Abstract
AIM: To investigate the complex relationships between resting energy expenditure (REE), eating psychopathology, and Hypothalamus Pituitary Adrenal axis functioning in patients with eating disorders.
METHODS: The study was designed as a cross-sectional survey, and it was planned by the Clinic for Eating Disorders of the University of Florence (Italy). The protocol was approved by the Ethics Committee of the Institution. Twenty two anorexia nervosa and twenty one Bulimia Nervosa patients were assessed by means of a clinical interview and the structured clinical interview for diagnostic and statistical manual of mental disorders, fourth edition. Eating attitudes and behaviour were specifically investigated by means of the eating disorder examination questionnaire (EDE-Q). Patients were also evaluated by means of the symptom checklist (SCL 90-R), REE was measured by means of indirect calorimetry, and blood cortisol morning levels were evaluated.
RESULTS: Both anorexia nervosa and bulimia nervosa patients showed a reduced REE as compared with predicted REE. Body mass index (BMI) was positively associated with resting energy expenditure in Bulimics, whereas a strong, negative association between BMI and REE was observed in Anorectics. The pattern of associations between variables supported a mediation model, where shape concern accounted for variations in REE and cortisol levels (mediator), and variations in the mediator significantly accounted for variations in REE. When these associations where taken into account together, the relationship between shape concern and REE was no longer significant, whereas the association between cortisol levels and REE retained its significance, showing strong evidence for a single, dominant mediator. Anorectics and Bulimics showed an opposite pattern of association between BMI and REE. In Anorectics only, a higher REE was associated with a more severe eating disorder specific psychopathology, and cortisol levels represent a possible mediating factor for this relationship.
CONCLUSION: The data supported a mediation model where cortisol levels mediated the relationship between eating psychopathology (concern about body shape) and REE.
Keywords: Anorexia nervosa, Bulimia nervosa, Cortisol, Psychopathology, Resting energy expenditure
Core tip: We have investigated the relationship between resting energy expenditure (REE), eating psychopathology, and hypothalamus adrenal axis in EDs. Twenty two anorexia nervosa (AN) and 21 bulimia nervosa (BN) patients were assessed. Both AN and BN showed a reduced REE as compared with predicted REE. AN and BN showed an opposite pattern of association between REE and Body Mass Index (BMI) which was positively associated with REE in BN, whereas a strong, negative association between BMI and REE was observed in AN. In AN only, a higher REE was associated with a more severe eating disorder psychopathology, and higher cortisol levels. The data supported a mediation model where cortisol levels mediated the relationship between eating psychopathology and REE.
INTRODUCTION
Chronic underfeeding and binge-purging behaviours can lead to alterations in metabolism and body composition. Several studies investigated energy metabolism in patients with anorexia nervosa (AN)[1-6], and the observed reduction of resting energy expenditure (REE) has been associated to the smaller volume of metabolically active tissues and to an adaptation to underfeeding[6-8]. Measured REE is significantly lower than predicted REE in AN subjects[9]. As far as bulimic normal weight patients are concerned, most of the published studies found that the resting metabolic rate of these patients was significantly lower than that of controls[10,11] , whereas Detzer et al[12] did not find significant differences between patients with bulimia nervosa (BN) and controls.
REE is one component of the total energy expenditure. It includes basal metabolic rate, which refers to the minimum part of energy required to maintain the organisms’ basic functions, and it is related to the amount of energy utilized when the body is at complete rest. It can be measured by means of indirect calorimetry, and it is often used in clinical settings. REE alterations found in eating disorders could be partly explained with the quantitative changes in cell mass[1,13]. Similarly, Vaisman et al[14] attributed the lowered resting metabolic rate to the reduction in lean body mass.
Alternatively, previous findings supported the relationship between stress induced cortisol levels and metabolic rate. In eating disorders, increased hypothalamus adrenal axis (HPA) arousal with abnormalities in its regulation is well proven[15,16]; HPA axis hyperactivity is well documented in AN and BN especially during the acute phase of the illness, even if the abnormalities showed by the BN patients are milder[17]. The HPA axis alterations can influence other biological systems involved in eating behaviour, such as leptin, endogenous opioids, thyroid, reproductive, immune and sympathetic nervous systems and the abnormalities in these systems could be considered to be involved in the onset and the maintenance of eating disorders[17]. HPA axis abnormalities seem to be associated with an history of stressful life events and an excess of traumatic life events, such as sexual and physical abuse are reported in patients with eating disorders. As the relationship between life events and HPA axis is well known[17-19], it is not unexpected that the HPA axis may also be functioning abnormally in eating disorders. Moreover cortisol is considered a catabolic hormone[20,21], and it has been found that weight gain in AN was associated with normalization of plasmatic and urinary cortisol levels[22], HPA functioning and the reduction of cortisol secretory burst[23].
Finally, few studies considered the relationship between eating disorder psychopathology and REE in eating disorders patients, with conflicting results[13,23,24].
The aims of the present study were as follows: (1) to evaluate the pattern of association between body mass index (BMI) and REE in AN and BN; (2) to investigate the possible mechanisms of REE alterations in AN and BN, analyzing the interaction between eating disorder psychopathology, HPA functioning and metabolic status.
MATERIALS AND METHODS
Sample and measures
The study was designed as a cross-sectional survey, and it was planned by the Clinic for Eating Disorders of the University of Florence (Italy). The protocol was approved by the Ethics Committee of the Institution. A written informed consent was obtained from each patient after the procedures of the study were fully explained. The study was performed on a series of 61 eating disorders patients attending the Clinic for Eating Disorders between January 2010 and July 2013, provided they met the following inclusion criteria: female, age between 18 and 60 years, diagnosis of AN restricting type and BN Binge/Purging type, assessed by means of the Structured Clinical Interview for Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV)[25,26]. Patients were included if they reported at least 1 year of a stable Eating Disorder diagnosis (at least 1 year with full diagnostic criteria for anorexia nervosa or bulimia nervosa according the DSM-IV criteria and without diagnostic crossover in the same year), at least 6 wk of a stable body weight, no intense physical exercise in the past six months (both assessed by means of a face-to face clinical interview). None of the patients were in a remission or recovery phase of disease. The exclusion criteria were as follows: comorbid schizophrenia or bipolar disorder, illiteracy, mental retardation, severe medical conditions, current use of psychoactive medications that can interfere with HPA Axis response (i.e., antidepressants use could increase human hippocampal neurogenesis by activating the glucocorticoid receptor[27].
Six patients refused to participate in the study, and 12 patients were excluded because of the following reasons: bipolar disorder (2 patient), illiteracy (1 patient), mental retardation (1 patient), severe medical conditions (3 patients with heart failure, 1 with renal failure), pharmacological treatments (4 patients taking SSRI). The final sample was composed of 43 female subjects, 22 with AN Restricting Type, and 21 with BN Binge/Purging type. The Structured clinical interview for DSM-IV[26] axis was used to confirm psychiatric diagnoses.
Psychological assessment
Psychopathological, behavioural and sociodemographic data were collected through a face-to-face interview on the first day of admission, by two expert psychiatrists (L.L., C.LS.). The structured clinical interview for DSM IV[26] was applied to evaluate diagnoses according to DSM-IV. Anthropometric measurements were made using standard calibrated instruments. Height (m) was measured using a wall-mounted stadiometer, weight (kg) using electronic scales. BMI was calculated. Eating attitudes and behaviour were specifically investigated by means of the eating disorder examination questionnaire (EDE-Q)[28]. The self-reported EDE-Q consists of 38 items, assessing the core psychopathological features of eating disorders, and contains 4 subscales: dietary restraint, eating concern, weight concern, and shape concern. Finally, patients were evaluated by means of the symptom checklist (SCL 90-R)[29], a psychometric instrument devoted to the identification of psychopathological distress.
Blood samples
Blood samples were drawn in the morning (8 am), after an overnight fast, for the determination of cortisol levels (mcg/L), TSH, and thyroid hormones levels.
Indirect calorimetry
REE was measured by means of indirect calorimetry using a canopy system (MMC Horizon, Sensor Medics, Anaheim, United States in a quiet environment, with the patients in the supine position for 20 min before measurement, because activities of daily living increase REE, but a short rest (20 min) before testing is sufficient for the effect to dissipate) and after a 12 h overnight fast. Measurement duration of 10 min with the first 5 min deleted and the remaining 5 min having a coefficient of variation < 10% gave accurate readings of REE[30]. Energy expenditure was derived from CO2 production and O2 consumption, with the appropriate Weir’s formula, neglecting protein oxidation[31]. The inter-day coefficient of variation of such measurements (as determined in six patients on subsequent days) was always less than 3%, without any sequence effect. Basal energy expenditure can be measured a number of different ways, but perhaps the most convenient way is by indirect calorimetry. This method is based on the assumption that metabolism is a reflection of energy expenditure. Since the oxidation of nutrients requires oxygen, by measuring oxygen consumption and carbon dioxide production, an estimate of energy production in kilocalories can be made. However, if indirect calorimetry is unavailable, the Harris-Benedict equations (multiple linear regression equations derived from a sample of normal individuals), based on height, weight, age, and sex, can be used clinically to estimate basal energy expenditure[8]. For females, the equation is: basal energy expenditure = 655.1 + (9.56 × body weight in kg) + (1.84 × height in cm) - (4.67 × age in years)[32,33]. REE was measured prior to start psychological and pharmacological interventions.
Statistical analysis
For between-group comparisons (AN vs BN), χ2 and Independent-Samples t test were applied, while Paired Sample t test was adopted to compare REE with predicted REE within each group. Correlation analyses (Pearson’s correlation), and subsequently linear regression analyses were performed in the whole sample, and within each group, to assess the associations between BMI, cortisol levels, eating specific and general psychopathology, and REE.
Subsequently, moderator and mediator effect analyses were performed. Whereas moderator variables specify when certain effects will hold, mediators consider how or why such effects occur (Baron and Kenny)[34]. The moderator function of third variables partitions a focal independent variable into subgroups, that establish its domains of maximal effectiveness in regard to a given dependent variable. Therefore, in order to evaluate whether the relationship between REE and BMI was different within Eating Disorders subgroups (first aim of the study), general linear model (GLM) was used to examine the moderating effect of diagnosis (AN vs BN) on the interaction between REE and BMI.
In order to evaluate the possible mechanisms that could explain the associations between REE and BMI (second aim of the study), mediators effect analyses were performed. The mediator function of a third variable represents the generative mechanism through which the focal independent variable is able to influence the dependent variable of interest.
RESULTS
Table 1 reported demographic, clinical and psychopathological variables for AN and BN patients. No significant difference was detected between AN and BN, with the exception of BMI and FT3, which were higher in BN as compared with AN patients. No significant difference was found between AN and BN, in terms of REE while predicted REE was significant lower in AN. REE was lower than predicted REE, in both AN (t = 2.27; P = 0.034) and BN (t = 5.82; P < 0.001) groups. These comparisons retained their significance even when adjusting for FT3 hormones.
Table 1.
General and clinical characteristics of the sample
| Anorexia Nervosa; n: 22 | Bulimia Nervosa; n: 21 | t | |
| Age (yr) | 31.73 ± 9.86 | 27.86 ± 7.12 | 1.46 |
| Education (yr) | 18.13 ± 3.78 | 18.66 ± 2.67 | 0.52 |
| BMI (kg/m2) | 15.43 ± 2.00 | 22.96 ± 1.49 | -13.8b |
| REE (kcal/d) | 1088 ± 174 | 1092 ± 207 | -0.06 |
| Predicted REE (kcal/d)a | 1196 ± 85 | 1421 ± 207 | 4.53b |
| Cortisol levels (mcg/L) | 513.65 ± 159.52 | 496.05 ± 133.22 | 0.37 |
| TSH (mU/L) | 2.14 ± 1.57 | 1.67 ± 0.70 | 1.22 |
| FT3 (pmol/L) | 3.82 ± 0.98 | 4.75 ± 0.81 | -3.35b |
| FT4 (pmol/L) | 14.10 ± 3.43 | 14.14 ± 2.29 | -0.03 |
| SCL-90 GSI | 1.59 ± 0.57 | 1.52 ± 0.70 | 0.31 |
| EDE-Q total | 3.24 ± 1.59 | 3.24 ± 1.16 | 0.08 |
| EDE-Q restraint | 3.26 ± 2.23 | 3.19 ± 1.33 | 0.11 |
| EDE-Q eating concern | 2.86 ± 1.65 | 2.96 ± 1.38 | -0.19 |
| EDE-Q weight concern | 3.36 ± 1.54 | 3.10 ± 1.53 | 0.54 |
| EDE-Q shape concern | 3.48 ± 1.66 | 3.74 ± 1.09 | -0.57 |
| Binge eating episodes (month frequency) | 9.7 ± 6.4 | ||
| Purging behaviours (month frequency) | 8.4 ± 5.9 |
Calculated by means of Harris-Benedict equation. Data are expressed as mean ± SD deviation; for between groups comparisons: Independent-Sample t test; aP < 0.05;
P < 0.01. REE: Resting energy expenditure; BMI: Body mass index; SCL-90 GSI: Symptom checklist (SCL 90-R) global severity index; EDE-Q: Eating disorder examination questionnaire.
GLM analysis (Figure 1; part A) was adopted to evaluate whether the relationship between REE and BMI was different according to EDs subgroups (AN and BN). GLM (age adjusted) showed a significant effect of REE by diagnosis on BMI (β = 0.32; P < 0.001). The significant interaction was confirmed even when adjusting for body surface area. Therefore, when the interaction was broken down (Figure 1; part B), an opposite pattern of association was found in AN and BN: the increased BMI was associated with higher REE in the BN group (age adjusted β = 0.64, P = 0.001), while in AN patients the reduced BMI was associated with higher REE (age adjusted β = 0.63, P = 0.003).
Figure 1.
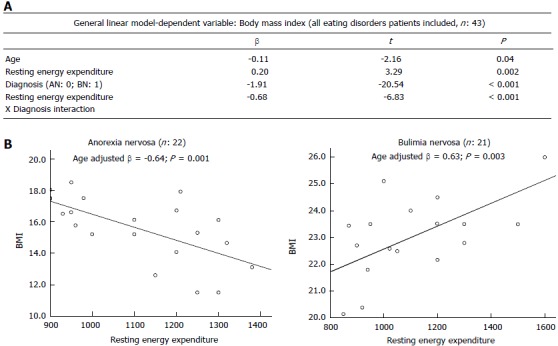
Moderators of the relationship between resting energy expenditure and body mass index. A: General linear model analysis (age adjusted) showed the effect of resting energy expenditure (REE) by diagnosis. Diagnosis was coded as dummy variables: 0 = Anorexia nervosa, 1 = Bulimia nervosa; B: The interaction showed in part A was broken down within diagnoses. Graphs reports results from linear regression analyses (age adjusted) of the opposite pattern of association between REE and body mass index (BMI) in anorexia nervosa and bulimia nervosa subjects.
To evaluate the possible underlying mechanisms for maintenance of REE in AN and BN, Person’s correlations were performed, considering psychopathological variables, cortisol levels, BMI and REE, within AN and BN patients (Table 2). No significant association was found in BN patients between REE and binge eating or purging behaviours, TSH or thyroid hormones levels. Considering AN patients, a strong positive association was found between REE and EDE-Q shape concern. Moreover, in the same group, significant positive correlations between EDE-Q shape concern and cortisol levels were also observed, as well as between cortisol levels and REE. Conversely, in BN group these associations were lacking or less significant than in AN.
Table 2.
Pearson’s correlations
| BMI | REE | Cortisol levels | SCL-90 GSI | EDE-Q total | EDE-Q restraint | EDE-Q EC | EDE-Q WC | EDE-Q SC | |
| Anorexia Nervosa patients; n: 22 | |||||||||
| Age | -0.11 | -0.07 | -0.05 | -0.58a | -0.12 | 0.06 | -0.13 | -0.12 | 0.02 |
| BMI | -0.63b | -0.49a | 0.13 | -0.32 | -0.20 | -0.42 | -0.25 | -0.46a | |
| REE | 0.83b | 0.10 | 0.39 | 0.17 | 0.33 | 0.45 | 0.63b | ||
| Cortisol levels | 0.68 | 0.33 | 0.09 | 0.28 | 0.34 | 0.68b | |||
| SCL-90 GSI | 0.40 | 0.34 | 0.35 | 0.35 | 0.76b | ||||
| Bulimia Nervosa patients; n: 21 | |||||||||
| Age | -0.16 | 0.14 | -0.04 | 0.09 | 0.24 | 0.38 | -0.12 | 0.42 | 0.13 |
| BMI | 0.59b | 0.77 | -0.20 | 0.16 | -0.21 | 0.45a | 0.15 | 0.2 | |
| REE | 0.24 | 0.00 | 0.43 | 0.33 | 0.42 | 0.19 | 0.46a | ||
| Cortisol levels | -0.10 | -0.05 | 0.33 | -0.12 | 0.12 | 0.23 | |||
| SCL-90 GSI | 0.37 | 0.28 | 0.40 | -0.02 | 0.30 | ||||
Data are Pearson’s correlation coefficients:
P < 0.05;
P < 0.01. REE: Resting energy expenditure; BMI: Body mass index; SCL-90 GSI: Symptom checklist (SCL 90-R) global severity index; EDE-Q: Eating disorder examination questionnaire; EC: Eating concern; WC: Weight concern; SC: Shape concern.
In order to explain the relationship between a more severe eating disorder psychopathology and a higher REE in AN, we hypothesized a possible mediating role of cortisol levels. According to Baron et al[34], we assumed a three variable system represented by a path diagram, where the dependent variable was REE (Figure 2). It included a direct impact (Path c) of the independent variable (EDE-Q shape concern), the impact of the mediator (cortisol levels; Path b), and the impact of the independent variable on the mediator (Path a). To test for mediation, we regressed the mediator on the independent variable, the dependent variable on the independent variable, the independent variable on the dependent variable (direct impact), and the dependent variable on both the independent variable and on the mediator. Separate coefficients were estimated and reported. According to this model, the following conditions supported mediation hypothesis: (1) variations in levels of the independent variable (EDE-Q shape concern) significantly accounted for variations in the mediator (cortisol levels; β = 0.47; P = 0.01); (2) variations in the mediator significantly accounted for variations in the dependent variable (REE; β = 0.80; P < 0.01); (3) variations in the independent variable significantly accounted for variations in the dependent variable (REE; β = 0.63; P < 0.01); (4) finally, when Paths a and b were controlled, this previously significant relation between EDE-Q shape concern and REE was no longer significant (β = 0.12; P = 0.53), while the relation between cortisol levels and REE retained its significance (β = 0.75; P = 0.002), showing strong evidence for a single, dominant mediator.
Figure 2.
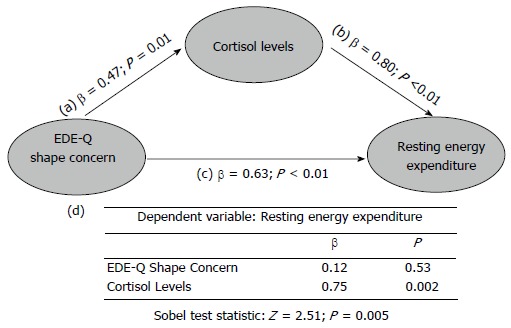
Mediator Model for the relationship between shape concern, cortisol levels and resting energy expenditure. The model included a direct impact (Path c) of the independent variable [eating disorder examination questionnaire (EDE-Q) shape concern]on the dependent variable (REE), the impact of the mediator (cortisol levels; Path b), and the impact of the independent variable on the mediator (Path a). The β values of separate regression analyses are reported in the graph. Table (d section of the figure) reported the regression analysis of the combined effect of EDE-Q shape concern, and cortisol levels on REE. The bottom part of the graph reports the results of the Sobel test, in order to calculate the indirect effect of shape concern on REE via the mediator (cortisol levels).
In order to calculate the indirect effect of EDE-Q shape concern on REE via the mediator (cortisol levels) we performed the Sobel test[35], which resulted to be significant (Z = 2.51; P = 0.005). The model described above was tested also for EDE-Q total and subscale scores (data not shown). The best fit for the data was represented by the model including EDE-Q shape concern scores.
DISCUSSION
To the best of our knowledge, this is the first study which evaluated REE in AN and BN, considering eating disorder specific psychopathology and HPA functioning as possible factors involved in the metabolic alterations.
According to our main results: (1) AN and BN patients showed an opposite pattern of association between BMI and REE. In AN patients, a higher REE was negatively associated with BMI, whereas BN patients showed a positive association between these two variables; (2) in AN patients only, a higher REE was associated with a more severe eating disorder specific psychopathology, and cortisol levels represented a possible mediating factor for this relationship.
According to previous findings[2,9,11,13], both AN and BN showed a reduced REE, which was significantly lower than the predicted REE. It has been suggested that dietary restraint places both AN and BN patients in a state of semi-starvation which is responsible for REE reduction, and it is partially compensated by binge eating behaviours in BN[7]. However, in the present study, AN patients did not show a reduced REE compared with BN, despite their lower BMI. In AN patients, we found that a higher REE was associated with a worse clinical condition, including lower BMI and higher eating disorder psychopathology.
It is generally assumed that AN patients maintain their low weight by severely restricting food intake, purging, and engaging in physical activity[36]. However, clinical observations consistently suggest that these patients gain weight with great difficulty, and they usually maintain their low body weight for a long time, as well. The physiological maintenance of their low body weight has been inferred from some clinical observations suggesting that AN patients apparently require high energy intake to gain weight[37]. This relative increase in energy expenditure could also account for the difficulties of AN subjects to gain weight when desired.
This pilot study provided evidences for possible explaining mechanisms of this phenomenon. We found that a severe underweight status in AN was associated with relative higher REE, and that these two conditions were both associated with an over concern about body shape, which represents the “core psychopathology” of AN.
In order to explain such a relationship, we hypothesized a mediating role of the HPA axis. According to mediation model by Baron et al[34], a given variable may be said to function as a mediator to the extent that it accounts for the relationship between the predictor and the criterion. In this study, the cortisol levels (mediator) explained how eating specific psychopathology accounted for REE variability in AN patients.
As a possible causal mechanisms, it has been supposed that increased plasma cortisol in AN is due to the higher corticotropin releasing hormone (CRH) production in the central nervous system. As already described, stress-which in this study was specifically related with eating psychopathology- may lead to the hypersecretion of CRH, which is known to be a potent anorexic agent[21].
Furthermore, previous findings supported the relationship between stress induced cortisol levels and REE. It has been suggested that, when in excess, cortisol is an overall catabolic hormone, which decreases lean body mass and muscle mass and increase energy expenditure[20,21]. Moreover, it increases availability of all fuel substrates by mobilization of glucose[38], free fatty acids[39], and amino acids from endogenous stores[40]. Given the cross-sectional design of the study, we are not able to derive final conclusion on causal relationship psychopathology and REE. The present mediation model support a strong mediator role of cortisol, suggesting a possible underlying mechanism. However, it does not explain all the variance of the mentioned association, which could be caused by other metabolic factors related to underweight in AN patients.
Some limitations of this pilot study should be considered. First of all we do not analyze the body composition of the patients, one of the determinant of the REE. Then, the cross- sectional design of the study does not allow any firm conclusion about causal relationships. Temperament and personality disorders information were not available. Finally the sample size is small, and it did not allow the generalization of the main findings. Moreover, it is possible that other psychopathological measures (e.g., EDE-Q weight concern) could reach significant associations with larger samples. Therefore, our results should be considered as preliminary, and larger, prospective researches are warranted in order to confirm or not these findings. The results of the present study supported the hypothesis that Cortisol levels in AN, through changes in REE, could represent a biological substrate for the capacity to maintain low body weight, and for the inefficiency at gaining weight. Safe and effective re-feeding strategies in Eating Disorders should carefully consider the complex mechanisms which can determine the energy balance impairment observed in AN and BN patients.
COMMENTS
Background
Metabolic changes in eating disorders patients may partially explain the obstacles of weight regain interventions. One of the reasons can be the resting energy expenditure alteration which appears to be correlated with the disorder severity in terms of both weight loss and eating disorder psychopathology. Cortisol levels, a measure of response to stress appear to be a putative biological mechanism underlying the association between eating psychopathology and resting energy expenditure.
Research frontiers
The present paper is in line with the recent advanced neurobiological and psychosomatic models based on the complex interaction between psychiatric conditions, stress response and subjective perception of symptoms.
Innovations and breakthroughs
Resting energy expenditure abnormalities have already been described in Anorexia Nervosa patients, but generally they do not consider the role of psychopathology, as a marker of severity of the disorder. The present study is original and innovative, since it proposes a model which includes resting expenditure, cortisol levels as a marker of stress, as well as eating disorder specific psychopathology.
Applications
The present study demonstrated that the severity of psychopathology represents not just an indicator of subjective perception of the eating disorder, but also of more compromised metabolic condition which could interfere with treatment process.
Terminology
Resting Energy Expenditure: it is based on the assumption that metabolism is a reflection of energy expenditure. Since the oxidation of nutrients requires oxygen, by measuring oxygen consumption and carbon dioxide production, an estimate of energy production in kilocalories can be made. Shape concern: it includes all the concerns and behaviors associated with once own body. It is the core psychopathological feature of eating disorders.
Peer review
This is, in summary, an interesting cross-sectional survey aimed to investigate the relationship between resting energy expenditure, eating psychopathology, and hypothalamus pituitary adrenal-axis functioning in twenty-two patients with eating disorders. The manuscript is interesting and well-written.
Footnotes
P- Reviewers: Akgül S, Inui A, Marchesini G, Serafini G S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Melchior JC, Rigaud D, Rozen R, Malon D, Apfelbaum M. Energy expenditure economy induced by decrease in lean body mass in anorexia nervosa. Eur J Clin Nutr. 1989;43:793–799. [PubMed] [Google Scholar]
- 2.Platte P, Pirke KM, Trimborn P, Pietsch K, Krieg JC, Fichter MM. Resting metabolic rate and total energy expenditure in acute and weight recovered patients with anorexia nervosa and in healthy young women. Int J Eat Disord. 1994;16:45–52. doi: 10.1002/1098-108x(199407)16:1<45::aid-eat2260160104>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Schebendach JE, Golden NH, Jacobson MS, Hertz S, Shenker IR. The metabolic responses to starvation and refeeding in adolescents with anorexia nervosa. Ann N Y Acad Sci. 1997;817:110–119. doi: 10.1111/j.1749-6632.1997.tb48200.x. [DOI] [PubMed] [Google Scholar]
- 4.Polito A, Fabbri A, Ferro-Luzzi A, Cuzzolaro M, Censi L, Ciarapica D, Fabbrini E, Giannini D. Basal metabolic rate in anorexia nervosa: relation to body composition and leptin concentrations. Am J Clin Nutr. 2000;71:1495–1502. doi: 10.1093/ajcn/71.6.1495. [DOI] [PubMed] [Google Scholar]
- 5.Russell J, Baur LA, Beumont PJ, Byrnes S, Gross G, Touyz S, Abraham S, Zipfel S. Altered energy metabolism in anorexia nervosa. Psychoneuroendocrinology. 2001;26:51–63. doi: 10.1016/s0306-4530(00)00036-6. [DOI] [PubMed] [Google Scholar]
- 6.Scalfi L, Marra M, De Filippo E, Caso G, Pasanisi F, Contaldo F. The prediction of basal metabolic rate in female patients with anorexia nervosa. Int J Obes Relat Metab Disord. 2001;25:359–364. doi: 10.1038/sj.ijo.0801547. [DOI] [PubMed] [Google Scholar]
- 7.de Zwaan M, Aslam Z, Mitchell JE. Research on energy expenditure in individuals with eating disorders: a review. Int J Eat Disord. 2002;32:127–134. doi: 10.1002/eat.10074. [DOI] [PubMed] [Google Scholar]
- 8.van Marken Lichtenbelt WD, Heidendal GA, Westerterp KR. Energy expenditure and physical activity in relation to bone mineral density in women with anorexia nervosa. Eur J Clin Nutr. 1997;51:826–830. doi: 10.1038/sj.ejcn.1600492. [DOI] [PubMed] [Google Scholar]
- 9.Kosmiski L, Schmiege SJ, Mascolo M, Gaudiani J, Mehler PS. Chronic starvation secondary to anorexia nervosa is associated with an adaptive suppression of resting energy expenditure. J Clin Endocrinol Metab. 2014;99:908–914. doi: 10.1210/jc.2013-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devlin MJ, Walsh BT, Kral JG, Heymsfield SB, Pi-Sunyer FX, Dantzic S. Metabolic abnormalities in bulimia nervosa. Arch Gen Psychiatry. 1990;47:144–148. doi: 10.1001/archpsyc.1990.01810140044007. [DOI] [PubMed] [Google Scholar]
- 11.Obarzanek E, Lesem MD, Goldstein DS, Jimerson DC. Reduced resting metabolic rate in patients with bulimia nervosa. Arch Gen Psychiatry. 1991;48:456–462. doi: 10.1001/archpsyc.1991.01810290068013. [DOI] [PubMed] [Google Scholar]
- 12.Detzer MJ, Leitenberg H, Poehlman ET, Rosen JC, Silberg NT, Vara LS. Resting metabolic rate in women with bulimia nervosa: a cross-sectional and treatment study. Am J Clin Nutr. 1994;60:327–332. doi: 10.1093/ajcn/60.3.327. [DOI] [PubMed] [Google Scholar]
- 13.Casper RC, Schoeller DA, Kushner R, Hnilicka J, Gold ST. Total daily energy expenditure and activity level in anorexia nervosa. Am J Clin Nutr. 1991;53:1143–1150. doi: 10.1093/ajcn/53.5.1143. [DOI] [PubMed] [Google Scholar]
- 14.Vaisman N, Rossi MF, Goldberg E, Dibden LJ, Wykes LJ, Pencharz PB. Energy expenditure and body composition in patients with anorexia nervosa. J Pediatr. 1988;113:919–924. doi: 10.1016/s0022-3476(88)80032-5. [DOI] [PubMed] [Google Scholar]
- 15.Brambilla F, Ferrari E, Brunetta M, Peirone A, Draisci A, Sacerdote P, Panerai A. Immunoendocrine aspects of anorexia nervosa. Psychiatry Res. 1996;62:97–104. doi: 10.1016/0165-1781(96)02992-7. [DOI] [PubMed] [Google Scholar]
- 16.Monteleone P, Luisi M, Colurcio B, Casarosa E, Monteleone P, Ioime R, Genazzani AR, Maj M. Plasma levels of neuroactive steroids are increased in untreated women with anorexia nervosa or bulimia nervosa. Psychosom Med. 2001;63:62–68. doi: 10.1097/00006842-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Lo Sauro C, Ravaldi C, Cabras PL, Faravelli C, Ricca V. Stress, hypothalamic-pituitary-adrenal axis and eating disorders. Neuropsychobiology. 2008;57:95–115. doi: 10.1159/000138912. [DOI] [PubMed] [Google Scholar]
- 18.Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. BIOL PSYCHIAT. 2008;63:1247–1254. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Voorhees E, Scarpa A. The effects of child maltreatment on the hypothalamic-pituitary-adrenal axis. Trauma Violence Abuse. 2004;5:333–352. doi: 10.1177/1524838004269486. [DOI] [PubMed] [Google Scholar]
- 20.Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271:E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- 21.Christiansen JJ, Djurhuus CB, Gravholt CH, Iversen P, Christiansen JS, Schmitz O, Weeke J, Jørgensen JO, Møller N. Effects of cortisol on carbohydrate, lipid, and protein metabolism: studies of acute cortisol withdrawal in adrenocortical failure. J Clin Endocrinol Metab. 2007;92:3553–3559. doi: 10.1210/jc.2007-0445. [DOI] [PubMed] [Google Scholar]
- 22.Misra M, Miller KK, Almazan C, Ramaswamy K, Aggarwal A, Herzog DB, Neubauer G, Breu J, Klibanski A. Hormonal and body composition predictors of soluble leptin receptor, leptin, and free leptin index in adolescent girls with anorexia nervosa and controls and relation to insulin sensitivity. J Clin Endocrinol Metab. 2004;89:3486–3495. doi: 10.1210/jc.2003-032251. [DOI] [PubMed] [Google Scholar]
- 23.Rigaud D, Verges B, Colas-Linhart N, Petiet A, Moukkaddem M, Van Wymelbeke V, Brondel L. Hormonal and psychological factors linked to the increased thermic effect of food in malnourished fasting anorexia nervosa. J Clin Endocrinol Metab. 2007;92:1623–1629. doi: 10.1210/jc.2006-1319. [DOI] [PubMed] [Google Scholar]
- 24.Konrad KK, Carels RA, Garner DM. Metabolic and psychological changes during refeeding in anorexia nervosa. Eat Weight Disord. 2007;12:20–26. doi: 10.1007/BF03327768. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed. ) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 27.Anacker C, Zunszain1 PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thure S, Price J, Pariante CM. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry. 2011:1–13. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord. 1994;16:363–370. [PubMed] [Google Scholar]
- 29.Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale--preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- 30.Compher C, Frankenfield D, Keim N, Roth-Yousey L; Evidence Analysis Working Group. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106:881–903. doi: 10.1016/j.jada.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Washington DC: Carnegie Institute; 1919. [Google Scholar]
- 33.Salisbury JJ, Levine AS, Crow SJ, Mitchell JE. Refeeding, metabolic rate, and weight gain in anorexia nervosa: a review. Int J Eat Disord. 1995;17:337–345. doi: 10.1002/1098-108x(199505)17:4<337::aid-eat2260170405>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 34.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 35.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equations models. In: Leinhart S, ed , editors. Sociological methodology. San Francisco: Jossey-Bass; 1982. pp. 290–312. [Google Scholar]
- 36.Obarzanek E, Lesem MD, Jimerson DC. Resting metabolic rate of anorexia nervosa patients during weight gain. Am J Clin Nutr. 1994;60:666–675. doi: 10.1093/ajcn/60.5.666. [DOI] [PubMed] [Google Scholar]
- 37.Kaye WH, Gwirtsman HE, Obarzanek E, George DT. Relative importance of calorie intake needed to gain weight and level of physical activity in anorexia nervosa. Am J Clin Nutr. 1988;47:989–994. doi: 10.1093/ajcn/47.6.989. [DOI] [PubMed] [Google Scholar]
- 38.Dinneen S, Alzaid A, Miles J, Rizza R. Metabolic effects of the nocturnal rise in cortisol on carbohydrate metabolism in normal humans. J Clin Invest. 1993;92:2283–2290. doi: 10.1172/JCI116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Djurhuus CB, Gravholt CH, Nielsen S, Mengel A, Christiansen JS, Schmitz OE, Møller N. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am J Physiol Endocrinol Metab. 2002;283:E172–E177. doi: 10.1152/ajpendo.00544.2001. [DOI] [PubMed] [Google Scholar]
- 40.Berneis K, Vosmeer S, Keller U. Effects of glucocorticoids and of growth hormone on serum leptin concentrations in man. Eur J Endocrinol. 1996;135:663–665. doi: 10.1530/eje.0.1350663. [DOI] [PubMed] [Google Scholar]


