Abstract
Context
Activating patients with heart failure (HF) to adhere to physician advice has not translated into clinical benefit, but past trials have had methodologic limitations.
Objective
To determine the value of self-management counseling plus HF education, over HF education alone, on the primary endpoint of death or HF hospitalization.
Design, Setting, and Patients
A single center behavioral efficacy trial in 902 patients with mild to moderate systolic or diastolic dysfunction, randomized between 2001–2004.
Interventions
All patients were offered 18 contacts and 18 HF educational tip sheets over the course of 1 year. Patients randomized to education received tip sheets in the mail and phone calls to check comprehension. Patients randomized to self-management received tip sheets in groups and were taught self-management skills to implement the advice.
Main Outcome Measure
Death or HF hospitalization, blindly adjudicated by cardiologists. Intent-to-treat results were analyzed as time-to-event and accelerated failure time models were used for non-proportional hazards.
Results
Patients were an average of 63.6 years, 47% female, 40% minority, 52% with family income <$30,000/year, and 23% with diastolic dysfunction. The self-management arm was no different from the education arm on the primary endpoint (Wilcoxon p=0.58). Post-hoc analyses on pre-specified subgroups revealed a significant income x treatment interaction (log-logistic estimate=0.64, p=0.02). Patients with income <$30,000 in self-management had a slower time to event than those in education (p=0.05) and were no different than higher income patients in either treatment arm.
Conclusions
The addition of self-management counseling to HF education does not reduce death or HF hospitalizations in patients with mild to moderate HF. Future trials should evaluate tailored outpatient HF management featuring ongoing education and comprehension checks for all, augmented by group-based skill development for those more economically disadvantaged. Such an approach may be a cost-effective, timely, and simple option for reducing HF costs.
The epidemic of heart failure (HF) is overburdening the health care system. Increased prevalence has translated into prolonged morbidity, particularly among women and the elderly.1 Fifty percent of those discharged with HF will be rehospitalized within 6 months.2 At an estimated rate of $9,400 per hospitalization,3 HF accounts for the largest single Medicare expenditure4 and a 2007 direct and indirect cost in the United States of $33.2 billion.5
Success in the control of the HF epidemic has come from advances in understanding effective, evidence-based medical therapies.6 Challenges remain, however, in the delivery of these therapies to patients. The range of patient non-adherence to HF drugs ranges is 30–60% and to lifestyle recommendations is 50–80%, with the higher rates occurring in the more socio-economically disadvantaged subgroups.7
To meet the challenge of delivering evidence-based therapies to HF patients, research has turned to the evaluation of disease management and patient self-management programs.8–11 Disease management programs extend medical care in the outpatient setting but keep patients in a passive role and, as such, raise questions about optimum duration and cost-effectiveness. In contrast, patient self-management programs activate patients to collaborate in their care by teaching them skills to implement medical advice. If these skills can be maintained over time, this is a potentially cost-effective approach to controlling HF costs.
Five HF patient self-management trials have been published to date.12–16 All are limited by small sample sizes (range: 70–197 patients), short durations (typically 2 to 3 contacts), and inadequate evaluations of maintenance of treatments effects (typically only 0–3 months). Only one trial showed benefit on a clinical endpoint (death or hospitalization)16 but baseline differences in prognostic factors limit interpretation. Thus, the value of patient self-management programs for patients with HF is questionable.
The Heart Failure Adherence and Retention Trial (HART) is the largest and most rigorous HF self-management trial to date. It was designed to evaluate the value of one year of self-management counseling plus HF education, versus HF education alone, on death or HF hospitalization in patients with mild to moderate systolic or diastolic HF.
METHODS
Design
The design and methods have been reported.17 HART was an efficacy trial with two clinically important composite primary outcomes: death or HF hospitalizations and death or all-cause hospitalizations. We focus only on death or HF hospitalization here because results were similar, but weaker, for the composite of death or all-cause hospitalization. We showed previously that self-management counseling plus HF education improved self-efficacy at self-management.18 We thus hypothesized that this benefit would extend to improvement in adherence to drug therapy, salt restriction, and depression which would, in turn, result in an improvement in the primary clinical endpoints.
Sample size was based upon the assumption that the self-management intervention would produce a 25% reduction in the primary endpoint, based upon the results of prior self-management trials.19–20 The base rate for the primary endpoint in the control group was assumed to be 15% per year, derived from rates in the treatment arms of positive drug trials with similar patients21 since these drugs would likely be the standard of care when HART ended. Dropouts and losses were estimated to be 15% and sample size was adjusted by approximately 3% to allow for interim analyses. Assuming a 2-sided alpha of 0.05 and 80% power, this led to a sample size of 900 patients, evenly distributed between 2 treatment arms.
Double-blinding in a behavioral trial is impossible as patients are aware of the treatment they are receiving. However, HART was a partially blinded trial. All staff, except for the senior investigators, and all participants were blinded to trial hypotheses by providing neutral names for the treatment arms (i.e., “Skills Training” for the self-management arm and “Enhanced Education” for the education arm). All investigators and staff, except for the data management team, were blinded to the randomization status of the participant. Treatment teams within each trial arm had no contact with patients in the other arm.
The protocol was approved by the institutional committees on human research at the 10 recruiting hospitals.
Trial Patients
Eligible patients had systolic or diastolic HF for not less than the prior 3 months, defined as either: (1) left ventricular ejection fraction ≤40% by echocardiography, radiographic ventriculography, or radionuclide ventriculography; or (2) diuretic therapy for at least 3 months plus one previous hospitalization for HF.
Exclusions were factors that would jeopardize the conduct or rigor of the trial. These included: (1) uncertain 12-month prognosis (i.e., New York Heart Association (NYHA) Class IV, likelihood of cardiac transplant over the next year, symptomatic or sustained ventricular tachycardia, or other illnesses that limit 12-month survival); (2) NYHA Class I who were unlikely to have a primary endpoint; (3) unlikely to benefit from the behavioral treatment (i.e., cognitive dysfunction, substance abuse, psychotic disorder, or active suicidal ideation); (4) HF symptoms that may be eliminated by surgery (e.g., severe aortic stenosis); (5) logistical issues (e.g., enrollment in a conflicting protocol, non-English speaking); (6) physicians refusal; (7) unwillingness to make lifestyle changes now or in the near future; and (8) unstable angina, myocardial infarction (MI), coronary artery bypass graft (CABG) surgery, or percutaneous transluminal coronary angioplasty (PTCA) within the last month (temporary exclusion).
Recruitment and Randomization
Recruitment of patients was conducted between October 2001 and October 2004 at 10 recruiting hospitals located throughout the Chicago metropolitan area. Each recruiting hospital had a local cardiologist who served as the local principal investigator for the trial (Appendix). Patients were recruited through inpatient and outpatient screening and referrals from cardiologists and internists, tailored to the features of the setting.
Informed consent was obtained from potential eligible patients and the baseline examination was conducted. At the conclusion of the examination, the nurse coordinator called the automated randomization service (Moffitt Cancer Center, University of South Florida) and treatment assignment was sent to an unblinded staff member who mailed a letter of notification to the patient. Follow-up by staff leading the relevant trial arm commenced approximately 2–5 days after receipt of the letter.
To make group treatment logistically feasible, randomization was conducted using a stratified block design with strata defined as four geographic locations and blocks of size 20. Once 20 patients from the same geographic area were recruited, randomization resulted in 10 patients assigned to a group held within their community.
Treatments
A detailed description of the treatments tested in HART has been reported.17 The self-management plus education treatment (called “self-management”) featured group-based HF education plus counseling to help patients develop mastery in problem-solving skills and in 5 self-management skills. Eighteen 2-hour group meetings of 5–10 patients were spread over the course of one year. At each meeting, education in the form of 18 Heart Failure Tip Sheets from the American Heart Association (http://www.hearthub.org/hc-heart-failure.htm) summarized basic elements of HF management including medication adherence, sudden weight gain, salt restriction, moderate physical activity, and stress management. Implementation of this advice was aided by training in five self-mangement skills: self-monitoring, environmental restructuring, elicitation of support from family and friends, cognitive restructuring, and the relaxation response. To foster proactivity in invoking these skills when needed, a problem-solving format was used where patients identified barriers to implementing the tips and the use of self-management skills to overcome them.
Groups were led by health professionals with advanced degrees, experience in conducting groups, willingness to follow a protocol, and demonstrated competency after a 2-day training session. The HART intervention team insured treatment fidelity by: (1) audiotaping all sessions, randomly selecting 5% for review, and providing needed feedback; (2) monitoring data on patients and group leaders provided by the data management team to identify and resolve problems; and (3) holding mandatory monthly group leader meetings to minimize drift.
The education arm (called “education”) was conceptualized as an attention control, representative of the standard of care in HF education that would be in place when HART ended. An attention control, rather than a usual care control, minimized such problems as a placebo effect in which any attention could promote benefit, the inability to mask patients to trial hypotheses, and the risk of an “underdog” effect that would promote differential dropout or treatment cross-over in the controls.
Patients randomized to education received the same 18 Heart Failure Tip Sheets, on the same schedule, as the group meetings in the self-management group, but delivered by mail. To insure receipt and check comprehension, a study coordinator contacted the patient by phone within 2–3 days of each mailing. If the Tip Sheet had not been read, the call was rescheduled. All questions about the Tip Sheets were answered but for questions about self-management skills or non-Tip Sheet concerns, the patient was referred back to his/her provider.
Study coordinators were trained on purpose, structure, content, data reporting responsibilities, and quality control procedures. Training included role playing to simulate phone call interactions. Ongoing training, as needed, took place in response to special problems.
Outcome Measures
Primary endpoints were assessed blindly by a team of cardiologists (Appendix). All patients or, in the case of death, their family members, were contacted every 3 months by telephone to ascertain occurrence of a death or hospitalization. Reports of death were confirmed by medical record, death certificate, emergency medical services record, or queries from the Social Security Death Index. HF admissions were adjudicated by the presence of either shortness of breath, peripheral edema, or chest x-ray evidence of pulmonary edema without evidence of another disease process accounting for symptoms or signs. HF admissions were confirmed if the patient responded to HF therapy or had a documented decrease in left ventricular function.
Baseline and annual outcome assessment has been described in detail previously.17 Briefly, examinations consisted of: (1) clinical assessment of height, weight, pulse, respiratory rate, blood pressure, 6-Minute Walk, and a blood draw; (2) medical history including medical conditions, medications, socio-demographic status, and risk factors; (3) and questionnaires completed via interview and self-report. The patient was asked to put a month's supply of an ace inhibitor (or beta blocker if the patient was not taking an ace inhibitor) into a Medication Event Monitoring System (MEMS) electronic pill cap container (MEMS V Trackcap, AARDEX, Zug, Switzerland) and was taught to use it for the ensuing month. Adherence to drug therapy was defined as the percentage of pills taken, relative to pills prescribed, with a goal of ≥80%.
Depression was assessed by the Geriatric Depression Scale wherein a score >10 is a sensitive and specific screen for major depressive symptoms.22 Salt intake was assessed as mg/day based upon the CALS Food Frequency Questionnaire which was developed specifically to include the main sources of sodium in the diet.23 Clinically significant salt intake is ≤2400 mg/day.24–25 The Self-Efficacy at Self-Management Scale, developed specifically for HART, included 5 items each of which assessed one of the 5 self-management skills that were the targets of the intervention on a 10-point scale and calculated as the average item response. Co-morbidities were assessed as the number of the following: previous myocardial infarction, high blood pressure, diabetes, cancer, stroke, renal disease, arthritis, lung disease, liver disease, depression, asthma, sleep apnea, and Parkinson's disease. The 0–13 range was categorized at the median of ≤3. Family income was dichotomized at the median of $30,000/year. Six-Minute Walk was dichotomized at the lowest tertile (≤620 ft.).
Statistical Analyses
Analyses were conducted in accordance with the pre-specified and DSMB-approved analyses plan. Comparisons of categorical variables were with chi-squared tests. Comparisons of continuous variables were with two-sample t-tests except where small sample sizes or skewed distributions warranted use of the Wilcoxon rank-sum statistic.
The impact of treatment on behavioral mediators was assessed on the subgroup for whom pre- and post-treatment measures were available using repeated measures analyses where a significant time effect (before vs. after the 1-year treatment) indicated that both arms changed and a significant treatment by time interaction indicated that one arm changed more than the other.
The impact of treatment on the primary endpoint was described as time-to-event within arm and compared using the Wilcoxon test since Kaplan-Meier curves indicated non-proportional hazards between treatment arms on the primary endpoint. Rates were calculated as events divided by at-risk minus censored, odds were the ratio of the proportion of at-risk patients with an event within treatment arm, and chi-square statistics were the analytic tool.
The impact of treatment on the primary endpoint in pre-specified subgroups was evaluated using a parametric log-logistic accelerated failure time multivariate model, appropriate for use with non-proportional hazard data.26–27 This model assumes explanatory variables act multiplicatively on the speed at which a subject proceeds to an event. Risk factors accelerate time to event (acceleration factor >1), protective factors decelerate time to event (acceleration factor <1), and the statistical significance of this acceleration/deceleration is calculated from the parameter estimate. A multivariate base model considered all pre-specified subgroup variables (i.e., age, gender, education, income, ethnicity, functional capacity, co-morbidities, and baseline drug adherence)17 and other predictors associated with the primary outcome from the literature (i.e., NYHA Class, depression, ace inhibitors or angiotensin receptor blockers, and beta blockers), and retained only those reaching a p-value ≤0.15 at each iteration using backward elimination. All pre-specified subgroup variables remaining in the base model were tested for their interaction with treatment arm.
HART featured a cluster design within the self-management arm only where patients were treated in 42 groups of 6–15 patients each. The impact of group assignment and group leader on the primary outcome within the self-management arm was found to be non-significant. Thus, clustering was not considered further in multivariate analyses.
RESULTS
Figure 1 presents the trial profile. Of 3,154 patients screened over 3 years, 902 were enrolled, resulting in a screening to enrollment ratio of 3.5 patients screened for every patient enrolled. Losses were minimal with 50 (11.1%) in the self-management group and 41 (9.1%) in the education group. Mean follow-up time was 771.8 days (769.1 in education; 774.4 in self-management).
Figure 1.
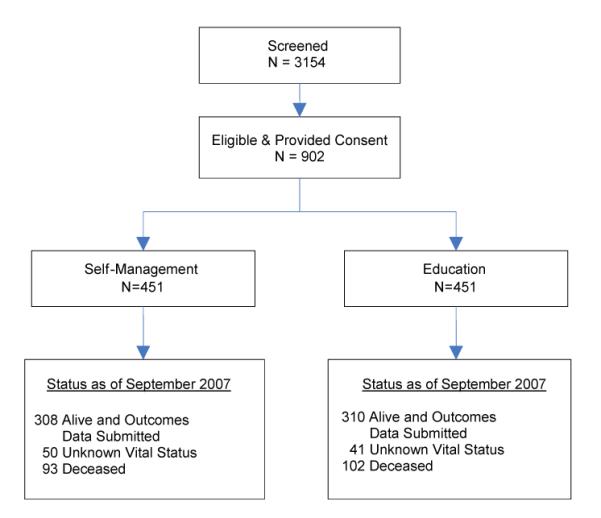
Trial Profile
Table 1 presents the baseline characteristics in the total cohort and within treatment arm. On average, the cohort was approximately 64 years of age, 47% female, 40% minority, and 23% with diastolic dysfunction. Patients were taking an average of 6.8 medications and 37% did not adhere to at least 80% of the prescribed dosage of either an ace inhibitor or beta blocker. Median salt intake was 3,338 mg/day, well over the recommended 2,400 mg/day (Chobanian03). Major depressive symptoms were evident in 29%.
Table 1.
Baseline Characteristics in the Total Cohort and by Treatment Arm
| OVERALL | TREATMENT GROUP |
||
|---|---|---|---|
| (N=902) | Self-Management (N=451) | Education (N=451) | |
| Demographics | |||
| Age, mean(sd) | 63.6 (13.5) | 63.8 (13.7) | 63.4 (13.3) |
| Female, N(%) | 427 (47.3) | 209 (46.3) | 218 (48.3) |
| Minority, N(%) | 362 (40.1) | 177 (39.2) | 185 (41.0) |
| ≤High School Education, N(%) | 394 (43.7) | 197 (43.7) | 197 (43.7) |
| Annual Family income < $30,000, N(%) | 428 (51.6) | 215 (51.9) | 213 (51.3) |
| Unmarried, N(%) | 503 (56.3) | 251 (56.3) | 252 (56.4) |
| Medical | |||
| Diastolic, N(%) | 208 (23.1) | 107 (23.8) | 101 (22.4) |
| NYHA Class III, N(%) | 285 (31.6) | 144 (31.9) | 141 (31.3) |
| 6-MinuteWalk(ft), md(iqr) | 843 (631.5) | 827 (610) | 880 (665) |
| Acute Physiology Score, mean(sd) | 2.6 (2.1) | 2.6 (2.0) | 2.6 (2.1) |
| History of Hypertension, N(%) | 676 (75.2) | 345 (76.8) | 331 (73.6) |
| History of Diabetes, N(%) | 362 (40.2) | 178 (39.6) | 184 (40.9) |
| Number of Co-Morbidities, mean(sd) | 3.2 (1.7) | 3.2 (1.7) | 3.3 (1.7) |
| Number of Medications, mean(sd) | 6.8 (3.0) | 6.8 (3.0) | 6.8 (3.0) |
| Current Use of, N(%) | |||
| Ace Inhibitor or ARB | 773 (85.7) | 383 (84.9) | 390 (86.5) |
| Beta Blocker | 636 (70.5) | 320 (71.0) | 316 (70.1) |
| Quality of Life: Physical Function, mean(sd)§ | 4.3 (1.0) | 4.3 (1.1) | 4.2 (1.0) |
| Adherence | |||
| Non-Adherence to Drug Therapy, N(%)* | 274 (36.5) | 143 (38.4) | 131 (34.6) |
| Salt Intake (mg/day), md(iqr) | 3338 (1619) | 3342 (1681) | 3309 (1558) |
| Current Smoker, N(%) | 85 (9.4) | 39 (8.6) | 46 (10.2) |
| Body Mass Index, mean(sd) | 31.0 (7.7) | 31.1 (7.3) | 30.9 (8.0) |
| Self-Efficacy at Self-Management, mean(sd)# | 7.6 (1.7) | 7.6 (1.8) | 7.6 (1.6) |
| Psychosocial | |||
| Major Depressive Symptoms, N(%) | 265 (29.4) | 136 (30.2) | 129 (28.7) |
| Social Support-Emotional, mean(sd)§ | 75.2 (22.2) | 74.3 (22.5) | 76.1 (21.9) |
| Purpose in Life, mean(sd)‡ | 4.5 (0.8) | 4.5 (0.8) | 4.5 (0.8) |
Range = 0–100 with higher score= greater quality of life or emotional support.
Non-Adherence = taking <80% of prescribed dose of ace inhibitor or beta blocker.
Range = 1–10 with higher score = greater self-efficacy.
Range = 1–6 with higher score = greater life purpose.
There were no statistically significant differences between treatment arms on any of the baseline variables.
Table 2 presents data on the receipt of the treatments in those who were alive by the end of the 1-year treatments. Approximately 13% of those in self-management failed to receive any treatment, compared to only 2% of those in education, due to the greater logistical difficulty of organizing a group relative to making phone calls.
Table 2.
Receipt of Treatment in Those Who Were Alive by the End of the 1-Year Treatment.
| 0% (0 sessions) | 1–24% (1–4 sessions) | 25–50% (5–9 sessions) | 51–80% (10–14 sessions) | 81–100% (15–18 sessions) | ||
|---|---|---|---|---|---|---|
| Treatment Group | N1 | N (%) | N (%) | N (%) | N (%) | N (%) |
| Self-Management | 418 | 54 (12.9) | 43 (10.3) | 41 (9.8) | 86 (20.6) | 194 (46.4) |
| Education | 416 | 8 (1.9) | 35 (8.4) | 48 (11.5) | 104 (25.0) | 221 (53.1) |
33 Self Management and 35 Education participants died before the end of treatment. A maximum of 18 sessions was offered.
The treatments had a variable impact on mediating behavioral risk factor targets. Both groups improved on self-efficacy at self-management, depression and salt restriction. Self-efficacy scores improved by 0.2 points in both groups (time: p=0.008) and major depressive symptoms decreased to 20% in the self-management group and 22% in the education group (time effect: p=0.008). Restricting salt to ≤2400 mg/day occurred in 28% in self-management and 18% in education (time effect: p=0.01) but there was more improvement in the self-management group (treatment*time interaction: p=0.05). However, even in the self-management group, salt intake remained high with 72% not meeting clinical guidelines. Non-adherence to the prescribed dosage of ace inhibitor or beta blocker increased in both groups by 7 percentage points (time effect: p=0.01).
Figure 2 presents the Kaplan-Meier estimates of the time to the primary endpoint of death or HF hospitalization by treatment arm. There was no benefit of self-management plus education over education alone (Wilcoxon p=0.58). Table 3 presents rates of the primary endpoints and their disaggregated components by treatment arm. There were no differences between groups on any of these outcomes.
Figure 2.
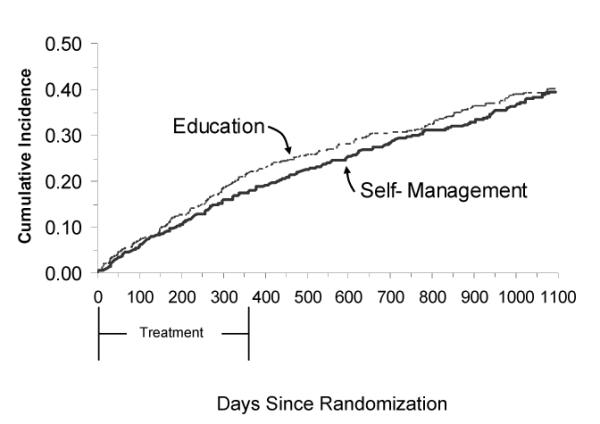
Time to Death or Heart Failure Hospitalization by Treatment Arm
Table 3.
Rates of Clinical Endpomts by Treatment Arm
|
|
||||
|---|---|---|---|---|
| Self-Management (N=451) | Education (N=451) | |||
| N(%) | N(%) | OR (95% CI)* | p-value* | |
| Death or Heart Failure Hospitalization | 163 (36.1) | 171 (37.9) | 0.95 (0.72, 1.26) | 0.74 |
| Death or All-Cause Hospitalization | 307 (68.1) | 327 (72.5) | 0.82 (0.60, 1.12) | 0.22 |
| Death | 88 (19.5) | 100 (22.2) | 0.87 (0.63, 1.21) | 0.41 |
| Heart Failure Hospitalization | 105 (23.3) | 105 (23.3) | 1.00 (0.72, 1.38) | >99 |
| All-Cause Hospitalization | 279 (61.9) | 287 (63.6) | 0.85 (0.62, 1.17) | 0.32 |
Based on different N's due to censoring.
Figure 3 presents the odds ratios for death or HF hospitalization in pre-specified baseline subgroups. At the design of HART, the hypothesized benefit of self-management over education was expected to be a 25% reduction in the primary endpoint (OR=0.75). The observed overall odds ratio was 0.93. However, for the subgroups of patients who were low-income, had poorer functional capacity, and poorer drug adherence at baseline, self-management treatment achieved the hypothesized treatment benefit of a 25% reduction in the primary endpoint.
Figure 3.
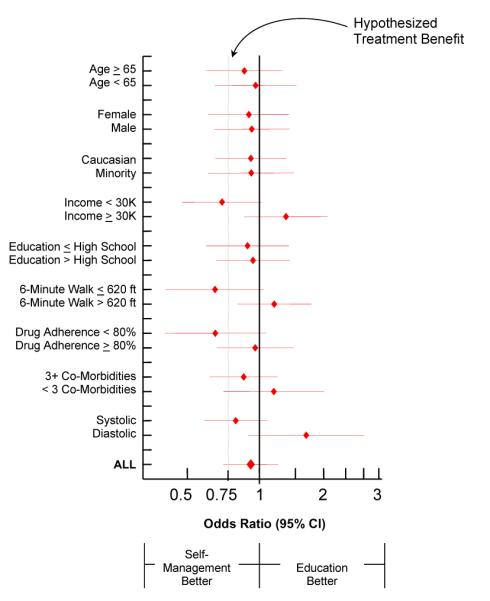
Time to Death or Heart Failure Hospitalization in Pre-Specified Baseline Subgroups
Table 4 presents the results of the multivariate model testing the statistical significance of treatment benefit in pre-specified subgroups. Adjusting for covariates and main effects, the income by treatment interaction was significant (parameter estimate=0.64, p=0.02). In patients with incomes <$30,000, those randomized to education had a 36% faster time to event compared to those randomized to self-management (p=0.05) but there were no differences between low-income patients randomized to self-management and high-income patients randomized to either arm. Thus, self-management counseling neutralized the effect that low income would have had on the primary endpoint. The effect of income in those randomized to education was that that those with income <$30,000 had a 54% faster time to event than their high-income counterparts (p=0.03, data not shown). Figure 4 presents this interaction visually, showing the benefits of self-management counseling only in the low-income subgroup.
Table 4.
Pre-Specified Independent Predictors of Death or Heart Failure Hospitalization1
| Predictor | Parameter Estimate | Acceleration Factor |
|---|---|---|
| Age ≥ 65 years | −0.45** | 1.57 |
| New York Heart Association Class III | −0.65*** | 1.92 |
| Major Depressive Symptoms | −0.28+ | 1.32 |
| ≥ 3 Co-Morbidities | −0.61*** | 1.84 |
| Minority | −0.29+ | 1.34 |
| Income | -- | -- |
| Treatment | -- | -- |
| Treatment *Income | 0.64* | -- |
| (Referent) Self-Management—Income<$30,000 | ||
| Education—Income<$30,000 | −0.36* | 1.44 |
| Self-Management—Income≥$30,000 | −0.21 | 1.23 |
| Education—Income<$30,000 | −0.36 | 1.44 |
| Education—Income≥$30,000 | 0.07 | 0.93 |
Statistics reported are derived from log-logistic accelerated failure time models
p<0.001
p<0.01
p<0.05
p<0.10
Figure 4.
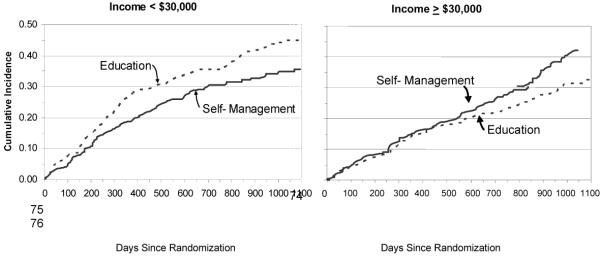
Time to Death or Heart Failure Hospitalization by Treatment Arm in Low- and High-Income Subgroups
COMMENT
Past trials have failed to support the efficacy of activating patients to manage their HF by learning self-management skills,12–16 but they have been limited by weak designs. The size, duration, methodological rigor, and representativeness of HART position it well to provide more conclusive results. We hypothesized that HF education, the standard of practice, was necessary but not sufficient to affect clinical outcomes. Like any chronic illness, HF should be managed collaboratively where the provider prescribes evidence-based therapy and an informed, proactive patient implements it.28–29 To achieve a proactive patient, we reasoned that HF education should be augmented by patient self-management skills training. This hypothesis was not supported. Consistent with past trials, self-management training plus HF education had no benefit over HF education alone in reducing death or HF hospitalizations in patients with mild to moderate systolic or diastolic HF.
We believe, however, that the results and implications of HART go beyond this primary finding. Post-hoc analyses of pre-specified baseline characteristics revealed that this hypothesis may have been supported in low-income patients. Testing the benefits of treatment in subgroups provides statistical challenges in trials powered to test benefit only in the total cohort. Thus, to maintain power, subgroup hypotheses were tested using treatment by subgroup interactions in multivariate models. This modeling showed that in low-income patients self-management counseling provided benefit over education alone and essentially eliminated the impact of low income on time to death or HF hospitalization. This supports a tailored approach to outpatient HF management in which patients with limited socioeconomic resources need more than standard HF education to improve outcomes.16,30
A second implication of HART is the possibility that ongoing education and telephone follow-up may be all that is needed to activate those patients with economic resources to self-manage their HF. Patients with income ≥ $30,000 had a lower than the expected 15%/year rate on the primary endpoint in years 2 (9.0%) and 3 (12.8%), and they improved on most of the behavioral risk factor targets. Although HF education is part of current outpatient management, the HART education group received this education over one year and the telephone follow-up from non-professional staff insured that the educational tips were read and comprehended. Thus, educational initiatives from the American Heart Association (http://www.hearthub.org/hc-heart-failure.htm) and others (http://www.hfsa.org/heart_failure_education_modules.asp) may be most potent when accompanied by extended and inexpensive phone follow-up.
Technology-assisted remote monitoring was not evaluated in this trial. However, a recent meta-analysis showed that remote telephone monitoring was as effective as remote technology-assisted monitoring in reducing HF hospitalizations, both of which achieved a 30% reduction in risk over usual care.31 Long-term cost-effectiveness, however, remains to be evaluated.31 Since technology-assisted monitoring puts all of the responsibility on the provider and the health care system, both of which incur costs, an important question to be answered in future trials is their cost-effectiveness, relative to the simple approaches studied in HART.
These unexpected subgroup findings must be interpreted with caution. Since they were observed on a post-hoc basis, they must be replicated in trials where hypotheses are specified a priori. Such trials should be conducted, however, because the findings from HART are clinically intuitive. To slow progression of HF and cut costs, patients may need only small but ongoing incentives to proactively maximize their health. However, the disadvantaged subgroup who disproportionately bear the brunt of HF events may benefit from additional support and skills training provided by self-management groups. Such groups could be cost-effective if they help these disadvantaged patients to stay out of the hospital and stay alive.
What makes this hypothesis innovative is that the proposed tailored treatments are simple and inexpensive. As we face a growing health care crisis stemming, in part, from over-reliance on expensive technology that we cannot afford, evaluating the efficacy and costs of simpler approaches may prove to be timely.
Acknowledgements
The HART investigators wish to thank the HART patients for the time and effort they devoted to this trial. No author has any conflicts of interest to disclose. All authors have had full access to the data in the trial and take responsibility for the integrity of the data and the accuracy of the data analyses.
Support for HART from HL065547. Clinical Trials Registration NCT00018005.
Appendix
Executive Committee
Co-authors listed on this paper.
Sponsor Contacts at NHLBI
Jared Jobe, PhD; Peter Kaufmann, PhD
Recruiting Hospital Principal Investigators and Collaborators
Cheryl Brody, DO: South Suburban Hospital/ Leslie Brookfield, MD, FACC: Advocate Lutheran General Hospital/ Stephanie Dunlap, MD: University of Illinois/ Philip Krause, MD, FACC: Rush North Shore Medical Center/Stuart Rosenbush, MD (PI), William Elliott, MD, PhD, Donald Tanis, MD: Rush University Medical Center/ Mitchell Saltzberg (PI), Maria Rosa Costanzo, MD: Midwest Heart Specialists/ Michael Shapiro (PI), Thomas Stamos, MD: John H. Stroger Hospital of Cook County/Marc A. Silver, MD: Advocate Christ Medical Center/ Michael Waligora, MD (PI), Randall Williams, MD: Evanston Northwestern Healthcare and Glenbrook Hospital.
Self-Management Group Leaders
Kristin J. Flynn (Supervisor); Andrea Kozak (Assistant Supervisor); Carl Thoresen, Virginia Price, Susan Gilkey (Trainers); Maureen Gecht; Jim Jarzenbowski; Kevin McClone; Carmen Lynas; Sybil Madison-Boyd; Marilynn Rochon; Bruce Bybarczyk; Sarah Sellergren; Stephen Tate; Tamara Gathright; Rocio Munoz; Cheryl S. Rucker-Whitaker; Lynda H. Powell; Kim Lebowitz; Patty Roberts; Ayesha Shaikh; Marilyn Vander Werf.
Education Control Group Leader
Kathleen L. Grady (supervisor)
Endpoints Adjudiction Committee
James E. Calvin, Jr. (Chair); Stamatis Dimitropoulos, MD; William Elliott, MD, PhD; Philip Kraus, MD; Payman Sattar, MD; Sujata Shanbhag, MD; Thomas Stamos, MD; Patricia Vassallo, MD.
Data and Safety Monitoring Board
Lawrence S. Cohen, MD (Chair); Baruch A. Brody, PhD (starting 2002); Byron W. Brown (through 2004); Nancy R. Cook, ScD (starting 2005); Julie Buring, ScD; Robert M. Kaplan; Lynn Warner Stevenson, MD; David Thomasma, PhD (through 2002).
REFERENCES
- 1.McCullough PA, Philbin EF, Spertus JA, et al. Confirmation of a heart failure epidemic: findings from the resource utilization among congestive heart failure (REACH) study. J Am Coll Cardiol. 2002;39:60–69. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 2.Aghababian RV. Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department. Rev Cardiovasc Med. 2002;3(suppl 4):S3–S9. [PubMed] [Google Scholar]
- 3.Russo CA, Ho K, Elixhauser A. HCUP Statistical Brief #26. Agency for Healthcare Research and Quality; Rockville, MD: Feb, 2007. Hospital stays for circulatory diseases, 2004. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb26.pdf. [PubMed] [Google Scholar]
- 4.Massie BM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J. 1997;133:703–712. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 5.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 Update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–3171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 6.Jessup M, Brozena S. Medical progress: heart failure. New Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 7.van der Wal MHL, Jaarsma T. Adherence in heart failure in the elderly: Problem and possible solutions. Intl J Cardiology. 2007 doi: 10.1016/j.ijcard.2007.10.011. doi:10.1016/j.ijcard.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Kozak AT, Rucker-Whitaker C, Basu S, et al. Elements of nonpharmacologic interventions that prevent progression of heart failure: a meta-analysis. Congestive Heart Failure. 2007;13:280–287. doi: 10.1111/j.1527-5299.2007.07236.x. [DOI] [PubMed] [Google Scholar]
- 9.McAlister FA, Stewart S, Ferrua S, et al. Multidisciplinary strategies for the management of heart failure patients at high risk for admission. A systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–819. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 10.Gwadry-Sridhar F, Elintoft V, Lee DS, et al. A systematic review and meta-analysis of studies comparing readmission rates and mortality rates in patients with heart failure. Arch Intern Med. 2004;164:2315–2320. doi: 10.1001/archinte.164.21.2315. [DOI] [PubMed] [Google Scholar]
- 11.Phillips CO, Wright SM, Kern DE, et al. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure. JAMA. 2004;291:1358–1367. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 12.Harrison MB, Browne GB, Roberts J, Tugwell P, Gafni A, Graham ID. Quality of life in individuals with heart failure. A randomized trial of the effectiveness of two models of hospital-to-home transition. Med Care. 2002;40:271–282. doi: 10.1097/00005650-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Sethares KA, Elliott K. The effect of a tailored message intervention on heart failure readmission rates, quality of life, and benefit and barrier beliefs in persons with heart failure. Heart Lung. 2004;33:249–260. doi: 10.1016/j.hrtlng.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Wright SP, Walsh H, Ingley KM, et al. Uptake of self-management strategies in a heart failure management programme. Europ J Heart Failure. 2003;5:371–380. doi: 10.1016/s1388-9842(03)00039-4. [DOI] [PubMed] [Google Scholar]
- 15.Prasun MA, Kocheril AG, Klass PH, Dunlap SH, Piano MR. The effects of a sliding scale diuretic titration protocol in patients with heart failure. J Cardiovas Nurs. 2005;20:62–70. doi: 10.1097/00005082-200501000-00012. [DOI] [PubMed] [Google Scholar]
- 16.DeWalt DA, Malone RM, Bryant ME, et al. A heart failure self-management program for patients of all literacy levels: A randomized, controlled trial [ISRCTN11535170] BMC Health Serv Res. 2006;6:1–10. doi: 10.1186/1472-6963-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powell LH, Calvin JE., Jr. Mendes de Leon CF et al. The Heart Failure Adherence and Retention Trial (HART): Design and rationale. Am Heart J. 2008;156:452–460. doi: 10.1016/j.ahj.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn KJ, Powell LH, Mendes de Leon CG, et al. Increasing self-management skills in heart failure patients: A pilot study. Congestive Heart Failure. 2005;11:297–302. doi: 10.1111/j.1527-5299.2005.04361.x. [DOI] [PubMed] [Google Scholar]
- 19.Friedman M, Thoresen CE, Gill JJ, et al. Alteration of Type A behavior and reduction in cardiac recurrences in post-myocardial infarction subjects. Am Heart J. 1984;108:237–248. doi: 10.1016/0002-8703(84)90606-9. [DOI] [PubMed] [Google Scholar]
- 20.Lorig KR, Sobel DS, Sewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health sttus while reducing hospitalization. A randomized trial. Med Care. 1999;37:5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 21.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1999;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 22.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiat Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 23.DeBusk RF, MULTIFIT A new approach to risk factor modification. Cardiol Clin. 1996;14:143–157. doi: 10.1016/s0733-8651(05)70267-8. [DOI] [PubMed] [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, et al. the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The JNC 7 Report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 25.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. JACC. 2009;53(15):e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Collett D. Modeling Survival Data in Medical Research. 2nd Chapman & Hall/CRC Press LLC; New York, NY: 2003. [Google Scholar]
- 27.Anderson K. A nonproportional hazards Weibull accelerated failure time regression model. Biometrics. 1991;47:281–288. [PubMed] [Google Scholar]
- 28.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74:511–544. [PubMed] [Google Scholar]
- 29.Von Korff M, Gruman J, Schaefer J, et al. Collaborative management of chronic illness. Ann Intern Med. 1997;127:1097–1102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Wagner EH. Deconstructing heart failure disease management. Ann Intern Med. 2004;141:644–646. doi: 10.7326/0003-4819-141-8-200410190-00015. [DOI] [PubMed] [Google Scholar]
- 31.Klersy C, DeSilvestri A, Gabutti G, Regoli F, Auricchio A. A meta-analysis of remote monitoring of heart failure patients. JACC. 2009;54:1683–1694. doi: 10.1016/j.jacc.2009.08.017. [DOI] [PubMed] [Google Scholar]


