Abstract
This review is focused on the evolution and function of alveolar proteins. The lung faces physical and environmental challenges, due to changing pressures/volumes and foreign pathogens, respectively. The pulmonary surfactant system is integral in protecting the lung from these challenges via two groups of surfactant proteins – the small molecular weight hydrophobic SPs, SP-B and -C, that regulate interfacial adsorption of the lipids, and the large hydrophilic SPs, SP-A and -D, which are surfactant collectins capable of inhibiting foreign pathogens. Further aiding pulmonary host defence are non-surfactant collectins and antimicrobial peptides that are expressed across the biological kingdoms. Linking to the first symposium session, which emphasised molecular structure and biophysical function of surfactant lipids and proteins, this review begins with a discussion of the role of temperature and hydrostatic pressure in shaping the evolution of SP-C in mammals. Transitioning to the role of the alveolus in innate host defence we discuss the structure, function and regulation of antimicrobial peptides, the defensins and cathelicidins. We describe the recent discovery of novel avian collectins and provide evidence for their role in preventing influenza infection. This is followed by discussions of the roles of SP-A and SP-D in mediating host defence at the alveolar surface and in mediating inflammation and the allergic response of the airways. Finally we discuss the use of animal models of lung disease including knockouts to develop an understanding of the role of these proteins in initiating and/or perpetuating disease with the aim of developing new therapeutic strategies.
Keywords: Surfactant proteins, SP-A, SP-C, SP-D, Binding affinity, Oligomerization, Deficiency, Alveolar proteins, Collectins, Cathelicidins, Defensins, Innate host defence, Hemagglutination inhibition activity, Bacterial aggregation, Pro-inflammatory response, Anti-inflammatory response, Airways, Allergic response, Sensitization, Surfactant homeostasis, Alveolar lipoproteinosis, Type II cell hypertrophy and hyperplasia, Lamellar body size and number
1. Introduction
This review summarises the contributions in the second session of the alveolar biology symposium, which emphasised the evolution and function of various alveolar proteins. These included the small molecular weight surfactant proteins SP-B and SP-C, that are involved in surface tension regulatory functions; the large hydrophilic surfactant collectin proteins, SP-A and SP-D, that are involved in host defence; and various non-surfactant collectins, specifically newly discovered avian collectins and antimicrobial peptides, defensins and cathelicidins.
The lung is unique in that it faces both complex physical challenges associated with dynamically changing pressures and volumes, as well as environmental challenges associated with the vast array of foreign pathogens and particles to which the lung is exposed. Each of these two very diverse challenges is a consequence of the immense surface area of this dynamic fluid-lined organ. The pulmonary surfactant system is uniquely situated at the air–liquid interface of this large internal surface, such that it is able to play a role both in dynamically regulating the interfacial surface tension with changing lung volumes as well as actively inhibiting and inactivating a broad spectrum of foreign pathogens.
These roles are achieved by two separate groups of surfactant proteins. The small molecular weight hydrophobic SPs, SP-B and -C, are intricately associated with the surfactant lipid film at the air–liquid interface and function to regulate: (1) the initial interfacial adsorption of the lipids; (2) the reversible sequestration of the lipids into the surfactant reservoir (multilayer membrane aggregates in the hypophase that are associated with the interfacial film); and finally (3) the recruitment of lipids from the reservoir to the surface film to spread over an expanding surface area (Fig. 1). In this way they regulate the structure, integrity and composition of the surface lipid film, such that it optimally controls interfacial surface tension (Possmayer et al., 2001; Perez-Gil, 2008; Zuo et al., 2008). The large hydrophilic SPs, SP-A and -D, are members of a family of collagenous carbohydrate binding proteins, known as collectins, or calcium-dependent (C-type) lectins. Collectins consist of oligomers of trimeric subunits that are capable of recognizing, inhibiting and inactivating a broad spectrum of foreign pathogens, making them important effector molecules of the innate immune system (Haagsman and Diemel, 2001).
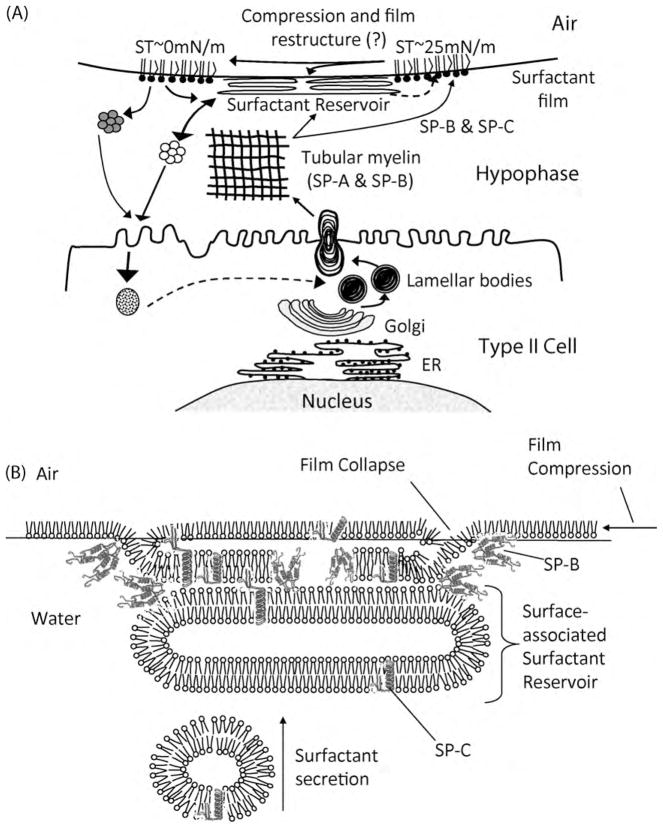
Fig. 1.
(A) Schematic diagram of the life cycle of pulmonary surfactant. Pulmonary surfactant is packaged in lamellar bodies (LB) that are secreted into the liquid lining the alveoli (hypophase) via exocytosis across the type II cell plasma membrane. Here the lamellar bodies swell and unravel, forming a crosshatched structure, termed tubular myelin (TM), which consists of lipids and proteins, in particular SP-A and SP-B. This structure supplies lipids to the surface film at the air–liquid interface as well as the surfactant reservoir, which is a multilayer structure associated with the surface film. The adsorption of the lipids to the air–liquid interface is mediated by the surfactant proteins, SP-B and -C. As the mixed molecular film is compressed, lipids are squeezed out of the film into the reservoir, and the film undergoes a restructuring through as yet unknown molecular mechanisms, which renders it capable of reducing surface tension (ST) to near 0 mN/m. Upon reexpansion, some lipids from the reservoir re-enter the surface film. Lipids from the surface film and the reservoir are eventually recycled and taken back up by the type II cell via endocytosis. Figure reproduced with modifications from (Foot et al., 2006) with permission from Elsevier.
(B) Hypothetical model of the surfactant film and surface-associated surfactant reservoir demonstrating film collapse under dynamic compression. The interaction of the two hydrophobic surfactant proteins (SP-B and -C) with the lipid mono- and bilayers is indicated. This interaction aids in the regulation of movement of lipids between the interfacial film and the surface-associated reservoir. Figure adapted from the original (Perez-Gil and Keough, 1998) and reproduced with modifications from (Foot et al., 2006) with permission from Elsevier.
However, the surfactant collectins are not the only proteins involved in pulmonary innate defence. Recently, four novel collectin proteins, all highly expressed in the respiratory tract, have been described in the chicken, including an SP-A homologue named chicken lung lectin (cLL, an SP-A like protein lacking collagen) and three other chicken collectins (cCL1-3) homologous to human collectins CL-L1, CL-K1 and CL-P1, respectively (Hogenkamp et al., 2006). In addition, there is a range of antimicrobial peptides, including the two main families – the defensins and the cathelicidins. Antimicrobial peptides are ancient molecules having been found in plants, insects, mammals and birds (van Dijk et al., 2008). They are small, cationic and often amphipathic and display a variety of activities related to host defence functions, including direct antimicrobial activity against various microbial pathogens (Hiemstra, 2007).
This review summarises the diverse contributions to this symposium, and begins with a discussion of the role of temperature and hydrostatic pressure in shaping the evolution of one of the hydrophobic surfactant proteins, SP-C, in mammals. This topic served as a link to the first session which emphasised the molecular structure and biophysical function of the surfactant lipids and proteins, and discusses the possible implications of alterations in SP-C structure for the regulation of surface activity at the air–liquid interface under extreme environmental conditions. The transition to discussions on the role of the alveolus in innate host defence is achieved by a discussion of the structure, function and regulation of the ancient antimicrobial peptides, the defensins and cathelicidins. We describe the recent discovery of novel avian collectins in the chicken respiratory tract and provide evidence for their role in fighting influenza infection. This is followed by a series of contributions on SP-A and SP-D, beginning with their respective roles in mediating host defence at the alveolar surface. As these proteins are not only restricted to the air–liquid interface, we also discuss the role of SP-D in mediating airway inflammation and the airway allergic response. Finally, we discuss the use of animal models of lung disease including SP-A and SP-D knockouts to develop an understanding of the role of these proteins in initiating and/or perpetuating disease with the aim of developing new therapeutic strategies.
2. The role of temperature and pressure in shaping the evolution of SP-C (S. Orgeig)
The hydrophobic proteins SP-B and SP-C promote the surface tension lowering functions of surfactant, as they enhance lipid adsorption to the air–liquid interface and regulate the movement of the lipids between the surfactant film and the surface-associated phase (Schürch et al., 1998; Perez-Gil, 2001, 2008; Possmayer et al., 2001; Zuo et al., 2008) (Fig. 1B). Although the exact mechanism by which SP-C promotes interfacial adsorption is not understood (Perez-Gil, 2008), SP-C is able to promote the transfer of phospholipids between membrane vesicles and the monolayer at the air–liquid interface. Specifically this function requires the positive charges of the residues lysine and arginine, which signal the boundary between the N-terminal extramembrane domain and the alpha-helical transmembrane domain (Creuwels et al., 1995) (see Fig. 2). Many of the functions of SP-C are attributed to the very dynamic N-terminal domain, which is capable of interacting with and perturbing the lipid packing of phospholipid membranes and monolayer films (Plasencia et al., 2001a,b, 2004, 2005). Specifically, SP-C is able to stabilise the surfactant film during dynamic compression/expansion cycles, a function that is attributed to the palmitoylation of the two cysteine residues in the N-terminal domain of the protein (Qanbar et al., 1996; Gustafsson et al., 2000) (see Fig. 2). It is this palmitoylation that is essential for the close association of SP-C with highly compressed and presumably highly ordered surfactant films, as depalmitoylation of N-terminal-mimicking peptides caused the peptides to be lost from the monolayer at high pressures (Bi et al., 2002). Palmitoylation also appears important in stabilising interdigitated-like phospholipid structures (Plasencia et al., 2008) such as those potentially involved as intermediates in bilayer-to-monolayer conversions or in bilayer–bilayer fusion (Perez-Gil, 2008). Hence, it is likely that palmitoylation directly enables SP-C to link the monolayer at the air–liquid interface to an adjacent bilayer and to link two bilayers together, thus allowing SP-C to promote the formation and stabilisation of the surface-associated surfactant reservoir (ten Brinke et al., 2002). It is these functions and the fact that SP-C deficiency leads to severe respiratory complications in the long term and under adverse conditions (Lawson et al., 2005; Mulugeta and Beers, 2006) that suggest that SP-C is important for lung function during lung injury or in cases of alveolar instability and collapse (ten Brinke et al., 2002).
Fig. 2.
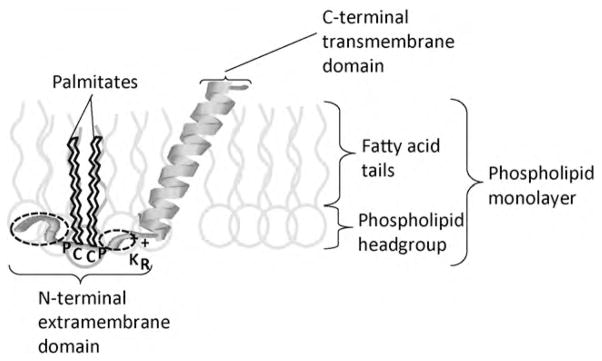
Schematic diagram of an SP-C molecule in a surfactant phospholipid film. The α-helix within the C-terminal transmembrane domain is embedded at an angle in the phospholipid fatty acid tails and the N-terminal extramembrane domain is associated with the hydrophilic phospholipid head groups. The two palmitate groups that are covalently linked to two cysteine residues (C) are anchored within the phospholipid fatty acid tails. On either side of the cysteine residues is a proline residue (P) which changes the orientation of the protein chain, probably enabling appropriate orientation of the palmitates and the N-terminal segment with the phospholipid headgroups. The two + signs indicate two positively charged residues, Lys (K) and Arg (R) at the boundary between the more polar N-terminal domain and the hydrophobic C-terminal domain. The two dotted circles indicate the location of site 2 and sites 9 and 10 that are evolutionarily labile and appear to be under positive selection in the diving mammals. In cetaceans and sirenians, the charge and polarity of these residues may be important, possibly leading to stronger binding of the N-terminal tail to the phospholipid headgroups, leading to greater stability of the lipid–protein complex during the high compression forces during diving. In pinnipeds, the hydrophobicity at sites 9 and 10 is consistent and therefore appears to be important, possibly leading to greater interactions with the palmitic acid residues linked to the cysteines (C), and possibly aiding in the adsorption of SP-C/lipid complexes to the air–liquid interface upon resurfacing after a dive. Figure reproduced with modifications from (Foot et al., 2006) with permission from Elsevier.
It was therefore hypothesised that SP-C may be critical for effective lung function in marine mammals that regularly collapse their lungs during deep dives when they experience high hydrostatic pressures, requiring frequent and alternating collapse and reformation of the surfactant film at the air–liquid interface (Foot et al., 2007). In addition, SP-C may be important in maintaining surfactant function at cold temperatures in animals that undergo periods of torpor or hibernation to promote rapid adsorption of lipids to the air–liquid interface (Potter et al., 2007). Hence, SP-C is a good candidate to identify the presence of adaptive evolutionary changes in primary amino acid sequence in selected groups of mammals, e.g. marine mammals or heterothermic mammals, which would enable them to cope with the problems of deep diving or variable body temperatures.
In two separate studies, phylogenetic analysis by maximum likelihood were used to estimate rates of non-synonymous (amino acid-changing) to synonymous (silent) substitutions among nucleotide and inferred amino acid sequences of the sftpc gene from a range of mammals. An excess of non-synonymous over synonymous nucleotide substitutions indicates positive selection. Closely related heterothermic and homeothermic species were compared to determine the role of body temperature regulation in shaping the evolution of SP-C (Potter et al., 2007) and closely related groups of aquatic versus terrestrial mammalian species were compared to determine whether diving has acted as an evolutionary selection pressure on the primary sequence of SP-C (Foot et al., 2007).
Mode of body temperature regulation did not appear to influence the evolution of SP-C, as there were no amino acid sites under positive selection. Instead the protein sequence of SP-C is highly conserved with synonymous or highly conservative amino acid substitutions being predominant. Hence, SP-C in heterothermic mammals is under purifying selection, which ensures maintenance of surfactant function despite the variability in mode of mammalian body temperature regulation. Heterothermic animals do not employ modulation of SP-C primary sequence to regulate surfactant function under torpid conditions.
On the other hand, in marine mammals there was evidence of positively selected sites, particularly in the N-terminal extramembrane domain of SP-C (Foot et al., 2007). Unlike the hydrophobic C-terminal transmembrane domain which forms an alpha-helix that is embedded in the fatty acid tails of the lipid mono- or bilayers of surfactant, the N-terminal domain is more polar and is closely associated with the polar headgroups of the phospholipid layers (Fig. 2). Different amino acid sites in the N-terminal domain of SP-C demonstrate positive selection in the three diving lineages: site 2 in the cetaceans (whales and dolphins), sites 7, 9 and 10 in the pinnipeds (seals and sea lions) and sites 2, 9 and 10 in the sirenians (dugongs and manatees). The biophysical properties that appeared influential in determining the amino acid substitutions were isoelectric point, chemical composition of the side chain, polarity and hydrophobicity (Foot et al., 2007). At sites 2 and 10 there is a tendency for more polar and/or more charged residues, as they are involved in polar interactions with the hydrophilic head groups of the phospholipid layer (Fig. 2). An increase in charge and polarity is likely to lead to improved binding of this part of the protein to the lipid layer, thereby increasing stability of the lipid–protein complex, which may be highly desirable during the extreme compression of the lung that occurs during deep diving. In addition, at site 9 there is a tendency for more hydrophobic residues particularly in the pinnipeds. As this site is immediately adjacent to the Pro-Cys-Cys-Pro motif (or the Pro-Cys-Phe-Pro motif in most carnivores), it is possible that there are some hydrophobic interactions with the palmitic acid residues covalently linked to the Cys residues, or to the hydrophobic aromatic ring of the Phe residue. In order for a protein such as SP-C to promote adsorption to an air–liquid interface, the N-terminal region may need to partially unfold. The degree of unfolding and potentially the rate of adsorption are correlated with the number and regular distribution of non-polar, hydrophobic amino acid residues within the protein chain (Schürch et al., 1998). Generally, within the N-terminal domain of SP-C there is a regular distribution of non-polar residues interspersed between polar residues. Hence, it is possible that positive selection among the pinnipeds of a hydrophobic Val residue at position 9 to replace the more polar Ser in most of the terrestrial carnivores is an adaptation to the regular collapse of the surfactant film during diving and may possibly enable more efficient adsorption of the lipids and proteins after surfacing and with the expansion of the lung. For example, such a property could be beneficial when the lungs need to be reinflated rapidly upon resurfacing after a dive. Previous studies have shown SP-C is more effective than SP-B in maintaining compression–expansion cycling with overcompression (i.e. surface area reduction by 95%) of surfactant organic extract films (Qanbar et al., 1996). The palmitates associated with the two cysteines in the N-terminus appear important for this function. Consequently, positive selection in the N-terminal extramembrane domain of SP-C in diving mammals may reflect adaptations to the repeated collapse and reinflation of the lung upon diving and resurfacing (Foot et al., 2007). The positively selected amino acid changes in this region appear to be the first evidence that SP-C, a protein important in regulating the surface activity of surfactant at the air–liquid interface, could be selectively modified in specific evolutionary lineages in response to a functional selection pressure associated with living in an extreme environment.
3. The role of defensins and cathelicidins in host defence in the human lung (P.S. Hiemstra)
The lung epithelium constitutes an enormous surface area that is continuously exposed to inhaled substances, including gases, particles and potentially pathogenic micro-organisms. The efficient host defence system that is present in the lung explains why severe lung infections are rare in healthy subjects, despite this high exposure to pathogens. The epithelium of the airways and alveoli is a central player in this host defence system. This epithelium constitutes a physical barrier, the airway epithelium removes particles and micro-organisms by mucociliary clearance mechanisms and it produces a range of mediators, including chemokines, cytokines and growth factors, that activate and recruit other cells with the ultimate aim to clear infections (Bals and Hiemstra, 2004). In this way neutrophils and macrophages, as well as other inflammatory immune cells, can be recruited to directly clear the respiratory pathogens or mount an adaptive immune response. In addition, epithelial cells produce antimicrobial reactive oxygen and nitrogen intermediates, as well as antimicrobial peptides and proteins. These act as endogenous broad-spectrum antibiotics and thus form an important element of the innate immune system. Antimicrobial peptides represent an ancient evolutionary conserved group of proteins that are found in all biological kingdoms, including bacteria, plants, and animals (both invertebrates and vertebrates) (Zasloff, 2002; Yeaman and Yount, 2007). Epithelial cells use the release of antimicrobial peptides to kill micro-organisms, whereas neutrophils (that also are an important cellular source of antimicrobial peptides) use these peptides mainly to kill ingested micro-organisms present in the phagolysosome or present in specialized extracellular structures, termed neutrophil extracellular traps (Brinkmann and Zychlinsky, 2007). Most antimicrobial peptides are characterised by a cationic charge and broad-spectrum antimicrobial activity against a range of micro-organisms. The widespread distribution of antimicrobial peptides demonstrates the importance of this innate defence system in species with and without an adaptive immune system.
The main families of antimicrobial peptides present in human airway secretions are the defensins and the cathelicidins (Bals and Hiemstra, 2004). Whereas various defensins can be found in airway secretions, humans only express one member of the cathelicidin family of antimicrobial peptides, hCAP-18/LL-37. hCAP-18 is the precursor of the cathelicidin peptide LL-37, that is released from hCAP-18 by proteolytic cleavage (Burton and Steel, 2009). Two defensin families can be distinguished based on their structure (pairing of disulfide bridges): the alpha- and the beta-defensins. Both alpha- and beta-defensins are present in airway secretions (Bals and Hiemstra, 2004). In addition to AMPs, a range of larger antimicrobial proteins is present in the lung including lysozyme, lactoferrin and small cationic proteinase inhibitors such as secretory leukocyte protease inhibitor. The main cellular sources of antimicrobial peptides and proteins are the epithelial cells and neutrophils. Whereas neutrophils store antimicrobial peptides in their granules, synthesis of these peptides by epithelial cells is a tightly regulated process (Bals and Hiemstra, 2004). A wide range of processes and mediators have been shown to regulate epithelial expression of antimicrobial peptides, including a range of microbial products, growth factors involved in wound repair, cytokines released during inflammation and the active form of vitamin D (1,25(OH2)D3) (Fig. 3). This information is important to understand the role of antimicrobial peptides in inflammatory and infectious lung diseases, but also for the development of new treatments based on enhancing endogenous antimicrobial peptide production. Indeed, local application of vitamin D to skin or oral administration has been demonstrated to increase expression of hCAP18/LL-37 in skin (Weber et al., 2005; Hata et al., 2008).
Fig. 3.
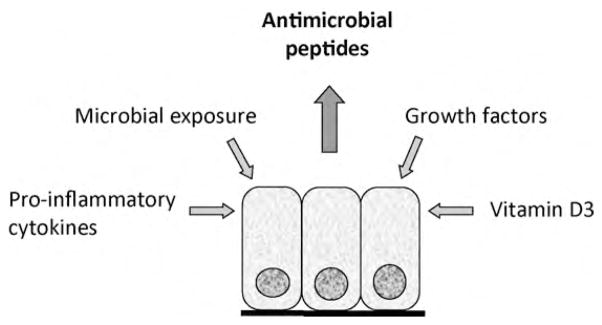
Regulation of antimicrobial peptide expression in airway epithelial cells. A variety of mechanisms and mediators may increase expression of antimicrobial peptides in epithelial cells of the lung. Although most studies have been performed using bronchial epithelial cells, similar mechanisms appear to be operative at the alveolar surface.
Defensins and LL-37 are cationic peptides that act on microbial membranes, disturb their integrity and kill the micro-organisms. In addition to this direct antimicrobial activity, defensins and LL-37 also display functions in inflammation, immunity and wound repair. These defensins and LL-37 activities include the induction of pro-inflammatory cytokines and chemokines for example in epithelial cells, chemotactic activity for inflammatory and immune cells, and mitogenic and chemotactic activities on epithelial cells resulting in enhanced wound repair (Oppenheim and Yang, 2005; Hiemstra, 2006). Structure–function studies are beginning to unravel the structural elements that are involved in these various activities, as demonstrated for LL-37 (Burton and Steel, 2009). Such insight might prove useful for the design of peptides with reduced toxicity and enhanced antimicrobial activity. We have used such an approach to develop an antimicrobial peptide based on the structure of LL-37, that has similar antimicrobial and LPS-neutralizing activity and reduced pro-inflammatory (neutrophil chemotactic) activity when compared to native LL-37 (Nell et al., 2006). Such peptides could be particularly useful as novel drugs for the treatment of infections involving pathogens that are resistant to conventional antibiotics (Hancock and Sahl, 2006).
Clinical studies confirm a role for defensins and cathelicidins in respiratory infections and chronic lung inflammatory disorders such as asthma, chronic obstructive pulmonary disease and cystic fibrosis. This is demonstrated by studies showing disease-related expression patterns in the lung as well as associations of gene polymorphisms with these conditions. These studies also reveal that inflammatory processes and tissue destruction may affect the activity of AMPs, as demonstrated for LL-37 in cystic fibrosis (Bergsson et al., 2009). Therefore, increased knowledge of the role of defensins and cathelicidins in the lung can be relevant for both our understanding of the pathogenesis of respiratory diseases, as well as for the development of novel treatments. Such novel treatments may include the administration of AMPs and AMP-derived peptides, the enhancement of endogenous AMP production, but also strategies to prevent the degradation of AMPs and other inhibitory mechanisms for example in purulent secretions.
4. The role of avian collectins in host defence (E.J.A. Veldhuizen)
In addition to the antimicrobial peptides and proteins, the lung also includes in its host defence arsenal a group of proteins known as collectins. Collectins are members of the family of vertebrate C-type lectins and are characterised by a C-type carbohydrate recognition domain (CRD), which is able to recognise and bind to certain carbohydrates. On a larger evolutionary scale, proteins containing a CRD are a subfamily of a superfamily of proteins containing C-type lectin-like domains that do not necessarily recognise saccharides but may recognise certain protein motifs. Such C-type proteins are represented and highly conserved even amongst invertebrates where they also function in the immune system (Haagsman and Diemel, 2001).
Collectins are important effector molecules of the innate immune system and the roles specifically of SP-A and SP-D in lung defence have been well studied in mammals. While SP-A is present and highly conserved amongst most major taxa of airbreathing vertebrates (Sullivan et al., 1998), the presence of SP-D in other vertebrates has not been widely investigated. However, recent studies in the chicken have shown that in contrast to mammals, SP-D appears to be absent (Hogenkamp et al., 2006). Rather, in addition to chicken SP-A, a second SP-A-like gene called chicken lung lectin (cLL) is present in the chicken genome. The presence of two chicken SP-A-like genes is thought to have arisen through gene duplication after the separation of avian and mammalian lineages (Hughes, 2007). Both SP-A and cLL genes are highly and almost exclusively expressed throughout the respiratory tract (Hogenkamp et al., 2006) including the trachea, lung, and airsacs (Fig. 4). In addition, three other chicken collectins (cCL1-3) homologous to human collectins CL-L1, CL-K1 and CL-P1, respectively, were also expressed in lung tissue.
Fig. 4.
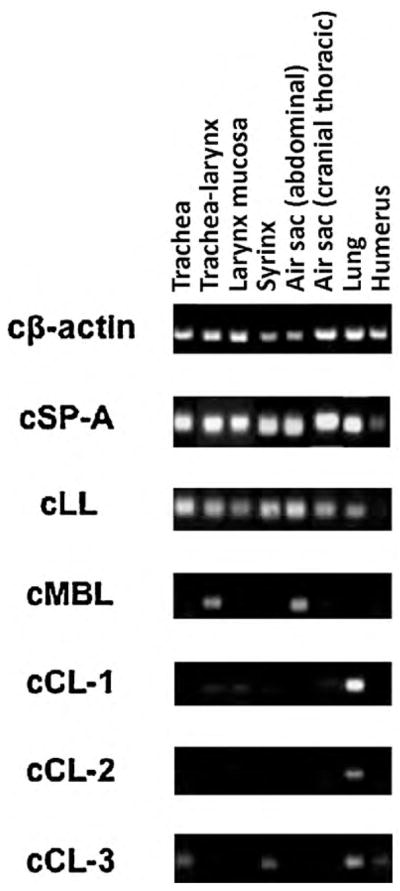
Gene expression profile of collectins and cLL in the respiratory tract of chicken (Hogenkamp et al., 2006).
An interesting feature of the two chicken SP-A homologues is their lack of a collagen-like region, since cSP-A contains only three Gly-X-Y repeats while cLL lacks even this small collagen-like region (hence the name lung lectin). It seems obvious that the characteristic octadecamer ‘bouquet of flowers’ form of mammalian SP-As (Fig. 5) cannot be formed by these chicken homologues. However, the collagen-like domain is not needed for trimerization of collectins, and hence a trimer could be the active form of cLL. Chemical cross-linking experiments have indeed shown that recombinant cLL in solution is oligomerized, although the exact number of monomers involved was not determined (Hogenkamp et al., 2008). The role of the collagen domain of mammalian SP-A has not been completely elucidated but it seems to be involved in stabilization of the protein and may act as a spacer to facilitate interactions between different phospholipid structures, for example, in tubular myelin and for agglutination of microbes (Palaniyar et al., 2001). In addition, it was shown that the collagen-like region is also involved in the interaction of SP-A with various immune cells responsible for phagocytosis and clearance of micro-organisms (Tenner, 1999). It will be interesting to determine how the biophysical and immunological function of the collagen-free chicken SP-As compare to their mammalian counterparts.
Fig. 5.
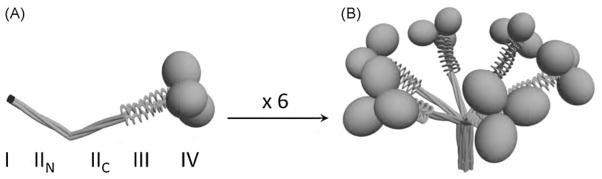
Three-dimensional models of trimeric (A) and oligomeric (B) forms of SP-A. The four structural domains of the human SP-A polypeptide chain are shown: I) NH2 -terminal segment; II) collagen-like domain with a sequence irregularity, which divides the collagen-like domain in two parts: NH2 -terminal (IIN ) and COOH-terminal (IIC ) portions; III) neck region between the collagen and the globular domain; and IV) COOH-terminal globular domain. Figure modified from Sanchez-Barbero et al. (2005, 2007).
In order to elucidate the possible involvement of chicken collectins in innate defence, the effect of H9N2 influenza A virus infection on the gene expression levels of chicken collectins was determined in trachea and lung tissue. Interestingly within 24 h a downregulation was observed in lung tissue, while collectins were generally upregulated in trachea, indicating differential expression depending on the location within the respiratory tract (Reemers et al., 2009). In addition, recombinantly expressed cLL showed viral hemagglutination inhibition activity in vitro (Hogenkamp et al., 2008). These first results on avian collectins indicate that they could be important in innate defence of the chicken lung against (viral) infections. Further elucidation of the role of chicken collectins is important to understand the immunology of the chicken respiratory tract and could lead to strategies that prevent infectious diseases in poultry. Considering the potential danger of zoonoses, including avian influenza, this is also of major importance for public health.
5. SP-A mediated host defence at the alveolar surface (C. Casals)
In addition to the above-mentioned six avian lectin/collectins, there are nine different collectins identified so far in mammals. Surfactant proteins SP-A and SP-D belong to the non-serum mammalian collectins, secreted to the alveolar fluid or mucosal surfaces (Wright, 2005). SP-A constitutes the major protein component of pulmonary surfactant by mass in mammals. In contrast to SP-D, SPA is mainly associated with surfactant membranes (Casals, 2001), which facilitates its location at the air–liquid interface of the lung, the initial defence barrier against inhaled pathogens or toxins. SPA has some properties related to its ability to bind and aggregate surfactant membranes: (1) Together with SP-B, contributes to the formation of tubular myelin; (2) improves the rate of surfactant adsorption to an air–liquid interface; and (3) protects surfactant membranes against inactivation by transudated serum proteins (Casals, 2001; Casals and Garcia-Verdugo, 2005). At the same time, SP-A participates in alveolar innate immune defence, together with SP-D and other proteins and peptides, by direct killing of micro-organisms or by indirect killing through enhancing the uptake of pathogens by phagocytes (Casals and Garcia-Verdugo, 2005; Wright, 2005; Kuroki et al., 2007; Haczku, 2008).
SP-A can bind to some micro-organisms such as Gram negative bacteria (Wu et al., 2003; Kuroki et al., 2007). This results in either bacterial killing and/or bacterial aggregation. Bacterial aggregation facilitates phagocytosis mediated by the collagen tails of SP-A, initiating a pro-inflammatory response (Wright, 2005; Haczku, 2008). The binding of SP-A to receptors via the globular heads results in an anti-inflammatory response (Yamada et al., 2006; Janssen et al., 2008) and prevents the persistence of inflammation, which is detrimental to the lung. SP-A immune functions may depend on whether a pro-inflammatory response is needed to combat infection or whether an anti-inflammatory action is required to limit inflammation and avoid tissue damage.
SP-A functions depend on SP-A binding capabilities (to lipids, carbohydrates, and proteins), which in turn depend on its complex structure (Casals and Garcia-Verdugo, 2005). Mammalian mature SP-A consists of 18 subunits (Fig. 5), each of which consists of four structural domains: (I) a 7–10 residue N-terminal segment involved in intermolecular disulfide bond formation; (II) a 79 residue collagen-like domain characterised by 23 Gly-X-Y repeats with an interruption near the midpoint of the domain; (III) a 35 amino acid segment with high alpha-helical propensity, which constitutes the neck region between the collagen and the globular domain; and (IV) a 115 residue C-terminal globular domain involved in lipid binding and in Ca2+-dependent binding of oligosaccharides (Casals, 2001; Casals and Garcia-Verdugo, 2005; Wright, 2005). SP-A is modified after translation (cleavage of the signal peptide, proline hydroxylation, and N-linked glycosylation) and assembled into a complex oligomeric structure that resembles a flower bouquet (Fig. 5) (Casals, 2001; Casals and Garcia-Verdugo, 2005; Wright, 2005). SP-A assembly is an intracellular process that can be conceptualised in two parts: the folding of monomeric subunits into trimers (Fig. 5A) and the association of six trimers into an octadecamer (Casals and Garcia-Verdugo, 2005) (Fig. 5B). Supratrimeric assembly of SP-A depends on inter-chain disulfide bonds and noncovalent intermolecular forces in the microfibrillar N-terminal piece (Casals and Garcia-Verdugo, 2005; Sanchez-Barbero et al., 2005).
Mammalian SP-A is not only assembled in supratrimeric oligomers but also forms multimers by self-association of the protein in the presence of Ca2+. An intact collagen domain is required for the formation of supratrimeric oligomers and multimers (Casals and Garcia-Verdugo, 2005). An important structural difference between mammalian and avian SP-A resides in the significant size reduction (cSP-A) or the lack (cLL) of the collagen-like region (Hogenkamp et al., 2006). It is believed that the collagen domain of mammalian SP-A is required for the formation of SP-A supraquaternary structure adsorbed to surfactant membranes and for the formation of tubular myelin (Casals and Garcia-Verdugo, 2005). Although information on the supraquaternary structure of avian SP-A is lacking, tubular myelin structures have not been observed in avian surfactant (Bernhard et al., 2001). This avian SP-A structural adaptation might be related to the fact that the unique avian lung structure is organized to maintain a unidirectional flow of air across the respiratory surfaces in the air capillaries. Avian lungs do not undergo cyclical change to surface area during respiration and their surfactant system does not lower surface tension under compression to the same extent as mammals (Daniels et al., 1998; Bernhard et al., 2001).
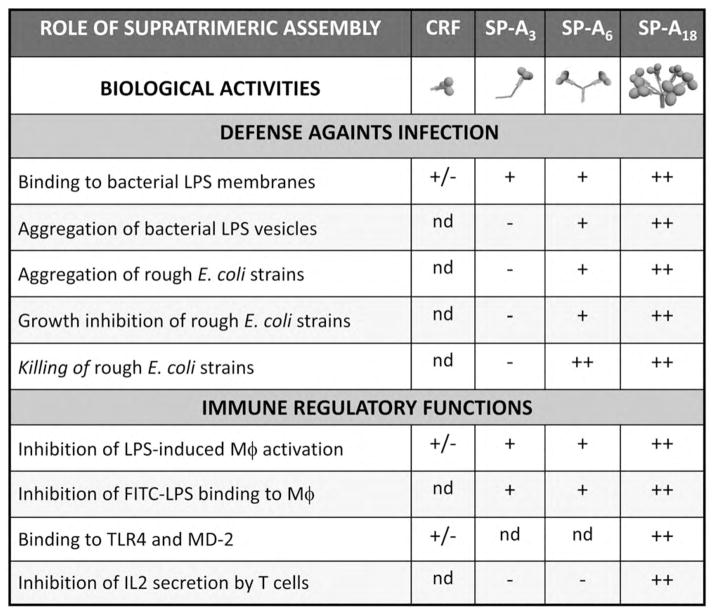
Supratrimeric oligomerization and multimerization of mammalian SP-A and other collectins appears to be needed for many of their functions. The binding affinity of a single SP-A lectin domain for carbohydrates is very low. However, the greater multiplicity of lectin domains found in higher-order oligomers and self-aggregated forms of SP-A is required to give high-affinity binding to carbohydrate-bearing surfaces (Casals and Garcia-Verdugo, 2005). The collagen-like domain of mammalian SP-A functions as scaffolding that amplifies the ligand binding activities of globular domains and, in addition, is responsible for the binding of SP-A to some receptors on the surface of alveolar macrophages and epithelial cells (Wright, 2005). In relation to the importance of the degree of SP-A oligomerization for some of its immune functions, Fig. 6 shows that antimicrobial activities of human SP-A require supratrimeric oligomerization (unpublished results). Hexamers at least are needed. On the other hand, while full-length trimers of human SP-A are able to inhibit LPS-induced macrophage activation, trimers are unable to inhibit interleukin secretion by T cells, which requires octadecameric structures (Sanchez-Barbero et al., 2005, 2007). Together, these studies strengthen the concept that supratrimeric oligomerization is important for the host defence function and the immunosuppressive activity of SP-A. It is possible that mutations in SP-A, not yet identified, compromise supratrimeric oligomerization and lead to increased susceptibility to bacterial and viral infections and/or chronic lung inflammation. These symptoms occur in immunocompromised individuals who are heterozygous or homozygous for the (Arg23Cys) variant of mannose binding protein (MBP). This mutation in the collagen-like region of serum MBP affects protein oligomerization (Wallis et al., 2004).
Fig. 6.
Role of the degree of oligomerization in antibacterial activities and immune regulatory functions of human SP-A. CRF is the collagenase-resistant fragment, which consists of three globular domains plus the neck domain of SP-A (Yamada et al., 2006); SP-A3 are full-length trimers of recombinant human SP-A expressed in mammalian cells, which result from site-directed substitution of serine for Cys6 and substitution of a functional signal peptide for the cysteine-containing SP-A signal sequence (Sanchez-Barbero et al., 2005). SP-A6 are full-length hexamers of recombinant human SP-A expressed in insect cells (Sanchez-Barbero et al., 2007). SP-A18 are octadecameric native or recombinant human SP-A expressed in mammalian cells (Sanchez-Barbero et al., 2005).
6. SP-D mediated host defence at the alveolar surface (H.W. Clark)
SP-D is the other hydrophilic surfactant protein and its monomer consists of four regions: a short N-terminal non-collagen sequence, a very long collagen domain of 59 Gly-X-Y repeats and a short linking domain, the ‘neck’ region that connects the collagen domain to the fourth region, the C-terminal carbohydrate recognition domain (CRD) (Fig. 7A). Unlike SP-A which has a ‘bunch of tulips’ orientation of oligomers (Fig. 6B), SP-D forms a cruciform structure in which four trimers self-associate at their N-termini to form highly ordered SP-D dodecamers (Fig. 7B), possessing a wider spacing of the CRDs (100 nm) than in SP-A. Both SP-A and SP-D interact with the hydroxyl groups on surface carbohydrates of pathogens via their CRDs. The basis of their distinguishing self from non-self is that the pattern of hydroxyl groups on surface carbohydrates of pathogens are more widely separated than the hydroxyls typically present on the surface carbohydrates of mammalian cells. Furthermore, the different geometry of the CRDs of SP-A and SP-D allows interactions with hydroxyl groups over different distances, which widens their combined range of pathogen recognition. Both SP-A and SP-D are considered primarily as molecules of the innate immune system, involved in the first line defence of the lungs from infection (Haagsman and Diemel, 2001).
Fig. 7.
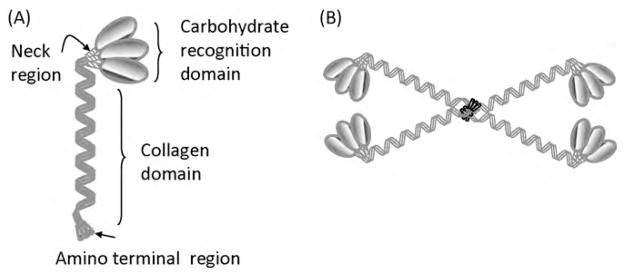
Schematic diagram of SP-D indicating the domain structure of the monomer (A) and the higher order dodecamer indicating the cruciform structure (B). This figure is adapted from Haagsman et al. (2008), Haczku (2006, 2008) and Haczku et al. (2004).
SP-D plays a multifaceted role in innate immunity in the alveolus by decreasing inflammation and promoting clearance of pathogens from the respiratory tract without recourse to stimulating a secondary immune response. The collectins maintain an inflammation free lung by promoting homeostatic clearance of apoptotic cells (Clark et al., 2002, 2003). Moreover, SP-A and SP-D may inhibit the release of pro-inflammatory cytokines by binding to signal inhibitory peptide on macrophages (SIRP-alpha) via their CRDs. However, when the CRDs are interacting with pathogens, they are unavailable to stimulate SIRP-alpha and instead the collagen tails bind CD91 and calreticulin, which leads to an appropriate pro-inflammatory signal in the presence of invading pathogens (Gardai et al., 2003). To study pathogen recognition by SP-A and SP-D, in more detail, examination of SP-A and SP-D interactions with the model target pathogen Haemophilus influenzae was undertaken. It is known that the lipopolysaccharide (LPS) on the surface of Haemophilus is an important pathogenic factor. By using mutant strains of the H. influenzae Eagan, it was demonstrated that the binding affinity of collectins for the LPS of Haemophilus strains was dependent on the extent of the arborisation of surface monosaccharides arising from heptose branch chains from the core region of the LPS (Fig. 8). It became apparent that native human SP-D (and a fragment of SP-D consisting of the neck and head of the CRD and only a short part of the collagenous domain) bound best to a mutant strain (Eagan 4A) with only one heptose branch chain in which access to the core region of the LPS was unimpeded. There was a less avid interaction with mutant strains with two branch chain heptoses (Eagan CA7) and even less in the pathogenic native strain Eagan which had three branch chain heptoses. LPS from a further strain 7004 Eagan with four branch chain heptoses was similarly resistant to SP-D binding (Mackay et al., 2006).
Fig. 8.
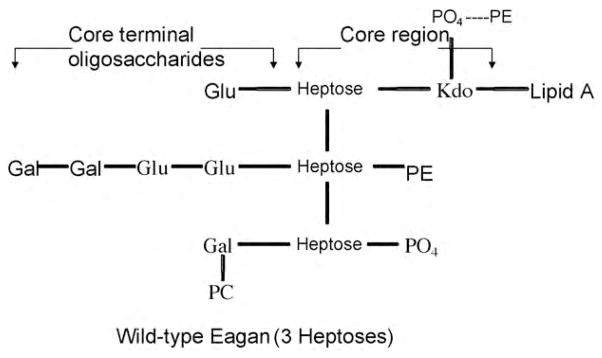
Diagramatic structure of lipopolysaccharide (LPS) from Haemophilus influenzae Eagan. This wild type structure shows three heptose branch chains from which monosaccharides arise. Strains with only one heptose (Eagan 4A strain) or two heptoses (Eagan CA7 strain) show greater binding to SP-D due to the increased accessibility of the target heptose residues which are less shielded by the presence of fewer terminal oligosaccharides than in wild-type. PE: Phosphatidylethanolamine; PO4 : Phosphate; PC: Phosphatidylcholine; Glu: Glucose; Gal: galactose; Kdo: 3-deoxy-d-manno-octulosonic acid. Adapted from Masoud et al. (1997).
The differences in binding to purified LPS from these strains was reflected in the binding to live bacteria from these strains and the pathogenicity in vivo in murine pulmonary infection was inversely related to collectin binding. The strains with at least three heptose branch chains were more successful in reproducing in the murine respiratory tract and induced a stronger inflammatory response resulting in increased recruitment of both neutrophils and macrophages to the lung (Mackay et al., 2006). These findings suggest that H. influenzae strains have developed variation in complexity of their carbohydrate surface structures and the successful pathogenic strains subvert the potential of collectin opsonisation by shielding the target core region. These results demonstrate in vivo that the important interaction is with the core oligosaccharides and not the core terminal oligosaccharides as previously reported (Sahly et al., 2002).
Recently the interaction of SP-D with LPS was characterised in some detail using infrared reflection–absorption spectroscopy which provided molecular structure information from films at the air–water interface, where protein adsorption to LPS monolayers serves as a model for protein–lipid interaction (Wang et al., 2008). Sophisticated imaging techniques have demonstrated that SP-D likely binds to these core heptose sugars, consistent with our own findings. These studies neatly demonstrate how these phylogenetically ancient microbial molecules may have adapted by developing surface structures which obscure or hinder collectin binding to targets on the pathogen surface and thus successfully subvert the innate immune response.
7. The role of SP-D in airway inflammation and the allergic airway response (A. Haczku)
Epithelial changes are a major characteristic of airway remodeling in chronic inflammation and epithelial cells have also been shown to regulate the inflammatory changes in the lung. The acute inflammatory airway response is characterised by a time-dependent onset followed by active resolution. Emerging evidence suggests that epithelial cells of the proximal and distal air spaces release host defence mediators, viz. the hydrophilic surfactant proteins SP-A and SP-D, that can facilitate both the initiation and the resolution part of inflammatory airway changes.
The immune regulatory effects of the lung collectins were extensively studied in collectin knockout mice. SP-A−/− mice appear normal under baseline conditions. However, SP-D−/− and double knockout mice show serious signs of constitutive activation of the immune system indicating that presence of SP-D in the lung is important for immunoprotection. This immunoprotective function is paired with a role in pathogen clearance as demonstrated by the fact that upon injury or infection, in spite of an exaggerated inflammatory response, mice lacking SP-D have impaired host defence (Botas et al., 1998; LeVine et al., 2000; Hawgood et al., 2001, 2002; Yoshida and Whitsett, 2006). Immune cells in the lung of SP-D−/− mice display multiple abnormalities including altered morphology and constitutive release of pro-inflammatory mediators. SP-D−/− mice also display spontaneous development of emphysema and heightened susceptibility to inflammatory stimuli, infections or allergic sensitization.
That SP-D protects against pro-inflammatory mediator release including the ones that favor development of the allergic ‘Th2-type’ inflammation was recently confirmed in a model of airway sensitization. Culture of alveolar macrophages from ovalbumin sensitised mice together with SP-D and allergen resulted in increased production of the immunosuppressive IL-10 as well as IL-12, and IFNγ cytokines, unfavorable for development of Th2-type changes (Takeda et al., 2003). SP-D also directly inhibited Th2 cytokine release in allergen-stimulated splenic mononuclear cells in vitro, derived from mice sensitised with Aspergillus fumigatus (Haczku et al., 2006).
Further studies also suggested that lack of SP-D in the lung of SP-D−/− mice resulted in a dendritic cell population with a pro-inflammatory, myeloid phenotype and constitutive TNFα expression. In contrast, administration of SP-D to bone marrow dendritic cell cultures suppressed the activation marker and TNFα expression (Hortobagyi et al., 2008), indicating that native SP-D inhibits maturation and activation of the pro-inflammatory dendritic cells.
Thus, SP-D can alter the differentiation and inhibit activation of immature dendritic cells through inhibition of TNFα (Hortobagyi et al., 2008). In addition, dendritic cells in the lung of SP-D−/− mice were unable to migrate to the regional lymph nodes and showed an abnormal accumulation in the airway submucosal tissue, possibly due to a markedly increased expression of a chemokine (CCL17) (Haczku et al., 2006). In SP-D−/− animals, increased percentage of both CD4+ and CD8+ T cells were found together with an increased expression of the activation markers CD69 and CD25 in the bronchoalveolar lavage fluid (BAL) (Fisher et al., 2002; Haczku et al., 2006). That the presence of SP-D can directly suppress T-cell function was demonstrated in vitro on isolated lymphocytes in a number of studies (Borron et al., 1998; Vass et al., 2004; Haczku et al., 2006; Haczku, 2008). The in vivo consequences of SP-D interaction with T-cell dependent inflammatory events were demonstrated in various models of allergic airway sensitization. Treatment of allergen-sensitised mice with recombinant or purified SP-D was effective in reducing the eosinophilic inflammation and specific IgE production (Madan et al., 2001a,b; Kishore et al., 2002; Strong et al., 2002; Singh et al., 2003; Liu et al., 2005).
Thus, SP-D directly modulates macrophage and dendritic cell function as well as T-cell dependent inflammatory events. SP-D (and SP-A) has a unique, dual functional capacity to induce pathogen elimination on the one hand and control pro-inflammatory mechanisms on the other, suggesting a potential suitability for therapeutic prevention and treatment of chronic airway inflammation without compromising host defence function of the airways.
8. The role of SP-A and SP-D in the healthy lung – lessons learnt from lung disease and knockout models (L. Knudsen)
The availability of knockout mouse models for both SP-A and SP-D has opened the door to understanding the physiological function of these molecules in the normal lung and potentially provides insight into the roles they may play in various lung diseases. For example, the SP-A knock-out mouse demonstrates a normal pulmonary phenotype, except that tubular myelin, an active ultrastructural subtype of intra-alveolar surfactant, is lacking. Surface activity appears relatively unaffected and, contrary to what one might expect from in vitro studies, which show that SP-A inhibits secretion of surfactant by alveolar type II cells and promotes uptake and recycling of surfactant material from the alveolar space (Hawgood and Poulain, 2001), surfactant homeostasis is undisturbed (Korfhagen et al., 1996). However, it could be demonstrated that SP-A-independent compensatory pathways, based on actin-and clathrin-mediated endocytosis of phospholipids by alveolar type II cells, maintain the intra-alveolar surfactant pool in the absence of SP-A. These mechanisms might be easily overrun under challenging conditions (Bates et al., 2008). When challenged, the surfactant of the SP-A deficient mice is more readily inactivated by plasma proteins and the animals are more vulnerable to pulmonary infections (Korfhagen et al., 1996). In humans, it was demonstrated that lowered levels of SP-A in BAL precede the development of acute respiratory distress syndrome, indicating that the loss of SPA might contribute to the inactivation of surfactant in this disease (Greene et al., 1999).
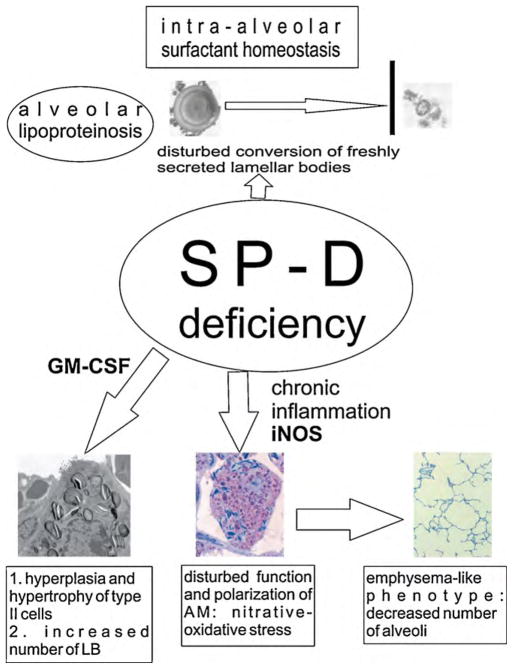
In contrast, the SP-D knockout mouse develops marked disturbances of surfactant homeostasis leading to an alveolar lipoproteinosis and an increase of the intracellular surfactant pool, which could not be anticipated from in vitro data (Hawgood and Poulain, 2001). The alveolar lipoproteinosis was attributed to an impaired uptake of ultrastructurally aberrant surfactant forms from the alveolar space by type II cells, ascribing a novel role to SP-D for modifying freshly secreted surfactant material (Ikegami et al., 2005, 2009). No causal link between human pulmonary alveolar lipoproteinosis and SP-D deficiency has been traced so far, although a recent report on lysinuric protein intolerance-related alveolar proteinosis found a low bioavailability of SP-D in the airways accompanied by high amounts of either degraded or entrapped SP-D within unusual surfactant lipid forms (Douda et al., 2009). Regarding the intracellular surfactant pool, defined as the total amount of lamellar bodies, there is an increase in the number of lamellar bodies per cell accompanied by a hypertrophy and hyperplasia of alveolar type II cells in SP-D knockout mice. Furthermore, the lungs of these mice develop an emphysema-like phenotype (Ochs et al., 2004) and are in a chronic inflammatory state. This is characterised, among other things, by elevated levels of pro-inflammatory mediators such as granulocyte-macrophage colony stimulating factor (GM-CSF) as well as an inappropriate activation of alveolar macrophages with oxidative-nitrative stress due to an excessive expression of inducible nitric oxide synthase (iNOS) (Wert et al., 2000; Hawgood et al., 2001; Atochina et al., 2004) (Fig. 9).
Fig. 9.
SP-D is needed for proper cellular processing of freshly secreted surfactant material. Absence of SP-D leads to an alveolar lipoproteinosis due to inability of alveolar type II cells to take up ultrastructural aberrant surfactant forms. A chronic inflammation is found in SP-D knockout mice. While elevated GM-CSF levels mediate hyperplasia and hypertrophy of alveolar type II cells accompanied by an increase of the number of lamellar bodies (LB), the increased iNOS-expression of activated alveolar macrophages (AM) is at least in part responsible for the development of pulmonary emphysema.
As shown by analysis of the GM-CSF/SP-D double knock-out mouse, hypertrophy and hyperplasia of alveolar type II cells as well as disturbances of the intracellular surfactant homeostasis are at least partly mediated by GM-CSF, whereas the remodelling process leading to the emphysema-like phenotype is not (Ochs et al., 2004). Pharmacological inhibition of iNOS, furthermore, was shown to decrease inflammatory markers (Atochina-Vasserman et al., 2007) and the additional ablation of the iNOS-gene in SP-D knock-out mice attenuated the degree of pulmonary emphysema, indicating an important role of iNOS for the pathogenesis in this model (Knudsen et al., unpublished observations).
In an attempt to rescue the phenotype of SP-D knock-out mice by substitution of different fragments of SP-D, intranasal delivery of a recombinant fragment of human SP-D consisting of a short tail of the collagen-like domain, the neck, and the carbohydrate recognition domain is sufficient to correct emphysema and surfactant disturbances (Clark et al., 2002, 2003; Knudsen et al., 2007). However, a fragment without the collagen-like tail does not display these therapeutic effects, pointing to an important role of the collagen-like domain for the protein’s function (Kingma et al., 2006; Knudsen et al., 2009). Human lung diseases characterised by chronic inflammation and low levels of SP-D in BAL include cystic fibrosis (in particular during acute exacerbation) (Postle et al., 1999; Noah et al., 2003) and smoke-related COPD (Honda et al., 1996; Betsuyaku et al., 2004). However, the role of these relative SP-D deficiencies in the pathogenesis of human lung diseases needs further investigations.
The important role of SP-D in maintaining an adequate immune response was recently demonstrated clinically by findings that children suffering from recurrent broncho-pulmonary infections demonstrated a lack of SP-D in their BAL (Griese et al., 2008). The increased vulnerability towards infections due to SP-D deficiency could also be confirmed in SP-D knockout mice (Jounblat et al., 2005).
9. Conclusions
The aim of this review was to highlight the diversity of function of the pulmonary surfactant system driven primarily by its unique set of surfactant proteins. Aiding particularly the pulmonary host defence function is a further diverse suite of proteins and peptides, some of which have performed their function for many millions of years.
We provide an evolutionary flavour through discussions of the potential environmental forces that may have led to primary sequence adaptations in a surfactant protein, through a discussion of the ancient groups of antimicrobial peptides and the discovery of a novel set of avian collectin proteins. However, the main focus was on diversity of function of pulmonary host defence proteins, on unravelling the mechanisms through structure–function relationships and on utilising the unique properties to develop new therapeutic tools, particularly a new generation of antimicrobial drugs.
We provided evidence that the primary sequence of SP-C may be selectively modified in specific evolutionary lineages in response to a functional selection pressure associated with living in an extreme environment. These specific sequence modifications require rigorous structure–function analyses in model membrane systems.
We have described an ancient and diverse set of antimicrobial peptides that are capable of directly killing micro-organisms through disruption of microbial membranes, but that also display functions in inflammation, immunity and wound repair. It is hoped that structure–function studies aimed at unravelling the structural elements that are involved in these various activities will provide insight to design peptides with reduced toxicity and enhanced antimicrobial activity.
We have described the discovery of a novel set of avian collectins that are expressed throughout the respiratory tract including trachea, lung, and airsacs of the chicken. We have provided evidence for anti-viral activity of chicken lung lectin in vitro, as well as a differential gene expression response of chicken collectins in response to influenza A virus infection, depending on the location within the respiratory tract. Further structure–function and immunological studies are required to elucidate the host defence mechanisms of these proteins, which may lead to strategies to prevent infectious diseases in poultry and potentially benefit public health.
We have described in detail the structure and function of the surfactant collectins SP-A and SP-D. Both are capable of directly killing micro-organisms as well as mobilising other arms of the immune system to carry out this function. Furthermore, both have complex immunoregulatory functions and can either promote or resolve inflammation. We provide evidence that for SP-A supratrimeric oligomerization to produce octadecameric structures is crucial to elicit the full range of host defence functions. Detailed structure–function studies examining the interaction of SP-D or fragments of SP-D with various native and mutant bacterial strains have elucidated the mechanism of binding between the protein’s carbohydrate binding domain and the pathogen’s lipopolysaccharide coat, providing an excellent example of the evolutionary arms race between host and pathogen.
A series of elegant in vivo and in vitro studies using both SP-D knockout mice and various cell culture systems has demonstrated that SP-D has important immunoregulatory functions in the airways. SP-D is able to directly modulate macrophage and dendritic cell function as well as T-cell dependent inflammatory events. Hence, SP-D has a dual function, capable of eliminating pathogens on the one hand and controlling pro-inflammatory mechanisms on the other. This suggests that SP-D represents a potentially suitable target for therapeutic prevention and treatment of chronic airway inflammation without compromising host defence function of the airways.
SP-A and SP-D knockout mice in combination with other regulatory gene ablations have provided important clues to potential mediators of the pathogenic phenotype seen in these model organisms. In addition knockout mice are being used to test the ability of certain recombinant fragments of the surfactant collectins to rescue the disease phenotype. These studies pave the way to elucidating the mechanistic links between SP-A or SP-D deficiency and the specific pathogenesis in various diseases, e.g. acute respiratory distress, cystic fibrosis or smoke-related COPD.
Finally, it is hoped that this review exemplifies the intended aim for the International Congress of Respiratory Science, which is to achieve vertical and horizontal integration and to foster interdisciplinary interaction and collaboration.
Acknowledgments
The authors would like to thank the organisers of the 2nd International Congress of Respiratory Science for an excellent conference and for facilitating this publication volume.
Footnotes
This paper is part of the “ICRS Supplement”, guest-edited by Dr. Steven F. Perry.
References
- Atochina-Vasserman EN, Beers MF, Kadire H, Tomer Y, Inch A, Scott P, Guo CJ, Gow AJ. Selective inhibition of inducible NO synthase activity in vivo reverses inflammatory abnormalities in surfactant protein D-deficient mice. J Immunol. 2007;179:8090–8097. doi: 10.4049/jimmunol.179.12.8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atochina EN, Beers MF, Hawgood S, Poulain F, Davis C, Fusaro T, Gow AJ. Surfactant protein-D, a mediator of innate lung immunity, alters the products of nitric oxide metabolism. Am J Respir Cell Mol Biol. 2004;30:271–279. doi: 10.1165/rcmb.2003-0091OC. [DOI] [PubMed] [Google Scholar]
- Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- Bates SR, Dodia C, Tao JQ, Fisher AB. Surfactant protein-A plays an important role in lung surfactant clearance: evidence using the surfactant protein-A gene-targeted mouse. Am J Physiol Lung Cell Mol Physiol. 2008;294:L325–L333. doi: 10.1152/ajplung.00341.2007. [DOI] [PubMed] [Google Scholar]
- Bergsson G, Reeves EP, McNally P, Chotirmall SH, Greene CM, Greally P, Murphy P, O’Neill SJ, McElvaney NG. LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline. J Immunol. 2009;183:543–551. doi: 10.4049/jimmunol.0803959. [DOI] [PubMed] [Google Scholar]
- Bernhard W, Gebert A, Vieten G, Rau GA, Hohlfeld JM, Postle AD, Freihorst J. Pulmonary surfactant in birds: coping with surface tension in a tubular lung. Am J Physiol Regul Integr Comp Physiol. 2001;281:R327–R337. doi: 10.1152/ajpregu.2001.281.1.R327. [DOI] [PubMed] [Google Scholar]
- Betsuyaku T, Kuroki Y, Nagai K, Nasuhara Y, Nishimura M. Effects of ageing and smoking on SP-A and SP-D levels in bronchoalveolar lavage fluid. Eur Respir J. 2004;24:964–970. doi: 10.1183/09031936.04.00064004. [DOI] [PubMed] [Google Scholar]
- Bi X, Flach CR, Perez-Gil J, Plasencia I, Andreu D, Oliveira E, Mendelsohn R. Secondary structure and lipid interactions of the N-terminal segment of pulmonary surfactant SP-C in Langmuir films: IR reflection-absorption spectroscopy and surface pressure studies. Biochemistry. 2002;41:8385–8395. doi: 10.1021/bi020129g. [DOI] [PubMed] [Google Scholar]
- Borron PJ, Crouch EC, Lewis JF, Wright JR, Possmayer F, Fraher LJ. Recombinant rat surfactant-associated protein D inhibits human T lymphocyte proliferation and IL-2 production. J Immunol. 1998;161:4599–4603. [PubMed] [Google Scholar]
- Botas C, Poulain F, Akiyama J, Brown C, Allen L, Goerke J, Clements J, Carlson E, Gillespie AM, Epstein C, Hawgood S. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc Natl Acad Sci USA. 1998;95:11869–11874. doi: 10.1073/pnas.95.20.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- Burton MF, Steel PG. The chemistry and biology of LL-37. Nat Prod Rep. 2009;26:1572–1584. doi: 10.1039/b912533g. [DOI] [PubMed] [Google Scholar]
- Casals C. Role of surfactant protein A (SP-A)/lipid interactions for SP-A functions in the lung. Pediatr Pathol Mol Med. 2001;20:249–268. [PubMed] [Google Scholar]
- Casals C, Garcia-Verdugo I. Molecular and functional properties of surfactant protein A. In: Nag K, editor. Developments in Lung Surfactant Dysfunction in Lung Biology in Health and Disease. Marcel Dekker Inc; New York: 2005. pp. 55–84. [Google Scholar]
- Clark H, Palaniyar N, Hawgood S, Reid KBM. A recombinant fragment of human surfactant protein D reduces alveolar macrophage apoptosis and pro-inflammatory cytokines in mice developing pulmonary emphysema. Annals N Y Acad Sci; 5th International Conference on Apoptosis from Signalling Pathways to Therapeutic Tools; Luxembourg. 2003. pp. 113–116. [DOI] [PubMed] [Google Scholar]
- Clark H, Palaniyar N, Strong P, Edmondson J, Hawgood S, Reid KBM. Surfactant protein D reduces alveolar macrophage apoptosis in vivo. J Immunol. 2002;169:2892–2899. doi: 10.4049/jimmunol.169.6.2892. [DOI] [PubMed] [Google Scholar]
- Creuwels LA, Boer EH, Demel RA, van Golde LM, Haagsman HP. Neutralization of the positive charges of surfactant protein C. Effects on structure and function. J Biol Chem. 1995;270:16225–16229. doi: 10.1074/jbc.270.27.16225. [DOI] [PubMed] [Google Scholar]
- Daniels CB, Lopatko OV, Orgeig S. Evolution of surface activity related functions of vertebrate pulmonary surfactant. Clin Exp Pharmacol Physiol. 1998;25:716–721. doi: 10.1111/j.1440-1681.1998.tb02283.x. [DOI] [PubMed] [Google Scholar]
- Douda DN, Farmakovski N, Dell S, Grasemann H, Palaniyar N. SP-D counteracts GM-CSF-mediated increase of granuloma formation by alveolar macrophages in lysinuric protein intolerance. Orphanet J Rare Dis. 2009;4:29. doi: 10.1186/1750-1172-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JH, Larson J, Cool C, Dow SW. Lymphocyte activation in the lungs of SP-D null mice. Am J Respir Cell Mol Biol. 2002;27:24–33. doi: 10.1165/ajrcmb.27.1.4563. [DOI] [PubMed] [Google Scholar]
- Foot NJ, Orgeig S, Daniels CB. The evolution of a physiological system: the pulmonary surfactant system in diving mammals. Special issue: frontiers in comparative physiology II: respiratory rhythm, pattern and responses to environmental change. Respir Physiol Neurobiol. 2006;154:118–138. doi: 10.1016/j.resp.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Foot NJ, Orgeig S, Donnellan S, Bertozzi T, Daniels CB. Positive selection in the N-terminal extramembrane domain of lung surfactant protein C (SP-C) in marine mammals. J Mol Evol. 2007;65:12–22. doi: 10.1007/s00239-006-0083-1. [DOI] [PubMed] [Google Scholar]
- Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRP alpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- Greene KE, Wright JR, Steinberg KP, Ruzinski JT, Caldwell E, Wong WB, Hull W, Whitsett JA, Akino T, Kuroki Y, Nagae H, Hudson LD, Martin TR. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999;160:1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- Griese M, Steinecker M, Schumacher S, Braun A, Lohse P, Heinrich S. Children with absent surfactant protein D in bronchoalveolar lavage have more frequently pneumonia. Pediatr Allergy Immunol. 2008;19:639–647. doi: 10.1111/j.1399-3038.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson M, Palmblad M, Curstedt T, Johansson J, Schurch S. Palmitoylation of a pulmonary surfactant protein C analogue affects the surface associated lipid reservoir and film stability. Biochim Biophys Acta. 2000;1466:169–178. doi: 10.1016/s0005-2736(00)00198-x. [DOI] [PubMed] [Google Scholar]
- Haagsman HP, Diemel RV. Surfactant-associated proteins: functions and structural variation. Comp Biochem Physiol A. 2001;129:91–108. doi: 10.1016/s1095-6433(01)00308-7. [DOI] [PubMed] [Google Scholar]
- Haagsman HP, Hogenkamp A, van Eijk M, Veldhuizen EJA. Surfactant collectins and innate immunity. Neonatology. 2008;93:288–294. doi: 10.1159/000121454. [DOI] [PubMed] [Google Scholar]
- Haczku A. Role and regulation of lung collectins in allergic airway sensitization. Pharmacol Therapeut. 2006;110:14–34. doi: 10.1016/j.pharmthera.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Haczku A. Protective role of the lung collectins surfactant protein A and surfactant protein D in airway inflammation. J Allergy Clin Immunol. 2008;122:861–879. doi: 10.1016/j.jaci.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haczku A, Cao Y, Vass G, Kierstein S, Nath P, Atochina-Vasserman EN, Scanlon ST, Li L, Griswold DE, Chung KF, Poulain FR, Hawgood S, Beers MF, Crouch EC. IL-4 and IL-13 form a negative feedback circuit with surfactant protein-D in the allergic airway response. J Immunol. 2006;176:3557–3565. doi: 10.4049/jimmunol.176.6.3557. [DOI] [PubMed] [Google Scholar]
- Haczku A, Vass G, Kierstein S. Surfactant protein D and asthma. Clin Exp Allergy. 2004;34:1815–1818. doi: 10.1111/j.1365-2222.2004.02134.x. [DOI] [PubMed] [Google Scholar]
- Hancock REW, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, Kanada K, Yomasaki K, Alexandrescu D, Gallo RL. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008;122:829–831. doi: 10.1016/j.jaci.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawgood S, Akiyama J, Brown C, Allen L, Li G, Poulain FR. GM-CSF mediates alveolar macrophage proliferation and type II cell hypertrophy in SP-D gene-targeted mice. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1148–L1156. doi: 10.1152/ajplung.2001.280.6.L1148. [DOI] [PubMed] [Google Scholar]
- Hawgood S, Ochs M, Jung AJ, Akiyama J, Allen L, Brown C, Edmondson J, Levitt S, Carlson E, Gillespie AM, Villar A, Epstein CJ, Poulain FR. Sequential targeted deficiency of SP-A and -D leads to progressive alveolar lipoproteinosis and emphysema. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1002–L1010. doi: 10.1152/ajplung.00118.2002. [DOI] [PubMed] [Google Scholar]
- Hawgood S, Poulain FR. The pulmonary collectins and surfactant metabolism. Annu Rev Physiol. 2001;63:495–519. doi: 10.1146/annurev.physiol.63.1.495. [DOI] [PubMed] [Google Scholar]
- Hiemstra PS. Defensins and cathelicidins in inflammatory lung disease: beyond antimicrobial activity. Biochem Soc Trans. 2006;34:276–278. doi: 10.1042/BST20060276. [DOI] [PubMed] [Google Scholar]
- Hiemstra PS. The role of epithelial β-defensins and cathelicidins in host defense of the lung. Exp Lung Res. 2007;33:537–542. doi: 10.1080/01902140701756687. [DOI] [PubMed] [Google Scholar]
- Hogenkamp A, Isohadouten N, Reemers SSN, Romijn RA, Hemrika W, White MR, Tefsen B, Vervelde L, van Eijk M, Veldhuizen EJA, Haagsman HP. Chicken lung lectin is a functional C-type lectin and inhibits haemagglutination by influenza A virus. Vet Microbiol. 2008;130:37–46. doi: 10.1016/j.vetmic.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Hogenkamp A, van Eijk M, van Dijk A, van Asten AJAM, Veldhuizen EJA, Haagsman HP. Characterization and expression sites of newly identified chicken collectins. Mol Immunol. 2006;43:1604–1616. doi: 10.1016/j.molimm.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Honda Y, Takajashi H, Kuroki Y, Akino T, Abe S. Decreased contents of pulmonary surfactant proteins A and D in BAL fluids of healthy smokers. Chest. 1996;109:1006–1009. doi: 10.1378/chest.109.4.1006. [DOI] [PubMed] [Google Scholar]
- Hortobagyi L, Kierstein S, Krytska K, Zhu XP, Das AM, Poulain F, Haczku A. Surfactant protein D inhibits TNF-alpha production by macrophages and dendritic cells in mice. J Allergy Clin Immunol. 2008;122:521–528. doi: 10.1016/j.jaci.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. Evolution of the lung surfactant proteins in birds and mammals. Immunogenetics. 2007;59:565–572. doi: 10.1007/s00251-007-0218-6. [DOI] [PubMed] [Google Scholar]
- Ikegami M, Grant S, Korfhagen T, Scheule RK, Whitsett JA. Surfactant protein-D regulates the postnatal maturation of pulmonary surfactant lipid pool sizes. J Appl Physiol. 2009;106:1545–1552. doi: 10.1152/japplphysiol.91567.2008. [DOI] [PubMed] [Google Scholar]
- Ikegami M, Na CL, Korfhagen TR, Whitsett JA. Surfactant protein D influences surfactant ultrastructure and uptake by alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L552–L561. doi: 10.1152/ajplung.00142.2004. [DOI] [PubMed] [Google Scholar]
- Janssen WJ, McPhillips KA, Dickinson MG, Linderman DJ, Morimoto K, Xiao YQ, Oldham KM, Vandivier RW, Henson PM, Gardai SJ. Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha. Am J Respir Crit Care Med. 2008;178:158–167. doi: 10.1164/rccm.200711-1661OC. Epub 2008 Apr 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jounblat R, Clark H, Eggleton P, Hawgood S, Andrew PW, Kadioglu A. The role of surfactant protein D in the colonisation of the respiratory tract and onset of bacteraemia during pneumococcal pneumonia. Respir Res. 2005;6:126. doi: 10.1186/1465-9921-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma PS, Zhang LQ, Ikegami M, Hartshorn K, McCormack FX, Whitsett JA. Correction of pulmonary abnormalities in Sftpd(−/−) mice requires the collagenous domain of surfactant protein D. J Biol Chem. 2006;281:24496–24505. doi: 10.1074/jbc.M600651200. [DOI] [PubMed] [Google Scholar]
- Kishore U, Madan T, Sarma PU, Singh M, Urban BC, Reid KBM. Protective roles of pulmonary surfactant proteins, SP-A and SP-D, against lung allergy and infection caused by Aspergillus fumigatus. Immunobiology. 2002;205:610–618. doi: 10.1078/0171-2985-00158. [DOI] [PubMed] [Google Scholar]
- Knudsen L, Ochs M, MacKay R, Townsend P, Deb R, Muehlfeld C, Richter J, Gilbert F, Hawgood S, Reid K, Clark H. Truncated recombinant human SP-D attenuates emphysema and type II cell changes in SP-D deficient mice. Respir Res. 2007;8:70. doi: 10.1186/1465-9921-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen L, Wucherpfennig K, MacKay RM, Townsend P, Muhlfeld C, Richter J, Hawgood S, Reid K, Clark H, Ochs M. A recombinant fragment of human surfactant protein D lacking the short collagen-like stalk fails to correct morphological alterations in lungs of SP-D deficient mice. Anat Rec Adv Integr Anat Evol Biol. 2009;292:183–189. doi: 10.1002/ar.20830. [DOI] [PubMed] [Google Scholar]
- Korfhagen TR, Bruno MD, Ross GF, Huelsman KM, Ikegami M, Jobe AH, Wert SE, Stripp BR, Morris RE, Glasser SW, Bachurski CJ, Iwamoto HS, Whitsett JA. Altered surfactant function and structure in SP-A gene targeted mice. Proc Natl Acad Sci USA. 1996;93:9594–9599. doi: 10.1073/pnas.93.18.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 2007;9:1871–1879. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- Lawson WE, Polosukhin VV, Stathopoulos GT, Zoia O, Han W, Lane KB, Li B, Donnelly EF, Holburn GE, Lewis KG, Collins RD, Hull WM, Glasser SW, Whitsett JA, Blackwell TS. Increased and prolonged pulmonary fibrosis in surfactant protein C-deficient mice following intratracheal bleomycin. Am J Pathol. 2005;167:1267–1277. doi: 10.1016/S0002-9440(10)61214-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine AM, Whitsett JA, Gwozdz JA, Richardson TR, Fisher JH, Burhans MS, Korfhagen TR. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J Immunol. 2000;165:3934–3940. doi: 10.4049/jimmunol.165.7.3934. [DOI] [PubMed] [Google Scholar]
- Liu CF, Chen YL, Shieh CC, Yu CK, Reid KBM, Wang JY. Therapeutic effect of surfactant protein D in allergic inflammation of mite-sensitized mice. Clin Exp Allergy. 2005;35:515–521. doi: 10.1111/j.1365-2222.2005.02205.x. [DOI] [PubMed] [Google Scholar]
- Mackay RM, Deadman M, Moxon ER, Clark H. A recombinant fragment of human SP-D promotes phagocytosis of Haemophilus influenzae strains whose in vivo pathogenicity is inversely related to SP-D binding. Early Hum Dev. 2006;82:617–1617. [Google Scholar]
- Madan T, Kishore U, Singh M, Strong P, Clark H, Hussain EM, Reid KBM, Sarma PU. Surfactant proteins A and D protect mice against pulmonary hypersensitivity induced by Aspergillus fumigatus antigens and allergens. J Clin Invest. 2001a;107:467–475. doi: 10.1172/JCI10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan T, Kishore U, Singh M, Strong P, Hussain EM, Reid KB, Sarma PU. Protective role of lung surfactant protein D in a murine model of invasive pulmonary aspergillosis. Infect Immun. 2001b;69:2728–2731. doi: 10.1128/IAI.69.4.2728-2731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoud H, Moxon ER, Martin A, Krajcarski D, Richards JC. Structure of the variable and conserved lipopolysaccharide oligosaccharide epitopes expressed by Haemophilus influenzae serotype b strain Eagan. Biochemistry. 1997;36:2091–2103. doi: 10.1021/bi961989y. [DOI] [PubMed] [Google Scholar]
- Mulugeta S, Beers MF. Surfactant protein C: its unique properties and emerging immunomodulatory role in the lung. Microbes Infect. 2006;8:2317–2323. doi: 10.1016/j.micinf.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Nell MJ, Tjabringa GS, Wafelman AR, Verrijk R, Hiemstra PS, Drijfhout JW, Grote JJ. Development of novel LL-37 derived antimicrobial peptides with LIPS and LTA neutralizing and antimicrobial activities for therapeutic application. Peptides. 2006;27:649–660. doi: 10.1016/j.peptides.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Noah TL, Murphy PC, Alink JJ, Leigh MW, Hull WM, Stahlman MT, Whitsett JA. Bronchoalveolar lavage fluid surfactant protein-A and surfactant protein-D are inversely related to inflammation in early cystic fibrosis. Am J Respir Crit Care Med. 2003;168:685–691. doi: 10.1164/rccm.200301-005OC. [DOI] [PubMed] [Google Scholar]
- Ochs M, Knudsen L, Allen L, Stumbaugh A, Levitt S, Nyengaard JR, Hawgood S. GM-CSF mediates alveolar epithelial type II cell changes, but not emphysema-like pathology, in SP-D-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1333–L1341. doi: 10.1152/ajplung.00137.2004. [DOI] [PubMed] [Google Scholar]
- Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Palaniyar N, Ikegami M, Korfhagen T, Whitsett J, McCormack FX. Domains of surfactant protein A that affect protein oligomerization, lipid structure and surface tension. Comp Biochem Physiol A. 2001;129:109–127. doi: 10.1016/s1095-6433(01)00309-9. [DOI] [PubMed] [Google Scholar]
- Perez-Gil J. Lipid–protein interactions of hydrophobic proteins SP-B and SP-C in lung surfactant assembly and dynamics. Pediatr Pathol Mol Med. 2001;20:445–469. doi: 10.1080/pdp.20.6.445.469. [DOI] [PubMed] [Google Scholar]
- Perez-Gil J. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid–protein interactions. Biochim Biophys Acta Biomembr. 2008;1778:1676–1695. doi: 10.1016/j.bbamem.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Perez-Gil J, Keough KM. Interfacial properties of surfactant proteins. Biochim Biophys Acta. 1998;1408:203–217. doi: 10.1016/s0925-4439(98)00068-4. [DOI] [PubMed] [Google Scholar]
- Plasencia I, Baumgart F, Andreu D, Marsh D, Perez-Gil J. Effect of acylation on the interaction of the N-Terminal segment of pulmonary surfactant protein SP-C with phospholipid membranes. Biochim Biophys Acta Biomembr. 2008;1778:1274–1282. doi: 10.1016/j.bbamem.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Plasencia I, Cruz A, Casals C, Perez-Gil J. Superficial disposition of the N-terminal region of the surfactant protein SP-C and the absence of specific SP-B-SP-C interactions in phospholipid bilayers. Biochem J. 2001a;359:651–659. doi: 10.1042/0264-6021:3590651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasencia I, Keough KM, Perez-Gil J. Interaction of the N-terminal segment of pulmonary surfactant protein SP-C with interfacial phospholipid films. Biochim Biophys Acta. 2005;1713:118–128. doi: 10.1016/j.bbamem.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Plasencia I, Rivas L, Casals C, Keough KM, Perez-Gil J. Intrinsic structural differences in the N-terminal segment of pulmonary surfactant protein SP-C from different species. Comp Biochem Physiol A. 2001b;129:129–139. doi: 10.1016/s1095-6433(01)00310-5. [DOI] [PubMed] [Google Scholar]
- Plasencia I, Rivas L, Keough KM, Marsh D, Perez-Gil J. The N-terminal segment of pulmonary surfactant lipopeptide SP-C has intrinsic propensity to interact with and perturb phospholipid bilayers. Biochem J. 2004;377:183–193. doi: 10.1042/BJ20030815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possmayer F, Nag K, Rodriguez K, Qanbar R, Schurch S. Surface activity in vitro: role of surfactant proteins. Comp Biochem Physiol A. 2001;129:209–220. doi: 10.1016/s1095-6433(01)00317-8. [DOI] [PubMed] [Google Scholar]
- Postle AD, Mander A, Reid KB, Wang JY, Wright SM, Moustaki M, Warner JO. Deficient hydrophilic lung surfactant proteins A and D with normal surfactant phospholipid molecular species in cystic fibrosis. Am J Respir Cell Mol Biol. 1999;20:90–98. doi: 10.1165/ajrcmb.20.1.3253. [DOI] [PubMed] [Google Scholar]
- Potter S, Orgeig S, Donnellan S, Daniels CB. Purifying selection drives the evolution of surfactant protein C (SP-C) independently of body temperature regulation in mammals. Comp Biochem Physiol D. 2007;2:165–176. doi: 10.1016/j.cbd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Qanbar R, Cheng S, Possmayer F, Schurch S. Role of the palmitoylation of surfactant-associated protein C in surfactant film formation and stability. Am J Physiol. 1996;271:L572–L580. doi: 10.1152/ajplung.1996.271.4.L572. [DOI] [PubMed] [Google Scholar]
- Reemers SS, Veldhuizen EJA, Fleming C, van Haarlem DA, Haagsman H, Vervelde L. Transcriptional expression levels of chicken collectins are affected by avian influenza A virus inoculation. Vet Microbiol. 2009 doi: 10.1016/j.vetmic.2009.09.026. [DOI] [PubMed] [Google Scholar]
- Sahly H, Ofek I, Podschun R, Brade H, He YC, Ullmann U, Crouch E. Surfactant protein D binds selectively to Klebsiella pneumoniae lipopolysaccharides containing mannose-rich O-antigens. J Immunol. 2002;169:3267–3274. doi: 10.4049/jimmunol.169.6.3267. [DOI] [PubMed] [Google Scholar]
- Sanchez-Barbero F, Rivas G, Steinhilber W, Casals C. Structural and functional differences among human surfactant proteins SP-A1, SP-A2 and co-expressed SP-A1/SP-A2: role of supratrimeric oligomerization. Biochem J. 2007;406:479–489. doi: 10.1042/BJ20070275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Barbero F, Strassner J, Garcia-Canero R, Steinhilber W, Casals C. Role of the degree of oligomerization in the structure and function of human surfactant protein A. J Biol Chem. 2005;280:7659–7670. doi: 10.1074/jbc.M410266200. [DOI] [PubMed] [Google Scholar]
- Schürch S, Green FHY, Bachofen H. Formation and structure of surface films: captive bubble surfactometry. Biochim Biophys Acta. 1998;1408:180–202. doi: 10.1016/s0925-4439(98)00067-2. [DOI] [PubMed] [Google Scholar]
- Singh M, Madan T, Waters P, Parida SK, Sarma PU, Kishore U. Protective effects of a recombinant fragment of human surfactant protein D in a murine model of pulmonary hypersensitivity induced by dust mite allergens. Immunol Lett. 2003;86:299–307. doi: 10.1016/s0165-2478(03)00033-6. [DOI] [PubMed] [Google Scholar]
- Strong P, Reid KBM, Clark H. Intranasal delivery of a truncated recombinant human SP-D is effective at down-regulating allergic hypersensitivity in mice sensitized to allergens of Aspergillus fumigatus. Clin Exp Immunol. 2002;130:19–24. doi: 10.1046/j.1365-2249.2002.01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LC, Daniels CB, Phillips ID, Orgeig S, Whitsett JA. Conservation of surfactant protein A: evidence for a single origin for vertebrate pulmonary surfactant. J Mol Evol. 1998;46:131–138. doi: 10.1007/pl00006287. [DOI] [PubMed] [Google Scholar]
- Takeda K, Miyahara N, Rha YH, Taube C, Yang ES, Joetham A, Kodama T, Balhorn AM, Dakhama A, Duez C, Evans AJ, Voelker DR, Gelfand EW. Surfactant protein D regulates airway function and allergic inflammation through modulation of macrophage function. Am J Respir Crit Care Med. 2003;168:783–789. doi: 10.1164/rccm.200304-548OC. [DOI] [PubMed] [Google Scholar]
- ten Brinke A, van Golde LM, Batenburg JJ. Palmitoylation and processing of the lipopeptide surfactant protein C. Biochim Biophys Acta. 2002;1583:253–265. doi: 10.1016/s1388-1981(02)00248-2. [DOI] [PubMed] [Google Scholar]
- Tenner AJ. Membrane receptors for soluble defense collagens. Curr Opin Immunol. 1999;11:34–41. doi: 10.1016/s0952-7915(99)80007-7. [DOI] [PubMed] [Google Scholar]
- van Dijk A, Veldhuizen EJA, Haagsman HP. Avian defensins. Vet Immunol Immunopathol. 2008;124:1–18. doi: 10.1016/j.vetimm.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass G, Scanlon ST, Beers MF, Haczku A. Surfactant protein (SP)-D suppresses antigenic and mitogenic T cell activation in vitro. J Allergy Clin Immunol. 2004;113(2) Supplement 1:S252. [Google Scholar]
- Wallis R, Shaw JM, Uitdehaag J, Chen CB, Torgersen D, Drickamer K. Localization of the serine protease-binding sites in the collagen-like domain of mannose-binding protein – indirect effects of naturally occurring mutations on protease binding and activation. J Biol Chem. 2004;279:14065–14073. doi: 10.1074/jbc.M400171200. [DOI] [PubMed] [Google Scholar]
- Wang L, Brauner JW, Mao GR, Crouch E, Seaton B, Head J, Smith K, Flach CR, Mendelsohn R. Interaction of recombinant surfactant protein D with lipopolysaccharide: conformation and orientation of bound protein by IRRAS and simulations. Biochemistry. 2008;47:8103–8113. doi: 10.1021/bi800626h. [DOI] [PubMed] [Google Scholar]
- Weber G, Heilborn JD, Jimenez CIC, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124:1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- Wert SE, Yoshida M, LeVine AM, Ikegami M, Jones T, Ross GF, Fisher JH, Korfhagen TR, Whitsett JA. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci USA. 2000;97:5972–5977. doi: 10.1073/pnas.100448997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- Wu H, Kuzmenko A, Sijue W, Schaffer L, Weiss A, Fisher JH, Kwang Sik K, McCormack FX. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest. 2003;111:1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada C, Sano H, Shimizu T, Mitsuzawa H, Nishitani C, Himi T, Kuroki Y. Surfactant protein a directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response – importance of supratrimeric oligomerization. J Biol Chem. 2006;281:21771–21780. doi: 10.1074/jbc.M513041200. [DOI] [PubMed] [Google Scholar]
- Yeaman MR, Yount NY. Unifying themes in host defence effector polypeptides. Nat Rev Microbiol. 2007;5:727–740. doi: 10.1038/nrmicro1744. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Whitsett JA. Alveolar macrophages and emphysema in surfactant protein-D-deficient mice. Respirology. 2006;11:S37–S40. doi: 10.1111/j.1440-1843.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zuo YY, Veldhuizen RAW, Neumann AW, Petersen NO, Possmayer F. Current perspectives in pulmonary surfactant – inhibition, enhancement and evaluation. Biochimica et Biophysica Acta (BBA) – Biomembranes. 2008;1778:1947–1977. doi: 10.1016/j.bbamem.2008.03.021. [DOI] [PubMed] [Google Scholar]