Abstract
Objective
The liver is the key organ involved in systemic inflammation, but the relation between hepatic inflammation and atherogenesis is poorly understood. Since nuclear factor-κB (NF-κB) is a central regulator of inflammatory processes, we hypothesized that chronically enhanced hepatic NF-κB activation, through hepatocyte-specific expression of IκB kinase-β (IKKβ) (LIKK), will aggravate atherosclerosis development in APOE*3-Leiden (E3L) mice.
Methods and Results
E3L.LIKK and E3L control littermates were fed a Western-type diet for 24 weeks. E3L.LIKK mice showed a 2.3-fold increased atherosclerotic lesion area and more advanced atherosclerosis in the aortic root with less segments without atherosclerotic lesions (11 vs. 42%), and more segments with mild (63% vs. 44%) and severe (26% vs. 14 %) lesions. Expression of LIKK did not affect basal levels of inflammatory parameters, but plasma cytokine levels tended to be higher in E3L.LIKK mice after lipopolysaccharide (LPS) administration. E3L.LIKK mice showed transiently increased plasma cholesterol levels, confined to (V)LDL. This transient character resulted in a mild (+17%) increased cumulative plasma cholesterol exposure.
Conclusion
We conclude that selective activation of NF-κB in hepatocytes considerably promotes atherosclerosis development which is (at least partly) explained by an increased sensitivity to proinflammatory triggers and transiently increased plasma cholesterol levels.
Keywords: NF-κB, atherosclerosis, mouse models, liver, hepatocyte, inflammation, lipid metabolism
1. Introduction
Increased inflammation, in addition to disturbances in lipid metabolism, is the other main contributor to the development of atherosclerosis [1]. Nuclear factor-κB (NF-κB) has been identified as the most important transcription factor in the regulation of inflammatory processes during atherosclerosis development [2]. In unstimulated cells, NF-κB p65/p50 dimer is kept inactive by its inhibitory protein: inhibitor of κB (IκB). A wide range of extracellular stimuli, including cytokines, microbial components, and also free fatty acids, induce activation of the IκB kinase complex, which consists of two kinases (IKKα and -β) and a regulatory subunit, NEMO/IKKγ. This complex mediates the phosphorylation of IκB, resulting in its ubiquitination and degradation, leaving the NF-κB dimer free to translocate to the nucleus and activate its target genes [2].
While general inhibition of the NF-κB pathway by pharmacological agents reduces atherosclerosis development in mice [3,4], the relative contribution of NF-κB may differ at cellular- or tissue-specific level. Suppression of the NF-κB pathway in endothelial cells by ablation of NEMO/IKKγ has been shown to decrease atherosclerosis development [5]. In murine bone marrow transplantation models, inhibition of the NF-κB pathway at distinct levels in hematopoietic cells can have different outcomes, i.e. deficiency of the NF-κB p50 subunit resulted in smaller atherosclerotic lesions [6], whereas deletion of IKKβ increased atherosclerosis development [7]. Surprisingly, the role of the NF-κB pathway in hepatocytes on atherosclerosis development has not been investigated thus far.
The liver plays a central role in both lipid metabolism [8] and inflammation [9]. Disturbances in lipid metabolism and increased inflammation are the two main risk factors for atherogenesis [1]. Hepatocytes form the largest population of cells in the liver and execute most of its important functions. During inflammation, acute phase proteins are mainly synthesized by the hepatocytes [10]. Interestingly, hepatocyte-specific deficiency of gp130, a receptor component of IL-6 signaling which signals independent of the NF-κB pathway, decreases atherosclerosis in apoe−/− mice [11], suggesting that reduced hepatic inflammation is associated with less atherosclerosis development.
Despite ample evidence implicating the involvement of NF-κB in atherogenesis, the hepatocyte-specific role of NF-κB in atherosclerosis has not been investigated directly. Therefore, in this study we aimed to investigate whether chronic activation of hepatocyte-specific NF-κB aggravates atherosclerosis development. We used transgenic mice with hepatocyte-specific expression of human IKKβ (Liver-specific IKKβ or LIKK mice), resulting in an increase of active NF-κB [12], crossbred with atherosclerosis-prone APOE*3-Leiden (E3L) mice. E3L mice exhibit a human-like lipoprotein distribution on a cholesterol-rich diet due to transgenic expression of a human mutant of the APOE3 gene, and are therefore susceptible to atherosclerosis development [13]. Collectively, our results show that hepatocyte-specific NF-κB activation markedly aggravates atherosclerosis development in E3L mice.
2. Methods
Brief descriptions of the most important procedures of this study are provided in this section. An expanded description is available in the supplemental data (available online at http://atherosclerosisjournal. com).
2.1 Animals
Transgenic LIKK mice expressing constitutively active human IKKβ in hepatocytes under the control of an albumin promoter [12] were crossbred with E3L mice [13] to generate heterozygous E3L.LIKK and control E3L littermates, as described before [14]. Ten-12 weeks old female mice were fed a Western-type diet for 24 weeks. Blood was drawn every 4 weeks after a 4-hour fast.
2.2 Plasma analysis
Plasma levels of serum amyloid A (SAA), inflammatory cytokines, total cholesterol (TC), triglycerides (TG) and phospholipids (PL) levels were determined.
2.3 Lipopolysaccharide stimulation
Mice were injected i.v. with Salmonella minnesota Re595 lipopolysaccharide (LPS) (50 mg/kg body weight). Blood was collected 90 min after injection and plasma was assayed for cytokines.
2.4 Atherosclerosis quantification
The extent of atherosclerosis was assessed in the aortic root area. After staining with hematoxylin-phloxine- saffron (HPS), atherosclerotic lesion severity and area were determined.
3. Results
3.1 LIKK causes low-grade inflammation
The overall appearance of E3L and E3L.LIKK mice during the study was similar. To assess whether expression of LIKK affects body weight gain, we measured food intake and body weight weekly. Both were not different between E3L.LIKK and E3L control mice (Supplemental Fig. 1A–B). The liver- and spleen weight and histological morphology of the liver were also comparable between E3L.LIKK and E3L mice (data not shown). To gain more insight in the effects of LIKK on inflammation, we determined whether LIKK expression increased the inflammatory state of the liver and systemic inflammatory markers in E3L.LIKK mice on a Western-type diet. We confirmed previous findings [14] showing that the enhanced expression of hepatocyte-specific human IKKβ (Supplemental Fig. 2A) resulted in a 1.4-fold increased hepatic NF-κB activation, as shown by an increase in the phosphorylated p65 subunit (pNF-κBSer536) (Supplemental Fig. 2B). IKKβ kinase phosphorylates subunit p65 of NF-κB at the position Ser536, which activates the transcriptional activity of NF-κB [15]. The transgenic expression of human IKK² mRNA was present only in E3L.LIKK mice and did not alter murine IKK² mRNA expression (Supplemental Fig. 2C–D). The enhanced hepatic NF-κB activation in E3L.LIKK mice did not result in increased IL-6 expression in whole liver, but did result in a tendency towards increased IL-1β expression (P=0.085) and a significant increase in MCP-1 expression (Supplemental Table 2).
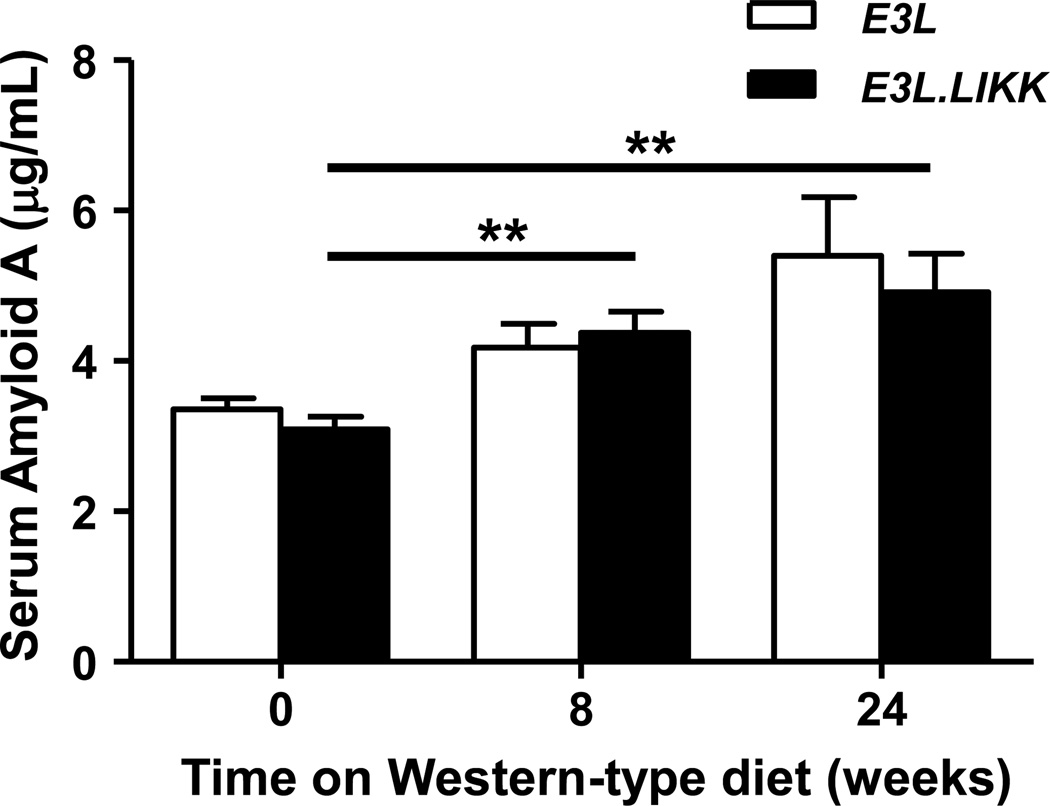
To evaluate whether the increased hepatocyte-specific NF-κB activation in E3L.LIKK mice enhanced the systemic inflammatory state, we determined the plasma inflammation marker SAA and plasma cytokines under basal conditions. LIKK expression did not affect SAA before (3.1 ±0.17 vs. 3.4 ±0.15 µg/mL) and after 8 weeks (4.4 ±0.28 vs. 4.2 ±0.31 µg/mL) and 24 weeks (4.9 ±0.51 vs. 5.4 ±0.78 µg/mL) of Western-type diet feeding (Fig. 1), and neither the determined plasma cytokine levels (Supplemental Fig. 3A–F). SAA levels increased with Western-type diet feeding in both E3L and E3L.LIKK mice, but this difference only reached statistical significance in E3L.LIKK mice (Fig. 1).
Figure 1. LIKK does not increase plasma SAA levels.
SAA levels were determined in plasma from E3L.LIKK (black bars) and E3L (white bars) mice fed a Western-type diet for 0, 8 and 24 weeks. Values are means ±SEM; n=15/group; **P<0.01.
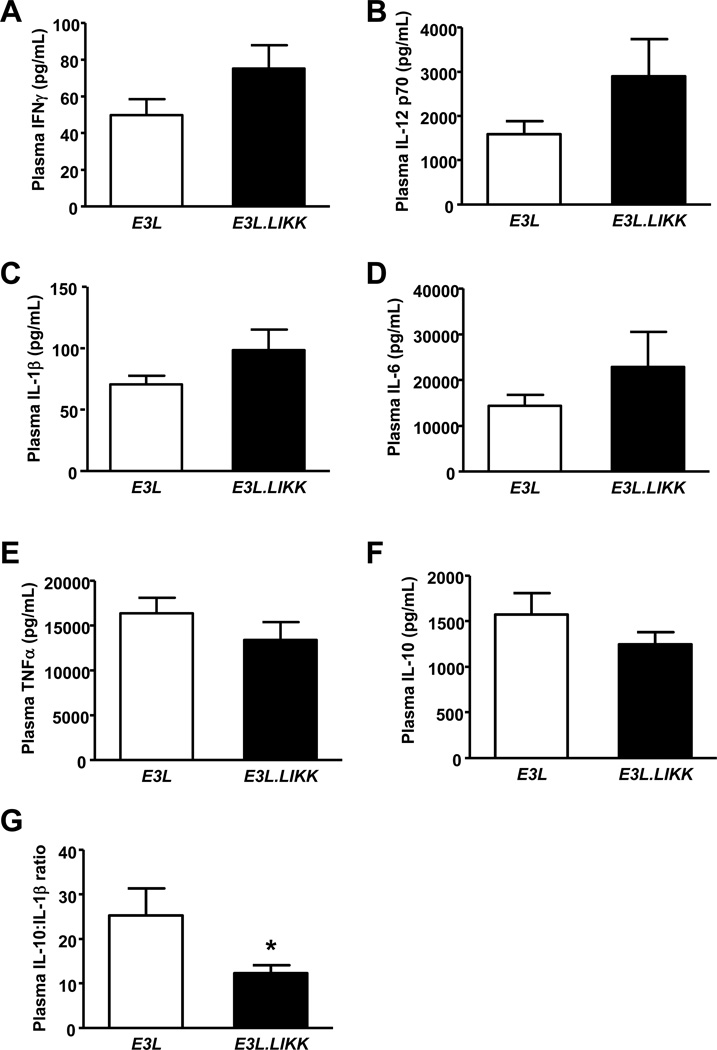
Since we did not observe a clear increased systemic proinflammatory state under basal conditions, we challenged the mice with LPS to boost the inflammatory response. Interestingly, after injection of LPS, proinflammatory cytokines (e.g. IL-1β, IFNγ) showed a tendency towards increased plasma levels in E3L.LIKK mice as compared to E3L mice (Fig. 2A–F). The anti-inflammatory IL-10:IL-1β ratio was significantly lower in E3L.LIKK mice (Fig. 2G). Overall, these data indicate that E3L.LIKK mice are more sensitive to proinflammatory triggers compared to their E3L littermates.
Figure 2. LIKK tends to enhance plasma cytokines after LPS stimulation.
LPS was injected intravenously in E3L.LIKK (black bars) and E3L (white bars) mice. Cytokine levels were measured 90 minutes after LPS injection (A-F). The IL-10:IL-1β ratio was calculated (G). Values are means ±SEM; n=7/group; *P<0.05.
To study whether this chronic low-grade inflammation in E3L.LIKK mice also resulted in increased inflammatory cell counts in liver and plasma, we determined the hepatic mRNA expression of various cell-type markers of inflammatory cells present in the liver, which are likely to influence atherogenesis [16], and the number of circulating monocytes. Hepatic mRNA expression of CD68 (Kupffer cells), CD3 ((NK)T cells), and Vα14 (NKT cells) were not different between the genotypes (Supplemental Table 2), neither were the total number of circulating monocytes, the proinflammatory Ly6C-hi monocyte subset, the intermediate Ly6C-med monocyte subset and the less inflammatory Ly6Clo monocyte subset (Supplemental Fig. 4A–D). Together, the above findings indicate that the enhanced hepatocyte-specific NF-κB activation in E3L.LIKK mice results in a tendency towards a mildly enhanced hepatic proinflammatory state and an elevated sensitivity to proinflammatory stimuli as compared to E3L littermates.
3.2 LIKK transiently enhances VLDL cholesterol levels
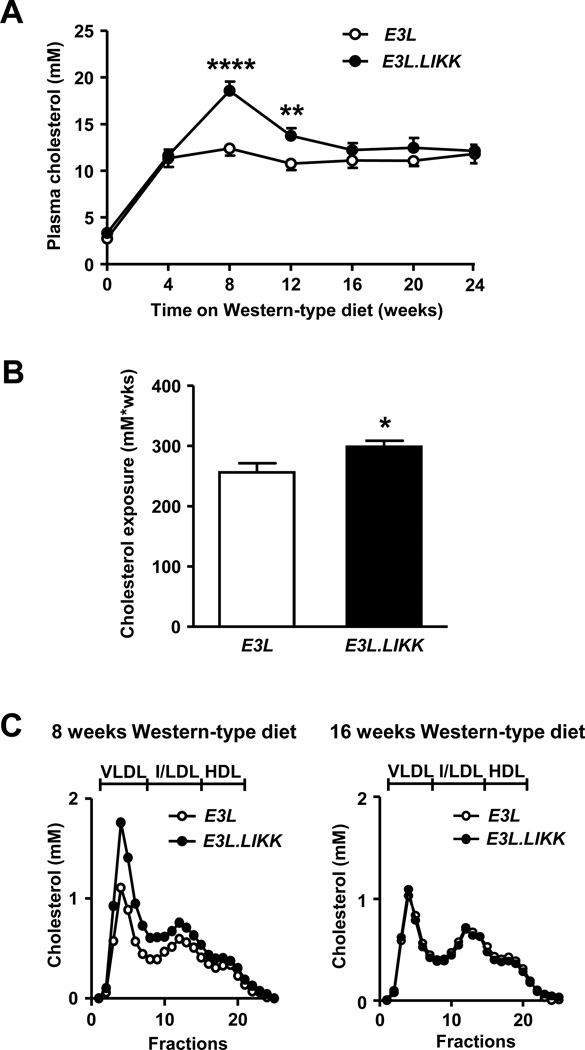
To assess the effect of hepatocyte-specific NF-κB activation on plasma lipid levels, TC, TG and PL concentrations were determined every 4 weeks in E3L.LIKK and E3L mice. LIKK expression caused a transient increase of plasma TC levels only at 8 weeks (+50%; P<0.0001) and 12 weeks (+28%; P<0.05) of Western-type diet feeding (Fig. 3A). Accordingly, the cumulative total cholesterol exposure was higher in E3L.LIKK than in E3L mice (+17%; P<0.05; Fig. 3B). A similar transient increase was found for plasma TG and PL levels (Supplemental Fig. 5A–B).
Figure 3. LIKK transiently increases (V)LDL.
Plasma cholesterol levels of E3L.LIKK (black symbols) and E3L (white symbols) mice fed a Western-type diet were assessed (A), and cumulative total cholesterol exposure was calculated (B). Lipoprotein profiles were determined at 8 (left) and 16 (right) weeks (C). Values are means ±SEM; n=15/group; *P<0.05, **P<0.01, ****P<0.0001.
To determine which lipoproteins contribute to the transient elevated plasma TC levels, lipoproteins were size-fractionated by FPLC, and cholesterol was measured in the individual fractions. The transient increase in plasma TC levels at 8 weeks of Western-type diet feeding in E3L.LIKK mice was confined to (V)LDL, whereas at 16 weeks the lipoprotein distribution in the E3L.LIKK mice was similar to that of the E3L mice, in line with the plasma lipid levels (Fig. 3C). Consistent with our previous finding that expression of LIKK increased the VLDL production in male mice on chow diet [14], we found that expression of LIKK increased, albeit not significantly, the VLDL-TG production rate (+24%) (Supplemental Fig. 6A), and tended to increase the VLDL-apolipoprotein B (apoB) production rate (+33%) (Supplemental Fig. 6B). No differences were observed in the liver lipid content between E3L.LIKK and E3L mice (Supplemental Fig. 7A–E). Taken together, these findings indicate that hepatocyte-specific NF-κB activation results in a modest and transient increase in plasma lipid levels in E3L mice.
3.3 LIKK enhances atherosclerosis development
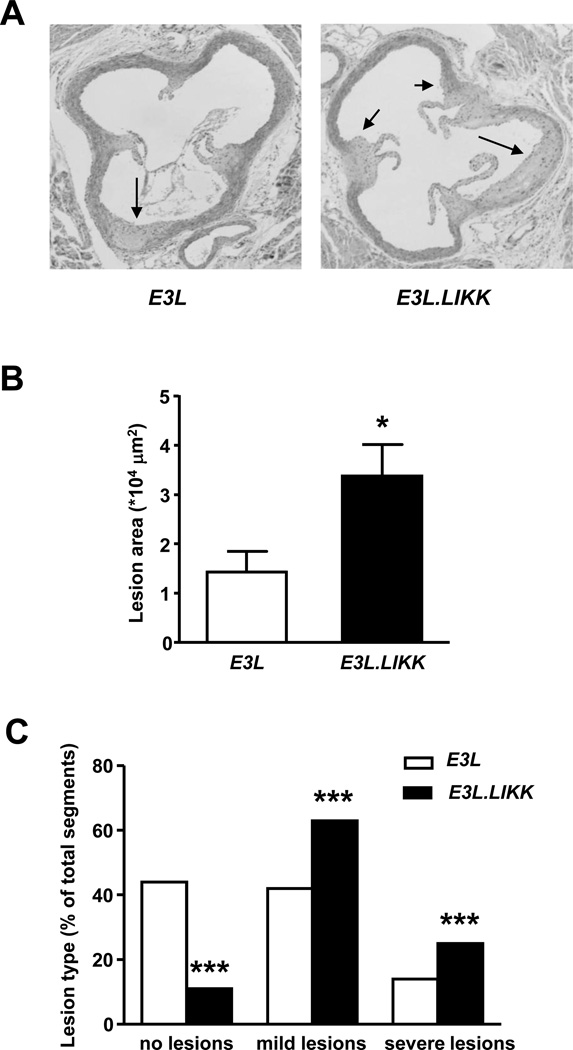
To investigate the effect of LIKK expression on atherosclerosis development, E3L.LIKK and E3L mice were sacrificed after 24 weeks of Western-type diet feeding, and lesion size and severity were measured in the aortic root. Representative pictures of both groups are shown in Fig. 4A. E3L.LIKK mice developed more than 2-fold larger atherosclerotic lesions (+131%; P<0.05; Fig. 4B) as compared to their E3L littermates. This increased lesion area coincided with more advanced lesion progression, since we found markedly fewer segments without atherosclerotic lesions (11% vs. 42%; P<0.001) and more segments with mild (63% vs. 44%; P<0.001) and severe lesions (26% vs. 14%; P<0.001) as compared to E3L mice (Fig. 4C). Examples of mild and severe lesions are shown in Supplemental Figure 8. These data indicate that chronic hepatocyte-specific NF-κB activation severely augments atherosclerosis development in E3L mice.
Figure 4. LIKK aggravates atherosclerotic lesion area and severity.
After 24 weeks of Western-type diet feeding, E3L.LIKK (black bars) and E3L (white bars) mice were sacrificed and cross-sections of aortic roots were stained with HPS. Representative pictures are shown. Arrows indicate lesions (A). Total lesion area was assessed in 4 sections of the aortic root (B) and lesion severity was determined separately in each of the 3 segments between the aortic valves of the 4 sections (C). Statistical analysis for lesion area was performed by Mann-Whitney U test, for lesion severity was determined by the χ2 test. Values are means ±SEM; n=15/group; *P<0.05, ***P<0.001.
3.4 LIKK aggravates atherosclerotic lesion composition
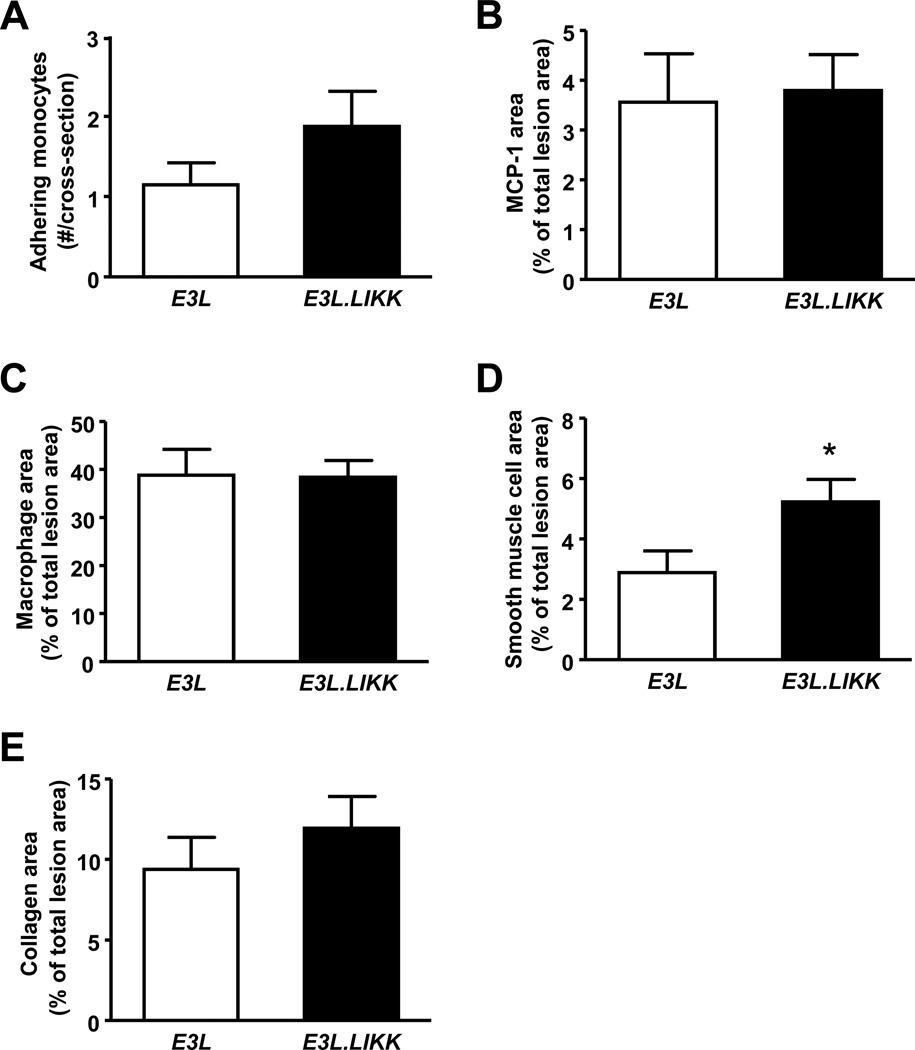
We next evaluated whether LIKK expression would affect monocyte adherence and recruitment to the vascular wall, as well as the composition of the atherosclerotic lesions with respect to the macrophage, smooth muscle cell, and collagen content of the lesions. Adherence of monocytes to the vessel wall and the content of the chemokine monocyte chemoattractant protein-1 (MCP-1) of the atherosclerotic lesions were not significantly enhanced in E3L.LIKK mice as compared to E3L mice (Fig. 5A–B). LIKK expression did not affect the relative macrophage and collagen content of the lesions (Fig. 5C+E), but did result in an increased smooth muscle cell content of the lesions (+79%, P<0.05; Fig. 5D).
Figure 5. LIKK induces more advanced atherosclerotic lesions.
In the sections obtained as described in Fig. 4, the number of adhering monocytes (A), monocyte chemoattractant protein-1 (MCP-1) content (B), 20 macrophage content (C), smooth muscle content (D) and collagen content (E) was determined. Values are means ±SEM; n=15/group; *P<0.05, **P<0.01.
3.5 Aggravated atherosclerosis development in E3L.LIKK mice does not solely depend on the transient increase in plasma cholesterol levels
In E3L mice on Western-type diet, the cumulative plasma cholesterol exposure is highly predictive for the atherosclerotic lesion area (unpublished data, J.F.P. Berbée, P.C.N. Rensen). To verify if the transient increase in plasma TC levels (Fig. 3) alone could account for the aggravation in atherosclerosis development observed in E3L.LIKK mice, or whether additional mechanism(s) could contribute, including the low-grade systemic inflammation, we assessed the correlation between the cumulative plasma total cholesterol exposure and the atherosclerotic lesion area of the E3L.LIKK and E3L mice. As expected, there was a significant positive logarithmic correlation between the atherosclerotic lesion area and the cumulative plasma cholesterol exposure in the control E3L mice (Supplemental Fig. 9A; r2=0.757, P=0.002). However, we did not observe such a correlation in the E3L.LIKK mice (Supplemental Fig. 9B; r2=−0.250, P=0.369), indicating that in addition to the transient increase in plasma TC levels in E3L.LIKK mice, additional mechanism(s), most likely the increased sensitivity to proinflammatory stimuli, contributed to the aggravated atherosclerosis development in these mice.
4. Discussion
NF-κB is regarded as a potential therapeutic target in atherosclerosis [3, 4] and studying tissue- and cellspecific effects of NF-κB in atherogenesis will expand our knowledge in the comprehensive actions of NF-κB on atherosclerosis development. The present study demonstrates for the first time that chronic, hepatocyte-specific expression of IKK² (LIKK) and subsequent activation of NF-κB aggravates atherosclerosis development in E3L mice. In addition, the atherosclerotic lesion composition with respect to the macrophage and collagen content was not affected by LIKK, but in line with the presence of more advanced lesions, the smooth muscle cell content was increased. Expression of LIKK resulted in transiently increased plasma cholesterol levels and an enhanced sensitivity to proinflammatory triggers, which both are likely to have contributed to the increased atherosclerotic lesion size and severity. Since lesion size and severity are often correlated in atherosclerosis studies with different murine models [13, 17], the increased lesion severity in E3L.LIKK mice is likely to be mainly attributed to the larger size of the lesions.
Expression of LIKK in E3L mice increased the activation of the NF-κB pathway in the liver, in line with our previous report [14]. In addition, hepatic mRNA expression of inflammatory parameters was increased or tended to be increased in E3L.LIKK mice, indicating that inflammatory mediators at local tissue level were enhanced in E3L.LIKK mice. This enhanced activation of hepatocyte-specific NF-κB in E3L.LIKK mice, however, did not result in a significant increased systemic proinflammatory state under basal conditions as compared to their E3L littermates. Importantly, Cai et al. [12] demonstrated that in LIKK mice on a wild-type background, systemic levels of IL-6 were only mildly elevated, while IL-1β and TNFα levels were similar as in wild-type mice. Our results show that LIKK expression on an E3L background resulted in a less pronounced hepatic inflammatory state as compared to LIKK expression on a wild-type background as described by Cai et al. [12], as reflected in a smaller increase in active NF-κB (1.4- vs. 2.2-fold) and mRNA levels of proinflammatory cytokines levels in the liver. Furthermore, under basal conditions E3L mice have lower levels of active NF-κB present in the liver as compared to wild-type mice (unpublished data, J.A. van Diepen, M.C. Wong, P.J. Voshol). This implies that E3L mice have a lower chronic inflammatory state than wild-type mice, which could interfere with the proinflammatory effects caused by expression of LIKK in the present study. Also, in comparison with other murine atherosclerosis models, e.g. the apoe−/− and ldlr−/− mice, E3L mice display a milder phenotype with respect to hyperlipidemia and increased inflammation [18, 19]. In the current study, basal circulating levels of some cytokines were at borderline of the detection limit of current assays (Supplemental Fig. 3) and, as expected, the cytokine levels increased approximately 5- to 3700-fold after LPS injection (Fig. 2). Furthermore, after stimulation with LPS, E3L.LIKK mice showed a tendency towards a higher systemic inflammatory state than E3L mice.
There is a strong interaction between inflammation and lipid metabolism [20]. For example, lowering inflammation using salicylate did not only reduced NF-κB activation, but concomitantly also reduced circulating cholesterol levels in E3L mice [21]. In line with this observation, in the present study we found higher plasma lipid levels at 8 weeks of Western-type diet feeding in female E3L.LIKK compared to E3L mice, which were confined to (V)LDL. We hypothesize that the increased lipid levels at this time point are accompanied by increased systemic inflammation, which is in line with previous findings showing that lipid metabolism and inflammation strongly influence each other [20]. A possible cause for the increased plasma lipid levels at 8 weeks of diet is therefore a more enhanced inflammation in the liver, possibly due to an increased activation of the NF-κB pathway in the liver.
We recently reported that male E3L.LIKK mice on chow diet also showed enhanced (V)LDL levels as a result of an increased hepatic VLDL-TG production rate [14], and found in the current study a trend towards an enhanced VLDL-apoB production in female E3L.LIKK mice on Western-type diet, with a similar effect-size. Possible reasons for the less apparent increase of VLDL-TG production in females compared to males are differences in gender and/or diet. Although the increase in VLDL-TG production is more apparent in male E3L.LIKK mice, we used female mice in the present study. The main reason for this is that female E3L mice are more susceptible to develop atherosclerosis. In order for male E3L mice to become similarly atherosclerosis-prone they need to be fed Western-type diets not only with higher percentages of cholesterol, but also containing cholate. In addition, fructose was added to the drinking water to further raise their (V)LDL-cholesterol levels [22]. The increase in (V)LDL levels in females in the current study was only transient at 8 weeks of Western-type diet feeding and disappeared at 16 weeks. Since no differences in plasma lipid levels and hepatic mRNA expression of genes involved in lipid metabolism were detected between both groups at 24 weeks of diet, the increased VLDL-TG production at 8 weeks of diet is likely to be transient. At present, we cannot explain the transient nature of this increase in (V)LDL levels, but it may be the result of a progressive negative feedback mechanism to reduce the hepatic VLDL production which takes place during long-term Western-type diet feeding.
Dyslipidemia is regarded as the classical risk factor for atherosclerosis development. The transiently enhanced total cholesterol levels, resulting in a modest increase (+17%) in cumulative total cholesterol exposure upon LIKK expression, thus likely contributed to the enhanced atherosclerosis development. Previous diet-induced atherosclerosis studies in E3L mice have consistently demonstrated that there is a positive logarithmic relation between the cumulative cholesterol exposure during the study and the atherosclerotic lesion area (J.F.P. Berbée, P.C.N. Rensen, unpublished data). In agreement with these previous observations, we did observe such a significant logarithmic relation in E3L mice but not in E3L.LIKK mice. This suggests that the increase in atherosclerotic lesion area in E3L.LIKK mice can only partly be attributed to the transiently enhanced plasma cholesterol levels and that additional mechanisms are involved.
Inflammation is the second main risk factor for atherosclerosis. Enhanced extravascular or systemic inflammation, by the periodontal pathogen Porphyromonas gingivalis [23] or by repeated administration of LPS [24], respectively, promotes atherosclerosis development. In addition, in humans, low-grade systemic inflammation is associated with enhanced risk of coronary artery disease [25, 26]. It is thus likely that, as discussed above, the increased sensitivity for proinflammatory triggers such as LPS in E3L.LIKK mice also directly contributed to the enhanced atherosclerotic lesion formation.
We excluded higher circulating levels of proinflammatory Ly6C-himonocytes as being another possible contributor to the aggravated atherosclerosis development in E3L.LIKK mice. Adhesion of monocytes to endothelial cells and subsequent migration into the vessel wall is one of the crucial steps in atherosclerotic lesion formation. Ly6C-hi monocytes are more prone to adhere to activated endothelium than Ly6C-lomonocytes and are, therefore, associated with enhanced atherosclerosis development [27]. We found that E3L.LIKK mice had similar levels of circulating subsets of monocytes as compared to their E3L controls, which is consistent with the observed similar number of adhering monocytes to the vascular wall.
In line with the enhanced atherosclerosis development that we observed in E3L.LIKK mice, Luchtefeld et al. [11] have reported that gp130-deficient mice with defective IL-6 signaling specifically in hepatocytes, develop less atherosclerosis, indicating that modulation of hepatic inflammation can have profound effects on atherogenesis. These studies also underscore that enhanced inflammation in the liver, e.g. due to viral hepatitis or steatohepatitis, may augment atherosclerosis development. Indeed, in several clinical studies, such hepatic pathological conditions are associated with an elevated occurrence of CVD [28, 29, 30]. Even after adjustment for classical risk factors for CVD, such as LDL cholesterol levels, chronic hepatitis C infection was still significantly associated with increased atherosclerosis in a cross-sectional study [30]. Together, these findings suggest that there is a direct effect of hepatic inflammation on atherosclerosis development, independent of systemic lipid levels. Moreover, they suggest that in addition to the currently used lipid-targeted drugs such as statins, reducing NF-κB activity in the liver may be a promising additive therapeutic strategy against atherosclerosis development.
In conclusion, we have shown that hepatocyte-specific activation of NF-κB leads to larger and more advanced atherosclerotic lesions. Our studies furthermore suggest that both the transient elevated (V)LDL cholesterol levels as well as the increased sensitivity to proinflammatory stimuli are most likely responsible for this aggravating effect on atherosclerosis. These findings contribute to the present understanding of the role of the liver, and more specifically the role of hepatic NF-κB, in atherosclerosis development and may help to develop new innovative anti-atherosclerotic strategies.
Supplementary Material
Acknowledgments
Funding
This work was supported by grants from the Netherlands Organization for Scientific Research (NWO Mosaic; 017.003.83 [to L.H.]) and F.R. Nieuwenkamp stichting. P.C.N. Rensen is an Established Investigator of the Netherlands Heart Foundation (Grant 2009T038).
Abbreviations used
- ApoB
apolipoprotein B
- CE
cholesteryl ester
- Cpt1a
carnitine palmitoyltransferase 1a
- Cyclo
cyclophilin
- E3L
APOE*3-Leiden
- Fas
fatty acid synthase
- FC
free cholesterol
- FPLC
fast performance liquid chromatography
- Gapdh
glyceraldehyde-3-phosphate dehydrogenase
- Hmgcr
HMG-CoA reductase
- Hprt
hypoxanthine-guanine phosphoribosyl transferase
- HPS
haematoxylin phloxine saffron
- IFNγ
interferon γ
- IκB
inhibitor of κB
- IKKβ
IκB kinase-β
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IL-10
interleukin-10
- IL-12p70
interleukin-12p70
- LIKK
liver-specific IKKβ
- MCP-1
monocyte chemoattractant protein-1
- MTTP
microsomal triglyceride transfer protein
- NF-κB
nuclear factor-κB
- PL
phospholipid
- RT-PCR
real-time PCR
- SAA
serum amyloid A
- Srebp-1c
sterol-regulatory element binding protein
- TC
total cholesterol
- TG
triglyceride
- TNFα
tumor necrosis factor α
- VLDL
very-low-density lipoprotein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None
Supplementary data
Supplementary data associated with this article can be found in the online version at http://atherosclerosisjournal.com.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.de Winther MP, Kanters E, Kraal G, et al. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 3.Chiba T, Kondo Y, Shinozaki S, et al. A selective NFkappaB inhibitor, DHMEQ, reduced atherosclerosis in ApoE-deficient mice. J Atheroscler Thromb. 2006;13:308–313. doi: 10.5551/jat.13.308. [DOI] [PubMed] [Google Scholar]
- 4.Cuaz-Perolin C, Billiet L, Bauge E, et al. M. Antiinflammatory and Antiatherogenic Effects of the NF-kappaB Inhibitor Acetyl-11-Keto-beta-Boswellic Acid in LPS-Challenged ApoE−/− Mice. Arterioscler Thromb Vasc Biol. 2007;28:272–277. doi: 10.1161/ATVBAHA.107.155606. [DOI] [PubMed] [Google Scholar]
- 5.Gareus R, Kotsaki E, Xanthoulea S, et al. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Kanters E, Gijbels MJ, van der Made I, et al. Hematopoietic NF-kappaB1 deficiency results in small atherosclerotic lesions with an inflammatory phenotype. Blood. 2004;103:934–940. doi: 10.1182/blood-2003-05-1450. [DOI] [PubMed] [Google Scholar]
- 7.Kanters E, Pasparakis M, Gijbels MJ, et al. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavoie JM, Gauthier MS. Regulation of fat metabolism in the liver: link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell Mol Life Sci. 2006;63:1393–1409. doi: 10.1007/s00018-006-6600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 10.Trautwein C, Boker K, Manns MP. Hepatocyte and immune system: acute phase reaction as a contribution to early defence mechanisms. Gut. 1994;35:1163–1166. doi: 10.1136/gut.35.9.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luchtefeld M, Schunkert H, Stoll M, et al. Signal transducer of inflammation gp130 modulates atherosclerosis in mice and man. J Exp Med. 2007;204:1935–1944. doi: 10.1084/jem.20070120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westerterp M, van der Hoogt CC, de Haan W, et al. Cholesteryl ester transfer protein decreases high-density lipoprotein and severely aggravates atherosclerosis in APOE*3-Leiden mice. Arterioscler Thromb Vasc Biol. 2006;26:2552–2559. doi: 10.1161/01.ATV.0000243925.65265.3c. [DOI] [PubMed] [Google Scholar]
- 14.van Diepen JA, Wong MC, Guigas B, et al. Hepatocyte-specific IKK-beta activation enhances VLDL-triglyceride production in APOE*3-Leiden mice. J Lipid Res. 2011 doi: 10.1194/jlr.M010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki CY, Slemenda CF, Ghosh P, et al. Traf1 induction and protection from tumor necrosis factor by nuclear factor-kappaB p65 is independent of serine 536 phosphorylation. Cancer Res. 2007;67:11218–11225. doi: 10.1158/0008-5472.CAN-07-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 17.Lutgens E, de Muinck ED, Heeneman S, et al. Compensatory enlargement and stenosis develop in apoE(−/−) and apoE*3-Leiden transgenic mice. Arterioscler Thromb Vasc Biol. 2001;21:1359–1365. doi: 10.1161/hq0801.093669. [DOI] [PubMed] [Google Scholar]
- 18.Zadelaar S, Kleemann R, Verschuren L, et al. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol. 2007;27:1706–1721. doi: 10.1161/ATVBAHA.107.142570. [DOI] [PubMed] [Google Scholar]
- 19.Kleemann R, Verschuren L, van Erk MJ, et al. Atherosclerosis and liver inflammation induced by increased dietary cholesterol intake: a combined transcriptomics and metabolomics analysis. Genome Biol. 2007;8:R200. doi: 10.1186/gb-2007-8-9-r200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khovidhunkit W, Kim MS, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.de Vries-van der Weij, Toet K, Zadelaar S, et al. Anti-inflammatory salicylate beneficially modulates pre-existing atherosclerosis through quenching of NF-kappaB activity and lowering of cholesterol. Atherosclerosis. 2010;213:241–246. doi: 10.1016/j.atherosclerosis.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Trion A, de Maat MP, Jukema JW, et al. No effect of C-reactive protein on early atherosclerosis development in apolipoprotein E*3-leiden/human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol. 2005;25:1635–1640. doi: 10.1161/01.ATV.0000171992.36710.1e. [DOI] [PubMed] [Google Scholar]
- 23.Gibson FC, III, Yumoto H, Takahashi Y, et al. Innate immune signaling and Porphyromonas gingivalis-accelerated atherosclerosis. J Dent Res. 2006;85:106–121. doi: 10.1177/154405910608500202. [DOI] [PubMed] [Google Scholar]
- 24.Westerterp M, Berbée JF, Pires NM, et al. Apolipoprotein C-I is crucially involved in lipopolysaccharide-induced atherosclerosis development in apolipoprotein E-knockout mice. Circulation. 2007;116:2173–2181. doi: 10.1161/CIRCULATIONAHA.107.693382. [DOI] [PubMed] [Google Scholar]
- 25.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang L, Wei H, Mak KH, et al. Markers of low-grade inflammation and soluble cell adhesion molecules in Chinese patients with coronary artery disease. Can J Cardiol. 2004;20:1433–1438. [PubMed] [Google Scholar]
- 27.Swirski FK, Libby P, Aikawa E, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishizaka N, Ishizaka Y, Takahashi E, et al. Increased prevalence of carotid atherosclerosis in hepatitis B virus carriers. Circulation. 2002;105:1028–1030. doi: 10.1161/hc0902.105718. [DOI] [PubMed] [Google Scholar]
- 29.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49:600–607. doi: 10.1016/j.jhep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Mostafa A, Mohamed MK, Saeed M, et al. Hepatitis C infection and clearance: impact on atherosclerosis and cardiometabolic risk factors. Gut. 2010;59:1135–1140. doi: 10.1136/gut.2009.202317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.