Abstract
Introduction
The multitude of symptoms associated with facioscapulohumeral muscular dystrophy (FSHD) disease burden are of varying importance. The extent of these symptoms and their cumulative effect on the FSHD population is unknown.
Methods
We conducted interviews with adult FSHD patients to identify which symptoms have the greatest effect on their lives. Each interview was recorded, transcribed, coded, and analyzed using a qualitative framework technique, triangulation, and 3-investigator consensus approach.
Results
1375 quotes were obtained through 20 patient interviews. 251 symptoms of importance were identified representing 14 themes of FSHD disease burden. Symptoms associated with mobility impairment, activity limitation, and social role limitation were most frequently mentioned by participants.
Conclusions
There are multiple themes and symptoms, some previously under-recognized, that play a key role in FSHD disease burden.
Keywords: Facioscapulohumeral muscular dystrophy, quality of life, disease burden, neuromuscular disorders, symptoms
Introduction
Facioscapulohumeral muscular dystrophy (FSHD) is an autosomal dominant disorder characterized by bifacial, scapulohumeral, truncal, and lower extremity weakness with relative sparing of the deltoid and forearm muscles.1,2 FSHD is the third most common muscular dystrophy with an estimated prevalence between 1:15,000 and 1:20,000.3,4 In addition to muscular weakness, individuals with FSHD may have pain associated with impaired joint stabilization or nerve stretch, hearing loss,5 retinal vascular abnormalities (Coat syndrome),6–8 and atrial arrhythmias.9,10
Here we report findings from a series of open-ended FSHD interviews which identify and quantify the most critical issues and symptoms to FSHD patients.
Materials and Methods
We selected subjects over the age of 21 with either genetically confirmed FSHD or symptoms consistent with the diagnosis of FSHD with an affected and genetically confirmed first degree family member. A purposive sampling strategy was utilized to capture a wide range of age and disability.
We conducted in-depth interviews with subjects by telephone. Participants were asked to identify which symptoms of FSHD have the greatest impact on their quality of life (QOL) and describe how their lives are affected by having the disease. All interviews were audio-recorded and transcribed verbatim for later analysis.
Following transcription, 3 investigators independently analyzed the interviews using a framework technique for qualitative analysis.11,12 Each interview quote was familiarized, indexed, charted, mapped, matched and interpreted by a team consensus approach.11,12 Similar quotes across participants were recorded, and the quote frequency for each symptom was determined. Like symptoms were classified into symptomatic themes of FSHD health, and each theme was further categorized as a physical, mental, social, or disease-specific aspect of FSHD health. Lastly, a model and frequency table was created based on cumulative participant data to highlight the most critical areas of patient-identified FSHD health.
The entire study was conducted with informed consent and institutional review board approval.
Results
Twenty individuals participated in this study. The age of onset, deletion size, education level and occupational status was available for 13 members of our sample (supplemental table 1).
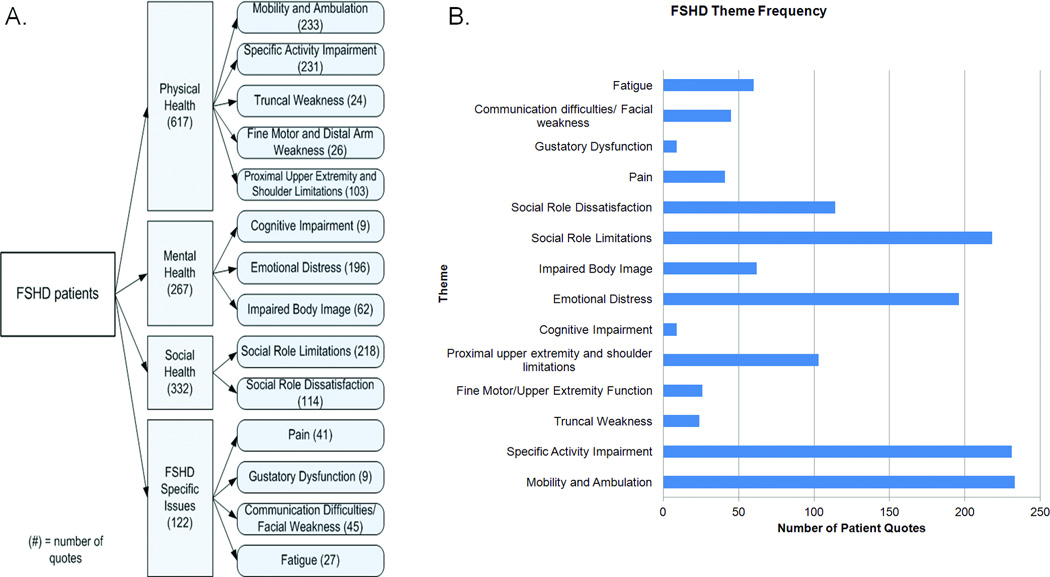
From the 20 FSHD participants, 1375 direct quotes were coded identifying 251 symptoms of importance. These symptoms represented 14 symptomatic themes and 4 major domains (figure 1). The most frequently identified symptoms were “impaired walking” followed by “difficulty with stairs”, “reliance on family members”, “difficulty lifting objects”, “limitations physically on what one can do”, and “difficulty getting places without handicap accessibility”(supplemental table 2). “Difficulty with mobility and ambulation” and “FSHD-specific activity impairment” were the symptomatic themes that had the highest number of representative quotes (figure 1). A full list of participant-identified symptoms, quote frequency, and theme classification is provided (supplemental table 2).
Figure 1.
A. Conceptual Model of Quality of Life in FSHD patients as represented by domains, subdomains and themes. Quote frequencies are provided in parentheses. B. Quote frequencies subdivided by subdomain.
Discussion
This study uses extensive patient interviews to identify the symptoms of highest importance to a sample of FSHD patients. In many instances, these identified symptoms are underreported in the FSHD literature and represent potential areas of therapeutic intervention.
Participants identified poor mobility as a theme that has a major impact on their lives. Advances in orthotic devices as well as improved accessibility to buildings may play a substantial role in further relieving future FSHD disease burden.
The effect FSHD has on a patient’s social role and emotional status is often overlooked in the clinical setting, yet these issues have a paramount importance to patients as highlighted through our interviews. Indeed, the frequency that patients mentioned the importance of social role limitations was greater than the frequency of quotes involving the cardinal features of FSHD (fascioscapulohumeral weakness). This is in agreement with other studies that have highlighted the need to address social and emotional factors in muscle diseases and those that have noted a relationship between emotional distress and the perception of disease severity.13–15 The emotional and social burdens of FSHD patients should not be neglected, as these aspects may be treatable through social services, social networking programs, education, anti-depressants, psychotherapy, and counseling.
The results from our study supplements prior work evaluating QOL in FSHD.13–24 Previous studies have largely used generic instruments (such as the SF-36 and the CIS-fatigue subscale) to characterize the impact of FSHD on patient QOL.13–24 Unfortunately, generic instruments may not be suitable for some neuromuscular diseases, as they can exclude questions that are highly relevant to a neuromuscular population while including questions that have limited relevance.14 While prior studies primarily utilize generic QOL measures to identify a global reduction in overall QOL, increased pain, and worsening fatigue in FSHD, our study further qualifies and quantifies these findings using patients’ own words and perspectives.
Previous work has also created and tested generic neuromuscular instruments by combining patient input from diverse neuromuscular populations.25,26 The Individualized Neuromuscular QOL measure was initially created using combined interview data from no fewer than 8 distinct neuromuscular populations.25 In our study all interview data came exclusively from FSHD patients. Not surprisingly, there is both overlap and uniqueness when comparing data between studies. For instance, our sample of FSHD patients did not identify “locking” or myotonia as an issue of importance, whereas a combined neuromuscular population including myotonic patients did.25 In addition, compared to myotonic dystrophy type-1 (DM1), FSHD participants report greater difficulty with proximal arm weakness, truncal weakness, facial weakness, and impaired body image.27 Our FSHD participants also identified 113 unique issues not reported by DM1 patients through similar interviews.27 These findings highlight some of the differences between neuromuscular populations and the potential need for disease-specific QOL outcome measures.28–31
This qualitative study was limited by its sample size. Future studies will need to validate the issues identified here using a larger and more diverse FSHD patient sample. All interviews were conducted by telephone. Due to this constraint, strength and functional testing were not performed. Future research will need to determine the association between patient reported disease burden and functional measures as well as age, gender, duration of symptoms, and genetics.
In conclusion, FSHD participants identify a wide array of symptoms that impact their QOL. These symptoms help to define the disease from the patient’s perspective and are potentially amendable to future therapeutic intervention.
Supplementary Material
Acknowledgements
The project described was supported by the Muscular Dystrophy Association and by Grant #1K23AR055947 from NIAMS/NIH. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the supporting agencies.
Abbreviations
- FSHD
Facioscapulohumeral muscular dystrophy
- QOL
Quality of life
- DM1
myotonic dystrophy type-1
References
- 1.Padberg G. Thesis. Leiden, the Netherlands: University of Leiden; 1982. Fascioscapulohumeral disease. [Google Scholar]
- 2.Tyler FH, Stephens FE. Studies in disorders of muscle. II Clinical manifestations and inheritance of facioscapulohumeral dystrophy in a large family. Ann Intern Med. 1950;32:640–660. doi: 10.7326/0003-4819-32-4-640. [DOI] [PubMed] [Google Scholar]
- 3.Flanigan KM, Coffeen CM, Sexton L, Stauffer D, Brunner S, Leppert MF. Genetic characterization of a large, historically significant Utah kindred with facioscapulohumeral dystrophy. Neuromuscul Disord. 2001;11:525–529. doi: 10.1016/s0960-8966(01)00201-2. [DOI] [PubMed] [Google Scholar]
- 4.Mostacciuolo ML, Pastorello E, Vazza G, Miorin M, Angelini C, Tomelleri G, et al. Facioscapulohumeral muscular dystrophy: epidemiological and molecular study in a north-east Italian population sample. Clin Genet. 2009;75:550–555. doi: 10.1111/j.1399-0004.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 5.Trevisan CP, Pastorello E, Ermani M, Angelini C, Tomelleri G, Tonin P, et al. Facioscapulohumeral muscular dystrophy: a multicenter study on hearing function. Audiol Neurootol. 2008;13:1–6. doi: 10.1159/000107431. [DOI] [PubMed] [Google Scholar]
- 6.Bass SJ, Sherman J, Giovinazzo V. Bilateral Coats' response in a female patient leads to diagnosis of facioscapulohumeral muscular dystrophy. Optometry. 2011;82:72–76. doi: 10.1016/j.optm.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Bindoff LA, Mjellem N, Sommerfelt K, Krossnes BK, Roberts F, Krohn J, et al. Severe fascioscapulohumeral muscular dystrophy presenting with Coats' disease and mental retardation. Neuromuscul Disord. 2006;16:559–563. doi: 10.1016/j.nmd.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Shields CL, Zahler J, Falk N, Furuta M, Eagle RC, Jr, Espinosa LE, et al. Neovascular glaucoma from advanced Coats disease as the initial manifestation of facioscapulohumeral dystrophy in a 2-year-old child. Arch Ophthalmol. 2007;125:840–842. doi: 10.1001/archopht.125.6.840. [DOI] [PubMed] [Google Scholar]
- 9.Statland JM, Tawil R. Facioscapulohumeral muscular dystrophy: molecular pathological advances and future directions. Curr Opin Neurol. 2011;24:423–428. doi: 10.1097/WCO.0b013e32834959af. [DOI] [PubMed] [Google Scholar]
- 10.Laforet P, de Toma C, Eymard B, Becane HM, Jeanpierre M, Fardeau M, et al. Cardiac involvement in genetically confirmed facioscapulohumeral muscular dystrophy. Neurology. 1998;51:1454–1456. doi: 10.1212/wnl.51.5.1454. [DOI] [PubMed] [Google Scholar]
- 11.McColl E. Developing questionnaires. In: Fayers P, Hays R, editors. Assessing Quality of Life in Clinical Trials. Oxford: Oxford Press; 2005. pp. 9–25. [Google Scholar]
- 12.Ritchie J, Spencer L. Anonymous Analyzing Qualitative Data. Routledge: 1994. Qualitative data analysis for applied research; pp. 173–194. [Google Scholar]
- 13.Graham CD, Rose MR, Grunfeld EA, Kyle SD, Weinman J. A systematic review of quality of life in adults with muscle disease. J Neurol. 2011;258:1581–1592. doi: 10.1007/s00415-011-6062-5. [DOI] [PubMed] [Google Scholar]
- 14.Burns TM, Graham CD, Rose MR, Simmons Z. Quality of life and measures of quality of life in patients with neuromuscular disorders. Muscle & Nerve. 2012 doi: 10.1002/mus.23245. in press. [DOI] [PubMed] [Google Scholar]
- 15.Rose MR, Sadjadi R, Weinman J, Akhatar T, Pandya S, Kissel JT, et al. The role of disease severity, illness perceptions and mood on quality of life in muscle disease. Muscle & Nerve. 2012 doi: 10.1002/mus.23320. in press. [DOI] [PubMed] [Google Scholar]
- 16.Abresch RT, Carter GT, Jensen MP, Kilmer DD. Assessment of pain and health-related quality of life in slowly progressive neuromuscular disease. Am J Hosp Palliat Care. 2002;19:39–48. doi: 10.1177/104990910201900109. [DOI] [PubMed] [Google Scholar]
- 17.Kalkman JS, Schillings ML, van der Werf SP, Padberg GW, Zwarts MJ, van Engelen BG, et al. Experienced fatigue in facioscapulohumeral dystrophy, myotonic dystrophy, and HMSN-I. J Neurol Neurosurg Psychiatry. 2005;76:1406–1409. doi: 10.1136/jnnp.2004.050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalkman JS, Schillings ML, Zwarts MJ, van Engelen BG, Bleijenberg G. The development of a model of fatigue in neuromuscular disorders: a longitudinal study. J Psychosom Res. 2007;62:571–579. doi: 10.1016/j.jpsychores.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Kalkman JS, Zwarts MJ, Schillings ML, van Engelen BG, Bleijenberg G. Different types of fatigue in patients with facioscapulohumeral dystrophy, myotonic dystrophy and HMSN-I. Experienced fatigue and physiological fatigue. Neurol Sci. 2008;29(Suppl 2):S238–S240. doi: 10.1007/s10072-008-0949-7. [DOI] [PubMed] [Google Scholar]
- 20.Minis MA, Kalkman JS, Akkermans RP, Engels JA, Huijbregts PA, Bleijenberg G, et al. Employment status of patients with neuromuscular diseases in relation to personal factors, fatigue and health status: a secondary analysis. J Rehabil Med. 2010;42:60–65. doi: 10.2340/16501977-0482. [DOI] [PubMed] [Google Scholar]
- 21.Padua L, Aprile I, Frusciante R, Iannaccone E, Rossi M, Renna R, et al. Quality of life and pain in patients with facioscapulohumeral muscular dystrophy. Muscle Nerve. 2009;40:200–205. doi: 10.1002/mus.21308. [DOI] [PubMed] [Google Scholar]
- 22.Winter Y, Schepelmann K, Spottke AE, Claus D, Grothe C, Schroder R, et al. Health-related quality of life in ALS, myasthenia gravis and facioscapulohumeral muscular dystrophy. J Neurol. 2010;257:1473–1481. doi: 10.1007/s00415-010-5549-9. [DOI] [PubMed] [Google Scholar]
- 23.Jensen MP, Hoffman AJ, Stoelb BL, Abresch RT, Carter GT, McDonald CM. Chronic pain in persons with myotonic dystrophy and facioscapulohumeral dystrophy. Arch Phys Med Rehabil. 2008;89:320–328. doi: 10.1016/j.apmr.2007.08.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miro J, Raichle KA, Carter GT, O'Brien SA, Abresch RT, McDonald CM, et al. Impact of biopsychosocial factors on chronic pain in persons with myotonic and facioscapulohumeral muscular dystrophy. Am J Hosp Palliat Care. 2009;26:308–319. doi: 10.1177/1049909109335146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent KA, Carr AJ, Walburn J, Scott DL, Rose MR. Construction and validation of a quality of life questionnaire for neuromuscular disease (INQoL) Neurology. 2007;68:1051–1057. doi: 10.1212/01.wnl.0000257819.47628.41. [DOI] [PubMed] [Google Scholar]
- 26.Sansone VA, Panzeri M, Montanari M, Apolone G, Gandossini S, Rose MR, et al. Italian validation of INQoL, a quality of life questionnaire for adults with muscle diseases. Eur J Neurol. 2010;17:1178–1187. doi: 10.1111/j.1468-1331.2010.02992.x. [DOI] [PubMed] [Google Scholar]
- 27.Heatwole CR, Bode R, Johnson NE, Quinn C, Martens WB, McDermott MP, et al. Patient Reported Impact of Symptoms in Myotonic Dystrophy Type-1: (PRISM-1) Neurology. 2012 doi: 10.1212/WNL.0b013e318260cbe6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Med Care. 1989;27:S217–S232. doi: 10.1097/00005650-198903001-00018. [DOI] [PubMed] [Google Scholar]
- 29.Wiklund I, Dimenas E, Wahl M. Factors of importance when evaluating quality of life in clinical trials. Control Clin Trials. 1990;11:169–179. doi: 10.1016/0197-2456(90)90011-p. [DOI] [PubMed] [Google Scholar]
- 30.Birbeck GL, Kim S, Hays RD, Vickrey BG. Quality of life measures in epilepsy: how well can they detect change over time? Neurology. 2000;54:1822–1827. doi: 10.1212/wnl.54.9.1822. [DOI] [PubMed] [Google Scholar]
- 31.Fletcher A, Gore S, Jones D, Fitzpatrick R, Spiegelhalter D, Cox D. Quality of life measures in health care. II: Design, analysis, and interpretation. BMJ. 1992;305:1145–1148. doi: 10.1136/bmj.305.6862.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.