Abstract
Current approaches to reprogram human somatic cells to pluripotent iPS cells utilize viral transduction of different combinations of transcription factors. These protocols are highly inefficient because only a small fraction of cells carry the appropriate number and stoichiometry of proviral insertions to initiate the reprogramming process. Here we have generated genetically homogeneous “secondary” somatic cells, which carry the reprogramming factors as defined doxycycline (DOX)-inducible transgenes. These cells were obtained by infecting fibroblasts with DOX-inducible lentiviruses, isolating “primary” iPS cells in the presence of the drug, and finally differentiating to “secondary” fibroblasts. When “secondary” fibroblast lines were cultured in the presence of DOX without further viral infection, up to 2% of the cells were reprogrammed to pluripotent “secondary” human iPS cells. This system will facilitate the characterization of the reprogramming process and provides a unique platform for genetic or chemical screens to enhance reprogramming or replace individual factors.
Keywords: pluripotency, reprogramming, iPS, OCT4, SOX2, KLF4, C-MYC, three factor reprogramming, four factor reprogramming
INTRODUCTION
Reprogramming of somatic cells to a pluripotent state has been achieved in mouse and human cells by viral transduction of the four transcription factors Oct4, Sox2, C-myc and Klf4 (Lowry et al., 2008; Maherali et al., 2007; Okita et al., 2007; Park et al., 2008; Takahashi et al., 2007; Takahashi and Yamanaka, 2006; Wernig et al., 2007). More recently it has been shown that mouse and human somatic cells can also be reprogrammed in the absence of C-myc with longer latency and substantially decreased efficiency (Nakagawa et al., 2008; Wernig et al., 2008b). The random integration of retroviral copies leads to genetic heterogeneity in the infected somatic cells with only 0.001 to 0.1% of the infected cells eventually becoming reprogrammed to a pluripotent state. These induced pluripotent (iPS) cells may carry 15 or more proviral inserts (Wernig et al., 2007), suggesting that high levels or a certain stoichiometry of factor expression is required to initiate reprogramming. This need for high titer retroviral vectors and the stochastic nature of the reprogramming process complicates mechanistic studies as well as efforts to screen for small molecules that could replace the virally transduced transcription factors.
In the mouse system we recently developed a DOX-inducible “secondary” reprogramming system to efficiently generate iPS cells without additional viral infections. This approach helped to gain insight into the dynamics of reprogramming and was critical for the generation of iPS cells from cell types that are known to be difficult to culture and/or refractory to direct infection (Hanna et al., 2008; Wernig et al., 2008a).
In order to adapt this approach to the human system, we derived human iPS cells from fibroblasts infected with DOX-inducible lentiviral vectors, which transduced either the three or four reprogramming factors. These iPS cells were differentiated in vitro in the absence of DOX into “secondary” fibroblast-like cells, which are genetically homogenous and carry the number of proviral insertions which are favorable to drug-induced activation. When cultured in the presence of DOX without subsequent virus infection, these secondary fibroblast-like cells reprogrammed with high efficiency into pluripotent secondary iPS cells.
RESULTS
Reprogramming of human fibroblasts by DOX-inducible lentivirus vectors
We and others have previously shown that DOX-inducible lentiviral transduction of the transcription factors Oct4, Sox2, C-myc and Klf4 can efficiently reprogram mouse fibroblasts to iPS cells (Brambrink et al., 2008; Stadtfeld et al., 2008). In the following experiments we used DOX-inducible lentiviral vectors carrying the mouse (Brambrink et al., 2008) or the human cDNAs encoding the four transcription factors OCT4, SOX2, C-MYC and KLF4. Human fibroblasts were transduced with either four (OCT4, SOX2, C-MYC, KLF4, referred to as “4 factor” cells) or three (OCT4, SOX2, KLF4, referred to as “3 factor” cells) reprogramming factors. These cells were simultaneously infected with a constitutively active lentivirus transducing the reverse tetracycline transactivator (FUW-M2rtTA). The infected cells were cultured in the presence of DOX and iPS cells with a human ES cell-like morphology were detected after approximately 4 weeks in 4 factor and after 8 weeks in 3 factor fibroblast cultures.
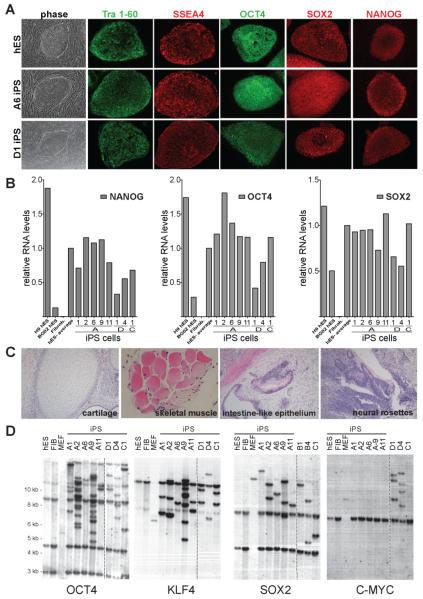
Colonies were expanded into stable iPS cell lines and maintained in the absence of DOX for more than 30 passages. All iPS lines showed a morphology characteristic of human ES (hES) cells and uniformly expressed the pluripotency markers Tra1-60, SSEA4, OCT4, SOX2 and NANOG (Fig. 1A). Furthermore, quantitative RT-PCR showed that the iPS cells had reactivated the endogenous NANOG, OCT4, and SOX2 genes to levels comparable to those found in hES cells (Fig. 1B). IPS cells derived with DOX-inducible viruses showed a normal karyotype (Fig. S1 and data not shown).
Figure 1. Characterization of DOX-inducible iPS cells derived from human fibroblasts.
A. Phase contrast picture and immunofluorescence staining of human ES (hES) cells and iPS cell lines A6 and D1 for pluripotency markers SSEA4, Tra 1-60, OCT4, SOX2 and NANOG.
B. Quantitative RT-PCR for the reactivation of the endogenous pluripotency related genes NANOG, OCT4 and SOX2 in independent iPS cell lines, hES cells and primary fibroblasts. Relative expression levels were normalized to the average expression of the two control hES cell lines.
C. Hematoxilin and eosin staining of a teratoma sections generated from A1 iPS cells.
D. Southern blot analysis of parental GM01660 fibroblasts, hES cells and iPS cells for proviral integrations of xbaI digested genomic DNA using 32P-labeld DNA probes against OCT4, KLF4, SOX2 and C-MYC.
To verify that the derived iPS cells had acquired pluripotency, 10 independent iPS cell lines were subcutaneously injected into SCID mice and shown to form tumors after 6 to 8 weeks (Lensch et al., 2007). Histological analysis revealed teratomas comprised of tissues of all three germ layers including cartilage, skeletal muscle (mesoderm), neural rosettes (ectoderm) and intestinal epithelium (endoderm) (Fig. 1C). In addition, DNA fingerprinting analysis genetically matched iPS cell lines to the parental fibroblast cell lines (data not shown). Southern blot analysis probing for proviral integrations showed a unique pattern for each iPS cell line demonstrating that each of these lines were derived from independently infected fibroblasts (Fig.1D). Furthermore, this analysis revealed that 2 out of the 6 independent 3 factor iPS cell lines (A6 and A11) carried only a few lentiviral integrations (Fig. 1D). This low number of proviral integrations is in contrast to murine iPS cell lines, which typically carry between 10 and 20 proviral integrations (Wernig et al., 2007). Supplemental Table 1 summarizes the characteristics of iPS cell lines derived from different donor cells using different vector combinations. Using human cDNAs, we isolated six 3 factor iPS cell lines from GM01660 fibroblasts (A1, A2 A6, A9, A11 and C1) and two 4 factor iPS cell lines from MRC5 fibroblasts (D1 and D4). To assess drug-inducible factor expression from the integrated lentiviruses, we compared vector-specific transcript levels in primary infected fibroblasts and in iPS cells cultured in the presence or absence of DOX. Two out of the three iPS cell lines tested induced transgene expression to levels comparable to or higher than those found in primary infected fibroblasts (Fig. S2). However, one out of three lines did not show strong transgene induction, which could suggest partial viral silencing in some iPS cells. Transgene expression was strictly dependent on the presence of the drug, as only few or no viral transcripts were detected in the absence of DOX.
DOX inducible secondary fibroblasts
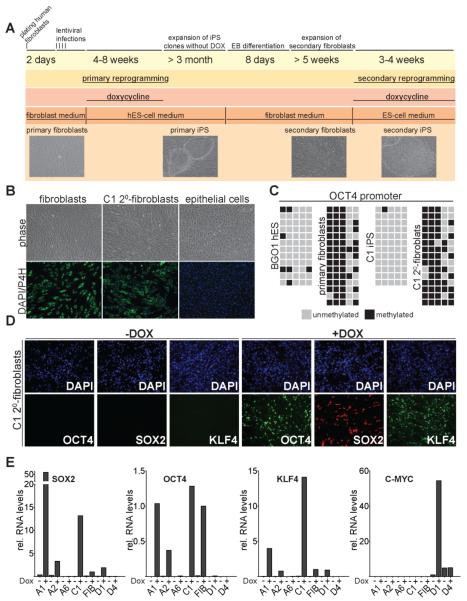
Previously, we generated genetically homogenous secondary mouse fibroblasts that were efficiently converted to a pluripotent state upon the addition of DOX but did not require infection with viral vectors to induce the reprogramming process (Hanna et al., 2008; Wernig et al., 2008a). In order to establish a similar system in human cells we differentiated DOX-independent primary iPS cells into fibroblast like cells (see scheme in Fig. 2A). Following established protocols for differentiating human ES cells (Xu et al., 2004), iPS cells were differentiated in the absence of DOX and in the presence of high serum concentrations into embryoid bodies and subsequently cultured under fibroblast growth conditions. To assure elimination of undifferentiated iPS cells, which cannot be propagated as single cells on conventional cultures dishes (Takahashi et al., 2007), fibroblast-like cells were passaged at least four times using trypsin. The secondary cells acquired a homogenous fibroblast-like morphology (Fig. 2B) and were positive for prolyl-4-hydroxylase beta expression (P4H), an enzyme required for collagen synthesis (Fig. 2B), confirming differentiation into fibroblast-like cells. These cells will subsequently be referred to as secondary fibroblasts. We failed to detect cells positive for NANOG or OCT4 by immunostaining in any of the secondary fibroblast lines consistent with complete differentiation of the primary iPS cells (data not shown). In addition to these in vitro derived fibroblasts, we established fibroblast-like cultures from human iPS-derived teratomas explanted into culture.
Figure 2. Characterization of genetically identical secondary fibroblasts derived from iPS cells.
A. Timeline depicting the generation and reprogramming of secondary iPS cell-derived fibroblasts.
B. Phase contrast picture and immunostaining of C1 secondary fibroblasts, primary fibroblasts and epithelial cells (EpRas) for the fibroblast marker prolyl-4-hydroxylase beta (P4H).
C. Methylation analysis of the OCT4 promoter region. Light gray squares indicate unmethylated, and black squares indicate methylated CpGs in the OCT4 promoter of iPS cells, hES cells, primary, and secondary fibroblasts.
D. Immunostaining for DOX-induced transgene expression of OCT4, KLF4 and SOX2 in C1 secondary fibroblasts 48 hours after drug administration.
E. Quantitative RT-PCR for DOX-dependent transgene expression of OCT4, KLF4, SOX2 and C-MYC in secondary fibroblasts and primary infected fibroblasts. Relative expression levels were normalized to DOX-induced expression in primary infected fibroblasts.
In order to confirm differentiation of the iPS cells to a somatic epigenetic state at the molecular level, we determined the methylation levels of the OCT4 and NANOG promoters in secondary fibroblasts. While human iPS cells showed the expected hypomethylation associated with active OCT4 and NANOG genes, secondary fibroblasts showed increased promoter methylation similar to that found in the parental fibroblast cell line (Fig. 2C and S3). Together these results indicate that the secondary fibroblasts have the molecular and morphological characteristics of somatic cells.
To assess inducible expression of the reprogramming factors, secondary fibroblasts were treated with DOX and stained for expression of the reprogramming factors. Most cells stained positive for OCT4, SOX2 and KLF4 48 hours after DOX addition (Fig. 2D). However, a fraction of cells failed to activate the factors, possibly because of vector silencing. We used quantitative RT-PCR with specific primers for the viral transgenes to quantify vector activation. We detected robust DOX-dependent factor expression in some secondary fibroblast lines including A1 and C1 but less in other lines such as A2 and D1 (Fig. 2E). Variegated vector expression within and variation of vector induction between different secondary fibroblast lines has also been observed in the mouse system (Wernig et al, 2008a) suggesting that the chromosomal position of the proviruses affects drug-inducible expression.
High reprogramming efficiency in secondary human fibroblasts
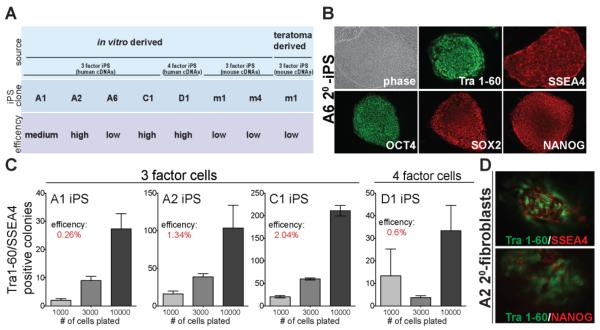
To assess whether addition of DOX to secondary fibroblasts was sufficient to induce reprogramming, we plated the cells in the presence of the drug under hES cell culture conditions. Within 72 hours after DOX-induction, a significant fraction of cells underwent cell death, which was more pronounced in cell lines with high levels of transgene expression, particularly in cell line A1. After approximately 8 days of drug induction, granulated colonies, which did not show hES cell-like morphology, appeared in the 4 factor secondary fibroblasts but were not seen in the 3 factor fibroblasts. This closely resembled changes that we and others observed after primary infection (Nakagawa et al., 2008; Takahashi et al., 2007). After 20 to 25 days of DOX treatment, colonies with typical hES cell-like morphology emerged which could be expanded into secondary iPS cell lines. No hES cell-like colonies appeared in any of the secondary fibroblast lines in the absence of DOX, indicating that derivation of secondary iPS cells was strictly dependent on DOX-induced transgene expression. Secondary iPS cell lines were obtained from eight different secondary fibroblast lines including one teratoma-derived line (Fig 3A). All of these lines could be maintained in the absence of DOX and expressed the pluripotency markers Tra 1-60, SSEA4, OCT4, SOX2 and NANOG (Fig. 3B).
Figure 3. DOX-induced reprogramming of secondary fibroblast lines.
A. Summary of reprogramming efficiency of in vitro differentiated and teratoma-derived secondary fibroblasts. Reprogramming efficiencies are categorized into “low”, indicating efficiency of< 0.1%, “medium”, 0.1–0.5%, and “high”, >0.5%.
B. Phase contrast picture and immunofluorescence staining of secondary iPS cell line A6 for pluripotency markers SSEA4, Tra 1-60, OCT4, SOX2 and NANOG.
C. Reprogramming efficiency of secondary fibroblasts A1, A2, C1 and D1 at 28 days (A1, A2) or 20 days (C1, D1) after DOX-induction as determined by immunostaining for Tra 1-60 and SSEA4. Efficiencies were calculated as the fraction of Tra 1-60/SSEA4 positive colonies to cells seeded. Error bars indicate the SEM generated from triplicates of the same experiment.
D. Immunostaining of C1 secondary iPS colonies for Tra 1-60/SSEA4 and Tra 1-60/NANOG 20 days after DOX-induction.
In order to compare reprogramming efficiencies of the different cell lines, we plated the 3 factor secondary fibroblast cell lines A1, A2 and C1, and the 4 factor line D1 at three different densities onto feeder cells. Reprogramming efficiencies were determined after 20 or 28 days of DOX treatment by counting SSEA4/Tra 1-60 double positive colonies (Fig. 3C). To ascertain whether Tra-1-60 and SSEA4 double positive cells were valid markers for iPS cells, we performed additional co-immunostaining for Tra 1-60 and NANOG on a subset of the cells (Fig. 3D). More than 80% of the Tra 1-60 positive colonies also stained positive for NANOG demonstrating that Tra 1-60/SSEA4 double positive staining can be used as a valid marker for reprogrammed iPS cells. Using this criterion we found that independent secondary fibroblast lines had reprogramming efficiencies between 0.26% and 2.0%, which is in the same range as reprogramming efficiencies of secondary mouse fibroblasts (Wernig et al., 2008a). Reprogramming efficiencies of different secondary cell lines appeared to be positively correlated with high transgene expression after DOX induction. However, A1 secondary fibroblasts, which displayed high transgene expression particularly for SOX2 (Fig. 2E), showed only a moderate reprogramming efficiency. This may have been due to the increased cell death observed in this cell line after addition of DOX.
Transgene expression is required for at least 8 days to induce reprogramming
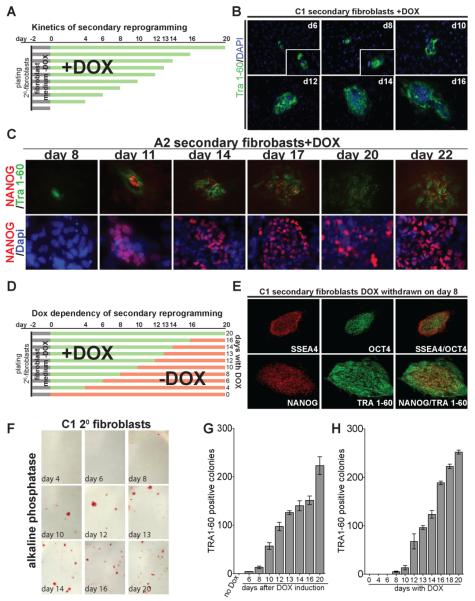
To quantitatively determine the kinetics of reprogramming, we stained secondary fibroblasts at different times after addition of DOX for the expression of different pluripotency markers (experimental overview in Fig. 4A). The first cells staining positive for Tra 1-60 appeared after 6 days in C1 and 8 days in A2 secondary fibroblasts (Fig. 4B,C), whereas NANOG positive cells were first detected at 11 days after drug exposure (Fig. 4C). The number of Tra 1-60 positive colonies increased with prolonged exposure to DOX (Fig. 4G).
Figure 4. Kinetics and requirement for transgene expression of secondary fibroblast reprogramming.
A. Experimental outline of reprogramming kinetics experiment. Secondary fibroblasts were plated in DOX-free fibroblast medium on day -2 (black bars). After two days (day 0) medium was changed to hES medium containing DOX (green bars). Cells were fixed at the indicated time points and stained for pluripotency markers.
B. Immunostaining of C1 secondary fibroblasts for Tra 1-60 at indicated days after DOX induction. Inset shows magnification of the same picture.
C. Immunostaining of A2 secondary fibroblasts for Tra 1-60 and NANOG at the indicated days after DOX induction (upper panel). Lower panel shows a magnification of NANOG and DAPI staining of the same area.
D. Experimental outline of the DOX withdrawal experiment. Secondary fibroblasts were plated in fibroblast medium without DOX on day -2 (black bars). After two days (day 0) medium was changed to human ES cell medium containing DOX (green bars). Medium was changed to hES medium without DOX at the indicated time points (red bars). Secondary iPS colonies were picked on day 20 for all DOX conditions.
E. Immunostaining of secondary C1 iPS cells for SSEA4, Tra 1-60, OCT4 and NANOG, which were derived by DOX-induction for 8 days.
F. Alkaline phosphatase staining of C1 secondary fibroblasts at day 20 after exposure to DOX. The day indicates the time when DOX was withdrawn from the culture medium.
G. Number of reprogrammed colonies at different times after addition of DOX. The number of colonies on the indicated day was determined by immunostaining for Tra 1-60. Error bars indicate the SEM generated from triplicates of the same experiment.
H. Number of reprogrammed colonies at day 20 after DOX addition. DOX was withdrawn from the culture medium at the indicated time points. The number of colonies was determined by immunostaining for Tra 1-60. Error bars indicate the SEM generated from triplicates of the same experiment.
To determine how long after DOX induction cells acquire a self-sustaining state independent of transgene expression, we cultured two independent secondary fibroblast lines (A2 and C1) in the presence of DOX and withdrew the drug at different times after induction (Fig. 4D). Reprogramming was assessed 20 days after initial DOX addition. Colonies with hES cell-like morphology appeared in C1 secondary fibroblasts exposed to DOX for 8 days, while A2 secondary fibroblasts required at least 14 days of DOX. These time points are approximately 2 days after the appearance of Tra 1-60 and NANOG positive cells in each of these lines. No colonies were detected on plates that were not exposed to DOX or were treated for less than 8 days. Individual colonies from line C1 treated with DOX for 8 days and from line A2 treated with DOX for 14 days were picked and expanded (n=10) and shown to give rise to secondary iPS lines. All secondary iPS lines expressed the pluripotency specific markers SSEA4, Tra 1-60, OCT4, SOX2 and NANOG (Fig. 4E and S4). The number of reprogrammed cells increased with prolonged exposure to DOX as shown by alkaline phosphatase (AP) staining (Fig. 4F) and quantification of Tra 1-60 positive colonies (Fig. 4H). Interestingly, the number of reprogrammed colonies found after 20 days was similar to the number of Tra 1-60 positive colonies detected on the day when DOX was withdrawn (compare Fig. 4G and 4H). This correlation suggests that as early as 8 days after DOX addition Tra 1-60 positive cells are primed to become pluripotent.
In summary, our results demonstrate that secondary fibroblasts can generate iPS cells when cultured in the presence of DOX with up to 2% efficiency. This is more than two orders of magnitude higher than achieved by conventional viral transduction.
DISCUSSION
Current protocols for reprogramming human somatic cells are based on viral vector-mediated transduction of the reprogramming factors and are highly inefficient. This is especially true for 3 factor reprogramming events, without the oncogene C-MYC, which were previously reported to occur with a frequency of <0.001% (Nakagawa et al., 2008). In this paper we developed a secondary reprogramming system in human somatic cells, based on an analogous approach in the mouse system, to overcome some of the limitations of current reprogramming protocols. Human fibroblasts infected with DOX-inducible vectors transducing either the 3 or 4 reprogramming factors gave rise to iPS cells with approximately the same low efficiency as reported previously. These primary iPS cells were differentiated in the absence of DOX into secondary fibroblasts. When cultured in the presence of DOX, these genetically distinct secondary fibroblast lines, carrying different combinations of proviruses, gave rise to secondary iPS cells with efficiencies ranging from 0.26 to 2%; an improvement over the reported efficiencies found by primary infections by more than two orders of magnitude. The fact that we observed a slightly faster time course of reprogramming in the secondary compared to the primary system is likely the result of the very low efficiency and stochastic nature of primary reprogramming with 3 factors. The time course of reprogramming that we observed in the secondary system is in agreement with previous reports of primary reprogramming (Nakagawa et al., 2008; Park et al., 2008; Takahashi et al., 2007; Yu et al., 2007).
Reprogrammed secondary iPS cells expressed pluripotency markers such as AP, Tra 1-60, OCT4 and NANOG. The finding that in secondary fibroblasts, OCT4 and NANOG promoters are hypermethylated to a similar extent as primary fibroblasts, suggests that the reactivation of OCT4 and NANOG during secondary reprogramming depends on the reestablishment of the auto-regulatory loop involving activation of the four endogenous pluripotency factors OCT4, NANOG, SOX2 and TCF3 (Jaenisch and Young, 2008). This argues that the same molecular process drives primary and secondary reprogramming.
In contrast to mouse cells, nuclear transfer-mediated reprogramming of human cells has not yet been achieved. Similarly, conventional reprogramming of human cells is vastly less efficient than that of mouse cells. The reasons for these differences are not clear and could be due to inherent differences between human and mouse cells and/or to technical issues such as differential susceptibility of the cells to culture conditions. However, the reprogramming efficiency of human secondary fibroblasts was in the same range as that of mouse secondary fibroblasts arguing against inherent differences of mouse and human cells to epigenetic reprogramming.
The first reprogrammed cells from secondary fibroblasts, although very infrequent, appeared already at 8 to 11 days after DOX exposure as indicated by AP and Tra 1-60 expression. However, we noted that during reprogramming of human secondary fibroblasts, AP and Tra 1-60 were activated only slightly earlier than NANOG, which is different from the sequential appearance of these markers in mouse cells (Brambrink et al., 2008; Stadtfeld et al., 2008; Wernig et al., 2008a). The human secondary system, with its greatly increased efficiency, will allow detailed study of the role and importance of NANOG and other pluripotency markers during the reprogramming process of human cells. Nevertheless, it seems prudent to also investigate the kinetics of NANOG activation in human cells after primary infection.
The drug-inducible system described here represents a novel, predictable, and highly reproducible platform to study the kinetics of iPS cell generation. This should facilitate the study of early molecular events leading to epigenetic reprogramming of human somatic cells. In addition, the genetic homogeneity of secondary cells makes chemical and genetic screening approaches to enhance reprogramming efficiency feasible. Since the reprogrammed state is not dependent on continued expression of the exogenous factors, the transgenes can be genetically engineered and excised allowing the generation of secondary cells that lack a particular reprogramming factor (Hanna et al., 2007). This will enable screens for compounds that can replace the original reprogramming factors.
MATERIALS and METHODS
Lentiviral infection and iPS cell derivation
VSVG coated lentiviruses were generated in 293 cells as described previously (Brambrink et al., 2008). Briefly, culture medium was changed 12 hours post-transfection and virus-containing supernatant was collected 60–72 hours post transfection. Viral supernatant was filtered through a 0.45μm filter. Virus-containing supernatants were pooled for 3 and 4 factor infections and supplemented with FUW-M2rtTA virus and an equal volume of fresh culture medium. 1×106 human fibroblasts were seeded 24 hours before transduction in T75 flaks. Four consecutive infections were performed over a period of 48 hours in the presence of 2μg/ml of polybrene. Culture medium was changed 12 hours after the last infection. Five days after transduction, fibroblasts were passaged using trypsin and re-plated at different densities between 5 × 104 and 5 × 105 cells per 10 cm on gelatin coated dishes or MEF feeder layers. To induce reprogramming, culture medium was replaced 48 hours later by human ES cell medium supplemented with doxycycline (Sigma-Aldrich; 2 μg/ml). Human iPS cells colonies were picked manually based on morphology between 4 and 8 weeks after doxycycline induction and manually maintained and passaged according to human ES cell protocols in the absence of doxycycline.
Derivation of secondary fibroblast-like cells
Human iPS cells were differentiated into fibroblast-like cells as described previously (Xu et al., 2004). IPS cells were differentiated by embryoid body formation in fibroblast medium for 5 days, and subsequently plated onto adherent tissue culture dishes and passaged according to primary fibroblast protocols using trypsin for at least 4 passages before the start of experiments. For the derivation of secondary iPS cells, secondary fibroblasts were plated at densities between 1 × 103 and 1 × 104 per 35 mm on gelatin-coated dishes or MEF feeder layers. 48 hours later, fibroblast medium was replaced by human ES cell medium supplemented with doxycycline (Sigma-Aldrich; 2 μg/ml). For the kinetics experiments (Fig. 4D–F and 4H), cells were fixed at the indicated time points and immunostained after the completion of the experiment (see Supplementary Materials for immunostaining procedures). For DOX-withdrawal experiments (Fig. 4B,C and 4G), medium was replaced with human ES medium without DOX at the indicated times.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Raaji Alagappan and Ping Xu for support with tissue culture and Jessica Daussman, Ruth Flannery and Dongdong Fu for their help with animal husbandry and processing of teratomas. We thank all the members of the Jaenisch lab for helpful discussions and comments on the manuscript. D.H. is a Merck Fellow of the Life Science Research Foundation. R.J. was supported by National Institute of Health grants RO1-HD045022 and R37-CA084198.
Footnotes
Additional Material and Methods can be found in the Supplemental Materials.
REFERENCES
- Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensch MW, Schlaeger TM, Zon LI, Daley GQ. Teratoma formation assays with human embryonic stem cells: a rationale for one type of human-animal chimera. Cell Stem Cell. 2007;1:253–258. doi: 10.1016/j.stem.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc NatlAcadSci U S A. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Wernig M, Lengner CJ, Hanna J, Lodato MA, Steine E, Foreman R, Staerk J, Markoulaki S, Jaenisch R. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat Biotechnol. 2008a doi: 10.1038/nbt1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008b;2:10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- Xu C, Jiang J, Sottile V, McWhir J, Lebkowski J, Carpenter MK. Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cell growth. Stem Cells. 2004;22:972–980. doi: 10.1634/stemcells.22-6-972. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.