Abstract
This review summarizes an expanding body of knowledge indicating that failure to resolve inflammation and altered immune processes underlie the development of pulmonary arterial hypertension. The chemokines and cytokines implicated in pulmonary arterial hypertension that could form a biomarker platform are discussed. Pre-clinical studies that provide the basis for dysregulated immunity in animal models of the disease are reviewed. In addition we present therapies that target inflammatory/immune mechanisms that are currently enrolling patients and discuss others in development. We show how genetic and metabolic abnormalities are inextricably linked to dysregulated immunity and adverse remodeling in the pulmonary arteries.
Keywords: Tertiary lymphoid follicle, Macrophage and macrophage migration inhibitory factor, Elastase, Leukotriene B4, Pulmonary hypertension
Introduction
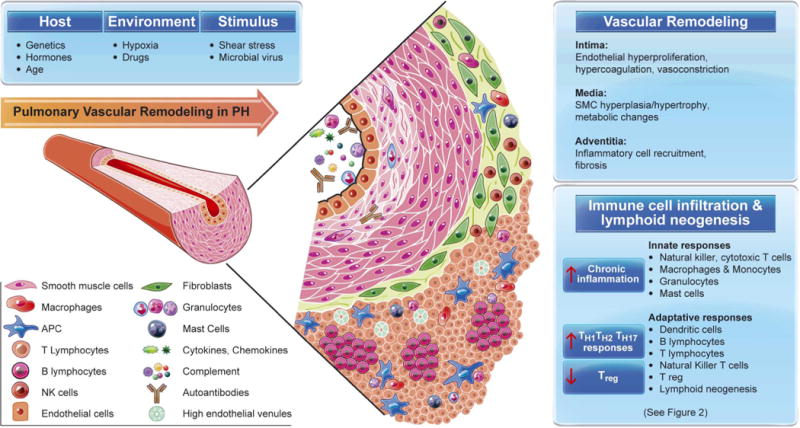
Pulmonary arterial hypertension (PAH) is a progressive cardiopulmonary disease in which extensive obliterative changes are prevalent in the small to mid-sized pulmonary arterioles. Alterations in structure and function of the endothelium occur in conjunction with growth of neointimal, medial and adventitial layers, culminating in an occlusive arteriopathy associated with high resistance to blood flow and right heart failure and death. Currently approved PAH therapies focus on dilating the partially occluded vessels, and are weak anti-proliferative agents. However, they have not resulted in a strategy that is effective in reversing vascular remodeling and preventing deterioration and the need for a lung transplant. In recent years, greater attention has focused on the frequently-observed perivascular inflammation in patients with all forms of PAH, from idiopathic (I) PAH to PAH associated with systemic autoimmune diseases1-4. An expanding body of knowledge has related genetic susceptibility, inflammation and metabolic (glycolytic) shifts in vascular cells to PAH pathogenesis. In fact, the inflammatory processes are inextricably linked to altered vascular and inflammatory cell metabolism5. Thus there is a strong rationale to identify genetic factors that predispose to impaired resolution of inflammation and to determine how immune-mediated vascular injury initiates and propagates alterations in metabolic function and in the phenotype of PAH vascular cells. Based on clinical and animal studies, described below, there is now reason to suggest that advanced vascular remodeling may be reversed by approaches that address specific inflammatory and immune processes.
Histopathology and Biomarker Evidence of Inflammation in Pulmonary Arterial Hypertension (PAH)
Pulmonary vascular lesions occurring in patients with PAH as well as in animal models of PH are characterized by varying degrees of perivascular inflammatory infiltrates, comprising T- and B-lymphocytes, macrophages, dendritic cells (DCs) and mast cells. (By convention, animal models are still referred to as having PH rather than PAH). Figure 1 illustrates representative histopathology and the observed inflammatory cells implicated in PAH. Recently, correlations of the average perivascular inflammation score with intima plus media and adventitia thickness, respectively, and with mean pulmonary arterial pressure have been reported; these associations supports a role for perivascular inflammation in the processes of pulmonary vascular remodeling6. Moreover, these studies also indicate that in the presence of a mutation in bone morphogenetic protein type 2 receptor (BMPR2),, the inflammatory pathology was more advanced. The fact that inflammation precedes vascular remodeling in experimental PH suggests that altered immunity is a cause rather than a consequence of vascular disease7. Beyond increased perivascular immune cells accumulation and intravascular infiltration, circulating levels of certain cytokines and chemokines are abnormally elevated. These include interleukin (IL)-1β, IL-6, IL-8, monocyte chemoattractant protein (MCP)-1, fractalkine, CCL5/RANTES and tumor necrosis factor (TNF)-α. Some of these cytokines and chemokines correlate with a worse clinical outcome in PAH patients and may serve as biomarkers of disease progression. Some, like IL-1β and TNF-α have been related to an accumulation of extracellular matrix proteins such as fibronectin8, observed in PAH lesions9 and others like IL-6 have been related to the proliferation of smooth muscle cells10. The cytokines implicated in PAH, e.g., in 11, 12 and in related studies are summarized in Table 1.
Figure 1. Pulmonary vascular changes in PAH includes infiltrating adaptive and innate immune cells.
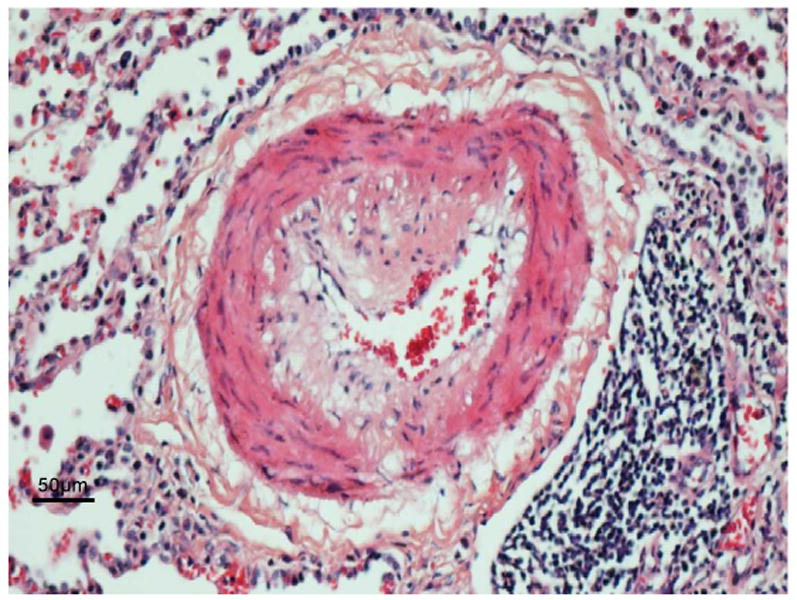
In the top panel is a representative histophathology of a vessel with severe neointimal formation represented below by a diagrammatic illustration. The histopathology shows a single endothelial layer and an eccentric neointima (pale pink) that contains cells that have markers of inflammatory cells and others that stain with markers of smooth muscle but appear poorly differentiated. The medial muscular layer is expanded and there is an abundant adventitial layer. This vessel is decorated with complement and autoantibodies, infiltrated by neutrophils in the lumen attacking the vessel wall and other inflammatory cells binding to the endothelium and infiltrating. The neointima is comprised of pale cells and matrix and infiltrating T and B cells and in the adventitia there are dendritic cells, macrophages and mast cells and in the periadventitial space tertiary lymphoid follicles characterized by T cells, B cells and plamacytoid dendritic cells (APC).
Table 1. Serum Cytokine/ Chemokine Levels in Patients with Pulmonary Arterial Hypertension (PAH).
Chemokines, Cytokines and Pulmonary Arterial Hypertension
Recent investigations provide evidence that both pulmonary vascular cells and inflammatory cells are important local sources of chemokines and cytokines that can lead to pulmonary vascular remodeling in PAH. Indeed the increased expression of cytokines and chemokines contribute to exaggerated contractility and proliferation of vascular cells. IL-1 can induce fibroblast growth factor (FGF)-213 and both FGF-2 and IL-6 and play an integral role in mediating the proliferative response in the smooth muscle like cells and fibroblasts of the pulmonary vasculature in PAH14-19. Mutations in BMPR2 seen in heritable PAH20,21 and dysfunction of the BMPR2 signaling pathway seen in all forms of PAH22 can lead to inappropriate expression of growth factors and pro-inflammatory responses in vascular cells as described both in experimental and human PAH 23-26. For example, in PA endothelial cells, loss of BMPR2 causes repression of apelin19 and this reduces a microRNA that normally represses FGF-215. However, in addition, increased p-p38 signaling resulting from loss of BMPR2 in vascular cells, causes heightened production of IL-627. Loss of BMPR2 also results in the enhanced secretion of GM-CSF in response to TNF-α. The mechanism is related to the increased translation of GM-CSF mRNA associated with subverted stress granule formation. Infusion of GM-CSF in rats can exaggerate, and administration of neutralizing antibodies to GM-CSF can prevent hypoxia induced PH28. It is also known that BMPR2 signaling plays an important role in the development of T cells from thymocytes29. BMP-2/4 (ligands for BMPR2) and TGF-β have a synergistic effect on the induction of Foxp3+ regulatory T (Treg) cells. BMP-2/4 affects non-Smad signaling molecules, including phosphorylated ERK and JNK, which may promote the differentiation of Foxp3+ Treg induced by TGF-β30. These cells protect against autoimmune responses that lead to severe PH in the experimental and clinical setting31.
Several cytokines can directly control cell proliferation, migration and differentiation of pulmonary vascular cells. IL-6 is prominent among these multifunctional pro-inflammatory cytokines and has been linked to the pathogenesis of PAH. Delivery of recombinant IL-6 protein in rodents is sufficient to cause pulmonary vascular remodeling and to exaggerate the pulmonary hypertensive response to chronic hypoxia32, 33. Furthermore, IL-6 overexpressing mice spontaneously develop PH and pulmonary vascular remodeling and an obliterative form of remodeling in hypoxia resembling human disease, whereas IL-6 knockout mice are more resistant to the development of PH induced by chronic hypoxia16, 17. IL-6 also induces pulmonary artery smooth muscle cell proliferation via induction of FGF-2 by the transcription factor KLF-510.
Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) has recently been identified as playing a key role in apoptosis of endothelial cells and proliferation of smooth muscle cells in a number of PH experimental models and its inhibition is related to prevention of disease pathology34. In addition osteoprotegerin a protein regulated by BMP and serotonin and IL-1 signaling is highly expressed in smooth muscle cells and serum of patients with pulmonary hypertension35 and can stimulate their migration and proliferation36.
Recent data from our group 37 demonstrated that increased production of macrophage migration inhibitory factor (MIF) plays a pivotal role in the pathogenesis of PAH. MIF is a critical upstream inflammatory mediator with pleiotropic actions partly explained by its binding to the extracellular domain of CD74. In endothelial cells, a MIF-CD74 interaction can lead to the activation of Src-family kinase, MAPK/ERK, PI3K/Akt and NF-κB pathways, and to apoptotic resistance via elevated BCL2 and BCL-xL and repressed p5338-43. In addition, MIF can bind to CXCR2 and CXCR4 and lead to the proliferation of pulmonary artery smooth muscle cells and contribute to hypoxic PH44-47. In addition to elevated production of cytokines and chemokines, phenotypic alterations and functional defects in cytotoxic T and natural killer (NK) cells are linked to human PAH and experimental PH as well as pulmonary veno-occlusive disease48, 49. Recent data show deposition of complement C3 in idiopathic PAH patients and the protective effect of complement depletion in experimental models of PH50, emphasizing the relevance of exploring complement-mediated vascular injury in the pathobiology of PAH.
Immune Dysregulation, T Cells, B Cells and Dendritic Cells
Further analyses of immunity in PAH support the notion that maladaptation of the immune response exists and may explain both the accumulation of perivascular inflammatory cells and the overabundance of cytokines and chemokines. Indeed, a delicate balance between immunity and tolerance exists and any disturbance may result in chronic inflammation or autoimmunity. Several types of autoantibodies directed against antinuclear antigens, endothelial cells (EC) and fibroblasts have been found in idiopathic and systemic sclerosis-associated PAH51-54. These autoantibodies may play an important role in EC apoptosis and in the expression of cell adhesion molecules55-57 but further studies are necessary to characterize their pathogenic importance.
The role of T cells and more specifically of Treg cells in the control of self-tolerance is well-established58-60, and altered Treg function has been demonstrated in patients with PAH7, 61. Tregs not only control other T-cells but also regulate monocytes, macrophages, dendritic cells, natural killer cells and B cells; decreased Treg function may predispose individuals to PAH, as it does in animals. For example, conditions associated with PAH, such as HIV, systemic sclerosis, systemic lupus erythematosus, Hashimoto's thyroiditis, Sjögren's syndrome and the anti-phospholipid syndrome, are characterized by abnormal CD4+ T-cell number and function62-66. In animals with a congenital absence of T cells (athymic nude rats), vascular injury causes the lungs to become infiltrated with macrophages, mast cells and B cells, similar to human PAH lesions, and disease is prevented with Treg reconstitution7,32. Similarly, NK cells have recently been implicated as having a beneficial effect on the pathogenesis of PH48 but a phenotypic switch results in their impaired production of interferon gamma and elevated levels of MMP9.
In experimental PH and clinical PAH, accumulation of immature dendritic cells (DCs) in remodeled pulmonary arteries has been demonstrated, suggesting that they may contribute to PAH immunopathology67. In addition, a recent study described lymphoid neogenesis in lungs from patients with idiopathic (I) PAH4, 68. In this study, pulmonary tertiary lymphoid tissues were identified, ranging from small lymphoid aggregates to large accumulations of lymphocytes resembling highly organized lymphoid follicles. Thus, the presence of pulmonary tertiary lymphoid tissues in IPAH lungs could provide a structural basis for a local autoimmune response occurring in this apparently idiopathic disease. In addition circulating autoantibodies are commonly detected in IPAH patients without evidence of an associated autoimmune condition51-54. Moreover, several autoimmune and infectious diseases such as systemic sclerosis, systemic lupus erythematous and HIV, herpes, and schistosomiasis infection are recognized causes of PAH. In many of these conditions, the PAH is not reversible with the treatment of the causal disease69-71. The sequence of events from initiation or recurrence of inflammation to pulmonary vascular disease development remains unknown in idiopathic and even in autoimmune and infectious forms of PAH. A recent study, indicated that passive transfer of IgG from rats following monocrotaline injection and documentation of anti-fibroblast antibodies, resulted in pulmonary hypertension and vascular changes in naïve rats 72.
Macrophages and Pulmonary Vascular Pathobiology
Recent attention has focused on the role of perivascular macrophages macrophage infiltrating pulmonary arterioles. The role of the macrophage in processes as diverse as limb or cardiac regeneration and pathogen defense indicates that it is important to comprehend the behavior of this cell in the context of the vascular milieu. CD68+ macrophages are prominent in advanced obliterative plexiform lesions observed in experimental and clinical PAH1, 73-78 and macrophage depletion or inactivation prevents PAH in several model systems including experimentally induced hypoxic PH and portopulmonary hypertension75, 79.
Macrophages have been implicated in infectious etiologies such as HIV-associated experimental PAH80, and human herpes virus infection is associated with vascular remodeling, perivascular macrophages and lung fibrosis81. However, even in patients with IPAH 82, recruitment of lung macrophages is evident and has been associated with the unfolded protein response. Rare conditions, such as the vHL-Chuvash mutation, are characterized by the association of PAH with macrophage infiltration83. Activation of macrophages is also closely linked to epigenetic changes that stimulate and induce proliferation of vascular fibroblasts in patients and in experimental models of PAH84. These features involve HDAC1 mediated activation of a host of pro-inflammatory cytokines. Altered metabolism involving a switch to glycolysis, fatty acid oxidation, and production of reactive oxygen species underlies the abnormal interaction of fibroblasts and macrophages. Reversing the metabolic phenotype can also reverse the pathologic features of PH in terms of macrophage recruitment and activation. There is recent evidence for macrophage GM-CSF and LTB4 signaling pathways in PAH development, consistent with a paradigm of cooperative interaction between these two pathways in regulating gene expression in inflammatory macrophages85.
The absence of normal Treg activity in athymic rats leads to activated macrophage recruitment following vascular injury with the vascular endothelial growth factor receptor (VEGFR) inhibitor, SU5416; this macrophage recruitment occurs in close association with the development of PH. As described79, these macrophage secrete LTB4 that induces pulmonary artery EC injury and apoptosis as well as pulmonary artery smooth muscle cell proliferation/hypertrophy. Blocking macrophage-derived LTB4 biosynthesis or signal transduction reverses experimental PH, and depleting CD68+ macrophages prevents PH from developing in this model79. A useful oversimplification of the athymic/immune dysregulation model is that following vascular injury in animals with a healthy immune systems, normal Treg activity skews macrophage responses to promote injury resolution, but that without this Treg activity (as observed in T-cell deficient athymic rats) pulmonary macrophages become activated and participate in injury evolution (Figure 2)86.
Figure 2. Distinct roles for reparative and dysregulated immunity following vascular injury.
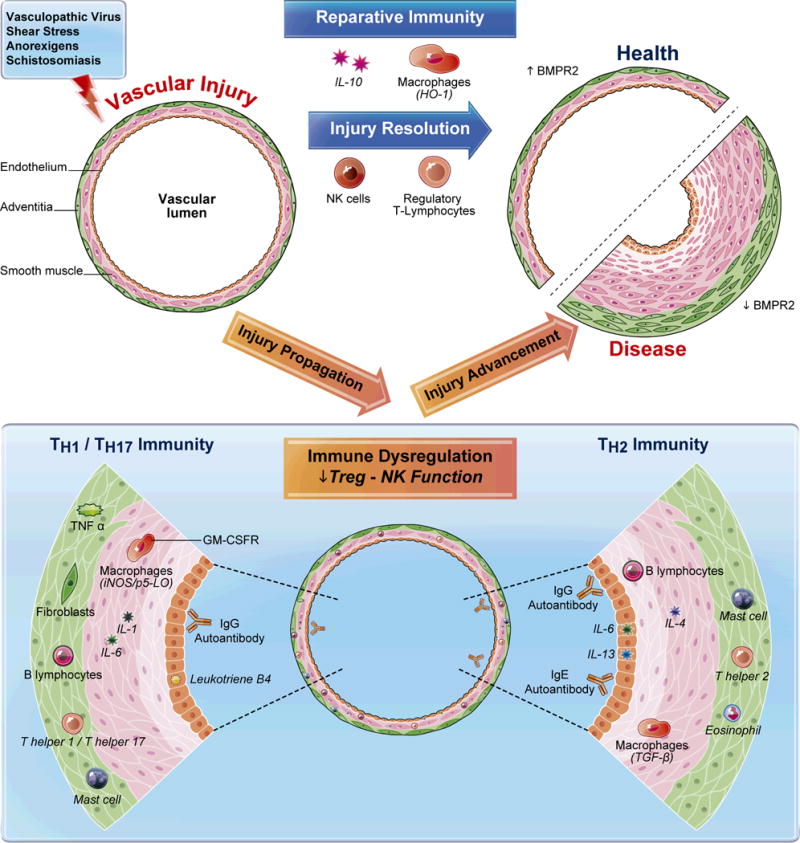
A variety of stimuli can be injurious to pulmonary arterioles; a health-promoting immune response will resolve inflammation after the stimuli has been contained and eliminated. With normal reparative immunity, inflammation is prevented from becoming inappropriately-exuberant and prolonged by the regulatory activity of NK cells and Tregs. In processes not well understood, adaptive immune responses (T cells, B cells) driven by antigen-specific signals coordinate with evolutionarily-ancient innate immune responses (NK cells, macrophages) to dampen immunity once the ‘danger' of the vascular injury has passed. This process may involve the skewing of immune responses with anti-inflammatory molecules, such as IL-10 and HO-1, and the induction of BMPR2; the result is the restoration of vascular health. However, when there is a genetic or acquired predisposition to immune dysregulation, or if the vascular injury itself drives a poorly regulated response (e.g., schistosomiasis infection), vascular injury is not quickly resolved. A useful oversimplification of this situation divides the dysregulated response into two subtypes: TH1/TH17 and TH2 immunity. In the TH1/TH17-skewed response, the arteriole may be invaded by mononuclear cells, including cytotoxic T cells, autoreactive B cells, autoantibodies, mast cells, and activated macrophages expressing GM-CSFR, iNOS and LTB4. Injurious cytokines liberated in this milieu include TNF-α, IL-1 and IL-6. Additionally, adventitial fibroblasts may play a unique role in skewing and maintaining dysregulated immunity. In TH2-driven responses, there are unique inflammatory patterns. TGF-β-mediated immunity, linked closely with enhanced IL-4 and IL-13 activity, drive a destructive eosinophil and mast cell-rich perivascular infiltrate. IL-6 can be vasoprotective in this setting in contrast to the TH1/TH17-skewed response, where it is likely harmful.90 With unmitigated immune dysregulation, inflammation is not resolved in a health-promoting manner, and pulmonary vascular disease ensues.
TH1/TH17 and TH2 Immunity in PAH
The effector responses of CD4+ T cells are roughly divided into TH1, TH2 and TH17 responses; all are important in the pathogenesis of autoimmune disorders87. TH17 effector cells are induced in parallel to TH1 (producing IFN-γ, TNFs and IL-2), and, like TH1, polarized TH17 cells have the capacity to cause inflammation and autoimmune disease. Both TH1 and TH17 co-localize regionally and may require each other for recruitment into the region88. In addition to IL-17, Th17 cells produce IL-6, TNF-α, GM-CSF, IL-21, and IL-22. By distinction, Th2 cells, which produce the cytokines IL-4, IL-5, and IL-13, are involved in allergic responses and the clearance of extracellular pathogens, such as worms. When immune dysregulation favors TH1/TH17 immunity reactions, TNF-α and IL-6 appear as harmful mediators promoting vascular remodeling16, 89, whereas IL-6 may exert a protective effect in pulmonary vascular injury induced by Schistosomiasis90. Plasma IL-13 is associated with PAH in systemic sclerosis91 and overexpressing IL-13 induces experimental PH92. Figure 2 postulates that when immune dysregulation occurs, perhaps due to a deficiency in normal Treg and/or regulatory NK function, there can be a skewing towards TH1/TH17 immunity including a predominance of LTB4-secreting GM-CSFR+ macrophages that can directly induce vascular injury by inducing EC apoptosis, and smooth muscle hypertrophy and proliferation. Alternatively, such as in the case of Schistosomiasis, TH2 immunity also induces vascular remodeling, but apparently by unique pathways.
Neutrophils and Neutrophil Elastase
Little attention has been given to the neutrophil in the pathogenesis of PAH, but it is evident both in experimental and clinical studies that neutrophil elastase can influence pathogenesis. We have recently shown enhanced neutrophil elastase in smooth muscle cells from patients with IPAH93 and experimental models of PH, including genetic models such as the S100A4/Mts1 overexpressing mouse, where the elastase inhibitor elafin repressed the development and progression of PH93. Elevated neutrophil elastase is also present in pulmonary artery smooth muscle cells from mice exposed to chronic hypoxia94. Repressing neutrophil elastase both arrests progression and induces regression of experimentally induced PH secondary to injection of the toxin monocrotaline95. In addition to the release of biologically active growth factors from the extracellular matrix by elastase96,97, elastin and fibronectin fragments that result from elastase activity are highly chemoattractant98, and elastase can also activate components of the complement system, further contributing to the immune inflammatory response99.
Therapeutic Considerations
While more clearly needs to be understood with respect to the immune/inflammatory component of PAH, a number of therapeutic approaches may prove sufficient to prevent progression of disease. Elastase inhibition is one strategy that has proven effective in reversing advanced PH in the inflammatory monocrotaline model, and a highly selective elastase inhibitor, human recombinant elafin, has received FDA approval as an orphan drug to treat PAH. Elafin also suppresses NFkB activation and the subsequent inflammatory response associated with experimental PH100. We have recently shown that low dose FK506 not only is an immunosuppressant, but also can reverse the severe PH in the SUGEN/hypoxia model, in keeping with its other function of activating the BMPR2 receptor signaling by removing FKBP12 from the BMPR type 1 co-receptor101. In human endothelial cells from patients with PAH, low dose FK506101 improved function by restoring BMPR2 signaling as evidenced by elevated pSMAD, Id1 and apelin. FK506 activated this pathway in PAH cells whereas the normal ligand BMP4, failed to do so Inhibition of the hydrolase that produces leukotriene B4 by bestatin can in fact reverse PH in the athymic rat/SUGEN and monocrotaline models79, and this compound has a long record of clinical use with minimal toxicity102, 103. B cell depletion with rituximab is another strategy that is being investigated in patients with PAH associated with systemic sclerosis, in a currently-enrolling NIH trial. Apelin can promote endothelial cell homeostasis and is in the clinic to treat heart failure104. Apelin, like elafin and like naturally occurring PPARγ adducts (nitro fatty acids)19, can also inhibit NFkB105, and induce Nrf2, the transcription factor for antioxidant genes106 such as hemoxygenase 1. Antioxidants that revert vascular cells to a normal metabolic phenotype, such as dicholoroacetate, show promise. HDAC1 inhibitors, tested so far in the experimental setting can revert activated to normal fibroblasts and can prevent adverse interactions with macrophages84. Although inherently anti-inflammatory, TGF-β is a pleiotropic cytokine, that is also associated with TH2 immunity, endothelial injury, and now implicated in the development of PH associated with Schistosomiasis mansoni infection90. Other experimental studies previously described in this review, have used GM-CSF inhibition to show that suppression of macrophage recruitment can prevent hypoxia induced pulmonary hypertension28. Table 2 highlights pre-clinical studies that have found a pathogenic role for inflammation in the development of PH and the responsiveness to therapeutics. Table 3 highlights ongoing clinical trials employing immunotherapies to reverse pulmonary hypertension.
Table 2. Selected Pre-Clinical Studies Demonstrating Role for Immunity in PH.
| Pre-Clinical ImmuneTarget | Drug | PH Model | Prevented PH | Reversed PH | Ref. | |
|---|---|---|---|---|---|---|
| IL-6 | n/a | Spontaneous PH in Mice Overexpressing IL-6 | n/a | n/a | 16 | |
| TNF-α | n/a | Spontaneous PH in Mice Overexpressing TNF-α | n/a | n/a | 108 | |
| OX-40L | n/a | Spontaneous PH in Mice Overexpressing OX-40L | n/a | n/a | 131 | |
| CD20 | Anti-Rat CD20 | SU5416/Ovalbumin Rat | X | 132 | ||
| IL-1 | IL-1 receptor antagonist | Monocrotaline Rat | X | 133 | ||
| TGF-β | TGF-β | 1D11 | Schistosoma Mouse | X | 90 | |
| ALK5 | SB431542/LY364947 | |||||
| Purine synthesis | Mycophenolate mofetil | Monocrotaline Rat | X | 134 | ||
| Target(s) of Steroids | Dexamethasone | Monocrotaline Rat | X | X | 135, 136 | |
| Target of Rapamycin (TOR) | Rapamycin | Monocrotaline Pneumonectomy Rat | X | X | 137 | |
| NFAT | Cyclosporine | Moncrotaline Rat, Hypoxia Rat | X | X | 138, 139 | |
| FK506 | SU5416/Hypoxia Rat | X | X | 101 | ||
| TRAIL | TRAIL-antibodies | Monocrotaline Rat, Hypoxia Mouse, ApoE-/- Mouse | X | 140 | ||
| 5-LO/FLAP | MK886 | Hypoxic Mouse, Monocrotaline Adeno-5-LO Rat | X | 141, 142 | ||
| Zileuton | Monocrotaline/Adeno-5-LO Rat | X | 141 | |||
| LTB4 | BLT1 | LY293111/ONO4057 | SU5416/Athymic Rat, Monocrotaline Rat | X | X | 79, 143 |
| LTA4H | Bestatin | SU5416/Athymic Rat, Monocrotaline Rat | X | 79 | ||
| JNJ-26993135 | ||||||
Table 3. Currently-Enrolling Clinical Trials in Pulmonary Hypertension that Target Immunity.
| Drug Intervention | Drug Target | Patient Population | Clinical Trial Sponsor and Collaborator |
|---|---|---|---|
| FK506 | NFAT Inhibitor | PAH | Stanford University |
| Rituximab | CD20+ B cells | Systemic Sclerosis PAH | National Institute of Allergy and Infectious Disease/Division of Allergy, Immunology, and Transplantation |
| Anakinra | Interleukin-1 Receptor Antagonist | PAH | Virginia Commonwealth University |
While the targets of harmful immunity are numerous in PAH, some may prove ineffective, and, for others, the risk/benefit ratio is likely unacceptable. For example, targeting TGF-β, may limit certain TH2-mediated processes90, but could, if given chronically, be pro-inflammatory and harmful. On the other hand, directly addressing TH2 cytokines IL-4, IL-5 and IL-13 could, in the correct PAH subtype, prove highly useful as suggested by protection from disease in genetic knockout studies (e.g. Graham et al.107). While TNF-α appears to be clearly implicated in PAH pathogenesis108, the results of antagonizing this cytokine has been mixed in pre-clinical studies109, 110 and it predisposes patients to severe infectious complications such as tuberculosis. Some approaches that are putatively useful for limited duration, such as i.v. cyclophosphamide or high dose steroids, must be balanced against the risks of profound systemic immunosuppression if considered as a more chronic therapy. Finally, different subtypes of PAH are characterized by distinct inflammatory profiles, a phenomenon which suggests that immunotherapies should be tailored to each patient's disease. In this respect, the addition of adjunctive immunotherapies will likely require higher selectivity than what is currently required for vasodilators. Targeting IL-13 may be appropriate in a helminthic disease causing TH2 immunity whereas therapies addressing B cell immunity will likely be more effective for a disease process dominated by pathogenic antibodies (and so on).
Factors Linking Genetics, Inflammation and Metabolism
Recently, a call for a unifying theory of PAH pathogenesis was made to the research community to consolidate the ever growing diversity of scientific findings. To this end, we here consider immunity as a unifying hypothesis explaining this disease. Why does inflammation cause PAH, and how is it linked to all the other non-inflammatory and genetic factors implicated in disease pathogenesis? There are many ways of looking at this. If we start with the BMPR2 mutation, we now know that downstream effectors of BMPR2 signaling in both endothelial and smooth muscle cells can activate PPARγ mediated gene regulation111,19. The targets of PPARγ such as apelin and PGC-1α profoundly affect inflammation (macrophage recruitment)112 and metabolism113. Dysregulated immunity (as illustrated in Figure 2) can arise from other genetic causes (such as, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED))114, viruses (such as HIV), or may be an idiopathic process (such as with the connective-tissue diseases). Dysregulated immunity may occur when the normal means of controlling inflammation via Tregs is functionally impaired7. When there is a predilection to harmful immunity, an injury to the pulmonary circulation, whether induced by a vasculopathic virus or through altered shear stress or by local hypoxia, transforms a self-limited inflammatory response into a propagated injurious process. For some conditions, inflammation is limited to the lungs, such as in idiopathic PAH, while for other conditions, such as in systemic sclerosis, the vasculopathy is systemic. It is possible that the differences observed between local and systemic presentations reflect processes that are, variably, either antigen-specific or non-antigen specific. A clear case of localized injury is the TH2 adaptive immune response mounted against Schistosomiasis eggs physically lodged in the pulmonary circulation whereas a non-antigen specific cause may possibly apply to anorexigen-induced PAH. Inflammation confined to lungs suggests that the injury occurs there specifically due to unique aspects of the pulmonary circulation and/or autoantigen localization. In scleroderma associated with PAH there is likely a global physiologic derangement perhaps reflecting more widespread autoantigen presence. In this manner, the concept of inherited or acquired immune dysregulation could explain the protean manifestations of PAH.
Two themes form the focus of this PAH compendium: inflammation and metabolism. A cogent case has been made for how abnormal metabolism is a separate and credible unifying theory of PAH pathogenesis115, and because the two unifying theories of inflammation and metabolism in PAH aren't mutually exclusive, an important future goal of research will be to show the relationship between dysregulated immunity and altered metabolism in the development of this disease. Inflamed tissues are characterized by significant changes in metabolic activity that are attributable to the recruitment of monocytes and neutrophils, in addition to locally-proliferating lymphocyte populations116, 117. Immune cells, implicated in PAH pathogenesis, obtain energy by different means. Lymphocytes predominantly use oxidative phosphorylation, whereas myeloid lineage cells derive their energy almost exclusively from glycolysis118, 119. Lymphocytes divide and quiesce in accordance with strictly controlled levels of essential metabolites that support anabolic growth118, whereas innate immune cell survival is dependent on different metabolic cues. For example, neutrophil mitochondria maintain a transmembrane potential via the glycerol-3-phosphate shuttle leading to increased aerobic glycolysis (a peculiar mitochondrial phenotype also observed in the lung vasculature and right ventricle of PAH patients); this pattern of increased aerobic glycolysis is acquired during neutrophil differentiation from myeloid precursors116. The differences in the metabolic requirements of adaptive and innate immune cells can help shape the characteristics of the immune response in a specific microenvironment. Dysregulated immunity observed in PAH may directly lead to alterations in mitochondrial metabolism, and, conversely, altered mitochondrial energetics in cardiopulmonary tissue may directly influence participating immune cells. Beyond cell energy utilization, inflammation is linked to other facets of metabolic derangement including insulin resistance. Macrophages are highly implicated in PAH pathogenesis28,79, and their infiltration into adipose tissue is associated with insulin resistance120, 121, which is also a clinical feature of PAH122. In summary, these studies cumulatively illustrate how bridging research in the fields of genetics, metabolism and immunity will yield a more holistic theory of PAH pathogenesis with perhaps stronger insights than those afforded by separate approaches to the problem.
What does this mean for the patient or clinician?
The focus on inflammation and altered immunity in pulmonary arterial hypertension lends itself to a whole new strategy for preventing and potentially reversing disease progression
Supplementary Material
A Patient Asks Questions….
I read that there is a lot of inflammation in my blood and in my lungs, as if I had an infection, but I don't have fever. What does this mean and how important is it for my condition?
Inflammation is now increasingly recognized to be very important for the development of PAH. Although some viruses have been implicated as potential triggers of PAH, this inflammation is not due to infection. Rather, it is due to activation of white blood cells by still unknown causes. Yet, we do now know that inflammation in the blood and around the lung vessels in PAH patients (i.e. accumulation of activated white blood cells around and within the lung blood vessels) may explain many of the features of the disease, including the overgrowth of the cells in the wall of the lung blood vessels. Blood cells that go through the lungs are activated and secrete substances (called cytokines) designed to organize our defense and response to infections. In this case however they have adverse effects in the lung blood vessels. It is not known why in PAH they may affect the lung blood vessels but not blood vessels outside the lungs. This activation of white blood cells is important but low grade, perhaps explaining why PAH patients do not have a fever. It may however cause weakness and malaise, similar to the malaise one feels during a viral infection, contributing to the PAH clinical symptoms.
Animal work has shown that normalizing the function of some of these white blood cells can also normalize PAH, opening a new window in the treatment of the disease. It is also promising in terms of developing accurate tools to monitor the activity of the disease. Clinical trials are ongoing with several approaches designed to selectively inhibit the function of white blood cells and hold promise that they may reverse PAH without increasing susceptibility to infections in the treated patients.
Together with the “metabolic theory” –see previous article- this field is in the cutting edge of PAH research. Like in the case with cancer –discussed in the previous article- the PAH researchers can take advantage of important clues discovered from inflammation research in other more common and intensely researched diseases, like infection, autoimmune diseases or treatment of transplanted patients.
For the case description, see introductory article by E.D. Michelakis, page xxx
Acknowledgments
Sources of Funding: Funded in part by ANR Starting Grant 2012: “EPINE” ANR-12-JSV1-0004-01 (C Guignabert), NIH grants HL082662 (MR Nicolls) and NHLBI-HV-10-05 (MR Nicolls, M Rabinovitch).
Footnotes
Disclosures: The authors have no conflicts of interest to disclose
Contributor Information
Marlene Rabinovitch, Cardiovascular Institute and Department of Pediatrics, LabEx LERMIT, Centre Chirurgical Marie Lannelongue, Le Plessis-Robinson and Université Paris-Sud, School of Medicine, Le Kremlin-Bicêtre.
Christophe Guignabert, Department of Medicine, Stanford University School of Medicine, LabEx LERMIT, Centre Chirurgical Marie Lannelongue, Le Plessis-Robinson and Université Paris-Sud, School of Medicine, Le Kremlin-Bicêtre.
Marc Humbert, INSERM UMR_S 999, LabEx LERMIT, Centre Chirurgical Marie Lannelongue, Le Plessis-Robinson and Université Paris-Sud, School of Medicine, Le Kremlin-Bicêtre.
Mark R Nicolls, AP-HP, Service de Pneumologie, Centre de Référence de l'Hypertension Pulmonaire Sévère, DHU Thorax Innovation, Hôpital de Bicêtre, France.
References
- 1.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 2.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: A perspective. Eur Respir J. 2005;26:1110–1118. doi: 10.1183/09031936.05.00045705. [DOI] [PubMed] [Google Scholar]
- 3.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Teng X, Hassoun PM, Yang SC, Champion HC, Tuder RM, Johns RA. Resistin-like molecule-{beta} (relm{beta}) in scleroderma-associated pulmonary hypertension. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2008-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perros F, Dorfmuller P, Montani D, Hammad H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S, Cohen-Kaminsky S, Humbert M, Lambrecht BN. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:311–321. doi: 10.1164/rccm.201105-0927OC. [DOI] [PubMed] [Google Scholar]
- 5.Sutendra G, Dromparis P, Bonnet S, Haromy A, McMurtry MS, Bleackley RC, Michelakis ED. Pyruvate dehydrogenase inhibition by the inflammatory cytokine tnfalpha contributes to the pathogenesis of pulmonary arterial hypertension. J Mol Med (Berl) 89:771–783. doi: 10.1007/s00109-011-0762-2. [DOI] [PubMed] [Google Scholar]
- 6.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamosiuniene R, Tian W, Dhillon G, Wang L, Sung YK, Gera L, Patterson AJ, Agrawal R, Rabinovitch M, Ambler K, Long CS, Voelkel NF, Nicolls MR. Regulatory t cells limit vascular endothelial injury and prevent pulmonary hypertension. Circulation research. 2011;109:867–879. doi: 10.1161/CIRCRESAHA.110.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molossi S, Clausell N, Rabinovitch M. Reciprocal induction of tumor necrosis factor-α and interleukin-1β activity mediates fibronectin synthesis in coronary artery smooth muscle cells. J Cell Physiol. 1995;163:19–29. doi: 10.1002/jcp.1041630104. [DOI] [PubMed] [Google Scholar]
- 9.Jones PL, Cowan KN, Rabinovitch M. Tenascin-c, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. The American journal of pathology. 1997;150:1349–1360. [PMC free article] [PubMed] [Google Scholar]
- 10.Courboulin A, Tremblay VL, Barrier M, Meloche J, Jacob MH, Chapolard M, Bisserier M, Paulin R, Lambert C, Provencher S, Bonnet S. Kruppel-like factor 5 contributes to pulmonary artery smooth muscle proliferation and resistance to apoptosis in human pulmonary arterial hypertension. Respir Res. 2011;12:128. doi: 10.1186/1465-9921-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151:1628–1631. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 12.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J, Morrell NW. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–927. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 13.Lee JG, Kay EP. Common and distinct pathways for cellular activities in fgf-2 signaling induced by il-1beta in corneal endothelial cells. Investigative ophthalmology & visual science. 2009;50:2067–2076. doi: 10.1167/iovs.08-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izikki M, Guignabert C, Fadel E, Humbert M, Tu L, Zadigue P, Dartevelle P, Simonneau G, Adnot S, Maitre B, Raffestin B, Eddahibi S. Endothelial-derived fgf2 contributes to the progression of pulmonary hypertension in humans and rodents. J Clin Invest. 2009;119:512–523. doi: 10.1172/JCI35070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, McLean DL, Park H, Comhair SA, Greif DM, Erzurum SC, Chun HJ. An endothelial apelin-fgf link mediated by mir-424 and mir-503 is disrupted in pulmonary arterial hypertension. Nature medicine. 2013;19:74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circulation research. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. 228p following 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savale L, Tu L, Rideau D, Izziki M, Maitre B, Adnot S, Eddahibi S. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res. 2009;10:6. doi: 10.1186/1465-9921-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu L, De Man FS, Girerd B, Huertas A, Chaumais MC, Lecerf F, Francois C, Perros F, Dorfmuller P, Fadel E, Montani D, Eddahibi S, Humbert M, Guignabert C. A critical role for p130cas in the progression of pulmonary hypertension in humans and rodents. Am J Respir Crit Care Med. 2012;186:666–676. doi: 10.1164/rccm.201202-0309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alastalo TP, Li M, de Jesus Perez V, Pham D, Sawada H, Wang JK, Koskenvuo M, Wang L, Freeman BA, Chang HY, Rabinovitch M. Disruption of ppargamma/beta-catenin-mediated regulation of apelin impairs bmp-induced mouse and human pulmonary arterial ec survival. J Clin Invest. 2011;121:3735–3746. doi: 10.1172/JCI43382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene pph1) is caused by mutations in the bone morphogenetic protein receptor-ii gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Loyd JE, III, Nichols WC, Trembath RC. Heterozygous germline mutations in bmpr2, encoding a tgf-beta receptor, cause familial primary pulmonary hypertension. Nature Genetics. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type ii bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 23.Park SH, Chen WC, Hoffman C, Marsh LM, West J, Grunig G. Modification of hemodynamic and immune responses to exposure with a weak antigen by the expression of a hypomorphic bmpr2 gene. PLoS One. 2013;8:e55180. doi: 10.1371/journal.pone.0055180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mushaben EM, Hershey GK, Pauciulo MW, Nichols WC, Le Cras TD. Chronic allergic inflammation causes vascular remodeling and pulmonary hypertension in bmpr2 hypomorph and wild-type mice. PLoS One. 2012;7:e32468. doi: 10.1371/journal.pone.0032468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies RJ, Holmes AM, Deighton J, Long L, Yang X, Barker L, Walker C, Budd DC, Upton PD, Morrell NW. Bmp type ii receptor deficiency confers resistance to growth inhibition by tgf-beta in pulmonary artery smooth muscle cells: Role of proinflammatory cytokines. Am J Physiol Lung Cell Mol Physiol. 2012;302:L604–615. doi: 10.1152/ajplung.00309.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song Y, Coleman L, Shi J, Beppu H, Sato K, Walsh K, Loscalzo J, Zhang YY. Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous bmpr2-mutant mice. American journal of physiology. Heart and circulatory physiology. 2008;295:H677–690. doi: 10.1152/ajpheart.91519.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagen M, Fagan K, Steudel W, Carr M, Lane K, Rodman DM, West J. Interaction of interleukin-6 and the bmp pathway in pulmonary smooth muscle. American journal of physiology. Lung cellular and molecular physiology. 2007;292:L1473–1479. doi: 10.1152/ajplung.00197.2006. [DOI] [PubMed] [Google Scholar]
- 28.Sawada H, Saito T, Nickel NP, Alastalo TP, Glotzbach JP, Chan R, Haghighat L, Fuchs G, Januszyk M, Cao A, Lai YJ, de Jesus Perez V, Kim YM, Wang L, Chen PI, Speikerkoetter E, Mitani Y, Gurtner GC, Sarnow P, Rabinovitch M. Reduced bmpr2 expression induces gm-csf translation and macrophage recruitment in humand and mice to exacerbate pulmonary hypertension. J Exp Med. 2014 doi: 10.1084/jem.20111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hager-Theodorides AL, Outram SV, Shah DK, Sacedon R, Shrimpton RE, Vicente A, Varas A, Crompton T. Bone morphogenetic protein 2/4 signaling regulates early thymocyte differentiation. J Immunol. 2002;169:5496–5504. doi: 10.4049/jimmunol.169.10.5496. [DOI] [PubMed] [Google Scholar]
- 30.Lu L, Ma J, Wang X, Wang J, Zhang F, Yu J, He G, Xu B, Brand DD, Horwitz DA, Shi W, Zheng SG. Synergistic effect of tgf-beta superfamily members on the induction of foxp3+ treg. Eur J Immunol. 2010;40:142–152. doi: 10.1002/eji.200939618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, Scerbavicius R, Burns N, Cool C, Wood K, Parr JE, Boackle SA, Voelkel NF. Absence of t cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med. 2007;175:1280–1289. doi: 10.1164/rccm.200608-1189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golembeski SM, West J, Tada Y, Fagan KA. Interleukin-6 causes mild pulmonary hypertension and augments hypoxia-induced pulmonary hypertension in mice. Chest. 2005;128:572S–573S. doi: 10.1378/chest.128.6_suppl.572S-a. [DOI] [PubMed] [Google Scholar]
- 33.Miyata M, Sakuma F, Yoshimura A, Ishikawa H, Nishimaki T, Kasukawa R. Pulmonary hypertension in rats. 2. Role of interleukin-6. Int Arch Allergy Immunol. 1995;108:287–291. doi: 10.1159/000237166. [DOI] [PubMed] [Google Scholar]
- 34.Cracowski JL, Chabot F, Labarère J, Faure P, Degano B, Schwebel C, Chaouat A, Reynaud-Gaubert M, Cracowski C, Sitbon O, Yaici A, Simonneau G, Humbert M. Proinflammatory cytokine levels are linked with death in pulmonary arterial hypertension. European Respiratory Journal. 2013 doi: 10.1183/09031936.00151313. [DOI] [PubMed] [Google Scholar]
- 35.Condliffe R, Pickworth JA, Hopkinson K, Walker SJ, Hameed AG, Suntharaligam J, Soon E, Treacy C, Pepke-Zaba J, Francis SE, Crossman DC, Newman CM, Elliot CA, Morton AC, Morrell NW, Kiely DG, Lawrie A. Serum osteoprotegerin is increased and predicts survival in idiopathic pulmonary arterial hypertension. Pulm Circ. 2012;2:21–27. doi: 10.4103/2045-8932.94819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawrie A, Waterman E, Southwood M, Evans D, Suntharalingam J, Francis S, Crossman D, Croucher P, Morrell N, Newman C. Evidence of a role for osteoprotegerin in the pathogenesis of pulmonary arterial hypertension. The American journal of pathology. 2008;172:256–264. doi: 10.2353/ajpath.2008.070395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu L, Huertas A, Le Hiress M, Ricard N, Phan C, Fadel E, Seferian A, Montani D, Simonneau G, Humbert M, Guignabert C. Mif/cd74-dependent interleukin-6 and monocyte chemoattractant protein-1 secretion by pulmonary endothelial cells in idiopathic pulmonary hypertension. American Thoracic Society. 2013:A1739. [Google Scholar]
- 38.Damico R, Simms T, Kim BS, Tekeste Z, Amankwan H, Damarla M, Hassoun PM. P53 mediates cigarette smoke-induced apoptosis of pulmonary endothelial cells: Inhibitory effects of macrophage migration inhibitor factor. Am J Respir Cell Mol Biol. 2011;44:323–332. doi: 10.1165/rcmb.2009-0379OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guignabert C, Montani D. Key roles of src family tyrosine kinases in the integrity of the pulmonary vascular bed. Eur Respir J. 2013;41:3–4. doi: 10.1183/09031936.00091912. [DOI] [PubMed] [Google Scholar]
- 40.Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. Mif signal transduction initiated by binding to cd74. J Exp Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagaraj C, Tang B, Balint Z, Wygrecka M, Hrzenjak A, Kwapiszewska G, Stacher E, Lindenmann J, Weir EK, Olschewski H, Olschewski A. Src tyrosine kinase is crucial for potassium channel function in human pulmonary arteries. Eur Respir J. 2013;41:85–95. doi: 10.1183/09031936.00211811. [DOI] [PubMed] [Google Scholar]
- 42.Stein R, Mattes MJ, Cardillo TM, Hansen HJ, Chang CH, Burton J, Govindan S, Goldenberg DM. Cd74: A new candidate target for the immunotherapy of b-cell neoplasms. Clin Cancer Res. 2007;13:5556s–5563s. doi: 10.1158/1078-0432.CCR-07-1167. [DOI] [PubMed] [Google Scholar]
- 43.Tu L, Dewachter L, Gore B, Fadel E, Dartevelle P, Simonneau G, Humbert M, Eddahibi S, Guignabert C. Autocrine fibroblast growth factor-2 signaling contributes to altered endothelial phenotype in pulmonary hypertension. Am J Respir Cell Mol Biol. 2011;45:311–322. doi: 10.1165/rcmb.2010-0317OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. Mif is a noncognate ligand of cxc chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 45.Zhang B, Luo Y, Liu ML, Wang J, Xu DQ, Dong MQ, Liu Y, Xu M, Dong HY, Zhao PT, Gao YQ, Li ZC. Macrophage migration inhibitory factor contributes to hypoxic pulmonary vasoconstriction in rats. Microvasc Res. 2012;83:205–212. doi: 10.1016/j.mvr.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Talwar A, Tsang D, Bruchfeld A, Sadoughi A, Hu M, Omonuwa K, Cheng KF, Al-Abed Y, Miller EJ. Macrophage migration inhibitory factor mediates hypoxia-induced pulmonary hypertension. Mol Med. 2012;18:215–223. doi: 10.2119/molmed.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang B, Shen M, Xu M, Liu LL, Luo Y, Xu DQ, Wang YX, Liu ML, Liu Y, Dong HY, Zhao PT, Li ZC. Role of macrophage migration inhibitory factor in the proliferation of smooth muscle cell in pulmonary hypertension. Mediators Inflamm. 2012;2012:840737. doi: 10.1155/2012/840737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ormiston ML, Chang C, Long LL, Soon E, Jones D, Machado R, Treacy C, Toshner MR, Campbell K, Riding A, Southwood M, Pepke-Zaba J, Exley A, Trembath RC, Colucci F, Wills M, Trowsdale J, Morrell NW. Impaired natural killer cell phenotype and function in idiopathic and heritable pulmonary arterial hypertension. Circulation. 2012;126:1099–1109. doi: 10.1161/CIRCULATIONAHA.112.110619. [DOI] [PubMed] [Google Scholar]
- 49.Perros F, Cohen-Kaminsky S, Gambaryan N, Girerd B, Raymond N, Klingelschmitt I, Huertas A, Mercier O, Fadel E, Simonneau G, Humbert M, Dorfmuller P, Montani D. Cytotoxic cells and granulysin in pulmonary arterial hypertension and pulmonary veno-occlusive disease. Am J Respir Crit Care Med. 2013;187:189–196. doi: 10.1164/rccm.201208-1364OC. [DOI] [PubMed] [Google Scholar]
- 50.Bauer EM, Zheng H, Comhair S, Erzurum S, Billiar TR, Bauer PM. Complement c3 deficiency attenuates chronic hypoxia-induced pulmonary hypertension in mice. PLoS One. 2011;6:e28578. doi: 10.1371/journal.pone.0028578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dib H, Tamby MC, Bussone G, Regent A, Berezne A, Lafine C, Broussard C, Simonneau G, Guillevin L, Witko-Sarsat V, Humbert M, Mouthon L. Targets of anti-endothelial cell antibodies in pulmonary hypertension and scleroderma. Eur Respir J. 2012;39:1405–1414. doi: 10.1183/09031936.00181410. [DOI] [PubMed] [Google Scholar]
- 52.Tamby MC, Humbert M, Guilpain P, Servettaz A, Dupin N, Christner JJ, Simonneau G, Fermanian J, Weill B, Guillevin L, Mouthon L. Antibodies to fibroblasts in idiopathic and scleroderma-associated pulmonary hypertension. Eur Respir J. 2006;28:799–807. doi: 10.1183/09031936.06.00152705. [DOI] [PubMed] [Google Scholar]
- 53.Rich S, Kieras K, Hart K, Groves BM, Stobo JD, Brundage BH. Antinuclear antibodies in primary pulmonary hypertension. J Am Coll Cardiol. 1986;8:1307–1311. doi: 10.1016/s0735-1097(86)80301-1. [DOI] [PubMed] [Google Scholar]
- 54.Tamby MC, Chanseaud Y, Humbert M, Fermanian J, Guilpain P, Garcia-de-la-Pena-Lefebvre P, Brunet S, Servettaz A, Weill B, Simonneau G, Guillevin L, Boissier MC, Mouthon L. Anti-endothelial cell antibodies in idiopathic and systemic sclerosis associated pulmonary arterial hypertension. Thorax. 2005;60:765–772. doi: 10.1136/thx.2004.029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bordron A, Dueymes M, Levy Y, Jamin C, Leroy JP, Piette JC, Shoenfeld Y, Youinou PY. The binding of some human antiendothelial cell antibodies induces endothelial cell apoptosis. J Clin Invest. 1998;101:2029–2035. doi: 10.1172/JCI2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carvalho D, Savage CO, Black CM, Pearson JD. Igg antiendothelial cell autoantibodies from scleroderma patients induce leukocyte adhesion to human vascular endothelial cells in vitro. Induction of adhesion molecule expression and involvement of endothelium-derived cytokines. J Clin Invest. 1996;97:111–119. doi: 10.1172/JCI118377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arends SJ, Damoiseaux JG, Duijvestijn AM, Debrus-Palmans L, Vroomen M, Boomars KA, Brunner-La Rocca HP, Reutelingsperger CP, Cohen Tervaert JW, van Paassen P. Immunoglobulin g anti-endothelial cell antibodies: Inducers of endothelial cell apoptosis in pulmonary arterial hypertension? Clin Exp Immunol. 2013;174:433–440. doi: 10.1111/cei.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishizuka Y, Sakakura T. Thymus and reproduction: Sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–755. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 59.Kim JM, Rasmussen JP, Rudensky AY. Regulatory t cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 60.Sakaguchi S. Naturally arising cd4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 61.Huertas A, Tu L, Gambaryan N, Girerd B, Perros F, Montani D, Fabre D, Fadel E, Eddahibi S, Cohen-Kaminsky S, Guignabert C, Humbert M. Leptin and regulatory t-lymphocytes in idiopathic pulmonary arterial hypertension. Eur Respir J. 2012;40:895–904. doi: 10.1183/09031936.00159911. [DOI] [PubMed] [Google Scholar]
- 62.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in hiv infection. Chest. 1991;100:1268–1271. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 63.Radstake TR, van Bon L, Broen J, Wenink M, Santegoets K, Deng Y, Hussaini A, Simms R, Cruikshank WW, Lafyatis R. Increased frequency and compromised function of t regulatory cells in systemic sclerosis (ssc) is related to a diminished cd69 and tgfbeta expression. PLoS One. 2009;4:e5981. doi: 10.1371/journal.pone.0005981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonelli M, Savitskaya A, Steiner CW, Rath E, Smolen JS, Scheinecker C. Phenotypic and functional analysis of cd4+ cd25- foxp3+ t cells in patients with systemic lupus erythematosus. J Immunol. 2009;182:1689–1695. doi: 10.4049/jimmunol.182.3.1689. [DOI] [PubMed] [Google Scholar]
- 65.Covas MI, Esquerda A, Garcia-Rico A, Mahy N. Peripheral blood t-lymphocyte subsets in autoimmune thyroid disease. J Investig Allergol Clin Immunol. 1992;2:131–135. [PubMed] [Google Scholar]
- 66.Mandl T, Bredberg A, Jacobsson LT, Manthorpe R, Henriksson G. Cd4+ t-lymphocytopenia--a frequent finding in anti-ssa antibody seropositive patients with primary sjogren's syndrome. J Rheumatol. 2004;31:726–728. [PubMed] [Google Scholar]
- 67.Perros F, Dorfmuller P, Souza R, Durand-Gasselin I, Mussot S, Mazmanian M, Herve P, Emilie D, Simonneau G, Humbert M. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J. 2007;29:462–468. doi: 10.1183/09031936.00094706. [DOI] [PubMed] [Google Scholar]
- 68.Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin Immunol. 2008;20:26–42. doi: 10.1016/j.smim.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tcherakian C, Rivaud E, Zucman D, Metivier AC, Couderc LJ. Curing hiv-associated pulmonary arterial hypertension. Eur Respir J. 2012;39:1045–1046. doi: 10.1183/09031936.00138211. [DOI] [PubMed] [Google Scholar]
- 70.Degano B, Guillaume M, Savale L, Montani D, Jais X, Yaici A, Le Pavec J, Humbert M, Simonneau G, Sitbon O. Hiv-associated pulmonary arterial hypertension: Survival and prognostic factors in the modern therapeutic era. AIDS. 2010;24:67–75. doi: 10.1097/QAD.0b013e328331c65e. [DOI] [PubMed] [Google Scholar]
- 71.Jais X, Launay D, Yaici A, Le Pavec J, Tcherakian C, Sitbon O, Simonneau G, Humbert M. Immunosuppressive therapy in lupus- and mixed connective tissue disease-associated pulmonary arterial hypertension: A retrospective analysis of twenty-three cases. Arthritis Rheum. 2008;58:521–531. doi: 10.1002/art.23303. [DOI] [PubMed] [Google Scholar]
- 72.Colvin KL, Cripe PJ, Ivy DD, Stenmark KR, Yeager ME. Bronchus-associated lymphoid tissue in pulmonary hypertension produces pathologic autoantibodies. Am J Respir Crit Care Med. 2013;188:1126–1136. doi: 10.1164/rccm.201302-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 74.Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, Klussmann S, Ghofrani HA, Weissmann N, Klepetko W, Banat GA, Seeger W, Grimminger F, Schermuly RT. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:897–908. doi: 10.1164/rccm.201202-0335OC. [DOI] [PubMed] [Google Scholar]
- 75.Thenappan T, Goel A, Marsboom G, Fang YH, Toth PT, Zhang HJ, Kajimoto H, Hong Z, Paul J, Wietholt C, Pogoriler J, Piao L, Rehman J, Archer SL. A central role for cd68(+) macrophages in hepatopulmonary syndrome. Reversal by macrophage depletion. Am J Respir Crit Care Med. 2011;183:1080–1091. doi: 10.1164/rccm.201008-1303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Overbeek MJ, Mouchaers KT, Niessen HM, Hadi AM, Kupreishvili K, Boonstra A, Voskuyl AE, Belien JA, Smit EF, Dijkmans BC, Vonk-Noordegraaf A, Grunberg K. Characteristics of interstitial fibrosis and inflammatory cell infiltration in right ventricles of systemic sclerosis-associated pulmonary arterial hypertension. International journal of rheumatology. 2010;2010 doi: 10.1155/2010/604615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. The American journal of pathology. 2006;168:659–669. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation. 2011;123:1986–1995. doi: 10.1161/CIRCULATIONAHA.110.978627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tian W, Jiang X, Tamosiuniene R, Sung YK, Qian J, Dhillon G, Gera L, Farkas L, Rabinovitch M, Zamanian RT, Inayathullah M, Fridlib M, Rajadas J, Peters-Golden M, Voelkel NF, Nicolls MR. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med. 2013;5:200ra117. doi: 10.1126/scitranslmed.3006674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Porter KM, Walp ER, Elms SC, Raynor R, Mitchell PO, Guidot DM, Sutliff RL. Human immunodeficiency virus-1 transgene expression increases pulmonary vascular resistance and exacerbates hypoxia-induced pulmonary hypertension development. Pulm Circ. 2013;3:58–67. doi: 10.4103/2045-8932.109915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calabrese F, Kipar A, Lunardi F, Balestro E, Perissinotto E, Rossi E, Nannini N, Marulli G, Stewart JP, Rea F. Herpes virus infection is associated with vascular remodeling and pulmonary hypertension in idiopathic pulmonary fibrosis. PLoS One. 2013;8:e55715. doi: 10.1371/journal.pone.0055715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yeager ME, Reddy MB, Nguyen CM, Colvin KL, Ivy DD, Stenmark KR. Activation of the unfolded protein response is associated with pulmonary hypertension. Pulm Circ. 2012;2:229–240. doi: 10.4103/2045-8932.97613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hickey MM, Richardson T, Wang T, Mosqueira M, Arguiri E, Yu H, Yu QC, Solomides CC, Morrisey EE, Khurana TS, Christofidou-Solomidou M, Simon MC. The von hippel-lindau chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J Clin Invest. 2010;120:827–839. doi: 10.1172/JCI36362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, Strassheim D, Meyrick B, Yeager ME, Flockton AR, McKeon BA, Lemon DD, Horn TR, Anwar A, Barajas C, Stenmark KR. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol. 2011;187:2711–2722. doi: 10.4049/jimmunol.1100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Serezani CH, Kane S, Collins L, Morato-Marques M, Osterholzer JJ, Peters-Golden M. Macrophage dectin-1 expression is controlled by leukotriene b4 via a gm-csf/pu.1 axis. J Immunol. 2012;189:906–915. doi: 10.4049/jimmunol.1200257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Damsker JM, Hansen AM, Caspi RR. Th1 and th17 cells: Adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a th17 or a th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, Augustin A, Mason RJ. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. American journal of physiology. Lung cellular and molecular physiology. 2001;280:L39–49. doi: 10.1152/ajplung.2001.280.1.L39. [DOI] [PubMed] [Google Scholar]
- 90.Graham BB, Chabon J, Kumar R, Kolosionek E, Gebreab L, Debella E, Edwards M, Diener K, Shade T, Bifeng G, Bandeira A, Butrous G, Jones K, Geraci M, Tuder RM. Protective role of il6 in vascular remodeling in schistosoma-pulmonary hypertension. Am J Respir Cell Mol Biol. 2013 doi: 10.1165/rcmb.2012-0532OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Christmann RB, Hayes E, Pendergrass S, Padilla C, Farina G, Affandi AJ, Whitfield ML, Farber HW, Lafyatis R. Interferon and alternative activation of monocyte/macrophages in systemic sclerosis-associated pulmonary arterial hypertension. Arthritis Rheum. 2011;63:1718–1728. doi: 10.1002/art.30318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cho WK, Lee CM, Kang MJ, Huang Y, Giordano FJ, Lee PJ, Trow TK, Homer RJ, Sessa WC, Elias JA, Lee CG. Il-13 receptor alpha2-arginase 2 pathway mediates il-13-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2013;304:L112–124. doi: 10.1152/ajplung.00101.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim YM, Haghighat L, Spiekerkoetter E, Sawada H, Alvira CM, Wang L, Acharya S, Rodriguez-Colon G, Orton A, Zhao M, Rabinovitch M. Neutrophil elastase is produced by pulmonary artery smooth muscle cells and is linked to neointimal lesions. The American journal of pathology. 2011;179:1560–1572. doi: 10.1016/j.ajpath.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zaidi SHE, You XM, Ciura S, Husain M, Rabinovitch M. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation. 2002;105:516–521. doi: 10.1161/hc0402.102866. [DOI] [PubMed] [Google Scholar]
- 95.Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M. Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nature medicine. 2000;6:698–702. doi: 10.1038/76282. [DOI] [PubMed] [Google Scholar]
- 96.Thompson K, Kobayashi J, Childs T, Wigle D, Rabinovitch M. Endothelial and serum factors which include apolipoprotein a1 tether elastin to smooth muscle cells inducing serine elastase activity via tyrosine kinase-mediated transcription and translation. J Cell Physiol. 1998;174:78–89. doi: 10.1002/(SICI)1097-4652(199801)174:1<78::AID-JCP9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 97.Thompson K, Rabinovitch M. Exogenous leukocyte and endogenous elastases can mediate mitogenic activity in pulmonary artery smooth muscle cells by release of extracellular-matrix bound basic fibroblast growth factor. J Cell Physiol. 1996;166:495–505. doi: 10.1002/(SICI)1097-4652(199603)166:3<495::AID-JCP4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 98.Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980;66:859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vogt W. Cleavage of the fifth component of complement and generation of a functionally active c5b6-like complex by human leukocyte elastase. Immunobiology. 2000;201:470–477. doi: 10.1016/S0171-2985(00)80099-6. [DOI] [PubMed] [Google Scholar]
- 100.Butler MW, Robertson I, Greene CM, O'Neill SJ, Taggart CC, McElvaney NG. Elafin prevents lipopolysaccharide-induced ap-1 and nf-kappab activation via an effect on the ubiquitin-proteasome pathway. J Biol Chem. 2006;281:34730–34735. doi: 10.1074/jbc.M604844200. [DOI] [PubMed] [Google Scholar]
- 101.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, Haghighat L, de Jesus Perez V, Wang L, Reddy S, Zhao M, Bernstein D, Solow-Cordero DE, Beachy PA, Wandless TJ, Ten Dijke P, Rabinovitch M. Fk506 activates bmpr2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123:3600–3613. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ota K, Kurita S, Yamada K, Masaoka T, Uzuka Y, Ogawa N. Immunotherapy with bestatin for acute nonlymphocytic leukemia in adults. Cancer Immunol Immunother. 1986;23:5–10. doi: 10.1007/BF00205548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ichinose Y, Genka K, Koike T, Kato H, Watanabe Y, Mori T, Iioka S, Sakuma A, Ohta M Group NKLCS. Randomized double-blind placebo-controlled trial of bestatin in patients with resected stage i squamous-cell lung carcinoma. J Natl Cancer Inst. 2003;95:605–610. doi: 10.1093/jnci/95.8.605. [DOI] [PubMed] [Google Scholar]
- 104.Barnes GD, Alam S, Carter G, Pedersen CM, Lee KM, Hubbard TJ, Veitch S, Jeong H, White A, Cruden NL, Huson L, Japp AG, Newby DE. Sustained cardiovascular actions of apj agonism during renin-angiotensin system activation and in patients with heart failure. Circulation. Heart failure. 2013;6:482–491. doi: 10.1161/CIRCHEARTFAILURE.111.000077. [DOI] [PubMed] [Google Scholar]
- 105.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koenitzer JR, Freeman BA. Redox signaling in inflammation: Interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Ann N Y Acad Sci. 2010;1203:45–52. doi: 10.1111/j.1749-6632.2010.05559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Graham BB, Mentink-Kane MM, El-Haddad H, Purnell S, Zhang L, Zaiman A, Redente EF, Riches DW, Hassoun PM, Bandeira A, Champion HC, Butrous G, Wynn TA, Tuder RM. Schistosomiasis-induced experimental pulmonary hypertension: Role of interleukin-13 signaling. The American journal of pathology. 2010;177:1549–1561. doi: 10.2353/ajpath.2010.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fujita M, Mason RJ, Cool C, Shannon JM, Hara N, Fagan KA. Pulmonary hypertension in tnf-alpha-overexpressing mice is associated with decreased vegf gene expression. J Appl Physiol. 2002;93:2162–2170. doi: 10.1152/japplphysiol.00083.2002. [DOI] [PubMed] [Google Scholar]
- 109.Wang Q, Zuo XR, Wang YY, Xie WP, Wang H, Zhang M. Monocrotaline-induced pulmonary arterial hypertension is attenuated by tnf-alpha antagonists via the suppression of tnf-alpha expression and nf-kappab pathway in rats. Vascul Pharmacol. 2013;58:71–77. doi: 10.1016/j.vph.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 110.Henriques-Coelho T, Brandao-Nogueira A, Moreira-Goncalves D, Correia-Pinto J, Leite-Moreira AF. Effects of tnf-alpha blockade in monocrotaline-induced pulmonary hypertension. Rev Port Cardiol. 2008;27:341–348. [PubMed] [Google Scholar]
- 111.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative bmp-2/ppargamma/apoe axis in human and murine smcs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leeper NJ, Tedesco MM, Kojima Y, Schultz GM, Kundu RK, Ashley EA, Tsao PS, Dalman RL, Quertermous T. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. American journal of physiology. Heart and circulatory physiology. 2009 doi: 10.1152/ajpheart.01341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mazzucotelli A, Ribet C, Castan-Laurell I, Daviaud D, Guigne C, Langin D, Valet P. The transcriptional co-activator pgc-1alpha up regulates apelin in human and mouse adipocytes. Regul Pept. 2008;150:33–37. doi: 10.1016/j.regpep.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 114.Korniszewski L, Kurzyna M, Stolarski B, Torbicki A, Smerdel A, Ploski R. Fatal primary pulmonary hypertension in a 30-yr-old female with apeced syndrome. Eur Respir J. 2003;22:709–711. doi: 10.1183/09031936.03.00018203. [DOI] [PubMed] [Google Scholar]
- 115.Sutendra G, Dromparis P, Wright P, Bonnet S, Haromy A, Hao Z, McMurtry MS, Michalak M, Vance JE, Sessa WC, Michelakis ED. The role of nogo and the mitochondria-endoplasmic reticulum unit in pulmonary hypertension. Sci Transl Med. 3:88ra55. doi: 10.1126/scitranslmed.3002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol. 2010;184:4062–4068. doi: 10.4049/jimmunol.0903002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johnson AR, Milner JJ, Makowski L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunological reviews. 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: Energy metabolism and the t-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 119.van Raam BJ, Sluiter W, de Wit E, Roos D, Verhoeven AJ, Kuijpers TW. Mitochondrial membrane potential in human neutrophils is maintained by complex iii activity in the absence of supercomplex organisation. PLoS One. 2008;3:e2013. doi: 10.1371/journal.pone.0002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, Rabinovitch M, Doyle RL. Insulin resistance in pulmonary arterial hypertension. Eur Respir J. 2009;33:318–324. doi: 10.1183/09031936.00000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanchez O, Marcos E, Perros F, Fadel E, Tu L, Humbert M, Dartevelle P, Simonneau G, Adnot S, Eddahibi S. Role of endothelium-derived cc chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2007;176:1041–1047. doi: 10.1164/rccm.200610-1559OC. [DOI] [PubMed] [Google Scholar]
- 124.Balabanian K, Foussat A, Dorfmuller P, Durand-Gasselin I, Capel F, Bouchet-Delbos L, Portier A, Marfaing-Koka A, Krzysiek R, Rimaniol AC, Simonneau G, Emilie D, Humbert M. Cx(3)c chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:1419–1425. doi: 10.1164/rccm.2106007. [DOI] [PubMed] [Google Scholar]
- 125.Perros F, Dorfmuller P, Souza R, Durand-Gasselin I, Godot V, Capel F, Adnot S, Eddahibi S, Mazmanian M, Fadel E, Herve P, Simonneau G, Emilie D, Humbert M. Fractalkine-induced smooth muscle cell proliferation in pulmonary hypertension. Eur Respir J. 2007;29:937–943. doi: 10.1183/09031936.00104706. [DOI] [PubMed] [Google Scholar]
- 126.Cracowski JL, Chabot F, Labarere J, Faure P, Degano B, Schwebel C, Chaouat A, Reynaud-Gaubert M, Cracowski C, Sitbon O, Yaici A, Simonneau G, Humbert M. Proinflammatory cytokine levels are linked with death in pulmonary arterial hypertension. Eur Respir J. 2013 doi: 10.1183/09031936.00151313. [DOI] [PubMed] [Google Scholar]
- 127.Dorfmuller P, Zarka V, Durand-Gasselin I, Monti G, Balabanian K, Garcia G, Capron F, Coulomb-Lhermine A, Marfaing-Koka A, Simonneau G, Emilie D, Humbert M. Chemokine rantes in severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:534–539. doi: 10.1164/ajrccm.165.4.2012112. [DOI] [PubMed] [Google Scholar]
- 128.Heresi GA, Aytekin M, Hammel JP, Wang S, Chatterjee S, Dweik RA. Plasma interleukin-6 adds prognostic information in pulmonary arterial hypertension. Eur Respir J. 2013 doi: 10.1183/09031936.00164713. [DOI] [PubMed] [Google Scholar]
- 129.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, Grunig E, Grunig G. Pulmonary arterial remodeling induced by a th2 immune response. J Exp Med. 2008;205:361–372. doi: 10.1084/jem.20071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hecker M, Zaslona Z, Kwapiszewska G, Niess G, Zakrzewicz A, Hergenreider E, Wilhelm J, Marsh LM, Sedding D, Klepetko W, Lohmeyer J, Dimmeler S, Seeger W, Weissmann N, Schermuly RT, Kneidinger N, Eickelberg O, Morty RE. Dysregulation of the il-13 receptor system: A novel pathomechanism in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:805–818. doi: 10.1164/rccm.200909-1367OC. [DOI] [PubMed] [Google Scholar]
- 131.Rabieyousefi M, Soroosh P, Satoh K, Date F, Ishii N, Yamashita M, Oka M, McMurtry IF, Shimokawa H, Nose M, Sugamura K, Ono M. Indispensable roles of ox40l-derived signal and epistatic genetic effect in immune-mediated pathogenesis of spontaneous pulmonary hypertension. BMC Immunol. 2011;12:67. doi: 10.1186/1471-2172-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mizuno S, Farkas L, Al Husseini A, Farkas D, Gomez-Arroyo J, Kraskauskas D, Nicolls MR, Cool CD, Bogaard HJ, Voelkel NF. Severe pulmonary arterial hypertension induced by su5416 and ovalbumin immunization. Am J Respir Cell Mol Biol. 2012;47:679–687. doi: 10.1165/rcmb.2012-0077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Voelkel NF, Tuder RM, Bridges J, Arend WP. Interleukin-1 receptor antagonist treatment reduces pulmonary hypertension generated in rats by monocrotaline. Am J Resp Cell Mol Biol. 1994;11:664–675. doi: 10.1165/ajrcmb.11.6.7946395. [DOI] [PubMed] [Google Scholar]
- 134.Suzuki C, Takahashi M, Morimoto H, Izawa A, Ise H, Hongo M, Hoshikawa Y, Ito T, Miyashita H, Kobayashi E, Shimada K, Ikeda U. Mycophenolate mofetil attenuates pulmonary arterial hypertension in rats. Biochem Biophys Res Commun. 2006;349:781–788. doi: 10.1016/j.bbrc.2006.08.109. [DOI] [PubMed] [Google Scholar]
- 135.Price LC, Montani D, Tcherakian C, Dorfmuller P, Souza R, Gambaryan N, Chaumais MC, Shao DM, Simonneau G, Howard LS, Adcock IM, Wort SJ, Humbert M, Perros F. Dexamethasone reverses monocrotaline-induced pulmonary arterial hypertension in rats. Eur Respir J. 2011;37:813–822. doi: 10.1183/09031936.00028310. [DOI] [PubMed] [Google Scholar]
- 136.Wang W, Wang YL, Chen XY, Li YT, Hao W, Jin YP, Han B. Dexamethasone attenuates development of monocrotaline-induced pulmonary arterial hypertension. Mol Biol Rep. 2011;38:3277–3284. doi: 10.1007/s11033-010-0390-x. [DOI] [PubMed] [Google Scholar]
- 137.Nishimura T, Faul JL, Berry GJ, Veve I, Pearl RG, Kao PN. 40-o-(2-hydroxyethyl)-rapamycin attenuates pulmonary arterial hypertension and neointimal formation in rats. Am J Respir Crit Care Med. 2001;163:498–502. doi: 10.1164/ajrccm.163.2.2006093. [DOI] [PubMed] [Google Scholar]
- 138.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated t cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci U S A. 2007;104:11418–11423. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koulmann N, Novel-Chate V, Peinnequin A, Chapot R, Serrurier B, Simler N, Richard H, Ventura-Clapier R, Bigard X. Cyclosporin a inhibits hypoxia-induced pulmonary hypertension and right ventricle hypertrophy. Am J Respir Crit Care Med. 2006;174:699–705. doi: 10.1164/rccm.200512-1976OC. [DOI] [PubMed] [Google Scholar]
- 140.Hameed AG, Arnold ND, Chamberlain J, Pickworth JA, Paiva C, Dawson S, Cross S, Long L, Zhao L, Morrell NW, Crossman DC, Newman CM, Kiely DG, Francis SE, Lawrie A. Inhibition of tumor necrosis factor-related apoptosis-inducing ligand (trail) reverses experimental pulmonary hypertension. J Exp Med. 2012;209:1919–1935. doi: 10.1084/jem.20112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jones JE, Walker JL, Song Y, Weiss N, Cardoso WV, Tuder RM, Loscalzo J, Zhang YY. Effect of 5-lipoxygenase on the development of pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol. 2004;286:H1775–1784. doi: 10.1152/ajpheart.00281.2003. [DOI] [PubMed] [Google Scholar]
- 142.Voelkel NF, Tuder RM, Wade K, Hoper M, Lepley RA, Goulet JL, Koller BH, Fitzpatrick F. Inhibition of 5-lipoxygenase-activating protein (flap) reduces pulmonary vascular reactivity and pulmonary hypertension in hypoxic rats. J Clin Invest. 1996;97:2491–2498. doi: 10.1172/JCI118696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tabata T, Ono S, Song C, Noda M, Suzuki S, Tanita T, Fujimura S. role of leukotriene b4 in monocrotaline-induced pulmonary hypertension. Nihon Kyobu Shikkan Gakkai Zasshi. 1997;35:160–166. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


