Abstract
Objective
Development of new animal lung cancer models that are relevant to human lung carcinogenesis is important for lung cancer research. Previously we have shown the induction of lung tumor in ferrets (Mustela putorius furo) exposed to both tobacco smoke and a tobacco carcinogen (4-(N-Methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone, NNK). In the present study, we investigated whether NNK treatment alone induces both preneoplastic and neoplastic lesions in the lungs of ferrets.
Methods
We exposed ferrets to NNK by i.p. injection of NNK (50 mg/kg BW) once a month for four consecutive months and then followed up for 24, 26 and 32 weeks. The incidences of pulmonary preneoplastic and neoplastic lesions were assessed by histopathological examination. The expressions of α7 nicotinic acetylcholine receptor (α7 nAChR, which has been shown to promote lung carcinogenesis) and its related molecular biomarkers in lungs were examined by immunohistochemistry and/or Western blotting analysis.
Results
Ferrets exposed to NNK alone developed both preneoplastic lesions (squamous metaplasia, dysplasia and atypical adenomatous hyperplasia) and tumors (squamous cell carcinoma, adenocarcinoma and adenosquamous carcinoma), which are commonly seen in humans. The incidence of tumor induced by NNK was time-dependent in the ferrets (16.7%, 40.0% and 66.7% for 24, 26 and 32 weeks, respectively). α7 nAChR is highly expressed in the ferret bronchial/bronchiolar epithelial cells, and alveolar macrophages in ferrets exposed to NNK, and in both squamous cell carcinoma and adenocarcinoma of the ferrets. In addition, we observed the tendency for an increase in phospho-ERK and cyclin D1 protein levels (p = 0.081 and 0.080, respectively) in the lungs of ferrets exposed to NNK.
Conclusion
The development of both preneoplastic and neoplastic lesions in ferret lungs by injecting NNK alone provides a simple and highly relevant non-rodent model for studying biomarkers/molecular targets for the prevention, detection and treatment of lung carcinogenesis in humans.
Keywords: ferret, tobacco carcinogen, NNK, lung cancer, α7 nicotinic acetylcholine receptor
1. Introduction
Lung cancer is the leading cause of cancer death in the United States and worldwide. While several factors increase the risk for developing lung cancer, cigarette smoking is the single most important factor associated with 90% of lung cancer cases [1-3]. As cigarette smoke contains more than 60 carcinogens (particularly nitrosamines), the tobacco-specific nitrosamines 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N’-nitrosonornicotine (NNN) are by far the most prevalent carcinogens in tobacco products [2, 3], and have been shown to induce lung carcinogenesis in vivo [4, 5]. Various animal models for lung cancer induced by cigarette smoke and/or carcinogens in cigarette smoke have been reported [5-7].
While rodent models are easier to use, the lung structure and bronchial branching of mice is different from humans [6, 8-10]. Further, there are limitations with regard to the relevance of tumors that the rodents develop compared with human tumors. For example, mice typically develop hyperplasia of alveolar epithelium in response to carcinogens, whereas squamous differentiation is more common in the human lung [6]; the most common subtypes of lung cancer in humans are squamous cell carcinomas and adenocarcinomas [11]. By contrast, the most common neoplastic subtypes resulting from carcinogen induction in mice are adenomas and, to a lesser extent, adenocarcinomas [8]. Although it is rare to observe a combination of different histological types in a single mouse tumor, such observations occur in 30-60% of human lung tumors [6, 8]. Furthermore, mice treated with carcinogens tend to develop multiple tumors which rarely metastasize; in humans, multiple tumors are uncommon and lung cancer metastasis is frequent [6, 8]. Given the need to develop better detection and treatment options for lung cancer, it is essential to develop animal models that are more representative of lung carcinogenesis in humans.
The ferret (Mustela putorius furo) has been shown to be a useful model for pulmonary research studies since the pulmonary structure and airways of ferrets are similar to humans [12]. The ferret is commonly used for studies of infectious lung disease since it is susceptible to infection from a large number of pathogens [13]. We previously reported that the ferrets exposed to both cigarette smoke and NNK develop adenocarcinomas and squamous cell carcinomas, the most common lung tumors in humans [14]. The morphological characteristics of ferret lung tumors are very similar to those of humans, further suggesting that the ferret may be a relevant model to study lung carcinogenesis [14]. However, the requirement for a smoking exposure device for ferrets has limited the wide use of the ferret model for lung carcinogenesis, and it is unknown whether both preneoplastic and neoplastic lesions could be induced by simple NNK injections in ferrets.
In the present study, we investigated the appropriateness of the ferret as a model for human lung cancer. We describe here the incidence of preneoplastic and neoplastic lesions and histopathological types of lung tumors in ferrets exposed to NNK alone for 24 to 32 weeks.
2. Materials and Methods
2.1. Animals, study group and carcinogen treatment
Male ferrets (1.1 – 1.7 kg, aged between 3 – 5 months old) were obtained from Marshall Farms (North Rose, NY). In order to avoid potential variation due to the dietary components and to make it easier for the future dietary/chemopreventive intervention study, the animals were fed a semi-purified ferret diet (Research Diets, Inc, New Brunswick, NJ: D90001 Diets contains the following components: Casein 34.5%; L-Arginine 0.5%; L-Methionine 0.3%; Maltodextrin 7.5%; Corn starch 26.5%; Cellulose 5.0%; Corn oil 10.5%; Lard 10.5%; Salt mix (S90002) 3.5%; Vitamin mix (V90002) 1.0%; Choline birartrate 0.2%), and had access to food and water ad libitum throughout all experiment periods. After acclimation, 22 ferrets were given intraperitoneal (i.p.) injections of NNK (Toronto Research Chemicals, Ontario, Canada) at a dose of 50 mg/kg BW, once a month for four consecutive months and then maintained for 24 weeks (n=6), 26 weeks (n=10) and 32 weeks (n=6). Control animals (n=8) received a sham injection of normal saline and observed for 26 weeks. At the end of each experimental period, ferrets were killed by terminal exsanguination under deep isoflurane (Isothesia, Butler Schein, Dublin, OH) anesthesia. Tissues and plasma were collected and stored at −80 °C until analyzed. This study protocol was approved by the Animal Care and Use Committee at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University.
2.2. Histopathology
The upper right lobe of each ferret lung was inflated post-mortem and fixed by an intratracheal instillation of 10% formalin, as previously described [15]. The lungs were embedded in paraffin, sectioned and stained with hematoxylin and eosin for histopathological examination. The incidence of preneoplastic lesions (squamous metaplasia and dysplasia and atypical adenomatous hyperplasia) and neoplastic lesions (adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, and lymphoma) were assessed. Histology slides were examined at both 10X and 40X magnification and observations were confirmed by two independent investigators who were blinded to the treatment groups.
2.3. Immunohistochemistry
The localization of α7 nicotinic acetylcholine receptor (nAChR) expression in lung, pulmonary cancerous bodies and macrophages was examined by immunohistochemistry using the avidin-biotin complex immunoperoxidase methods according to previously described method [16, 17]. Briefly, four μm thick sections from formalin-fixed, paraffin-embedded lung tissue blocks were immunostained for α7 nAChR (AChRα7 (319): sc-58607, Santa Cruz Biotechnology, Santa Cruz, CA) with dilutions of 1:50. After then, the sections were incubated with biotinylated second goat anti-rat antibody (Vector Laboratories, Inc., Burlingame, CA) diluted 1:250, and further incubated with VECTASTAIN Elite ABC reagent (Vector Laboratories Inc., Burlingame, CA). The sections were subsequently processed with peroxidase substrate solution (Vector Laboratories Inc., Burlingame, CA). The sections were then counterstained with hematoxylin.
2.4. Western blots analysis
Western blotting was performed with whole cell lysate of lung tissues by using a previously described method [18]. Antibodies against α7 nAChR and cyclin D1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against total-Akt, phospho-Akt, total-ERK1/2 and phospho-ERK1/2 were purchased from Cell Signaling (Beverly, MA). Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from Millipore (Milford, MA) was used as a loading control. Results were quantified using a densitometer and expressed as fold change in the treatment group vs. the control group. The dilutions of each antibody were as follows: α7 nAChR 1:300; cyclin D1 1:1000; total-Akt 1:2000; phospho-Akt (Ser473) 1:500; total-ERK1/2 1:1000; phospho-ERK1/2 1:500; GAPDH 1:20000.
2.5. Statistical analysis
All results are expressed as means ± SEM. Comparisons of data between two groups were made using unpaired Student’s t-test. Results were considered statistically significant at P<0.05.
3. Results
3.1. Mortality of ferrets exposed to NNK
NNK has been widely used as a carcinogen for inducing lung tumorigenesis. NNK is also a toxic chemical and the LD50 has been reported to be 1,000 mg/kg BW by the i.p. injection to mice [19]. In the present study, 50 mg/kg BW of NNK was given i.p. at 4-week intervals for a total of four doses over 4 months so as to minimize the toxicity of NNK. This total dose of NNK (200 mg) used in the ferrets was based on that the mink, receiving a single of dose (150 mg) of tobacco-carcinogen N-nitrosoamine (NNN), did not show any toxic effects [20]. Three ferrets died in the NNK treated group during the experiment period (between 11 – 14 weeks after the first injection of NNK) without any obvious pathology. Although it was unlikely, we cannot exclude the possibility that the death of these ferrets was due to the NNK injection. There were no significantly differences in the body weight between the control (1.57 ± 0.14 kg) and the NNK treated (1.72 ± 0.35 kg) groups at 24 weeks.
3.2. Incidence of lung preneoplastic lesions, neoplastic lesions and lung pathologic types in the lungs of ferrets exposed to NNK
We observed 83.3 to 100% incidences of preneoplastic lesions in ferrets exposed to NNK after 24 to 32 weeks. NNK resulted in various histopathological types of preneoplastic lesions, including squamous metaplasia, squamous dysplasia and atypical adenomatous hyperplasia in ferret lungs (Table 1, Fig. 1). In addition, we observed a 16.7% incidence of neoplastic pulmonary tumors (squamous cell carcinoma, adenosquamous carcinoma and adenocarcinoma) in ferrets exposed to NNK for 24 weeks; the incidence of such tumors increased to 40.0% or 66.7% in ferrets treated with NNK and maintained for 26 or 32 weeks, respectively (Table 2, Fig. 1). Although there were no statistically significant differences in the incidence between the different time points because of the small sample size, these data suggested that NNK could induce lung tumors in a time dependent manner. NNK treatment alone, for different periods of time, predominantly induced adenocarcinomas and, to a smaller extent, resulted in the induction of squamous cell carcinomas and adenosquamous carcinomas. We also observed a lymphoma in a ferret treated with NNK alone for only 32 weeks. Therefore, it appears that NNK treatment by itself can induce various tumor types normally seen with exposure to both NNK and cigarette smoke.
Table 1.
Incidences of preneoplastic lesions with pathological subtypes in the lungs of ferrets with or without NNK exposure for 24 - 32 weeksa).
| Group | NNK (mg/kg BW) |
Time (weeks) |
n | Preneoplastic lesions |
No. of preneoplastic lesion subtypes |
|||
|---|---|---|---|---|---|---|---|---|
| Positive animal | % Incidence | SMb) | SDc) | AAHd) | ||||
| 1 | None | 26 | 8 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| 2 | 200 | 24 | 6 | 6 | 100 | 2 | 3 | 4 |
|
| ||||||||
| 3 | 200 | 26 | 10 | 10 | 100 | 4 | 8 | 6 |
|
| ||||||||
| 4 | 200 | 32 | 6 | 5 | 83.3 | 1 | 1 | 3 |
Including the data of dead animals during the experiment period. Some ferrets developed more than one type of pathologic lesions.
SM: squamous metaplasia.
SD: squamous dysplasia.
AAH: atypical adenomatous hyperplasia.
Fig. 1.
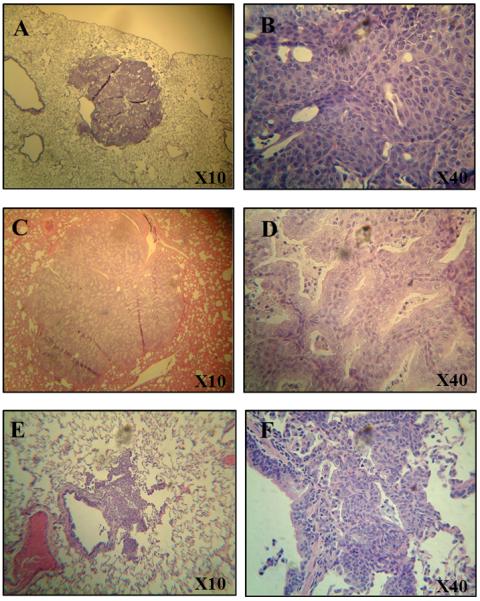
Representative image of squamous cell carcinoma (Panels A and B), adenocarcinoma (Panels C and D) and preneoplastic lesions (Panels E and F) at 10X and 40X magnifications in the lung of ferrets exposed to NNK for 24 - 32 weeks.
Table 2.
Incidences of neoplastic lesions with pathological subtypes in the lungs of ferrets with or without NNK exposure for 24 - 32 weeksa).
| Group | NNK (mg/kg BW) |
Time (weeks) |
n | Neoplastic lesions |
No. of neoplastic lesion subtypes |
||||
|---|---|---|---|---|---|---|---|---|---|
| Positive animal | % Incidence | ADCb) | SQCc) | ADSQCd) | Le) | ||||
| 1 | None | 26 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| |||||||||
| 2 | 200 | 24 | 6 | 1 | 16.7 | 1 | 0 | 0 | 0 |
|
| |||||||||
| 3 | 200 | 26 | 10 | 4 | 40.0 | 3 | 1 | 1 | 0 |
|
| |||||||||
| 4 | 200 | 32 | 6 | 4 | 66.7 | 2 | 1 | 0 | 1 |
Including the data of dead animals during the experiment period. Some ferrets developed more than one type of pathologic lesions.
ADC: Adenocarcinoma.
SQC: Squamous cell carcinoma.
ADSQC: Adenosquamous carcinoma.
L: Lymphoma.
3.3. α7 nAChR protein expressions in ferret lung exposed to NNK by immunohistochemistry and western blotting
Components of cigarette smoke, including nicotine and NNK, can induce cell proliferation, angiogenesis and apoptosis resistance by exerting its cellular functions through nAChRs [21]. Among the nAChRs, α7 nAChR plays an important role in the development and progression of lung cancer [21-23]. Since NNK is known to be a high-affinity ligand for α7 nAChR [24], we examined whether NNK induces protein levels of α7 nAChR, which mediates the tumor growth signaling pathway, in the lungs of ferrets. Since we only had the control group at 26 weeks, we assessed the protein expressions of α7 nAChR in the lung of ferrets with or without NNK exposure for 26 weeks. As the result, NNK exposure significantly increased α7 nAChR protein levels in whole lung homogenates (Fig. 2A). In addition, the α7 nAChR was highly expressed in the ferret bronchial/bronchiolar epithelial cells, exposed to NNK exposure (Fig. 3B), as compared with that of the ferrets without NNK injection (Fig. 3A). In addition, α7 nAChR was greatly expressed in both adenocarcinoma and squamous cell carcinoma of the ferrets (Fig. 3D, 3E) as well as in alveolar macrophages and tumor associated macrophages (Fig. 3C, 3F). Akt protein levels were not significantly changed (Fig. 2B) between the groups. However, we observed the tendency for an increase in phospho-ERK and cyclin D1 protein levels (p = 0.081 and 0.080, respectively; Fig. 2C, 2D), which were mainly involved in activation of cell proliferation. Since we observed two bands in cyclin D1 (Fig. 2D) in ferrets which were almost in the same location of cyclin D1 protein as that of human BEAS-2B and were strongly dose-dependent (correlation factor: R2> 0.85) (data not shown), we concluded that both bands were proteins of cyclin D1.
Fig. 2.
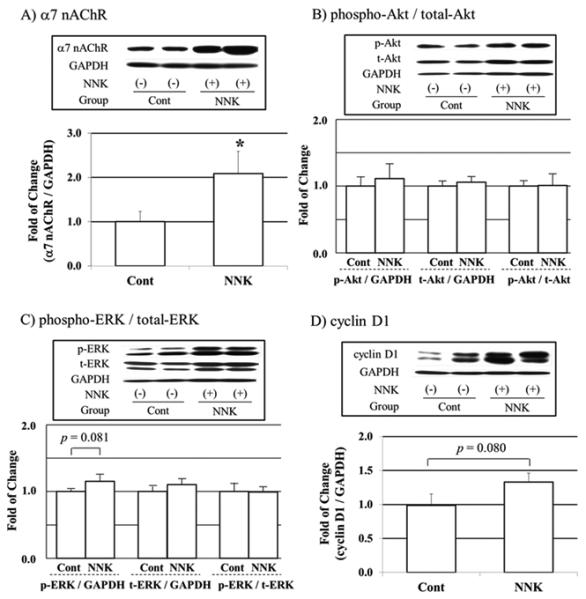
The expression of α7 nAChR (A), phospho-Akt/total-Akt (B), phospho-ERK/total-ERK (C) and cyclin D1 (D) protein levels in the lung of ferrets that were non-exposed (Cont) and exposed to NNK (NNK) for 26 weeks, measured by western blotting from whole lung homogenate. GAPDH was chosen as internal control for protein equal loading. Values are expressed Mean ± SEM. *p < 0.05.
Fig. 3.
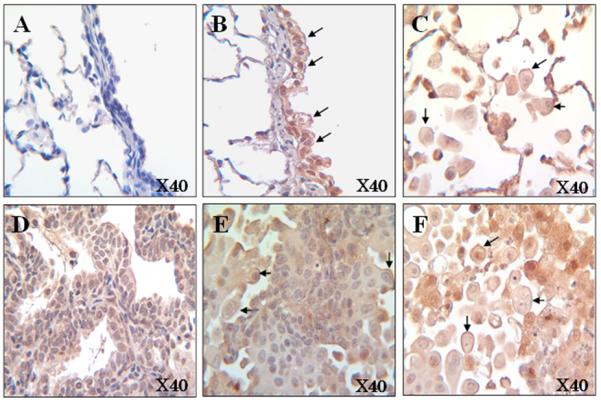
Representative image of α7 nAChR immunohistochemistry in the lung of ferrets that were non-exposed (Panel A) and exposed to NNK for 26 weeks [bronchiolar epithelial cells (Panel B), alveolar macrophage (Panel C), adenocarcinoma (Panel D), squamous cell carcinoma (Panel E), and tumor associated macrophage (Panel F)] at 40X magnifications. The arrows indicated the expression of α7 nAChR (strained in reddish-purple) on the cell membranes.
4. Discussion
In the present study, we demonstrated that the ferret developed both preneoplastic and neoplastic lesions in the lungs by intraperitoneal injecting NNK alone, indicating that the ferret was a highly relevant non-rodent model of lung carcinogenesis to humans. The preneoplastic lesions of ferrets exposed to NNK alone included squamous metaplasia, squamous dysplasia and atypical adenomatous hyperplasia (Table 1). These preneoplastic lesions morphologically closely resembled squamous carcinoma or adenocarcinoma, supporting the notion that ferret’s lung carcinomas derived from squamous metaplasia/dysplasia and atypical adenomatous hyperplasia. As we further evaluated the type of lung tumors that ferrets developed after NNK exposure, we detected adenocarcinomas, squamous cell carcinomas and adenosquamous carcinomas in the lungs of ferrets exposed to NNK (Table 2). As previously discussed, the most common types of human lung cancer are squamous cell carcinomas and adenocarcinomas [11], which are rarely seen in mouse models, emphasizing the relevance of the ferret models to human tumors. Furthermore, NNK treatment induced adenosquamous carcinomas, containing the combined phenotypes of both glandular and squamous differentiation. These combinations of different phenotypes are common in human lung tumors, but are not seen in mouse models of lung cancer [6]. We also observed a lymphoma in the lungs of a ferret exposed to NNK for 32 weeks (Table 2). Ferrets can spontaneously develop lymphomas [25, 26]. However, there was a previous study indicating that NNK treatment induces lymphomas in vivo [27]. Since the lymphoma observed in this study occurred in the lungs and occupied almost two-third of the right side of lung, it seems that this lymphoma would be induced by the NNK treatment.
Cigarette smoking is a major risk factor for the development of lung cancer in both men and women. There is no information regarding sex predilection on cancer development of ferret [28, 29]. We have previously reported that the induction of lung tumor exposed to both tobacco smoke and NNK in male ferrets (Table 3). In order to compare with the effect of NNK treatment with or without smoke-exposure, we used the same male ferrets to investigate whether NNK treatment alone induce lung tumor in the present study. We found that NNK induced lung tumors in a time-dependent manner, with the highest incidence (66.7%) after 32 weeks subsequent to NNK treatment. Interestingly, when we compared the incidence of lung preneoplastic and neoplastic lesions in ferrets treated with NNK and/or exposed to cigarette smoke from previous and current studies (Table 3), we observed that ferrets exposed to NNK alone for 24 weeks had a 16.7% incidence of lung tumors; the ferrets treated with the same dose of NNK and exposed to two doses of cigarette smoke (equivalent to 1/4 or 1.0 consumer packs of cigarettes/day) in our previous studies had an increased incidence of lung tumors (25% and 50%, for 22 and 24 weeks, respectively) [14, 30]. Ferrets treated with the higher dose of smoke-exposure alone (equivalent to 2.0 consumer packs of cigarettes/day) had precancerous lesions without incidence of lung tumors at 24 weeks post-treatment [31, 32]. In our previous ferret studies exposed to both smoke and NNK [14, 17, 30], there was an increased inflammatory response suggested by the clinical signs including cough, shortness of breath, excessive salivation and unhealthy fur in the ferrets. We did not observed these clinical signs in the ferrets with NNK alone in the present study. There were no significantly differences in the body weights between the control and the NNK treated groups at 24 weeks, however, we observed that ferrets exposed to NNK alone had less incidences of lung tumors, as compared with the ferrets treated with the same dose of NNK and exposed to cigarette smoke. In addition, we observed the presence of pathological lesions (including inflammatory cell infiltration and obstructive bronchiolitis) in the lungs of ferrets exposed to both smoke and NNK. Therefore, we believe that the smoke-induced inflammatory responses promote the progression of tumors after initiation by NNK in the ferret.
Table 3.
Comparison of the incidence of lung preneoplastic and neoplastic lesions in ferrets treated with NNK and/or exposed to cigarette smoke by present and previous studies.
| Treatment | Time (weeks) |
n | Preneoplastic Lesionsa) (% Incidence) |
Neoplastic Lesionsb) (% Incidence) |
Ref. | |
|---|---|---|---|---|---|---|
| NNK (mg/kg BW) |
Smoking Exposure (min/d) |
|||||
| 200 | None | 24 | 6 | 100 % | 16.7 % | Present study |
| 200 | None | 26 | 10 | 100 % | 40.0 % | Present study |
| 200 | None | 32 | 6 | 83.3 % | 66.7 % | Present study |
| 200 | 15 | 22 | 16 | 87.5 % | 25.0 % | 30 |
| 200 | 60 | 24 | 12 | 83.3 % | 50.0 % | 14 |
| None | 60 | 6 | 9 | 44.4 % | NDc) | 31 |
| None | 120 | 24 | 6 | 33.3 % | NDc) | 31 |
| None | 120 | 24 | 8 | 62.5 % | NDc) | 32 |
| None | 120 | 9 | 6 | 100 % | NDc) | 17, 38 |
Including squamous dysplasia, squamous metaplasia and atypical adenomatous hyperplasia.
Including adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma and lymphoma.
ND: not detected.
One of the interesting observations in this study was that the α7 nAChR is highly expressed on the membranes of bronchial/bronchiolar epithelial cells, and alveolar macrophages in ferrets exposed to NNK (Fig. 3). These observations consisted with the previous reports indicating that nAChRs were localized on the membrane of cells [33]. In addition, α7 nAChR was expressed in both squamous cell carcinoma and adenocarcinoma of the ferrets (Fig. 3). As we pointed above, NNK is known to be a high-affinity ligand for α7 nAChR [24], we believe that NNK can initiate lung carcinogenesis and also promote lung tumor development by activating α7 nAChR. This observation was supported by previous reports that α7 nAChR expression was higher in smoking patients with squamous carcinoma than those with adenocarcinoma [34], the demonstration that administration of nicotine (either by i.p. injection or through dermal patches) could promote tumor growth and metastasis in immunocompetent mice [35], and that the increased expression of α7 nAChR has been described in the lung Type-II and epithelial cells by NNK treatment in A/J mice [36].
Although the ferret model exposed to NNK alone could be applicable to the assay of the expression of α7 nAChR, we only observed the tendency to increase α7 nAChR’s down-stream protein levels such as phospho-ERK and cyclin D1 (Fig. 2). Since we have previously shown that NNK and cigarette smoke treatment resulted in increased ERK activation [37] and proliferating cellular nuclear antigen [31, 38-40], this could be due to the limited sample size or NNK treatment alone (without smoke exposure) in the present study. It has been reported that the expression of α7 nAChR was related to the major activation of the Rb-Raf-1/phospho-ERK pathway and was associated with proliferation of lung cancer cells [34, 41]. Meanwhile, nicotine induced an increase in cell proliferation and a decrease of apoptosis in cancer cell lines, mediated by α7 nAChR and their down-stream products, not only ERK but also those of the PI3K/AKT pathways [42].
In summary, ferrets injected with NNK alone developed morphological characteristics of pulmonary neoplasia and biomarkers related to carcinogenesis which are typically seen in smokers and lung cancer patients. Additionally, ferrets provide ample plasma samples to monitor biomarkers and the concentration of chemopreventive agents, both during the experiment and at its end. Ferrets also provide ample tissue quantities with which one can investigate molecular mechanisms at the protein level. Furthermore, the ferret is an excellent animal model for studying effects of micronutrient such as carotenoids, because of the similarities between ferrets and human in their absorption and accumulation [43]. In our previous studies [17, 30-32, 37-40], we have investigated the chemopreventive effects and potential biological mechanisms by dietary antioxidants supplementation, such as β-carotene, lycopene, β-cryptoxanthin, and both vitamins E and C, on cigarette-smoke and NNK exposure induced lung carcinogenesis using ferrets. Since this is the first report to establish the ferret as a model of pulmonary carcinogenesis by NNK exposure alone, we are currently investigating potential chemopreventive effects of carotenoids using this ferret model. Recently, the first ferret CFTR-knockout model of cystic fibrosis demonstrated many of the characteristics of human cystic fibrosis and provided a useful genetically-engineered ferret model for studying cystic fibrosis [44]. The ferret genome sequencing project is ongoing and has recently identified close to 16,000 partial ferret genes [45]. Therefore, the ferret could prove to be a valuable model to identify biomarkers/molecular targets for the prevention, detection and treatment of lung carcinogenesis.
Acknowledgement
This study was supported by the NIH/NCI CA104932 grant, and partially supported by the fund from Kagome Co., Ltd. (Japan). Any opinions, findings, conclusions, and recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the sponsors.
The abbreviations used are
- BW
body weight
- ERK
extracellular signal-regulated kinase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- LD50
lethal dose 50
- nAChR
nicotinic acetylcholine receptor
- NNK
4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone
- NNN
N’-nitrosonornicotine
- PI3K
Phosphoinositide 3-kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None declared.
References
- 1.American Cancer Society . Cancer Facts & Figures 2013. American Cancer Society; Atlanta: 2013. [Google Scholar]
- 2.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3(10):733–44. doi: 10.1038/nrc1190. PMID: 14570033. [DOI] [PubMed] [Google Scholar]
- 3.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131(12):2724–32. doi: 10.1002/ijc.27816. PMID: 22945513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11(6):559–603. doi: 10.1021/tx980005y. PMID: 9625726. [DOI] [PubMed] [Google Scholar]
- 5.Zheng HC, Takano Y. NNK-Induced Lung Tumors: A Review of Animal Model. J Oncol. 2011 doi: 10.1155/2011/635379. Article ID 635379. PMID: 21559252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn FF, Gigliotti AP, Hutt JA, March TH, Mauderly JL. A review of the histopathology of cigarette smoke-induced lung cancer in rats and mice. Int J Toxicol. 2007;26(4):307–13. doi: 10.1080/10915810701483450. PMID: 17661221. [DOI] [PubMed] [Google Scholar]
- 7.Coggins CR. A further review of inhalation studies with cigarette smoke and lung cancer in experimental animals, including transgenic mice. Inhal Toxicol. 2010;22(12):974–83. doi: 10.3109/08958378.2010.501831. PMID: 20698816. [DOI] [PubMed] [Google Scholar]
- 8.Nikitin AY, Alcaraz A, Anver MR, Bronson RT, Cardiff RD, Dixon D, et al. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer Res. 2004;64(7):2307–16. doi: 10.1158/0008-5472.can-03-3376. PMID: 15059877. [DOI] [PubMed] [Google Scholar]
- 9.Coggins CR. An updated review of inhalation studies with cigarette smoke in laboratory animals. Int J Toxicol. 2007;26(4):331–8. doi: 10.1080/10915810701490190. PMID: 17661224. [DOI] [PubMed] [Google Scholar]
- 10.Green FH, Vallyathan V, Hahn FF. Comparative pathology of environmental lung disease: an overview. Toxicol Pathol. 2007;35(1):136–47. doi: 10.1080/01926230601132055. PMID: 17325982. [DOI] [PubMed] [Google Scholar]
- 11.Kwak I, Tsai SY, DeMayo FJ. Genetically engineered mouse models for lung cancer. Annu Rev Physiol. 2004;66:647–63. doi: 10.1146/annurev.physiol.66.032102.134301. PMID: 14977417. [DOI] [PubMed] [Google Scholar]
- 12.Vinegar A, Sinnett EE, Kosch PC, Miller ML. Pulmonary physiology of the ferret and its potential as a model for inhalation toxicology. Lab Anim Sci. 1985;35(3):246–50. PMID: 4021438. [PubMed] [Google Scholar]
- 13.Cmp JV, Svensson TL, McBrayer A, Jonsson CB, Liljeström P, Bruder CE. De-novo transcriptome sequencing of a normalized cDNA pool from influenza infected ferrets. PLoS One. 2012;7(5):e37104. doi: 10.1371/journal.pone.0037104. PMID: 22606336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, Liu XS, Liu C, Smith DE, Russell RM, Wang XD. Induction of pulmonary neoplasia in the smoke-exposed ferret by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK): a model for human lung cancer. Cancer Lett. 2006;234(2):209–19. doi: 10.1016/j.canlet.2005.03.052. PMID: 15894421. [DOI] [PubMed] [Google Scholar]
- 15.Wang XD, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell M. Retinoid signaling and activator protein-1 expression in ferrets given beta-carotene supplements and exposed to tobacco smoke. J Natl Cancer Inst. 1999;91(1):60–6. doi: 10.1093/jnci/91.1.60. PMID: 9890171. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Wang XD, Mucci L, Gaziano JM, Zhang SM. Modulation of lung molecular biomarkers by β-carotene in the Physicians’ Health Study. Cancer. 2009;115(5):1049–58. doi: 10.1002/cncr.24061. PMID: 19142877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C, Bronson RT, Russell RM, Wang XD. β-Cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage, and squamous metaplasia in ferrets. Cancer Prev Res (Phila) 2011;4(8):1255–66. doi: 10.1158/1940-6207.CAPR-10-0384. PMID: 21421799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iskandar AR, Liu C, Smith DE, Hu KQ, Choi SW, Ausman LM, et al. β-Cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev Res (Phila) 2013;6(4):309–20. doi: 10.1158/1940-6207.CAPR-12-0368. PMID: 23275008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padma PR, Amonkar AJ, Bhide SV. Mutagenic and cytogenetic studies of N’-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Lett. 1989;46(3):173–80. doi: 10.1016/0304-3835(89)90127-4. PMID: 2670197. [DOI] [PubMed] [Google Scholar]
- 20.Koppang N, Rivenson A, Dahle HK, Hoffmann D. A study of tobacco carcinogenesis, LIII: carcinogenicity of N’-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in mink (Mustela vison) Cancer Lett. 1997;111(1-2):167–71. doi: 10.1016/s0304-3835(96)04507-7. PMID: 9022142. [DOI] [PubMed] [Google Scholar]
- 21.Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29(3):151–8. doi: 10.1016/j.tips.2007.12.006. PMID: 18262664. [DOI] [PubMed] [Google Scholar]
- 22.Grando SA. Basic and clinical aspects of non-neuronal acetylcholine: biological and clinical significance of non-canonical ligands of epithelial nicotinic acetylcholine receptors. J Pharmacol Sci. 2008;106(2):174–9. doi: 10.1254/jphs.fm0070087. PMID: 18285656. [DOI] [PubMed] [Google Scholar]
- 23.Improgo MR, Tapper AR, Gardner PD. Nicotinic acetylcholine receptor-mediated mechanisms in lung cancer. Biochem Pharmacol. 2011;82(8):1015–21. doi: 10.1016/j.bcp.2011.05.020. PMID: 21640716. [DOI] [PubMed] [Google Scholar]
- 24.Schuller HM, Orloff M. Tobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cells. Biochem Pharmacol. 1998;55(9):1377–84. doi: 10.1016/s0006-2952(97)00651-5. PMID: 10076528. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Fox JG, Padrid PA. Neoplastic diseases in ferrets: 574 cases (1968-1997) J Am Vet Med Assoc. 1998;212(9):1402–6. PMID: 9589126. [PubMed] [Google Scholar]
- 26.Ammersbach M, Delay J, Caswell JL, Smith DA, Taylor WM, Bienzle D. Laboratory findings, histopathology, and immunophenotype of lymphoma in domestic ferrets. Vet Pathol. 2008;45(5):663–73. doi: 10.1354/vp.45-5-663. PMID: 18725471. [DOI] [PubMed] [Google Scholar]
- 27.Anderson LM, Hecht SS, Dixon DE, Dove LF, Kovatch RM, Amin S, et al. Evaluation of the transplacental tumorigenicity of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in mice. Cancer Res. 1989;49(14):3770–5. PMID: 2736518. [PubMed] [Google Scholar]
- 28.Miwa Y, Kurosawa A, Ogawa H, Nakayama H, Sasai H, Sasaki N. Neoplasitic diseases in ferrets in Japan: a questionnaire study for 2000 to 2005. J Vet Med Sci. 2009;71(4):397–402. doi: 10.1292/jvms.71.397. PMID: 19420840. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Fox JG, Padrid PA. Neoplastic diseases in ferrets: 574 cases (1968-1997) J Am Vet Med Assoc. 1998;212(9):1402–6. PMID: 9589126. [PubMed] [Google Scholar]
- 30.Veeramachaneni S. Interaction between escalating dose of lycopene supplementation and exposure to alcohol/smoke and carcinogen in vivo. A dissertation of Tufts Univ. 2008 Jun;:116–141. [Google Scholar]
- 31.Liu C, Wang XD, Bronson RT, Smith DE, Krinsky NI, Russell RM. Effects of physiological versus pharmacological β-carotene supplementation on cell proliferation and histopathological changes in the lungs of cigarette smoke-exposed ferrets. Carcinogenesis. 2000;21:2245–2253. doi: 10.1093/carcin/21.12.2245. PMID: 11133814. [DOI] [PubMed] [Google Scholar]
- 32.Kim Y, Chongviriyaphan N, Liu C, Russell RM, Wang XD. Combined α-tocopherol and ascorbic acid protects against smoke-induced lung squamous metaplasia in ferrets. Lung Cancer. 2012;75(1):15–23. doi: 10.1016/j.lungcan.2011.05.017. PMID: 21665318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29(3):151–8. doi: 10.1016/j.tips.2007.12.006. PMID: 18262664. [DOI] [PubMed] [Google Scholar]
- 34.Paleari L, Catassi A, Ciarlo M, Cavalieri Z, Bruzzo C, Servent D, et al. Role of alpha7-nicotinic acetylcholine receptor in human non-small cell lung cancer proliferation. Cell Prolif. 2008;41(6):936–59. doi: 10.1111/j.1365-2184.2008.00566.x. PMID: 19040571. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Davis R, Rizwani W, Banerjee S, Kovacs M, Haura E, Coppola D, et al. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer. PLoS One. 2009;4(10):e7524. doi: 10.1371/journal.pone.0007524. doi: 10.1371. PMID: 19841737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Razani-Boroujerdi S, Sopori ML. Early manifestations of NNK-induced lung cancer: role of lung immunity in tumor susceptibility. Am J Respir Cell Mol Biol. 2007;36(1):13–9. doi: 10.1165/rcmb.2005-0330OC. PMID: 16873770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim Y, Chongviriyaphan N, Liu C, Russell RM, Wang XD. Combined antioxidant (beta-carotene, alpha-tocopherol and ascorbic acid) supplementation increases the levels of lung retinoic acid and inhibits the activation of mitogen-activated protein kinase in the ferret lung cancer model. Carcinogenesis. 2006;27(7):1410–9. doi: 10.1093/carcin/bgi340. PMID: 16401635. [DOI] [PubMed] [Google Scholar]
- 38.Liu C, Lian F, Smith DE, Russell RM, Wang XD. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res. 2003;63(12):3138–44. PMID: 12810641. [PubMed] [Google Scholar]
- 39.Liu C, Russell RM, Wang XD. Exposing ferrets to cigarette smoke and a pharmacological dose of beta-carotene supplementation enhance in vitro retinoic acid catabolism in lungs via induction of cytochrome P450 enzymes. J Nutr. 2003;133(1):173–9. doi: 10.1093/jn/133.1.173. PMID: 12514286. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y, Lian F, Yeum KJ, Chongviriyaphan N, Choi SW, Russell RM, et al. The effects of combined antioxidant (beta-carotene, alpha-tocopherol and ascorbic acid) supplementation on antioxidant capacity, DNA single-strand breaks and levels of insulin-like growth factor-1/IGF-binding protein 3 in the ferret model of lung cancer. Int J Cancer. 2007;120(9):1847–54. doi: 10.1002/ijc.22320. PMID: 17278094. [DOI] [PubMed] [Google Scholar]
- 41.Shi D, Guo W, Chen W, Fu L, Wang J, Tian Y, et al. Nicotine promotes proliferation of human nasopharyngeal carcinoma cells by regulating α7AChR, ERK, HIF-1α and VEGF/PEDF signaling. PLoS One. 2012;7(8):e43898. doi: 10.1371/journal.pone.0043898. doi: 10.1371. PMID: 22952803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cucina A, Dinicola S, Coluccia P, Proietti S, D’Anselmi F, Pasqualato A, et al. Nicotine stimulates proliferation and inhibits apoptosis in colon cancer cell lines through activation of survival pathways. J Surg Res. 2012;178(1):233–41. doi: 10.1016/j.jss.2011.12.029. PMID: 22520577. [DOI] [PubMed] [Google Scholar]
- 43.Wang XD. Can smoke-exposed ferrets be utilized to unravel the mechanisms of action of lycopene? J Nutr. 2005;135(8):2053S–6S. doi: 10.1093/jn/135.8.2053S. PMID: 16046740. [DOI] [PubMed] [Google Scholar]
- 44.Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120(9):3149–60. doi: 10.1172/JCI43052. PMID: 20739752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruder CE, Yao S, Larson F, Camp JV, Tapp R, McBrayer A, et al. Transcriptome sequencing and development of an expression microarray platform for the domestic ferret. BMC Genomics. 2010;11:251. doi: 10.1186/1471-2164-11-251. doi: 10.1186/1471-2164-11-251. PMID: 20403183. [DOI] [PMC free article] [PubMed] [Google Scholar]


