Abstract
The current study reports the effects of NIDCAP (Newborn Individualized Developmental Care and Assessment Program) at 8 years of age for a randomized controlled trial of 38 very early born (≤29 weeks postmenstrual age), high-risk preterm infants. It was hypothesized that the experimental group at school age in comparison with the control group would perform significantly better neuropsychologically and neuroelectrophysiologically. Twenty-two (11 control, 11 experimental) children of the original 38 (18 control, 20 experimental) participants were studied at school age with a detailed neuropsychological battery and with EEG spectral coherence measures. Results indicated significantly better right hemisphere and frontal lobe function in the experimental group than the control group, both neuropsychologically and neurophysiologically. Neurobehavioral and physiological results in the newborn period successfully predicted the beneficial brain function effects at age 8 years. Results support the conclusion that the NIDCAP intervention has lasting effects into school age.
Keywords: IDCAP, prematurity, neuropsychological function, EEG, spectral coherence
The incidence of prematurity is on a steady increase in the Western world. Of the 4.1 million live births in the United States each year, 12.7% (~520,700) are born prematurely. This represents a 20% increase since the year 2000.1 With advances in perinatology and neonatology survival rates of very low birth weight infants (VLBW, i.e., <1,250 grams) also have significantly increased. Prematurely born infants experience a range of physical, behavioral, and intellectual challenges,2 and recent research suggests that as they mature and both academic and life challenges increase in complexity, the gap between them and their full-term peers widens on many measures of cognition, educational achievement, behavior, and social adaptation.3,4 Preterm-born children are overrepresented among those requiring early intervention and special education services. As the demands of the learning environment become steadily more complex, they typically require an increase in educational services.1 The impact of IQ differences and learning problems intensifies in the face of increased demands on abstract integrative abilities requiring adequate memory, spatial, numerical, verbal, and phonological processing, as well as more nuanced mood, social interaction, and affect regulation. School-age preterm-born children are characterized by a range of mental control and executive function difficulties that include poor planning and problem solving and impaired working memory and inhibitory controls, all abilities associated with poor educational performance.5,6 The lifetime costs associated with prematurity far exceed the costs of newborn intensive care and include increased costs for educational, social, and emotional resources.2,7
Investigations of early brain development increasingly yield information that aids the understanding of the disabilities of preterm-born infants and children. Early brain-based differences in preterm infants8-11 are thought to contribute to the long-term disabilities, especially in terms of mild cerebral palsy and motor in-coordination,12 as well as in deficits of learning, school achievement and affect regulation.13-16
Although some of the integrative developmental differences might be explained by the cumulative effect of minor medical complications such as transient temperature control and glucose metabolism instabilities inevitably associated with preterm birth,17 the infant's sensory experience in the environment of the newborn intensive care unit (NICU), with its exposure to bright lights, high sound levels, and frequent stressful interventions clearly exert additional harmful if not damaging effects on the immature brain, and thus alter its subsequent development.18-24 The sensory environment outside the womb is an unexpected challenge for the preterm infant during a very sensitive period of rapid brain growth and differentiation.25,26 Brain science's realizations have prompted exploration of opportunities for very early developmental support and preventive intervention immediately after birth in the NICU to minimize the effects of the brain– environment mismatch and to improve the long-term outcome for preterm-born children. The Newborn Individualized Developmental Care and Assessment Program (NIDCAP)27 was created in an effort to build a system of care and environmental structure in the NICU that is specifically supportive of preterm infants’ early brain development. The synactive theory28 that underlies this approach proposes that care and environmental structures that take into account the infants’ sensory experience thresholds from functional modulation to disorganization may be supportive of long-term brain development and outcome. NIDCAP is based on the assumption that all infants, no matter how early-born, display reliably observable behaviors in the form of autonomic and visceral responses (e.g., respiration patterns, color fluctuations, spitting, hiccoughing, bowel movement strains), motor system patterns (e.g., postures; tone of trunk, extremities and face, and movements—finger splays, arching, grimacing), and state behaviors (e.g., diffuse sleep, strained wakefulness, and aroused upset),29-33 which express the current appropriateness for the individual infant of the environment (sound, light, activity, affective climate), as well as of the timing and quality of all care giving and all social interactions.27,33,34 Each infant's behavior in turn may aid the caregiving professionals in the reliable identification of the infant's restfulness, comfort, and well-being, as well as the infant's impending stress responses. Detailed observations of an infant's behavior during daily care interactions are thought to provide the essential basis for recommendations and adaptations in how best to minimize stress and optimize an individual infant's neurodevelopment. The NIDCAP studies to date have shown that the care team benefits from information and education, direct support and coaching at the bedside, and opportunity for supportive reflection in implementing such infant-behavior-based recommendations. With such education and support, the care team welcomes collaboration with and integration of the infant's family into the 24-hour schedule of regulatory and calming support that the preterm infant needs—a collaboration that parents are eager to provide, that benefits the child and that maximizes the allocation of nursing care.
Several NIDCAP randomized controlled trials (RCTs) have shown positive results in both behavioral and electrophysiological brain functioning of very early born infants (<30 weeks gestational age) at high risk for serious organ damage such as chronic lung disease and intraventricular hemorrhage.18, 20-22,35,36 Improved outcomes were not only medical in nature but also included improved neurodevelopment, as was recently reviewed.37 A meta-analysis of the NIDCAP RCTs published to date nevertheless concluded that there was as yet not sufficient evidence to broadly recommend NIDCAP as a standard of clinical care,38 stating that not only did the RCTs all involve small sample sizes, but moreover they lacked follow-up into school age. The longest follow-up published since the meta-analysis’ publication extended to age 5 years and showed significantly fewer children with severe disabilities in the NIDCAP group than in the control group.36 The goal of the current study was to explore the continuity of NIDCAP effectiveness into school age, and to test the predictability of newborn period brain function measures to school age neuropsychological results.
Methods
Design
Follow-up at 8 years of age corrected for prematurity (CA) was conducted for the preterm-born children of a 2-group (control, C and experimental, E group) RCT. The original study has been reported elsewhere.18 The follow-up study protocol reported here was approved by the review board for research with human subjects at the hospital where the school age outcome assessments occurred. All outcome assessment personnel were blind to the children's group assignments.
Subjects
The original RCT sample consisted of 43 eligible pre-term infants. Four infants were not deemed viable by the attending neonatologists; 1 family refused participation. The sample submitted to analysis consisted of 38 participants (18C, 20E). The infants were delivered at a large urban tertiary care center with a regional high-risk peri-natal service. Soon after birth, all infants were admitted to and cared for at the hospital's NICU, a 48-bed, level III nursery with an exclusively inborn population. All infants, as described in the original report18 met the following criteria: (1) gestational age at birth ≤29 weeks;39 (2) birth weight ≤1,250 grams (g); (3) conceived spontaneously; (4) singleton; (5) mechanical ventilation onset within the first 3 hours after birth; and (6) lasting for more than 24 hours in the first 48 hours; (7) alive at 48 hours; (8) absence of chromosomal or other major genetic anomalies and congenital infections; (9) at least 1 family member with some English language facility; and (10) living within the greater urban area. Families whose infants met the study criteria were recruited as soon as an eligible infant passed the 24-hour mechanical ventilation mark. All recruitments were completed within the first 3 days after delivery. Of the original 38 study infants’ families 24 (13C, 11E) continued to reside in the study's greater urban area and were contacted once the children reached 8 years corrected age (8yCA). Prior to school entry, 2 of the control group children (both Caucasian boys) had succumbed to the sequelae of very significant lung disease stemming back to their NICU stays. The remaining 22 families and their school-age children when contacted agreed to participate in the follow-up study. The background comparability of the returning 8yCA children and those who could not be located at age 8 was examined using medical and demographic background variables of the children when newborns. Analysis of variance (ANOVA) and χ2 statistics identified no differences. Furthermore, no differences were identified in the medical or the demographic background variables of the 11 C-group children when compared with the 11 E-group children participating in the study at 8yCA.
Control and Experimental Group Experience
As described in the original publication,18 the intervention that the E-group infants received was NIDCAP.40 Intervention began from admission to the NICU to 2 weeks CA (2wCA). The standard of care for the C-group infants at the time of study included efforts toward primary care nursing, individual staff dependent parent inclusion, and the by then standard and uniform shielding of incubators with blankets; dressing of infants in T-shirts, the use of side and foot rolls; and liberal provision of pacifiers. Encouragement of holding and breast feeding an infant were also staff dependent. No efforts were made in the original study to prevent spillover and contamination effects from experimental to control group care. Therefore, any significant experimental effects identified have to be interpreted as conservative, since they exceed all control group contamination effects.
NIDCAP in the original study was carried out by 2 certified NIDCAP professionals, 1 NICU nurse, and 1 psychologist, who worked with the E-group infants’ care teams and families to jointly plan and implement individualized care and to structure individualized environments supportive of each infant. More specifically, the individualized intervention consisted of weekly neurobehavioral observations, starting with the infant's initial stabilization and ending at 2wCA, by which time, as described in the original study's publication, most infants were at home with their families. The weekly reports based on the observations provided suggestions for parents and staff in terms of ways to support an infant's development. The goal was assurance of restfulness, calm breathing and well-modulated color; calm digestive tract; well-modulated face, extremity, and trunk tone; comfortable restful positions; individualized adjustment of all timing and implementation of procedures, and provision of well-supported relaxation periods. The NIDCAP professionals provided daily support and guidance for the caregivers in understanding the E-group infants’ stress and comfort signals and in adjusting care accordingly, as well as in conceptualizing the infants as active participants in the care delivered. Parents were supported in caring for their infant, encouraged to nurse and hold their infant for as much of the 24-hour cycle as they desired, as well as to cradle their infant when the infant had to be in the incubator during stressful and difficult procedures. Staff members were encouraged to offer parents comfortable recliner chairs available as part of the study to relax in and to hold and sleep with their infant in restful close contact for prolonged periods of time. Several accessories specifically designed to support the E-group infants when in the incubator or crib included natural sheepskins, terrycloth buntings, hammocks, soft special size-appropriate body and hugging pillows, and soft, special pacifiers. Furthermore, parents were encouraged to personalize their infant's bed area.
Original Medical and Demographic Background and Outcome Assessment and Results
Medical and demographic information had been obtained from the study participants’ NICU medical records, the medical record of the community hospitals and the infants’ pediatricians. Data were abstracted in a double blind fashion by the study's coordinator. The information was coded into pre-defined variables. Demographic and parent/infant/child medical history information not accessible from medical records was obtained from parent interview at 2wCA by the study's senior psychologist, who was naive to infant/child group membership. No group differences were identified in background measures for the original study sample, yet highly significant differences in a large number of medical outcome variables were found—all in favor of the E-group infants. These included among others, as published, significant reduction in the severity of chronic lung disease as well as in the incidence of intraventricular hemorrhage, earlier independent feeding, better weight gain, and earlier discharge home from the hospital.
Original Neurobehavioral and Neurophysiological Outcome Assessments and Results at 2wCA
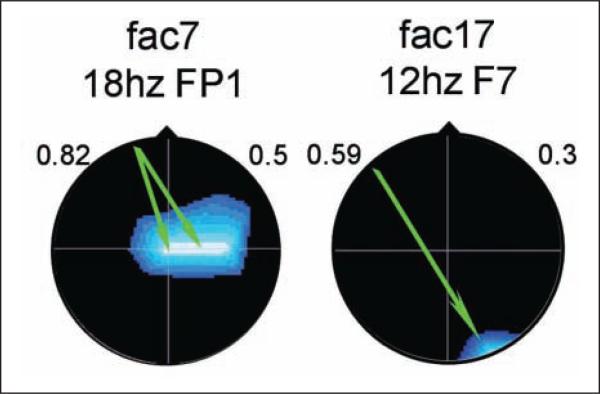
All infants at 2wCA had been assessed neurobehavior-ally with the APIB (Assessment of Preterm Infants’ Behavior),28,29 which yields 6 behavioral system scores and 12 summary variables. Results showed improved autonomic system regulation, motor system organization, and self-regulation, as well as better symmetry in orientation and in motor responses, autonomic stability, and modulation of tone, movement, and posture. The infants were also assessed neurophysiologically with electroencephalography (EEG) in sleep for which 20 scalp electrodes were employed, as described in the original publication. EEG information was analyzed with quantified methods that involved topographic mapping and group difference assessment by significance probability mapping (SPM).41 For the current follow-up study, a more advanced EEG technique, namely spectral coherence of sleep EEG, was employed. Spectral coherence between 2 EEG electrodes is generally taken as a measure of cortical coupling between the brain areas underlying the electrodes. Coherence measures have been used to assess the neural functions responsible for complex cognitive and affective regulatory processes.42-45 The EEG spectral coherence data were represented by 40 coherence factors, derived from an independent normative sample (N = 312) that also had been studied at 2wCA.8 These factors have proven successful in predicting postmenstrual age at birth, degree of medical compromise, and newborn behavioral competence.8 The EEG coherence results at 2wCA showed significant group differences on coherence factors 7 and 17,8 as depicted in Figure 1.
Figure 1.
Coherence factors at 2 weeks corrected age (2wCA), control versus experimental. Arrow color indicates E-group coherence: green = decreased; red = increased. Background color indicates loading on original principal components analysis: blue = decreased; red-orange = increased. Frequency and index electrode above. Head in vertex view, nose above, left ear to left.
Both factors differentiated the C group from the E group with probabilities of P < .02. Factor 7 involves significantly higher connectivity between mid-central to frontal brain regions in the beta frequency band. At later ages, these areas are thought to involve connections important for allocation of attention, coordination of verbal memory, temporal organization of thought and action in accordance with internal goals, reactivation of emotional states, social executive control, decision making, and social judgment.46-48 Higher connectivity in this system, evidenced by the experimental group, likely indicates greater engagement of the neural pathway subserving this specialized functioning.49-52 Factor 17 involved higher connectivity between right occipital to left anterior temporal regions in the alpha frequency band, again favoring the experimental group. This connectivity at later ages is thought to involve storage and processing of semantic information and general verbal memory functions.53-55
Medical and Demographic Background Assessment at 8yCA
Demographic and medical history information at 8yCA was obtained from parent interview by the study's senior psychologist, who was naive to infant/child group membership. With parent permission additional information was obtained from the pediatricians’ offices and Children's Hospital medical records as indicated. Medical record data were abstracted in a double blind fashion by the study's coordinator. Demographic and medical information was coded into predefined variables, including socioeconomic status of the family;56 ethnicity; gender; special school services, including resource room, reading specialists, and so on; diagnosis of cerebral palsy; and hearing loss. All children also were measured and weighed, and age at testing was also assessed. Given the recent literature as to the lifelong continuity of sequelae of prematurity,2 age was corrected for prematurity.
Neuropsychological Outcome Measures at 8yCA
To test the current study's hypotheses that NIDCAP has long-term effects and that brain-based measures in the newborn period predict to later developmental outcome, the children were assessed at 8yCA with a comprehensive battery of neuropsychological measures. The battery included the following: (1) the Wechsler Intelligence Scale for Children–Revised (WISC-R),57 which – measures verbal, performance, and full scale IQ (X̄= 100; SD = 15); (2) the Kaufman Assessment Battery for Children (K-ABC),58 which yields 4 standard scores (X̄= 100; SD = 15)—Sequential Processing, Simultaneous Processing, Mental Processing Composite (Sequential and Simultaneous), and Achievement—and is known to successfully distinguish cognitive processes from achievement, to assess hemispheric specialization, and to measure a child's neurocognitive strategies in solving problems and in processing information; (3) the Kaufman Test of Educational Achievement (K-TEA),59 an aca demic battery, which assesses skills in reading and mathematics; (4) the Expressive One-Word Picture Vocabulary Test (EOWPVT)60 and the Peabody Picture Vocabulary Test (PPVT), 61 – both widely used standard ized tests (X̄ = 100; SD = 15), to assess receptive and expressive vocabulary respectively; (5) the Test of Auditory Analysis Skills (TAAS),62 which measures oral word analysis skills; (6) Rapid Automatized Naming (RAN),63 which is a diagnostic indicator test of reading disability; (7) the Developmental Test of Visual Motor Integration (VMI)64 and the Benton Facial Recognition Test (BFRT),65 which test perception, memory, and motor planning; (8) the Rey-Osterrieth Complex Figure Test (ROCFT),66 which assesses perceptual organization and visual memory (copy and recall) and yields 4 age-referenced parameters—Organization, Style, Accuracy, and Error Score (Developmental Scoring System [DSSROCF]),67 all of which are sensitive to prematurity; (9) the Draw-A-Child Test,68 which measures perception and – fine motor skills and yields a Motor Index score (X̄ = 50; SD = 10); (10) the Test of Mental Control,69 which measures executive functioning; (11) the Laterality Test70 and the Right–Left Orientation Task,71 which assess laterality; and finally (12) the Structured Pediatric Psychosocial Inventory (SPPI),72 which measures social adaptation.
All neuropsychological assessments were performed by an experienced psychologist, purposefully blinded as to the subjects’ group identities. All studies were performed at a specially designed neurobehavioral laboratory outfitted with a 1-way mirror window and 2 hidden cameras; all assessments were videotaped for reliability check. Parent(s), if they so chose and their children agreed, watched the assessments through the 1-way mirror window. Rest breaks were interspersed as indicated. All neuropsychological variables derived from the assessments were coded and double-checked by 1 of 2 experienced blinded coders.
Given the small sample size and the large number of variables, the comprehensive battery of neuropsycho-logical measures was reduced by factor analysis into 6 independent orthogonal factors, which accounted for 89% of the variance. These 6 factors, an acceptable number of variables for sample size,73 were used for data analysis.
Neurophysiological Outcome Measures at 8yCA
Following the neuropsychological assessment, the children were assessed neurophysiologically (EEG) in the Eyes Closed (ECL) alert state. ECL was chosen for analysis because eye movement contamination is minimal. Paroxysmal epochs of eye or muscle artifact were visually identified by the senior neurophysiologist and excluded from subsequent analysis. ECL EEG was collected in 2-minute segments for a total of 12 minutes by a registered EEG technologist with expertise in pediatric EEG. Both the neurophysiologist and technologist were blinded as to the subjects’ study and group identities. All neurophysiologic assessments were performed in a specially outfitted research EEG suite immediately adjacent to the neurobehavioral laboratory. The parent(s), if they chose and their children agreed, watched the EEG assessments through a 1-way mirror window within the EEG suite.
EEG data were collected from 20 EEG channels at a 256 Hz sampling rate with subsequent bandpass filtering from 1 to 50 Hz and a 60 Hz notch filter to minimize environmentally induced artifacts such as data contamination from nearness to electrical power mains. Analyses were based upon data placed in the Laplacian, reference electrode free, format which is primarily sensitive to underlying cortex, relatively insensitive to deep/remote EEG sources, and unrelated to the particular reference used during data collection.74,75 Spectral analysis, including spectral coherence calculation as outlined by Saltzberg et al,42 was performed using a Nicolet software package. Using a spectral resolution of 2 Hz per data point (16 points over 32 Hz), among all 20 channels, 3,040 individual coherence variables were created. A multivariate regression analysis described by Semlitsch et al76 was used which, by identifying a signal proportional to a known source of artifact (e.g., prefrontal slow delta for eye blink artifact and fast beta for temporal muscle artifact) effectively removes remaining artifactual contributions. As previously described, these coherence variables— now artifact-free—were reduced in number by using in-house developed77 principal components analysis (PCA) software that includes varimax rotation and is suited to factoring of asymmetrical matrices. Forty resulting factors, representing 48% of the original coherence variance, were used for subsequent analyses.73,78 Using the rules generated by the above PCA, corresponding factors were created on the current C-group and E-group 8-year-old participants and represented them in the subsequent analyses.
Data Analysis
All statistical analyses were performed using BMDP 2007 software.79 Continuous variables were submitted to multivariate analysis of variance (MANOVA; BMDP4V), with subsequent exploratory univariate analysis of variance (ANOVA; BMDP7D).80 In cases of unequal variance, the Browne–Forsythe test of variance (F*) was used. Categorical variables were submitted either to Fisher's exact probability test (FET) for 2 × 2 tables; or to the χ2 test with Yates's correction.79,81 For all analyses, an a priori probability level of P ≤ .05, 2-tailed, was selected. The sample size assured detection at a ≤.05 probability level, of medium to large effects, which account for between 23% and 69% of the variance.82
Stepwise discriminant analysis (DSC; BMDP7M) was employed for the behavioral and the electrophysio-logical domains, at 2wCA and 8yCA. Wilks's Λ83 and jackknifed84,85 classification were performed to ascertain 2-group classification success per domain and across domains at each of the 2 age points. Canonical correlation analysis (BMDP6M) was used to explore the relationships among the behavioral and electrophysiological domains at 8yCA, as well as the data domain relationships from 2wCA to 8yCA.
Results
Medical and Demographic Variables at 8yCA
Table 1 shows the medical and demographic characteristics of the school-age children at time of testing.
Table 1.
Demographic and Medical Characteristics of the Control and Experimental Groups
| Characteristic | Control (N = 11) | Experimental (N = 11) | Test Statistic | df | P Valuea |
|---|---|---|---|---|---|
| Male/female | 6/5 | 3/8 | FET | 1 | .39 |
| Special school services | 6 | 7 | FET | 1 | 1.00 |
| Cerebral palsy | 1 | 0 | FET | 1 | 1.00 |
| Hearing loss | 1 | 1 | FET | 1 | 1.00 |
| Socioeconomic status | χ2 = 0.41 | 2 | .82 | ||
| I-II | 6 | 7 | |||
| III | 2 | 1 | |||
| IV-V | 3 | 3 | |||
| Ethnicity | FET | 1 | 1.00 | ||
| African American | 2 | 2 | |||
| Other | 9 | 9 | |||
| Age at testing | F*b = 5.42 | 1, 17 | .03 | ||
| Mean | 8 years 3.5 months | 8 years 1.2 months | |||
| SD | 2.7 months | 1.7 months |
Abbreviations: df, degrees of freedom; FET, Fisher's exact test; SD, standard deviation.
All P values are 2-tailed.
F* = Brown–Forsythe 1-way analysis of variance.
Gender, ethnicity, and social class (socioeconomic status or SES)56 distribution was comparable for the 2 groups. Aside from the 2 C-group children who had died, one other C-group child was diagnosed with cerebral palsy. Incidence of hearing loss and of requirement for special support services in school was comparable for C-group and E-group children. Growth measurement in terms of weight, height, and head circumference and their age-corrected percentiles were also comparable.
At the time of testing at 8yCA, the C-group was significantly older than the E-group. The direction of the age difference presented a bias against the experimental group and against the primary hypothesis. Given the small sample size and reduction in power when using a covariate, the age difference was not entered into further analyses as covariate. The C-group and E-group children tested were considered comparable medically and demographically.
Neuropsychological Outcome at 8yCA
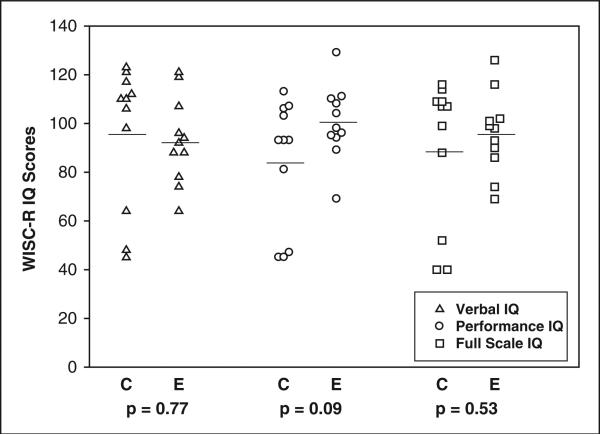
On the WISC-R,57 the E-group children showed a trend toward better Performance IQ (PIQ) scores than the C-group children, as Table 2 shows.
Table 2.
WISC-R IQ Scores for Control and Experimental Groups at 8 Years Corrected Age
| Mean (SD) | |||||
|---|---|---|---|---|---|
| IQ Scores | Control (N = 11) | Experimental (N = 11) | F a | df | P Valueb |
| Verbal | 95.82 (29.11) | 92.73 (17.72) | 0.10 | 1, 17 | .77 |
| Performance | 84.18 (26.23) | 100.27 (15.18) | 3.10 | 1, 16 | .09 |
| Full Scale | 89.27 (30.12) | 95.82 (16.53) | 0.40 | 1, 16 | .53 |
Abbreviations: WISC-R, Wechsler Intelligence Scale for Children–Revised; SD, standard deviation; df, degrees of freedom.
F = Brown–Forsythe 1-way analysis of variance.
All P values are 2-tailed.
Verbal and Full Scale IQ (VIQ and FSIQ) showed no differences between the 2 groups.
Table 3 displays the individual children's distributions of Verbal, Performance, and Full Scale IQ. Scores are arranged to show first the control and secondly the experimental group children in sequence of their initial recruitment.
Table 3.
Individual Test Scores of Wechsler Intelligence Scale for Children–Revised at 8 Years Corrected Age
| Subject | Group | Verbal IQ | Performance IQ | Full Scale IQ |
|---|---|---|---|---|
| CA | C | 123 | 93 | 109 |
| AC | C | 112 | 106 | 114 |
| TD | C | 64 | 47 | 52 |
| BD | C | 106 | 93 | 99 |
| JF C | 110 | 107 | 109 | |
| MH | C | 98 | 81 | 89 |
| BM | C | 121 | 93 | 107 |
| BP | C | 45 | 45 | 40 |
| MS | C | 110 | 103 | 107 |
| OT | C | 117 | 113 | 116 |
| SW | C | 48 | 45 | 40 |
| MA | E | 94 | 110 | 101 |
| AB | E | 78 | 98 | 86 |
| DS | E | 107 | 96 | 102 |
| MC | E | 95 | 104 | 99 |
| RD | E | 119 | 129 | 126 |
| KK | E | 88 | 111 | 98 |
| KN | E | 92 | 95 | 93 |
| AR | E | 88 | 94 | 90 |
| ES | E | 121 | 108 | 116 |
| MT | E | 64 | 89 | 74 |
| CW | E | 74 | 69 | 69 |
Abbreviations: C, control group; E, experimental group.
The distribution of IQ scores as displayed in graphic format in Figure 2 highlights the relative standing of the control and experimental group children.
Figure 2.
Wechsler Intelligence Scale for Children–Revised IQ distribution at 8 years corrected age (8yCA). C = control group; E = experimental group. Horizontal line denotes mean value.
The overall distribution is in keeping with the follow-up literature of very preterm-born children at high risk for lung disease and intraventricular hemorrhage.
The profile reflected by the standardized IQ score results is further supported by the findings of the factor analysis of the full neuropsychological battery. The 6 orthogonal factors derived from the full behavioral battery, which accounted for 89% of the data variance, significantly separated the 8yCA C-group from the E-group children by MANOVA (P < .05), as shown in Table 4.
Table 4.
Group (Control vs Experimental) Differences on Neuropsychological Factors at 8 Years Corrected Age
| Factors | P Value |
|---|---|
| Factor 1: Verbal and language abilities | .40 |
| Factor 2: Visual and spatial abilities | .01 |
| Factor 3: Automatized verbal abilities | .60 |
| Factor 4: Perceptual organization and visual memory | .68 |
| Factor 5: Verbal expression and memory | .14 |
| Factor 6: School achievement | .31 |
| MANOVA, F* = 2.52; df = 6, 15; P < .05 |
Abbreviations: MANOVA, multivariate analysis of variance; df, degrees of freedom.
Discriminant function analysis (DSC) selected 2 neuro-psychological factors (factors 2 and 5), which separated the C and E groups with 77.3% accuracy (jackknifed),84,85 Wilks's Λ = .61; F = 6.16; df = 2, 19; P = .01). The E group showed significantly better performance on factor 2, an index of spatial visualization and mental control, than the C group. Individual subtests within factor 2, evaluated descriptively, showed significant group differences in Coding (WISC-R; P < .02), Picture Completion (WISC-R; P < .04), and RAN-Colors (P < .04). These findings indicate significantly better functioning of the E-group children in mental control, attention, and integrative processing in the visual spatial domain, that is, in predominantly right hemispheric and frontal lobe functions. Factor 5, an index of memory and attentional abilities, although not significantly different between the C and E groups, nevertheless showed a trend toward better performance in the E group.
Neurophysiological Outcome at 8yCA
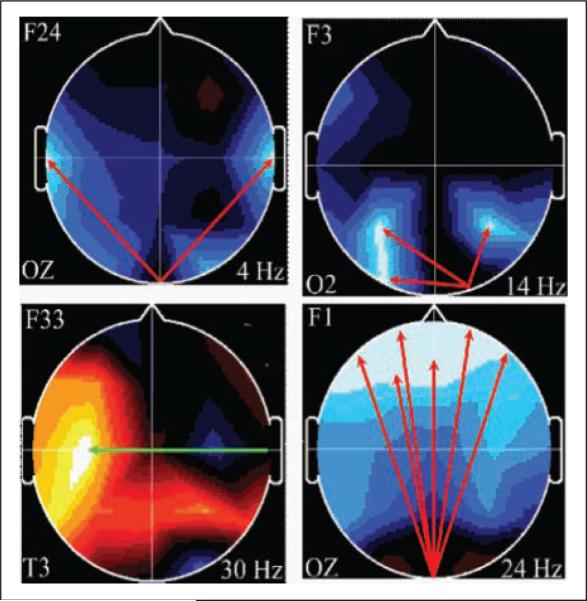
Of the 22 children studied at 8yCA, 19 children (9C, 10E) had complete electrophysiological data sets usable for coherence analysis. Of the 40 coherence factors in the ECL state, generated on the large independent sample of 275 children as described above, PCA identified 4 coherence factors (F24, F3, F33, and F1) that best differentiated the C-group children from the E-group children, as depicted in Figure 3.
Figure 3.
Coherence factors at 8 years corrected age (8yCA), control versus experimental. Arrow color indicates E-group coherence: green = decreased; red = increased. Background color indicates loading on original principal components analysis: blue = decreased; red-orange = increased. Index electrode lower left; frequency lower right. Head in vertex view, nose above, left ear to left.
These 4 factors correctly classified (jackknifed) the children with 94.7% accuracy (Wilks's Λ = .116; F = 26.57; df = 4, 14; P = .001). Coherence factor F24 involves broad connectivity between mid-occipital to bihemispheric mid-temporal regions in the 4-Hz theta band. The brain regions involved are implicated in word finding, verbal format storage, and retrieval functions (left temporal), in visual non-verbal format storage and retrieval operations (right temporal), and in organization and recall of discrete components of memory for order and recall (occipital).86 Coherence factor F3 represents connectivity between right occipital, an adjacent right parietal, a left parietal and a left occipital region in the 14-Hz beta frequency band. These brain regions are involved in visual input to the multimodal sensory association cortices bilaterally of the parietal lobes. Coherence factor F33 represents connectivity between right mid-temporal and left-central regions at 30-Hz beta frequency band. The E group's decrease in connectivity in these regions likely suggests pruning of cross-hemispheric temporal inputs to the left hemisphere. Coherence factor F1 represents connection between the mid-occipital and broad bilateral frontal association cortices at 24-Hz beta frequency band. These brain regions suggest functional transfer of visual information to frontal premotor cortices and to frontal regions involved in decision making as it relates to visual input.
Relationship of Neuropsychological and Neurophysiological Functioning at 8yCA
Canonical correlation employed to explore the relationship of the 6 neuropsychological and the 4 coherence factors at 8yCA showed a significant relationship, as identified with Bartlett's test (χ2 = 114.62; df = 72; P < .001). Neuropsychological factors 2 and 4 and coherence factors 24 and 36 showed the highest correlations to the canonical variable. Neuropsychological factor 2, as explained earlier, is a visual motor and visual spatial factor; neuropsychological factor 4, which mainly loaded on the Rey-Osterrieth Complex Figure Test,66 also implicates complex visual spatial processing, as well as visual spatial memory, executive functioning, planning, and organization. Coherence factor F24 as described above involves broad connectivity between mid-occipital to bihemispheric mid-temporal regions in the 4-Hz theta band. Coherence factor F36, not involved in the C and E-group discrimination, involves short distance connectivity between the right central and parietal regions in the slow beta range at 14 Hz. Loadings on the canonical variates indicate that the children with better visual-spatial processing and better performance on the ROCFT had enhanced connectivity between the occipital and both mid-temporal regions (factor F24) and increased connectivity between the central and parietal regions (factor F36) over the right hemisphere. The relationship between the neuropsycho-logical and neurophysiological variables thus involved the right hemisphere more than the left. The DSC results indicate that although correlated, the 2 sets of variables were not redundant.
Relationships Between 2wCA and Neuropsychological Functioning at 8yCA
Canonical correlation analysis employed to identify global correlations between variable sets in the newborn period (2wCA) and at 8yCA by Bartlett's test, failed to demonstrate any significant canonical correlations between newborn medical/demographic background or newborn medical outcome variables and the neuropsychological factors at 8yCA. However, Bartlett's test successfully identified a significant pair of canonical variates between the 18 APIB behavioral variables in the newborn period and the 6 neuropsychological factors at 8yCA (χ2 = 73.53; df = 48; P < .01). Identified were APIB variable 2 (motor system organization) and neuro-psychological factor 2 (visual and spatial abilities), as well as neuropsychological factor 6 (achievement). Similarly, canonical correlation employed to explore the relationship of the 40 neurophysiological coherence factors at 2wCA and the 6 neuropsychological factors at 8yCA revealed a significant relationship as identified with Bartlett's test (χ2 = 43.45; df = 24; P < .009). Newborn neurophysiological factor 21 represents increased long-distance interhemispheric connectivities between right frontal regions and broad bilateral occipital regions of the left hemisphere more than the right hemisphere, in the 10-Hz frequency band. These brain regions later on are involved in planning and executive function as related to visual information processing. Thus, although both the neurobehavioral and the neurophysiological measures from the newborn period are related to the neuropsychological functioning at 8yCA, none of the demographic, medical background, or medical outcome measures demonstrates any significant relationship.
Discussion
The results support the hypothesis that NIDCAP in the NICU significantly enhances neuropsychological and electrophysiological function in the E group at age 8yCA, as compared with the control group. The results are strengthened by the experimental group's younger ages at 8yCA testing. Path analysis87-89 demonstrated that neither intraventricular hemorrhage nor bipolar disorder significantly mediated the intervention effects at 8yCA.
At 2wCA, as published elsewhere18 E-group infants had shown significantly better medical outcome as well as better behavioral functioning than C-group infants, with strong differences in autonomic system regulation, motor system organization, and self-regulation, as well as better symmetry in orientation and in motor responses, autonomic stability, and modulation of tone, movement, and posture. EEG coherence results at 2wCA, as evaluated here, showed that E-group infants demonstrated enhanced cortical function in areas related to later attention, verbal memory, semantic processing, executive control, and organization of thought and action. Results are internally consistent and speak to the effectiveness of the experimental intervention and its importance in support of the immature infant's brain development from birth. These results provide compelling evidence for the effectiveness of intervention in the immediate newborn period.
When the study children reached 8yCA, those who had been in the NIDCAP group demonstrated significantly better spatial visualization and mental control than those who received standard NICU care. The EEG-derived measures of cortical connectivity also successfully differentiated E-group from C-group children at 8 years of age and corroborated the neuropsychological findings in terms of the neural pathways implicated. Furthermore, the neurobehavioral and neurophysiological measures in the newborn period better predicted neuro-psychological functioning at 8yCA than the medical/ demographic background and medical outcome variables, which failed to uncover significant relationships. The generalizability of these findings, although potentially limited by the small sample size, has important implications for the amelioration of the specific learning disabilities with which so many very early born children struggle.90-92
The study's findings validate the hypothesis that underlies NIDCAP, namely that the fetal brain in the late second semester and throughout the third trimester is differentially sensitive to the repeated stressful events experienced in the NICU; and that this vulnerability may be compensated for by consistent individualized developmental care implementation. Although the specific pathways of NIDCAP's effectiveness are not fully understood, protection against untoward changes in white matter architecture, as well as protection of programmed cell death, intercortical connectivity, and myelination, all may guard against compromised brain development. It is postulated that NIDCAP processes may involve stabilization of the NMDA (N-methyl-l-aspartate) axis and assurance of appropriate cell death (apoptosis), protection against neurocytotoxic damage, lowered sensory and pain thresholds, and increased stability with modulated thresholds of reactivity and sensitivity.93,94 Other potential mechanisms, inferred from results of differential mothering and sensory experience experiments in animal models, suggest that continuity and reliability of maternal care promotes hippocampal synaptogenesis, spatial learning and memory through systems known to mediate experience-dependent neural development.95,96 All of these effects may enhance NIDCAP group preterm infants’ capacity to take advantage of environmental offers in and beyond discharge from the stressful NICU environment.97
In the NIDCAP model of caregiving, the infant's behavior guides the caregiver in improving the infant's comfort and in decreasing the infant's stress. The NIDCAP approach focuses on individualization of care in the NICU as based on each infant's behavioral cues, to support each infant's strengths and to reduce the level of stress and pain experienced by the infant. Conscious, deliberate, and thoughtful care giving and the consistent familiar presence of and intimate contact with the parents and the family support the infant to be more calm and self-regulated. This in turn appears to facilitate brain development.98-100 The results from this study validate the differential vulnerability of the frontal regions of the preterm infant's brain and show that this vulnerability may be counteracted by NIDCAP care.19,23
The data, showing that NIDCAP may demonstrate beneficial effects for experimental group children not only in infancy but also into school age, are very encouraging. Interpretation of the findings nonetheless requires much caution. Further substantiation by larger longitudinal follow-up studies into school age is necessary to corroborate the results of this preliminary—but promising— small study.
Advances in newborn intensive care and medical practice since the time of this study have implications for the interpretation of the results, which are bound to their time. As younger infants survive, ever more vulnerable infants must experience the NICU. Furthermore, changes in care practice that were not yet an issue at the time of this study such as enforcement of “back to sleep” recommendations for sudden infant death syndrome,101 and the increasing life stresses of modern young families warrant further investigation and follow-up.
Although the exact underlying mechanisms of NIDCAP effectiveness remain to be discovered, evidence from this study points to the intervention's possible long-term effectiveness. The study's results demonstrate that NIDCAP improves health, brain development, and functional competence for preterm-born children. Future research will be required to validate the encouraging results here reported and to determine the intervention's effectiveness into adolescence and early adulthood.
Acknowledgments
This study was supported by U.S. Department of Education (NIHR, NIDRR, and NCRI-ECI) grants HO24S90003, HO23C970032, HI33G20203, and R305T990294; National Institutes of Health grant RO1HD3826, an I. B. Harris Foundation grant to H. Als, as well as a National Institute of Health grant P30HD18655 to J. J. Volpe and M. Greenberg. The authors thank the nurses and neonatologists for their support in the NICU, and foremost the infants and families for their participation and commitment in the study.
References
- 1.U.S. Department of Health and Human Services, Centers for Disease Control . National Vital Statistics Reports: Births: Final Data for 2004. U.S. Department of Health and Human Services; Washington, DC: 2005. [Google Scholar]
- 2.Behrman RE, Stith Butler A. Preterm Birth: Causes, Consequences, and Prevention. National Academies Press; Washington, DC: 2007. [PubMed] [Google Scholar]
- 3.Hack M, Taylor HG, Drotar D, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990s. JAMA. 2005;294:318–325. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- 4.Saigal S, Stoskopf B, Streiner D, et al. Transition of extremely low-birth-weight infants from adolescence to young adulthood: comparison with normal birth-weight controls. JAMA. 2006;295:667–675. doi: 10.1001/jama.295.6.667. [DOI] [PubMed] [Google Scholar]
- 5.Anderson P, Doyle L, Victorian Infant Collaborative Study Group Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pedia. 2004:50–57. doi: 10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Taylor H, Klein N, Drotar D, Schluchter M, Hack M. Consequences and risks of <1000-g birth weight for neuropsychological skills, achievement, and adaptive functioning. J Dev Behav Pedaitr. 2006:459–470. doi: 10.1097/00004703-200612000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Petrou S, Mehta Z, Hockley C, Cook-Mozaffari P, Henderson J, Goldacre M. The impact of preterm birth on hospital inpatient admissions and costs during the first 5 years of life. Pediatrics. 2003;112:1290–1297. doi: 10.1542/peds.112.6.1290. [DOI] [PubMed] [Google Scholar]
- 8.Duffy FH, Als H, McAnulty GB. Infant EEG spectral coherence data during quiet sleep: unrestricted principal components analysis—relation of factors to gestational age, medical risk, and neurobehavioral status. Clin Electroencephalogr. 2003;34:54–69. doi: 10.1177/155005940303400204. [DOI] [PubMed] [Google Scholar]
- 9.Mewes AU, Hüppi PS, Als H, et al. Regional brain development in serial MRI of low-risk preterm infants. Pediatrics. 2006;118:23–33. doi: 10.1542/peds.2005-2675. [DOI] [PubMed] [Google Scholar]
- 10.Mewes AU, Zöllei L, Hüppi PS, et al. Displacement of brain regions in preterm infants with non-synostotic dolichocephaly investigated by MRI. Neuroimage. 2007;36:1074–1085. doi: 10.1016/j.neuroimage.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constable RT, Ment LR, Vohr B, et al. Prematurely born children demonstrate white matter miscrostructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121:306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- 12.Bhushan V, Paneth N, Kiely J. Impact of improved survival of very low birth weight infants on recent secular trends in the prevalence of cerebral palsy. Pediatrics. 1993;91:1094–1100. [PubMed] [Google Scholar]
- 13.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 14.Marlow N, Hennessy E, Bracewell M, Wolke D. EPICure Study Group. Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120:793–804. doi: 10.1542/peds.2007-0440. [DOI] [PubMed] [Google Scholar]
- 15.Hemgren E, Persson K. Associations of motor co-ordination and attention with motor-perceptual development in 3-year-old preterm and full-term children who needed neonatal intensive care. Child Care Health Dev. 2007;22:11–21. doi: 10.1111/j.1365-2214.2006.00625.x. [DOI] [PubMed] [Google Scholar]
- 16.Wolke D, Samara M, Bracewell M, Marlow N, EPICure Study Group Specific language difficulties and school achievement in children born at 25 weeks of gestation or less. J Pediatr. 2008;152:256–262. doi: 10.1016/j.jpeds.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Duffy FH, Als H, McAnulty GB. Behavioral and electrophysiological evidence for gestational age effects in healthy preterm and full-term infants studied 2 weeks after expected due date. Child Dev. 1990;61:1271–1286. [PubMed] [Google Scholar]
- 18.Als H, Lawhon G, Duffy FH, McAnulty GB, Gibes-Grossman R, Blickman JG. Individualized developmental care for the very low birthweight preterm infant: Medical and neurofunctional effects. JAMA. 1994;272:853–858. [PubMed] [Google Scholar]
- 19.Buehler DM, Als H, Duffy FH, McAnulty GB, Liederman J. Effectiveness of individualized developmental care for low-risk preterm infants: behavioral and electrophysiological evidence. Pediatrics. 1995;96:923–932. [PubMed] [Google Scholar]
- 20.Fleisher BF, VandenBerg KA, Constantinou J, et al. Individualized developmental care for very-low-birth-weight premature infants. Clin Pediatr (Phila) 1995;34:523–529. doi: 10.1177/000992289503401003. [DOI] [PubMed] [Google Scholar]
- 21.Westrup B, Kleberg A, von Eichwald K, Stjernqvist K, Lagercrantz H. A randomized controlled trial to evaluate the effects of the Newborn Individualized Developmental Care and Assessment Program in a Swedish setting. Pediatrics. 2000;105:66–72. doi: 10.1542/peds.105.1.66. [DOI] [PubMed] [Google Scholar]
- 22.Kleberg A, Westrup B, Stjernqvist K, Lagercrantz H. Indications of improved cognitive development at one year of age among infants born very prematurely who received care based on the Newborn Individualized Developmental Care and Assessment Program (NIDCAP). Early Hum Dev. 2002;68:83–91. doi: 10.1016/s0378-3782(02)00014-2. [DOI] [PubMed] [Google Scholar]
- 23.Als H, Duffy F, McAnulty GB, et al. Early experience alters brain function and structure. Pediatrics. 2004;113:846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 24.Limperopoulos C, Gauvreau K, O'Leary H, et al. Cerebral hemodynamic changes during intensive care of preterm infants. Pediatrics. 2008;122:e1006–e1013. doi: 10.1542/peds.2008-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffy FH, Als H. Research Accomplishments and New Frontiers. Paul H. Brookes; Baltimore, MD: 1988. Neural plasticity and the effect of a supportive hospital environment on premature newborns. In: Kavanagh JF, ed. Understanding Mental Retardation. pp. 179–206. [Google Scholar]
- 26.Als H. Reading the premature infant. In: Goldson E, editor. Developmental Interventions in the Neonatal Intensive Care Nursery. Oxford University Press; New York, NY: 1999. pp. 18–85. [Google Scholar]
- 27.Als H. Program Guide—Newborn Individualized Developmental Care and Assessment Program (NIDCAP): An Education and Training Program for Health Care Professionals. NIDCAP Federation International; Boston, MA: 1986. revised 2008. 11th revision. [Google Scholar]
- 28.Als H. Toward a synactive theory of development: promise for the assessment of infant individuality. Inf Mental Health J. 1982;3:229–243. [Google Scholar]
- 29.Als H, Lester BM, Tronick EZ, Brazelton TB. Manual for the assessment of preterm infants' behavior (APIB). In: Fitzgerald HE, Lester BM, Yogman MW, editors. Theory and Research in Behavioral Pediatrics. Vol. 1. Plenum Press; New York, NY: 1982. pp. 65–132. [Google Scholar]
- 30.Als H. Infant individuality: Assessing patterns of very early development. In: Call J, Galenson E, Tyson RL, editors. Frontiers of Infant Psychiatry. Basic Books; New York, NY: 1983. pp. 363–378. [Google Scholar]
- 31.Als H. Manual for the Naturalistic Observation of the Newborn (Preterm and Fullterm) The Children's Hospital; Boston, MA: 1984. Revision. [Google Scholar]
- 32.Als H, Lawhon G, Brown E, et al. Individualized behavioral and environmental care for the very low birth weight preterm infant at high risk for bronchopulmonary dysplasia: neonatal intensive care unit and developmental outcome. Pediatrics. 1986;78:1123–1132. [PubMed] [Google Scholar]
- 33.Als H. Encyclopedia on Early Childhood Development. Centre of Excellence for Early Childhood Development Web site.; Individualized developmental care for preterm infants. http://www.child-encyclopedia.com/en-ca/home.html. Accessed April 14, 2009. [Google Scholar]
- 34.Gilkerson L, Als H. Role of reflective process in the implementation of developmentally supportive care in the newborn intensive care unit. Infants Young Child. 1995;7:20–28. [Google Scholar]
- 35.Als H, Gilkerson L, Duffy FH, et al. A three-center randomized controlled trial of individualized developmental care for very low birth weight preterm infants: medical, neurodevelopmental, parenting and caregiving effects. J Dev Behav Pediatr. 2003;24:399–408. 36. doi: 10.1097/00004703-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Westrup B, Böhm B, Lagercrantz H, Stjernqvist K. Pre-school outcome in children born very prematurely and cared for according to the Newborn Individu alized Developmental Care and Assessment Program (NIDCAP). Acta Paediatrica. 2004;93:498–507. doi: 10.1080/08035250410023548. [DOI] [PubMed] [Google Scholar]
- 37.Lawhon G, Hedlund R. Newborn Individualized Developmental Care and Assessment Program training and education. J Perinat Neonatal Nurs. 2008;22:133–144. doi: 10.1097/01.JPN.0000319100.90167.9f. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs S, Sokol J, Ohlsson A. The newborn individualized developmental care and assessment program is not supported by meta-analyses of the data. J Pediatr. 2002;140:699–706. doi: 10.1067/mpd.2002.123667. [DOI] [PubMed] [Google Scholar]
- 39.Ballard JL. A simplified assessment of gestational age. Pediatr Res. 1977;11:374. [Google Scholar]
- 40.Als H, Gibes R. Training Guide. Children's Hospital; Boston, MA: 1990. Newborn Individualized Developmental Care and Assessment Program (NIDCAP). Revised 1995. [Google Scholar]
- 41.Duffy F, Bartels P, Burchfiel J. Significance probability mapping: an aid in the topographic analysis of brain electrical activity. Electroencephalogr Clin Neurophysiol. 1981;51:455–462. doi: 10.1016/0013-4694(81)90221-2. [DOI] [PubMed] [Google Scholar]
- 42.Saltzberg B, Burton WD, Burch NR, Fletcher J, Michaels R. Electrophysiological measures of regional neural interactive coupling. Linear and non-linear dependence relationships among multiple channel electroencephalographic recordings. Int J Biomed Comput. 1986;18:77–87. doi: 10.1016/0020-7101(86)90050-4. [DOI] [PubMed] [Google Scholar]
- 43.Thatcher RW. Cyclic cortical reorganization during early childhood. Brain Cogn. 1992;20:24–50. doi: 10.1016/0278-2626(92)90060-y. [DOI] [PubMed] [Google Scholar]
- 44.Duffy FH, Jones KH, McAnulty GB, Albert MS. Spectral coherence in normal adults: unrestricted principal components analysis—relation of factors to age, gender, and neuropsychologic data. Clin Electroencephalogr. 1995;26:30–46. doi: 10.1177/155005949502600106. [DOI] [PubMed] [Google Scholar]
- 45.Bell MA, Fox NA. Crawling experience is related to changes in cortical organization during infancy: evidence from EEG coherence. Dev Psychobiol. 1996;29:551–561. doi: 10.1002/(SICI)1098-2302(199611)29:7<551::AID-DEV1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 46.Fuster JM. Executive frontal functions. Exp Brain Res. 2000;133:66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- 47.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 48.D'Esposito M, Postle BR. The organization of working memory function in lateral prefrontal cortex: evidence from event-related functional MRI. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford University Press; London, UK: 2002. pp. 168–187. [Google Scholar]
- 49.Gevins AS, Bressler SL, Morgan NH, et al. Event-related covariances during a bimanual visuomotor task. I. Methods and analysis of stimulus- and response-locked data. Electroencephalogr Clin Neurophysiol. 1989;74:58–75. doi: 10.1016/0168-5597(89)90052-x. [DOI] [PubMed] [Google Scholar]
- 50.Gevins AS, Cutillo BA, Bressler SL, et al. Event-related covariances during a bimanual visuomotor task. II. Preparation and feedback. Electroencephalogr Clin Neurophysiol. 1989;74:147–160. doi: 10.1016/0168-5597(89)90020-8. [DOI] [PubMed] [Google Scholar]
- 51.Gevins A. Dynamic functional topography of cognitive tasks. Brain Topogr. 1989;2:37–56. doi: 10.1007/BF01128842. [DOI] [PubMed] [Google Scholar]
- 52.Gevins AS, Bressler SL, Cutillo BA, et al. Effects of prolonged mental work on functional brain topography. Electroencephalogr Clin Neurophysiol. 1990;76:339–350. doi: 10.1016/0013-4694(90)90035-i. [DOI] [PubMed] [Google Scholar]
- 53.Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hart J, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol. 1990;27:226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- 55.Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- 56.Hollingshead AB. Four Factor Index of Social Status. Working Paper. Yale University; New Haven, CT: 1975. [Google Scholar]
- 57.Wechsler D. Wechsler Intelligence Scale for Children–Revised, WISC-R. Psychological Corporation; New York, NY: 1974. [Google Scholar]
- 58.Kaufman AS, Kaufman NL. Kaufman Assessment Battery for Children, K-ABC. American Guidance Service; Circle Pines, MN: 1983. [Google Scholar]
- 59.Kaufman AS, Kaufman NL. Kaufman Test of Educational Achievement, Comprehensive Form, K-TEA. American Guidance Service; Circle Pines, MN: 1985. [Google Scholar]
- 60.Gardner MF. Expressive One-Word Picture Vocabulary Tests–Revised. Academic Therapy Publications; Novato, CA: 1990. [Google Scholar]
- 61.Dunn LM, Dunn LM. Peabody Picture Vocabulary Test–Revised. American Guidance Service; Circle Pines, MN: 1981. [Google Scholar]
- 62.Rosner J. Helping Children Overcome Learning Difficulties. 2nd ed. Walker; New York, NY: 1979. [Google Scholar]
- 63.Denckla MB, Rudel RG. Rapid “automatized” naming of pictured objects, colors, letters and numbers by normal children. Cortex. 1974;10:186–202. doi: 10.1016/s0010-9452(74)80009-2. [DOI] [PubMed] [Google Scholar]
- 64.Beery K, Buktenica N. Developmental Test of Visual-Motor Integration. Follett; Chicago, IL: 1967. [Google Scholar]
- 65.Benton A, Van Allen M. Test of Facial Recognition. Manual. University of Iowa, Neurosensory Center; Iowa City, IA: 1973. [Google Scholar]
- 66.Osterrieth PA. Le test de copie d'une figure complexe. Arch Psychologie. 1944;30:206–356. [Google Scholar]
- 67.Bernstein JH, Waber DP. Developmental Scoring System for the Rey-Osterrieth Complex Figure. Psychological Assessment Resources; Odessa, FL: 1996. [Google Scholar]
- 68.McCarthy D. Manual for the McCarthy Scales of Children's Abilities. The Psychological Corporation; New York, NY: 1972. [Google Scholar]
- 69.Luria AR. Higher Cortical Functions in Man. Basic Books; New York, NY: 1980. [Google Scholar]
- 70.Newton M, Thomson M. Aston Index Revised. Learning Development Aids; Cambridge, UK: 1982. [Google Scholar]
- 71.Benton AL, Hamsher KD, Varney NR, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. Oxford University Press; Oxford, UK: 1983. [Google Scholar]
- 72.Webb TE, Van Devere CA. Structured Pediatric Psychosocial Interview. Fourier; Akron, OH: 1985. [Google Scholar]
- 73.Foley DH. Consideration of sample and feature size. IEEE Trans Inform Theory. 1972;IT-18:618–626. [Google Scholar]
- 74.Nunez PL. Electric Fields of the Brain. Oxford University Press; New York, NY: 1981. [Google Scholar]
- 75.Nunez PL, Pilgreen KL. The spline-Laplacian in clinical neurophysiology: a method to improve EEG spatial resolution. J Clin Neurophysiol. 1991;8:397–413. [PubMed] [Google Scholar]
- 76.Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Pschophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 77.Duffy F, Jones K, Bartels P, McAnulty G, Albert M. Unrestricted principal components analysis of brain electrical activity: issues of data dimensionality, artifact, and utility. Brain Topogr. 1992;4:291–307. doi: 10.1007/BF01135567. [DOI] [PubMed] [Google Scholar]
- 78.Bartels PH. Numerical evaluation of cytologic data III. Selection of features for discrimination. Anal Quant Cytol. 1979;1:153–159. [PubMed] [Google Scholar]
- 79.Dixon WJ. BMDP Statistical Software Manual. University of California Press; Berkeley, CA: 1988. [Google Scholar]
- 80.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Pub Health. 1996;86:726–728. doi: 10.2105/ajph.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yates F. The analysis of multiple classifications with unequal numbers in the different classes. J Am Stat. 1934;29:51–66. [Google Scholar]
- 82.Cohen J. Statistical Power for Analysis for the Behavioral Sciences. Academic Press; New York, NY: 1969. [Google Scholar]
- 83.Rao CR. Advanced Statistical Methods in Biometric Research. Hafner Press; New York, NY: 1974. [Google Scholar]
- 84.Lachenbruch PA. Discriminant Analysis. Hafner Press; New York, NY: 1975. [Google Scholar]
- 85.Lachenbruch PA, Mickey RM. Estimation of error rates in discriminant analysis. Technometrics. 1968;10:1–11. [Google Scholar]
- 86.Lezak MD. Neuropsychological Assessment. 2nd ed. Oxford University Press; New York, NY: 1983. [Google Scholar]
- 87.Duncan OD. Path analysis: sociological examples. Am J Sociol. 1966;72:1–16. [Google Scholar]
- 88.Wright S. Correlation and causation. J Agric Res. 1921;20:557–585. [Google Scholar]
- 89.Wright S. Path coefficients and path regressions: alternative or complementary concepts? Biometrics. 1960;16:189–202. [Google Scholar]
- 90.Cherkes-Julkowski M. Learning disability, attention-deficit disorder, and language impairment as outcomes of prematurity: a longitudinal descriptive study. J Learn Disabil. 1998;31:294–306. doi: 10.1177/002221949803100309. [DOI] [PubMed] [Google Scholar]
- 91.Resnick MB, Gomatam SV, Carter RL, et al. Educational disabilities of neonatal intensive care graduates. Pediatrics. 1998;102(2 pt 1):308–314. doi: 10.1542/peds.102.2.308. [DOI] [PubMed] [Google Scholar]
- 92.Aylward GP. Cognitive and neuropsychological outcomes: more than IQ scores. Mental Retard Dev Disabil Res Rev. 2002;8:234–240. doi: 10.1002/mrdd.10043. [DOI] [PubMed] [Google Scholar]
- 93.Anand KJS, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonat. 2000;77:69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- 94.Bhutta AT, Anand KJ. Vulnerability of the developing brain. Neuronal mechanisms. Clin Perinatol. 2002;29:357–372. doi: 10.1016/s0095-5108(02)00011-8. [DOI] [PubMed] [Google Scholar]
- 95.Liu D, Diorio J, Day JC, Francis DD, Meaney M. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 96.Bourgeois J. Synaptogenesis, heterochrony and epigenesis in the mammalian neocortex. Acta Paediatr Suppl. 1997;422:27–33. doi: 10.1111/j.1651-2227.1997.tb18340.x. [DOI] [PubMed] [Google Scholar]
- 97.Wolke D. Psychological development of prematurely born children. Arch Dis Child. 1998;78:567–570. doi: 10.1136/adc.78.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shore R. Rethinking the Brain: New Insights Into Early Development. Families and Work Institute. New York, NY: 1997. [Google Scholar]
- 99.Gunnar MR. Quality of Care and the Buffering of Stress Physiology: Its Potential in Protecting the Developing Human Brain. University of Minnesota Institute of Child Development; Minneapolis, MN: 1996. [Google Scholar]
- 100.Perry BD, Pollard RA, Blakley TL, Baker WL, Vigilante D. Childhood trauma, the neurobiology of adaptation, and “use dependent” development of the brain: how “states” become “traits”. Infant Mental Health J. 1995;259:271–291. [Google Scholar]
- 101.American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Policy statement. Pediatrics. 2005;116:1245–1255. doi: 10.1542/peds.2005-1499. [DOI] [PubMed] [Google Scholar]