Abstract
This paper reports the case history of a patient who had bisphosphonate-associated osteonecrosis of the jaw (ONJ) in which adjunctive treatment with teriparatide was used. The patient was treated for 5 years with alendronate for osteoporosis and developed ONJ after extraction of maxillary teeth. An implant was placed at the site of the extracted teeth. The pathology report confirmed the clinical diagnosis of ONJ; treatment was changed from alendronate to teriparatide and the ONJ resolved. To our knowledge, this is the third case history reported in the literature in which teriparatide was successfully used as adjunct therapy in ONJ because it has an anabolic effect and presumed role in accelerating bone healing. ONJ is a serious but infrequent condition that has been recently associated with nitrogen-containing bisphosphonate therapy.
Teriparatide may be a useful adjunctive therapy when ONJ develops.
Introduction
Osteonecrosis of the jaw (ONJ) associated with bisphosphonate therapy was initially described1 in 2003 among patients receiving parenteral bisphosphonates for lytic bone lesions and cancer-associated hypercalcemia. Since then, at least 865 case reports of bisphosphonate-associated ONJ involving the use of oral and parenteral bisphosphonates have been reported in the English-language literature.2 The rise in the number of published cases can be explained not only by the increased recognition of this condition by clinicians,3 but also by cumulative doses of bisphosphonates over time.4 Although most reports of ONJ involve patients receiving intravenous aminobisphosphonates for hypercalcemia of malignancy and for the prevention and treatment of bone metastases, data are accumulating regarding lesions in patients treated with oral bisphosphonates for osteoporosis.5,6
The prevalence of ONJ appears to vary widely depending on the underlying indication for, and potency and duration of, the bisphosphonate treatment. Various study designs and methodologies have been used to determine the epidemiology of ONJ,5,7 including Web-based surveys,8 reports from clinical trials,9 retrospective chart reviews,10 and population-based studies.11 It is important to recognize that these individual studies used different diagnostic criteria and study designs which may contribute to the heterogeneity of the results.
Recent postmarketing reports of two oral bisphosphonates used for patients with osteoporosis showed an ONJ incidence of less than 1.2 per 100,000 patient-years for risedronate use, and of less than 0.5–2.5 per 100,000 patient- years for alendronate.7,12,13 The criteria used to confirm the diagnosis of ONJ in these studies were heterogeneous. In contrast, the frequency of ONJ in patients with multiple myeloma, breast cancer, and prostate cancer who have been treated with intravenous bisphosphonates varied between 1% and 15%.8,14-18
ONJ prevalence seems to be related to the duration of bisphosphonate therapy in most subjects.7 In 2004–2005, a postal survey11 of Australian Oral and Maxillofacial Surgeons identified 158 cases of ONJ among patients taking bisphosphonates for multiple indications. The frequency of ONJ in patients with osteoporosis, most of them on weekly alendronate, was 1 in 2,260 to 1 in 8,470, approximately 100 times lower than in patients on IV bisphosphonates but still much higher than previous estimates among patients with osteoporosis. When dental extractions were done in this population, the calculated frequency of ONJ is estimated to be between 1 in 296 and 1 in 1,130. The median time to onset of ONJ was 12 months for zoledronate and 24 months in the case of pamidronate and alendronate. However, the diagnosis of ONJ in this study was not accompanied by formal validation of a case definition.11
Sedghizadeh et al.19 conducted an extensive search in the electronic medical record system of a single center for patients with a history of alendronate use who were also being treated for ONJ in the Oral Surgery clinic and the Orofacial Pain and Oral Medicine clinic at the University of Southern California. Inclusion criteria were that subjects had clinical features of ONJ, had a bisphosphonate history consistent with a diagnosis of ONJ which was defined as exposed necrotic bone (sequestra) in subjects with pain, infection, and a pathological fracture; extraoral fistula; or osteolysis extending to the inferior border of the mandible or floor of the sinonasal cavity—stage 2 or 3 in the ONJ staging system according to Ruggiero et al.20 The authors found that prevalence of ONJ secondary to oral alendronate therapy was approximately 4% and more common than previously suggested.19
A key limitation of this 4% prevalence estimate is that the study population was from a tertiary care referral center of patients receiving dental services and is therefore not population based. However, in a large controlled trial, the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) Pivotal Fracture Trial,9 almost 4,000 osteoporotic women were treated with 5 mg of zoledronate annually for 3 years, and a similar number received a placebo. Despite a rigorous search for any potential cases of bisphosphonate-associated ONJ—defined as an exposed bone with delayed healing despite 6 weeks of appropriate medical care and adjudicated by a blinded panel of experts—only two possible cases were found: one in the placebo group and one in the treatment group (a case of osteomyelitis that preceded any treatment with zoledronate).9,21 No ONJ lesions were seen in the HORIZON Recurrent Fracture Trial,22 which included more than 2,000 subjects who had suffered hip fracture. Both of these studies were funded by the maker of zoledronate and the patients’ follow-up was relatively short (3 years).
The risk of developing ONJ while taking intravenous pamidronate or zoledronate in cancer patients was further evaluated in a population-based cohort study23 using data from the Surveillance, Epidemiology and End Results (SEER) program linked to Medicare claims.
Because there was no billing diagnosis code specific for ONJ, surrogate International Disease Classification, 9th edition (ICD9) and Current Procedural Terminology (CPT) codes thought to represent ONJ lesions were used in the claims data. These ICD9 and CPT codes identified diagnoses of inflammatory conditions or osteomyelitis of the jaw or facial bone surgery.23 This study included 14,349 intravenous bisphosphonate users. The use of pamidronate and zoledronate was associated with an increased risk for facial bone surgery (hazard ratio [HR] 3.15, 95% CI 1.86–5.32) and an increased risk for diagnosis of inflammatory conditions or osteomyelitis of the jaw (HR 11.48, 95% CI 6.49–20.33).23
Predisposing factors
Both oral and systemic factors are thought to predispose individuals to develop ONJ.24-27 The oral risk factors include dental and periodontal disease, dental surgery, oral trauma, and poor dental hygiene. Systemic factors include dose, duration and type of bisphosphonate therapy, concomitant therapies (chemotherapy, corticosteroids), alcohol use, smoking, advanced age, and other underlying medical conditions (diabetes mellitus or peripheral vascular disease).
Pathogenesis
Bisphosphonates are pyrophosphate analogues characterized by their ability to localize to the bone where they inhibit osteoclast function.28 After being taken in by the skeleton, they continue to persist in the bone, due to their very long half-lives related to their strong mineral binding affinity.29 The precise molecular mechanism by which bisphosphonates may predispose to the development of ONJ is not well understood. Similarly, the effect of bisphosphonates on dental implant osseointegration is still under debate. Although dental implant failures associated with oral bisphosphonate therapy have been reported in the literature,30 both animal and human studies have shown no adverse effect of bisphosphonates on dental implant osseointegration.31-33 A longitudinal single-blind controlled study34 compared implant success in 25 postmenopausal women receiving oral bisphosphonate (102 implants) to 25 age-matched women (108 implants). At the end of the 3-year observation period, there were no cases of ONJ and the implant success was greater than 99% in both groups. Currently, the American Society for Bone and Mineral Research (ASBMR) does not consider prior oral bisphosphonate therapy as a contraindication to dental implant placement, nor are routine dental examinations recommended before beginning oral bisphosphonate therapy for osteoporosis.7
Clinical manifestations
An ASBMR task force panel defined ONJ as the presence of exposed bone in the maxillofacial region that does not heal for at least 8 weeks after its identification by a clinician in a patient exposed to bisphosphonates, but without prior radiation therapy to the craniofacial region.7
Patients with ONJ may be asymptomatic and the diagnosis often is made by the serendipitous discovery of exposed bone in the oral cavity during a regular dental examination.35 If sites become secondarily infected or there is trauma to the soft tissue by the sharp edges of exposed bone, symptoms such as pain, soft tissue swelling and infection, loose teeth, and purulent drainage may develop. Atypical complaints may include numbness, jaw heaviness, and dysesthesias. Less-specific signs and symptoms that could herald the development of clinical osteonecrosis are sudden changes in the health of periodontal and oral tissues, failure of oral mucosa to heal, undiagnosed oral pain, loose teeth, and soft tissue infections.
In three published case series’24,25,36 that included at least 50 patients with ONJ, the mandible was involved in 50% to 68% of the cases, the maxilla exclusively in 24% to 37% of the patients, and both the mandible and the maxilla in 4% to 13% of the cases. Both the mandible and the maxilla can be unilaterally or bilaterally involved.24 To our knowledge, there is only a single report37 of bisphosphonate-related osteonecrosis involving a site outside the oral cavity, which was in the auditory canal.
Diagnosis
The diagnosis of ONJ is primarily clinical, consisting of clinical signs and symptoms in relation to treatment with bisphosphonates. Radiography, computed tomography, or functional imaging such as bone scans are not necessary for diagnosis but may be used to exclude other conditions such as cysts or impacted teeth, depending on the patient’s symptoms.35
Two expert panels, one convened by the American Dental Association35 and another multidisciplinary team of medical oncologists, hematologists, urologists, and stomatologists from Spain,38 recommended that tissue biopsy should be performed only if metastatic disease was suspected.
Management
Management strategies need to consider both prevention and treatment for this condition. For patients with malignancy, pretreatment dental examinations are important. In a retrospective survey of patients treated with zoledronate, Ripamonti et al.39 showed that a dental examination and the application of preventive measures led to a sustained reduction in occurrence of ONJ from 7.8% to 1.7% (p = 0.016). Reducing bisphosphonate dose could be a preventive measure in oncology practice, and early results suggest that administration of intravenous bisphosphonate every 3 months is safer than monthly administration.40 Avoidance of invasive dental procedures is also an important preventive strategy. In addition, recent research41 suggests that antibiotic prophylaxis before dental procedures in patients with multiple myeloma treated with bisphosphonates may prevent the occurrence of ONJ after dental procedures.
However, recent recommendations for managing the care of patients receiving oral bisphosphonate therapy by the American Dental Association (ADA) Council on Scientific Affairs42 indicated that risk of developing ONJ apparently remained low for patients receiving oral bisphosphonates. Therefore, routine dental treatment should not be modified solely because the patient is taking oral bisphosphonates. Furthermore, given the morbidity and mortality associated with osteoporosis and the proven benefits of oral bisphosphonate therapy, patients should not alter their use of these medications without first consulting their physicians. In contrast to the American Society for Bone and Mineral Research (ASBMR) guidelines,7 the ADA guidelines42 recommend that all patients who are prescribed oral bisphosphonates should receive routine dental examinations before or during the early portion of their bisphosphonate therapeutic regimen. Oral healthcare providers should be informed if any problems develop in the oral cavity during oral bisphosphonate therapy.
The standard of care for the management of ONJ includes symptom palliation, treatment of dental and periodontal infections, and conservative surgical intervention.7 Wide surgical debridement is not recommended, although sharp bone edges and sequestra should be removed to prevent trauma to soft tissues.7 Some investigators have advocated the use of hyperbaric oxygen, but its efficacy appears doubtful.7 The value of stopping bisphosphonate therapy remains unclear, given the long half-life of these compounds, although some experts believe that it could help hasten healing.7 However, the optimal duration of bisphosphonate discontinuation lacks any evidence at this time.
Recently, teriparatide was reported as an adjunctive treatment modality for ONJ.43,44 Teriparatide contains the 1–34 N-terminal amino acid residues of the human parathyroid hormone and is approved for treatment of osteoporosis in men and postmenopausal women.45 Unlike bisphosphonates, which are antiresorptive agents, teriparatide has anabolic effects and promotes osteoblast differentiation and activity.45,46 The intermittent delivery of parathyroid hormone leads to an early decrease of receptor activator of nuclear factor-kappa B ligand mRNA and increase of osteoprotegerin mRNA,47 resulting in an increase in bone formation. If indeed bisphosphonate-associated ONJ results from oversuppression of bone turnover, teriparatide would seem to be beneficial by exerting its physiologic effect to promote bone formation and anabolic activity against a background of the suppressed turnover common to long-term bisphosphonate use. Recently, a prospective trial48 examined the early anabolic effects of teriparatide in postmenopausal women with osteoporosis previously treated with alendronate or risedronate. Bone turnover markers including N-terminal propeptide (P1NP), bone-specific alkaline phosphatase activity (BAP), osteocalcin (OC), serum C telopeptide (CTX), and N-telopeptide to urine creatinine ratio (NTX) were measured at baseline, 0.5, 1–6, and 12 months. All bone turnover markers increased from baseline for both groups as early as 2 weeks after beginning teriparatide therapy. The results from these studies support the hypothesis that administration of parathyroid hormone after discontinued bisphosphonate treatment can stimulate bone formation and may help restore the bone tissues in the oral cavity. However, there is no specific evidence for the benefit of teriparatide therapy on the course of bisphosphonate-associated ONJ except for a few anecdotal reports of benefit such as this one.
This review was based on a search of PubMed for English language papers published from 1966 to October 2008 using these keywords: osteonecrosis of jaw, bisphosphonate, teriparatide, osteoporosis, malignancy, epidemiology, pathogenesis and treatment. In this overview paper, we also present a patient with osteoporosis who was treated with alendronate who developed ONJ following a surgical procedure. The necrotic bone showed significant healing after treatment with teriparatide for four months.
Case report
A 63-year-old Caucasian male physician presented to a periodontist complaining of pain in the maxillary right second premolar and maxillary left first premolar and requested extraction of the symptomatic teeth and placement of dental implants. His medical history was significant for osteoporosis and erosive osteoarthritis of the hands. He had a wrist fracture at age 53 and also had several thoracic spine fractures. His medications included calcium citrate, a multivitamin, and alendronate, which he had been taking orally for five years. He also reported the following list of medications: esomeprazole, glucosamine, chondroitin, atorvastatin, aspirin, and mirtazapine. He denied a history of glucocorticoid exposure and did not use tobacco products or alcohol.
Fifteen months after the teeth were extracted, the patient underwent one- stage titanium tooth implants placed at both sites (Figure 1A). Four days later, the patient returned, complaining of pain in the area of the maxillary left canine. An assessment of the intraoral radiograph revealed possible impingement of the adjacent maxillary left first premolar implant body on the periodontal ligament of the maxillary left canine. The implant was removed and a collagen plug was placed in the osteotomy site. One month later, the maxillary left first premolar implant site had not healed and the surrounding tissue was inflamed. The implant at the maxillary right second premolar site was asymptomatic, but probing depths around it were 12 mm instead of the normal 2–3 mm. An evaluation of the intraoral radiograph showed bone loss around the implant body (Figure 1B). Three weeks later, the remaining implant was removed and the area thoroughly degranulated. Around the implant, we noted advanced bone loss that extended through the buccal cortical plate to the adjacent premolar tooth. During the procedure, the maxillary left first premolar implant was also degranulated and specimens were sent for histopathologic examination. After debridement, bone allografts were placed in both sites and covered with resorbable collagen membranes. Chlorhexidine rinse was prescribed and utilized both before and after implant removal.
Figure 1.
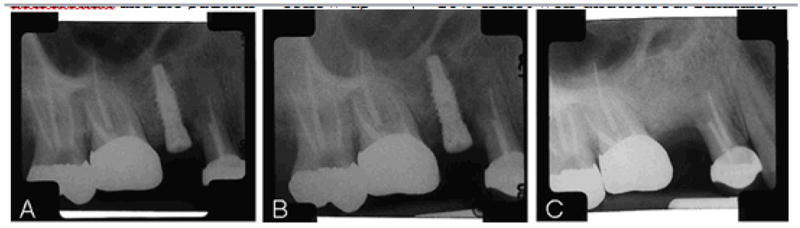
(A) Maxillary right second premolar immediately after implant placement. (B) Failing implant, maxillary right second premolar. Notice the radiolucent area around the metal. (C) Healed implant site 8 months after implant removal and 4 months after teriparatide infusions were started.
The biopsy report stated that there was nonvital bone and focal inflammation at the implant sites. With the patient’s lack of symptoms, the radiographic appearance, and the history of alendronate use, a clinical and pathologic diagnosis of bisphosphonate-associated ONJ was made. The large amount of bone loss precluded the possibility of delayed healing, whereas absence of pain and new hyperostosis made primary osteomyelitis unlikely.
The patient was referred to our rheumatology/osteoporosis clinic for further management. The patient’s previous diagnosis of osteoporosis complicated by prior wrist and vertebral fractures and the histopathologic evidence of bisphosphonate-associated ONJ were taken into consideration and the patient was started on teriparatide 20 μg subcutaneously daily and alendronate was discontinued. Four months after the teriparatide infusions were started, an evaluation of the intraoral radiographs of the implant sites showed osseous regeneration with increased bone density (Figure 1C). The patient is currently awaiting replacement of the dental implants at these sites.
Discussion
In our patient, given the history of osteoporosis with multiple fragility fractures, the decision was made to switch from alendronate to teriparatide. His alveolar bone showed significant healing 4 months after the start of teriparatide treatment. Although bone healing may have occurred with discontinuation of bisphosphonate therapy and surgery alone, based on our patient, intermittent administration of teriparatide might be useful in the clinical setting of osteoporosis and ONJ.
A critical evaluation of the current literature and on the published case reports shows that the risk of developing ONJ as a result of oral bisphosphonate therapy is significantly lower compared with the risk associated with intravenous bisphosphonate therapy in patients with cancer.42 Multiple risk factors such as dental and periodontal disease, dental surgery, poor dental hygiene, duration and type of bisphosphonate therapy, and underlying medical conditions may also play a role. The diagnosis of ONJ is mostly based on clinical findings. Although some experts recommend stopping bisphosphonate therapy in patients who develop ONJ, there is insufficient evidence to support this practice. Our patient’s necrotic bone showed significant healing 4 months after the start of teriparatide treatment. It is likely that because of its anabolic effects on the bone, administration of this medication may be useful in the clinical setting of osteoporosis and ONJ. Clinical trials are needed to test this hypothesis.
Footnotes
Financial disclosures
Pongthorn Narongroeknawin, Maria I. Danila, Lewis G. Humphreys, and Andrei Barasch: none; Jeffrey R. Curtis: consultancies, speaking fees, and honoraria from Roche, Eli Lilly, Merck, Novartis, Procter, and Gamble.
References
- 1.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–7. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 2.Brooks JK, Gilson AJ, Sindler AJ, et al. Osteonecrosis of the jaws associated with use of risedronate: report of 2 new cases. Oral Surg Oral Med Oral Path Oral Radiol Endod. 2007;103:780–6. doi: 10.1016/j.tripleo.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Malden NJ, Pai AY. Oral bisphosphonate associated osteonecrosis of the jaws: three case reports. Br Dent J. 2007;203:93–7. doi: 10.1038/bdj.2007.636. [DOI] [PubMed] [Google Scholar]
- 4.Bilezikian JP. Osteonecrosis of the jaw—do bisphosphonates pose a risk? N Engl J Med. 2006;355:2278–81. doi: 10.1056/NEJMp068157. [DOI] [PubMed] [Google Scholar]
- 5.Khan AA, Sándor GK, Dore E, et al. Bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2009;36:478–90. doi: 10.3899/jrheum.080759. [DOI] [PubMed] [Google Scholar]
- 6.Silverman SL, Landesberg R. Osteonecrosis of the jaw and the role of bisphosphonates: a critical review. Am J Med. 2009;122:S33–45. doi: 10.1016/j.amjmed.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–91. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 8.Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005;353:99–102. doi: 10.1056/NEJM200507073530120. [DOI] [PubMed] [Google Scholar]
- 9.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 10.Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–36. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate- associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg. 2007;65:415–23. doi: 10.1016/j.joms.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 12.American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc. 2006;137:1144–50. doi: 10.14219/jada.archive.2006.0355. [DOI] [PubMed] [Google Scholar]
- 13.Reid IR, Cundy T. Osteonecrosis of the jaw. Skeletal Radiol. 2009;38:5–9. doi: 10.1007/s00256-008-0549-x. [DOI] [PubMed] [Google Scholar]
- 14.Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23:8580–7. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos MA, Kastritis E, Anagnostopoulos A, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica. 2006;91:968–71. [PubMed] [Google Scholar]
- 16.Pozzi S, Marcheselli R, Sacchi S, et al. Bisphosphonate-associated osteonecrosis of the jaw: a review of 35 cases and an evaluation of its frequency in multiple myeloma patients. Leuk Lymphoma. 2007;48:56–64. doi: 10.1080/10428190600977690. [DOI] [PubMed] [Google Scholar]
- 17.Ortega C, Faggiuolo R, Vormola R, et al. Jaw complications in breast and prostate cancer patients treated with zoledronic acid. Acta Oncol. 2006;45:216–7. doi: 10.1080/02841860500341173. [DOI] [PubMed] [Google Scholar]
- 18.Zervas K, Verrou E, Teleioudis Z, et al. Incidence, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: a single-centre experience in 303 patients. Brit J Haematol. 2006;134:620–3. doi: 10.1111/j.1365-2141.2006.06230.x. [DOI] [PubMed] [Google Scholar]
- 19.Sedghizadeh PP, Stanley K, Caligiuri M, et al. Oral bisphosphonate use and the prevalence of osteonecrosis of the jaw: an institutional inquiry. J Am Dent Assoc. 2009;140:61–6. doi: 10.14219/jada.archive.2009.0019. [DOI] [PubMed] [Google Scholar]
- 20.Ruggiero SL, Fantasia J, Carlson E. Bisphosphonate-related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:433–41. doi: 10.1016/j.tripleo.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Grbic JT, Landesberg R, Lin SQ, et al. Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the health outcomes and reduced incidence with zoledronic acid once yearly pivotal fracture trial. J Am Dent Assoc. 2008;139:32–40. doi: 10.14219/jada.archive.2008.0017. [DOI] [PubMed] [Google Scholar]
- 22.Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799–809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson GS, Kuo YF, Freeman JL, Goodwin JS. Intravenous bisphosphonate therapy and inflammatory conditions or surgery of the jaw: a population-based analysis. J Natl Cancer Inst. 2007;99:1016–24. doi: 10.1093/jnci/djm025. [DOI] [PubMed] [Google Scholar]
- 24.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–34. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–75. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Badros A, Weikel D, Salama A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol. 2006;24:945–52. doi: 10.1200/JCO.2005.04.2465. [DOI] [PubMed] [Google Scholar]
- 27.Yarom N, Yahalom R, Shoshani Y, et al. Osteonecrosis of the jaw induced by orally administered bisphosphonates: incidence, clinical features, predisposing factors and treatment outcome. Osteoporos Int. 2007;18:1363–70. doi: 10.1007/s00198-007-0384-2. [DOI] [PubMed] [Google Scholar]
- 28.Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 29.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–61. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 30.Starck WJ, Epker BN. Failure of osseointegrated dental implants after diphosphonate therapy for osteoporosis: a case report. Int J Oral Maxillofac Implants. 1995;10:74–8. [PubMed] [Google Scholar]
- 31.Duarte PM, de Vasconcelos Gurgel BC, Sallum AW, et al. Alendronate therapy may be effective in the prevention of bone loss around titanium implants inserted in estrogen-deficient rats. J Periodontol. 2005;76:107–14. doi: 10.1902/jop.2005.76.1.107. [DOI] [PubMed] [Google Scholar]
- 32.Narai S, Nagahata S. Effects of alendronate on the removal torque of implants in rats with induced osteoporosis. Int J Oral Maxillofac Implants. 2003;18:218–23. [PubMed] [Google Scholar]
- 33.Chacon GE, Stine EA, Larsen PE, et al. Effect of alendronate on endosseous implant integration: an in vivo study in rabbits. J Oral Maxillofac Surg. 2006;64:1005–9. doi: 10.1016/j.joms.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Jeffcoat MK. Safety of oral bisphosphonates: controlled studies on alveolar bone. Int J Oral Maxillofac Implants. 2006;21:349–53. [PubMed] [Google Scholar]
- 35. [June 16, 2009];Expert Panel Recommendations for the Prevention, Diagnosis, and Treatment of Osteonecrosis of the Jaws. 2004 Jun; at http://www.ada.org/prof/resources/topics/topics_osteonecrosis_whitepaper.pdf. [PubMed]
- 36.Magopoulos C, Karakinaris G, Telioudis Z, et al. Osteonecrosis of the jaws due to bisphosphonate use. A review of 60 cases and treatment proposals. Am J Otolaryngol. 2007;28:158–63. doi: 10.1016/j.amjoto.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Polizzotto MN, Cousins V, Schwarer AP. Bisphosphonate-associated osteonecrosis of the auditory canal. Br J Haematol. 2006;132:114. doi: 10.1111/j.1365-2141.2005.05833.x. [DOI] [PubMed] [Google Scholar]
- 38.Bagán J, Blade J, Cozar JM, et al. Recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw (ONJ) in cancer patients treated with bisphosphonates. Med Oral Patol Oral Cir Bucal. 2007;12:E336–40. [PubMed] [Google Scholar]
- 39.Ripamonti CI, Maniezzo M, Campa T, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 2009;20:137–45. doi: 10.1093/annonc/mdn526. [DOI] [PubMed] [Google Scholar]
- 40.Corso A, Varettoni M, Zappasodi P, et al. A different schedule of zoledronic acid can reduce the risk of the osteonecrosis of the jaw in patients with multiple myeloma. Leukemia. 2007;21:1545–8. doi: 10.1038/sj.leu.2404682. [DOI] [PubMed] [Google Scholar]
- 41.Montefusco V, Gay F, Spina F, et al. Antibiotic prophylaxis before dental procedures may reduce the incidence of osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates. Leuk Lymphoma. 2008;49:2156–62. doi: 10.1080/10428190802483778. [DOI] [PubMed] [Google Scholar]
- 42.Edwards BJ, Hellstein JW, Jacobsen PL, et al. Updated recommendations for managing the care of patients receiving oral bisphosphonate therapy: an advisory statement from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2008;139:1674–7. doi: 10.14219/jada.archive.2008.0110. [DOI] [PubMed] [Google Scholar]
- 43.Wang HL, Weber D, McCauley LK. Effect of long-term oral bisphosphonates on implant wound healing: literature review and a case report. J Periodontol. 2007;78:584–94. doi: 10.1902/jop.2007.060239. [DOI] [PubMed] [Google Scholar]
- 44.Harper RP, Fung E. Resolution of bisphos phonate-associated osteonecrosis of the mandible: possible application for intermittent low-dose parathyroid hormone [rhPTH(1–34)] J Oral Maxillofac Surg. 2007;65:573–80. doi: 10.1016/j.joms.2006.10.076. [DOI] [PubMed] [Google Scholar]
- 45.Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357:905–16. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 46.Kawane T, Takahashi S, Saitoh H, et al. Anabolic effects of recombinant human parathyroid hormone (1–84) and synthetic human parathyroid hormone (1–34) on the mandibles of osteopenic ovariectomized rats with maxillary molar extraction. Horm Metab Res. 2002;34:293–302. doi: 10.1055/s-2002-33257. [DOI] [PubMed] [Google Scholar]
- 47.Locklin RM, Khosla S, Turner RT, Riggs BL. Mediators of the biphasic responses of bone to intermittent and continuously administered parathyroid hormone. J Cell Biochem. 2003;89:180–90. doi: 10.1002/jcb.10490. [DOI] [PubMed] [Google Scholar]
- 48.Miller PD, Delmas PD, Lindsay R, et al. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab. 2008;93:3785–93. doi: 10.1210/jc.2008-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]


