Abstract
Hypoxia is the major hindrance to successful radiation therapy of tumors. Attempts to increase the oxygen (O2) tension (PO2) of tissue by delivering more O2 have been clinically disappointing, largely due to the way O2 is transported and released by the hemoglobin (Hb) within the red blood cells (RBCs). Systemic manipulation of O2 transport increases vascular resistance due to metabolic autoregulation of blood flow to prevent over oxygenation. This study investigates a new technology to increase O2 delivery to a target tissue by decreasing the Hb-O2 affinity of the blood circulating within the targeted tissue. As the Hb-O2 affinity decreases, the tissue PO2 to satisfy tissue O2 metabolic needs increases, without increasing O2 delivery or extraction. Paramagnetic nanoparticles (PMNPs) synthetized using gadolinium oxide, were coated with the cell permeable Hb allosteric effector, L35 (3,5-trichlorophenylureido-phenoxy-methylpropionic acid). L35 decreases Hb affinity for O2 and favors the release of O2. The L35-coaded PMNPs (L35-PMNPs) were intravenously infused (10 mg/kg) to hamster instrumented with the dorsal window chamber model. Magnetic field of 3 mT was applied to localize the effects of the L35-PMNPs to the window chamber. Systemic O2 transport characteristics and microvascular tissue oxygenation were measured after L35-PMNPs administration with and without magnetic field. The tissue PO2 untreated control animals was 25.2 mmHg. L35-PMNP without magnetic field decreased tissue PO2 to 23.4 mmHg, increased blood pressure and reduced blood flow, largely due to systemic modification of Hb-O2 affinity. L35-PMNP with magnetic field increased tissue PO2 to 27.9 mmHg, without systemic or microhemodynamics changes. These results indicate that localized modification of Hb-O2 affinity can increase PO2 of target tissue, without affecting systemic O2 delivery or triggering O2 autoregulation mechanisms. This technology can be used to treat local hypoxia and to increase O2 in tumors enhancing the efficacy of radiation therapies.
Keywords: paramagnets, hemoglobin, tissue oxygen, oxygen delivery, microcirculation, hypoxia
BACKGROUND
Localized increase of tissue oxygen (O2) tensions (PO2) is a biomedical challenge with important clinical implications [1]. Therapies to increase tissue PO2 often include vasoactive drugs to increase of blood flow and O2 supplementation by increasing the fraction of inspired O2 (FiO2). Increased blood flow augments O2 delivery, but not tissue PO2, as tissue PO2 gradients between the blood and the tissue remain unaltered, as well as reduces blood tissue transit time. Increased FiO2 augments alveolar and arterial PO2, however the additional O2 is mostly dissolved in the plasma and due to the limited O2 solubility in plasma, the dissolved O2 in plasma is insignificant compared to the O2 bound to the Hb in the RBCs. Therefore, increasing the FiO2 does not necessarily translate into an increase of O2 released to tissue. The clinical applicability of targeted increase in PO2 spans from the treatment of focal ischemia to the treatment of cancer. The hypoxic environment of tumors is often associated with limited therapy efficacy (e.g. radiation) [2]. Increasing the O2 tension in tumors would allow for increased production of cytotoxic reactive oxygen species (ROS) [3].
Oxygen is transported and delivered through its reversible binding to Hb contained in the red blood cells (RBCs). Normal Hb-O2 binding is controlled by allosteric effectors (e.g. 2,3-diphosphoglycerate, 2,3 DPG) which facilitates loading and offloading of O2 by tuning Hb-O2 binding properties. Hb-O2 binding properties control O2 delivery, which determines tissue PO2 for tissues with O2 consumption remains unaltered. The flux of O2 leaving a blood vessel is determined by the O2 consumption of the tissue surrounding the blood vessel. This flux of O2 from arterioles and capillaries to the tissues is driven by the PO2 gradients between the blood and adjacent cells [4]. Therefore, modifying Hb-O2 affinity can be used to manipulate the PO2 gradients required to preserve the tissue O2 consumption while manipulating tissue PO2 [5]. For example, allosteric effectors that increase Hb-O2 affinity can decrease tissue PO2, as change in Hb-O2 saturation occur at lower PO2; whereas decreasing Hb-O2 affinity can increase tissue PO2 by achieving the required changes in Hb-O2 saturation at higher PO2. Our previous studies showed that a systemic increase in Hb-O2 affinity is beneficial during anemic and hypoxic conditions by increasing O2 captured in the lungs, increasing arterial Hb-O2 saturation, and preserving O2 delivery to the microcirculation [6-7]. Systemic decreases in Hb-O2 affinity facilitates the release of O2 to the microcirculation which triggers O2 autoregulation mechanisms, producing vasoconstriction and preventing O2 from being transported and offloaded to tissues [8]. To prevent metabolic O2 autoregulation mechanisms in response to tissue hyper-oxygenation, the decrease in Hb-O2 affinity has to occur after the arterioles in the capillary microcirculation.
A fundamental feature of the Hb-O2 affinity and cooperativity is the action of allosteric effectors in Hb quaternary equilibrium favoring the low-affinity T or the high-affinity R state. Hemoglobin is a sophisticated molecular machine able to maintain cooperative ligand binding over a large range of Hb-O2 affinities. L35 (3,5-trichlorophenylureido-phenoxymethylpropionic acid) is a potent Hb allosteric effector [9-10]. L35 synthesizes has been described in detail before [9], and L35 binding to Hb and L35 effects on Hb structured were documented before [10]. L35 favors Hb low-affinity state and increasing the PO2 were 50% of the Hb is saturated with O2 (P50). L35 binds toward the αα end of the Hb central cavity and works synergistically with 2,3 DPG and other organic phosphate-based allosteric effectors to decrease Hb-O2 affinity, since they bind to the ββ end of the Hb central cavity [11]. L35 easily penetrates the RBC membrane without damaging the cell membrane; and decreases the Hb-O2 affinity of RBCs suspensions [9].
This study builds on previous studies in which oleic acid was used to coat paramagnetic gadolinium oxide nanocrystals to produce paramagnetic nanoparticles (PMNPs) [12-13]. The oleic acid on the PMNPs, can be loaded with a wide variety of hydrophobic drugs, and technically the paramagnetic properties of the gadolinium are used to localize the effects of the drugs to a specific site by applying a magnetic fields. This study utilizes L35 loaded PMNPs (L35-PMNPs) to evaluate whether localized decrease of Hb-O2 affinity increases PO2 in a targeted area of tissue. We tested the hypothesis that the release of the allosteric effectors (L35) from L35-PMNPs can be concentrated to a specific site using a magnetic field, decreasing the Hb-O2 affinity of the RBCs circulating the site, increasing tissue PO2, and preventing hemodynamic changes due to metabolic autoregulation. The L35-PMNPs were given as a single IV dose (10 mg/kg) to Golden Syrian hamsters instrumented with the dorsal window chamber model. One group of animals was exposed to a strong magnetic field (3 mT) applied to the window chamber area, whereas the other group was not exposed to magnetic field. Control animals did not receive L35-PMNPs. Oxygen tensions were measured using phosphorescence quenching microscopy (PQM) and focuses on healthy tissues with tissue PO2 are well regulated.
MATERIALS AND METHODS
L35-PMNPs preparation
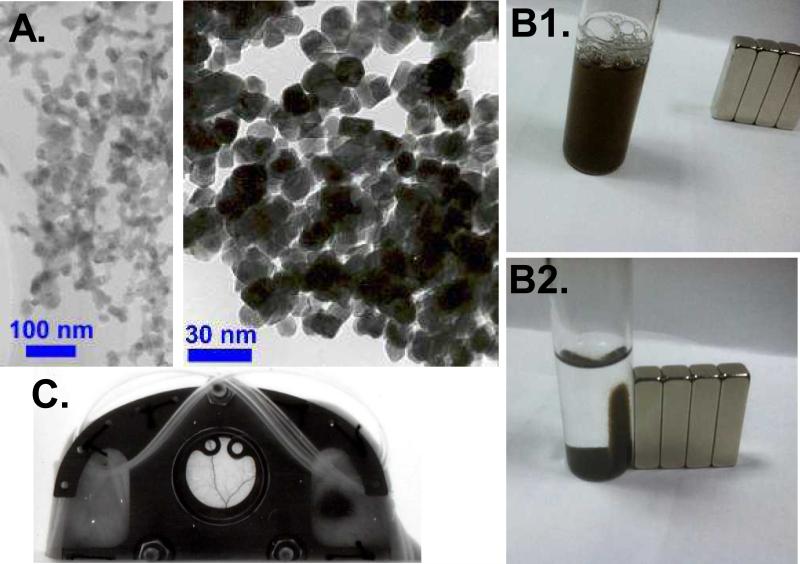
The preparation of the L35 loaded PMNP (L35-PMNP) is based on a protocol developed by the Einstein team to create Adriamycin coated PMNPs for targeted treatment of tumors. The general process started with the preparation of the oleic acid coated paragnetic nanocrystalline core. 10 mg of commercially purchased Gadolinium Oxide nanocrystals (Nanostructured & Amorphous Materials, Inc., Houston, TX), with average diameter of 30 nm (Figure 1A) were washed several times in 5 mL of deionized (DI) water and then centrifuged. 300 μL of oleic acid in DMSO (1:19) was mixed with water-free spun down particles followed by vigorous sonication for 1 hr. in a cold water bath and then left on Lab rotor overnight. The resulting particles were spun down and washed several times with DI water, dried and then lyophilized for storage. They were reconstituted by mixing with an aqueous solvent and sonicating briefly. The resulting suspension is stable with no detectable aggregates forming over an extended several day periods. Dynamic light scattering measurements indicate that the resulting particles were less that 100 nm. The drug loading process consists of mixing 1 mg/mL of L35 (obtained through a generous gift from Dr's Iraj and Parviz Lalezari, L35 synthesizes was described before [9]) solubilized in 1 mL of DMSO with 10 mg/mL of oleic acid coated gadolinium oxide based paramagnetic nanoparticles (PMNPs) at room temperature. After the DMSO mix was allowed to remain for 24 hours in the dark at room temperature. Then, the suspension was centrifuged, the brown color of the L-35 was localized, the DMSO was poured off, and the brown colored PMNPs were washed three times with PBS buffer and vortexed to re-suspend the particles. After last wash, the excess buffer was poured off and the remaining brown material was lyophilized. The L35 coated PMNPs were then PEGylated by adding a small aliquot of m-PEG-DSPE (Nanocs inc. New York, NY) dissolved in DMSO (derived from a stock solution of 1 mg of m-PEG-DSPE dissolved in 1ml of DMSO). Typically, 10-50μL of the m-PEG-DSPE DMSO stock solution was used to PEGylate 50-100mg of L35-PMNPs. Other drugs have been loaded PMNP's prepared in a similar manner (e.g. adriamycin, curcumin, melanin). Exposing a cuvette containing melanin loaded PMNPs suspension to magnet, results in the accumulation of the nanoparticles adjacent to the position of the magnet (Figure 1B).
Figure 1.
A. Paramagnetic nanoparticles (PMNPs). Exposing the suspension of PMNPs to powerful magnet resulted in the accumulation of the brown particles to the location were the magnetic field was applied. Other drugs have been loaded to PMNP's (e.g. adriamycin, curcumin and melanin). B1. Brown color solution of melanin-PMNPs in PBS buffer (without magnetic field). B2. Melanin-PMNPs suspended in PBS buffer with a magnetic field applied. Pictures B1 and B2 were taken with melanin-PMNPs, as they are darken brown and easier to photograph. C. Hamster window chamber model. Implanted titanium frames on the dorsal skinfold for microvascular studies (18 mm window diameter). Intact tissue is studied in the absence of surgical trauma or anesthesia. Catheters are implanted and exteriorized at the dorsal side and secured to the frames. A cylindrical strong magnet was used to localize the effects of the L35-PMNM to the dorsal window area.
Rhodamine labeled particles
The fluorescent dye rhodamine 110 (Sigma-Aldrich, St Louis, MO) was bound to the surface of the L35-PMNPs by linking the dye to carboxyl groups. Briefly, a concentrated dispersion of L35-PMNPs was prepared in a 25 mM aqueous (2-N-morpholino ethanesulfonic acid, Sigma-Aldrich) buffer. To the L35-PMNPs dispersion, 4 mmol of 1-3-(dimethylaminopropyl)-3-ethylcarbodiimidehydrochloride (Sigma-Aldrich) followed by 0.25 mmol of rhodamine 110 dye were added, and the reaction vial was vortexed for 4 h protected from the light at room temperature. The resulting rhodamine labeled L35-PMNPs were washed twice times through alternate cycles of vortexing and centrifugation to remove any unbound rhodamine dye.
Animal preparation
Male Golden Syrian Hamsters 55 - 65 g (Charles River Laboratories, Boston, MA) were fitted with a dorsal window chamber. Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals and the experimental protocol was approved by the local animal care committee. The hamster window chamber model is widely used for microvascular studies in the unanaesthetized state, and the complete surgical technique is described in detail elsewhere [14-15]. Briefly, the animal was prepared for chamber implantation with a 50 mg/kg ip injection of pentobarbital sodium anesthesia. After hair removal, sutures were used to lift the dorsal skin away from the animal, and one frame of the chamber was positioned on the animal's back. A chamber consisted of two identical titanium frames with a 15-mm circular window. With the aid of backlighting and a stereomicroscope, one side of the skin fold was removed following the outline of the window until only a thin layer of retractor muscle and the intact subcutaneous skin of the opposing side remained. The intact skin of the other side was exposed to the ambient environment. Animals were allowed 2 days for recovery (Figure 1C). Finally, catheters (PE-50) were implanted in the carotid artery and jugular vein. Catheters were tunneled under the skin, exteriorized at the dorsal side of the neck, and securely attached to the window frame.
Inclusion criteria
Hamsters were suitable for the experiments if: 1) systemic parameters were within normal range, namely, heart rate (HR) > 340 beat/min, mean arterial blood pressure (MAP) > 80 mmHg, systemic hematocrit (Hct) > 45%, and arterial PO2 pressure (PaO2) > 50 mmHg; and 2) microscopic examination of the tissue in the chamber observed under x650 magnification did not reveal signs of edema or bleeding. Hamsters are a fossorial species with a low arterial PO2 compared to other rodents; however, the PO2s in the hamster window chamber are similar to the PO2s in other rodents (e.g. mice) [16].
Systemic parameters
MAP and heart rate (HR) were recorded continuously (MP 150, Biopac System; Santa Barbara, CA). Hct was measured from centrifuged arterial blood samples taken in heparinized capillary tubes. Hb content was determined spectrophotometrically (B-Hemoglobin, Hemocue, Stockholm, Sweden). Arterial blood was collected in heparinized glass capillaries (50 μl) and immediately analyzed for PO2, PCO2, base excess (BE) and pH (Rapidlab 248, Bayer, Norwood, MA). Arterial Hb saturations were measured on the IL482 CO-Oximeter System (Instrumentation Laboratory, Lexington, MA).
Blood oxygen equilibrium curve
Oxygen equilibrium curves for Hamster blood were obtained by deoxygenation of O2-equilibrated samples in a Hemox buffer at 37°C, using a Hemox Analyzer (TCS Scientific Corporation, New Hope, PA). The Hemox buffer pH was adjusted to match the arterial blood pH of the animals using Tris and BisTris buffers. Tris and BisTris buffers were prepared by titrating the reagents with HCI before adjusting the pH of the solutions to keep Cl- ion concentration equal to the buffer at the pH values.
Experimental Setup
The window chamber was studied using a custom intravital microscope. Briefly, the restrained animals were fixed to the microscopic stage for transillumination (BX51WI, Olympus, New Hyde Park, NY). Measurements were carried out using a 40X (LUMPFL-WIR, numerical aperture 0.8, Olympus) water immersion objective. Tissue image was projected onto a charge-coupled device camera (COHU 4815, San Diego CA), viewed on a TV monitor, and analyzed online using the following techniques.
Microhemodynamics
A video image-shearing method was used to measure vessel diameter (D) [17]. Changes in arteriolar and venular diameter from baseline were used as indicators of a change in vascular tone. Arteriolar and venular centerline velocities were measured on-line using the photodiode cross-correlation method (Photo Diode/Velocity Tracker Model 102B, Vista Electronics, San Diego, CA). The measured centerline velocity (V) was corrected according to vessel size to obtain the mean RBC velocity [18]. Blood flow (Q) was calculated from the measured values as Q = π × V (D/2)2.
Microvascular oxygen tensions
High resolution non-invasive microvascular PO2 measurements were made using phosphorescence quenching microscopy (PQM) [19]. PQM is based on the relationship between the decay rate of excited Palladium-mesotetra-(4-carboxyphenyl) porphyrin (Frontier Scientific Porphyrin Products, Logan, UT) bound to albumin and the O2 concentration according to the Stern-Volmer equation [20]. PO2 measurements by PQM were obtained by illuminating the tissue with a pulsed light at 420 nm to excite the probe into its triplet state. The emitted phosphorescence (680 nm) was collected and analyzed to yield the phosphorescence lifetime, which was then converted into PO2. The phosphorescence lifetimes are concentration independent, which permit extravascular fluid PO2 measurements. Animals received a 50 μl IV of 10 mg/dL Palladium- porphyrin complex (Frontier Scientific Porphyrin Products, Logan, UT). Porphyrin was allows to distribute for 15 min before measurements were taken. Intravascular PO2 were measured for the same blood vessels selected for hemodynamic analysis.
Experimental protocol
Awake hamsters, instrumented with the window chamber, were restrained in an acrylic tube with perforations to allow access of air. The window chamber protruded out of a slit on the tube, and was used to secure the animal to the microscope stage. Animals were given 20 min to adjust to the tube environment before any measurement. Then, detailed mappings were made of the chamber vasculature. Blood vessels were chosen based on distinctive anatomic landmarks and optical clarity. The same blood vessels were studied at baseline and follwing time points. Six to eight arterioles and venules were selected in each preparation. Tissue PO2 was measured in consecutive spots, mapping the entire center of the window in locations free of blood vessels. Each row consisted of 35-40 measurements, and covered an area of 7.3 mm2. Rows of measurements were spaced 100 μm to prevent repeated measurements from the same spot, and 15 to 22 rows were measured in each window. L35-PMNPs were suspended in saline and vortexed for 1 min. After baseline measurements (hemodynamics and PO2s), 10 mg per kg of body weight of L35-PMNPs were administered in 100 μL volume. The magnetic field was applied around the window chamber area before L35- PMNPs infusion. The time points studied were baseline (before any treatment) and 1 hour after treatment. All measurements were completed within 30 mins.
Experimental groups
A total of 24 animals were used for the study. Animals were assigned randomly to each experimental group. Groups were labeled based on experimental conditions: 1) L35-PMNPs with magnetic field ON (n=8), animals received L35-PMNPs and a applied magnetic field; 2) L35-PMNPs with magnetic field OFF (n=8), animals received L35-PMNPs and no applied magnetic field; 3) Untreated control animals (n=8) did not receive the L35-PMNPs or applied magnetic field.
Oxygen saturations
Intravascular Hb-O2 saturations were calculated using the Hb-O2 equilibrium curves measured, and the intravascular PO2s measured with the PQM. The O2 equilibrium curves were measured at arterial blood pH, as described above. Intravascular Hb-O2 saturation for the animals treated with L35-PMNPs and magnetic field ON were assumed to follow the changes in Hb-O2 equilibrium curves measured in untreated animals. This assumption may over-estimates venular Hb-O2 saturations, as the local change in Hb-O2 equilibrium curves of the blood leaving the tissue may be shifted to the right. However, micro-sampling blood from small venules is extremely difficult.
Oxygen delivery and extraction
The microvascular methodology used in our studies allows a detailed analysis of O2 supply in the tissue. Calculations are made using equation 1 and 2:
| (1) |
| (2) |
Where, RBCHb is the total Hb [gHb/dlblood], γ is the O2 carrying capacity of saturated Hb [1.34 mlO2/gHb], SA is the arteriolar blood O2 saturation, A-V indicates the arteriolar/venular differences, and Q is the average microvascular blood flow (arterioles and venules). Intravascular Hb O2 saturations were calculated as described above.
Biodistribution in vivo imaging studies
The biodistribution imaging studies were performed on hamsters (n = 3), similar size as used for the microvascular study. Animals received 50 mg/kg IP injection of pentobarbital sodium anesthesia. After hair removal, sutures were used to lift the dorsal skin away from the animal and a catheter was implanted in the jugular vein. Rhodamine labeled L35-PMNPs (10 mg/kg) were injected intravenously and strong magnet was placed under the skin. Dorsal skin fluorescence images were acquired on a Maestro II imaging system (Perkin Elmer, Norwalk, CT), one hour after injection using a 500-720 nm emission filter with exposure time of 1 sec.
Tissue accumulation study
Hamsters (n=2), instrumented with the window chamber, were restrained and secured to the intravital microscope (BX51WI, Olympus, New Hyde Park, NY), as described before. Rhodamine labeled L35-PMNPs (10 mg/kg suspended in 100 μL of physiological saline solution, 0.9% NaCl) were injected intravenously. Images before and after application of the magnetic field were recorded using a low light video camera (ORCA 9247, Hamamatsu, Tokyo, Japan). Images were collected using a 20X (LUMPFL-WIR, numerical aperture 0.6, Olympus) water immersion objective and the fluorescence filter XF100-2 (emission: 520nm and excitation: 495nm; Omega Optical, Inc Brattleboro, VT). The magnetic field was maintained for an hour, and then the animals were placed back in their cages. Additional hamsters (n=2), instrumented with the window chamber, received the rhodamine labeled L35-PMNPs, but were not exposed to magnetic field. 24 hours after injection, major organs and the window chamber were dissected for ex vivo imaging. Microscopic fluorescence images were taken to identify the presence of rhodamine labeled L35-PMNPs in the tissues.
Data analysis
Results are presented as mean ± standard deviation. Data within each group was analyzed using analysis of variance for repeated measurements (ANOVA, Kruskal-Wallis test). When appropriate, post hoc analyses were performed with the Dunn's multiple comparison test. Data comparison between groups was analyzed using two-way analysis of variance (Two-way ANOVA test). When appropriate, post-test analyses were performed with the Bonferroni post-tests comparison. Tissue PO2s frequency distribution of each groups tested for normality according to the Shapiro-Wilk test. Microhemodynamic data are presented as absolute values. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, Inc., San Diego, CA). Changes were considered statistically significant if P<0.05.
RESULTS
Twenty-four animals were used in the study divided into three groups: L35-PMNPs with magnetic field ON (n=8), L35-PMNPs with magnetic field OFF (n=8), untreated controls (n=8). All animals tolerated the entire protocol without signs of discomfort or stress. Seven (n=7) additional animals were used for the biodistribution imaging (n=3) and the tissue accumulation study (n=4). All animals passed the Grubbs' test, ensuring that all the measured values at baseline were within a similar population (P<0.05). Similarities between groups at baseline for hamsters were statistically verified between groups (P>0.30).
Blood chemistry and oxygen affinity
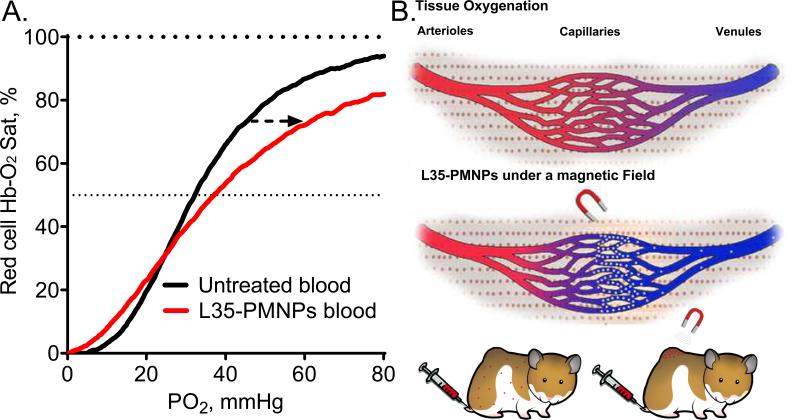
Blood basic hematology, arterial gases, and Hb-O2 affinity parameters for all groups are presented in Table 1. Infusion of L35-PMNPs with magnetic field ON did not affect blood gas parameters. However, infusion of L35-PMNPs with magnetic field OFF increased arterial PO2, decreased arterial PCO2, and increased blood pH. Effects of L35-PMNPs in blood Hb-O2 affinity with magnetic field ON were not statistically significant; whereas, with the magnetic field OFF, there was a significant increase in P50 and reduce Hb-O2 cooperativity (hill number). Arterial O2 saturations were not different between groups, as the increase in arterial PO2 in the group with magnetic field OFF compensated for the decrease on Hb-O2 affinity. In vitro effects of L35-PMNPs effect in blood Hb-O2 affinity are presented in Figure 2A. Based on the L35-PMNPs dose given to the animals (10 mg/kg) and the animal's blood volume (4.2 mL for a 60g hamster), 0.3 mg of L35-PMNPs were mixed with 2 mL of hamster fresh blood and incubated for 30 min. Figure 2B illustrates the potential mechanisms tested in this studies. Effect in RBC survival and long term effects in blood Hb-O2 affinity are presented in Supplemental Table 1. There were not significant effects in RBC survival detected 24 hour after administration L35-PMNP with or without magnetic field. Additionally, the change in blood Hb-O2 affinity produced by L35 disappeared in vivo after 24 hours, suggesting that L35 is rapidly metabolized.
Table 1.
Blood Oxygen Transport Characteristics
| L35-PMNP | ||||
|---|---|---|---|---|
| Baseline | Magnetic field ON | Magnetic field OFF | Untreated | |
| Hematocrict, % | 48 ± 3 | 47 ± 2 | 48 ± 2 | 47 ± 2 |
| Hemoglobin, g/dl | 14.7 ± 0.4 | 14.5 ± 0.5 | 14.8 ± 0.4 | 14.5 ± 0.3 |
| P50, mmHg | 32.6 ± 1.4 | 33.1 ± 1.7 | 38.4 ± 1.4†‡# | 32.6 ± 1.4 |
| Cooperativity | 2.96 ± 0.10 | 2.84 ± 0.12 | 2.62 ± 0.16†‡# | 2.92 ± 0.10 |
| Arterial Pressure, mmHg | 107 ± 7 | 110 ± 8 | 119 ± 6† | 113 ± 8 |
| Heart rate, bpm | 442 ± 27 | 433 ± 29 | 401 ± 22 | 448 ± 25 |
| PaO2, mmHg | 59.9 ± 4.1 | 57.1 ± 5.2 | 67.2 ± 6.2†‡# | 58.6 ± 7.2 |
| PaCO2, mmHg | 51.8 ± 4.3 | 51.2 ± 6.0 | 47.6 ± 6.0 | 50.2 ± 4.4 |
| pHa | 7.335 ± 0.017 | 7.331 ± 0.026 | 7.364 ± 0.027 | 7.343 ± 0.029 |
Values are means ± SD. P50, PO2 at which Hb is half saturated with O2; Cooperativity or Hill number, describes the affinity of the Hb-O2 dissociation curve. PaO2, arterial partial O2 pressure; PaCO2, arterial partial pressure of CO2; pHa, arterial blood pH.
P<0.05 compared to baseline
P<0.05 compared to with magnetic field ON
P<0.05 compared to Untreated control.
Figure 2.
A. Modified Hb-O2 dissociation curve. The allosteric effect L35-PMNPs (0.3 mg added to 2 mL of fresh blood, and incubated for 30min) decreases the Hb-O2 affinity which is evidenced by a shift in the dissociation curve to the right. B. Illustration of the mechanism proposed to increase tissue PO2 evaluated in this manuscript.
Blood pressure and heart rate
Systemic hemodynamics (MAP and HR) are presented in Table 1. Infusion of L35-PMNPs with magnetic field ON did not affect MAP or HR. Infusion of L35-PMNPs with magnetic field OFF increased MAP and reduced HR compared to baseline.
Microhemodynamics
Changes in arteriolar and venular diameters and blood flow are presented in Figure 3. Arterioles with diameters ranging between 40 and 64 μm and venules with diameter ranging between 50 and 72 μm were included in the study. Infusion of L35-PMNPs with magnetic field ON did not affect vessel diameter, while L35-PMNPs with magnetic field OFF produced significant arteriolar and venular vasoconstriction compared to baseline. Arteriolar and venular blood flows were preserved after infusion of L35-PMNPs with magnetic field ON. The vasoconstriction induced by L35-PMNPs with magnetic field OFF significantly reduced arterial blood flow compared to baseline, and to the magnetic field ON and untreated control groups.
Figure 3.
Microhemodynamics. A. Arteriolar diameters and volumetric flow rates and B. Venular diameters and flow rates at baseline and after treatment. †, P<0.05 relative to baseline. A metabolic autoregulatory response to early release of O2 from systemically decreased Hb-O2 affinity in the microcirculation produced significant reduction in arteriolar and venular diameters. Application of a magnetic field prevented the hemodynamic changes.
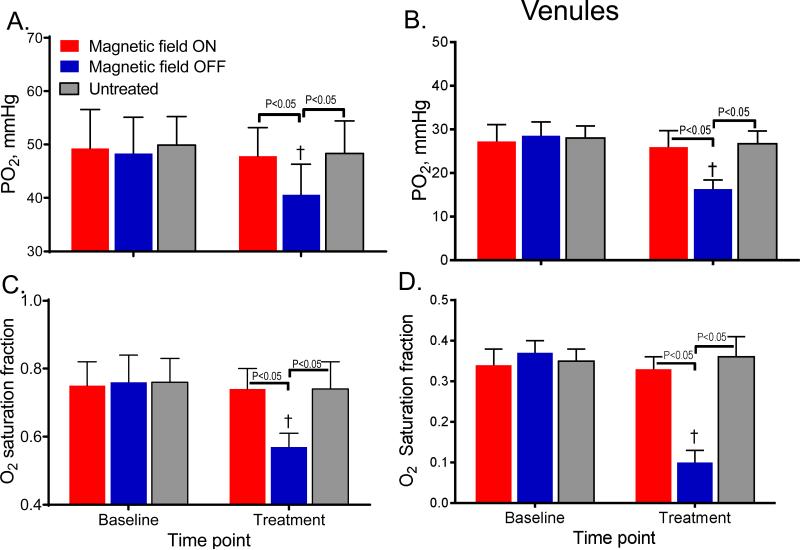
Intravascular PO2 and O2 saturations
Changes in arteriolar and venular PO2 and SO2 are presented in Figure 4. Arteriolar PO2 and SO2 of L35-PMNPs with magnetic field ON were not different compared to baseline or to untreated control group. However, the arteriolar PO2 and SO2 of L35-PMNPs with magnetic field OFF were significantly decreased compared to baseline, and to the magnetic field ON and the untreated control groups. Similarly, venular PO2 and SO2 for the magnetic field ON were not different compared to baseline, but they were significantly decreased compared to baseline, and to the magnetic field ON and untreated control groups.
Figure 4.
A. Arteriolar oxygen pressure, B. Venular oxygen pressure and C. Arteriolar Hb-O2 saturation and D. Venular Hb-O2 saturation. †, P < 0.05 relative to baseline. Both arteriolar and venular PO2 and Hb-O2 saturation in the microcirculation decreased in the group with systemic decreased Hb-O2 affinity (magnetic field OFF). Intravascular Hb-O2 saturation for the group treated with L35-PMNPs with magnetic field ON were assumed to similar to untreated animals. This assumption over-estimates venular Hb-O2 saturations, as the blood treated locally with L35 should have a decreased Hb-O2 affinity than untreated blood.
Distribution of tissue oxygen tension
Histograms of tissue PO2s for all groups are represented in Figure 6. Results include all measurements from the animals included in each group. Tissue PO2 did not follow a normal distribution. The median value for the untreated control group was 25.9 (CI: 24.4:27.6) mmHg. The median value for the L35-PMNPs with magnetic field ON increased to 27.2 (CI: 24.2:29.7) mmHg, and it was significantly higher compared to the untreated control and to the group with the magnetic field OFF. Conversely, the median value for the L35-PMNPs with magnetic field OFF decreased to 23.4 (CI: 19.6:26.3) mmHg, it was significantly lower compared to the untreated control group.
Figure 6.
A. Oxygen delivery, B. Oxygen extraction, and C. Oxygen extraction ratio for baseline and after treatment. The O2 delivery for the group with the magnetic field was OFF was significantly lower than the other groups, due to low O2 arteriolar saturation and reduced arteriolar blood flow in the microcirculation. The systemically decreased Hb-O2 affinity in the microcirculation increased O2 extraction ration to preserve O2 extraction (consumption).
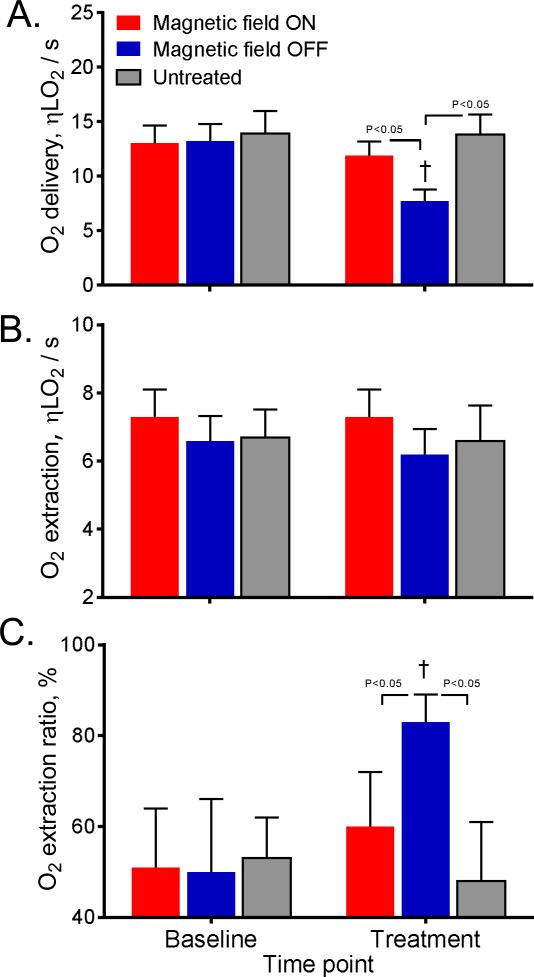
Microvascular oxygenation
Calculated O2 delivery, extraction and extraction ration are presented in Figure 5. The O2 delivery of L35-PMPNs with magnetic field ON was not different compared to baseline and to the untreated control group. On the other hand, O2 delivery of L35-PMNPs with magnetic field OFF was significantly lower compared to baseline, and to the group with magnetic field ON and the untreated control. The O2 extraction was not significantly different between groups. The O2 extraction ratio of L35-PMNPs with magnetic field OFF increased from 48% at baseline to 82%, and it was significantly increased compared to baseline, and to the L35-PMNPs with magnetic field ON and the untreated control groups.
Figure 5.
Measured tissue PO2 distributions for all groups. When the L35-PMNP were localized to the tissue area of the window model using a magnetic field, the median PO2 increased from 27.2 mmHg compared to 25.9 mmHg in the untreated animals, whereas in the absence of the magnetic field the median PO2 dropped to 23.4 mmHg. In normal condition, it is difficult to modify tissue PO2, since tissue PO2 is the result of the balance between O2 delivery and O2 consumption by the tissue.
Biodistribution and tissue accumulation
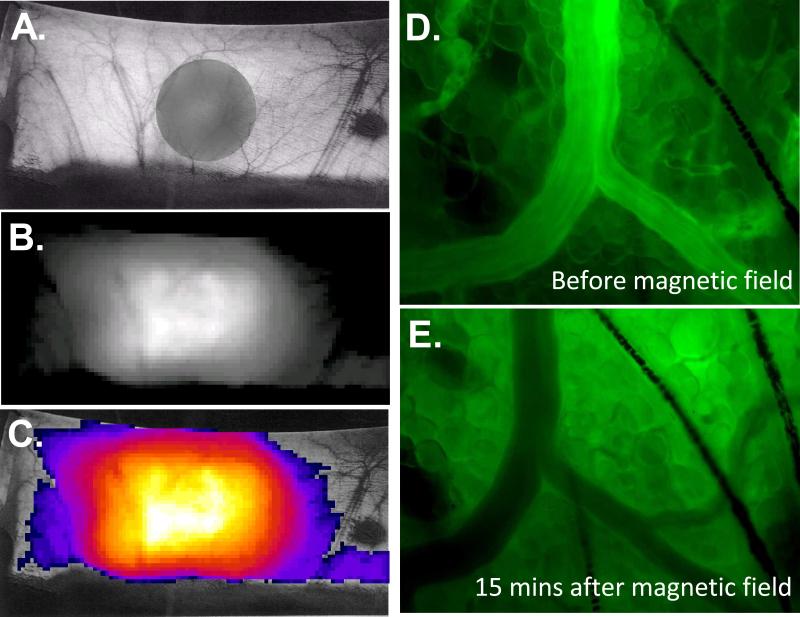
Rhodamine labeled L35-PMNPs were injected into hamsters and accumulation of fluorescently labeled L35-PMNPs was observed in the area were the magnetic field was applied (Figure 7A, 7B and 7C). Microcirculation images of the target tissue of the window chamber models also reveled that the rhodamine labeled L35-PMNPs accumulated in the area were the magnetic field was applied, (Figure 7D and 7E). 24 hours after injection, fluorescent was detected predominantly in the window chamber of animals exposed to magnetic field. In animals no exposed to magnetic field, fluorescent was not observed in the window tissue. Supplemental Table 2 presents the presence of fluorescence in major organs after ex vivo imaging. Fluorescence was detected in the kidneys, liver and lung, independently of the exposure to magnetic field.
Figure 7.
A. Hamster dorsal depilated skin, gray shadow projected by the circular magnet placed underneath the lifted skin. B. Intensity collected from rhodamine labeled L35-PMNPs one hour after injection using a 500-720 nm emission filter with exposure time of 1 sec. C. Pseudo-color accumulation of rhodamine labeled L35-PMNPs. D. Intravascular fluorescent image after infusion of rhodamine labeled L35-PMNPs before application of magnetic field. E. Intravascular fluorescent image after 15 min of application of magnetic field. Microscopic fluorescence images were taken to identify the presence of rhodamine labeled L35-PMNPs in the tissues.
DISCUSION
The principal finding of the study is that the paramagnetic nanoparticles can be used for localized drug delivery of L35, a potent Hb allosteric effector, to decrease Hb-O2 affinity of the circulating blood in the vicinity of the tissue where a magnetic field is applied. The decrease in Hb-O2 affinity favored the release of O2 and increased tissue PO2, without affecting autoregulatory mechanism or having a systemic change in Hb-O2 affinity. This study confirms that reduction in Hb-O2 affinity increase tissue PO2 without increasing the O2 extracted. Our results also indicate that systemic decrease of Hb-O2 favors the release O2 before the microcirculation, reducing arteriolar PO2 and O2 content. Systemic decrease of Hb-O2 triggered O2 metabolic autoregulation to prevent hyper-oxygenation, reducing the diameter and blood flow of arterioles and increasing vascular resistance. The systemic decrease of Hb-O2 induced respiratory adjustments to maintain arterial O2 saturation, which produced respiratory alkalosis. Although the P50 with L35-PMNPs without magnetic field decreased only by 6 mmHg, it caused significant systemic hemodynamic changes, increased blood pressure and vasoconstriction, and ventilation to maintain arterial Hb-O2 saturation.
In the pass, research attempted to pharmacologically shift the Hb-O2 dissociation curve to the right in order to facilitate the offloading of O2 in tissues [21]. The idea was based on classical physiology of high-altitude natives, which showed that Andean communities had a decreased Hb-O2 affinity [22]. Pharmacological manipulation of the Hb-O2 dissociation curve to increase tissue oxygenation can be important for some clinical circumstances, and future research with L35-PMNPs may answer this interesting question. Few studies have been reported to decrease Hb-O2 affinity of intact RBCs, Teisseire et al. reported a method for increasing P50 using inositol hexaphosphate (IP6) ex vivo, which function was evaluated in anesthetized piglets after exchange transfusion [23-24]. They found that erythrocytes loaded with IP6 released more O2 based on arterial and venular saturation differences, although, exchange transfusion of IP6 loaded RBCs also decreased cardiac output, increased vascular resistance and produced respiratory alkalosis. As most of the Hb allosteric effector can not diffuse through the erythrocyte membrane, they have to be introduced into the RBCs using a reverse osmoticlysis process or electroporation ex vivo [25-26]. Using these processes reduces RBCs survival in the circulation, and Teisseire et al. reported that IP6 loaded RBC had only half life of less than 24 hours [23-24]. In our approach, L35-PMNPs had a systemic effect in vivo, and RBCs survival was not affect at 24 hour with or without the magnetic field applied (supplemental material). Interestingly, in the absence of the magnetic field the acute hemodynamic changes produced by L35-PMNPs were similar to the results presented by others who shifted the Hb-O2 dissociation curve to the right. We hypothesized that the hemodynamics and respiratory changes produced by decreasing Hb-O2 affinity in the hamster window chamber model are more pronounced since the previous studies were attained in anesthetized experimental models [23-24]. In addition, the lack of anesthesia in our experimental model makes harder to disturb oxygenation homeostasis to increase tissue PO2 above normal.
Regulatory mechanisms operate to maintain tissue oxygenation homeostasis and to set tissue PO2 levels within a certain range [4]. Under normal conditions, the sigmoidal shape of the Hb-O2 dissociation curve facilitates O2 transport, as virtually all Hb is loaded with O2 in the lungs, while O2 is offloaded in the tissues at a relatively high PO2s. Therefore, in normal conditions O2 delivery is not limited by blood flow, thereby optimizing the cardiac output and the circulatory load. In theory, changes in the Hb-O2 affinity should have important effects on the blood O2 transport. Previous studies showed that shitting Hb-O2 affinity to the right cause an excessive tissue O2 supply, resulting in a vasoconstrictor response [27]. The most significant O2 release to the tissues under normal conditions is reported to occur in the range of arterioles study here [4], systemic changes in Hb-O2 affinity produced also vasoconstriction in these blood vessels, which suggest their important role in O2 homeostasis. However, modification of Hb-O2 affinity down the stream from resistance arterioles by magnetically localized release of L35 did not produce vasoconstriction and increased tissue PO2. Moreover, when the P50 of the RBCs was increased locally, there was not increase in microvascular O2 delivery or extraction, while during when the P50 was changed systemically, the microvascular O2 delivery decreased and the O2 extraction remained constant, increasing O2 extraction ratio. This result demonstrates that O2 extraction cannot be increased beyond the requirements determined by the normal metabolic demand, likely since over oxygenation may lead to detrimental free radical formation. The systemic decrease in O2 affinity caused a strong autoregulatory response, limiting O2 supply and causing vasoconstriction and reducing blood flow. On the other hand, reduced blood flow increases RBC transit time in the microcirculation and increased O2 extraction ratio. This result supports the role of Hb-O2 availability per se as a determinant of local blood flow in the peripheral circulation. Our results suggest that allosteric effectors, that decrease Hb-O2 affinity, should not be used to increase O2 availability to the tissue under conditions where O2 supply is compromised.
Oxygen transport from blood into tissue occurs by passive diffusion, thus the maximum distance that O2 can diffuse from the blood into surrounding tissue decreases within a few micrometers. Our tissue PO2 distribution histograms confirm tissue PO2 heterogeneity. Observations in hypoxic tumors have shown a substantial increase in O2 saturation in the venular blood, of the order of 15 to 30%, showing that O2 extraction is incomplete [28]. L35-PMNPs with magnetic field can shift the Hb-O2 affinity to the right, increasing the PO2 of the microenvironment were the O2 exchange takes place, without the need for affecting tumor O2 consumption. However, tumors also reflect diffusive limitation to O2 transport, even when the PO2 gradients are favorable for O2 release [28]. L35 is by far the most potent allosteric effector of Hb ever described. At a concentration of 0.1 mM, it increases the P50 of a suspension of RBCs by 50%; at 0.2 mM it raises the P50 by 2.5-fold [9]. L35-PMNPs might find clinical use to increase PO2 of hypoxic tissues or to tumors before irradiation; although, the effects of L35 are acute, as L35 appears to be metabolized and loses its activity within 24 hours (see supplemental material).
Biodistribution and tissue accumulation study showed the magnetic localization of the L35-PMNPs in the target tissue. Fluorescence microscopy revealed extravasation of the fluorescence from the labeled L35-PMNPs after application of the magnetic field, likely due to increased extra-cellular accumulation of fluorophore conjugate to the L35-PMNPs (Figure 7C and 7D). In vivo imaging studies found accumulation of fluorescence in the tissue of the window chamber within 15 mins (Figure 7D). In addition, to the target tissue, fluorescence was also found in the liver, lung, spleen and kidney, which can be attributed to PMNP uptake by the Kupffer cells and macrophages. Kidney could be a target, because of its role in elimination of xenobiotics. Particle size may be a major factor in the accumulation of PMNP in various organs. Studies on the biological effects of nanoparticles had found similar results [29]. Application a magnetic field after injection of PMNP increased the translocation process across biological barriers. The transport of PMNPs from the blood and their deposit in target organs can exert potential toxic effects, which needs to be study in detail. Preliminary studies have shown that magnetic localization of the PMNPs is greater in tumor tissues by the combination of magnetic localization and leaky tumor vasculature. It can be anticipated that the magnet induced localization of the L35-PMNPs at a tumor site will produce a more profound increase in tumor PO2.
CONCLUSION
This study indicates that localize delivery of a pharmaceutical agent using nonmagnetic nanoparticles is possible; the hydrophobic nature of L35 the localization with the PMNP facilitates the target release of cell permeable drugs. The L35 loaded PMNP was able to effectively modify the Hb-O2 systemically, and localized, when a magnetic field was applied. Systemic modifications on Hb-O2 affinity triggered autoregulatory mechanism, which hindered tissue oxygenation. Localized decrease in Hb-O2 affinity did not activate regulatory mechanism and increased tissue PO2, without affecting O2 delivery and extraction. Therefore, localized decreases in Hb-O2 affinity only affect the PO2 gradient between the blood and the tissue, without affecting O2 utilization. There are significant clinical implications of localize increase in PO2, however study of PMNP pharmacokinetic and long-term safety have to be study before.
ACKNOWLEDGMENTS
This work was partially supported by Bioengineering Research Partnership grant Program project P01-HL071064, and grants R01-HL52684, R01-HL62354, and R01-HL62318. The authors thank F. Barra and C. Walser for the surgical preparation of the animals. The authors also thank Dr's Iraj and Parviz Lalezari for generously gifting the L35 used in the study.
REFERENCES
- 1.Tsai AG, Cabrales P, Hangai-Hoger N, Intaglietta M. Oxygen distribution and respiration by the microcirculation. Antioxidants & redox signaling. 2004;6:1011–8. doi: 10.1089/ars.2004.6.1011. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. Journal of Clinical Oncology. 2013;31:2205–18. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshimura M, Itasaka S, Harada H, Hiraoka M. Microenvironment and radiation therapy. BioMed research international. 20122013 doi: 10.1155/2013/685308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai AG, Johnson PC, Intaglietta M. Oxygen gradients in the microcirculation. Physiol Rev. 2003;83:933–63. doi: 10.1152/physrev.00034.2002. [DOI] [PubMed] [Google Scholar]
- 5.Hsia CC. Respiratory function of hemoglobin. N Engl J Med. 1998;338:239–47. doi: 10.1056/NEJM199801223380407. [DOI] [PubMed] [Google Scholar]
- 6.Cabrales P, Tsai AG, Intaglietta M. Modulation of perfusion and oxygenation by red blood cell oxygen affinity during acute anemia. Am J Respir Cell Mol Biol. 2008;38:354–61. doi: 10.1165/rcmb.2007-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villela NR, Cabrales P, Tsai AG, Intaglietta M. Microcirculatory effects of changing blood hemoglobin oxygen affinity during hemorrhagic shock resuscitation in an experimental model. Shock. 2009;31:645–52. doi: 10.1097/SHK.0b013e31818bb98a. [DOI] [PubMed] [Google Scholar]
- 8.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–55. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalezari I, Lalezari P, Poyart C, Marden M, Kister J, Bohn B, Fermi G, Perutz MF. New effectors of human hemoglobin: structure and function. Biochemistry. 1990;29:1515–23. doi: 10.1021/bi00458a024. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Lalezari I, Nagel RL, Hirsch RE. Liganded hemoglobin structural perturbations by the allosteric effector L35. Biophys J. 2005;88:2057–67. doi: 10.1529/biophysj.104.046136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalezari I, Lalezari P, Poyart C, Marden M, Kister J, Bohn B, Fermi G, Perutz M. New effectors of human hemoglobin: structure and function. Biochemistry. 1990;29:1515–23. doi: 10.1021/bi00458a024. [DOI] [PubMed] [Google Scholar]
- 12.Soderlind F, Pedersen H, Petoral RM, Jr., Kall PO, Uvdal K. Synthesis and characterisation of Gd2O3 nanocrystals functionalised by organic acids. J Colloid Interface Sci. 2005;288:140–8. doi: 10.1016/j.jcis.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 13.Faucher L, Tremblay M, Lagueux J, Gossuin Y, Fortin MA. Rapid synthesis of PEGylated ultrasmall gadolinium oxide nanoparticles for cell labeling and tracking with MRI. ACS Appl Mater Interfaces. 2012;4:4506–15. doi: 10.1021/am3006466. [DOI] [PubMed] [Google Scholar]
- 14.Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol. 1984;246:H508–H17. doi: 10.1152/ajpheart.1984.246.4.H508. [DOI] [PubMed] [Google Scholar]
- 15.Endrich B, Asaishi K, Götz A, Messmer K. Technical report: A new chamber technique for microvascular studies in unanaesthetized hamsters. Res Exp Med. 1980;177:125–34. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- 16.Cabrales P, Tsai AG, Frangos JA, Intaglietta M. Role of endothelial nitric oxide in microvascular oxygen delivery and consumption. Free Radic Biol Med. 2005;39:1229–37. doi: 10.1016/j.freeradbiomed.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res. 1973;5:309–12. doi: 10.1016/0026-2862(73)90042-3. [DOI] [PubMed] [Google Scholar]
- 18.Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 19.Kerger H, Groth G, Kalenka A, Vajkoczy P, Tsai AG, Intaglietta M. pO2 measurements by phosphorescence quenching: characteristics and applications of an automated system. Microvasc Res. 2003;65:32–8. doi: 10.1016/s0026-2862(02)00027-4. [DOI] [PubMed] [Google Scholar]
- 20.Vanderkooi JM, Maniara G, Green TJ, Wilson DF. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J Biol Chem. 1987;262:5476–82. [PubMed] [Google Scholar]
- 21.Kunert MP, Liard JF, Abraham DJ, Lombard JH. Low-affinity hemoglobin increases tissue PO2 and decreases arteriolar diameter and flow in the rat cremaster muscle. Microvasc Res. 1996;52:58–68. doi: 10.1006/mvre.1996.0043. [DOI] [PubMed] [Google Scholar]
- 22.Reynafarje C, Hurtado A. Adaptation to high altitude. N Engl J Med. 1971;285:862. doi: 10.1056/nejm197110072851520. [DOI] [PubMed] [Google Scholar]
- 23.Benesch R, Edalji R, Benesch RE. The allosteric effect of inositol hexasulfate on oxygen binding by hemoglobin. Biochemistry. 1976;15:3396–8. doi: 10.1021/bi00660a035. [DOI] [PubMed] [Google Scholar]
- 24.Gibson QH, Gray RD. The reaction of inositol hexaphosphate with hemoglobin. Biochem Biophys Res Commun. 1970;41:415–20. doi: 10.1016/0006-291x(70)90520-6. [DOI] [PubMed] [Google Scholar]
- 25.Mouneimne Y, Barhoumi R, Myers T, Slogoff S, Nicolau C. Stable rightward shifts of the oxyhemoglobin dissociation curve induced by encapsulation of inositol hexaphosphate in red blood cells using electroporation. FEBS Lett. 1990;275:117–20. doi: 10.1016/0014-5793(90)81453-u. [DOI] [PubMed] [Google Scholar]
- 26.Bruggemann U, Roux EC, Hannig J, Nicolau C. Low-oxygen-affinity red cells produced in a large-volume, continuous-flow electroporation system. Transfusion. 1995;35:478–86. doi: 10.1046/j.1537-2995.1995.35695288766.x. [DOI] [PubMed] [Google Scholar]
- 27.Ledingham JM. Autoregulation in hypertension: a review. J Hypertens Suppl. 1989;7:S97–104. discussion S5. [PubMed] [Google Scholar]
- 28.Helmlinger G, Sckell A, Dellian M, Forbes NS, Jain RK. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin Cancer Res. 2002;8:1284–91. [PubMed] [Google Scholar]
- 29.Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci. 2007;97:163–80. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]