Abstract
Background
Given that nicotine modulates amino acid neurotransmission, we sought to examine the impact of nicotine on cortical γ-aminobutyric acid (GABA) levels in male and female smokers.
Methods
Healthy nicotine-dependent men (n = 10) and women (n = 6) underwent proton magnetic resonance spectroscopy (1H-MRS) to measure occipital cortex GABA concentrations. A subset of the smoking men (n = 5) underwent 1H-MRS scans before and after 48 hours of smoking abstinence, whereas each of the women were scheduled to undergo pre- and postabstinence scans during the follicular and luteal phases of one menstrual cycle. Healthy nonsmoking men (n = 7) and women (n = 13) underwent 1H-MRS for comparison.
Results
Short-term abstinence had no significant effect on cortical GABA concentrations in either men or women. There was, however, a significant effect of sex, diagnosis (smoker/nonsmoker), and menstrual cycle phase on cortical GABA levels, such that female smokers experienced a significant reduction in cortical GABA levels during the follicular phase and no cyclicity in GABA levels across the menstrual cycle, whereas cortical GABA levels were similar in smoking and nonsmoking men.
Conclusions
Taken together with previous 1H-MRS data showing abnormalities in occipital cortex GABA concentrations in several affective disorders, our preliminary finding that nicotine modulation of GABA levels varies by sex provides a further rationale for investigating the impact of nicotine on central GABAergic function as a potential risk factor for women to experience depressive symptoms during smoking cessation.
Keywords: Nicotine, smoking, abstinence, menstrual cycle, GABA, sex
There are several noteworthy differences between men and women with regard to smoking behavior, but ultimately none are as worrisome as the disparity in ability to quit smoking. Many explanations as to why women are less successful in their abstinence attempts have been proffered; however, the observation that women are more likely to experience the emergence of depressive symptoms during smoking cessation (Killen et al 2003; Levine et al 2003; Smith et al 2003), a known risk factor for smoking relapse (Benowitz and Hatsukami 1998; Glassman et al 1990; Murphy et al 2003; Pomerleau et al 2001), might be a critical contributor to this sex-specific recidivism.
Although nicotine has pronounced effects on a number of neurotransmitter systems (for review, see Mansvelder and McGehee 2002), recent findings that γ-aminobutyric acid (GABA) neuronal dysregulation might play a role in the pathogenesis of several affective disorders, including major depression (melancholic sub-type) and premenstrual dysphoric disorder (PMDD) (Epperson et al 2002; Sanacora et al 1999, 2002), suggest that nicotinic modulation of GABAergic function might mediate, albeit in part, nicotine's effects on mood.
Nicotine modulation of nicotinic acetylcholine receptors on GABAergic interneurons induces GABA release (Fuxe et al 1989), which could lead to downstream alterations in GABA synthesis and/or GABAA receptor activity. Nicotine, at high doses, also enhances neurosteroidogenesis in rodents, leading to increased production of the potent GABAA receptor agonist allopregnanolone (ALLO) (Porcu et al 2003). Chronic exposure to ALLO results in downregulation and desensitization of the GABAA receptor (Smith et al 1998). Finally, nicotine alters metabolism of the ovarian hormone estradiol (Irwin et al 1994), which has excitatory effects in the central nervous system (Mellon and Griffin 2002).
Therefore, we sought to determine the impact of nicotine and a brief period of smoking abstinence on central GABAergic function, using proton magnetic resonance spectroscopy (1HMRS) to measure occipital GABA concentrations in a group of healthy smoking men and women. Given that women experience menstrual cycle–related fluctuations in cortical GABA levels and have greater peripheral sources of progesterone for conversion to ALLO (Epperson et al 2002), we studied women across the menstrual cycle and anticipated that female smokers would be more vulnerable to potential nicotine-induced alterations in cortical GABA levels than male smokers.
Methods and Materials
Subjects
Ten men (aged 37 ± 18 years [mean ± SD]) and six women (aged 35 ± 13 years) who smoked 20–40 cigarettes daily for at least 1 year and who had no present or past history of psychiatric or substance (excepting nicotine) abuse/dependence disorders according to the Structured Clinical Interview for Diagnosis–Nonpatient version (SCID-NP; Spitzer et al 1990) were enrolled. Subjects were recruited through paid advertisements and gave written informed consent for participation, using forms and procedures approved by the medical school's Human Investigation Committee. Of the 16 subjects, 13 were Caucasian, 2 were Hispanic, and 1 was African American. The age of onset of smoking was similar between men (19.4 ± 4.2 years) and women (17.2 ± 2.9 years). The mean score for the entire group on the Fagerstrom Test of Nicotine Dependence was 4.69 ± 2.0 and on the Clinical Epidemiology Scale for Depression was 3.6 ± 3.5. Mean baseline cotinine levels were 227.5 ng/mL.
A group of healthy nonsmoking men (n = 7) underwent 1H-MRS concurrent with the smokers, whereas for women, GABA data collected from a historical sample of nonsmoking women (n = 13) with identical MRS techniques was used for comparison. The healthy nonsmoking men and women were aged 33.8 ± 5.9 years and 30.8 ± 5.9 years, respectively. Healthy subjects had no personal or family history (first-degree relatives) of psychiatric or substance abuse disorders, according to a structured clinical interview with the SCID-NP and per subject report, respectively.
Timing of 1H-MRS Scans
Magnetic resonance spectroscopy scans were conducted before and after 48 hours of smoking abstinence (confirmed by expired CO level of <10 ppm, tested three times per day, and cotinine levels of <60 ng/mL, measured at the time of scanning). In order to examine the impact of smoking abstinence by menstrual cycle phase, women smokers underwent abstinence twice, once during the follicular phase (days 3–7; with first day of menstrual flow designated as day 1) and the midluteal phase (days 19–24; 5–9 days after ovulation as determined by urine lutenizing hormone test kit [Answer; Church and Dwight, Princeton, New Jersey]). Thus, female smokers were scheduled for four scans (once pre and post abstinence in the follicular and mid-luteal phases), and 5 of the 10 smoking men were, who were recruited to undergo abstinence, were scheduled for two scans (once pre and post abstinence). The remaining 5 male smokers and the 7 male nonsmokers underwent one 1H-MRS scan. Female nonsmokers from the historical sample were scanned in both the follicular and mid-luteal phases. Scan scheduling for the female smokers was counterbalanced with respect to menstrual cycle phase.
1H-MRS Methods
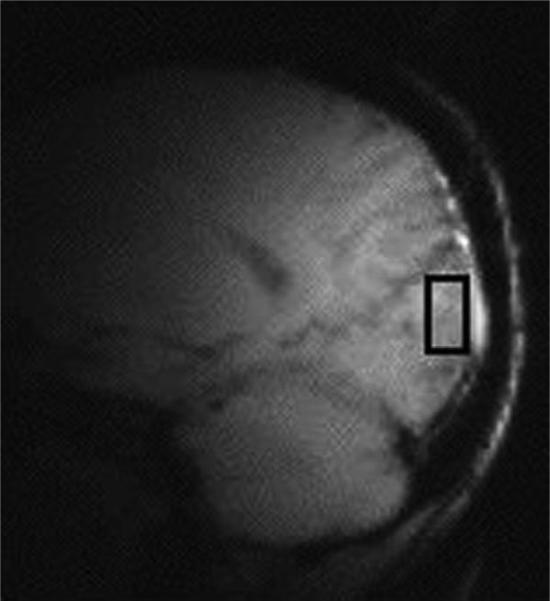
Proton MRS studies were performed with a 2.1-T magnet (Oxford Magnetic Technology, Oxford, England) with a 1-m bore and a Bruker Avance spectrometer (Bruker Instruments, Billerica, Massachusetts). Subjects lay supine with the occipital cortex against a 7-cm surface coil tuned to the 1H-MRS frequency of 89.67 MHz. Gradient-echo scout images of the subject's brain were obtained for subject positioning, and a 1.5 × 3 × 3-cm3 voxel centered on the midline of the occipital cortex, 2 cm deep from the dura, was chosen for MRS (Figure 1).
Figure 1.
Scout image of voxel in occipital cortex. Gradient-echo scout images of the subject's brain were obtained for subject positioning, and a 1.5 × 3 × 3-cm3 voxel centered on the midline of the occipital cortex, 2 cm deep from the dura, was chosen for magnetic resonance spectroscopy.
Automated first- and second-order shimming was applied in the volume of interest. Detection of the 3.0-ppm GABA C4 resonance was performed for 20 min with J-editing (Rothman et al 1993). Briefly, pairs of subspectra were obtained, one with a frequency-selective inversion pulse applied to the GABA C3 resonance, and one without the inversion pulse. The subspectra were subtracted to obtain difference spectra that contained GABA (total), which is the combined measure of GABA and the GABA-containing dipeptide homocarnosine. Localization was achieved with selective excitation, three-dimensional, image-selected, in vivo spectroscopy, outer volume suppression, and a surface spoiler coil. The spectral acquisition parameters were as follows: repetition time = 3.39 sec; echo time = 68 msec; sweep width = 15,000 Hz; and acquisition time = 510 msec. The free induction decay was zero-filled to 32 K, processed with 3-Hz Lorentzian broadening, and Fourier-transformed. The GABA signal was then integrated over a .30-ppm bandwidth at 3.00 ppm, and the creatine signal was integrated over a .20-ppm bandwidth at 3.00 ppm in the GABA-inverted spectrum. The GABA concentration was calculated by comparison of the GABA and creatine resonance areas, adjusting for differences in the efficiency of detection.
Steroid and Cotinine Measurements
Serum estradiol and progesterone levels were measured on each scan day to confirm menstrual cycle phase in all smoking and nonsmoking women. Estradiol and progesterone were assayed in a commercial laboratory by competitive immunoassay with a chemiluminescent substrate in a commercially available kit provided by Diagnostic Products (Los Angeles, California). Blood was obtained to measure cotinine, a major metabolite of nicotine, on each scan day. Exposure to nicotine is the major determinant of plasma or serum cotinine concentration. Because cotinine has a much longer half-life than nicotine, plasma cotinine concentrations are used as a measure of chronic nicotine exposure and less exactly to quantify smoking history. Cotinine levels were assayed with reversed-phase high-performance liquid chromatography. Between-day coefficients of variation, in routine use, at concentrations of 20 μg/L and 200 μg/L, were 11.6% and 6.6%, respectively.
Statistical Analysis
The impact of abstinence on GABA levels was assessed by paired t test, with pre–post differences for all subjects (male and female) combined. Gamma-aminobutyric acid data were analyzed to determine between sex differences by two-way analysis of variance (ANOVA) with smoking and sex as between-subject factors. To examine the differences in GABA levels between smoking and nonsmoking women across the menstrual cycle, we used a mixed-effects model with smoking as a between-subject factor, phase (follicular, midluteal) as a within-subject factor, and a subject random effect. Mixed-effects analysis allows for use of all available data on each subject (Brown and Prescott 1999).
Results
Impact of Abstinence on Cortical GABA Levels
Gamma-aminobutyric acid levels, expressed as millimoles per kilogram of brain tissue, were 1.13 ± .23 before abstinence and 1.15 ± .27 after abstinence in the five men who underwent smoking abstinence. Of the six smoking women enrolled in the study, GABA measurements were obtained from the follicular-phase preabstinence scans in three women (.97 ± .04) and from postabstinence scans in four women (.88 ± .13). Luteal-phase GABA levels were measured in all six women both before (1.01 ± .07) and after (1.07 ± .2) abstinence; however, luteal-phase GABA data from one woman had to be dropped because her progesterone level of .78 ng/mL indicated that she had not successfully ovulated. There was no significant difference between GABA levels before and after 48 hours of abstinence in men and women [t (10) = .15, p = .88]. Mixed-model analysis also confirmed that there were no differences pre- versus post-abstinence within each sex and when controlling for phase of menstrual cycle in women.
Impact of Sex on Cortical GABA Concentration
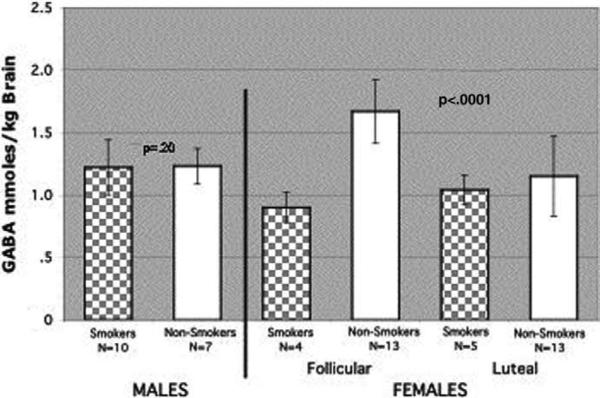
For these analyses, GABA levels from pre- and postabstinence scans were averaged for those individuals with data from both scans and either the pre- or postabstinence data were used when subject movement limited the ability to quantify GABA levels during one of the scans (Figure 2). In the two-way ANOVA of sex and smoking effects on GABA levels, there was a statistically significant sex × smoking interaction [F(1,29) = 23.37, p < .0001]. If we test for simple effects of sex among smokers and nonsmokers, we see that GABA levels in smoking women are significantly lower than in smoking men [t (29) = 2.56, p < .02], whereas for nonsmokers it is the reverse [t (29) = –4.55, p = .0001]. Post hoc comparisons showed that follicular-phase GABA levels were significantly higher in nonsmoking women (n = 13; 1.67 ± .25) than in smoking women (n = 4; .90 ± .32) [t (29) = 6.4, p < .0001] and that GABA levels were similar between smoking (n = 10; 1.22 ± .22) and nonsmoking men (n = 7; 1.23 ± .14) [t (29) = .1, p = .92] (Figure 3).
Figure 2.
Occipital cortex γ-aminobutyric acid (GABA) levels measured by proton magnetic resonance spectroscopy in male and female smokers and nonsmokers. Cortical GABA levels were obtained from 10 men. GABA levels from 5 of the 10 men with pre- and postabstinence scans were averaged. Follicular- and luteal-phase GABA levels for 6 female smokers represent the average of the pre- and postabstinence scans. GABA levels were obtained in 4 of the 6 women during the follicular phase and from 5 of the 6 women during the luteal phase. Two-way analysis of variance of sex and smoking effects on GABA levels revealed a significant sex × smoking interaction (p < .0001). Cortical GABA levels did not differ significantly between smoking and nonsmoking men (p = .2). Mixed-model analysis showed a significant menstrual cycle phase × smoking interaction (p = .0001).
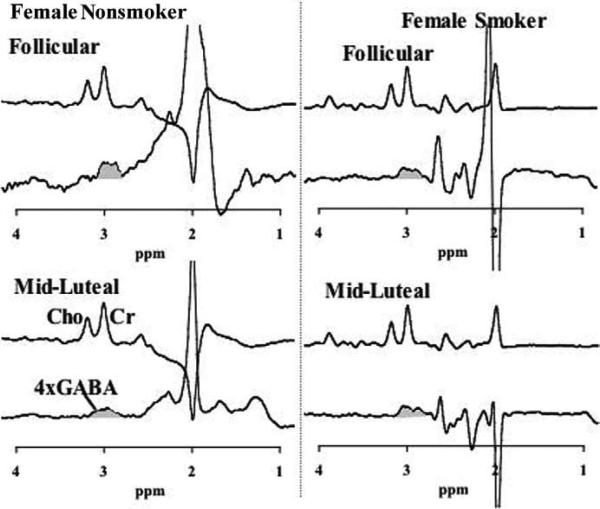
Figure 3.
Representative γ-aminobutyric acid (GABA) spectra in smoking and nonsmoking women. Representative spectra from a nonsmoking woman (left) and a smoking woman (right), for comparison of GABA in the follicular phase (top) and the midluteal phase (bottom). For each phase of the menstrual cycle, a subspectrum from the J-editing sequence is shown so that the creatine (Cr) resonance can be compared with the GABA resonance at 3.0 ppm, which is shaded grey. Cho, choline.
There was a significant interactive effect of sex and smoking [F(1,27) = 16.6, p = .0004] with respect to percentage of tissue in the voxel (% tissue). Female smokers had significantly lower × tissue (89.4% ± 2.2%) than female nonsmokers [94.7% ± 3.0; t (27) = –3.3, p = .003]. Conversely, male smokers (89.2% ± 2.5%) had significantly higher × tissue in the voxel than male nonsmokers [84.9% ± 3.6%; t (27) = –2.5, p = .02]. With respect to percentage of gray matter (% GM), there was a significant main effect of smoking [F(1,27) = 11.8, p = .002], with significantly lower % GM in smokers (47.5% ± 3.6%) than in nonsmokers (55.1% ± 5.5%). As expected given the % GM differences, there was significant sex × smoking interaction for percentage of white matter (% white matter) also [F(1,27) = 7.3, p = .01]. Male smokers (42.4% ± 4.4%) had significantly higher × white matter than male nonsmokers [30.5% ± 5.7%; t (27) = 4.0, p = .0004], whereas female smokers (40.1% ± 4.6%) were not significantly different from female nonsmokers [39.4% ± 5.3%; t (27) = .3, p = .8].
Impact of Menstrual Cycle Phase on Cortical GABA Concentrations
Menstrual cycle phase was confirmed by serum estradiol and progesterone levels measured on the day of each 1H-MRS scan. For the four women with GABA data in the follicular phase, mean estradiol and progesterone levels were 26.1 ± 5.8 pg/mL and .47 ± .06 ng/mL, respectively. For the five of the six women with successful GABA measurements during the luteal phase, mean estradiol and progesterone levels were 68.2 ± 38.7 pg/mL and 7.6 ± 4.4 ng/mL, respectively. In the mixed-model analysis, there was a statistically significant phase of menstrual cycle × smoking status interaction [F(1,9.5) = 12.9, p = .005]. Follicular-phase occipital cortex GABA concentrations (.90 ± .12) were similar to luteal-phase levels (.97 ± .16) [t (9.3) = .45, p = .67] in healthy smoking women. Healthy nonsmoking women, however, experienced a significant decline in cortical GABA levels from the follicular (1.67 ± .25) to the midluteal (1.15 ± .32) phase [t (10) = 5.70, p = .0002].
There were significant main effects of smoking for % tissue [F(1,18.5) = 22.5, p = .0002] in the voxel and for % GM [F(1,18.6) = 4.4, p = .05]. The interactions between phase and smoking were not significant [F(1,14.4) = .01, p = .93 and F(1,13.1) = .23, p = .64, respectively]. Smoking women had significantly lower % tissue in the voxel (90.2% ± 1.9%) than nonsmoking women (95.2% ± 2.7%) and significantly lower % GM (49.6% ± 4.2%) than nonsmoking women (54.9% ± 6.3%) across the menstrual cycle.
Cotinine Levels and GABA
Cotinine levels in men were 193 ± 75 ng/mL, whereas in the follicular and luteal phases they were 323 ± 164 ng/mL and 282 ± 196 ng/mL, respectively. Baseline cotinine levels were not significantly different between men and women in the follicular phase [two-sample unequal variances t test: t (4.01) = 1.46, p = .22] or luteal phase. Cortical GABA levels were not correlated with plasma cotinine levels in men or women (Spearman's correlation coefficient = .53, p = .22).
Discussion
Although 48 hours of smoking abstinence had no significant impact on GABA levels in the occipital cortex, baseline differences in GABA levels between smoking men and women were striking even in this small sample. During the follicular phase, when hormonal conditions are most like those found in men, cortical GABA levels were reduced in female smokers compared with male smokers. Although the preliminary nature of these findings warrants caution, the difference between smoking men and women compared with their sex-matched, nonsmoking counterparts is intriguing.
Compared with nonsmoking men, nicotine-dependent men did not seem to experience a change in cortical GABA concentrations secondary to nicotine dependence. In contrast, female smokers experienced a dramatic reduction in cortical GABA concentration, particularly in the follicular phase, and exhibited a complete loss of menstrual cycle cyclicity in cortical GABA concentrations. Because there was no sex difference in the number of cigarettes smoked per day, duration of nicotine addiction, or severity of depressive symptoms at baseline, these factors are unlikely to have contributed significantly to these findings. Furthermore, our confirmation of menstrual cycle phase by serum estradiol and progesterone levels concurrent with GABA measurements suggests that failure to detect changes in cortical GABA levels across the menstrual cycle in smoking women can not be attributed to a lack of hormonal cyclicity in that group.
Our finding of differences in % tissue in the voxel and % GM between smokers and nonsmokers could suggest that chronic nicotine exposure has an impact on the integrity of brain tissue; however, the relationship between male and female smokers and their nonsmoking counterparts with respect to % tissue was in the opposite direction for men and women, which makes the findings of tissue composition difficult to interpret. Although GABA concentrations are low in white matter (.05 mmol/kg) (Perry et al 1990), and creatine and phosphocreatine concentrations differ between grey and white matter (Hetherington et al 1994; Kreis et al 1993; Michaelis et al 1993; Petroff et al 1989; Pfefferbaum et al 1990), there were no menstrual cycle phase differences in the % tissue composition, % GM, or % white matter in the voxel of interest, which could explain the lack of menstrual cycle cyclicity in GABA levels in female smokers. In addition, the sum of creatine and phosphocreatine has been shown to remain constant even after death (Pontén et al 1973). Thus, it is highly unlikely that nicotine would change creatine levels (our chosen reference point for GABA) by 40% in one phase of the menstrual cycle and not another.
Several potential mechanisms for nicotine-induced alterations in GABA levels and cyclicity in female smokers could include alteration of estradiol metabolism (Irwin et al 1994) and/or induction of neurosteroidogenesis, with a resultant increase in ALLO levels (Porcu et al 2003). Regarding the latter, it is conceivable that women are more susceptible to nicotine's effects on neurosteroid production than men secondary to luteal-phase increases in the substrate (progesterone) for conversion to ALLO. That acute benzodiazepine administration (Goddard et al, in press) and luteal-phase increases in ALLO (Epperson et al 2002) are associated with reductions in cortical GABA levels, as measured by 1H-MRS in healthy subjects, suggests that agonist modulation of the GABAA receptor is likely to result in a downregulation of GABA synthesis.
Similar to our previous report of cortical GABA levels in women with PMDD, female smokers differ from control subjects most dramatically in the follicular phase. However, women who smoke do not experience an increase in GABA levels from the follicular to luteal phases. That only one of the PMDD subjects in our previous report was a light smoker (5–7 cigarettes per day), and none of the smoking women reported significant premenstrual mood changes (retrospectively or prospectively) suggests that the follicular-phase reductions in GABA seen in women with PMDD and female smokers can not be attributed to nicotine dependence or PMDD, respectively.
Although the mechanism for potential sex differences in nicotine modulation of cortical GABA levels cannot be determined by 1H-MRS alone, these data provide the rationale for future investigations examining the impact of sex and menstrual cycle phase on the relationship between nicotine dependence and central GABAergic function. Although it is not usually considered in mood disorders or substance dependence, the occipital cortex has been seen to be affected by many conditions, including nicotine administration (Domino et al 2000; Ghatan et al 1998), depression (Kumar et al 1993), and cocaine abuse (Hetherington et al 1999). Thus, as 1H-MRS techniques for sequential measurement of GABA levels are advanced, they should be applied to other brain areas known to be modulated by nicotine administration. Given that our period of abstinence was insufficient to determine the impact of smoking cessation on mood and/or GABA concentrations, future studies should include not only a larger subject pool, with healthy control subjects scanned concurrently, but also a longer period of smoking abstinence to further elucidate the relationship between nicotine, depression, and sex-specific recidivism.
Acknowledgments
This study was funded by the National Institute of Mental Health (K23 MH01830-01 (CNE) and the National Institute of Drug Abuse (P50DA13334) (SO). Additional support for this study came from the Department of Veterans Affairs (National Center for Posttraumatic Stress Disorder, Alcohol Research Center, REAP RO1 AA11321) and the National Institute of Alcohol Abuse and Alcoholism (P50 AA12870-01, KO2 AA 00261-01) and National Alliance for Research on Schizophrenia and Depression (GFM) and the Dana Foundation (CNE).
References
- Benowitz NL, Hatsukami D. Sex differences in the pharmacology of nicotine addiction. Addiction. 1998;3:383–404. doi: 10.1080/13556219871930. [DOI] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied Mixed Models in Medicine. John Wiley & Sons; New York: 1999. [Google Scholar]
- Domino EF, Minoshima S, Guthrie SK, Ohl L, Ni L, Koeppe RA, et al. Effects of nicotine on regional cerebral glucose metabolism in awake resting tobacco smokers. Neuroscience. 2000;101:277–282. doi: 10.1016/s0306-4522(00)00357-2. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, et al. Cortical y-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: A 1HMRS study. Arch Gen Psychiatry. 2002;59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Andersson K, Eneroth P, Harfstrand A, Agnati LF. Neuroendocrine actions of nicotine and of exposure to cigarette smoke: Medical implications. Psychoneuroendocrinology. 1989;14:19–41. doi: 10.1016/0306-4530(89)90054-1. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, Wahren J. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology. 1998;136:179–189. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, et al. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–1549. [PubMed] [Google Scholar]
- Goddard AW, Mason GF, Appel M, Rothman DL, Gueorguieva R, Behar KL, Krystal JH. Reduced cortical GABA neuronal response to benzodiazepine administration in panic disorder. Neuropsychopharmacology. doi: 10.1176/appi.ajp.161.12.2186. in press. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Mason GF, Pan JW, Ponder SL, Vaughn JT, Twieg DB, Pohost GM. Evaluation of cerebral gray white matter metabolite difference by spectroscopic imaging at 4.1T. Magn Reson Med. 1994;32:565– 571. doi: 10.1002/mrm.1910320504. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Telang F, Pan JW, Sammi M, Schuhlein D, Molina P, Volkow ND. Spectroscopic imaging of the updtake kinetics of human brain ethanol. Magn Reson Med. 1999;42:1019–1026. doi: 10.1002/(sici)1522-2594(199912)42:6<1019::aid-mrm5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Irwin RP, Lin SZ, Rogawski MA, Purdy RH, Paul SM. Steroid potentiation and inhibition of N-methyl-D-aspartate receptor mediated intracellular Ca(( responses: Structure activity studies. J Pharmacol Exp Ther. 1994;271:677–682. [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Schatzberg A, Hayward C, Varady A. Onset of major depression during treatment for nicotine dependence. Addict Behav. 2003;28:461–470. doi: 10.1016/s0306-4603(01)00266-0. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Absolute quantification of water and metabolites in human brain: II. Metabolic concentrations. J Magn Reson Series B. 1993;102:9–19. [Google Scholar]
- Kumar A, Newberg A, Alavi A, Berlin J, Smith R, Reivich M. Regional cerebral glucose metabolism in late-life depression and alzheimer disease: A preliminary positron emission tomography study. Proc Natl Acad Sci U S A. 1993;90:7019–7023. doi: 10.1073/pnas.90.15.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MD, Marcus MD, Perkins KA. A history of depression and smoking cessation outcomes among women concerned about post-cessation weight gain. Nicotine Tob Res. 2003;5:69–76. doi: 10.1080/1462220021000060455. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: Biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Michaelis T, Merboldt KD, Hanicke W, Frahm J. Absolute concentrations of metabolites in the adult human brain in vivo: Quantification of localized proton MR spectra. Radiology. 1993;187:219–227. doi: 10.1148/radiology.187.1.8451417. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Horton NJ, Monson RR, Laird NM, Sobol AM, Leighton AH, et al. Cigarette smoking in relation to depression: Historical trends from the Stirling County study. Am J Psychiatry. 2003;160:1663–1669. doi: 10.1176/appi.ajp.160.9.1663. [DOI] [PubMed] [Google Scholar]
- Perry TL, Bergeron C, Steele JC, McLachlan DR, Hansen S. Brain amino acid contents are dissimilar in sporadic and Guamanian amyotrophic lateral sclerosis. J Neurol Sci. 1990;99:3–8. doi: 10.1016/0022-510x(90)90194-r. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Bergeron D, Alger JR, Prichard JW. High-field proton magnetic resonance spectroscopy of human cerebrum obtained during surgery for epilepsy. Neurology. 1989;39:1197–1202. doi: 10.1212/wnl.39.9.1197. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO. In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: Effects of normal aging. Magn Reson Med. 1990;42:276–284. doi: 10.1002/(sici)1522-2594(199902)41:2<276::aid-mrm10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Brouwer RJ, Pomerleau OF. Emergence of depression during early abstinence in depressed and non-depressed women smokers. J Addict Dis. 2001;20:73–80. doi: 10.1300/J069v20n01_07. [DOI] [PubMed] [Google Scholar]
- Pontén U, Ratcheson RA, Salford LG. Optimal freezing conditions for cerebral metabolites in rats. J Neurochem. 1973;21:1127–1138. doi: 10.1111/j.1471-4159.1973.tb07567.x. [DOI] [PubMed] [Google Scholar]
- Porcu P, Sogliano C, Cinus M, Purdy RH, Biggio G, Concas A. Nicotine induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol Biochem Behav. 2003;74:683–690. doi: 10.1016/s0091-3057(02)01065-1. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Petroff OAC, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-amoni acids butyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OAC, et al. Reduced cortical gamma-amino butyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu F, Markowitz RS, ffrench-Mullen JM, Li X. GABAA receptor alpha-4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–929. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, et al. Targeting smokers at increased risk for relapse: Treating women and those with a history of depression. Nicotine Tob Res. 2003;5:99–109. doi: 10.1080/1462220021000060437. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R-Non-Patient Edition, Version 1.0. American Psychiatric Press; Washington, DC: 1990. [Google Scholar]