Abstract
Through the descriptive and rheological characterization of worm-like micelles formed by N-hexadecyl-N-methylpyrrolidinium bromide and sodium laurate, the formation and properties of the worm-like micelles were affected by the concentrations of sodium laurate and temperature. Additionally, cryogenic transmission electron microscopy images further validated the formation of worm-like micelles.
Introduction
Numerous self-aggregates, such as micelles, bicelles, micro emulsions, liquid crystals and vesicles, are able to form by the association of surfactant molecules [1]–[5]. Among these diverse microstructures, worm-like micelles have attracted extensive attention because of excellent performance in terms of viscoelastic behavior [6], [7]. Worm-like micelles have found applications in various areas over the past two decades, such as clear fracturing fluids, heat-conducting fluids, personal care products and templates for nanomaterial synthesis [8]–[11].
In dilute solutions, surfactants generally form small spherical micelles spontaneously when the concentration of surfactant is higher than the critical micelle concentration (CMC) [12], [13]. Concurrently, under appropriate conditions of surfactant concentration, salinity and temperature, the spherical micelles may grow in one-dimension and form entangled worm-like structures that present viscoelastic performance similar to that of polymer solutions [14], [15]. In agreement with the Israelachvili prediction [16], the introduction of additives facilitates the self-assembly of surfactants via electrostatic repulsion force screening [17], [18]. However, in contrast to polymers that are connected by covalent bonds, worm-like micelles are also known as “living polymer systems” [19] because they join together through ionic bonds and can break and intertwine reversibly.
Ionic liquids (ILs) are also known as organic molten electrolytes due to their distinctive physical and chemical properties, such as high conductivity, low melting temperature, outstanding catalytic properties, and so on. ILs, therefore, have earned close attention from scientific researchers. Similar to ionic surfactants, surface active ionic liquids (SAILs) exist as a hydrophilic head and a hydrophobic chain simultaneously; hence, the self-assembled aggregates can be formed in aqueous solution. In recent years, the aggregation behaviors of SAILs and their structures, which can be manipulated by changing the cations, anions, substituent, etc., have been reported frequently [20], [21]. Zhao and Zheng synthesized N-alkyl-N-methylpyrrolidinium bromide (CnMPBr) and studied its multiple assembly behaviors [22], [23]. Dong and Zheng synthesized N-alkyl-N-methylimidazolium bromide (CnmimBr) and investigated its phase behavior [24]. Geng and Zheng studied the mutual interaction effects between bovine serum albumin and N-tetradecyl-N-methylimidazolium bromide [25].
Therefore, we were motivated to explore worm-like micelles formed by C16MPBr in the presence of sodium laurate (SL). Their molecular structures of C16MPBr and SL are shown in Figure 1. To study the shape and viscoelastic properties of worm-like micelles, rheometer and cryogenic-transmission electron microscopy (cyro-TEM) were utilized. These techniques were used to observe microscopic configuration, to calculate the rheological parameters and the flow activation energy. In addition, the effect of temperature on the morphology of worm-like micelles were also studied.
Figure 1. Chemical structure of C16MPBr (a) and Sodium Laurate (b).
Materials and Methods
Materials
Cationic surfactant C16MPBr was synthesized and purified as described previously [22], [23]. Anionic surfactant SL was an AR grade product of the Aladdin Chemistry Company and was used without purification. Deionized water was used to prepare all solutions.
Sample preparation
The solutions to be analyzed were prepared by simply mixing a variable concentration of SL with a fixed concentration of C16MPBr (70 mM). Samples were homogenized by mild heating and vortex mixed and then placed in a thermostatic bath at 25°C for at least one week to equilibrate before investigation.
Rheological measurements
The rheological properties were performed on a Physica MCR301 rheometer made by Anton Paar GmbH with a Rotor CC27 system. The shearing rate ranged from 0.01 to 1000 s−1 in the steady shear experiment. For the dynamic oscillatory measurements, the frequency region was set to a range of 0.01–100 rad·s−1, and the linear viscoelastic region was identified depending on a dynamic strain sweep test, in which the frequency was fixed at 1.0 Hz.
The Maxwell-fluid model with a single relaxation time was usually used to explain the aggregation behavior of worm-like micelle solutions. The elastic modulus (storage modulus) G′, the viscous modulus (loss modulus) G″ and the complex viscosity  are given by the following equations [1], [2]:
are given by the following equations [1], [2]:
| (1) |
| (2) |
| (3) |
Here, ω is the frequency.  is the relaxation time and is approximately equal to the reciprocal of
is the relaxation time and is approximately equal to the reciprocal of  , which is the crossover frequency when G′ and G″ intersect. G0 is the plateau modulus, which is estimated by reaching a plateau at high frequency.
, which is the crossover frequency when G′ and G″ intersect. G0 is the plateau modulus, which is estimated by reaching a plateau at high frequency.
Cryo-TEM
Specimens for examination were stored in a controlled environment vitrification system with temperature and humidity control functions. The preparation of the cryo-TEM samples was performed with the following sequence of operations. First, 5 mL of the sample was placed onto a perforated polymer film held by tweezers to insure that the formation of the thin film spanned the mesh hole. Second, after 10 seconds, the samples were immediately immersed into liquid ethane just above its freezing point of −183°C. Third, the samples were then stored in liquid nitrogen to protect against contamination before they were moved to a Gatan cryo Holder 626 and examined with a FEI Tecnai 20 TEM (200 kV) at about −174°C. The images were captured with a Gatan US1000 894 CCD and processed with Leginon software.
Results and Discussion
The formation of worm-like micelles
The shear viscosity of 70 mM C16MPBr with different concentrations of SL is measured by the rheometer and the results are plotted in Figure 2a. At low SL concentrations, the samples show the typical characteristics of a Newtonian fluid; the steady-shear viscosity does not change with the shear rate. When the concentration of SL exceeds 20 mM, the samples show a non-Newtonian flow phenomenon; the viscosity maintains a constant value (Newtonian plateau value) at low shear rates and decreases dramatically at high shear rates, deviating from the simple Maxwell Model. Flow instability resulting in shear-banding that occurred in the solutions is the main reason why differences appeared from low to high shear rates. Usually, this phenomenon is identified as evidence of the formation of worm-like micelles [26]. When the concentration of SL exceeds 50 mM, the color of the solutions change from transparent to milky due to the formation of precipitate, which is generally called the salting-out effect [12].
Figure 2. Steady rheology plots.
(a) The shear viscosity for 70 mM C16MPBr with different SL concentrations at T = 25°C; (b) Variations of zero-shear viscosity ( ) as a function of different SL concentrations for the 70 mM C16MPBr. The error bars represent standard deviations.
) as a function of different SL concentrations for the 70 mM C16MPBr. The error bars represent standard deviations.
The variation of zero-shear viscosity  , which can be calculated from the average viscosity of the low shear rate, as a function of SL concentration is shown in Figure 2b. At the beginning, the values of
, which can be calculated from the average viscosity of the low shear rate, as a function of SL concentration is shown in Figure 2b. At the beginning, the values of 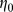 are approximately zero, indicating Newtonian fluid behavior. With the increase of SL concentration, the
are approximately zero, indicating Newtonian fluid behavior. With the increase of SL concentration, the  decreases sharply after reaching the peak value of 23.5 Pa·s at 30 mM. The value of the maximum
decreases sharply after reaching the peak value of 23.5 Pa·s at 30 mM. The value of the maximum  was typical for SAILs. An increase in the curvature energy of surfactant aggregates is induced by an increase in the salt concentration, causing the flexible worm-like micelles to form from the original spherical micelles, which schematic illustrate in figure 2b. The decrease in viscosity is generally attributed to a faster time-scale for reversible breakage which allows disentanglement to happen faster and the formation of branched micelles [27].
was typical for SAILs. An increase in the curvature energy of surfactant aggregates is induced by an increase in the salt concentration, causing the flexible worm-like micelles to form from the original spherical micelles, which schematic illustrate in figure 2b. The decrease in viscosity is generally attributed to a faster time-scale for reversible breakage which allows disentanglement to happen faster and the formation of branched micelles [27].
Rheological model of worm-like micelles
The variation profile of shear stress σ on the shear rate γ with different concentrations of SL at 70 mM C16MPBr is shown in Figure 3. The worm-like micellar solutions belong to the class of viscoelastic fluids with no yield stress, which can be inferred from the fact that all curves pass through the origin and show a linear relationship [28].
Figure 3. Shear stress with different concentration of SL at a fixed C16MPBr concentration of 70 Mm.
The viscoelasticity of worm-like micelles
To characterize the viscoelastic behavior, dynamic measurements were employed to test the C16MPBr/SL solution system. Before dynamic rheological experiments, the linear viscoelastic zone where G* (complex modulus) is independent of the applied stress was first determined through the stress sweep measurement. σ = 1.0 Pa was chosen in subsequent frequency sweep measurements according to the stress scanning of the 70 mM C16MPBr/30 mM SL solution.
Figure 4a shows the frequency (ω) dependence of the storage (G′) and loss (G″) modulus for the sample containing 70 mM C16MPBr/30 mM SL. With the increase of ω, both G′ and G″ increased in the low frequency region, and G′ continued to rise, while G″ declined after the crossover frequency  . The sample shows liquid-like behavior (G′<G″) before
. The sample shows liquid-like behavior (G′<G″) before  and solid-like behavior (G′>G″) thereafter. Finally, G′ reaches a plateau (G0), while G″ increases again after reaching a minimum (G″min). This trend fits with the viscoelastic characteristics of worm-like micelles that follow the single-element Maxwell’s model [29], [30]. The moduli G′ and G″ vary as ω2 and ω in the low frequency region, and deviation from the rules occurs in the high frequency region. These are two pieces of evidence that the formation of worm-like micelles and the deviation can be explained by breaking/recombining or the dynamic equilibrium theory [31].
and solid-like behavior (G′>G″) thereafter. Finally, G′ reaches a plateau (G0), while G″ increases again after reaching a minimum (G″min). This trend fits with the viscoelastic characteristics of worm-like micelles that follow the single-element Maxwell’s model [29], [30]. The moduli G′ and G″ vary as ω2 and ω in the low frequency region, and deviation from the rules occurs in the high frequency region. These are two pieces of evidence that the formation of worm-like micelles and the deviation can be explained by breaking/recombining or the dynamic equilibrium theory [31].
Figure 4. Dynamic oscillatory plots.
(a) Variations of G′ and G″ as a function of frequency (ω) in aqueous 70 mM C16MPBr/30 mM SL solution; (b) Cole-Cole plots (solid lines indicate the best fitting of Maxwell model).
To uncover how well the data are in accord with the semicircle characteristic of the Maxwell-fluid model, “Cole-Cole” plots of G″ versus G′ are described by [6]:
| (4) |
As shown in Figure 4b, the shape of the curve follows semicircular behavior perfectly, which indicates the formation of worm-like micelles at low and medium frequency, whereas the degree of deviation from the semicircular behavior at high frequency can be obtained by measuring the deviation of the data points.
In general, the steady-shear viscosity ( ) and the complex viscosity (
) and the complex viscosity ( ) of surfactant solutions with network structures, such as worm-like micelles, are equal or near-equal when shear rate (γ) and frequency (ω) are equivalent values. When
) of surfactant solutions with network structures, such as worm-like micelles, are equal or near-equal when shear rate (γ) and frequency (ω) are equivalent values. When  is significantly larger than
is significantly larger than  , if the structures can survive small oscillatory deformations but are ruptured by large deformations, we can draw the conclusion that the Cox–Merz empirical rule does not apply. The steady-shear viscosity as a function of
, if the structures can survive small oscillatory deformations but are ruptured by large deformations, we can draw the conclusion that the Cox–Merz empirical rule does not apply. The steady-shear viscosity as a function of  and the complex viscosity as a function of
and the complex viscosity as a function of  for the 70 mM C16MPBr/30 mM SL solution is shown in Figure 5. The coincidence of
for the 70 mM C16MPBr/30 mM SL solution is shown in Figure 5. The coincidence of  and
and  indicates that worm-like micelles that possess rigid network structure have already formed [32]. The curves deviate from the Cox–Merz empirical rule at high frequencies, a result that can be attributed to faster breakdown and reconnection of the aggregates.
indicates that worm-like micelles that possess rigid network structure have already formed [32]. The curves deviate from the Cox–Merz empirical rule at high frequencies, a result that can be attributed to faster breakdown and reconnection of the aggregates.
Figure 5. Shear-rate dependence of the steady-shear viscosity and frequency dependence of the complex viscosity for 70 mM C16MPBr/30 mM SL solution.
The rheological parameters related to the microstructures of C16MPBr solutions with different SL concentrations have been calculated according to the following formulas [1], [2], and the results are listed in Table 1.
 |
(5) |
| (6) |
| (7) |
Table 1. Various rheological parameters calculated for the aqueous solutions with different SL concentration at a fixed concentration of 7016MPBr.
| SL(mM) |

|

|

|

|

|

|

|

|
| 30 | 22.95±0.32 | 21.22±0.25 | 20.61±0.21 | 3.01±0.007 | 1.03±0.02 | 0.97±0.02 | 145±1.2 | 991±31.67 |
| 35 | 20.55±0.23 | 21.03±0.18 | 20.38±0.22 | 2.28±0.008 | 0.95±0.03 | 1.056±0.03 | 146±1.2 | 1304±24.57 |
The errors reported here were calculated from the standard deviation for all the measurements.
Here, le is the entanglement length, lp is the persistence length,  is the average contour length, kb is the Boltzmann constant, T is the absolute temperature,
is the average contour length, kb is the Boltzmann constant, T is the absolute temperature,  is the scission energy, and
is the scission energy, and 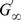 is obtained by the extrapolation of the Cole-Cole plots at high frequencies to the X-axis intercept or from the equation
is obtained by the extrapolation of the Cole-Cole plots at high frequencies to the X-axis intercept or from the equation  , where
, where  is the viscosity modulus at shear frequency ωc. Here, we set lp = 15 nm according to the literature [28]. From Table 1, it can be observed that
is the viscosity modulus at shear frequency ωc. Here, we set lp = 15 nm according to the literature [28]. From Table 1, it can be observed that  is largest and ωc is smallest for the sample at 35 mM SL. The 70 mM C16MPBr/35 mM SL solution has the longest worm-like micelles and the longest kinetic lifetimes [2].
is largest and ωc is smallest for the sample at 35 mM SL. The 70 mM C16MPBr/35 mM SL solution has the longest worm-like micelles and the longest kinetic lifetimes [2].
Effect of temperature
We also studied whether temperature has an impact on the formation of worm-like micelles. To show this effect quantitatively, rheological measurements of the 70 mM C16MPBr/30 mM SL solution at different temperatures, from 15°C to 30°C, and related rheological parameters are calculated in Table 2. The values of  ,
,  and
and  clearly decrease, but G0 alone and le are constant with temperature, albeit in an opposite manner. In the case of heating, the constant of G0 indicates that the degree of entanglement of the micellar network is unchanged, and a decline of
clearly decrease, but G0 alone and le are constant with temperature, albeit in an opposite manner. In the case of heating, the constant of G0 indicates that the degree of entanglement of the micellar network is unchanged, and a decline of  shows a lower viscosity of the sample. We speculate that the viscosity decrease is not only on account of the decrease of
shows a lower viscosity of the sample. We speculate that the viscosity decrease is not only on account of the decrease of  but also because of the amount of branching joints growing as a consequence of the rise in temperature. The growth of branching joints aggravate slide effects that accompany applied force, hence allowing a fast relaxation process and a decrement in viscosity under the condition of a maintained network structure [33].
but also because of the amount of branching joints growing as a consequence of the rise in temperature. The growth of branching joints aggravate slide effects that accompany applied force, hence allowing a fast relaxation process and a decrement in viscosity under the condition of a maintained network structure [33].
Table 2. Various rheological parameters calculated for the sample of 7016MPBr/30 mM SL at different temperatures.
| T(°C) |
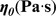
|

|

|

|
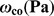
|

|

|

|
| 15 | 80.63±2.17 | 20.71±0.62 | 17.97±0.43 | 1.97±0.09 | 0.18±0.003 | 5.46±0.004 | 153±2.94 | 1394±70.77 |
| 20 | 50.31±1.03 | 20.74±0.57 | 19.52±0.54 | 2.38±0.12 | 0.44±0.02 | 2.25±0.07 | 148±1.23 | 1210±80.68 |
| 25 | 22.95±0.54 | 21.22±0.61 | 20.61±0.61 | 3.01±0.14 | 1.03±0.04 | 0.97±0.04 | 145±3.22 | 991±47.98 |
| 30 | 11.67±0.23 | 20.33±0.59 | 20.54±0.57 | 3.13±0.13 | 2.21±0.08 | 0.45±0.01 | 146±3.33 | 959±38.42 |
The errors reported here were calculated from the standard deviation for all the measurements.
The relaxation time fits the Arrhenius equation [34], resulting in a linear relationship, which can be observed from Figure 6a, which shows an Arrhenius plot of ln  versus 103/T as given by [1], [2]:
versus 103/T as given by [1], [2]:
| (8) |
Here, Ea is the flow activation energy, R is the gas constant and A is a constant. The value of Ea calculated from the slope is approximately 120kJ·mol−1, approximately equal to the values of worm-like micelle systems reported previously [35]. Figure 6b presents the plot of ln (G″min/G0) as a function of the reciprocal of the absolute temperature, according to Eq. (7). The value of  is equal to 228kJ·mol−1 for the 70 mM C16MPBr/30 mM SL solution indicates that it is more favorable to form elongated micelles with kinetic lifetime longer than conventional ionic surfactants such as CTAB [2], [12].
is equal to 228kJ·mol−1 for the 70 mM C16MPBr/30 mM SL solution indicates that it is more favorable to form elongated micelles with kinetic lifetime longer than conventional ionic surfactants such as CTAB [2], [12].
Figure 6. Temperature effect plots.
(a) An Arrhenius plot of lnτ R as a function of 1/T for the 70 mM C16MPBr/30 mM SL solution; (b) the plot of ln (G″min/G0) as a function of 1/T. The error bars represent standard deviations.
Cryo-TEM
Cryo-TEM is employed for the direct observation of the micelle morphology and micelle joints of C16MPBr/SL solutions. As above, the morphology of aggregation undergoes a transformation process from spherical aggregates to worm-like micelles. During this process, the value of  achieves a maximum value. Figure 7a and Figure 7b show that three-dimensional networks of worm-like micelles are observed in the 70 mM C16MPBr/30 mM SL solution and 70 mM C16MPBr/35 mM SL solution, respectively. The contour length could not be obtained on account of the unclear ends of the micelles. These worm-like micelles are found to overlap with each other and entangle into three-dimensional network structures, which corroborate the results of the rheology measurements, i.e., the appearance of a maximum
achieves a maximum value. Figure 7a and Figure 7b show that three-dimensional networks of worm-like micelles are observed in the 70 mM C16MPBr/30 mM SL solution and 70 mM C16MPBr/35 mM SL solution, respectively. The contour length could not be obtained on account of the unclear ends of the micelles. These worm-like micelles are found to overlap with each other and entangle into three-dimensional network structures, which corroborate the results of the rheology measurements, i.e., the appearance of a maximum  is attributed to the presence of worm-like micelles. Other than the entangled worm-like micelles, a few branched micelles with joints were also observed that can explicitly explain the temperature effect on the character of worm-like micelles.
is attributed to the presence of worm-like micelles. Other than the entangled worm-like micelles, a few branched micelles with joints were also observed that can explicitly explain the temperature effect on the character of worm-like micelles.
Figure 7. Cryo-TEM images.
(a) 70 mM C16MPBr/30 mM SL solution; (b) 70 mM C16MPBr/35 mM SL solution.
Conclusions
In summary, with the aid of sodium laurate, the aggregation behavior of worm-like micelles that fit the Maxwell model of a single stress relaxation mode has been observed in aqueous solution. These were formed by a novel surface active IL, N-hexadecyl-N-methylpyrrolidinium bromide. Rheological measurements revealed that the main factor affecting the viscoelastic properties of worm-like micelles are temperature and the concentrations of sodium laurate. Cryo-TEM imaging visually observed the formation of worm-like micelles and verified the influencing factors on the network structure of worm-like micelles. We believe that this work further explained the mechanism of formation for solutions of worm-like micelles with high viscoelasticity and serves as a possible utilization of ILs in colloidal systems.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was sponsored by the National Natural Science Foundation of China (number 51174221, 21303268), the Program for New Century Excellent Talents in University (number 20110226), China Postdoctoral Science Foundation funded project (number 2013T60689), Doctoral Fund from National Ministry of Education (number 20120133110010) and the Fundamental Research Funds for the Central Universities (number 14CX02041A). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cates ME, Candau S (1990) Statics and dynamics of worm-like surfactant micelles. Journal of Physics: Condensed Matter 2: 6869. [Google Scholar]
- 2. Rehage H, Hoffmann H (1991) Viscoelastic surfactant solutions: model systems for rheological research. Molecular Physics 74: 933–973. [Google Scholar]
- 3. Dvinskikh SV, Dürr UH, Yamamoto K, Ramamoorthy A (2007) High-resolution 2D NMR spectroscopy of bicelles to measure the membrane interaction of ligands. Journal of the American Chemical Society 129: 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chu Z, Dreiss CA, Feng Y (2013) Smart wormlike micelles. Chemical Society Reviews 42: 7174–7203. [DOI] [PubMed] [Google Scholar]
- 5. Anderson V, Pearson J, Boek E (2006) The rheology of worm-like micellar fluids. Rheology reviews 2006: 217–253. [Google Scholar]
- 6. Cates ME, Fielding SM (2006) Rheology of giant micelles. Advances in Physics 55: 799–879. [Google Scholar]
- 7. Granek R, Cates ME (1992) Stress relaxation in living polymers: Results from a Poisson renewal model. The Journal of Chemical Physics 96: 4758. [Google Scholar]
- 8. Xu J, Dürr UH, Im SC, Gan Z, Waskell L, et al. (2008) Bicelle-Enabled Structural Studies on a Membrane-Associated Cytochrome b5 by Solid-State MAS NMR Spectroscopy. Angewandte Chemie 120: 7982–7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dvinskikh SV, Yamamoto K, Dürr UH, Ramamoorthy A (2007) Sensitivity and resolution enhancement in solid-state NMR spectroscopy of bicelles. Journal of Magnetic Resonance 184: 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamamoto K, Soong R, Ramamoorthy A (2009) Comprehensive analysis of lipid dynamics variation with lipid composition and hydration of bicelles using nuclear magnetic resonance (NMR) spectroscopy. Langmuir 25: 7010–7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dvinskikh S, Dürr U, Yamamoto K, Ramamoorthy A (2006) A high-resolution solid-state NMR approach for the structural studies of bicelles. Journal of the American Chemical Society 128: 6326–6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davies TS, Ketner AM, Raghavan SR (2006) Self-assembly of surfactant vesicles that transform into viscoelastic wormlike micelles upon heating. Journal of the American Chemical Society 128: 6669–6675. [DOI] [PubMed] [Google Scholar]
- 13. Hoffmann H, Rauscher A, Gradzielski M, Schulz S (1992) Influence of ionic surfactants on the viscoelastic properties of zwitterionic surfactant solutions. Langmuir 8: 2140–2146. [Google Scholar]
- 14. Rehage H, Hoffmann H (1988) Rheological properties of viscoelastic surfactant systems. The Journal of Physical Chemistry 92: 4712–4719. [Google Scholar]
- 15. Moitzi C, Freiberger N, Glatter O (2005) Viscoelastic wormlike micellar solutions made from nonionic surfactants: structural investigations by SANS and DLS. The Journal of Physical Chemistry B 109: 16161–16168. [DOI] [PubMed] [Google Scholar]
- 16.Israelachvili JN (2011) Intermolecular and surface forces: revised third edition: Academic press.
- 17. Shikata T, Hirata H, Kotaka T (1987) Micelle formation of detergent molecules in aqueous media: viscoelastic properties of aqueous cetyltrimethylammonium bromide solutions. Langmuir 3: 1081–1086. [Google Scholar]
- 18. Candau S, Hirsch E, Zana R, Adam M (1988) Network properties of semidilute aqueous KBr solutions of cetyltrimethylammonium bromide. Journal of colloid and interface science 122: 430–440. [Google Scholar]
- 19. Candau S, Hirsch E, Zana R (1985) Light scattering investigations of the behavior of semidilute aqueous micellar solutions of cetyltrimethylammonium bromide: analogy with semidilute polymer solutions. Journal of colloid and interface science 105: 521–528. [Google Scholar]
- 20. Dong B, Li N, Zheng L, Yu L, Inoue T (2007) Surface adsorption and micelle formation of surface active ionic liquids in aqueous solution. Langmuir 23: 4178–4182. [DOI] [PubMed] [Google Scholar]
- 21. Welton T (1999) Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chemical reviews 99: 2071–2084. [DOI] [PubMed] [Google Scholar]
- 22. Zhao M, Zheng L (2011) Micelle formation by N-alkyl-N-methylpyrrolidinium bromide in aqueous solution. Physical Chemistry Chemical Physics 13: 1332–1337. [DOI] [PubMed] [Google Scholar]
- 23. Shi L, Zhao M, Zheng L (2012) Lyotropic liquid crystalline phases formed in ternary mixtures of N-alkyl-N-methylpyrrolidinium bromide/1-decanol/water. RSC Advances 2: 11922–11929. [Google Scholar]
- 24. Dong B, Zhao X, Zheng L, Zhang J, Li N, et al. (2008) Aggregation behavior of long-chain imidazolium ionic liquids in aqueous solution: micellization and characterization of micelle microenvironment. Colloids and Surfaces A: Physicochemical and Engineering Aspects 317: 666–672. [Google Scholar]
- 25. Geng F, Zheng L, Yu L, Li G, Tung C (2010) Interaction of bovine serum albumin and long-chain imidazolium ionic liquid measured by fluorescence spectra and surface tension. Process biochemistry 45: 306–311. [Google Scholar]
- 26. Ali AA, Makhloufi R (1999) Effect of organic salts on micellar growth and structure studied by rheology. Colloid and Polymer Science 277: 270–275. [Google Scholar]
- 27. Lin Z (1996) Branched worm-like micelles and their networks. Langmuir 12: 1729–1737. [Google Scholar]
- 28. Li J, Zhao M, Zheng L (2012) Salt-induced wormlike micelles formed by N-alkyl-N-methylpyrrolidinium bromide in aqueous solution. Colloids and Surfaces A: Physicochemical and Engineering Aspects 396: 16–21. [Google Scholar]
- 29. Lu T, Huang J, Li Z, Jia S, Fu H (2008) Effect of hydrotropic salt on the assembly transitions and rheological responses of cationic gemini surfactant solutions. The Journal of Physical Chemistry B 112: 2909–2914. [DOI] [PubMed] [Google Scholar]
- 30. Acharya DP, Hossain MK, Sakai T, Kunieda H (2004) Phase and rheological behaviour of viscoelastic wormlike micellar solutions formed in mixed nonionic surfactant systems. Physical Chemistry Chemical Physics 6: 1627–1631. [Google Scholar]
- 31. Pei X, Zhao J, Wei X (2011) Wormlike micelles formed by mixed cationic and anionic gemini surfactants in aqueous solution. Journal of colloid and interface science 356: 176–181. [DOI] [PubMed] [Google Scholar]
- 32. Magid L (1998) The surfactant-polyelectrolyte analogy. The Journal of Physical Chemistry B 102: 4064–4074. [Google Scholar]
- 33. Shrestha RG, Shrestha LK, Aramaki K (2007) Formation of wormlike micelle in a mixed amino-acid based anionic surfactant and cationic surfactant systems. Journal of colloid and interface science 311: 276–284. [DOI] [PubMed] [Google Scholar]
- 34. Kumar R, Kalur GC, Ziserman L, Danino D, Raghavan SR (2007) Wormlike micelles of a C22-tailed zwitterionic betaine surfactant: from viscoelastic solutions to elastic gels. Langmuir 23: 12849–12856. [DOI] [PubMed] [Google Scholar]
- 35. Shrestha RG, Tobita K, Aramaki K (2009) Rheological behavior of viscoelastic wormlike micelles in mixed N-dodecyl glutamic acid/poly (oxyethylene) hexadecyl ether systems in presence of salts. Colloids and Surfaces A: Physicochemical and Engineering Aspects 332: 103–111. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.









