Abstract
Fibroblast growth factor-23 (FGF-23) has been implicated in the renal phosphate wasting in X-linked hypophosphatemia, tumor-induced osteomalacia, and autosomal dominant hypophosphatemic rickets. Recently, we demonstrated that Hyp mice have greater urinary PGE2 levels compared with C57/B6 mice and that indomethacin administration in vivo and in vitro ameliorates the phosphate transport defect in Hyp mice. To determine further whether altered prostaglandin metabolism plays a role in the renal phosphate transport defect in Hyp mice, we incubated renal proximal tubules with arachidonic acid. We find that PGE2 production was higher in Hyp mice than in C57/B6 mice. Incubation of C57/B6 mouse renal proximal tubules with FGF-23R176Q, an active mutant form of FGR23, increased tubular PGE2 production, an effect that was inhibited by 50 μM PD-98059 and 10 μM SB-203580, inhibitors of the MAP kinase pathway. C57/B6 mice injected with FGF-23R176Q had a ~10-fold increase in PGE2 excretion 24 h after intraperitoneal injection of FGF-23R176Q compared with vehicle-treated controls. Finally, we show that PGE2 inhibited both phosphate and volume absorption in mouse proximal convoluted tubules perfused in vitro and reduced brush-border membrane vesicle NaPi-2a protein abundance from renal cortex incubated in vitro with PGE2. In conclusion, FGF-23 increases urinary and renal tubular PGE2 production via the MAP kinase pathway and PGE2 inhibits proximal tubule phosphate transport.
Keywords: volume absorption, in vitro microperfusion, NaPi-2a, rickets, prostaglandin E2
Tumor-induced osteomalacia, X-linked hypophosphatemia, and autosomal dominant hypophosphatemic rickets are characterized by hypophosphatemia, hyperphosphaturia, inappropriately normal 1,25(OH)2 vitamin D levels for the degree of hypophosphatemia, and defective bone mineralization. Serum fibroblast growth factor-23 (FGF-23) levels are increased in these three disorders. In tumor-induced osteomalacia, a rare paraneoplastic disorder seen in patients with mesenchymal tumors, there is renal phosphate wasting due to increased FGF-23 production (8, 9, 17, 37) and likely the secretion of other phosphaturic peptides previously known as phosphatonins (9). Tumor resection results in a reduction in FGF-23 to normal levels with concomitant correction of the disordered phosphate metabolism (39). X-linked hypophosphatemia is due to a mutation in the PHEX gene (11, 15, 24, 31). The PHEX gene is a phosphate-regulating gene with endopeptidase activity, which is located on the X chromosome. X-linked hypophosphatemia may be due to failure to inactivate normal circulating FGF-23 (7). However, others have found that FGF-23 is not a PHEX substrate (13, 20) and that in some way inactive PHEX results in increased production of FGF-23 (20). Most patients with X-linked hypophosphatemia have inappropriately high levels of FGF-23 (18, 33, 40), whereas patients with autosomal dominant hypophosphatemic rickets have a R176Q mutation in FGF-23 that resists proteolytic cleavage resulting in increased FGF-23R176Q serum levels (3, 7, 30, 36, 37).
We recently provided evidence that there is altered prostaglandin production in Hyp mice (5). We found that Hyp mice have higher urinary PGE2 excretion than C57/B6 mice. In addition, administration of indomethacin in vivo and in vitro normalized the phosphate transport defect in Hyp mice to levels comparable to those of C57/B6 mice. The present study examines whether Hyp mouse tubules have higher rates of PGE2 production than C57/B6 mice and whether this is mediated, at least in part, by FGF-23. We also examine whether PGE2 can inhibit phosphate transport in mouse proximal tubules.
METHODS
In vivo studies
We previously found that Hyp mice have higher urinary PGE2/creatine (Cr) levels than C57/B6 mice (5). To examine whether this was mediated by FGF-23, FGF-23R176Q (250 ng) was injected intraperitoneally and urine PGE2 and creatinine were measured 24 h later. FGF-23R176Q disrupts a consensus proteolytic cleavage motif but has the same action as FGF-23 (3, 27). Hyp mice are on the same genetic background as C57/B6 mice. These studies were approved by IACUC at the University of Texas Southwestern Medical Center and adhered to the APS's principles for the use of animals.
Proximal tubule isolation
C57/B6 and Hyp proximal tubules were prepared using the same protocol previously used by our laboratory (25, 26) and by others (32, 34, 35). Briefly, adult mice were sedated, killed, and their kidneys were removed and the cortex was dissected, minced, and placed in Krebs solution (4°C) that contained (in mM) 105 NaCl, 24 NaHCO3, 5 KCl, 1.5 CaCl2, 1.0 MgSO4, 2.0 Na2PO4, 5.0 glucose, 1.0 alanine, 4.0 lactate, 10.0 HEPES, 0.2% bovine serum albumin that was adjusted to 300 mosmol/kgH2O and gassed with 95% CO2-5% O2 (pH 7.4). The minced cortex was then incubated in a shaker bath for 45 min at 37°C after adding 150 mg/100 ml collagenase (Boehringer, Mannheim, Indianapolis, IN). Each prepa ration was comprised of the renal cortex from at least two mice. The solution was incubated in a shaker bath at 37°C for 45 min. The reaction was arrested by the addition of ice-cold Krebs solution (4°C), and the solution was filtered through four layers of gauze. The pellet was then washed three times and resuspended in a 45% Percoll solution (Pharmacia, Piscataway, NJ) containing (in mM) 100 NaCl, 26 NaHCO3, 3.4 KCl, 1.2 K2HPO4, 1.2 MgSO4, and 2.6 CaCl2 and centrifuged at 14,500 rpm for 30 min at 4°C to separate the proximal tubules (fourth layer) from glomeruli and distal nephron segments. The proximal tubules were washed and resuspended in a one-to-one mixture of Ham's F-12 and DMEM that contained 10−5 M arachidonic acid at a protein concentration of 2–4 mg/ml (1, 12, 16). Heparin (10 μg/ml) was added to all FGF-23R176Q incubation studies (and as vehicle in control studies) as it has been show to facilitate binding of FGF-23 to its receptor (22, 23). In addition, we found that the inhibition of FGF-23R176Q on proximal tubule phosphate transport requires heparin (6). Protein content was estimated using the BCA protein assay (Pierce, Rockford, IL). Tubules were incubated for 15 min in a rotary incubator at 37°C. After incubation, the reaction mixture was frozen in liquid nitrogen and thawed and sonicated three times to disrupt the cells. The number of individual tubule incubations is presented in the figures. There were at least three different tubule preparations for each group of experiments. PGE2 was measured using an ELISA assay (Amersham, Piscataway, NJ).
In vitro microperfusion flux studies
Isolated segments of C57/B6 mouse proximal convoluted tubules were perfused as previously described (5). Briefly, tubules were dissected in Hanks' balanced salt solution containing (in mM) 137 NaCl, 5 KCl, 0.8 MgSO4, 0.33 Na2HPO4, 0.44 KH2PO4, 1 MgCl2 10 Tris (hydroxymethyl) aminomethane hydrochloride, 0.25 CaCl2, 2 glutamine, and 2 l-lactate at 4°C. Dissected tubules were then transferred to a 1.2-ml temperature-controlled bath chamber and perfused using concentric glass pipettes at 38°C.
Proximal tubules were perfused at ~10 nl/min. The perfusion solution was an ultrafiltrate-like solution containing (in mM) 115 NaCl, 25 NaHCO3, 4.0 Na2HPO4, 10 Na acetate, 1.8 mM CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose, and 5 alanine. The bathing solution contained 6 g/dl bovine serum albumin. The osmolality of all solutions was adjusted to 295 mosmol/kgH2O. The pH and osmolality of the bathing solution were maintained constant by continuously changing the bath at a rate of at least 0.5 ml/min. Net volume absorption (JV, in nl•mm−1•min−1) was measured as the difference between the perfusion (VO) and collection (VL) rates (nl/min) normalized per millimeter of tubular length (L). [Methoxy-3H]inulin was added to the perfusate at a concentration of 75 μCi/ml so that the perfusion rate could be calculated. Inulin was dialyzed every 2 wk to eliminate dissociated [3H]methanol and then dried and stored at −20°C. The collection rate was measured with a 50-nl constant-volume pipette. The length (in mm) was measured with an eyepiece micrometer. Phosphate transport was determined using the following equation:
where Po is the phosphate concentration in the perfusate and Co* and CL* were the phosphate concentrations in the perfusate and collected fluids, respectively, in counts per minute per nanoliter.
Proximal tubules were incubated for at least 20 min before the initiation of the control period. Collections in the experimental period were initiated after 15 min of incubation. There were at least four collections in each period and the mean of these collections was used as the value for that tubule. In the experimental period, 10−6 M PGE2 was added to the bathing solution to determine whether PGE2 inhibits mouse proximal convoluted tubule phosphate transport. Previous studies demonstrated that 10−5 M PGE2 inhibits phosphate transport in the rabbit proximal straight tubule (10).
Tubule preparation and brush-border membrane vesicle isolation
C57/B6 mouse kidneys were used to determine whether PGE2 causes a reduction in NaPi-2a brush-border membrane abundance using a NaPi-2a-specific antibody, a generous gift from Dr. J. Biber (University of Zürich, Switzerland). C57/B6 mouse kidneys were removed, and the cortex was minced finely with a razor blade and then incubated in the above ultrafiltrate-like solution containing 0.5 g/dl albumin and 1.5 mg/ml collagenase (Worthington, Lakewood, NJ) for 45 min at 37°C. The resultant tissue was washed and then placed in DMEM containing 2 mM lactate and 2 mM butyrate and incubated in a 5% CO2 incubator with 10−6 M PGE2. The cortical tissue was continuously gently shaken for 3 h. The tissue was then placed in isolation buffer containing 300 mM mannitol, 16 mM HEPES, and 5 mM EGTA titrated to pH 7.4 with Tris. The isolation buffer contained aprotinin (2 μg/ml), leupeptin (2 μg/ml), and phenyl-methylsulfonyl fluoride (100 μg/ml). The cortical tissue was homogenized with 20 strokes of a Potter Elvehjem homogenizer at 4°C. Brush-border membrane vesicles (BBMV) were then isolated by differential centrifugation and magnesium precipitation as described previously (14). The final BBMV fraction was resuspended in isolation buffer. Protein was assayed using the Lowry method with crystalline BSA as the standard (21). The brush-border membrane preparation had about ninefold enrichment of the brush-border enzyme leucine amino peptidase over the initial renal cortical tissue.
SDS-PAGE and immunoblotting
Brush-border membrane proteins (25 μg/lane) were denatured and then separated on a 7.5% polyacryl-amide gel using SDS-PAGE as previously described (4, 5). The proteins were transferred overnight to a polyvinylidene diflouride membrane at 120–140 mA at 4°C. The blot was blocked with fresh Blotto (5% nonfat milk and 0.1% Tween 20 in PBS, pH 7.4) for 1 h followed by incubation with primary antibody to NaPi-2a. NaPi-2a antibody was added at 1:1,000 dilution overnight at 4°C. The blot was then washed extensively with Blotto. The secondary antibody, horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin, was added at 1:10,000 dilution and incubated in room temperature for 1 h. The blot was again washed with Blotto, and enhanced chemiluminescence was used to detect bound antibody (Amersham Life Science). The NaPi-2a protein abundance was quantitated using densitometry. Equal loading of the samples was confirmed using an antibody to β-actin at a 1:5,000 dilution (Sigma, St. Louis, MO).
Data are expressed as means ± SE. ANOVA with post hoc Student-Newman-Keuls was used to determine statistical significance in experiments with more than two groups. Paired Student's t-tests were used in microperfusion studies.
RESULTS
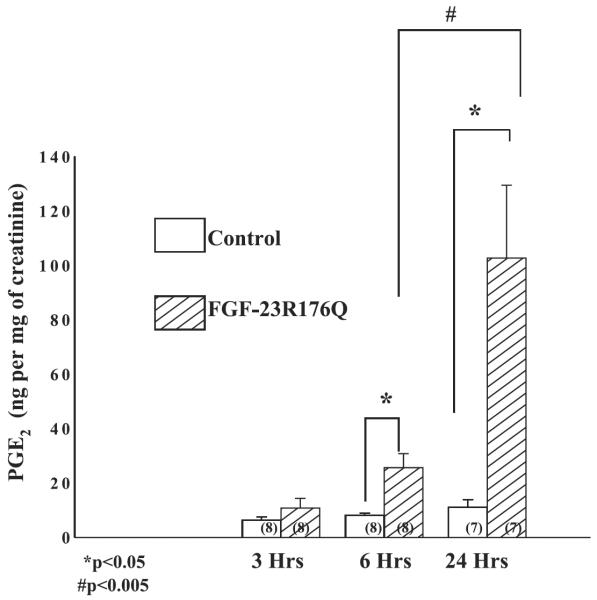
In the first series of experiments, we examined the effect of FGF-23R176Q on urinary PGE2 excretion. FGF-23R176Q (250 ng) or vehicle was injected intraperitoneally in C57/B6 mice and urine was obtained 3, 6, and 24 h later. The results of urine PGE2/Cr are shown in Fig. 1. FGF-23R176Q increased urinary PGE2/Cr levels in C57/B6 mice after 6 and 24 h.
Fig. 1.
Effect of FGF-23R176Q on urinary PGE2 excretion: 250 ng of FGF-23R176Q or vehicle were injected into the peritoneum of C57/B6 mice and urinary PGE2/creatine (Cr) was measured 3, 6, and 24 h after injection. FGF-23R176Q increased urinary PGE2/Cr (top), P < 0.05.
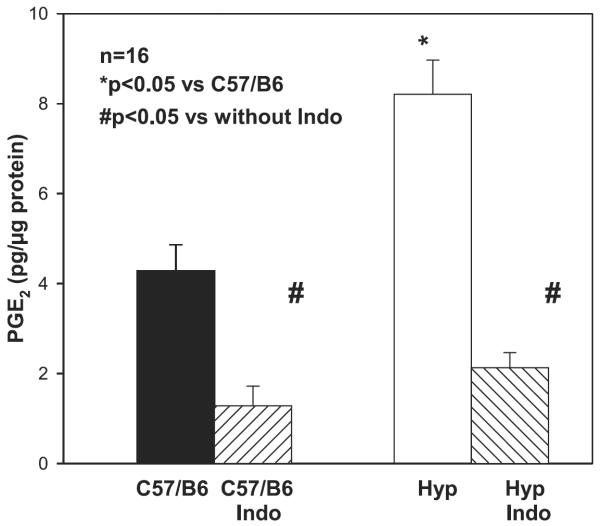
We previously demonstrated that Hyp mice have higher urinary PGE2/Cr ratios than C57/B6 mice. To examine whether proximal tubules of Hyp mice had higher rates of PGE2 production than C57/B6 mice, we incubated proximal tubules with 10−5 M arachidonic acid. As shown in Fig. 2, proximal tubules from Hyp mice had a higher PGE2 production after 15 min of incubation than proximal tubules from C57/B6 mice. PGE2 production was inhibited by indomethacin in both groups of tubules, P < 0.05.
Fig. 2.
Tubular PGE2 production in Hyp and C57/B6 mice: comparison of tubular PGE2 production in Hyp and C57/B6 mice. Proximal tubules were incubated in vitro in DMEM containing 10−5 M arachidonic acid for 15 min. PGE2 production was higher in Hyp than C57/B6 mouse proximal tubules (P < 0.05). PGE2 production was inhibited by indomethacin (P < 0.05).
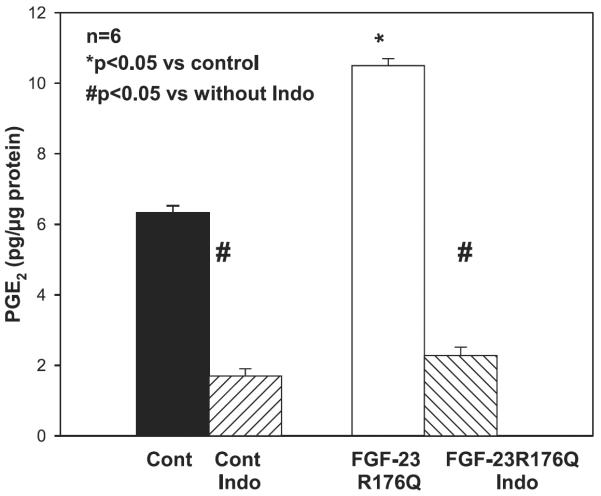
In the next series of experiments, we examined whether incubation of proximal tubules from C57/B6 mice with 20 ng/ml FGF-23R176Q would result in an increase in PGE2 production. This dose of FGF-23R176Q was chosen as it was comparable to the dose shown to produce maximal inhibition of phosphate transport in OKP cells and it is comparable to the level measured in patients with renal failure (19, 38). As shown in Fig. 3, incubation of proximal tubules from C57/B6 mice with FGF-23R176Q increased PGE2 production, an effect inhibited by indomethacin.
Fig. 3.
Effect of FGF-23R176Q on tubular PGE2 production in C57/B6 mice: comparison of tubular of 20 ng/ml FGF-23R176Q on tubular PGE2 production in C57/B6 mice. Proximal tubules were incubated in vitro in DMEM containing 10−5 M arachidonic acid for 15 min. PGE2 production was higher in proximal tubules incubated with FGF-23R176Q (P < 0.05). PGE2 production was inhibited by indomethacin (P < 0.05).
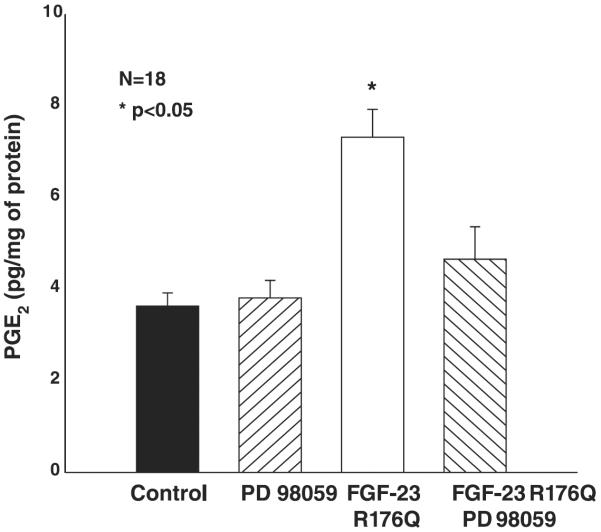
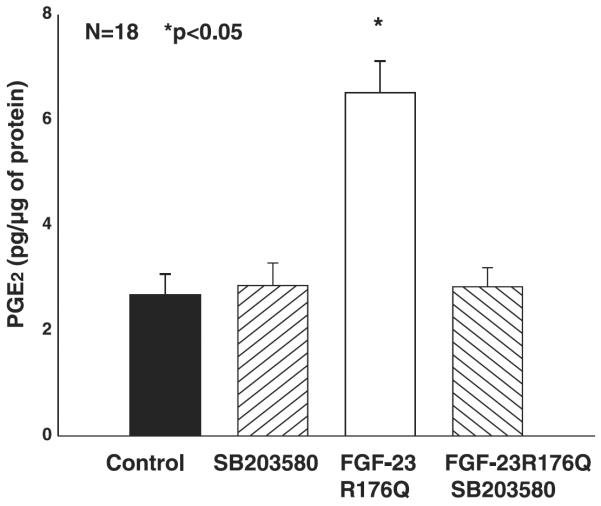
Previous studies demonstrated that the inhibition of phosphate transport by FGF-23 in OK cells was via the MAPK pathway and blocked by both PD-98059 and SB-203580. To examine whether the FGF-23-mediated increase in tubular PGE2 production is via the MAPK pathway, we incubated proximal tubules from C57/B6 mice with 50 μM PD-98059 (Fig. 4) or 10 μM SB-203580 (Fig. 5) with or without FGF-23R176Q. PGE2 production in the presence of both inhibitors was comparable to that in control C57/B6 proximal tubules. The FGF-23R176Q-mediated increase in PGE2 production was inhibited by both compounds.
Fig. 4.
Effect of FGF-23R176Q on PGE2 production in the presence of PD-98059. Proximal tubules were incubated in vitro in DMEM containing 10−5 M arachidonic acid and 20 ng/ml of FGF-23R176Q for 15 min. PD-98059 blocked the FGF-23R176Q-mediated increase in PGE2 production.
Fig. 5.
Effect of FGF-23R176Q on PGE2 production in the presence of SB-203580. Proximal tubules were incubated in vitro in DMEM containing 10−5 M arachidonic acid and 20 ng/ml of FGF-23R176Q for 15 min. SB-203580 had no effect of PGE2 production but blocked the FGF-23R176Q-mediated increase in PGE2 production.
We previously demonstrated that incubation of Hyp proximal tubules but not C57/B6 mouse proximal tubules with indomethacin resulted in an increase in phosphate transport consistent with endogenous PGE2 production mediating the inhibition in phosphate transport in Hyp mice (5). To examine directly whether PGE2 inhibits phosphate transport in mouse proximal tubule, we perfused C57/B6 mouse proximal tubules in vitro. During the experimental period, 10−6 M PGE2 was added to the bathing solution. As shown in Fig. 6, PGE2 inhibited phosphate transport in mouse proximal tubules from 12.6 ± 3.7 to 10.8 ± 3.3 pmol·mm−1·min−1, P < 0.05. PGE2 also inhibited volume absorption from 1.30 ± 0.20 to 1.00 ± 0.17 nl·mm−1·min−1, P < 0.05. A vehicle time control showed no change in volume absorption with time 0.83 ± 0.11 to 0.77 ± 0.27 nl·mm−1·min−1 (n = 6). There was actually a small increase in phosphate transport with time 14.3 ± 3.1 to 16.1 ± 3.2 pmol·mm−1·min−1, P < 0.05 (n = 6). Thus PGE2 inhibits phosphate and volume reabsorption in the mouse PCT as previously shown in the rabbit PST (10).
Fig. 6.
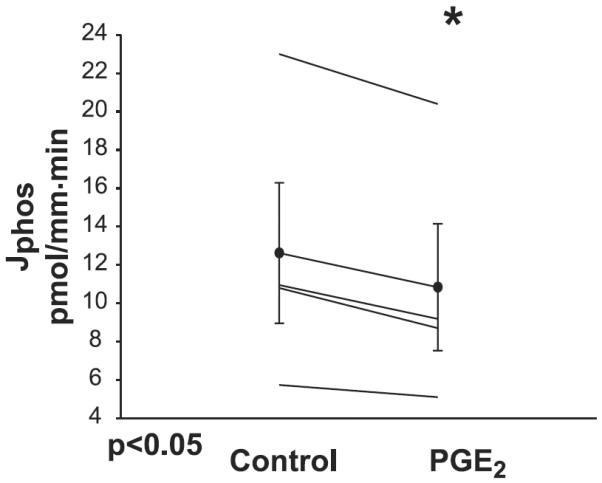
Effect of PGE2 on phosphate transport in C57/B6 mice: proximal convoluted tubules were perfused with an ultrafiltrate-like solution and bathed in a serum-like albumin solution. During the experimental period, 10−6 M PGE2 was added to the bathing solution which resulted in a decrease in phosphate transport (P < 0.05).
In the final series of experiments, we examined whether incubating a cortical tubular suspension with PGE2 would result in a reduction in BBMV NaPi-2a protein abundance. The results are shown in Fig. 7. PGE2 resulted in a 30% reduction in NaPi-2a/β-actin protein abundance (3.4 ± 0.3 vs. 2.4 ± 0.3, n = 7, P = 0.05). These results are consistent with PGE2 reducing phosphate transport, at least in part, by decreasing apical membrane NaPi-2a protein abundance.
Fig. 7.
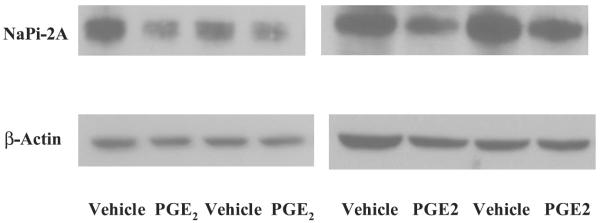
Effect of PGE2 on brush-border membrane vesicle (BBMV) NaPi-2a expression. Renal cortex from C57/B6 mice was minced and then incubated with vehicle or 10−6 M PGE2 for 3 h in tissue culture media. NaPi-2a and β-actin expression were determined from BBMV using immunoblot. The figure shows data from 2 different immunoblots. There was significantly less BBMV NaPi-2a expression in cells incubated with PGE2.
DISCUSSION
The present study demonstrated that Hyp mice proximal tubules have higher rates of PGE2 production than control mice. Injection of FGF-23R176Q into control mice resulted in an increase in PGE2 excretion and addition of FGF-23R176Q to proximal tubules in vitro increase the rates of PGE2 production, an effect mediated via the MAP kinase pathway. Finally, we demonstrate that PGE2 inhibits phosphate transport in proximal tubules by reducing apical expression of NaPi-2.
We recently provided evidence that Hyp mice have altered prostaglandin production that is involved in the defective proximal tubule phosphate transport (5). We found that urinary PGE2 was higher in Hyp mice compared with C57/B6 mice. Furthermore, indomethacin administered in vivo decreased urinary phosphate excretion and increased serum phosphate levels. This was accompanied by a normalization of BBMV NaPi-2a protein abundance. Finally, in Hyp tubules perfused in vitro, addition of indomethacin to the bathing media resulted in an increase in the rate of phosphate transport.
This study extends these findings. We find that administration of FGF-23R176Q results in an increase in urinary PGE2/creatinine excretion. In our previous study, we found that Hyp mice had a twofold greater urinary to PGE2/Cr level compared with C57/B6 mice (5). In this study, we show that 24 h after administration of FGF-23R176Q, there was a 10-fold increase in urinary PGE2/Cr level. This difference in magnitude is likely due pharmacological serum levels of FGF-23R176Q after intraperitoneal injection of 250 ng of FGF-23R176Q. In the present study, we also demonstrated that proximal tubules from Hyp mice produce more PGE2 than that of C57/B6 mice and that administration of FGF-23R176Q to C57/B6 mouse proximal tubules increases PGE2 production to levels comparable to that of Hyp mice. These observations further link FGF-23 to altered phosphate transport mediated by an increase in PGE2 production.
The time required for FGF-23 to mediate an increase in PGE2 production and inhibit phosphate transport is different in vivo and in vitro. We previously demonstrated that FGF-23R176Q inhibits phosphate transport in perfused tubules within 15–30 min after addition of the hormone to the bath (6), a similar time frame used in the perfused tubule studies examining the effect of PGE2 in these studies and for FGF-23R176Q to increase PGE2 production. Shimada et al. (28) found that 9 h after the intravenous injection of 4.5 μg of FGF-23, there was an increase in fractional excretion of phosphate and lower phosphate serum level in mice in vivo, a time comparable to our findings with an increase in PGE2 excretion. The causes for the difference in time in vivo and in vitro are unknown, but it may be due to other compensatory factors that regulate phosphate transport in vivo.
Previous studies in the rabbit showed that PGE2 can regulate transport in this segment; 10−5 M PGE2 has been shown to inhibit both volume absorption and phosphate transport in the rabbit proximal straight tubule (10). In this study, we confirm these findings and provide a further link between phosphate transport and prostaglandin production. In addition, we demonstrate that incubation of proximal tubules with PGE2 results in a reduction in BBMV NaPi-2a protein abundance.
We also examined the effect of 20 ng/ml of FGF-23R176Q on tubular PGE2 production in vitro. This dose of FGF-23R176Q was comparable to that used by others to produce maximal inhibition of phosphate transport when added to OK cells in vitro; however, lower concentrations of FGF-23 can inhibit phosphate transport in OK cells (38). We recently demonstrated that this concentration of FGF-23R176Q inhibits phosphate transport in rabbit proximal tubules perfused in vitro and causes a reduction in mouse BBMV NaPi-2 protein abundance from mouse cortex incubated in vitro (6). These levels of FGF-23R176Q may have physiological relevance as normal values or FGF-23 range between 10 and 50 ng/l (39) and are increased 100- to 1,000-fold in patients with end-stage renal disease (19). It should be noted, however, that the levels of FGF-23 in Hyp mice are only 10-fold higher than control mice (2).
FGF-23 inhibits phosphate transport in OK cells, a cell line with characteristics of the proximal tubule (7, 38). This effect of FGF-23 is mediated via an FGF receptor 3c which is linked to the MAPK pathway (38). The inhibition of phosphate transport by FGF-23 is blocked by PD-98059 and SB-203580 (38). In this study, we demonstrate that the FGF-23R176Q-mediated tubular production of PGE-2 is also inhibited by PD-98059 and SB-203580 without having an effect on basal PGE2 production.
The effect of FGF-23 on proximal tubular transport appears to be specific for phosphate transport. Injection of recombinant FGF-23 into mice resulted in a reduction in serum phosphate levels and an increase in urinary excretion of phosphate but did not cause an increase in glucose or amino acid excretion (29). In rabbit proximal tubules perfused in vitro, we found an inhibition of 20 ng/ml FGF-23R176Q on phosphate transport but not on glucose transport.
In conclusion, this study shows that FGF-23R176Q increases PGE2 excretion and tubular PGE2 production. The effect of FGF-23R176Q to increase FGF-23 is totally inhibited by blocking the MAPK pathway. Furthermore, we provide additional evidence that the increase in cellular PGE2 may mediate the inhibition in phosphate transport by FGF-23.
Acknowledgments
GRANTS This work was supported by National Institutes of Health Grant DK-065842.
REFERENCES
- 1.Alavi N, Lianos EA, Bentzel CJ. Prostaglandin and thromboxane synthesis by highly enriched rabbit proximal tubular cells in culture. J Lab Clin Med. 1987;110:338–345. [PubMed] [Google Scholar]
- 2.Aono Y, Shimada T, Yamaziki Y, Hino R, Takeuchi Y, Fujita T, Fukumoto S, Nagano N, Wada M, Yamashita T. The neutralization of FGF-23 ameliorates hypophosphatemia and rickets in Hyp mice. J Bone Miner Res. 2003;18:1056. [Google Scholar]
- 3.Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem. 2003;278:9843–9849. doi: 10.1074/jbc.M210490200. [DOI] [PubMed] [Google Scholar]
- 4.Baum M, Biemesderfer D, Gentry D, Aronson PS. Ontogeny of rabbit renal cortical NHE3 and NHE1: effect of glucocorticoids. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;268:F815–F820. doi: 10.1152/ajprenal.1995.268.5.F815. [DOI] [PubMed] [Google Scholar]
- 5.Baum M, Loleh S, Saini N, Seikaly M, Dwarakanath V, Quigley R. Correction of proximal tubule phosphate transport defect in Hyp mice in vivo and in vitro with indomethacin. Proc Natl Acad Sci USA. 2003;100:11098–11103. doi: 10.1073/pnas.1834060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum M, Schiavi S, Dwarakanath V, Quigley R. Effect of fibro-blast growth factor-23 on phosphate transport in proximal tubules. Kidney Int. 2005;68:1148–1153. doi: 10.1111/j.1523-1755.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 7.Bowe AE, Finnegan R, Jan de Beur SM, Cho J, Levine MA, Kumar R, Schiavi SC. FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun. 2001;284:977–981. doi: 10.1006/bbrc.2001.5084. [DOI] [PubMed] [Google Scholar]
- 8.De Beur SMJ, Bowe AE, Cho JY, Finnegan R, Kumar R, Levine MA, Schiavi SC. Demonstration that FGF-23, a factor produced by tumors associated with phosphate wasting, inhibits phosphate transport in vitro. J Bone Miner Res. 2001;16:S151. [Google Scholar]
- 9.De Beur SMJ, Finnegan RB, Vassiliadis J, Cook B, Barberio D, Estes S, Manavalan P, Petroziello J, Madden SL, Cho JY, Kumar R, Levine MA, Schiavi SC. Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J Bone Miner Res. 2002;17:1102–1110. doi: 10.1359/jbmr.2002.17.6.1102. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez JH, Pitts TO, Brown T, Puschett DB, Schuler F, Chen TC, Puschett JB. Prostaglandin E2 and parathyroid hormone: comparisons of their actions on the rabbit proximal tubule. Kidney Int. 1984;26:404–410. doi: 10.1038/ki.1984.189. [DOI] [PubMed] [Google Scholar]
- 11.Du L, Desbarats M, Viel J, Glorieux FH, Cawthorn C, Ecarot B. cDNA cloning of the murine Pex gene implicated in X-linked hypophosphatemia and evidence for expression in bone. Genomics. 1996;36:22–28. doi: 10.1006/geno.1996.0421. [DOI] [PubMed] [Google Scholar]
- 12.Farman N, Pradelles P, Bonvalet JP. PGE2, PGF2α, 6-keto-PGF1α, and TxB2 synthesis along the rabbit nephron. Am J Physiol Renal Fluid Electrolyte Physiol. 1987;252:F53–F59. doi: 10.1152/ajprenal.1987.252.1.F53. [DOI] [PubMed] [Google Scholar]
- 13.Guo R, Liu SG, Spurney RF, Quarles LD. Analysis of recombinant Phex: an endopeptidase in search of a substrate. Am J Physiol Endocrinol Metab. 2001;281:E837–E847. doi: 10.1152/ajpendo.2001.281.4.E837. [DOI] [PubMed] [Google Scholar]
- 14.Gupta N, Tarif SR, Seikaly M, Baum M. Role of glucocorticoids in the maturation of the rat renal Na+/H+ antiporter (NHE3) Kidney Int. 2001;60:173–181. doi: 10.1046/j.1523-1755.2001.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holm IA, Huang X, Kunkel LM. Mutational analysis of the PEX gene in patients with X-linked hypophosphatemic rickets. Am J Hum Genet. 1997;60:790–797. [PMC free article] [PubMed] [Google Scholar]
- 16.Imbert-Teboul M, Siaume S, Morel F. Sites of prostaglandin E2 (PGE2) synthesis along the rabbit nephron. Mol Cell Endocrinol. 1986;45:1–10. doi: 10.1016/0303-7207(86)90076-6. [DOI] [PubMed] [Google Scholar]
- 17.John MR, Wickert H, Zaar K, Jonsson KB, Grauer A, Ruppersberger P, Schmidt-Gayk H, Murer H, Ziegler R, Blind E. A case of neuroendocrine oncogenic osteomalacia associated with a PHEX and fibroblast growth factor-23 expressing sinusidal malignant schwannoma. Bone. 2001;29:393–402. doi: 10.1016/s8756-3282(01)00586-5. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson KB, Zahradnik R, Larsson T, White KE, Hampson G, Miyauchi A, Ljunggren O, Koshiyama H, Sugimoto T, Oba K, Yamamoto T, Imanishi Y, Econs M, Lavigne J, Jueppner H. FGF-23 is a circulating factor that is elevated in oncogenic osteomalacia and X-linked hypophosphatemic rickets. J Bone Miner Res. 2002;17:S158. [Google Scholar]
- 19.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Pellegrini L, Burke DF, von Delft F, Mulloy B, Blundell TL. Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature. 2000;407:1029–1034. doi: 10.1038/35039551. [DOI] [PubMed] [Google Scholar]
- 23.Raman R, Venkataraman G, Ernst S, Sasisekharan V, Sasisekharan R. Structural specificity of heparin binding in the fibroblast growth factor family of proteins. Proc Natl Acad Sci USA. 2003;100:2357–2362. doi: 10.1073/pnas.0437842100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowe PS, Oudet CL, Francis F, Sinding C, Pannetier S, Econs MJ, Strom TM, Meitinger T, Garabedian M, David A, Macher MA, Questiaux E, Popowska E, Pronicka E, Read AP, Mokrzycki A, Glorieux FH, Drezner MK, Hanauer A, Lehrach H, Goulding JN, O'Riordan JL. Distribution of mutations in the PEX gene in families with X-linked hypophosphataemic rickets (HYP) Hum Mol Genet. 1997;6:539–549. doi: 10.1093/hmg/6.4.539. [DOI] [PubMed] [Google Scholar]
- 25.Sakarcan A, Aricheta R, Baum M. Intracellular cystine loading causes proximal tubule respiratory dysfunction: effect of glycine. Pediatr Res. 1992;32:710–713. doi: 10.1203/00006450-199212000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Sakarcan A, Timmons C, Baum M. Intracellular distribution of cystine in cystine-loaded proximal tubules. Pediatr Res. 1994;35:447–450. [PubMed] [Google Scholar]
- 27.Segawa H, Kawakami E, Kaneko I, Kuwahata M, Ito M, Kusano K, Saito H, Fukushima N, Miyamoto K. Effect of hydrolysis-resistant FGF23-R179Q on dietary phosphate regulation of the renal type-II Na/Pi transporter. Pflügers Arch. 2003;446:585–592. doi: 10.1007/s00424-003-1084-1. [DOI] [PubMed] [Google Scholar]
- 28.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 29.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–3182. doi: 10.1210/endo.143.8.8795. [DOI] [PubMed] [Google Scholar]
- 31.The HYP Consortium A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 32.Vinay P, Gougoux A, Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol Renal Fluid Electrolyte Physiol. 1981;241:F403–F411. doi: 10.1152/ajprenal.1981.241.4.F403. [DOI] [PubMed] [Google Scholar]
- 33.Weber TJ, Liu SG, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–1234. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 34.Weinberg JM, Davis JA, Abarzua M, Kiani T, Kunkel R. Protection by glycine of proximal tubules from injury due to inhibitors of mitochondrial ATP production. Am J Physiol Cell Physiol. 1990;258:C1127–C1140. doi: 10.1152/ajpcell.1990.258.6.C1127. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg JM, Davis JA, Abarzua M, Smith RK, Kunkel R. Ouabain-induced lethal proximal tubule cell injury is prevented by glycine. Am J Physiol Renal Fluid Electrolyte Physiol. 1990;258:F346–F355. doi: 10.1152/ajprenal.1990.258.2.F346. [DOI] [PubMed] [Google Scholar]
- 36.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 37.White KE, Jonsson KB, Carn G, Hampson G, Spector TD, Mannstadt M, Lorenz-Depiereux B, Miyauchi A, Yang IM, Ljunggren O, Meitinger T, Strom TM, Juppner H, Econs MJ. The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypep-tide overexpressed by tumors that cause phosphate wasting. J Clin Endocrinol Metab. 2001;86:497–500. doi: 10.1210/jcem.86.2.7408. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita T, Konishi M, Miyake A, Inui K, Itoh N. Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J Biol Chem. 2002;277:28265–28270. doi: 10.1074/jbc.M202527200. [DOI] [PubMed] [Google Scholar]
- 39.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 40.Yamazaki Y, Shibata M, Okazaki R, Takeuchi Y, Fujita T, Yamashita T, Fukumoto S. FGF-23 protein is present in normal plasma and is increased in patients with tumor-induced osteomalacia. J Bone Miner Res. 2002;17:S159. [Google Scholar]