driven by stage-dependent social interactions? Seasonal or breeding stage variation in T may reflect the sum of all social and environmental effects on T; however, direct comparisons of seasonal androgen responses and male-male social androgen responses show that the two are not necessarily related (Goymann, 2009; Goymann et al., 2007), suggesting that other social or environmental effects on T may influence seasonal patterns of T secretion, independently of male-male challenges. For example, males have been known to elevate T when presented with a fertile female (see Goymann, 2009 and references therein). In our study, however, we found that experimental exposure to a simulated courtship interaction did not yield an acute hormonal response, even though males were motivated to interact with the female lure, as evidenced by our observation that all SCI-stimulated males performed courtship behaviors including low-amplitude vocalizations, ptiloerection, tail-spreading, etc. (Rosvall, pers. obs.). Thus, these simulated courtship interactions were not sufficient to bring about T elevation, though longer exposure times should be investigated in the future, particularly since there are other species in which females stimulate hormonal changes in males even when male-male interactions do not (Moore, 1982).
4.3 Implications for the evolution of T-mediated traits and social modulation of T
As described above, adding the dark-eyed junco to the list of exceptions to the acute (social) component of the challenge hypotheses is surprising in light of the species life history. One possibility for this discrepancy relates to the evolutionary history of the dark-eyed junco. The species complex is thought to have diversified rapidly in the last 10,000 years (Mila et al., 2007), and most subspecies breed in high altitude or high latitude forests. Reproductive timing has been shown to be quite plastic in other junco subspecies (Yeh and Price, 2004), and thus, it is feasible that multi-broodedness is a derived state in the junco, and hormonal mechanisms have not yet evolved alongside the long breeding seasons that now characterize this population and subspecies. Another interpretation is that breeding season length and the extent of male parental care may not drive evolutionary changes in social modulation of hormones. To date, however, attempts to relate interspecific variability to other life history variables have met with limited success (Goymann, 2009), highlighting the need for more comparative study.
The role of the junco in the field of evolutionary endocrinology is also worth considering in light of our findings. McGlothlin et al. (2008) found that the degree to which a male elevates T in response to a GnRH challenges was significantly and positively correlated with T levels measured immediately following an STI performed while females were re-nesting after simulated predation of their chicks. At the time, there were fewer known exceptions to the challenge hypothesis, and these results were interpreted as supporting the hypothesis that Rpotential is a good proxy for the natural elevation in T seen in response to male-male social challenges (Rmale-male). Other studies also showed that GnRH-induced T levels or the GnRH-induced rise in T predicts individual variation in a number of male phenotypes, including aggression (McGlothlin et al., 2008; McGlothlin et al., 2007), further suggesting that the ability to elevate T may underlie natural variation in phenotype.
Our current results show that social modulation of T is largely absent in the junco, except possibly during the period of time when females are re-nesting. Thus, the established links between Rpotential and aggression are unlikely to be caused by the lasting effects of prior social elevations of T that have primed animals to be more or less aggressive. We note, however, that individual variation in T levels and in neural sensitivity to T both co-vary with individual differences in aggression (Rosvall et al., 2012a), suggesting that T still plays a role in the regulation of aggression, even if T is not acutely regulated by male-male social interactions. Rather, it may be that aggressiveness co-varies with individual differences in how the HPG axis responds to seasonally varying circumstances (see Goymann, 2009; Goymann et al., 2007), such as shifting demands related to sperm production or mate guarding. In addition, genetic or developmental effects may shape both HPG axis reactivity and aggression, without a causal link between the two (McGlothlin et al., 2008; McGlothlin et al., 2010). Rpotential is repeatable among males (Jawor et al., 2006), it co-varies with the degree to which males elevate T in at least one naturally occurring life history transition (i.e. re-nesting after predation of chicks), and it relates to fitness in wild juncos (Cain and Ketterson, 2012; McGlothlin et al., 2010), suggesting that understanding variation in androgen responsiveness is still an important issue, even in species where socially mediated changes in T are at most highly context-specific, if not absent.
4.4 The role of corticosterone
Recent work suggests that some species that do not socially modulate T secretion might instead show a CORT response to an acute social challenge (Landys et al., 2007; Landys et al., 2010; Van Duyse et al., 2004). We did not detect social modulation of CORT in this study, though we note that latencies from capture to completion of blood sampling were marginally longer that what is ideal to assess baseline CORT (Angelier et al., 2010). Thus, values reported here may not reflect baseline, as indicated by the significant effect of handling time we observed on CORT levels. In addition, all control males were also captured using targeted playback, though prior work in this system has shown that short playbacks have no detectable effect on CORT signaling (Rosvall et al., 2012b). Further, in a post-hoc comparison using all Phase 3 STIs of various durations in which males were bled within 3.5 min of capture (i.e. a timeframe in which minimal handling-induced CORT elevation should occur), we found no apparent differences between CORT levels from controls, males captured after ~15 min of STI, and males captured after ~30 min of STI. Thus, while we cannot fully discount the possibility of transient or marginal social elevation in CORT, it is clear that juncos do not show the robust CORT response seen in some other species (e.g. Van Duyse et al., 2004). Importantly, juncos are not characterized by the life history traits that have been associated with social modulation of CORT in previous studies (Goymann, 2009; Goymann et al., 2007; Landys et al., 2007), and thus, the lack of CORT responses seen here is consistent with existing hypotheses regarding hormonal response to social challenge.
Our results also revealed that T levels from males captured on their territories and bled immediately thereafter (all social treatments and controls) were significantly higher than those from males that were transported to a central laboratory prior to blood sampling and GnRH challenge. We hypothesized that these differences were due to significant differences in handling times between these two sets of males (3.6 ± 0.2 min vs. 38.4 ± 4.3 min). Consistent with this view, pre-GnRH challenge CORT levels were high (Figure 3D), and we observed a significant negative relationship between T and CORT in the pre-GnRH challenge samples (Figure 4). On the other hand, CORT levels were unrelated to post-GnRH challenge T levels, suggesting that handling stress may not directly impact the ability to respond to GnRH, but instead may relate to stress-related effects on the balance between HPG activation versus clearance or metabolism of T. Indeed, the HPA axis is known to affect HPG axis functioning (e.g. Deviche et al., 2010), but more work is needed to understand these interactions.
Highlights.
When and why do males socially modulate testosterone (T)?
We staged interactions varying in sensory modality, timecourse, and sex of stimuli
We found that male juncos largely did not elevate T to staged social interactions
Acknowledgments
The authors thank the University of Virginia Mountain Lake Biological Station, Mountain Lake Hotel, and Jefferson National Forest for access to field sites; P Aleixandre, CM Bergeon Burns, NM Gerlach, B Milá, E Leibgold, R Stewart, S Wanamaker, and J Welklin for assistance in the field and/or lab; and JL Goodson, JW McGlothlin, RP Phillips, and G Hendrick for helpful feedback and discussion.
Funding: Support from NIH NRSA (F32HD068222) and R21 (HD073583) to KAR, NSF graduate research fellowship and DDIG (IOS-1011145) to DGR, NSF (IOS-0820055) to EDK, and the NIH training grant ‘Common Themes in Reproductive Diversity’ (T32HD049336) to EDK and MPP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis EA, Aaron DC, Vasquez RA, Wingfield JC. Seasonal Modulation of Testosterone during Breeding of the Rufous-Collared Sparrow (Zonotrichia capensis australis) in Southern Patagonia. Physiol Biochem Zool. 2013;86:782–790. doi: 10.1086/673868. [DOI] [PubMed] [Google Scholar]
- Angelier F, Tonra CM, Holberton RL, Marra PP. How to capture wild passerine species to study baseline corticosterone levels. Journal of Ornithology. 2010;151:415–422. [Google Scholar]
- Apfelbeck B, Goymann W. Ignoring the challenge? Male black redstarts (Phoenicurus ochruros) do not increase testosterone levels during territorial conflicts but they do so in response to gonadotropin-releasing hormone. Proceedings of the Royal Society B: Biological Sciences. 2011;278:3233–3242. doi: 10.1098/rspb.2011.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfelbeck B, Stegherr J, Goymann W. Simulating winning in the wild - The behavioral and hormonal response of black redstarts to single and repeated territorial challenges of high and low intensity. Horm Behav. 2011;60:565–571. doi: 10.1016/j.yhbeh.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Cain KE, Ketterson ED. Competitive females are successful females; phenotype, mechanism, and selection in a common songbird. Behav Ecol Sociobiol. 2012;66:241–252. doi: 10.1007/s00265-011-1272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisi RM, Diaz-Munoz SL, Wingfield JC, Bentley GE. Social and breeding status are associated with the expression of GnIH. Genes Brain Behav. 2011;10:557–564. doi: 10.1111/j.1601-183X.2011.00693.x. [DOI] [PubMed] [Google Scholar]
- Clotfelter ED, O'Neal DM, Gaudioso JM, Casto JM, Parker-Renga IM, Snajdr EA. Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Horm Behav. 2004;46:171–178. doi: 10.1016/j.yhbeh.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Cramer ERA. Are Androgens Related to Aggression in House Wrens? Ethology. 2012;118:975–983. [Google Scholar]
- Deviche P, Sharp PJ, Dawson A, Sabo J, Fokidis B, Davies S, et al. Up to the challenge? Hormonal and behavioral responses of free-ranging male Cassin's Sparrows, Peucaea cassinii to conspecific song playback. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Deviche P, Wingfield JC, Sharp PJ. Year-class differences in the reproductive system, plasma prolactin and corticosterone concentrations, and onset of prebasic molt in male dark-eyed juncos (Junco hyemalis) during the breeding period. Gen Comp Endocrinol. 2000;118:425–435. doi: 10.1006/gcen.2000.7478. [DOI] [PubMed] [Google Scholar]
- Deviche PJ, Hurley LL, Fokidis HB, Lerbour B, Silverin B, Sabo J, et al. Acute stress rapidly decreases plasma testosterone in a free-ranging male songbird: Potential site of action and mechanism. Gen Comp Endocrinol. 2010;169:82–90. doi: 10.1016/j.ygcen.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Dufty AM, Wingfield JC. Endocrine response of captive male brown-headed cowbirds to intrasexual social cues. Condor. 1990;92:613–620. [Google Scholar]
- Enstrom DA, Ketterson ED, Nolan V. Testosterone and mate choice in the dark-eyed junco. Anim Behav. 1997;54:1135–1146. [Google Scholar]
- Fokidis HB, Orchinik M, Deviche P. Context-specific territorial behavior in urban birds: No evidence for involvement of testosterone or corticosterone. Horm Behav. 2011;59:133–143. doi: 10.1016/j.yhbeh.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Gleason ED, Fuxjager MJ, Oyegbile TO, Marler CA. Testosterone release and social context: When it occurs and why. Front Neuroendocrinol. 2009;30:460–469. doi: 10.1016/j.yfrne.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Goymann W. Social modulation of androgens in male birds. Gen Comp Endocrinol. 2009;163:149–157. doi: 10.1016/j.ygcen.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Goymann W, Landys MM, Wingfield JC. Distinguishing seasonal androgen responses from male-male androgen responsiveness - Revisiting the Challenge Hypothesis. Horm Behav. 2007;51:463–476. doi: 10.1016/j.yhbeh.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Greives TJ, McGlothlin JW, Jawor JM, Demas GE, Ketterson ED. Testosterone and innate immune function inversely covary in a wild population of breeding Dark-Eyed Juncos (Junco hyemalis) Funct Ecol. 2006;20:812–818. [Google Scholar]
- Hegner RE, Wingfield JC. Effects of experimental manipulation of testosterone levels on parental investment and breeding success in male house sparrows. Auk. 1987;104:462–469. [Google Scholar]
- Hirschenhauser K, Oliveira RF. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim Behav. 2006;71:265–277. [Google Scholar]
- Hirschenhauser K, Winkler H, Oliveira RF. Comparative analysis of male androgen responsiveness to social environment in birds: the effects of mating system and paternal incubation. Horm Behav. 2003;43:508–519. doi: 10.1016/s0018-506x(03)00027-8. [DOI] [PubMed] [Google Scholar]
- Jawor JM, McGlothlin JW, Casto JM, Greives TJ, Snajdr EA, Bentley GE. Seasonal and individual variation in response to GnRH challenge in male dark-eyed juncos (Junco hyemalis) Gen Comp Endocrinol. 2006;149:182–189. doi: 10.1016/j.ygcen.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Atwell JW, McGlothlin JW. Phenotypic integration and independence: Hormones, performance, and response to environmental change. Integr Comp Biol. 2009;49:365–379. doi: 10.1093/icb/icp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V. Hormones and life histories - an integrative approach. Am Nat. 1992;140:S33–S62. doi: 10.1086/285396. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V, Sandell M. Testosterone in females: Mediator of adaptive traits, constraint on sexual dimorphism, or both? Am Nat. 2005;166:S85–S98. doi: 10.1086/444602. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V, Wolf L, Ziegenfus C. Testosterone and avian life histories - effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis) Am Nat. 1992;140:980–999. [Google Scholar]
- Landys MM, Goymann W, Raess M, Slagsvold T. Hormonal responses to male-male social challenge in the blue tit Cyanistes caeruleus: Single-broodedness as an explanatory variable. Physiol Biochem Zool. 2007;80:228–240. doi: 10.1086/510564. [DOI] [PubMed] [Google Scholar]
- Landys MM, Goymann W, Schwabl I, Trapschuh M, Slagsvold T. Impact of season and social challenge on testosterone and corticosterone levels in a year-round territorial bird. Horm Behav. 2010;58:317–325. doi: 10.1016/j.yhbeh.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Lynn SE. Behavioral insensitivity to testosterone: Why and how does testosterone alter paternal and aggressive behavior in some avian species but not others? Gen Comp Endocrinol. 2008;157:233–240. doi: 10.1016/j.ygcen.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Marler CA, Oyegbile TO, Plavicki J, Trainor BC. Response to Wingfield's commentary on "A continuing saga: The role of testosterone in aggression". Horm Behav. 2005;48:256–258. doi: 10.1016/j.yhbeh.2005.05.009. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Greives TJ, Casto JM, Phillips JL, Ketterson ED. Hormones and honest signals: males with larger ornaments elevate testosterone more when challenged. J Evol Biol. 2008;21:39–48. doi: 10.1111/j.1420-9101.2007.01471.x. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Ketterson ED. Natural variation in a testosterone-mediated trade off between mating effort and parental effort. Am Nat. 2007;170:864–875. doi: 10.1086/522838. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Ketterson ED. Hormone-mediated suites as adaptations and evolutionary constraints. Phil Trans Royal Soc B. 2008;363:1611–1620. doi: 10.1098/rstb.2007.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlothlin JW, Parker PG, Nolan V, Ketterson ED. Correlational selection leads to genetic integration of body size and an attractive plumage trait in dark-eyed juncos. Evolution. 2005;59:658–671. [PubMed] [Google Scholar]
- McGlothlin JW, Whittaker DJ, Schrock SE, Gerlach NM, Jawor JM, Snajdr EA. Natural selection on testosterone production in a wild songbird population. Am Nat. 2010;175:687–701. doi: 10.1086/652469. [DOI] [PubMed] [Google Scholar]
- Mila B, McCormack JE, Castaneda G, Wayne RK, Smith TB. Recent postglacial range expansion drives the rapid diversification of a songbird lineage in the genus Junco. Proceedings of the Royal Society B-Biological Sciences. 2007;274:2653–2660. doi: 10.1098/rspb.2007.0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore IT, Wada H, Perfito N, Buch DS, Hahn TP, Wingfield JC. Territoriality and testosterone in an equatorial population of rufous-collared sparrows, Zonotrichia capensis. Anim Behav. 2004a;67:411–420. [Google Scholar]
- Moore IT, Wingfield JC, Brenowitz EA. Plasticity of the avian song control system in response to localized environmental cues in an equatorial songbird. J Neurosci. 2004b;24:10182–10185. doi: 10.1523/JNEUROSCI.3475-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MC. Hormonal response of free-living male white-crowned sparrows to experimental manipulation of female sexual-behavior. Horm Behav. 1982;16:323–329. doi: 10.1016/0018-506x(82)90030-7. [DOI] [PubMed] [Google Scholar]
- Nolan V, Ketterson ED, Cristol DA, Rogers CM, Clotfelter ED, Titus RC, et al. Dark-eyed Junco (Junco hyemalis) In: Poole A, editor. The Birds of North America Online, Cornell Lab of Ornithology. Ithaca, NY. USA: 2002. [Google Scholar]
- Oliveira RF. Social behavior in context: Hormonal modulation of behavioral plasticity and social competence. Integr Comp Biol. 2009;49:423–440. doi: 10.1093/icb/icp055. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AVM. Social modulation of androgen levels in male teleost fish. Comp Biochem Physiol B. 2002;132:203–215. doi: 10.1016/s1096-4959(01)00523-1. [DOI] [PubMed] [Google Scholar]
- Reed WL, Clark ME, Parker PG, Raouf SA, Arguedas N, Monk DS. Physiological effects on demography: A long-term experimental study of testosterone's effects on fitness. Am Nat. 2006;167:667–683. doi: 10.1086/503054. [DOI] [PubMed] [Google Scholar]
- Reichard DG, Rice RJ, Schultz EM, Schrock SE. Low-amplitude songs produced by male dark-eyed juncos (Junco hyemalis) differ when sung during intra- and inter-sexual interactions. Behaviour. 2013;150:1183–1202. [Google Scholar]
- Reichard DG, Rice RJ, Vanderbilt CC, Ketterson ED. Deciphering Information Encoded in Birdsong: Male Songbirds with Fertile Mates Respond Most Strongly to Complex, Low-Amplitude Songs Used in Courtship. Am Nat. 2011;178:478–487. doi: 10.1086/661901. [DOI] [PubMed] [Google Scholar]
- Rosvall KA, Bergeon Burns CM, Barske J, Goodson JL, Schlinger BA, Sengelaub DR, et al. Neural sensitivity to sex steroids predicts individual differences in aggression: implications for behavioural evolution. Proceedings of the Royal Society B: Biological Sciences. 2012a;279:3547–3555. doi: 10.1098/rspb.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall KA, Reichard DG, Ferguson SM, Whittaker DJ, Ketterson ED. Robust behavioral effects of song playback in the absence of testosterone or corticosterone release. Horm Behav. 2012b;62:418–425. doi: 10.1016/j.yhbeh.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriba M, Goymann W. The decoy matters! Hormonal and behavioural differences in the reaction of territorial European robins towards stuffed and live decoys. Gen Comp Endocrinol. 2008;155:511–516. doi: 10.1016/j.ygcen.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Scriba MF, Goymann W. European robins (Erithacus rubecula) lack an increase in testosterone during simulated territorial intrusions. Journal of Ornithology. 2010;151:607–614. [Google Scholar]
- Van Duyse E, Pinxten R, Darras VM, Arckens L, Eens M. Opposite changes in plasma testosterone and corticosterone levels following a simulated territorial challenge in male great tits. Behaviour. 2004;141:451–467. [Google Scholar]
- Wikelski M, Hau M, Wingfield JC. Social instability increases plasma testosterone in a year round territorial neotropical bird. Proc Roy Soc B. 1999;266:551–556. [Google Scholar]
- Wingfield JC. Environmental and endocrine control of reproduction in the song sparrow, Melospiza melodia. 2. Agonistic interactions as environmental information stimulating secretion of testosterone. Gen Comp Endocrinol. 1984;56:417–424. doi: 10.1016/0016-6480(84)90084-4. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Ball GF, Dufty AM, Hegner RE, Ramenofsky M. Testosterone and aggression in birds. Am Sci. 1987;75:602–608. [Google Scholar]
- Wingfield JC, Farner DS. Some endocrine correlates of renesting after loss of clutch or brood in the white-crowned sparrow, Zonotrichia leucophrys gambelii. Gen Comp Endocrinol. 1979;38:322–331. doi: 10.1016/0016-6480(79)90066-2. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Goldsmith AR. Plasma levels of prolactin and gonadal steroids in relation to multiple-brooding and renesting in free-living populations of the song sparrow, Melospiza melodia. Horm Behav. 1990;24:89–103. doi: 10.1016/0018-506x(90)90029-w. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Ball GF. The Challenge Hypothesis - theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat. 1990;136:829–846. [Google Scholar]
- Wingfield JC, Hunt KE. Arctic spring: hormone-behavior interactions in a severe environment. Comp Biochem Physiol B. 2002;132:275–286. doi: 10.1016/s1096-4959(01)00540-1. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Lynn SE, Soma KK. Avoiding the 'costs' of testosterone: Ecological bases of hormone-behavior interactions. Brain Behav Evol. 2001;57:239–251. doi: 10.1159/000047243. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Moore MC. Hormonal, social, and environmental factors in the reproductive biology of free-living male birds. In: Crews D, editor. Psychobiology of reproductive behavior. An evolutionary perspective. Englewood Cliffs, New Jersey: Prentice Hall, Inc.; 1987. pp. 148–175. [Google Scholar]
- Wingfield JC, Newman AL, Hunt GL, Farner DS. Endocrine aspects of female-female pairing in the western gull (Larus occidentalis wymani) Anim Behav. 1982;30:9–22. [Google Scholar]
- Wingfield JC, Wada M. Changes in plasma-levels of testosterone during male-male interactions in the song sparrow, Melospiza melodia - time course and specificity of response. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 1989;166:189–194. [Google Scholar]
- Wolf L, Ketterson ED, Nolan V. Behavioral response of female dark-eyed juncos to the experimental removal of their mates - Implications for the evolution of male parental care. Anim Behav. 1990;39:125–134. [Google Scholar]
- Yeh PJ, Price TD. Adaptive Phenotypic Plasticity and the Successful Colonization of a Novel Environment. The American Naturalist. 2004;164:531–542. doi: 10.1086/423825. [DOI] [PubMed] [Google Scholar]