Abstract
The immune system is regulated by circadian clocks within the brain and immune cells. Environmental circadian disruption (ECD), consisting of a 6-h phase advance of the light:dark cycle once a week for 4 weeks, elevates the inflammatory response to lipopolysaccharide (LPS) both in vivo and in vitro. This indicates that circadian disruption adversely affects immune function; however, it remains unclear how the circadian system regulates this response under ECD conditions. Here, we develop an assay using ex vivo whole-blood LPS challenge to investigate the circadian regulation of immune responses in mice and to determine the effects of ECD on these rhythms. LPS-induced IL-6 release in whole blood was regulated in a circadian manner, peaking during subjective day under both entrained and free-running conditions. This LPS-induced IL-6 release rhythm was associated with daily variation in both white blood cell counts and immune cell responsiveness. ECD increased the overall level of LPS-induced IL-6 release by increasing immune cell responsiveness and not by affecting immune cell number or the circadian regulation of this rhythm. This indicates that ECD produces pathological immune responses by increasing the proinflammatory responses of immune cells. Also, this newly developed whole blood assay can provide a noninvasive longitudinal method to quantify potential health consequences of circadian disruption in humans.
Keywords: environmental circadian disruption, lipopolysaccharide, IL-6, whole blood
The immune system and its responses are regulated by circadian clocks within the brain and immune cells (Haus and Smolensky, 1999; Scheiermann et al., 2013). At the molecular level, circadian rhythms are generated by interlocking transcriptional/translational feedback loops involving a family of clock genes (e.g., Period1-3, Cryptochrome 1-2, Bmal1, Clock) that regulate cellular functions across the day (Ko and Takahashi, 2006). At the whole animal level, immune responses are regulated in a circadian manner, with daily rhythms in immune cell counts and cytokine levels evident in rodents and humans (Abo et al., 1981; Arjona and Sarkar, 2006; Born et al., 1997; Gibbs et al., 2012).Isolated macrophages exhibit rhythms in clock gene expression, phagocytic activity, and response to the bacterial endotoxin lipopolysaccharide (LPS) (Gibbs et al., 2012; Keller et al., 2009). Importantly, responses to specific antigens (Fortier et al., 2011; Shackelford and Feigin, 1973) and nonspecific bacterial products (Halberg et al., 1960) are under diurnal regulation. Thus, recent work indicates that circadian and immune functions are highly interconnected; however, the consequences of a disrupted circadian environment for proper immune functions remain unclear.
Environmental lighting conditions that produce circadian disruption lead to pathological immune responses (Golombek et al., 2013). Using an environmental circadian disruption (ECD) paradigm involving 4 weekly 6-h phase advances of the light:dark cycle, we have demonstrated that ECD elevates LPS-induced inflammatory responses in mice in vivo and in isolated macrophages ex vivo (Castanon-Cervantes et al., 2010). Furthermore, clock gene rhythms were disrupted in peritoneal macrophages of ECD mice, with changes in both Bmal1 and Per2 rhythms (Castanon-Cervantes et al., 2010). This suggests that pathological immune responses are associated with altered circadian functions in immune cells; however, it remains unclear whether LPS-induced cytokine release is clock controlled and how ECD affects this response.
Here, we develop an ex vivo whole-blood LPS challenge to investigate the circadian regulation of immune responses and the effects of ECD. LPS-induced IL-6 release in whole blood was robust and rhythmic under both entrained and free-running conditions. Circadian variation in this immune response was associated with daily variation in both white blood cell counts and immune cell responsiveness. ECD did not abolish this immune response rhythm but instead elevated overall LPS-induced IL-6 release by increasing immune cell responsiveness and not immune cell number.
MATERIALS AND METHODS
Male C57BL/6J mice (N = 144; age = 4-5 mo) (Jackson Laboratories, Bar Harbor, ME) were entrained for at least 10 days to a 12-h light:12-h dark cycle (LD12:12, lights-off at 1800 h EST) with light provided by fluorescent bulbs (200-400 lux at cage level). Mice were housed singly in polypropylene cages without a running wheel inside light-tight boxes with ad libitum access to water and food (rodent chow #5001, Purina, St. Louis, MO). Mice were either maintained on this schedule (LD control, n = 48), released from LD12:12 into constant darkness for 2 days (DD, n = 48), or exposed to the ECD schedule (ECD, n = 48) as previously described (Castanon-Cervantes et al., 2010). Briefly, mice were exposed once a week to a 6-h phase advance of the light:dark cycle for 4 weeks. For blood and cell assays, mice were euthanized with CO2, and ECD mice were euthanized on the seventh day after the last shift. Within text and figures, ZT/CT refers to the time of sacrifice and blood collection. All procedures and protocols were approved by the Morehouse School of Medicine's Institutional Animal Care and Use Committee.
Whole-Blood LPS Challenge
Trunk blood was collected into EDTA-coated tubes once every 3 h (n = 6 mice/time point/group). Blood (270 μL) was incubated for 4 h at 37 °C with 30 μL of LPS in RPMI-1640 medium supplemented with L-glutamine and 2 mM HEPES (final LPS concentration = 50 μg/mL). Following centrifugation (1000 g, 5 min), plasma was collected and frozen at –20 °C.
Blood Cell Counts
Blood was collected either 4 h after lights-on (i.e., ZT4; ECD, n = 10; LD controls, n = 10) or 4 h after lights-off (i.e., ZT16; LD controls, n =10). Small-volume complete blood count differential assay was performed by Charles River (Wilmington, MA).
LPS Stimulation in Isolated PBMCs
Undifferentiated peripheral-blood mononuclear cells (PBMCs) were separated from trunk blood at ZT4 using HISTOPAQUE 1083 (Sigma-Aldrich, St. Louis, MO). After mononuclear cell layer separation, cells were suspended in RPMI-1640 with 10% FBS, and approximately 2 × 106 cells were seeded before overnight incubation at 37 °C (5% CO2). Cells were then washed with PBS and provided LPS (1 μg/mL) in fresh RPMI-1640 with 10% FBS before being returned to the incubator. Medium (100 μL) was extracted from each dish after 6 h, 24 h, and 48 h of LPS stimulation and then frozen at –20 °C.
Macrophage Isolation and Stimulation
Peritoneal exudate cells (PECs) were collected at ZT4 and ZT16 as described previously (Castanon-Cervantes et al., 2010). After extraction and washing, approximately 1 × 106 cells were seeded in RPMI-1640 with 10% FBS. PECs were incubated overnight at 37 °C (5% CO2) before PBS wash and provision of LPS (1 μg/mL) in RPMI-1640 and 10% FBS. Supernatant (100 μL) was collected 24 h after stimulation and frozen at –20 °C.
ELISA
The proinflammatory cytokine IL-6 was selected as the primary immune response measure because it is an important mediator of the acute immune response that is associated with inflammatory diseases such as rheumatoid arthritis, autoimmunity, and multiple myeloma (Kishimoto, 2006), and ECD-induced changes in IL-6 are representative of changes in other proinflammatory cytokines (Castanon-Cervantes et al., 2010). IL-6 release was quantified with ELISA (product no. 550950, BD OptEIA, San Diego, CA) according to the manufacturer's protocol. Levels of IL-6 in plasma and supernatants were analyzed at 1:2 (plasma), 1:1 (PBMCs), and 1:10 (PECs) dilutions.
Statistics
Most statistical tests were conducted with JMP software (JMP 8, SAS Institute Inc., Cary, NC). LPS-induced IL-6 response rhythms were analyzed with CircWave software (Roelof A. Hut, University of Groningen), with group comparisons conducted using full factorial ANOVA and post hoc least squares mean (LSM) contrasts with Bonferroni correction for multiple comparisons. PBMC data were assessed using repeated-measures ANOVA followed post hoc by full factorial ANOVA and LSM contrasts. Immune cell count and PEC data were assessed using the Student t test. Group differences were considered significant at p ≤ 0.05.
RESULTS AND DISCUSSION
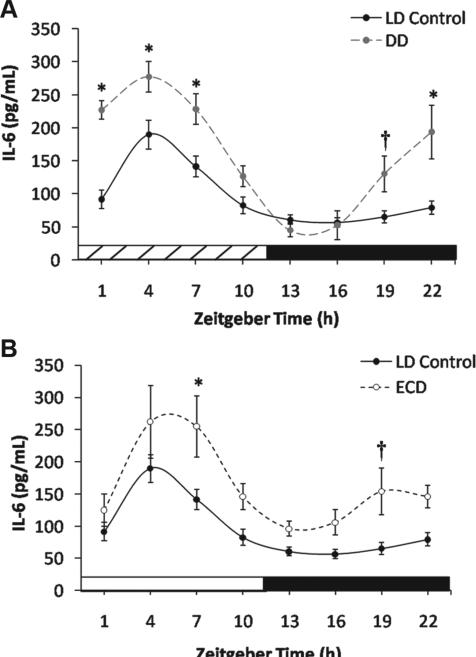
Blood collected from LD control mice exhibited a robust diurnal rhythm in LPS-induced IL-6 release (CircWave: F2,44 = 24.8, p < 0.0001) (Fig. 1A). The rhythm peaked during the day (center of gravity: ZT4.9 ± 2.8), which is similar to the phase of highest mortality after LPS challenge in vivo (Halberg et al., 1960; Marpegan et al., 2009). The LPS-induced IL-6 response rhythm in whole blood persisted after release into DD (CircWave: F2,45 = 44.8, p < 0.0001) (Fig. 1A), indicating that this rhythm is under circa-dian control. Similar to LD controls, the DD peak time occurred during subjective day (center of gravity: CT2.9 ± 2.5), but LPS-induced IL-6 values were higher at most time points in DD than LD controls (group: F1,79 = 44.7, p < 0.0001; group × time: F7,79 = 3.9, p < 0.005; LSM contrasts, p < 0.001) (Fig. 1A). This result is consistent with previous reports that indicate cytokine levels and inflammatory responses are increased under DD (Carlson and Chiu, 2008; Hansson et al., 1990; Monje et al., 2011). While Marpegan and colleagues (2009) did not observe persistence of the LPS-induced mortality rhythm in DD, this may reflect that DD mice had higher mortality responses than LD mice at each of the 2 time points investigated. The increase in immune responses under DD may indicate that daily light exposure under entrained conditions suppresses inflammatory responses; however, it remains to be determined whether this is an acute response to DD release and if it is associated with increased severity of LPS responses in vivo.
Figure 1.
(A) Mean LPS-induced IL-6 response (±SEM) of whole blood in LD control and DD groups (*p < 0.006; †p < 0.05; n = 6 per time point). (B) Mean LPS-induced IL-6 response (±SEM) of whole blood in LD control and ECD groups.
LPS-induced IL-6 responses were also rhythmic after ECD (CircWave: F2,45 = 6.5, p < 0.005) (Fig. 1B), with a daytime peak (center of gravity: ZT4.8 ± 3.1). Relative to LD controls, stimulated IL-6 release responses were elevated by ECD (group: F1,79 = 27.0, p < 0.0001; group × time: F7,79 = 0.6, p = 0.78; LSM contrasts, p < 0.0001) (Fig. 1B), consistent with the effect of ECD previously observed after LPS challenge at ZT4 for mice in vivo and for macrophages in vitro (Castanon-Cervantes et al., 2010). By constructing the LPS-induced IL-6 response rhythm, we find that this effect extends to other times of the day and that circadian regulation is not abolished as predicted based on the blunting of clock gene rhythms in macrophages (Castanon-Cervantes et al., 2010). Based on the time course of IL-6 release for cultured cells incubated with LPS (Castanon-Cervantes et al., 2010) (Fig. 3A), it is likely that a longer LPS incubation would have magnified the difference between LD control and ECD groups.
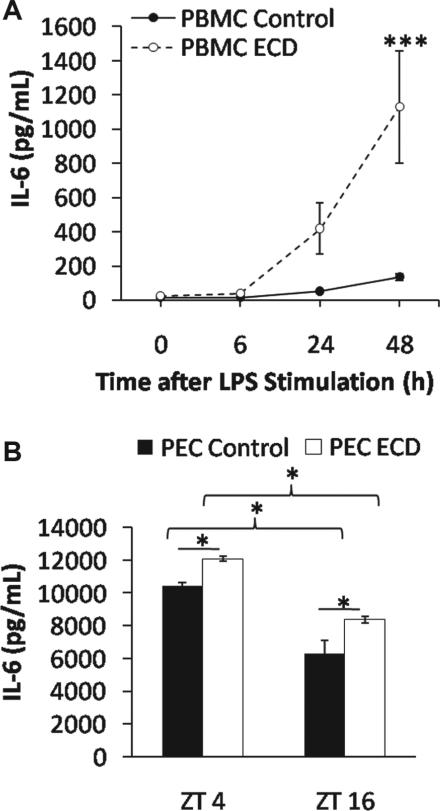
Figure 3.
(A) Mean LPS-induced IL-6 response (±SEM) of cultured PBMCs from LD control and ECD mice (***p < 0.0001). (B) Mean LPS-induced IL-6 response (±SEM) of diurnal PECs from LD control and ECD mice (*p < 0.01).
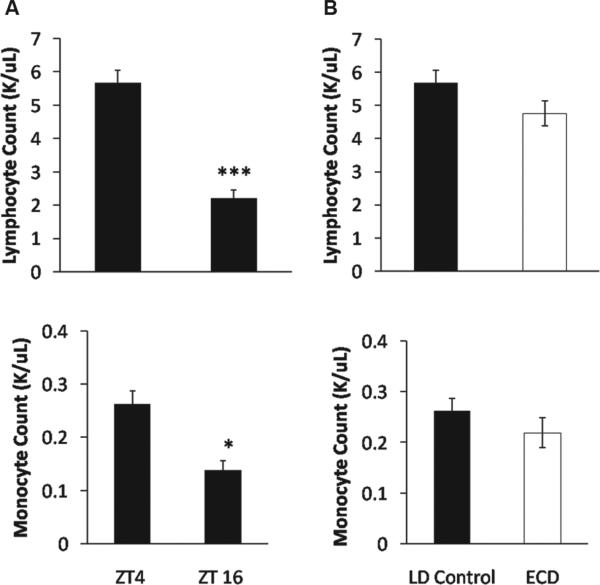
We next determined whether daily and ECD-induced changes were due to increased immune cell numbers and/or immune cell responsiveness. First, we collected blood at ZT4 and ZT16 for a complete blood count with differentials. Lymphocytes and monocytes, the cells most likely to be mounting the LPS response in our whole blood assay (Sabroe et al., 2002), were more prevalent in the blood at ZT4 than at ZT16 (lymphocyte: t18 = –7.7, p < 0.0001; monocyte: t18 = –4.2, p = 0.0005)(Fig. 2A), which may account for the daytime increase in immune response. In contrast, LD control and ECD groups did not differ in immune cell count at ZT4 (lymphocyte: t18 = –1.7, p = 0.1; monocyte: t18 = –1.2, p = 0.26) (Fig. 2B), which indicates that the ECD-induced elevation of the IL-6 response at ZT4 is not due to increased cell numbers. Next, to determine if ECD increases immune cell responsiveness, we isolated and cultured PBMCs from ECD and LD control mice before LPS challenge in vitro. Consistent with previous results using macrophages (Castanon-Cervantes et al., 2010), ECD increased the level of LPS-induced IL-6 release from PBMC cultures (LSM contrast, p < 0.01) (Fig. 3A). Last, we tested if PECs exhibited an LPS-induced IL-6 release rhythm that could account for the LPS sensitivity rhythm in vitro (Fig. 1A and 1B) and in vivo (Halberg et al., 1960; Marpegan et al., 2009). IL-6 release was significantly higher at ZT4 than at ZT16 (LSM contrast, p < 0.0001) (Fig. 3B), and ECD history augmented this response at both time points (LSM contrast, p < 0.005) (Fig. 3B), similar to results using whole-blood LPS challenge (Fig. 1B). Since immune cell number was controlled when testing for PEC responses, this further indicates that differences in cell numbers alone cannot account for time-of-day or ECD-induced changes in IL-6 release following LPS challenge.
Figure 2.
(A) Mean lymphocyte and monocyte cell counts (±SEM) of LD control mice at ZT4 and ZT16 (***p < 0.0001; *p < 0.005). (B) Mean lymphocyte and monocyte cell counts (±SEM) of LD control and ECD mice at ZT4.
After ECD, LPS-induced IL-6 responses remain rhythmic in both whole blood and PECs, which was an unexpected result given our previous observations indicating that ECD diminishes clock gene rhythmicity in macrophages (Castanon-Cervantes et al., 2010). While rhythmicity was not abolished, the overall level of the LPS-induced IL-6 response was elevated by ECD in whole blood, PBMCs, and PECs. Since macrophages can remain in the peritoneum for long periods of time, it is likely that the PEC cells tested here in vitro were exposed to ECD while in vivo. Conversely, PBMCs are only in circulation for a few days before migration and differentiation. Therefore, these newly derived cells were not exposed to ECD but yet, like PECs, display an ECD-induced increase in LPS responsiveness. Mechanisms for this ECD-induced increase in IL-6 response in these newly derived cells may include a systemic signal in circulation or epigenetic modifications to hematopoietic parent cells that are passed to daughter cells during replication and act on LPS-activated proinflammatory and/or anti-inflammatory pathways.
In summary, the circadian rhythm in the inflammatory response to LPS is related to both diurnal variation in immune cell number and responsiveness. ECD elevates the LPS-induced IL-6 release response without abolishing this immune rhythm. ECD-induced elevation of IL-6 release is associated with increased immune cell responsiveness rather than cell number. It remains to be determined whether the LPS-induced IL-6 release rhythm is altered during ECD but recovers when resynchronization is complete, which was the focus of this investigation. Also, it remains unknown whether humans exhibit pathological immune responses after circa-dian disruption. The whole blood assay developed here may provide a noninvasive longitudinal means to quantify inflammatory responses in human subjects across the circadian day and following laboratory- or real life–induced circadian disruption.
ACKNOWLEDGMENTS
This work was supported by NINDS grant U54NS060659, ACTSI pilot grant supported in part by PHS grants (UL1 RR025008, KL2 RR025009, or TL1 RR025010) from the Clinical and Translational Science Award program, and NCRR/RCMI grant G12-RR03034. The authors thank the MSM Center for Laboratory Animal Resources, the MSM Office of Graduate Studies, and Drs. Andrew Gewirtz, Ting-Chung Suen, and Gianluca Tosini.
Footnotes
CONFLICT OF INTEREST STATEMENT
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Abo T, Kawate T, Itoh K, Kumagai K. Studies on the bioperiodicity of the immune response, I: circadian rhythms of human T, B, and K cell traffic in the peripheral blood. J Immunol. 1981;126:1360–1363. [PubMed] [Google Scholar]
- Arjona A, Sarkar DK. Evidence supporting a circadian control of natural killer cell function. Brain Behav Immun. 2006;20:469–476. doi: 10.1016/j.bbi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–4464. [PubMed] [Google Scholar]
- Carlson DE, Chiu WC. The absence of circadian cues during recovery from sepsis modifies pituitary-adrenocortical function and impairs survival. Shock. 2008;29:127–132. doi: 10.1097/shk.0b013e318142c5a2. [DOI] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier EE, Rooney J, Dardente H, Hardy MP, Labrecque N, Cermakian N. Circadian variation of the response of T cells to antigen. J Immunol. 2011;187:6291–6300. doi: 10.4049/jimmunol.1004030. [DOI] [PubMed] [Google Scholar]
- Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon AS. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek DA, Casiraghi LP, Agostino PV, Paladino N, Duhart JM, Plano SA, Chiesa JJ. The times they're a-changing: effects of circadian desynchronization on physiology and disease. J Physiol Paris. 2013 doi: 10.1016/j.jphysparis.2013.03.007. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23545147. [DOI] [PubMed]
- Halberg F, Johnson EA, Brown BW, Bittner JJ. Susceptibility rhythm to E. coli endotoxin and bioassay. Proc Soc Exp Biol Med. 1960;103:142–144. doi: 10.3181/00379727-103-25439. [DOI] [PubMed] [Google Scholar]
- Hansson I, Holmdahl R, Mattsson R. Constant darkness enhances autoimmunity to type II collagen and exaggerates development of collagen-induced arthritis in DBA/1 mice. J Neuroimmunol. 1990;27:79–84. doi: 10.1016/0165-5728(90)90139-e. [DOI] [PubMed] [Google Scholar]
- Haus E, Smolensky MH. Biologic rhythms in the immune system. Chronobiol Int. 1999;16:581–622. doi: 10.3109/07420529908998730. [DOI] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8(Suppl 2):S2. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Marpegan L, Leone MJ, Katz ME, Sobrero PM, Bekinstein TA, Golombek DA. Diurnal variation in endotoxin-induced mortality in mice: correlation with proinflammatory factors. Chronobiol Int. 2009;26:1430–1442. doi: 10.3109/07420520903408358. [DOI] [PubMed] [Google Scholar]
- Monje FJ, Cabatic M, Divisch I, Kim EJ, Herkner KR, Binder BR, Pollak DD. Constant darkness induces IL-6-dependent depression-like behavior through the NF-kappaB signaling pathway. J Neurosci. 2011;31:9075–9083. doi: 10.1523/JNEUROSCI.1537-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabroe I, Jones EC, Usher LR, Whyte MK, Dower SK. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–4710. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford PG, Feigin RD. Periodicity of susceptibility to pneumococcal infection: influence of light and adrenocortical secretions. Science. 1973;182:285–287. doi: 10.1126/science.182.4109.285. [DOI] [PubMed] [Google Scholar]