SUMMARY
Chromosomal translocations disrupting MLL generate MLL-fusion proteins that induce aggressive leukemias. Unexpectedly, MLL-fusion proteins are rarely observed at high levels, suggesting excessive MLL-fusions may be incompatible with a malignant phenotype. Here, we used clinical proteasome inhibitors, bortezomib and carfilzomib, to reduce the turnover of endogenous MLL-fusions and discovered that accumulated MLL-fusions induce latent, context-dependent tumor suppression programs. Specifically, in MLL pro-B lymphoid, but not myeloid, leukemias, proteasome inhibition triggers apoptosis and cell cycle arrest involving activation cleavage of BID by Caspase-8 and upregulation of p27, respectively. Furthermore, proteasome inhibition conferred preliminary benefit to MLL-AF4 leukemia patients. Hence, feasible strategies to treat cancer-type and oncogene specific cancers can be improvised through harnessing inherent tumor suppression properties of individual oncogenic fusions.
INTRODUCTION
During tumorigenesis, the accumulation of genetic and epigenetic alterations is a key mechanism that contributes to the malignant phenotype and is a hallmark of cancer (Hanahan and Weinberg, 2011). Driver mutations appear important for tumorigenesis, and tumor cells frequently develop dependence on select oncogenes during cancer evolution for tumor maintenance and malignant progression, hence, develop “oncogene addiction” (Sharma and Settleman, 2007). In several exemplary cases, oncogene addiction can be broken by molecularly targeted agents aimed at therapeutic inhibition of the oncogenic signaling pathway or the oncoprotein itself (Luo et al., 2009).
Classically known oncogenes, such as MYC, RAS, and E2F1, induce transformation and promote tumorigenesis in various settings, but paradoxically incur cellular tumor suppression responses, such as cellular senescence and programmed cell death. Highlighting this phenomenon, MYC-induced lymphomagenesis depends upon an inhibition of apoptosis, often through upregulation of pro-survival proteins of the anti-apoptotic BCL-2 family. Like many oncogenes, MYC is not directly druggable, thereby posing challenges to cancer therapy development. Intriguingly, tumorigenesis by oncogenes may involve cell-type specificity and proper oncogene dosage (Lowe et al., 2004). Regarding oncogene dosage, for example, whereas moderate MYC expression leads to transformation, excess MYC accumulation triggers apoptosis. Thus, tumor suppressive surveillance may depend on a threshold of oncogene abundance.
The Mixed-Lineage Leukemia gene (MLL) encodes a large, 500 kDa nuclear protein that contains multiple conserved domains, including a SET domain endowed with methlytransferase activity that is used to methylate histone H3 at lysine 4 (H3K4), a mark associated with euchromatin and active transcription (Dou et al., 2006; Milne et al., 2002). MLL functions as heterodimeric complexes composed of its amino (MLLN320) and carboxy (MLLC180) terminal segments following site-specific proteolysis of its full-length precursor polypeptide by the Taspase1 protease (Hsieh et al., 2003; Oyama et al., 2013; Takeda et al., 2006; Takeda et al., 2013). Additional regulation of MLL function involves its biphasic accumulation and proteasome-mediated degradation coinciding with the cell cycle, coordinated by the ubiquitin-proteasome system E3 SCFSkp2 and APCCdc20 at S and M phases, respectively (Liu et al., 2007; Liu et al., 2010). Interestingly, MLL is also subject to proteasome-mediated degradation by the ECSASB2 E3 ligase (Wang et al., 2012).
Chromosome 11q23 translocations involving MLL account for ~80% of infant leukemias, ~10% of adult acute leukemias, and ~33% of therapy-related myelodysplastic syndrome/secondary acute leukemias (Liu et al., 2009). Leukemogenic MLL translocations fuse the common MLL 5’ part that encodes its N-terminal ~1,400 aa in frame with more than 60 translocation partner genes (TPGs) (Krivtsov and Armstrong, 2007; Liedtke and Cleary, 2009; Liu et al., 2009; Muntean and Hess, 2012; Yip and So, 2013). MLL translocations involving fusion of chromosome 11 with chromosomes 4 and 19 resulting in MLL-AF4 and MLL-ENL, respectively are prevalent in acute lymphoblastic leukemia (ALL) (Bhojwani et al., 2009) whereas its translocations with chromosomes 9 and 6 producing MLL-AF9 and MLL-AF6, respectively, are commonly associated with acute myeloid leukemia (AML). MLL-induced leukemias often show resistance to chemotherapies and consequently, patients with these malignancies typically undergo rapid relapse following these conventional treatments. The lack of adequate therapy for leukemias is likely due, in part, to the wide diversity of TPGs and potent oncogenic capacity of MLL-TPG fusion proteins. Hence, much remains to be learned about the activities of the MLL-fusion proteins.
Unexpectedly, expression of certain MLL-fusion products appears to compromise leukemia cell survival (Ayton and Cleary, 2001; Caslini et al., 2000; Xia et al., 2005), and clinical samples from leukemia patients rarely show high-level accumulation of these oncoproteins. Here, we investigated the hypothesis that accumulation of high levels of endogenous MLL-fusion proteins are detrimental to leukemia cell survival and proliferation.
RESULTS
MLL-Fusion Proteins Accumulate Upon Proteasome Inhibition
We previously observed that MLL-fusion proteins typically do not reach excessive levels in vivo (Liu et al., 2007; Liu et al., 2010), indicating that their high-levels may result in undesirable cellular consequences. To examine this further, we monitored the accumulation of MLL and MLL-fusion proteins upon proteasome inhibition in different human leukemia cell lines, including pro-B MLL leukemia cell lines RS4;11 and SEM, and pro-B non-MLL, i.e., without MLL translocation, leukemia lines JM1 and REH (Drexler et al., 2004) (Figure 1A). Before exposure to bortezomib, RS4;11 and SEM cell lines had detectable MLL-AF4 levels that were more abundant than MLL, which is consistent with the fact that MLL-fusion proteins exhibit reduced turnover by the cell cycle dependent ubiquitin proteasome system (Liu et al., 2007) and that MLL-fusions can reduce the levels of MLL (Liu et al., 2010). Significantly, upon exposure to bortezomib, the levels of MLL and MLL-fusion proteins increased in all tested leukemia cell lines (Figure 1B). In pro-B MLL leukemia cells, MLL-AF4 levels rose over the duration of bortezomib treatment, and a similar increase of MLL-AF9 was observed in treated myelogenous MLL cell lines THP-1 and NOMO-1 (Figure 1B). Furthermore, stability analysis demonstrated that MLL-AF4 has a longer protein half-life than MLL (Figure S1A). Therefore, MLL-fusion proteins in leukemia cells are continually turned over and their levels appear restricted from reaching an overabundance.
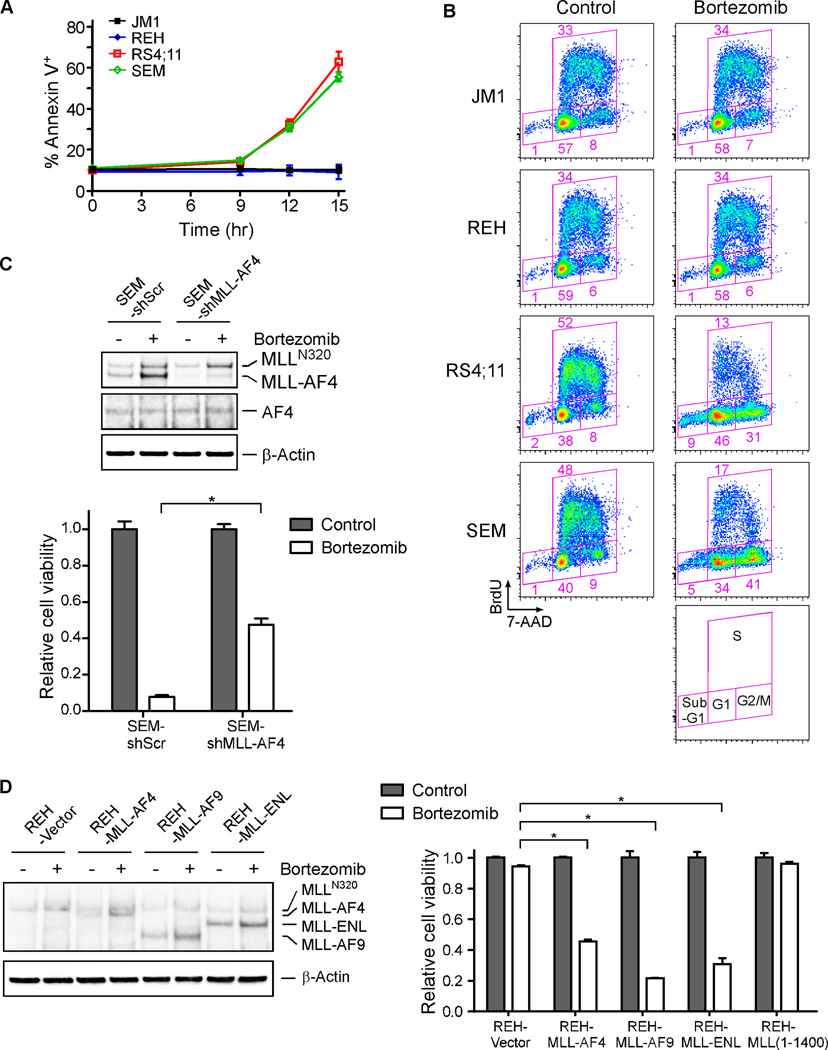
Figure 1. Pro-B MLL-AF4 leukemia cells display marked sensitivity to proteasome inhibitors.
A. Immunoblots of MLL (MLLN320) and the MLL-AF4 fusion protein in pro-B leukemia cell lines. Antibody against the shared amino-terminus of MLL among all MLL-fusions was utilized. The β-Actin blot is included to demonstrate similar loading.
B. Immunoblots of MLLN320 and MLL-fusion proteins in the indicated cell lines at 0, 3, 6, and 9 hours (hr) after treatment with 5 nM bortezomib.
C. Cell viability was measured by MTT assay 24 hours after the addition of bortezomib at the indicated concentrations. Upper panel showed viability plots of pro-B ALL cell lines; lower panel, of AML cell lines. Relative cell viability was calculated by the absorbance reading for bortezomib-treated cells normalized by untreated cells. Error bars reflect ± SEM measured from three independent experiments.
See also Figure S1.
Pro-B MLL Leukemia Cells Show Greater Sensitivity upon Proteasome Inhibition, Exhibiting Apoptosis and G2/M Cell Cycle Block
Next, we investigated what effect bortezomib treatment has on MLL leukemia cells. Importantly, the pro-B MLL leukemia cell lines RS4;11 and SEM showed a dosage dependent reduction in cell viability (Figure 1C). The reduction in cell viability observed in these lines was greater than that in non-MLL pro-B lines JM1 and REH cells (Figure 1C). The IC50 of bortezomib was determined to be approximately 3 nM in both RS4;11 and SEM cell lines, which was 10 times lower than that for the other cell lines tested (Figure 1C). This difference in sensitivity to proteasome inhibition was confirmed with carfilzomib, another FDA approved proteasome inhibitor (Demo et al., 2007) (Figure S1B). Interestingly, in AML, MLL leukemia lines MV4–11, MOLM-13, NOMO-1, and THP-1 were similarly resistant to bortezomib as non-MLL lines HL60 and U937 (Figure 1C). Notably, despite displaying significant sensitivity to bortezomib, RS4;11 and SEM cells displayed equivalent sensitivity as the other leukemia lines to common chemotherapeutic agents, including doxorubicin (DNA topoisomerase II inhibitor), etoposide (DNA topoisomerase II inhibitor), paclitaxel (microtubule stabilizing agent), cisplatin (DNA cross-linker), and dexamethasone (corticosteroid) (Figures S1C–G). Thus, these pro-B MLL-AF4 leukemia cells do not have an intrinsic cell survival impairment. We concluded that pro-B MLL-AF4 leukemia cells display a selective sensitivity to proteasome inhibition.
We sought to better understand the susceptibility to bortezomib in the pro-B MLL-AF4 leukemia cells. Annexin V staining of RS4;11, SEM, JM1, and REH cells following exposure to bortezomib confirmed that the pro-B MLL-AF4 leukemia cells were more prone to undergo cell death than their pro-B non-MLL leukemia counterparts (Figure 2A). Furthermore, cell cycle analysis demonstrated that compared to JM1 and REH, the RS4;11 and SEM cell lines displayed a reduction in the S phase and an accumulation in the G2/M phase (Figure 2B). These cells also displayed a greater ratio of sub-G1 ploidy (Figure 2B), indicative of increased DNA cleavage that associates with apoptosis. These results suggest that bortezomib selectively induces apoptosis and G2/M block in pro-B leukemia cells bearing the MLL-AF4 oncoprotein.
Figure 2. Proteasome inhibition induces apoptosis and cell cycle arrest in the pro-B leukemia cells harboring MLL-fusion proteins.
A. Cells were stained with Annexin V after treatment with 5 nM bortezomib.
B. Cell cycle profiles were analyzed upon a 12-hour exposure to 5 nM bortezomib. Cell cycle profiling was performed after a 30-minute pulse incorporation of BrdU, followed by flow cytometry analyses. Regions of the FACS plot delineating different cell cycle phases are specified.
C. Upper panel, immunoblots demonstrated shMLL-AF4-mediated specific knockdown of MLL-AF4 but not MLL (MLLN320) in SEM cells. The indicated cells were treated with 12 hours of 5 nM bortezomib. Lower panel, MTT assays of the indicated cells after a 24-hour exposure to 5 nM bortezomib were presented.
D. Left panel, immunoblots demonstrated exogenous expression of FLAG-tagged MLL-AF4, MLL-AF9, MLL-ENL, and MLL (aa 1–1400), determined by an anti-MLL amino-terminus antibody. Right panel, MTT assay were performed after a 24-hour treatment with 5 nM bortezomib.
Error bars reflect ± SEM calculated from three independent experiments. * indicates statistical significance (p < 0.05).
See also Figure S2.
To investigate that MLL-AF4 could be involved in the bortezomib-induced cytotoxicity, we performed shRNA-mediated knockdown studies. SEM and RS4;11 cells were retrovirally transduced with a shRNA specifically targeting the junction sequence of MLL-AF4 (shMLL-AF4) or a control shRNA. While the control shRNA had no effect on bortezomib-induced killing, shMLL-AF4 led to a reduction in bortezomib-triggered apoptosis (Figures 2C and S2A, S2B). Furthermore, ectopic expression of MLL-AF4 in SEM cells appeared to render increased sensitivity to bortezomib (Figure S2C). Conversely, since non-MLL pro-B leukemia cells were less susceptible to the bortezomib-stimulated cytotoxicity (Figure 1), we determined if ectopic expression of MLL-fusions in REH cells would alter their sensitivity to proteasome inhibition. To this end, we generated REH cell lines stably transduced with MLL-AF4, MLL-AF9 or MLL-ENL (Figure 2D). Remarkably, introduction of MLL-AF4, MLL-AF9 or MLL-ENL caused REH cells to become more susceptible to bortezomib (Figures 2D). Of note, reconstitution of the common MLL amino-terminus alone in REH cells (Figures 2D and S2D) or MLL-AF4 or MLL-ENL in HL60 myelogenous leukemia cells (Figure S2E) did not confer bortezomib sensitivity. Altogether, these results demonstrate that apoptosis induced by proteasome inhibition in the pro-B MLL leukemia cells is likely dependent on the MLL-fusion proteins. They also suggest that distinct leukemia cells are intrinsically equipped with an oncogenic fusion protein that could be retooled as a self-destructive weapon.
Bortezomib Triggers Apoptosis in Pro-B MLL Leukemia through BID Activation
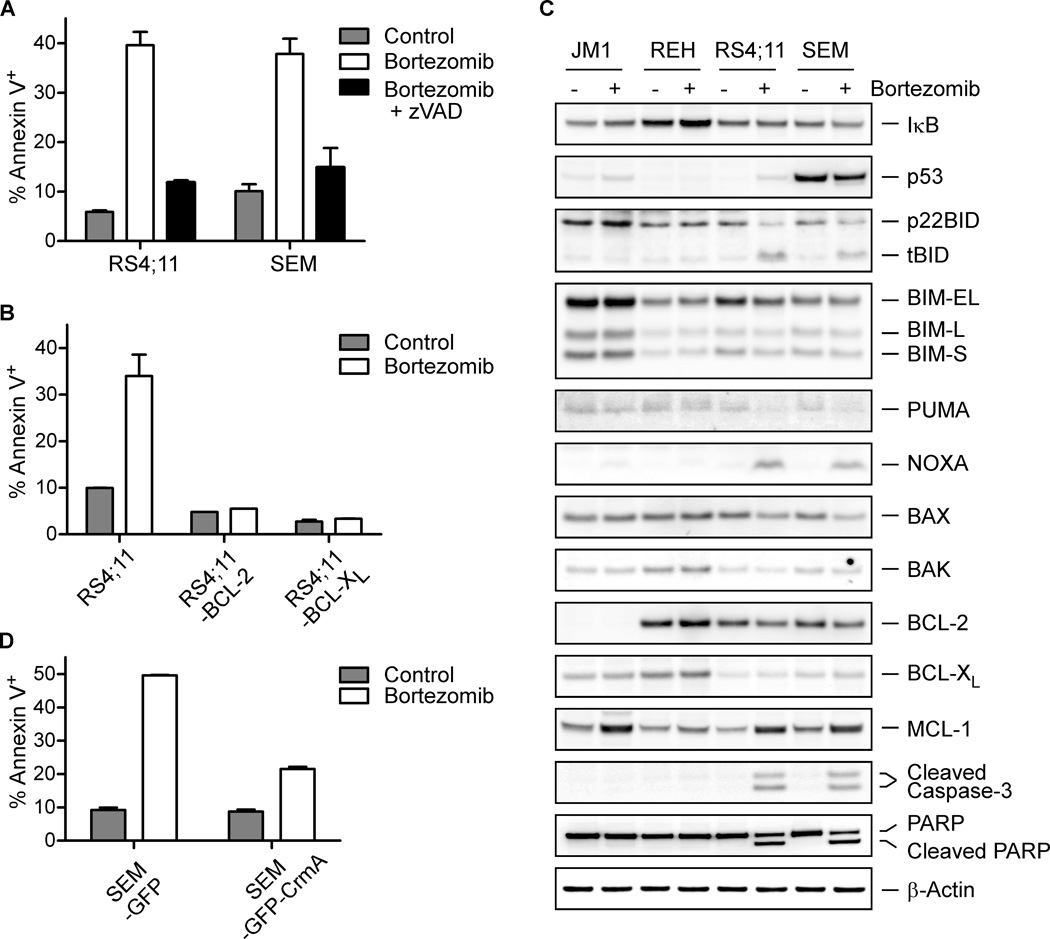
Multiple death stimuli culminate in the activation of pro-apoptotic BCL-2 cascade, resulting in mitochondrial outer membrane permeabilization (MOMP) and cytochrome c efflux into the cytosol for caspase activation (Danial and Korsmeyer, 2004; Kim et al., 2006; Kim et al., 2009; Ren et al., 2010). To determine whether caspase-mediated apoptosis is involved in bortezomib-induced cancer cell death, RS4;11 and SEM cells were co-treated with zVAD, a pan-caspase inhibitor, during their exposure to bortezomib. Co-treatment of zVAD efficiently reduced the number of Annexin V positive cells caused by bortezomib (Figures 3A and S3A). We next examined if anti-apoptotic BCL-2 family proteins BCL-2 or BCL-XL could suppress this death. In RS4;11 and SEM cells, retroviral transduction of BCL-2 or BCL-XL disrupted bortezomib-induced cell death (Figures 3B and S3B), indicating that proteasome inhibition induces cell death through the mitochondrion-dependent apoptotic pathway. Consistent with these pharmacological and genetic evidences of apoptosis, RS4;11 and SEM but not JM1 or REH cells displayed cleavage of Caspase-3 and PARP (poly ADP-ribose polymerase) (Figure 3C). Thus, in pro-B MLL leukemia cell lines, bortezomib induces mitochondrial apoptosis that can be blocked by anti-apoptotic BCL-2 and BCL-XL.
Figure 3. The cell death of pro-B leukemia cells induced upon proteasome inhibition involves the extrinsic apoptotic pathway.
A. The percentage of Annexin V positive cells was determined after a 12-hour exposure to 5 nM bortezomib with or without co-treatment of 50 µM zVAD.
B. Annexin V staining of the indicated cells was assessed after a 12-hour treatment with 5 nM bortezomib.
C. Immunoblots of key apoptosis regulators of the indicated pro-B leukemia cells before and after a 12-hour exposure to 5 nM bortezomib.
D. Annexin V staining of the indicated cells was assessed after a 12-hour treatment with 5 nM bortezomib.
Error bars reflect ± SEM calculated from three independent experiments.
See also Figure S3.
Bortezomib has been reported to trigger apoptosis in multiple myeloma cells through both NF-κB dependent and independent mechanisms (Adams, 2004; Cvek and Dvorak, 2011; Hideshima et al., 2009). However, in RS4;11 and SEM cells upon proteasome inhibition, no increase was seen in the levels of I-κB, a major regulatory protein of NF-κB complexes that is controlled by stimulus-dependent proteasome-mediated degradation (Figure 3C). This suggests that NF-κB inhibition is unlikely to be the main mechanism underlying bortezomib-induced apoptosis. The observation that bortezomib-induced apoptosis can be inhibited by BCL-2 and BCL-XL indicates the involvement of mitochondrion-dependent cell program. In response to death signals, activator BH3-only molecules, including truncated BID (tBID), BIM, and PUMA, directly interact with BAX and BAK to induce a stepwise structural reorganization and the ensuing oligomerization of BAX and BAK, leading to MOMP (Cheng et al., 2001; Kim et al., 2006; Kim et al., 2009; Ren et al., 2010). To interrogate further, we examined the levels of pro- and anti-apoptotic BCL-2 family proteins-key players that integrate death and survival signals at the mitochondria (Cvek and Dvorak, 2011; Fennell et al., 2008). In RS4;11 and SEM cells, but not JM1 or REH cells, conversion of BID to tBID p15 was observed upon proteasome inhibition (Figure 3C), suggesting that Caspase-8 is likely activated to cleave BID. BID normally resides in the cytosol, and, once cleaved by Caspase-8 (tBID), translocates to the mitochondrion where it activates BAX and BAK to induce cytochrome c release, serving as an amplification loop for Caspase-3 activation. Caspase-8 can be autoactivated within the Death-Inducing Signaling Complex (DISC) upon ligation of death receptors such as FAS. Indeed, quantitative RT-PCR (qRT-PCR) analysis of RS4;11 and SEM cells showed that bortezomib increased transcript levels of FAS, FASLG, and CASP8, likely contributing to the activation of Caspase-8, but not those of HOXA9 or MEIS1, two key downstream leukemogenic effectors of MLL-AF4 (Figures S3C–H). Furthermore, bortezomib-mediated transcriptional upregulation of CASP8, FAS, and FASLG was inhibited by knockdown of MLL-AF4 in SEM cells (Figures S3I–K). Proteasome inhibition was reported to increase the duration and amount of Caspase-8 activity upon death receptor engagement (Gonzalvez et al., 2012). To further interrogate the involvement of Caspase-8 activation in bortezomib-induced apoptosis in Pro-B MLL leukemia, we exogenously expressed CrmA, an inhibitor of Caspase-8 (Garcia-Calvo et al., 1998; Muzio et al., 1996), and found that CrmA expression significantly protected SEM cells from bortezomib-induced apoptosis (Figure 3D). As previously reported, MCL-1 protein, which is rapidly turned over by the ubiquitin-proteasome system, was stabilized by bortezomib (Figure 3C) (Perciavalle and Opferman, 2013). However, the potent inactivator of MCL-1, NOXA, was also highly induced by bortezomib (Figure 3C). Consequently, NOXA would prevent MCL-1 from sequestering tBID, allowing tBID to activate BAX and BAK (Kim et al., 2006). Although both NOXA and PUMA are known transcriptional targets of p53, only NOXA was induced upon proteasome inhibition. Furthermore, the levels of p53 did not correlate with bortezomib-induced apoptosis in these cell lines that carry wild-type p53 alleles based on the Cancer Cell Line Encyclopedia (Barretina et al., 2012) (Figure 3C). Hence, p53 is unlikely to play a role in proteasome inhibitor-induced apoptosis at our experimental setting. Taken together, these results are consistent with a model in which proteasome inhibition activates Caspase-8 to convert BID to tBID, which in turn, initiates BAX- and BAK-dependent mitochondrial apoptosis in pro-B MLL leukemia cells
Bortezomib Induces Cell Cycle Dysfunction in RS4;11 and SEM Cells through p27 Activation
Because bortezomib triggers both apoptosis and cell cycle arrest (Figures 2A and 2B), we examined whether these two processes are separable. First, we determined if the bortezomib-induced G2/M block is affected when apoptosis is abrogated. For this, anti-apoptotic BCL-XL was ectopically expressed in SEM cells to inhibit apoptosis. Despite acquiring a marked resistance to apoptosis, the BCL-XL-expressing SEM cells still exhibited a G2/M block upon proteasome inhibition (Figure 4A). Similarly, co-treatment of zVAD in SEM cells did not impact the G2/M block caused by bortezomib (Figure S4A). To further investigate, we performed immunoblotting to detect levels of cyclins and cyclin-dependent kinase inhibitors (CDKIs), key regulators of cell cycle progression. In RS4;11 and SEM cells, we observed a marked upregulation of p27 that correlated with the bortezomib-induced cell cycle arrest (Figure 4B). In contrast, the levels of p15, p16, p21, Cyclins D1, E2, A, B1, were unchanged by bortezomib, and hence these were unlikely to be directly involved in the bortezomib-induced cell cycle arrest (Figure 4B).
Figure 4. The p27 upregulation plays a key role in bortezomib-induced cell cycle arrest of pro-B leukemia cells expressing MLL-fusions.
A. Cell cycle profiles of the SEM-BCL-XL cells exposed to 5 nM bortezomib for 12 hours were obtained after a 30-minute BrdU pulse incorporation, followed by flow cytometry analyses. Percentages of gated cells were indicated.
B. Immunoblots of the indicated pro-B leukemic cells following a 12-hour treatment with 5 nM bortezomib.
C. Upper panel, cell cycle profiles of BCL-2 reconstituted SEM cells expressing shRNA-scramble (shScr) or shRNA-p27 (shp27) were obtained after a 12-hour exposure to 5 nM bortezomib. Lower left panel, immunoblots of p27 in knockdown cells. The percentage of the cells in G2/M phase is plotted in the lower right panel.
D. p27 mRNA levels were detected by qRT-PCR in pro-B MLL leukemia cells after a 12-hour treatment with 5 nM bortezomib. Values were normalized against GAPDH.
E. The changes of p27 mRNA and protein in the indicated SEM cells upon bortezomib treatment was determined.
F. The p27 protein level in REH cells expressing either MLL-AF4 or MLL-AF9 after a 12-hour exposure to 5 nM bortezomib.
G. ChIP analyses at promoter and exon 2 of the CDKN1B locus on the indicated cells upon bortezomib treatment. Assays were performed with the indicated antibodies and immunoprecipitates were subjected to quantitative PCR analyses using primers covering the depicted genomic regions. TSS, Transcription Start Site.
Error bars reflect ± SEM calculated from three independent experiments. * indicates statistical significance (p < 0.05).
See also Figure S4.
We next examined the role of p27 in the bortezomib-induced G2/M block. In SEM-BCL-2 cells, knockdown of p27 led to an attenuation of the bortezomib-induced G2/M block (Figure 4C), indicating that p27 is important for these pro-B MLL leukemia cells to undergo cell cycle arrest in response to proteasome inhibition. Levels of p27 can be controlled by protein degradation (Chu et al., 2008) and by direct transcriptional activation through MLL and MLL-AF4 (Milne et al., 2005; Xia et al., 2005). Thus, we examined if the upregulation of p27 upon bortezomib treatment depends on MLL-AF4. qRT-PCR analysis showed that the p27 mRNA level in SEM and RS4;11 but not JM1 or REH cells was increased by the bortezomib treatment (Figure 4D). Significantly, in SEM cells, specific knockdown of MLL-AF4 impeded p27 induction (Figure 4E). Conversely, enforced expression of MLL-AF4 or MLL-AF9 in REH cells rendered capability to upregulate p27 (Figure 4F). Taken together, these results suggest that bortezomib principally stabilizes MLL-AF4 or MLL-AF9, which in turn activates the transcription of the p27 gene (CDKN1B). Similarly, knockdown of MLL-AF4 in RS4;11 cells also compromised p27 induction but to a lesser degree, which could be due to a less efficient knockdown (Figure S4B). Intriguingly, concurrent knockdown of both MLL-AF4 and MLL by targeting the shared amino-terminus (shMLL-N) in RS4;11 cells seemed to further impair the p27 induction upon the bortezomib treatment, implicating a possible assistance of MLL in the MLL-AF4-dependent induction of p27 (Figure S4B), reminiscent of what was shown before that MLL-AF9-induced leukemogenesis required MLL (Thiel et al., 2010).
We further evaluated the mechanisms by which MLL-fusion proteins contribute to the p27 upregulation. Interestingly, MLL-fusion partner proteins, including AF4, AF9, ENL, and ELL, are functional components of the P-TEFb complex (Positive Transcription Elongation Factor b) that functions in transcriptional elongation (Mohan et al., 2010). Moreover, MLL-AF4, but not MLL, is able to recruit P-TEFb (Yokoyama et al., 2010). Therefore, we examined whether the bortezomib-stabilized MLL-AF4 recruits P-TEFb to enhance CDKN1B transcription. To this end, we performed chromatin immunoprecipitation (ChIP) assays. In bortezomib treated REH and SEM cells, anti-MLL N-terminus antibody detected an increased occupancy of MLL and/or MLL-AF4 at both the CDKN1B promoter and exon 2 (Figure 4G). Of note, this N-terminus antibody recognizes both MLL and MLL-fusions (Liu et al., 2010). Like the MLL-AF4 protein, Cyclin T1, a key component of the P-TEFb complex (Mohan et al., 2010), was also observed to have an increased promoter and gene body occupancy in SEM but not REH cells (Figures 4G). Altogether, the body of evidence indicates that accumulation of MLL-AF4 in response to bortezomib leads to recruitment of the P-TEFb complex to promote transcriptional processivity along CDKN1B, thereby increasing the production of p27 mRNA.
MLL-fusions Target the CDKN1B Locus through Binding B Cell Specific Transcription Factor PAX5
Recruitment of the P-TEFb complex to the CDKN1B locus by MLL-AF4 could explain how MLL-fusion proteins might stimulate the induction of p27. However, it could not explain why these MLL-fusions did not induce p27 in AML cells despite similarly increased protein abundance. MLL and MLL-fusion proteins do not consist of any apparent sequence-specific DNA binding domain (Liu et al., 2009). Therefore, we envisioned that MLL-fusion proteins may upregulate CDKN1B transcription through DNA binding protein partners, such as PAX5 and EBF1 (early B cell factor) transcription factors, that are selectively expressed in pro-B cells (Busslinger, 2004). By co-immunoprecipitation assays, MLL-AF4 was found to interact with PAX5, but not EBF1 (Figures 5A and S5A). Other MLL-fusion proteins, including MLL-AF9, MLL-ENL, and MLL-ELL, also interacted with PAX5 (Figure 5B), suggesting that the PAX5-interaction domain of the MLL-fusion proteins is located within the common MLL N-terminal 1,400 amino acids. Indeed, while the different MLL translocation partners failed to pull down PAX5 by themselves, the amino-terminus 1,400 amino acids of MLL was sufficient to pull down PAX5 (Figure S5B). Mapping experiments using individual MLL fragments demonstrated that the minimal interaction domain of MLL encompasses the first 400 amino acids (Figure 5C).
Figure 5. The recruitment of MLL-AF4 through PAX5 to the CDKN1B locus underlies the specific cytotoxicity upon proteasome inhibition in pro-B leukemia cells.
A. 293T cells were transfected with FLAG-MLL-AF4 and HA-PAX5 or HA-EBF1 expression constructs as indicated, subjected to anti-FLAG immunoprecipitation, and analyzed with the indicated antibodies.
B. 293T cells transfected with the indicated FLAG-MLL constructs and HA-PAX5 were subjected to anti-FLAG immunoprecipitation, and analyzed with immunoblots.
C. 293T cells transfected with the indicated FLAG-MLL constructs expressing individual MLL amino-terminus fragments and HA-PAX5 were subjected to anti-FLAG immunoprecipitation and analyzed with immunoblots.
D. An antibody that recognizes the common amino-terminal regional of MLL and MLL-fusions was used for immunoprecipitation and the precipitates were analyzed with the indicted antibodies.
E. SEM cells with the indicated knockdown were subjected to qRT-PCR analysis after a 12-hour treatment with 5 nM bortezomib.
F. PAX5, p27, and MLL-AF4 protein levels of the indicated SEM cells were determined after a 12-hour treatment with 5 nM bortezomib.
G. SEM cells of the indicated knockdown were subjected to ChIP after a 12-hour treatment with 5 nM bortezomib using the indicated antibodies and immunoprecipitates were subjected to quantitative PCR analyses.
Error bars reflect ± SEM calculated from three independent experiments.
See also Figure S5.
Using JM1, REH, RS4;11, and SEM pro-B leukemia cells, we confirmed by co-immunoprecipitation assays that endogenous MLL/MLL-AF4 interacted with PAX5 (Figure 5D). Functionally, in SEM cells, knockdown of PAX5 abrogated the ability of bortezomib to induce p27 mRNA and protein (Figures 5E, 5F and S5C). Furthermore, PAX5 knockdown did not affect MLL-AF4 stability (Figure 5F). We noted that PAX5 levels increased upon bortezomib treatment (Figure S5D), and this increase was also associated with an increased PAX5 occupancy at the CDKN1B promoter (Figure 5G). Moreover, the increased abundance of MLL/MLL-AF4 at the CDKN1B promoter was lost when PAX5 was knocked down (Figure 5G), indicating that PAX5 is required for MLL/MLL-AF4 to bind the CDKN1B promoter. Our collective data support a model in which the MLL-AF4 recruitment to the CDKN1B locus depends on PAX5, and the interaction between MLL-AF4 and PAX5 enhances transcriptional processivity of CDKN1B, resulting in the cell cycle arrest.
Since PAX5 appears to be the critical link for recruiting the MLL fusion protein to the promoters of cell cycle genes such as CDKN1B, we assessed the effect of enforced PAX5 expression in THP-1 cells an acute myelogenous MLL leukemia cell line. Interestingly, introduction of exogenous PAX5 did not restore the sensitivity of the MLL-AF9-harboring THP-1 cells to the proteasome inhibitors (Figures S5E and S5F). Furthermore, ChIP assays revealed that neither ectopically expressed PAX5 nor MLL or MLL fusion proteins could be recruited to the CDKN1B promoter (Figure S5G), suggesting that additional factors and/or the epigenetic status of the CDKN1B locus unique to pro-B leukemia cells are likely required for the recruitment of the PAX5/MLL-fusion complex to it.
Preclinical and Clinical Evidence Supports a Role of Proteasome Inhibition in Treating Pro-B MLL Leukemias
To investigate if the anti-cancer effects of proteasome inhibitors on pro-B MLL-leukemias extend to in vivo settings, we performed xenograft studies by transplanting luciferase-GFP-tagged REH or SEM cells into NOD-scid Il2rg−/− (NSG) recipient mice. Subsequently, engrafted mice were treated with bortezomib (Luker et al., 2003) and monitored by bioluminescent imaging over approximately 3 weeks. Mice transplanted with REH or SEM cells without bortezomib therapy developed rapid tumor progression that was apparent 10 days after transplantation and that aggressively pervaded nearly the whole animal over the ensuing 7 days (Figure 6A). Importantly, mice engrafted with SEM cells and then treated with bortezomib showed a striking resistance to leukemia accumulation and had only a minor detectable tumor burden during the same assay period, thus suggesting significant bortezomib responsiveness in vivo. In contrast, mice transplanted with REH showed little responsiveness to this form of therapy, and displayed a significant tumor burden (Figure 6A). Hence, similar to the results obtain from our in vitro studies, our xenograft studies strongly suggested that bortezomib treatment is an effective agent against pro-B MLL-AF4 leukemia cells in vivo.
Figure 6. The in vivo therapeutic benefits of proteasome inhibition in pro-B MLL leukemias.
A. NSG mice transplanted with luciferase-expressing REH or SEM cells were treated with bortezomib. On the indicated days after the xenograft, mice were imaged to assess for leukemia progression. Representative bioluminenscence images are shown in the left panels and the quantification of bioluminescence (photonic flux) over the duration of treatment was shown in the right panel. Error bars reflect ± SEM.
B. Pathological and clinical summary of five adult MLL leukemia patients treated with bortezomib.
C. H&E stains of bone marrow biopsies of patient #1 before (left) and after (right) bortezomib treatment. Scale bars, 100 µm.
See also Table S1.
Leukemia patients bearing MLL translocations generally have a poor overall prognosis, and this aggressive disease tends to be refractory to conventional anti-cancer therapies (Liedtke and Cleary, 2009). Indeed, MLL leukemia patients frequently suffer relapse after high dose chemotherapy and/or bone marrow transplantation. Regrettably, those who fail to respond to standard therapeutic regimens are often left with limited medical options. Few clinical trials exist due to the low overall prevalence of adult MLL-fusion leukemias. A group of five adult MLL leukemia patients, comprised of two pro-B, one bi-phenotypic, and two myeloid leukemia cases, were compassionately treated with bortezomib at standard dosing recommended for multiple myeloma (Figure 6B and Table S1). Remarkably, patient #1, a 21 year-old female with pro-B MLL-AF4 leukemia achieved a complete cytogenetic remission after two standard cycles of bortezomib (1.3 mg/m2 on days 1, 4, 8, and 11 of a 21 day cycle) (Figure 6C). Due to neurotoxicity, she was administered with reduced doses of bortezomib at 1 mg/m2 given once a week as maintenance and which was discontinued after 8 weeks. This patient remained in complete remission without further treatment for more than one year. Unfortunately, her leukemia eventually re-emerged and was minimally responsive to bortezomib, and she passed way soon thereafter. She had previously relapsed 6 months after allogeneic bone marrow transplant from a matched unrelated donor following Hyper-CVAD chemotherapy. Patient #2, another pro-B MLL-AF4 patient, exhibited nondurable hematologic improvement. Patient #3, a biphenotypic MLL leukemia patient, experienced nondurable reduction in bone marrow blasts. Consistent with our preclinical findings showing that bortezomib has no efficacy on myelogenous MLL leukemia cells, patients #4 and #5 derived no clinical benefit from bortezomib therapy.
DISCUSSION
Oncogenes, such as MYC, RAS, and E2F1, underlie various human malignancies and promote cancer formation in various experimental settings. Yet, despite acting as important drivers of tumorigenesis, these oncogenes can trigger tumor suppression in a cell-context and dose-dependent manner (Lowe et al., 2004). Indeed, while moderate oncogene levels induce cancer initiation and maintenance, high-level expression can inadvertently activate tumor suppression surveillance programs including programmed cell death (Murphy et al., 2008; Sarkisian et al., 2007). Most notably, excessive Myc overexpression, in a titratable Myc mouse model, triggers apoptosis through the induction of BIM and PUMA, “activator” BH3-only molecules (Egle et al., 2004; Hemann et al., 2005; Hemann et al., 2004). Hence, this mechanism serves as a powerful way to curb the emergence of cancer and acts as an organismal protective mechanism (Pelengaris et al., 2002). Similarly, E2F1-induced oncogenesis is antagonized by apoptosis in RB deficient cancer cells (Chen et al., 1999; Mendoza et al., 2003). However, while oncogenes can activate tumor suppression programs, it remains undetermined if such latent programs persist in tumor cells, and whether they can be reactivated and thereby render clinical benefit for cancer patients. There are obvious important issues limiting this application. First, in many cancers, driver oncogenes are overexpressed. Second, during tumor progression, cancer cells usually acquire additional mutations that either abolish or bypass inherent tumor suppression functions. Third, ideal targets should be different between cancer and surrounding normal cells. Here, we demonstrate that MLL-fusion proteins can be stabilized and reactivated upon proteasome inhibition, triggering latent tumor suppression programs (Figure 7).
Figure 7. Illustration depicts how proteasome inhibition induces specific cytotoxicity in pro-B MLL leukemia cells.
The transcriptional regulation of p27 by MLL, MLL-AF9, MLL-AF4 and MLL-ENL has been reported in various experimental settings and appears to be very complex (Caslini et al., 2000; Milne et al., 2005; Wang et al., 2008; Xia et al., 2005). Intriguingly, in Jurkat cells overexpression of MLL-AF4 induces p27 whereas in 293T cells it suppresses (Xia et al., 2005). Furthermore, in human MLL-AF5 leukemia cells KP-L-RY and MLL-ENL-transformed murine myeloid progenitors pharmacological inhibition of GSK3 induces p27 and yields preclinical therapeutic benefits (Wang et al., 2008). In U937 cells, overexpression of the MLL amino-terminus (aa 1–410) induces p27 (Caslini et al., 2000). In pancreatic neuroendocrine cells and transformed mouse embryonic fibroblasts, wild-type MLL complexes with Menin to suppress p27 (Milne et al., 2005), which further refines the Menin-p27 tumor suppressor axis in the pancreas (Karnik et al., 2005). Surprisingly, although the loss of Menin in MLL-ENL transformed myeloid leukemia blasts results in reduced Hoxa7 and Hoxa9 expression and thus compromises the leukemia phenotype, the Menin loss under this experimental setting did not induce Cdkn1b (Yokoyama et al., 2005). Taken together, most data including ours favors a positive correlation between MLL/MLL-fusion and p27 expression. This begs several outstanding questions: First, why do leukemia fusions activate p27? Is this a necessity or simply an unwanted byproduct? In favor of the first scenario, it has been suggested that low level of p27 induction by MLL-fusion proteins might prevent leukemia initiating cells from exhaustion (Zhang et al., 2013). In favor of the second scenario, MLL-fusions unavoidably acquire this context-dependent tumor suppressor activity through the common MLL amino-terminus. Nevertheless, this capacity appears tightly regulated and can be manipulated by pharmacological means, thereby offering specific therapeutic benefits to pro-B MLL leukemia patients.
Collectively, these in vitro results suggest that the latent tumor suppression activity of MLL-fusion proteins in pro-B MLL leukemia cells can be activated by proteasome inhibitors. In accordance with our in vitro results, proteasome inhibition rendered therapeutic benefit in an MLL leukemia xenograft mouse model. Proteasome inhibition also rendered complete remission to one pro-B MLL-AF4 leukemia patient whose disease eventually relapsed. Bortezomib treatment did not appear to benefit the three AML MLL patients. Notably, in multiple myeloma, a malignancy for which bortezomib is approved, the rate of response to bortezomib was found to be 38% (Richardson et al., 2005).
How pro-B MLL leukemia cells display differential sensitivity and acquire resistance to proteasome inhibition warrant future investigation. Since overexpression of BCL-2 or BCL-XL can abrogate bortezomib-induced apoptosis, apoptosis resistance might represent one refractory mechanism. To overcome resistance, the potential clinical administration of proteasome inhibitors in combination with other anti-cancer agents, such as cell death based agents including ABT-263 (Tse et al., 2008), warrant further investigation. ABT-263, a small molecule inhibitor of BCL-2, is in clinical trials and has shown effectiveness and selectivity against certain types of cancers (Davids and Letai, 2012; Walensky, 2012). In conclusion, our findings reveal latent tumor suppression programs that can be aroused through hyperactivating oncogenic fusion proteins. This arousal might be suitably exploited as a cancer-specific therapeutic strategy.
EXPERIMENTAL PROCEDURES
Reagents
Bortezomib (Velcade) was obtained from Millennium Pharmaceutical Inc. (Cambridge, MA). Carfizomib was obtained from Proteolix (South San Francisco, CA).
Plasmid constructs
FLAG tagged fragments consisting of MLL aa 1–400, 400–750, 1–750, 750–1400, 1400–2664, 1–1400 derived from wild-type MLL, were inserted into eukaryotic expression vectors pCI-neo (Promega) for transient transfection assays. FLAG tagged MLL fusion genes, including MLL-AF4 and MLL-AF9, MLL-ENL and MLL-ELL, in eukaryotic expression vector are described previously (Liu et al., 2007). MLL-AF4 and MLL-AF9 were also inserted into pMSCV-puro vector (Clontech) for retrovirus production. Full-length PAX5 and EBF1 were cloned from JM1 cell cDNA and inserted into the pCMV-HA vector (Clontech). Full-length BCL-2 and BCL-XL were inserted into the pMSCV-puro (Clontech) or pMIG vector (kindly provided by Dr. William Hahn) for retrovirus production. GFP-tagged CrmA was inserted into pMSCV-puro vector (Clontech) for retrovirus production.
shRNA-mediated knockdown
Target sequence (agaaaagcagacctactcc and ctttaagcagacctactcc) against human MLL-AF4 in SEM and RS4;11 cells based on published information (Thomas et al., 2005), target sequence (ttgctccacccatcaaacc) against human MLL N-terminus, target sequence (gaatggacatcctgtataa) against human p27, and target sequence (ggatgcttgtctatttcta and ggctccccctactattata) against human PAX5 were inserted into the pSUPER.retro.puro vector, according to the manufacturer’s protocol (Oligoengine). Generated retrovirus carrying indicated shRNA was used to infect target cells for 2 days, and the cells were subjected to puromycin selection at 2 µg/mL.
Immunoblots
The anti-N-terminus MLL (MO435) antibodies were generated using a synthetic peptide of MLL amino acids 752–949 as immunogen. Details of the commercially available antibodies used for immunoblots are available in the supplemental experimental procedures. Antibodies were detected using the enhanced chemiluminescence method (Western Lightning, PerkinElmer). Immunoblot signals were acquired with the LAS-3000 Imaging system (FujiFilm) and were analyzed by ImageGauge software (FujiFilm) as previously described (Liu et al., 2010).
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed using the Magna ChIP A Kit (Millipore) according to the manufacture’s protocol. One µg of pre-immune rabbit IgG, anti-MLL (MO435), anti-Cyclin T1 (Santa Cruz Biotechnology), or PAX5 (Santa Cruz Biotechnology) antibody was utilized for each ChIP reaction. Precipitated DNA was analyzed using ViiA 7 Real-Time PCR System (Applied Biosystems). Primers used for ChIP-PCR assay are: CDKN1B Promoter (-50 bp to +74 bp relative to TSS): forward, ccaatggatctcctcctctg, reverse, aaaacaccccgaaaagacg; CDKN1B coding region (+1441 bp to +1597 bp relative to TSS): forward, atttcccctgcgcttagatt, reverse, atcaacccaccgagctgtt.
Mouse studies and in vivo imaging
REH and SEM cells are transduced by lentivirus generated using the FUGW-FL lentiviral vector which simultaneously expresses GFP and luciferase (Smith et al., 2004). GFP positive cells were sorted using MoFlo (Beckman Coulter). NSG mice were purchased from Jackson Laboratory (Bar Harbor, Maine). One million luciferase-expressing cells were intravenously injected via the tail vein into NSG mice. NSG mice were then administered intravenously with bortezomib at 0.5 mg/kg on a twice weekly schedule beginning two days after the xenograft. Total body bioluminescence was quantified as previously described (Luker et al., 2003). All animal work was performed in accordance with a protocol approved by the Animal Studies Committee of Washington University in St. Louis.
Statistics
The Student t test was used to analyze the differences between the groups. A p value less than 0.05 was considered statistically significant.
Patients
Retrospective chart review was performed to identify adult MLL leukemia patients who were treated with bortezomib at the Barnes Jewish Hospital, Washington University in St. Louis, between 2007 and 2010. Institutional Review Board approval for the retrospective data collection was not required. Patients provided verbal consent for the off-label, off-protocol use of bortezomib for MLL leukemia patients who failed standard of care therapies. Medical decisions were made at the discretion of the patients' physicians.
Supplementary Material
Highlights.
MLL-fusion and cancer type specific hypersensitivity to proteasome inhibitors
Proteasome inhibitors engage extrinsic cell death pathway in Pro-B MLL leukemia cells
Bortezomib activates p27 through a Pro-B leukemia specific PAX5/MLL-fusion complex
In vivo efficacy against pro-B MLL leukemia was observed with proteasome inhibitors
SIGNIFICANCE.
The oncogene addiction hypothesis posits that cancers driven by select oncogenes are vulnerable to therapeutic inhibition of the culprit oncoprotein. Surprisingly, recent studies have demonstrated that oncogenes have cryptic intrinsic tumor suppression activity that requires downregulation for tumorigenesis. Here, we investigated whether MLL-fusions possess latent tumor suppression activity and if so, could it be redirected to inflict self-destruction. Indeed, we found pro-B MLL leukemia cells were sensitive to bortezomib and carfilzomib. We treated five MLL leukemia patients who failed standard therapies with bortezomib, we observed complete remission in one patient and hematological improvement in another, both had pro-B leukemia. Our study supports further exploration of latent tumor suppression properties of individual oncogenes for cancer treatment.
ACKNOWLEDGMENTS
We apologize to all the investigators whose research could not be appropriately cited owing to space limitation. This work is supported by CA119008, CA138505 and Scholar award of the American Cancer Society to J.J.H; and by CA125562 to E.H.C. H.L. is supported by the Chinese National Key Basic Research Project (973: 2013CB966801), the National Natural Science Foundation of China (81370651), the Thousand Young Talents program of China, the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, and the American Society of Hematology Scholar Award. We also thank Ms. Wenjing Wu at the MSK Department of Public Affairs for her expert illustration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- Ayton PM, Cleary ML. Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene. 2001;20:5695–5707. doi: 10.1038/sj.onc.1204639. [DOI] [PubMed] [Google Scholar]
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojwani D, Howard SC, Pui CH. High-risk childhood acute lymphoblastic leukemia. Clin Lymphoma Myeloma. 2009;3(9 Suppl):S222–S230. doi: 10.3816/CLM.2009.s.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55–79. doi: 10.1146/annurev.immunol.22.012703.104807. [DOI] [PubMed] [Google Scholar]
- Caslini C, Shilatifard A, Yang L, Hess JL. The amino terminus of the mixed lineage leukemia protein (MLL) promotes cell cycle arrest and monocytic differentiation. Proc Natl Acad Sci U S A. 2000;97:2797–2802. doi: 10.1073/pnas.040574897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YN, Sharma SK, Ramsey TM, Jiang L, Martin MS, Baker K, Adams PD, Bair KW, Kaelin WG., Jr Selective killing of transformed cells by cyclin/cyclin-dependent kinase 2 antagonists. Proc Natl Acad Sci U S A. 1999;96:4325–4329. doi: 10.1073/pnas.96.8.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- Cvek B, Dvorak Z. The ubiquitin-proteasome system (UPS) and the mechanism of action of bortezomib. Curr Pharm Des. 2011;17:1483–1499. doi: 10.2174/138161211796197124. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol. 2012;30:3127–3135. doi: 10.1200/JCO.2011.37.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN, Jiang J, Laidig GJ, Lewis ER, Parlati F, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, Allis CD, Roeder RG. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol. 2006;13:713–719. doi: 10.1038/nsmb1128. [DOI] [PubMed] [Google Scholar]
- Drexler HG, Quentmeier H, MacLeod RA. Malignant hematopoietic cell lines: in vitro models for the study of MLL gene alterations. Leukemia. 2004;18:227–232. doi: 10.1038/sj.leu.2403236. [DOI] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci U S A. 2004;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell DA, Chacko A, Mutti L. BCL-2 family regulation by the 20S proteasome inhibitor bortezomib. Oncogene. 2008;27:1189–1197. doi: 10.1038/sj.onc.1210744. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- Gonzalvez F, Lawrence D, Yang B, Yee S, Pitti R, Marsters S, Pham VC, Stephan JP, Lill J, Ashkenazi A. TRAF2 Sets a threshold for extrinsic apoptosis by tagging caspase-8 with a ubiquitin shutoff timer. Mol Cell. 2012;48:888–899. doi: 10.1016/j.molcel.2012.09.031. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hemann MT, Bric A, Teruya-Feldstein J, Herbst A, Nilsson JA, Cordon-Cardo C, Cleveland JL, Tansey WP, Lowe SW. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature. 2005;436:807–811. doi: 10.1038/nature03845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemann MT, Zilfou JT, Zhao Z, Burgess DJ, Hannon GJ, Lowe SW. Suppression of tumorigenesis by the p53 target PUMA. Proc Natl Acad Sci U S A. 2004;101:9333–9338. doi: 10.1073/pnas.0403286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideshima T, Ikeda H, Chauhan D, Okawa Y, Raje N, Podar K, Mitsiades C, Munshi NC, Richardson PG, Carrasco RD, et al. Bortezomib induces canonical nuclear factor-kappaB activation in multiple myeloma cells. Blood. 2009;114:1046–1052. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JJ, Cheng EH, Korsmeyer SJ. Taspase1: a threonine aspartase required for cleavage of MLL and proper HOX gene expression. Cell. 2003;115:293–303. doi: 10.1016/s0092-8674(03)00816-x. [DOI] [PubMed] [Google Scholar]
- Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, Meyerson M, Kim SK. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci U S A. 2005;102:14659–14664. doi: 10.1073/pnas.0503484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7:823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- Liedtke M, Cleary ML. Therapeutic targeting of MLL. Blood. 2009;113:6061–6068. doi: 10.1182/blood-2008-12-197061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Cheng EH, Hsieh JJ. Bimodal degradation of MLL by SCFSkp2 and APCCdc20 assures cell cycle execution: a critical regulatory circuit lost in leukemogenic MLL fusions. Genes Dev. 2007;21:2385–2398. doi: 10.1101/gad.1574507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Cheng EH, Hsieh JJ. MLL fusions: pathways to leukemia. Cancer Biol Ther. 2009;8:1204–1211. doi: 10.4161/cbt.8.13.8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Takeda S, Kumar R, Westergard TD, Brown EJ, Pandita TK, Cheng EH, Hsieh JJ. Phosphorylation of MLL by ATR is required for execution of mammalian S-phase checkpoint. Nature. 2010;467:343–346. doi: 10.1038/nature09350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Luker GD, Pica CM, Song J, Luker KE, Piwnica-Worms D. Imaging 26S proteasome activity and inhibition in living mice. Nat Med. 2003;9:969–973. doi: 10.1038/nm894. [DOI] [PubMed] [Google Scholar]
- Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza N, Fong S, Marsters J, Koeppen H, Schwall R, Wickramasinghe D. Selective cyclin-dependent kinase 2/cyclin A antagonists that differ from ATP site inhibitors block tumor growth. Cancer Res. 2003;63:1020–1024. [PubMed] [Google Scholar]
- Milne TA, Briggs SD, Brock HW, Martin ME, Gibbs D, Allis CD, Hess JL. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell. 2002;10:1107–1117. doi: 10.1016/s1097-2765(02)00741-4. [DOI] [PubMed] [Google Scholar]
- Milne TA, Hughes CM, Lloyd R, Yang Z, Rozenblatt-Rosen O, Dou Y, Schnepp RW, Krankel C, Livolsi VA, Gibbs D, et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci U S A. 2005;102:749–754. doi: 10.1073/pnas.0408836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan M, Lin C, Guest E, Shilatifard A. Licensed to elongate: a molecular mechanism for MLL-based leukaemogenesis. Nat Rev Cancer. 2010;10:721–728. doi: 10.1038/nrc2915. [DOI] [PubMed] [Google Scholar]
- Muntean AG, Hess JL. The pathogenesis of mixed-lineage leukemia. Annu Rev Pathol. 2012;7:283–301. doi: 10.1146/annurev-pathol-011811-132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ, Junttila MR, Pouyet L, Karnezis A, Shchors K, Bui DA, Brown-Swigart L, Johnson L, Evan GI. Distinct thresholds govern Myc's biological output in vivo. Cancer Cell. 2008;14:447–457. doi: 10.1016/j.ccr.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Oyama T, Sasagawa S, Takeda S, Hess RA, Lieberman PM, Cheng EH, Hsieh JJ. Cleavage of TFIIA by Taspase1 Activates TRF2-Specified Mammalian Male Germ Cell Programs. Dev Cell. 2013;27:188–200. doi: 10.1016/j.devcel.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–334. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- Perciavalle RM, Opferman JT. Delving deeper: MCL-1's contributions to normal and cancer biology. Trends Cell Biol. 2013;23:22–29. doi: 10.1016/j.tcb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 2007;21:3214–3231. doi: 10.1101/gad.1609907. [DOI] [PubMed] [Google Scholar]
- Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- Takeda S, Chen DY, Westergard TD, Fisher JK, Rubens JA, Sasagawa S, Kan JT, Korsmeyer SJ, Cheng EH, Hsieh JJ. Proteolysis of MLL family proteins is essential for taspase1-orchestrated cell cycle progression. Genes Dev. 2006;20:2397–2409. doi: 10.1101/gad.1449406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Liu H, Sasagawa S, Dong Y, Trainor PA, Cheng EH, Hsieh JJ. HGF-MET signals via the MLL-ETS2 complex in hepatocellular carcinoma. J Clin Invest. 2013;123:3154–3165. doi: 10.1172/JCI65566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel AT, Blessington P, Zou T, Feather D, Wu X, Yan J, Zhang H, Liu Z, Ernst P, Koretzky GA, et al. MLL-AF9-induced leukemogenesis requires coexpression of the wild-type Mll allele. Cancer Cell. 2010;17:148–159. doi: 10.1016/j.ccr.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Gessner A, Vornlocher HP, Hadwiger P, Greil J, Heidenreich O. Targeting MLL-AF4 with short interfering RNAs inhibits clonogenicity and engraftment of t(4;11)-positive human leukemic cells. Blood. 2005;106:3559–3566. doi: 10.1182/blood-2005-03-1283. [DOI] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- Walensky LD. From mitochondrial biology to magic bullet: navitoclax disarms BCL-2 in chronic lymphocytic leukemia. J Clin Oncol. 2012;30:554–557. doi: 10.1200/JCO.2011.37.9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Muntean AG, Hess JL. ECSASB2 mediates MLL degradation during hematopoietic differentiation. Blood. 2012;119:1151–1161. doi: 10.1182/blood-2011-06-362079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Smith KS, Murphy M, Piloto O, Somervaille TC, Cleary ML. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature. 2008;455:1205–1209. doi: 10.1038/nature07284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia ZB, Popovic R, Chen J, Theisler C, Stuart T, Santillan DA, Erfurth F, Diaz MO, Zeleznik-Le NJ. The MLL fusion gene, MLL-AF4, regulates cyclin-dependent kinase inhibitor CDKN1B (p27kip1) expression. Proc Natl Acad Sci U S A. 2005;102:14028–14033. doi: 10.1073/pnas.0506464102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip BH, So CW. Mixed lineage leukemia protein in normal and leukemic stem cells. Exp Biol Med (Maywood) 2013;238:315–323. doi: 10.1177/1535370213480717. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Lin M, Naresh A, Kitabayashi I, Cleary ML. A higher-order complex containing AF4 and ENL family proteins with P-TEFb facilitates oncogenic and physiologic MLL-dependent transcription. Cancer Cell. 2010;17:198–212. doi: 10.1016/j.ccr.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123:207–218. doi: 10.1016/j.cell.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Zhang J, Seet CS, Sun C, Li J, You D, Volk A, Breslin P, Li X, Wei W, Qian Z, et al. p27kip1 maintains a subset of leukemia stem cells in the quiescent state in murine MLL-leukemia. Mol Oncol. 2013;7:1069–1082. doi: 10.1016/j.molonc.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.