Introduction
…. is sure design'd, by fraud or force: trust not their presents, nor admit the horse.
Virgil, Aeneid
Human immunodeficiency virus type 1 (HIV-1), the lymphotropic virus that causes AIDS, has infected more than 60 million people worldwide since its clinical appearance in 1981. Despite intensive prevention efforts, the HIV/AIDS epidemic continues to spread, particularly in developing countries in sub-Saharan Africa, southeast Asia and the Caribbean, as well as the developed world [1]. Although HIV can be transmitted very efficiently parenterally, the advent of routine blood screening prior to transfusion and harm reduction programs for injection drug users, have made this mode of transmission much less common than mucosal transmission. Most new HIV infections are attributable to mucosal transmission: through genital and rectal mucosae in the case of sexual transmission and through oral or gastrointestinal mucosae in the case of mother-to-child transmission [2]. Much has been learned about HIV pathogenesis and infection mechanisms at the molecular level, but the scientific community has yet to develop an effective vaccine or microbicide for HIV prevention. Many unanswered questions remain concerning HIV-1 sexual transmission.
In 1983, barely 2 years into the AIDS epidemic, we hypothesized that the agent that was subsequently identified as HIV-1 may be sexually transmitted by infected ‘Trojan Horse’ leukocytes in semen [3]. This hypothesis was based on our knowledge at the time that human semen contains substantial numbers of T lymphocytes and macrophages, which could host a T-cell tropic virus, and the following assumptions: intracellular virus would be better protected than free virus from adverse effects of antiviral factors in the genital environment such as antiviral antibodies likely to be present in genital secretions of the virus-infected transmitter, as well as antimicrobial peptides that play an important role in genital innate immune defense; and virus-infected allogeneic cells could also escape early detection by major histocompatibility complex (MHC)-restricted cytotoxic T cells in a new host. Over the intervening 25+ years, others have also championed this cause [4,5], and convincing evidence has emerged from clinical research as well as in-vitro and animal studies that infected leukocytes indeed play a role in HIV transmission. Yet, most recent research on sexual HIV transmission has focused on cell-free HIV in genital secretions because of the wide availability of HIV RNA quantification assays. Furthermore, the majority of HIV vaccines and microbicides have been designed to block transmission of cell-free virus and have been tested in animal and in-vitro models that use cell-free virus as the only infectious inoculum. As the molecular events underlying cell-associated HIV transmission differ from those underlying cell-free virus transmission, many of the current vaccine and microbicide candidates might not be expected to protect against cell-associated HIV transmission. The failure of several recent vaccine and microbicide clinical trials may be due in part to this oversight. It should be possible to design strategies that block cell-associated HIV transmission as well as cell-free HIV transmission.
In this article, we present an overview of research that has been conducted on cell-associated HIV mucosal transmission and recommendations for future research. We focus on sexual HIV transmission, but this review also has relevance for mother-to-child HIV transmission, which may occur through mucosal transmission of cell-associated HIV from maternal genital or mammary gland secretions [6–8]. We review published reports that describe and enumerate HIV-infected cells in genital secretions, and compelling evidence from clinical, animal and in-vitro studies demonstrating that such cells can transmit HIV across genital tract epithelial surfaces; potential molecular mechanisms underlying cell-associated HIV transmission that could be specifically targeted by future HIV prevention strategies; and in-vitro and animal cell-associated HIV transmission models currently used for studies on cell-associated HIV transmission mechanisms and for testing vaccine and microbicide candidates. Using this information as a foundation, we discuss the evidence and probability that various current microbicide and vaccine approaches prevent cell-associated HIV transmission, and suggest additional microbicide and vaccine concepts and experiments that will move this field forward.
Putative cellular vectors of HIV mucosal transmission
Seminal leukocytes
Cell populations
The principal cell types in human semen are spermatozoa, immature germ cells, and white blood cells (WBCs) (Fig. 1). WBCs enter semen from various sites along the reproductive tract, including the rete testis, epididymis, prostate, and urethra, where they play an immunosurveillance role [9]. WBCs in semen have been enumerated and characterized by immunohistology and FACS analysis. Most of these studies indicate that semen from healthy non-HIV-infected men contains on the order of 10 WBCs/ml, the majority of which are polymorphonuclear leukocytes, although substantial numbers of macrophages and CD4+ T cells are also present [10– 14]. Macrophages usually outnumber CD4+ T cells in semen. This is especially the case in HIV-infected men in whom seminal CD4+ lymphocytes are depleted; in one study of 98 antiretroviral therapy (ART)-naive HIV-positive men, the median ratio of macrophages to CD4+ lymphocytes in semen was 22:1 [15] (Table 1). These data indicate that macrophages are the most abundant HIV-susceptible host cell in semen and a likely principal mediator of cell-associated HIV transmission.
Fig. 1. Leukocytes in human genital secretions, identified by immunohistochemistry.
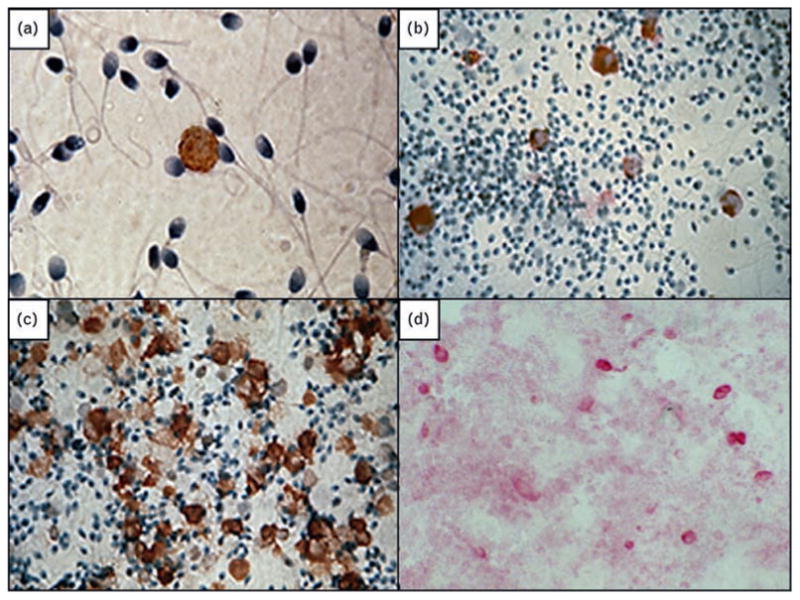
(a) CD4+ T cell in semen. (b) CD68+ macrophages in semen. (c) CD45+ leukocytes in semen from a man with leukocytospermia. (d) CD68+ macrophages in cervicovaginal secretions. Magnification × 400.
Table 1. White blood cell concentrations in semen.
Concentrations of WBCs in semen are highly variable. Leukocytospermia, an asymptomatic genital inflammatory condition characterized by more than 106 WBCs/ml semen [16,17] occurs in approximately 5–10% of healthy non-HIV-infected men [18–20] and as many as 24% of HIV-infected men [21]. Leukocytospermic semen contains substantially elevated concentrations of macrophages and CD4+ T cells [22]. In some HIV-positive leukocytospermic men, the seminal macrophage cell count has exceeded 107 cells/ml and the CD4 T-cell count exceeded 2 × 106 cells/ml (these cases are described in more detail below). HIV-infected cells have also been detected in pre-ejaculatory fluid, a urethral secretion secreted from the glands of Littre and Cowper glands during sexual stimulation, and these may also facilitate the sexual transmission of HIV [23,24].
Other important HIV-susceptible host cells such as dendritic and Langerhans cells have not been detected in semen, although it is possible that some viable HIV-infected Langerhans cells from penile skin, especially the inner foreskin [25–27], are shed in the vagina or rectum during intercourse.
Prevalence and quantity of HIV-infected leukocytes in semen
Most quantitative studies of HIV in semen have used commercially available HIV RNA assays to measure copy numbers of cell-free virions in seminal plasma; only a few have used HIV DNA PCR assays to assess the prevalence or number of HIV-infected cells in semen. In these studies, the prevalence of HIV proviral DNA in semen samples has ranged from 21 to 65% and the amount of HIV DNA has ranged from not detectable to 80 000 copies/ml (Table 2) [28–38]. Interestingly, in two of the larger studies that assessed both HIV RNA and DNA copy numbers in semen, these two parameters were not correlated [34,35]. Elevated proviral HIV DNA levels in semen have been associated with reduced peripheral CD4+ cell counts [32], acute HIV infection [39], leukocytospermia and recent sexually transmitted infection (STI) [32,40], and vasectomy [34]. After initiation of HAART, levels of both HIV RNA and DNA are reduced in semen, although HIV proviral DNA-bearing cells can persist in semen for several months [35,37] and have been shown to be infectious in vitro [33].
Table 2. Studies on HIV DNA in semen.
| Study | HIV DNA in semen | ||
|---|---|---|---|
|
| |||
| Prevalence | Copies/ml | ||
|
| |||
| Median | Range | ||
| Van Voorhis et al. [28] | 1/25 (4%)a | NA | NA |
| Mermin et al. [29] | 17/23 (74%)a | NA | NA |
| Hamed et al. [30] | 38/52 (73%)a,b | NA | NA |
| Quayle et al. [31] | 6/13 (46%)d | NA | ND to >1000 |
| Xu et al. [32] | 48/74 (65%)a | 36 | ND to 16667 |
| Zhang et al. [33] | 4/7 (57%)c | NA | ND to 90e |
| Krieger et al. [34] | 9/79 (11%)d | NA | NA |
| 58/117 (50%)f | NA | NA | |
| Mayer et al. [35] | 4/19 (21%)b | 0 | ND to 80000 |
| 4/18 (22%)c | 0 | ND to 3000 | |
| Tachet et al. [36] | 28/49 (57%)b | NA | ND to >100e |
| Ball et al. [14] | 15/32 (47%)b | <6e | ND to 2171e |
| Vernazza et al. [37] | 21/55 (38%)a | NA | NA |
| 11/67 (16%)c | NA | NA | |
| Ghosn et al. [38] | 3/5 (60%)a | 40e | ND to 401e |
| 5/15 (33%)c | ND | ND to 416e | |
NA, not available; ND, not detectable.
No ART.
ART.
HAART.
Therapy unknown.
Per 106 nonspermatozoal cells.
After vasectomy.
The percentage of HIV-infected WBCs in semen has not been previously determined. To perform this calculation, we returned to a database that was used in a publication on factors associated with elevated HIV proviral DNA levels in semen [32]. Semen from 38 HIV-positive men from this study had measurable levels of both HIV DNA and HIV-susceptible host cells (macrophages and CD4+ T cells, quantified by immunohistology); making assumptions that only a single HIV DNA copy was present in each infected cell and that only macrophages and CD4+ T cells were infected, the median infection rate of this seminal HIV-susceptible host cell population was 0.2% (range 0.002–16%).
Infectiousness of semen cells
Since the pioneering discovery in 1983 that HIV-1 could be cultured from seminal cells [41], a number of laboratories have cultured HIV from both seminal cells and cell-free seminal plasma (Table 3) [42–53]. Overall, the recovery rate of infectious HIV from seminal cells has been much higher (median 20%, range 4–55%) than that from seminal plasma (median 5.9%, range 3–11%, P < 0.0001). The relatively low HIV recovery rate from seminal plasma contrasts with quantitative PCR data indicating that HIV prevalence rates and viral copy numbers are higher in seminal plasma than in the semen cell fraction [14,34–36]. This discrepancy suggests that much of the cell-free HIV in semen is replication incompetent or inactivated. A number of factors have been identified in seminal plasma that may inactivate HIV, including anti-HIV antibodies [54,55], X4/R5 chemokines [20], SLPI, lactoferrin, and defensins [56]. The low culture rate could also reflect the toxicity of seminal plasma to peripheral blood mononuclear cell (PBMC) target cells used for culturing HIV [57–61].
Table 3. HIV culture rate from semen fractionsa.
| Study | Semen cells | Seminal plasma |
|---|---|---|
| O'Shea et al. [42] | 2/18 (11%) | – |
| Krieger et al. [43] | 13/55 (24%) | 6/55 (11%) |
| Krieger et al. [44] | 15/53 (28%) | 6/53 (11%) |
| Van Voorhis et al. [28] | 2/25 (8%) | 2/25 (8%) |
| Anderson et al. [45] | 4/95 (4%) | 5/95 (5%) |
| Vernazza et al. [46] | 18/33 (55%) | 1/33 (3%) |
| Krieger et al. [47] | 22/114 (19%) | 9/114 (8%) |
| Dyer et al. [48] | 22/65 (34%) | – |
| Vernazza et al. [49] | 29/98 (30%) | – |
| Vernazza et al. [50] | 16/43 (37%) | – |
| Coombs et al. [51] | 30/251 (12%) | 8/249 (3%) |
| Dulioust et al. [52] | 4/20 (20%) | – |
| Tachet et al. [36] | 9/35 (26%) | – |
| Nunnari et al. [53] | 5/28 (18%) | – |
| Overall culture rate | 191/933 (20.5%) | 37/624 (5.9%)* |
| Median culture rate | 21.8% | 7.9% |
| Mean culture rate | 23.2% | 7.1% |
Studies with >10 participants.
P < 0.0001.
Factors that affect the abundance and infectiousness of HIV-infected leukocytes in semen
Although WBCs can be detected in semen from virtually all men, several factors may affect the types, abundance, and infectiousness of WBCs in semen. Symptomatic bacterial genital tract infections and inflammation are often associated with increased urethral/seminal WBC numbers [62,63]. However, chronic asymptomatic genital viral infections do not generally produce elevated seminal WBC counts [64,65], and as mentioned above, HIV infection appears to deplete CD4+ and CD8+ lymphocytes in semen [15,66], an effect partially reversed by antiretroviral therapy [15].
Epidemiologic studies indicate that STIs substantially enhance HIV transmission [67,68]. Urethritis caused by Neisseria gonorrhoeae was associated with a 10-fold increase in HIV RNA copy numbers in semen, which declined following successful antibiotic treatment [69]. Other studies have demonstrated increased HIV RNA shedding from genital ulcers caused by various STI pathogens [70– 72]. Most of these studies have only measured cell-free HIV RNA, but because symptomatic infections and inflammation are associated with elevated WBC levels in semen, it is probable that the number of HIV-infected cells in semen is also increased. One study to date has shown that both HIV RNA and proviral DNA levels were elevated in semen from men with a recent STI [40]. Elevated polymorphonuclear leukocyte (PMN) counts and leukocytospermia have also been associated with increased levels of both cell-free and cell-associated HIV in semen [32,45,73], as well as increased levels of IL-1β, TNF-α, IL-6, and other proinflammatory cytokines that could activate HIV replication in infected cells [20,65,74].
Some men may be particularly contagious due to abnormally elevated seminal leukocyte counts. In one study from our laboratory, semen samples from two HIV-positive persons without STI symptoms contained 15–25 million macrophages and 2–6 million CD4+ T cells per ml (the average human ejaculate comprises 2.5 million per ml). In addition, their semen was highly infectious when cultured with PBMC target cells [15]. Both of these men had advanced HIV disease in the pre-HAART era and had high peripheral blood viral loads. Cases such as these may play an important role in the HIV epidemic. Men with acute HIV infection also have high levels of HIV RNA in semen, and epidemiologic studies indicate that they are highly infectious [75,76]. Only one study thus far has measured HIV proviral DNA levels in semen of acutely infected men; 10 out of 13 samples from three HIV-infected men within 80 days of initial infection tested positive for HIV DNA [39]. More research is needed to determine whether HIV-infected WBCs in semen contribute to the highly contagious profile of this group.
HIV transmission by spermatozoa
The question of whether spermatozoa transmit HIV infection has been controversial for several years [77–79]. HIV and simian immunodeficiency viruses (SIV) apparently infect testicular germ cells [80–82], and early electron microscopy and in-situ hybridization studies provided evidence that human spermatozoa may contain HIV viral particles or RNA [83–85]. However, these findings have not been confirmed [78,86], and most recent studies using PCR techniques have not detected HIV infection of viable spermatozoa [79,87].
Viable, motile spermatozoa from HIV-infected men, separated from other cell types in semen by density gradient centrifugation and/or swim-up techniques, rarely contain detectable amounts of HIV DNA or RNA [31,36,79,86,88–96]. Occasional positive results may be due to contamination of the sperm pellet with infected leukocytes or false-positive PCR reactions, or could indicate that HIV infection of sperm occurs but is exceedingly rare. We measured HIV DNA in isolated cell populations from semen of HIV-infected men and detected HIV DNA in immunobead-purified macrophage and CD4+ T-cell populations, but not in motile sperm [31]. In the same study, we also compared the relative infectiousness of cell populations from semen of HIV-positive men and found that isolated CD4+ T cells and macrophages were highly infectious when cultured with PBMC target cells in vitro, whereas motile sperm from the same participants were not infectious [31]. Reports from Assisted Reproduction Clinics that have used isolated motile sperm from HIV-infected men to inseminate HIV-uninfected partners provide further evidence that motile sperm are not infectious. Over 4500 inseminations have been performed with processed sperm from HIV-infected men without infection of the seronegative partners [96–105]. However, even in light of substantial data to the contrary, one cannot conclude that sperm never transmit HIV following natural intercourse. As mentioned above, occasional detection of HIV DNA in purified sperm preparations could indicate rare HIV infection of sperm. Furthermore, several groups have reported that HIV virions can bind to sperm through mannose or glycolipid receptors [85,106–111]. This interaction may be missed with processed sperm, as loosely-attached HIV may be stripped-off by gradient separation protocols, but this association could be relevant following normal intercourse as sperm could transport HIV to host cells in the lower as well as upper urogenital tract. In a recent study [112], abnormal/immotile ejaculated sperm from HIV-infected men were found to contain HIV DNA, suggesting that HIV-infected testicular germ cells produce immotile/nonviable sperm. These defective sperm could potentially introduce HIV to phagocytic macrophages or other cells in the female genital tract after intercourse [105,113].
Leukocytes in female genital secretions
Cell populations
Several studies have documented HIV-susceptible host cells in vaginal and cervical tissue (described below), but few have quantified or characterized these cell populations in human vaginal and cervical secretions. Macrophages and CD4+ T cells are often detectable but not numerous in cervicovaginal secretions from healthy uninfected [114,115] or HIV-infected women [116] (Table 4) [117]. The viability of lymphocytes in vaginal secretions from healthy women is usually poor, probably due to the toxic effects of low pH conditions commonly found in the human vagina [118].
Table 4. WBC concentrations in cervicovaginal secretions.
Leukocyte counts are elevated in cervicovaginal secretions of women with certain STIs. Neisseria gonorrhoeae and Chlamydia trachomatis infections can induce massive inflammatory infiltrates [119]. In contrast, bacterial vaginosis appears to have little or no effect on vaginal leukocyte counts [119–121], but these cells could have improved viability and higher infectiousness due to near neutral pH associated with this condition.
Prevalence and quantity of HIV-infected leukocytes in female genital secretions
Several studies on HIV in vaginal secretions have used qualitative HIV DNA assessment as an endpoint. An increased prevalence of HIV DNA in vaginal secretions has been associated with cervicitis, candidiasis, and STIs [122–133], hormonal contraception [129,134], and vitamin A or selenium deficiency [129,135–137]. The prevalence of HIV-infected cells in vaginal secretions is reduced in women on antiretroviral therapy [138,139].
Only a few studies have quantified HIV DNA in cells from cervicovaginal secretions [7,131,140–145] (Table 5). In these studies, maximum HIV proviral copies were on the order of 104 per lavage (103 copies/ml lavage fluid). Our laboratory quantified HIV RNA and DNA in cervicovaginal secretions from women in the WITS cohort during the third trimester of pregnancy; levels of HIV DNA, but not RNA, and proviral heterogeneity were positively associated with perinatal HIV transmission [7,140].
Table 5. Studies on HIV DNA in cervicovaginal secretions.
| Study | HIV DNA | ||
|---|---|---|---|
|
| |||
| Prevalence | Copy numberd | ||
|
| |||
| Median | Range | ||
| Iverson et al. [142] | 20/28 (71%)a,b,c | NA | ND to 95 |
| Panther et al. [140] | 7/7 (100%)b,g* | 3000e* | ND to 24000e |
| 9/17 (53%)b,h* | 300e* | ND to 60000e | |
| Debiaggi et al. [143] | 41/128 (32%)b,c | 28 | ND to 500 |
| Spinillo et al. [131] | 61/122 (50%)a,b,c | 48 | ND to 1500 |
| Andreoletti et al. [145] | 20/30 (67%)a | 7 | ND to 925 |
| Tuomala et al. [7] | 18/25 (72%)a,b,g* | 20e* | ND to 10202e |
| 22/51 (43%)a,b,h* | ND* | ND to 40074e | |
| Zara et al. [146] | 46/60 (77%)a,b,c | 69f | ND to 500 |
| Benki et al. [144] | 16/26 (62%)a,i | 40 | ND to 2220 |
| 6/26 (23%)a,j | ND | ND to 340 | |
NA, not available; ND, not detectable.
No ART.
ART.
HAART.
Per 105 cells or μg DNA unless noted.
Per lavage.
Mean.
Pregnant women who transmitted HIV to their infants.
Pregnant women who did not transmit HIV to their infants.
Endocervical secretions.
Vaginal secretions.
Significantly different, P <0.05.
Infectiousness of cervicovaginal leukocytes
Early studies on HIV isolation from cervical swabs did not separate cells from cell-free fractions; the culture rate averaged 43% [140,147–150]. Due to heavy contamination of vaginal lavages with endogenous bacteria and fungus, HIV culture is now usually conducted with filtered cell-free fractions, yielding culture rates ranging from 11 to 22% [147,151,152]. Only one study to date has compared the HIV culture rate from cell-free vs. cell-associated fractions of cervicovaginal lavage samples: HIV was cultured from 12 of 55 (22%) cell-free supernatants and five of 22 (23%) cell lysates [147]. Although correlates of HIV culture from cervicovaginal cell pellets have not been studied, it is possible that HIV-infected leukocytes from reproductive aged women with normal vaginal flora are inactivated by lactic acid produced by lactobacilli and are, therefore, less infectious [118]. We predict that HIV-infected genital leukocytes from women with neutral vaginal pH due to conditions such as bacterial vaginosis and low estrogen states [153] are more infectious than those from reproductive aged women with vaginal pH in the 3.5–5.0 range and are more capable of cell-associated HIV transmission.
Recently a sensitive short-term MAGI culture assay was used to improve the detection rate of infectious HIV in filtered female genital secretions. Although the overall culture rate was 51%, there was only a weak correlation between MAGI plaque (infectious virus) numbers and HIV RNA viral load. In addition, 10 out of 32 women with more than 10 000 HIV RNA copies/lavage had undetectable levels of infectious HIV in the MAGI plaque assay. The investigators speculated that the discrepancy may indicate inactivation of cell-free virus in genital secretions, possibly by neutralizing antibodies, low pH or innate immune mediators [152]. These data support the potential importance of cell-associated HIV transmission.
HIV target cells in genital mucosae
Following vaginal intercourse, HIV from an infectious partner enters an environment that contains a multitude of factors contributed from both male and female genital secretions. (The rectal environment is not as well studied but would be expected to contain many of the same components.) As discussed above, several factors in semen and cervicovaginal secretions (antimicrobial peptides, X4/R5 chemokines, anti-HIV antibodies) can inactivate cell-free HIV, but may not affect HIV-infected cells. Factors in this environment that have been determined to potentially affect cell-associated HIV transmission are mucins, large hydrophilic molecules that lubricate and protect genital mucosal epithelia, and endogenous vaginal lactobacilli that produce lactic acid to maintain a low pH [56]. In an in-vitro model system, lymphocytes and activated seminal leukocytes were able to traverse midcycle cervical mucus, although they failed to penetrate thicker substrates representing the viscosity of mucus present during the luteal phase of the menstrual cycle and pregnancy [154]. Macrophages and T cells were immobilized and eventually killed by low pH conditions commonly found in the human vagina [118]. However, after intercourse, the pH of cervicovaginal secretions is neutralized for several hours by the mild alkalinity of seminal plasma [155,156], providing seminal and cervicovaginal leukocytes a window of opportunity to reach the target genital epithelium. Furthermore, bacterial vaginosis and low-estrogen conditions underlying pre-menarchal, postpartum and postmenopausal states are also associated with elevated vaginal pH levels [153]. Thus, it appears probable that infected leukocytes in genital secretions can remain viable at least for several hours after intercourse in healthy reproductive aged women and longer in women with bacterial vaginosis and other conditions associated with elevated vaginal pH, and are capable of shuttling HIV through genital secretions to the epithelium.
Stratified squamous epithelial surfaces, such as those covering vaginal, ectocervical, rectal, and foreskin tissues, are comprised of a thick multicellular epithelial layer, whereas columnar epithelia such as those covering endocervical, penile urethra, and anal mucosae consist of a polarized monolayer of epithelial cells. In either case, unless the epithelial layer is compromised, infectious organisms such as HIV must traverse or find target cells within the epithelial layer. Transmission electron microscopy studies have demonstrated that HIV-infected T cells and monocytes readily bind to mucosal epithelial cells, and that their attachment induces directional budding of HIV toward the epithelial surface where virions can accumulate within intersynaptic clefts and enter endo-somal-like structures within epithelial cells (Fig. 2) [157,158]. Infectious virions may be sequestered by epithelial cells to await an opportunity to infect an appropriate target cell [159–161], which could be recruited to the site through release of chemokines or other proinflammatory signals by the infecting cell and/or affected epithelial cell [162], or virions may be transcytosed across columnar epithelial cells to infect cells in the lamina propria [163,164]. We and others have also shown that macrophages and T cells can infiltrate columnar and stratified epithelial layers (described in more detail below) and, therefore may, if infected with HIV, directly infect cells within or below the epithelium.
Fig. 2. HIV-infected leukocyte interaction with epithelial cells: attachment and directional viral shedding.
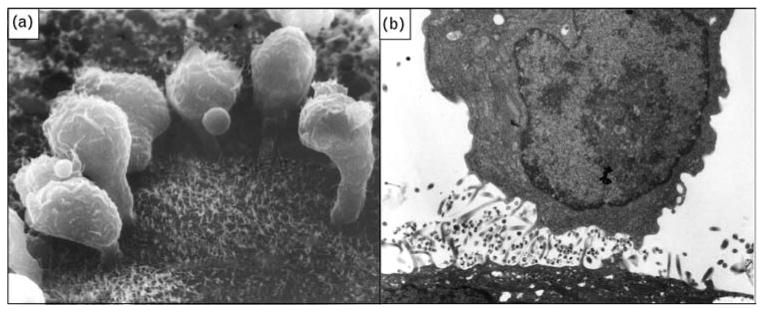
(a) Scanning electron micrograph showing HIV-infected lymphocytes adhering to the surface of an epithelial cell (magnification × 10000). (b) Transmission electron micrograph of HIV-infected macrophage from semen releasing virus after contact with a genital tract epithelial cell (magnification × 15 000). Original photographs provided by David M. Phillips with permission from the Population Council, New York. Part (a) reproduced from [4].
There is considerable regional, as well as interindividual and intraindividual variation in the density of the leukocyte cell populations that may serve as HIV target cells in genital mucosae [165,166]. There are usually few CD4+ T cells within the squamous epithelial layer, although they can be abundant under inflammatory conditions. However, macrophages and Langerhans cells are normally abundant within stratified squamous epithelia and can potentially be infected by HIV and/or transport virus to target cells in regional lymph nodes. The lamina propria that lies under the epithelial layer and dermal papillae that protrude into the stratified squamous epithelium, contain numerous HIV-susceptible host cells (CD4+ lymphocytes, macrophages, and dendritic cells). Transformation zones delineating the transition from stratified squamous to columnar epithelium (e.g., cervical os, rectal/anal junction, fossa navicularis at the opening of the penile urethra) contain an especially enriched population of HIV target cells [166]. HIV may also infect target cells in the uterine endometrium and fallopian tubes [167].
Concentrations of intraepithelial HIV target/host cells in the genital mucosa are substantially increased during infection/inflammation [166]. In addition, use of irritating compounds such as the spermicide Nonoxynol-9 (N-9) can damage the genital and rectal epithelium, resulting in inflammation and recruitment of lymphocytes, macrophages, PMNs, and other cells into the epithelial layer and secretions [168–171]. After intercourse, numbers of HIV-susceptible host cells are increased in cervicovaginal tissue and secretions, and potentially in rectal tissues and secretions, due to chemokines and other chemoattractants in semen and pro-inflammatory effects of semen on mucosal epithelial cells [172,173]. These conditions would be expected to enhance cell-associated HIV transmission.
Evidence for cell-associated HIV transmission
Clinical studies
The sexual transmission of HIV is a rare event: estimates for the probability of HIV transmission per unprotected coital act range from 1 in 200–2000 for male-to-female transmission, 1 in 200–10 000 for female-to-male transmission, and 1 in 10–1600 for male-to-male transmission [174]. Studies on the genetic composition of HIV recovered from blood of individuals newly infected with HIV-1 indicate that in the majority of cases, regardless of the transmission route, a single R5 tropic, CD4-dependent virus from an infected partner is responsible for productive clinical infections [175– 183]. This suggests that HIV is usually transmitted via a single HIV virion or infected cell. A different transmission pattern has been observed in studies of sex workers and STI patients, where multiple genetic variants can establish an infection in the recipient, probably due to compromise of the mucosal barrier and/or increased numbers of HIV target cells at the infection site [183– 186].
HIV quasispecies in semen often differ genetically from those in peripheral blood [187–191], and at least two studies provide evidence that genetic sequences of cell-free HIV differ from those of cell-associated HIV in semen [189,192]. It, therefore, should be possible to determine whether the initial transmission event is mediated by a cell-free virion or an HIV-infected cell. Investigators set out to distinguish between these possibilities in acute seroconverters and found that the genotype of the infecting virus matched that of HIV in semen cells of the transmitter in three out of five cases (one heterosexual and two male homosexual couples) [189]. More studies of this kind are needed to determine the prevalence and risk factors of cell-associated HIV transmission.
Other evidence that seminal leukocytes can cross the vaginal epithelium in humans is provided by a study that showed that unprotected heterosexual intercourse induces an allogeneic response in women that is specific for their sexual partner's human leukocyte antigen (HLA) [193]. This reaction was not observed in couples that always used condoms and is likely induced by exposure to seminal leukocytes because sperm do not express classical HLA antigens [194]. As the human vagina is a poor antigen induction site for systemic immune responses [195] and the allogeneic response was detected in peripheral blood, it is probable that the partners' leukocytes crossed the mucosal epithelium to stimulate an immune response in draining lymph nodes. Aweak but significant alloimmune response was also observed in the male partners and could be attributed to exposure to partner's vaginal leukocytes. In this study, PBMCs from women with allogeneic immunity inhibited HIV-1 infection of activated T cells from their partners, providing evidence that allogeneic immunity could protect against cell-associated HIV transmission.
Animal studies
Feline immunodeficiency virus model
The first animal model of cell-associated retroviral transmission across vaginal and rectal mucosal epithelia was the feline immunodeficiency virus (FIV) infection model. FIV, a lentivirus with characteristics similar to HIV, primarily infects T cells and causes an AIDS-like immunodeficiency disease in cats. FIV was one of the first animal models used to deduce mechanisms of HIV transmission and pathogenesis [196–198]. FIV can be transmitted via atraumatic instillation of infected T cells or cell-free virus onto vaginal or rectal mucosa [199,200], and this model was used to evaluate the efficacy of early topical microbicide candidates against vaginal and rectal transmission of cell-associated FIV [199,201]. The FIV model has also been used extensively for vaccine development; despite facing the numerous challenges of developing a vaccine to protect against a T-cell tropic retrovirus (genetic diversity, CD4+ T-cell depletion, immune-mediated enhancement of viral infection), two FIV vaccines based on inactivated virus and virus-infected cells are effective and commercially available [202,203]. More could be learned from these FIV vaccine models about immunological correlates of protection against cell-free and cell-associated retroviral transmission across mucosal surfaces.
Humanized mouse models
In 1997, two laboratories reported the intriguing observation that labeled mouse spleen mononuclear cells could cross the mouse vaginal epithelium following atraumatic instillation in the vaginal lumen; the cells were later detected within vaginal tissue and the draining lymph nodes [204,205]. Shortly thereafter, it was reported that HIV-infected human PBMCs could cross the intact vaginal epithelium in humanized severe combined immunodeficient (hu-SCID) mice to produce a systemic HIV infection [206,207], thus establishing a mouse model for studies on vaginal cell-associated HIV transmission. In the hu-SCID mouse model, cell-associated but not cell-free virus accomplished infection due to transepithelial migration of HIV-positive cells [207]. The hu-SCID model requires progesterone treatment of the animals to thin the vaginal epithelium, and infection is less reliable when HIV-infected cells are suspended in human seminal plasma before their introduction into the vaginal lumen [208]. Other immunodeficient mouse models have been reconstituted with human hematopoetic stem cells [i.e., bone marrow– liver–thymus (BLT), Rag2−/− gammac−/− (Rag-hu)]. Mucosal tissues of these mice are populated with Langerhans cells and other appropriate cell populations [209,210] and they are highly susceptible to infection following vaginal administration of cell-free HIV without any prior hormonal conditioning or mucosal abrasion [209,210]. However, one recent study failed to achieve HIV infection following rectal administration of cell-free and cell-associated HIV in Rag-hu mice [211]; no studies have been reported to date on vaginal cell-associated HIV transmission in the BLT and Rag-hu models. Thus, the hu-SCID model is the only proven mouse model to date for cell-associated HIV transmission studies and is appropriate for testing approaches to block binding of infected cells and their migration across a progesterone-thinned (columnar-like) vaginal epithelium.
Nonhuman primates
Higher apes can be infected with both HIV and SIV but are rarely used for HIV transmission research due to their endangered status. A study on HIV transmission in chimpanzees conducted in 1998 demonstrated that both HIV-1-infected cells and high titers of cell-free HIV-1 were independently capable of transmitting infection after atraumatic insertion into the vaginal cavity near the cervical os [212].
The most common model used for studies on mechanisms of HIV-1 sexual transmission has been the SIV/rhesus macaque model. Although most studies on vaginal transmission in macaques have used cell-free SIV recent studies have underscored the importance of cell-associated SIV transmission. An early study was unsuccessful at infecting female macaques with vaginal administration of cryopreserved SIV-infected PBMCs [213], but investigators at the Wisconsin National Primate Research Center achieved transvaginal infection using fresh SIV-infected macaque PBMCs. Infection was observed in animals with chemically induced vaginal ulcers and in intact animals following multiple low-dose exposures (7–2048 infectious cells/innoculum) [214,215]. Donor cells were detected in vaginal tissue and draining lymph nodes; viral RNA was detected in draining lymph nodes within one day of inoculation, and throughout lymphatic tissues within 5 days, which is faster than systemic spread of transvaginal cell-free SIV infection [216]. French investigators [217] recently reported that SIV-infected spleen cells (an enriched population of macrophages and memory T cells), harvested from acutely infected monkeys at the peak of viremia, also efficiently transmit HIV when placed in vaginas of DepoProvera-treated adult female macaques. Persistent systemic infection was achieved following atraumatic vaginal insemination with 107 cells containing 6.69 X 105 viral DNA copies. They found labeled infected cells in the vaginal lamina propria and draining lymph nodes 21 h after vaginal exposure.
These studies are significant because the amount of virus in the cell-associated viral inoculum is on the same order of magnitude as HIV introduced through natural seminal cell-associated HIV exposure (∼105 HIV proviral DNA copies have been detected in human semen). Cell-free vaginal SIV challenge studies use super-physiological doses of SIV (108–109 viral particles) that are several orders of magnitude higher than the median (102–103) and maximum (105) seminal HIV viral loads found in most large studies of ART-naive HIV-infected men [218]. These macaque cell-associated SIV models would be useful for preclinical testing of microbicide and vaccine candidates for efficacy against cell-associated HIV transmission.
In-vitro models
Various polarized primary epithelial cell monolayers have been used to study HIV transmission. HIV from infected cells can pass thorough monolayers of primary or transformed gastrointestinal columnar epithelial cells through a process called transcytosis to infect target cells below the epithelium [167,219]. Efficient cell-associated HIV transcytosis also occurs across polarized monolayers of transformed cervical epithelial cells [220,221]. In both of these models, HIV-infected leukocytes were much more efficient than cell-free virus in producing infection of subepithelial target cells. However, cell-associated HIV transcytosis was not observed in a stratified vaginal epithelial model [222] and was inefficient when infected cells were added to the apical surface of polarized primary cultures of human ectocervical and endocervical epithelia [223,224] or ectocervical and endocervical epithelial sheets [223], so the physiological relevance of this cell-associated HIV transmission mechanism is unclear. HIV-infected PM-1 T cells and TZM-bl (HeLa cervical carcinoma-derived) reporter cells are currently being used to test topical microbicides for efficacy against cell-associated HIV transmission [225]. However, as the TZM-bl cells, unlike normal genital epithelial cells, are engineered to express high levels of HIV receptors (CD4, CCR5, and CXCR4), they may be directly infected with free virions secreted from infected leukocytes.
Tissue explants have also been used for studies of cell-associated HIV transmission. In one study [226], cell-free or cell-associated HIV were placed on the luminal side of ectocervical explant tissue sealed in agarose, and viral transmission was detected by measuring HIV in the lower chamber at different time points. The addition of cell-associated and cell-free T-cell-tropic HIV and cell-free R5 virus resulted in transmission of the virus across the mucosa. In another study [160], labeled viable cells from semen were shown to bind to and penetrate the ectocervical epithelium, but failed to bind to endocervi-cal explants due to their entrapment in mucus secreted by these cells. We have used transmission and scanning electron microscopy to study interactions between macrophages and human endocervical tissue. When macrophages were added to the lumenal side of fresh human endocervical explant tissue, they attached to the surface and penetrated between epithelial cells (Fig. 3). We have also observed their penetration of an intact human vaginal epithelium model [227].
Fig. 3. Macrophage interactions with human endocervical tissue explants.
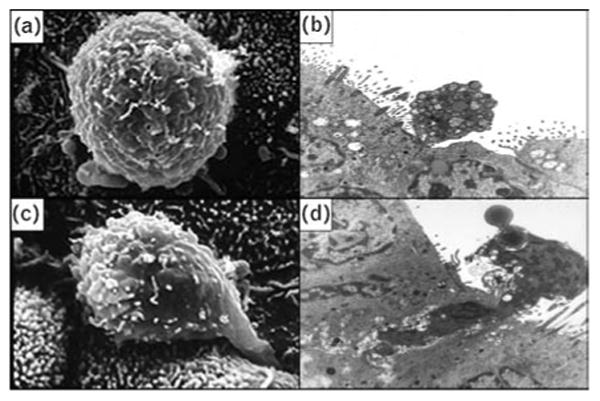
Scanning electron micrograph (a) and transmission electron micrograph (b) of human peripheral blood monocyte attached to epithelial intracellular junction. Scanning electron micrograph (c) and transmission electron micrograph (d) of human peripheral blood monocyte apparently infiltrating between two epithelial cells. Magnification: scanning electron micrograph, × 10000; transmission electron micrograph, × 15000.
Thus, in-vitro models are available for testing the efficacy of topical microbicides and vaccine-induced antibodies and cytotoxic T cells against several mechanisms underlying cell-associated HIV transmission including: viability and migration of infected cells through mucus, binding of infected cells to the epithelium, transcytosis of HIV across columnar epithelia, migration of infected cells through the epithelium, and cell-to-cell transfer of HIV.
Molecular events underlying cell-associated HIV transmission
The Trojan Horse HIV transmission hypothesis predicts that HIV-infected cells, deposited in the genital tract or rectal lumen during sexual intercourse, protect and transport virus to susceptible cells within or below the mucosal epithelium to infect a new host. The studies described above provide evidence that HIV-infected leukocytes are present in genital secretions and that they can indeed attach to the lumenal mucosal surface, infiltrate through the epithelium, and establish systemic infection.
On the basis of this information, HIV-infected cells from genital secretions may transmit virus across the genital mucosa of uninfected partners through at least three mechanisms. In the first mechanism, the genital epithelial cell plays a central role. HIV-infected leukocytes attach to the apical surface of epithelial cells and shed nascent virions toward the epithelial cell plasma membrane. These highly infectious viral particles may be sequestered by epithelial cells for subsequent transfer to HIV-susceptible host cells within the epithelium or transferred through the epithelial cell layer(s) by transcytosis to target cells in the lamina propria. The second mechanism entails direct transfer of virus from infected leukocytes to target cells within the epithelium, possibly through the formation of an infectious synapse; it is possible that target cells are attracted to infected leukocytes by chemokines released either by the infected cell or by epithelial cells that are activated by contact with the infected cell. Third, infected leukocytes may migrate through the epithelium to infect target cells in the lamina propria or draining lymph nodes (Fig. 4).
Fig. 4. Mechanisms underlying cell-associated HIV transmission.
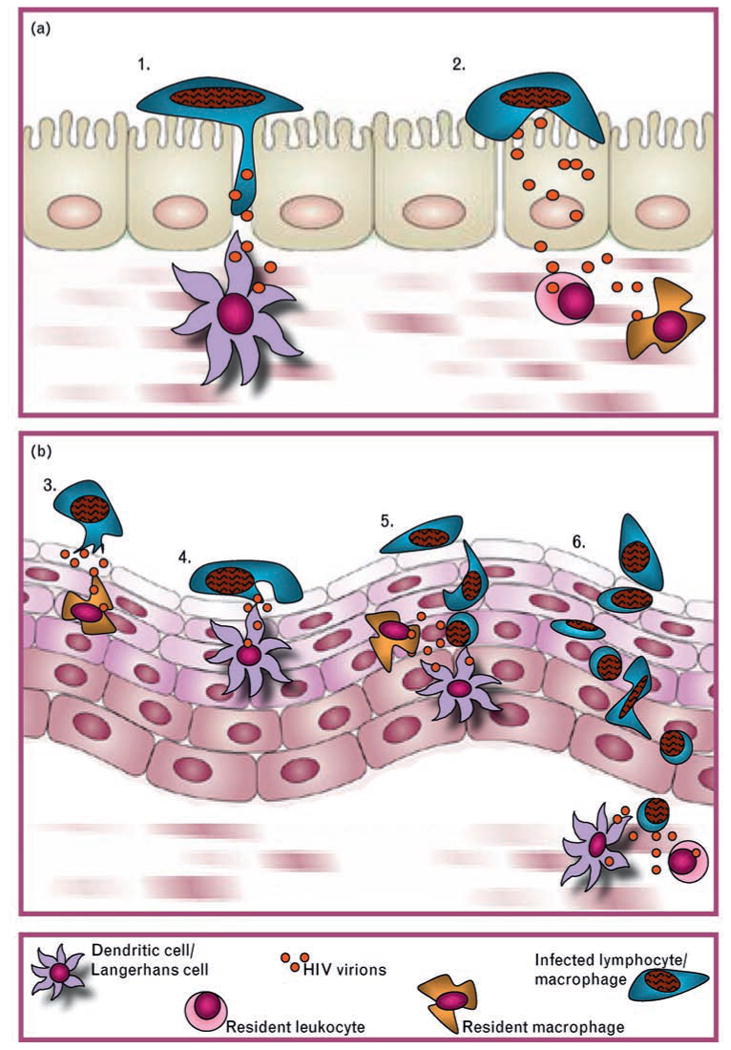
(a) Columnar epithelium: (1) Infected cell migrates between epithelial cells to infect susceptible host cells in the lamina propria or draining lymph nodes. (2) HIV trancytosis through epithelial cells to infect susceptible target cells in lamina propria. (b) Stratified squamous epithelium: (3) Transfer of HIV from infected leukocyte to epithelial cell, which transfers virus to intraepithelial or subepithelial target cells through (a) transcytosis or (b) attraction via release of chemokines. (4) Direct cell-to-cell transfer of HIV from infected leukocyte to intraepithelial target cell via viral synapses. (5) Transepithelial migration of infected leukocyte to infect intraepithelial target cells within the epithelium. (6) Transepithelial migration of infected cell to infect target cells in the subepithelium or draining lymph nodes.
Although recruitment and migration of immune cells from blood across the endothelium into the epithelium is well established, the reverse mechanism of apical-to-basal transepithelial migration that enables leukocytes to travel from the surface back into the body has yet to be fully elucidated [228]. Monoclonal antibody blocking studies have established a role for a number of adhesion molecules and their corresponding ligands in intracellular epithelial migration. In addition, much work has been done to understand the role of chemokines in cell trafficking and activation. This system is tightly regulated, but may be perturbed by inflammation and infection. Understanding the role of these components in leukocyte attachment and transepithelial migration is essential to understanding the mechanisms of cell-associated HIV transmission.
Adhesion to the epithelium
Scanning and transmission electron microscopy studies conducted in the early 1990s showed that HIV-infected monocytic cells attach to epithelial cells and directionally release viral particles toward the surface of epithelial cells, which appear to endocytose them [157]. HIV-infected monocytes were also observed to migrate between cervix-derived epithelial cells and release virions from pseudopods [158]. Sulfated polysaccharides, among the first topical microbicide candidates to have been studied in humans, inhibit these interactions between monocytes and epithelial cells [229].
Genital macrophages and T cells express a variety of adhesion molecules that enable their adherence to epithelial cells and HIV-susceptible host cells within the epithelium (Table 6). The β2 integrins LFA-1 αLβ2 (CD11a), ITGAM αMβ2 (CD11b), and ITGAX αXβ2 (CD11c) belong to a class of adhesion molecules present on the surface of macrophages and T cells. The presence of one or more of these receptors enables the cell to participate in a variety of immune-specific processes, such as migration and phagocytosis. LFA-1 has been recently implicated as the chief molecule involved in the trafficking of immune cells and the subsequent establishment of cell–cell contacts [230]. Its activation is tightly regulated; LFA-1 is not constitutively active but can be activated by inflammatory stimuli. The chemokine SDF-a is known to activate LFA-1 [231]; interestingly, this chemokine is present in high concentrations in semen and could activate LFA-1 on seminal leukocytes [20]. Upon activation, the LFA-1 receptor opens out in a ‘switchblade’ like manner into an extended conformation that allows it to bind intercellular adhesion molecules (ICAM-1, ICAM-2, and ICAM-3) [230].
Table 6. Integrin and chemokine ligand/receptor pairs potentially involved in cell-associated HIV transmission.
| Integrins | Integrin counter-receptors |
|---|---|
| CD11a (LFA-1)MΦ,T | ICAM-1MΦ,TE, JAM-AE |
| CD11b (MAC-1)MΦ | ICAM-1MΦ,TE, JAM-CE |
| αEb7T | E-cadherinE |
|
| |
| Chemokines | Chemokine receptors |
|
| |
| RANTES (CCL5)E | CCR5MΦ,T |
| MIP-1α (CCL3)E | CCR5MΦ,T |
| MIP-1β (CCL4)E | CCR5MΦ,T |
| MCP-1 (CCL2)E | CCR2MΦ |
| MCP-2 (CCL8)E | CCR2MΦ |
| SDF-1αE | CXCR4T |
| SDF-1βE | CXCR4T |
| MIFE | CXCR4T |
epithelial cell;
macrophage;
CD4+ T cell.
Inflammatory stimuli affect the activation state of β2 integrins, resulting in an increased affinity for ligand. Furthermore, inflammation is known to positively affect the availability of its receptors, particularly ICAMs. For example, interferon-γ (IFN-γ), produced by resident T and natural killer (NK) cells within the mucosal epithelium, upregulates ICAM-1 expression at the site of adhesion. It has been hypothesized that this upregulation is involved in IFN-γ-mediated recruitment of leukocytes following a local inflammatory stimulus [232].
The interaction between β2 integrins and ICAMs appears to play an important role in the establishment of HIV infection. In an endometrial cell line (HEC-1) model system, Carreno et al. [233] showed that trans-epithelial migration of HIV-infected monocytes is dependent on an initial interaction between LFA-1 and ICAM-2/3. Preincubation with anti-CD11a (LFA-1 α-chain) monoclonal antibodies blocked transmigration across the epithelium. However, transmigration was enhanced by first treating the HEC-1 cells with pro-inflammatory cytokines TNFα and IL-1β, causing an upregulation of both ICAM-2 and ICAM-3. It was also recently reported that interrupting the LFA-1/ICAM-1 interaction between CD4+ T cells and dendritic cells with anti-ICAM and LFA-1 monoclonal antibodies inhibited HIV transmission [234,235]. Several peptides derived from β2 subunits of LFA-1 and from the D1 region of ICAM-1 have been shown to block ICAM-1/LFA interactions. These peptides mimic the endogenous ligands and block the receptor, making it unavailable for binding. Of note, many of these peptides are internalized by T cells following binding to the receptor and are thus being investigated as potential vehicles to target drugs to cells [236].
Junctional adhesion molecules (JAMs) are also counter-receptors for β2 integrins. JAM-A is found exclusively in tight junctions and binds LFA-1 (CD11a) [237], whereas JAM-C is an important desmosomal component capable of binding Mac-1 (CD11c) [238]. We have detected the expression of both JAM-A and JAM-C by epithelial cells in normal vaginal and cervical tissue, suggesting that these counter-receptors may also play a role in macrophage infiltration of genital mucosa [227].
Not all leukocyte adhesion molecules belong to the β2 integrin family. Lymphocyte endothelial–epithelial cell adhesion molecules (LEEP–CAMs) are expressed in the vaginal mucosae and bind T cells, and, therefore, could play a crucial role in cell trafficking as well as the maintenance of intraepithelial lymphocytes (IELs) at this site [228]. Genital tract IELs also express the integrin αEβ7, which enables them to specifically adhere to epithelial-cadherin (E-cadherin) [165,228,239,240]. E-cadherin is present in epithelial cell junctions in the vaginal/ectocervical mucosa and has an important role in maintaining the integrity of the epithelium. Recently, it was shown that e-cadherin may also play a role in lymphocyte adhesion and transmigration; the integrin αEβ7, expressed on intraepithelial T cells, has a high specificity for E-cadherin and the affinity for this interaction is increased with antigenic stimulation [241]. Thus, pathogens that activate mucosal T cells via Toll-like receptor (TLR) or other receptors induce these cells to remain at the infection site where they may play a role in pathogen immune defense or, in the case of a lymphotropic virus such as HIV, serve as target cells for infection. The T-cell integrin α4β7 has recently been implicated in CD4+ T-cell depletion in the gut after HIV infection. α4β7 mediates the mucosal homing of T lymphocytes to the gut and when activated can bind the HIV protein gp120. This initiates a series of signaling events, including the activation of LFA-1, which increases HIV infectivity through intracellular viral synapses [242]. The T-cell α4β7 integrin is also expressed on T cells in the male and female genital tract and recent evidence shows that these T-cell populations are depleted following HIV infection, possibly through the same mechanism [15,243].
Apical-to-basal transepithelial migration
An important function of macrophages in mucosal tissues such as the lung and gut is to sample potential pathogens and other antigens in the lumenal cavity and report back to T cells within and below the epithelium [244,245]. They use adhesion molecules to stay attached to epithelial cells as they perform their surveillance function, and once activated, utilize cell junctions to migrate back into the tissue. Evidence presented above from animal and in-vitro studies suggest that SIV-infected and HIV-infected leukocytes are capable of apical-to-basal transepithelial migration in genital tissues. Once within or below the epithelial layer, they would encounter Langerhans and other dendritic cells, macrophages and CD4+ T cells, which could serve as the first targets of HIV infection within the host. Studies of SIV/HIV vaginal transmission in animal and in-vitro models using cell-free virus indicate that intraepithelial Langerhans cells, memory T cells, and macrophages are early viral targets [160,235– 249].
Infected leukocytes may be induced to migrate into the mucosal epithelium by a chemokine gradient. A number of chemokines have been documented in genital tract secretions [20,117]; some are produced by epithelial cells [172,250], whereas others are secreted by leukocytes residing in the epithelium [251]. The cervical and vaginal epithelia produce moderate levels of the granulocyte chemokine IL-8 and the CXCR4 ligand SDF-1α (a competitive inhibitor for X4 HIV cell entry) under normal conditions; under inflammatory conditions, levels of these chemokines are increased and secretion of other chemokines such as RANTES, MIP-1α, and MIP-1β are induced [250]. All three of these chemokines attract T cells and macrophages to sites of inflammation and have been implicated in chemotaxis of immune cells through endocervical tissues [252].
Leukocyte infiltration is potentially augmented by genital inflammation or infection. For example, TNFα produced in response to viral infection increases macrophage transmigration in the epithelial environment [253]. In addition, chemokines released by epithelial cells and resident leukocytes during an infection attract additional leukocytes to the infection site. Chlamydial infection of cervical and colonic epithelial cells induces upregulated secretion of the leukocyte chemokines IL-8 and GROα in addition to various cytokines [254]. It is, therefore, likely that cell-associated HIV transmission is enhanced under inflammatory conditions. Hormonal conditions may also affect cell migration through the epithelium and the composition and activation state of target cells within the epithelium [255].
Cell-to-cell HIV transfer
The immune system uses intercellular conduits to convey messages between cells without their having to cross the plasma membrane. Short-range connections take the form of gap junctions and synapses, whereas long-range connections are composed of nanotubules and filopodial bridges [256]. Pathogens including HIV have hijacked these structures to undergo cell-to-cell spread. Cell-to-cell HIV transmission is dependent on the formation of a structure between cells, termed the ‘virological synapse’ [257,258]. This mechanism of intercellular infection is very efficient and allows the virus to bypass a host immune response [4,259]. The initial events of virological synapse formation rely on the recruitment of β2 integrins to lipid rafts at the location of the forming synaptic cleft [260]. The integrins are responsible for positioning and binding at the point of intercellular contact. In the case of HIV-1 virological synapse formation, this recruitment is dependent on viral surface envelope glycoprotein (Env) and gp120 contact with target cell CD4 receptors [259]. Following the initial connection, other components of the recruited lipid raft microdomain, such as viral coreceptors (CCR5, CXCR4) and ICAMs, interact with Env, activated LFA-1, and other β2 integrins to ensure a firm connection. The actin cytoskeleton reorganization that accompanies this intercellular adherence determines the type of synapse created [261]. Formation can proceed with the fusion of cell membranes and the direct transfer of viral materials, or alternatively, with the maintenance of close contact and the establishment of a junction through which the virus can be passed by directional budding and endocytosis [154,262]. Corecruitment of viral transcripts and viral receptors to the intercellular junction, by effector and target cells, respectively, enables fast and efficient cell-to-cell transmission, as visualized by immunoelectron microscopy [259,263]. These synapses have been identified as a mode of virus transmission for monocyte-derived macrophages (MDMCs) and dendritic cells (MDDCs) as well as T lymphocytes [263]. Cell-to-cell HIV transmission may also occur via filopodial bridges [264] and nanotubules [265].
Clinical approaches to blocking cell-associated HIV transmission
The data reviewed above suggest that topical microbicides and vaccines should be developed that are effective against cell-associated HIV, as well as cell-free virus. There are several mechanisms by which this can be accomplished, and some of the microbicide and vaccine candidates undergoing preclinical and clinical assessments may be effective against this residual challenge in biomedical HIV prevention. Below is a summary of the preclinical studies that have been conducted to date with microbicide and vaccine candidates in cell-associated HIV transmission assays. It must be emphasized that this is an understudied area and that these data are preliminary. In the following section we predict, based on these data and theoretical considerations, which approaches will be effective against various events underlying cell-associated HIV transmission.
Preclinical trials conducted in cell-associated HIV transmission models
Table 7 summarizes animal and in-vitro models that can be used for cell-associated HIV transmission research. Several topical microbicide candidates have been tested in small animal cell-associated virus sexual transmission models. Nonoxynol-9 and WHI-07 blocked mucosal transmission of infected T cells in the FIV model [199,201]. Sulfated polysaccharides, in particular carrageenan, prevented macrophage trafficking from the vaginal cavity in mice [266] and several microbicide candidates have effectively blocked vaginal cell-associated HIV transmission in hu-SCID mice, including BufferGel [118], β-cyclodextrin [207], ICAM blockers [220], and the NNRTI TMCI-20 [267]. However, PRO2000 was ineffective in the hu-SCID rectal cell-associated HIV transmission model [221]. The only relevant vaccine trial was conducted in the FIV model. FIV vaccines based on whole inactivated virus or viral protein extracts suppressed viremia levels following vaginal challenge with FIV-infected cells but not following IV challenge with cell-free virus [268].
Table 7. Cell-associated HIV transmission models.
| Cervicovaginal | Rectal | |
|---|---|---|
| Animal models | ||
| FIV/feline | + | + |
| HIV/hu-SCID mice | + | + |
| SIV/macaque | + | − |
| In-vitro models | ||
| Polarized epithelial monolayers | + | − |
| Tissue explants | + | − |
| Organotypic cultures | + | − |
A few topical microbicides and passive immunization approaches have also been tested in in-vitro cell-associated HIV transmission models. Low doses of synthetic polymers (e.g., PVP, PEG) modified the fiber structure and mechanical properties of human cervical mucus and blocked the migration of monocytes through mucus [269]. In cervical and rectal epithelial monolayer models, which primarily measure HIV transcytosis, ICAM blockers [220], nonnucleoside reverse transcriptase inhibitors (NNRTIs) [221], and antibodies to HIV envelope proteins [222,270–273] were effective, whereas polyionic entry inhibitors (PRO2000, cellulose sulfate, polystyrene sulfate), the fusion inhibitor T-20 [221], and a panel of neutralizing monoclonal antibodies [274] were ineffective.
Potential topical microbicides and vaccine-induced protective mechanisms that could be effective at different stages of cell-associated HIV transmission
Microbicides of the surfactant class could disrupt the membranes of infectious cells in genital secretions and block cell-associated HIV transmission. An early topical microbicide candidate in the surfactant class, Nonoxynol-9 (N-9), lysed genital leukocytes in vitro [115] and blocked cell-associated FIV vaginal transmission [199]. However, it was found to be ineffective in an efficacy trial, with concerns raised that women who used the product most frequently, that is, those engaging in transactional sex for livelihood, were more likely to become infected when they used the product. Evidence now indicates that N-9 inactivates infected cells and cell-free HIV when present in optimal concentrations in vaginal secretions, but that it also induces an inflammatory reaction that recruits HIV target cells to the genital epithelium, which can enhance HIV transmission if HIV is introduced after N-9 levels decline to the point that they are no longer effective [169,275]. Other compounds in this class, such as C31G, have not completed efficacy trials [276,277], but it is conceivable that selective surfactants could be developed in the future, if potential safety concerns could be addressed in preclinical and animal studies.
Buffering agents are being developed as microbicides to maintain a protective low vaginal pH after intercourse and in women with other conditions such as bacterial vaginosis associated with neutral vaginal pH. This approach may be effective against both cell-free and cell-associated HIV transmission as HIV virions are rapidly inactivated and HIV-infected cells may be immobilized and killed by low pH. The first microbicide in this class to be tested in a clinical HIV efficacy trial, BufferGel, was ineffective [278], although adherence issues may have compromised the study. Other more potent acidifying agents in this class are under investigation and may be more effective. If so, acidifying agents could become important components of a combination agent topical microbicide. An alternative approach is to develop lactobacilli that overproduce lactic acid, maintaining a low vaginal pH in women with bacterial vaginosis and estrogen-deficient states, and after intercourse despite the buffering effect of semen [279]. In addition to enhancing the protective acidifying activity of lactobacilli, the organisms could be bioengineered to express fusion inhibitors [280] or soluble CD4+ molecules to prevent HIV binding [281].
Antibodies can trap cells in cervical mucus. This was first demonstrated in the infertility field with the finding that women with antisperm antibodies had immobilized (‘shaking’) sperm in their cervical mucus [282]. Therefore, it is possible that antibodies directed against surface antigens on HIV-infected cells in genital secretions could impede the migration of these cells through mucus. Monoclonal antibodies could be administered passively as a component of topical microbicide formulations [283]; candidate antigens include CD68, CCR5, LFA-1, Mac-1, and CD52g, a seminal plasma antigen that coats all cells in semen [284].
Antibodies and peptides that block LFA/ICAM and MAC-1/JAM C interactions and sulfated polysaccharides inhibit attachment of leukocytes to epithelial cells and, therefore, could inhibit cell-associated HIV transmission [285–287]. ICAMs are also important components of viral synapses; therefore, ICAM blockers could also block this cell-associated HIV transmission mechanism.
The directional shedding of HIV from infected leukocytes toward target cells in the epithelium provides another opportunity for intervention. Numerous approaches to blocking nascent HIV attachment are being studied, targeting each step of the viral binding process. The compounds under study range from monoclonal antibodies to aptimers and dendrimers to small molecule congeners [288–291]. Combinations of small molecules that specifically blocked HIV entry, BMS806/378806 and CMPD 167, showed synergistic activity in blocking cell-free SIV transmission in macaques [292]. Other compounds that may act against HIV binding or entry include cyanovirin-N and sulfonamide derivatives [225]. However, the entry inhibitor that has undergone the greatest level of clinical evaluation is PRO2000, a highly sulfated polyanion [293–295] that was associated with a modest 30% reduction in HIV transmission in HPTN 035 [278] and is being studied in a larger efficacy trial conducted by the UK Medical Research Council in east Africa. If the study findings corroborate HPTN 035, it will be the first demonstration of topical microbicide efficacy for HIV prevention.
Research teams are developing compounds that are congeners of RANTES and other HIVentry inhibitors to competitively inhibit HIV binding and fusion to target cells [296–298]. Such molecules could also block transepithelial migration of infected leukocytes if they can downregulate or block chemokine receptors either before the infected cells reach the epithelial surface or if they sufficiently penetrate the tissues to affect chemokine-mediated epithelial transmigration. They could also block the recruitment of target cells to sites of infection. Glycerol monolaurate (GML), an anti-inflammatory compound that inhibits immune activation and chemokine and cytokine production by human vaginal epithelial cells, was recently shown to prevent mucosal transmission of cell-free SIV by preventing attraction of HIV target cells to SIV infection foci in the cervical epithelium [162]. GML could also inhibit the transmigration of infected cells and target cells that mediate cell-associated HIV transmission. Other approaches to block intraepithelial migration of infected leukocytes include blocking leukocyte attachment to molecules constituting epithelial intracellular tight and desmesomal junctions, and fortifying these junctions.
Vaccine-generated HIV-specific cytotoxic T cells and antibody-dependent cellular cytotoxicity (ADCC) mediators could eliminate HIV-infected cells as they penetrate the genital epithelium [299,300]. Allogeneic immunization has also been considered for protection against both cell-free and cell-associated HIV transmission [301]. Topical application of TLR agonists induce type 1 interferons in the vaginal mucosa, which could protect against cell-associated HIV transmission; however, vaginal transmission of cell-free SIV was not inhibited by this approach possibly because the treatment also induced an inflammatory infiltrate that introduced more HIV-infectable host cells to the vaginal mucosa [302].
Animal studies have shown that HIV infection can be prevented when animals are given either topical or systemic chemoprophylaxis with antiretroviral drugs [303]. The most extensively studied compound thus far has been tenofovir, a nucleotide inhibitor of reverse transcriptase. Recent data suggest that tenofovir gel with or without emtricitabine (FTC) is highly effective in preventing retroviral vaginal and rectal transmission in macaque models [304–306]. Now that studies have demonstrated the safety of topical tenofovir and significant genital tract concentrations [307], large-scale efficacy trials of tenofovir gel as well as oral chemopro-phylaxis to prevent HIV transmission are currently underway [308]. Other reverse transcriptase agents are also being studied for their role in topical chemopro-phylaxis, including dapivirine (TMC-120), a nonnucleoside agent [267,309,310], and UC-781, a poorly absorbable reverse transcriptase agent [311–314]. Although reverse transcriptase inhibitors will only act after HIV has already entered cells, they act before proviral integration, and if they are highly bioavailable in genital secretions, it is feasible that they could be effective in limiting cell-associated viral infection of target cells in the genital mucosa, thereby limiting the likelihood of intracellular HIV replication resulting in productive HIV infection. Combinations of topical antiretrovirals could potentially be particularly effective in preventing cell-associated HIV transmission [315–317]. Topical microbicide candidates and mechanisms that could block cell-associated transmission are summarized in Table 8.
Table 8. Topical microbicide candidates that could prevent cell-associated HIV transmission by genital leukocytes.
| Class | Examples | Potential mechanisms |
|---|---|---|
| Membrane disrupters | C31 (Savvy)c Sodium lauryl sulfate (Invisible Condom) Nonoxynol-9c Ethanol in emollient gel |
Kill infected leukocytes, inactivate nascent HIV. However, nonspecific cytotoxicity could induce inflammation and enhance HIV transmission. |
| Acidifying agents | Carbopol 974Pc (BufferGel) AcidForm (Amphora) |
Kill/immobilize infected leukocytes; inactivate nascent HIV. |
| Entry inhibitors | ||
| Anionic polymers | Naphthalene sulfonate (PRO2000) Carrageenana,c (Carraguard) Cellulose sulfatec (Ushercell)) Cellulose acetate phthalate (CAP) Dendrimers [SPL7013 (Vivagel)] |
Negative charge could interfere with infected leukocyte: target cell interactions, entry of nascent HIV. |
| CCR5 blockers | PSC-RANTES CMPD167 Maraviroc Anti-CCR5 monoclonal antibodies |
Block chemotaxis of infected leukocytes and of target cells toward infected leukocytes; block binding of nascent HIV to target cells. |
| Fusion inhibitors | Cyanovirin-N | May interfere with infected leukocyte: target cell interactions, entry of nascent HIV. |
| Reverse transcriptase inhibitors | Tenofovir (PMPA)b Dapivirine (TMC 120) UC781 TMC120 MIV-150a |
Inhibit HIV replication in infected leukocytes and target cells. |
Alliances for Microbicide Development (www.microbicide.org). Cutler and Justman [317].
PC815 is co-formulation of Carrageenan and MIV-150.
Co-formulated with emtricitabine (FTC) in preclinical studies.
Not being studied at the present time in HIV prevention trials.
Conclusion
Mounting evidence from clinical, animal and in-vitro studies indicates that infected cells (‘Trojan Horse’ leukocytes) may be important vectors of HIV-1 mucosal transmission. This is an understudied topic in the field of HIV research, and a number of fundamental questions remain unanswered (see below).
What is the prevalence of cell-associated HIV transmission vs. cell-free HIV transmission, and what risk factors are associated with cell-associated HIV transmission?
What types of infected cells transmit HIV across mucosal surfaces?
What and where are the target cells of cell-associated HIV transmission; how does HIV reach them?
What is the survival time of infected leukocytes in the genital tract; how long does cell-associated HIV transmission take?
What are the molecular events underlying cell-associated HIV transmission that can be targeted by HIV-prevention strategies?
Preliminary studies using molecular sequencing of founder viruses in newly infected individuals have differentiated between cell-associated and cell-free HIV transmission. These studies should be expanded to determine the prevalence and risk factors associated with cell-associated HIV transmission in different populations. Animal and in-vitro models for studies of cell-associated HIV transmission have been introduced but require further optimization and standardization. These models could reveal critical information concerning molecular events underlying various stages of cell-associated HIV transmission: migration of HIV-infected cells through mucus and epithelial layers, their attachment to epithelial cells, directional HIV shedding and transcytosis, and cell-to-cell virus transfer. Insight into adhesion molecules, chemokines, and other factors that play a role in cell-associated HIV transmission could suggest new strategies for HIV prevention. To explore these mechanisms, the models should reflect natural physiological conditions as closely as possible and incorporate: mature HIV-infected macrophages, which are the predominant HIV-susceptible host cell in semen and cervicovaginal secretions; endogenous flora and low-pH conditions, which could suppress cell-associated HIV infection; inflammatory conditions or infections that could enhance cell-associated HIV infection; and different types of mucins found in genital and rectal secretions (including seminal plasma and cervical mucus), which can influence cell adhesion, migration, and HIV infectivity. Authentic physiologically relevant cell-associated HIV transmission models are needed for testing the efficacy of HIV vaccine and topical microbicide candidates during their pre-clinical trial assessment.
Acknowledgments
The authors thank Drs Jay Levy, Richard Cone, Thomas Moench, Rahm Gummuluru, Manish Sagar, and Greg Vigliante for helpful suggestions.
All of the authors contributed substantively to the writing of this article.
This work was supported by NIH grants R56AI071909 and R33AI076966 (D.J.A.) and the Lifespan CFAR (NIH Grant P30AI042853) (K.H.M.).
References
- 1.UNAIDS. AIDS epidemic update. Geneva: UNAIDS and WHO; 2007. [Google Scholar]
- 2.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 3.Anderson DJ, Yunis EJ. ‘Trojan Horse’ leukocytes in AIDS. N Engl J Med. 1983;309:984–985. [PubMed] [Google Scholar]
- 4.Phillips DM. The role of cell-to-cell transmission in HIV infection. AIDS. 1994;8:719–731. doi: 10.1097/00002030-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Levy JA. The transmission of AIDS: the case of the infected cell. JAMA. 1988;259:3037–3038. [PubMed] [Google Scholar]
- 6.Lehman DA, Farquhar C. Biological mechanisms of vertical human immunodeficiency virus (HIV-1) transmission. Rev Med Virol. 2007;17:381–403. doi: 10.1002/rmv.543. [DOI] [PubMed] [Google Scholar]
- 7.Tuomala RE, O'Driscoll PT, Bremer JW, Jennings C, Xu C, Read JS, et al. Cell-associated genital tract virus and vertical transmission of human immunodeficiency virus type 1 in antiretroviral-experienced women. J Infect Dis. 2003;187:375–384. doi: 10.1086/367706. [DOI] [PubMed] [Google Scholar]
- 8.John GC, Nduati RW, Mbori-Ngacha DA, Richardson BA, Panteleeff D, Mwatha A, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;183:206–212. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DJ, Pudney J. Mucosal immunology of the human male genital tract and experimental models. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal immunology. 3rd. New York: Elsevier Academic Press; 2005. pp. 1647–1659. [Google Scholar]
- 10.Wolff H, Anderson DJ. Immunohistologic characterization and quantitation of leukocyte subpopulations in human semen. Fertil Steril. 1988;49:497–504. [PubMed] [Google Scholar]
- 11.Gil T, Castilla JA, Hortas ML, Redondo M, Samaniego F, Garrido F, et al. Increase of large granular lymphocytes in human ejaculate containing antisperm antibodies. Hum Re-prod. 1998;13:296–301. doi: 10.1093/humrep/13.2.296. [DOI] [PubMed] [Google Scholar]
- 12.Tomlinson MJ, Barratt CL, Bolton AE, Lenton EA, Roberts HB, Cooke ID. Round cells and sperm fertilizing capacity: the presence of immature germ cells but not seminal leukocytes are associated with reduced success of in vitro fertilization. Fertil Steril. 1992;58:1257–1259. doi: 10.1016/s0015-0282(16)55583-6. [DOI] [PubMed] [Google Scholar]
- 13.Denny TN, Scolpino A, Garcia A, Polyak A, Weiss SN, Skurnick JH, et al. Evaluation of T-lymphocyte subsets present in semen and peripheral blood of healthy donors: a report from the heterosexual transmission study. Cytometry. 1995;20:349–355. doi: 10.1002/cyto.990200411. [DOI] [PubMed] [Google Scholar]
- 14.Ball JK, Curran R, Irving WL, Dearden AA. HIV-1 in semen: determination of proviral and viral titres compared to blood, and quantification of semen leukocyte populations. J Med Virol. 1999;59:356–363. doi: 10.1002/(sici)1096-9071(199911)59:3<356::aid-jmv16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Politch JA, Mayer KH, Anderson DJ. Depletion of CD4+ T cells in semen during HIV infection and their restoration following antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;50:283–289. doi: 10.1097/QAI.0b013e3181989870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 3. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 17.WHO. WHO laboratory manual for the examination of human semen and sperm-cervical interaction. 4. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 18.Tomlinson MJ, White A, Barratt CL, Bolton AE, Cooke ID. The removal of morphologically abnormal sperm forms by phagocytes: a positive role for seminal leukocytes? Hum Reprod. 1992;7:517–522. doi: 10.1093/oxfordjournals.humrep.a137682. [DOI] [PubMed] [Google Scholar]
- 19.Yanushpolsky EH, Politch JA, Hill JA, Anderson DJ. Antibiotic therapy and leukocytospermia: a prospective, randomized, controlled study. Fertil Steril. 1995;63:142–147. [PubMed] [Google Scholar]
- 20.Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod. 2007;22:2928–2935. doi: 10.1093/humrep/dem281. [DOI] [PubMed] [Google Scholar]
- 21.Anderson DJ, Politch JA, O'Brien WX, Xu C, Bowman FP, Mayer KH. Persistent HIV-1 RNA shedding in semen of men on HAART is associated with high risk sexual behavior. 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Cape Town, South Africa. 2009. [Google Scholar]
- 22.Wolff H, Anderson DJ. Male genital tract inflammation associated with increased numbers of potential human immunodeficiencyvirushostcellsinsemen. Andrologia. 1988;20:404–410. [PubMed] [Google Scholar]
- 23.Pudney J, Oneta M, Mayer K, Seage G, 3rd, Anderson D. Preejaculatory fluid as potential vector for sexual transmission of HIV-1. Lancet. 1992;340:1470. doi: 10.1016/0140-6736(92)92659-4. [DOI] [PubMed] [Google Scholar]
- 24.Ilaria G, Jacobs JL, Polsky B, Koll B, Baron P, MacLow C, et al. Detection of HIV-1 DNA sequences in preejaculatory fluid. Lancet. 1992;340:1469. doi: 10.1016/0140-6736(92)92658-3. [DOI] [PubMed] [Google Scholar]
- 25.Donoval BA, Landay AL, Moses S, Agot K, Ndinya-Achola JO, Nyagaya EA, et al. HIV-1 target cells in foreskins of African men with varying histories of sexually transmitted infections. Am J Clin Pathol. 2006;125:386–391. [PubMed] [Google Scholar]
- 26.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–1495. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- 27.Patterson BK, Landay A, Siegel JN, Flener Z, Pessis D, Chaviano A, Bailey RC. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol. 2002;161:867–873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Voorhis BJ, Martinez A, Mayer K, Anderson DJ. Detection of human immunodeficiency virus type 1 in semen from seropositive men using culture and polymerase chain reaction deoxyribonucleic acid amplification techniques. Fertil Steril. 1991;55:588–594. [PubMed] [Google Scholar]
- 29.Mermin JH, Holodniy M, Katzenstein DA, Merigan TC. Detection of human immunodeficiency virus DNA and RNA in semen by the polymerase chain reaction. J Infect Dis. 1991;164:769–772. doi: 10.1093/infdis/164.4.769. [DOI] [PubMed] [Google Scholar]
- 30.Hamed KA, Winters MA, Holodniy M, Katzenstein DA, Merigan TC. Detection of human immunodeficiency virus type 1 in semen: effects of disease stage and nucleoside therapy. J Infect Dis. 1993;167:798–802. doi: 10.1093/infdis/167.4.798. [DOI] [PubMed] [Google Scholar]
- 31.Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997;176:960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 32.Xu C, Politch JA, Tucker L, Mayer KH, Seage GR, 3rd, Anderson DJ. Factors associated with increased levels of human immunodeficiency virus type 1 DNA in semen. J Infect Dis. 1997;176:941–947. doi: 10.1086/516539. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Dornadula G, Beumont M, Livornese L, Jr, Van Uitert B, Henning K, et al. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339:1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 34.Krieger JN, Nirapathpongporn A, Chaiyaporn M, Peterson G, Nikolaeva I, Akridge R, et al. Vasectomy and human immunodeficiency virus type 1 in semen. J Urol. 1998;159:820–825. discussion 825–826. [PubMed] [Google Scholar]
- 35.Mayer KH, Boswell S, Goldstein R, Lo W, Xu C, Tucker L, et al. Persistence of human immunodeficiency virus in semen after adding indinavir to combination antiretroviral therapy. Clin Infect Dis. 1999;28:1252–1259. doi: 10.1086/514775. [DOI] [PubMed] [Google Scholar]
- 36.Tachet A, Dulioust E, Salmon D, De Almeida M, Rivalland S, Finkielsztejn L, et al. Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. AIDS. 1999;13:823–831. doi: 10.1097/00002030-199905070-00012. [DOI] [PubMed] [Google Scholar]
- 37.Vernazza PL, Troiani L, Flepp MJ, Cone RW, Schock J, Roth F, et al. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study AIDS. 2000;14:117–121. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- 38.Ghosn J, Viard JP, Katlama C, de Almeida M, Tubiana R, Letourneur F, et al. Evidence of genotypic resistance diversity of archived and circulating viral strains in blood and semen of pretreated HIV-infected men. AIDS. 2004;18:447–457. doi: 10.1097/00002030-200402200-00011. [DOI] [PubMed] [Google Scholar]
- 39.Tindall B, Evans L, Cunningham P, McQueen P, Hurren L, Vasak E, et al. Identification of HIV-1 in semen following primary HIV-1 infection. AIDS. 1992;6:949–952. doi: 10.1097/00002030-199209000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Atkins MC, Carlin EM, Emery VC, Griffiths PD, Boag F. Fluctuations of HIV load in semen of HIV positive patients with newly acquired sexually transmitted diseases. BMJ. 1996;313:341–342. doi: 10.1136/bmj.313.7053.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho DD, Schooley RT, Rota TR, Kaplan JC, Flynn T, Salahuddin SZ, et al. HTLV-III in the semen and blood of a healthy homosexual man. Science. 1984;226:451–453. doi: 10.1126/science.6208608. [DOI] [PubMed] [Google Scholar]
- 42.O'Shea S, Cordery M, Barrett WY, Richman DD, Bradbeer C, Banatvala JE. HIV excretion patterns and specific antibody responses in body fluids. J Med Virol. 1990;31:291–296. doi: 10.1002/jmv.1890310409. [DOI] [PubMed] [Google Scholar]
- 43.Krieger JN, Coombs RW, Collier AC, Ross SO, Chaloupka K, Cummings DK, et al. Recovery of human immunodeficiency virus type 1 from semen: minimal impact of stage of infection and current antiviral chemotherapy. J Infect Dis. 1991;163:386–388. doi: 10.1093/infdis/163.2.386. [DOI] [PubMed] [Google Scholar]
- 44.Krieger JN, Coombs RW, Collier AC, Koehler JK, Ross SO, Chaloupka K, et al. Fertility parameters in men infected with human immunodeficiency virus. J Infect Dis. 1991;164:464–469. doi: 10.1093/infdis/164.3.464. [DOI] [PubMed] [Google Scholar]
- 45.Anderson DJ, O'Brien TR, Politch JA, Martinez A, Seage GR, 3rd, Padian N, et al. Effects of disease stage and zidovudine therapy on the detection of human immunodeficiency virus type 1 in semen. JAMA. 1992;267:2769–2774. [PubMed] [Google Scholar]
- 46.Vernazza PL, Eron JJ, Cohen MS, van der Horst CM, Troiani L, Fiscus SA. Detection and biologic characterization of infectious HIV-1 in semen of seropositive men. AIDS. 1994;8:1325–1329. doi: 10.1097/00002030-199409000-00017. [DOI] [PubMed] [Google Scholar]
- 47.Krieger JN, Coombs RW, Collier AC, Ho DD, Ross SO, Zeh JE, Corey L. Intermittent shedding of human immunodeficiency virus in semen: implications for sexual transmission. J Urol. 1995;154:1035–1040. [PubMed] [Google Scholar]
- 48.Dyer JR, Gilliam BL, Eron JJ, Jr, Grosso L, Cohen MS, Fiscus SA. Quantitation of human immunodeficiency virus type 1 RNA in cell free seminal plasma: comparison of NASBA with Amplicor reverse transcription-PCR amplification and correlation with quantitative culture. J Virol Methods. 1996;60:161–170. doi: 10.1016/0166-0934(96)02063-0. [DOI] [PubMed] [Google Scholar]
- 49.Vernazza PL, Gilliam BL, Dyer J, Fiscus SA, Eron JJ, Frank AC, Cohen MS. Quantification of HIV in semen: correlation with antiviral treatment and immune status. AIDS. 1997;11:987–993. [PubMed] [Google Scholar]
- 50.Vernazza PL, Gilliam BL, Flepp M, Dyer JR, Frank AC, Fiscus SA, et al. Effect of antiviral treatment on the shedding of HIV-1 in semen. AIDS. 1997;11:1249–1254. doi: 10.1097/00002030-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Coombs RW, Speck CE, Hughes JP, Lee W, Sampoleo R, Ross SO, et al. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177:320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 52.Dulioust E, Tachet A, De Almeida M, Finkielsztejn L, Rivalland S, Salmon D, et al. Detection of HIV-1 in seminal plasma and seminal cells of HIV-1 seropositive men. J Reprod Immunol. 1998;41:27–40. doi: 10.1016/s0165-0378(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 53.Nunnari G, Otero M, Dornadula G, Vanella M, Zhang H, Frank I, et al. Residual HIV-1 disease in seminal cells of HIV-1-infected men on suppressive HAART: latency without ongoing cellular infections. AIDS. 2002;16:39–45. doi: 10.1097/00002030-200201040-00006. [DOI] [PubMed] [Google Scholar]
- 54.Belec L, Georges AJ, Steenman G, Martin PM. Antibodies to human immunodeficiency virus in the semen of heterosexual men. J Infect Dis. 1989;159:324–327. doi: 10.1093/infdis/159.2.324. [DOI] [PubMed] [Google Scholar]
- 55.Wolff H, Mayer K, Seage G, Politch J, Horsburgh CR, Anderson D. A comparison of HIV-1 antibody classes, titers, and specificities in paired semen and blood samples from HIV-1 seropositive men. J Acquir Immune Defic Syndr. 1992;5:65–69. [PubMed] [Google Scholar]
- 56.Anderson DJ. Genitourinary immune defense. In: Holmes KK, Sparling PF, Piot P, Wasserheit JN, Corey L, Cohen M, editors. Sexually transmitted diseases. 4th. New York: McGraw-Hill; 2007. [Google Scholar]
- 57.James K, Harvey J, Bradbury AW, Hargreave TB, Cullen RT. The effect of seminal plasma on macrophage function: a possible contributory factor in sexually transmitted disease. AIDS Res. 1983;1:45–57. doi: 10.1089/aid.1.1983.1.45. [DOI] [PubMed] [Google Scholar]
- 58.James K, Hargreave T. Immunosuppression by seminal plasma and its possible clinical significance. Immunol Today. 1984;5:357–363. doi: 10.1016/0167-5699(84)90079-3. [DOI] [PubMed] [Google Scholar]
- 59.Allen RD, Roberts TK. Role of spermine in the cytotoxic effects of seminal plasma. Am J Reprod Immunol Microbiol. 1987;13:4–8. doi: 10.1111/j.1600-0897.1987.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 60.Stites DP, Erickson RP. Suppressive effect of seminal plasma on lymphocyte activation. Nature. 1975;253:727–729. doi: 10.1038/253727a0. [DOI] [PubMed] [Google Scholar]
- 61.Okamoto M, Byrn R, Eyre RC, Mullen T, Church P, Kiessling AA. Seminal plasma induces programmed cell death in cultured peripheral blood mononuclear cells. AIDS Res Hum Retroviruses. 2002;18:797–803. doi: 10.1089/08892220260139549. [DOI] [PubMed] [Google Scholar]
- 62.Barratt CL, Harrison PE, Robinson A, Kessopoulou E, Cooke ID. Seminal white blood cells in men with urethral tract infection. A monoclonal antibody study. Br J Urol. 1991;68:531–536. doi: 10.1111/j.1464-410x.1991.tb15399.x. [DOI] [PubMed] [Google Scholar]
- 63.Lomas DA, Natin D, Stockley RA, Shahmanesh M. Chemo-tactic activity of urethral secretions in men with urethritis and the effect of treatment. J Infect Dis. 1993;167:233–236. doi: 10.1093/infdis/167.1.233. [DOI] [PubMed] [Google Scholar]
- 64.Eggert-Kruse W, Reuland M, Johannsen W, Strowitzki T, Schlehofer JR. Cytomegalovirus (CMV) infection: related to male and/or female infertility factors? Fertil Steril. 2009;91:67–82. doi: 10.1016/j.fertnstert.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 65.Bezold G, Politch JA, Kiviat NB, Kuypers JM, Wolff H, Anderson DJ. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil Steril. 2007;87:1087–1097. doi: 10.1016/j.fertnstert.2006.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denny TN, Skurnick JH, Garcia A, Perez G, Passannante MR, Colon J, et al. Lymphocyte immunoregulatory cells present in semen from human immunodeficiency virus (HIV)-infected individuals: a report from the HIV Heterosexual Transmission Study. Cytometry. 1996;26:47–51. doi: 10.1002/(SICI)1097-0320(19960315)26:1<47::AID-CYTO7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 67.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 68.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 70.Dyer JR, Eron JJ, Hoffman IF, Kazembe P, Vernazza PL, Nkata E, et al. Association of CD4 cell depletion and elevated blood and seminal plasma human immunodeficiency virus type 1 (HIV-1) RNA concentrations with genital ulcer disease in HIV-1-infected men in Malawi. J Infect Dis. 1998;177:224–227. doi: 10.1086/517359. [DOI] [PubMed] [Google Scholar]
- 71.Plummer FA, Wainberg MA, Plourde P, Jessamine P, D'Costa LJ, Wamola IA, Ronald AR. Detection of human immunodeficiency virus type 1 (HIV-1) in genital ulcer exudate of HIV-1-infected men by culture and gene amplification. J Infect Dis. 1990;161:810–811. doi: 10.1093/infdis/161.4.810. [DOI] [PubMed] [Google Scholar]
- 72.Schacker T, Ryncarz AJ, Goddard J, Diem K, Shaughnessy M, Corey L. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA. 1998;280:61–66. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- 73.Speck CE, Coombs RW, Koutsky LA, Zeh J, Ross SO, Hooton TM, et al. Risk factors for HIV-1 shedding in semen. Am J Epidemiol. 1999;150:622–631. doi: 10.1093/oxfordjournals.aje.a010061. [DOI] [PubMed] [Google Scholar]
- 74.Comhaire F, Bosmans E, Ombelet W, Punjabi U, Schoonjans F. Cytokines in semen of normal men and of patients with andrological diseases. Am J Reprod Immunol. 1994;31:99–103. doi: 10.1111/j.1600-0897.1994.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 75.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 76.Pilcher CD, Joaki G, Hoffman IF, Martinson FE, Mapanje C, Stewart PW, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007;21:1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Piomboni P, Baccetti B. Spermatozoon as a vehicle for HIV-1 and other viruses:a review. Mol Reprod Dev. 2000;56:238–242. doi: 10.1002/(SICI)1098-2795(200006)56:2+<238::AID-MRD5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 78.Pudney J, Nguyen H, Xu C, Anderson DJ. Microscopic evidence against HIV-1 infection of germ cells or attachment to sperm. J Reprod Immunol. 1999;44:57–77. doi: 10.1016/s0165-0378(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 79.Quayle AJ, Xu C, Tucker L, Anderson DJ. The case against an association between HIV-1 and sperm: molecular evidence. J Reprod Immunol. 1998;41:127–136. doi: 10.1016/s0165-0378(98)00053-9. [DOI] [PubMed] [Google Scholar]
- 80.Roulet V, Satie AP, Ruffault A, Le Tortorec A, Denis H, Guist'hau O, et al. Susceptibility of human testis to human immunodeficiency virus-1 infection in situ and in vitro. Am J Pathol. 2006;169:2094–2103. doi: 10.2353/ajpath.2006.060191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nuovo GJ, Becker J, Simsir A, Margiotta M, Khalife G, Shev-chuk M. HIV-1 nucleic acids localize to the spermatogonia and their progeny. A study by polymerase chain reaction in situ hybridization. Am J Pathol. 1994;144:1142–1148. [PMC free article] [PubMed] [Google Scholar]
- 82.Muciaccia B, Filippini A, Ziparo E, Colelli F, Baroni CD, Stefanini M. Testicular germ cells of HIV-seropositive asymptomatic men are infected by the virus. J Reprod Immunol. 1998;41:81–93. doi: 10.1016/s0165-0378(98)00050-3. [DOI] [PubMed] [Google Scholar]
- 83.Bagasra O, Farzadegan H, Seshamma T, Oakes JW, Saah A, Pomerantz RJ. Detection of HIV-1 proviral DNA in sperm from HIV-1-infected men. AIDS. 1994;8:1669–1674. doi: 10.1097/00002030-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 84.Baccetti B, Benedetto A, Burrini AG, Collodel G, Elia C, Piom-boni P, et al. HIV particles detected in spermatozoa of patients with AIDS. J Submicrosc Cytol Pathol. 1991;23:339–345. [PubMed] [Google Scholar]
- 85.Dussaix E, Guetard D, Dauguet C, D'Almeida M, Auer J, Ellrodt A, et al. Spermatozoa as potential carriers of HIV. Res Virol. 1993;144:487–495. doi: 10.1016/s0923-2516(06)80064-6. [DOI] [PubMed] [Google Scholar]
- 86.Persico T, Savasi V, Ferrazzi E, Oneta M, Semprini AE, Simoni G. Detection of human immunodeficiency virus-1 RNA and DNA by extractive and in situ PCR in unprocessed semen and seminal fractions isolated by semen-washing procedure. Hum Reprod. 2006;21:1525–1530. doi: 10.1093/humrep/del004. [DOI] [PubMed] [Google Scholar]
- 87.Pudney J, Nguyen H, Xu C, Anderson DJ. Microscopic evidence against HIV-1 infection of germ cells or attachment to sperm. J Reprod Immunol. 1998;41:105–125. doi: 10.1016/s0165-0378(98)00052-7. [DOI] [PubMed] [Google Scholar]
- 88.Kato S, Hanabusa H, Kaneko S, Takakuwa K, Suzuki M, Kuji N, et al. Complete removal of HIV-1 RNA and proviral DNA from semen by the swim-up method: assisted reproduction technique using spermatozoa free from HIV-1. AIDS. 2006;20:967–973. doi: 10.1097/01.aids.0000222067.07255.2d. [DOI] [PubMed] [Google Scholar]
- 89.Kim LU, Johnson MR, Barton S, Nelson MR, Sontag G, Smith JR, et al. Evaluation of sperm washing as a potential method of reducing HIV transmission in HIV-discordant couples wishing to have children. AIDS. 1999;13:645–651. doi: 10.1097/00002030-199904160-00004. [DOI] [PubMed] [Google Scholar]
- 90.Politch JA, Xu C, Tucker L, Anderson DJ. Separation of human immunodeficiency virus type 1 from motile sperm by the double tube gradient method versus other methods. Fertil Steril. 2004;81:440–447. doi: 10.1016/j.fertnstert.2003.06.028. [DOI] [PubMed] [Google Scholar]
- 91.Kashima K, Takakuwa K, Suzuki M, Makino M, Kaneko S, Kato S, et al. Studies of assisted reproduction techniques (ART) for HIV-1-discordant couples using washed sperm and the nested PCR method: a comparison of the pregnancy rates in HIV-1-discordant couples and control couples. Jpn J Infect Dis. 2009;62:173–176. [PubMed] [Google Scholar]
- 92.Lasheeb AS, King J, Ball JK, Curran R, Barratt CL, Afnan M, Pillay D. Semen characteristics in HIV-1 positive men and the effect of semen washing. Genitourin Med. 1997;73:303–305. doi: 10.1136/sti.73.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hanabusa H, Kuji N, Kato S, Tagami H, Kaneko S, Tanaka H, Yoshimura Y. An evaluation of semen processing methods for eliminating HIV-1. AIDS. 2000;14:1611–1616. doi: 10.1097/00002030-200007280-00017. [DOI] [PubMed] [Google Scholar]
- 94.Chrystie IL, Mullen JE, Braude PR, Rowell P, Williams E, Elkington N, et al. Assisted conception in HIV discordant couples: evaluation of semen processing techniques in reducing HIV viral load. J Reprod Immunol. 1998;41:301–306. doi: 10.1016/s0165-0378(98)00066-7. [DOI] [PubMed] [Google Scholar]
- 95.Pasquier C, Daudin M, Righi L, Berges L, Thauvin L, Berrebi A, et al. Sperm washing and virus nucleic acid detection to reduce HIV and hepatitis C virus transmission in serodiscordant couples wishing to have children. AIDS. 2000;14:2093–2099. doi: 10.1097/00002030-200009290-00004. [DOI] [PubMed] [Google Scholar]
- 96.Marina S, Marina F, Alcolea R, Exposito R, Huguet J, Nadal J, Verges A. Human immunodeficiency virus type 1-serodiscordant couples can bear healthy children after undergoing intrauterine insemination. Fertil Steril. 1998;70:35–39. doi: 10.1016/s0015-0282(98)00102-2. [DOI] [PubMed] [Google Scholar]
- 97.Semprini AE, Fiore S, Pardi G. Reproductive counselling for HIV-discordant couples. Lancet. 1997;349:1401–1402. doi: 10.1016/S0140-6736(05)63250-3. [DOI] [PubMed] [Google Scholar]
- 98.Semprini AE, Levi-Setti P, Bozzo M, Ravizza M, Taglioretti A, Sulpizio P, et al. Insemination of HIV-negative women with processed semen of HIV-positive partners. Lancet. 1992;340:1317–1319. doi: 10.1016/0140-6736(92)92495-2. [DOI] [PubMed] [Google Scholar]
- 99.Sauer MV. Sperm washing techniques address the fertility needs of HIV-seropositive men: a clinical review. Reprod Biomed Online. 2005;10:135–140. doi: 10.1016/s1472-6483(10)60815-2. [DOI] [PubMed] [Google Scholar]
- 100.Sauer MV, Chang PL. Establishing a clinical program for human immunodeficiency virus 1-seropositive men to father seronegative children by means of in vitro fertilization with intracytoplasmic sperm injection. Am J Obstet Gynecol. 2002;186:627–633. doi: 10.1067/mob.2002.122125. [DOI] [PubMed] [Google Scholar]
- 101.Pena JE, Thornton MH, Sauer MV. Complications of in vitro fertilization with intracytoplasmic sperm injection in human immunodeficiency virus serodiscordant couples. Arch Gynecol Obstet. 2003;268:198–201. doi: 10.1007/s00404-003-0507-8. [DOI] [PubMed] [Google Scholar]
- 102.Ohl J, Partisani M, Wittemer C, Schmitt MP, Cranz C, Stoll-Keller F, et al. Assisted reproduction techniques for HIV serodiscordant couples: 18 months of experience. Hum Re-prod. 2003;18:1244–1249. doi: 10.1093/humrep/deg258. [DOI] [PubMed] [Google Scholar]
- 103.Gilling-Smith C, Nicopoullos JD, Semprini AE, Frodsham LC. HIV and reproductive care: a review of current practice. BJOG. 2006;113:869–878. doi: 10.1111/j.1471-0528.2006.00960.x. [DOI] [PubMed] [Google Scholar]
- 104.Savasi V, Ferrazzi E, Lanzani C, Oneta M, Parrilla B, Persico T. Safety of sperm washing and ART outcome in 741 HIV-1-serodiscordant couples. Hum Reprod. 2007;22:772–777. doi: 10.1093/humrep/del422. [DOI] [PubMed] [Google Scholar]
- 105.Bujan L, Sergerie M, Kiffer N, Moinard N, Seguela G, Mercadier B, et al. Good efficiency of intrauterine insemination programme for serodiscordant couples with HIV-1 infected male partner: a retrospective comparative study. Eur J Obstet Gynecol Reprod Biol. 2007;135:76–82. doi: 10.1016/j.ejogrb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 106.Scofield VL. Sperm as infection-potentiating cofactors in HIV transmission. J Reprod Immunol. 1998;41:359–372. doi: 10.1016/s0165-0378(98)00071-0. [DOI] [PubMed] [Google Scholar]
- 107.Bandivdekar AH, Velhal SM, Raghavan VP. Identification of CD4-independent HIV receptors on spermatozoa. Am J Re-prod Immunol. 2003;50:322–327. doi: 10.1034/j.1600-0897.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 108.Brogi A, Presentini R, Moretti E, Strazza M, Piomboni P, Costantino-Ceccarini E. New insights into the interaction between the gp120 and the HIV receptor in human sperm (human.sperm/gp120/galactoglycerolipid/antigalactosylcer-amide/seminolip id/spermatogonia) J Reprod Immunol. 1998;41:213–231. doi: 10.1016/s0165-0378(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 109.Fanibunda SE, Velhal SM, Raghavan VP, Bandivdekar AH. CD4 independent binding of HIV gp120 to mannose receptor on human spermatozoa. J Acquir Immune Defic Syndr. 2008;48:389–397. doi: 10.1097/QAI.0b013e318179a0fb. [DOI] [PubMed] [Google Scholar]
- 110.Gadella BM, Hammache D, Pieroni G, Colenbrander B, van Golde LM, Fantini J. Glycolipids as potential binding sites for HIV: topology in the sperm plasma membrane in relation to the regulation of membrane fusion. J Reprod Immunol. 1998;41:233–253. doi: 10.1016/s0165-0378(98)00061-8. [DOI] [PubMed] [Google Scholar]
- 111.Cardona-Maya W, Lopez-Herrera A, Velilla-Hernandez P, Rugeles MT, Cadavid AP. The role of mannose receptor on HIV-1 entry into human spermatozoa. Am J Reprod Immunol. 2006;55:241–245. doi: 10.1111/j.1600-0897.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 112.Muciaccia B, Corallini S, Vicini E, Padula F, Gandini L, Liuzzi G, et al. HIV-1 viral DNA is present in ejaculated abnormal spermatozoa of seropositive subjects. Hum Reprod. 2007;22:2868–2878. doi: 10.1093/humrep/dem288. [DOI] [PubMed] [Google Scholar]
- 113.Sievers-Altermann R, Engelbrecht DV. Entry of spermatozoa into the cervical mucosa and transmission of the AIDS virus. S Afr Med J. 1990;77:319. [PubMed] [Google Scholar]
- 114.Wah RM, Anderson DJ, Hill JA. Asymptomatic cervicovaginal leukocytosis in infertile women. Fertil Steril. 1990;54:445–450. [PubMed] [Google Scholar]
- 115.Hill JA, Anderson DJ. Human vaginal leukocytes and the effects of vaginal fluid on lymphocyte and macrophage defense functions. Am J Obstet Gynecol. 1992;166:720–726. doi: 10.1016/0002-9378(92)91703-d. [DOI] [PubMed] [Google Scholar]
- 116.Bardeguez AD, Skurnick JH, Perez G, Colon JM, Kloser P, Denny TN. Lymphocyte shedding from genital tract of human immunodeficiency virus-infected women: immunophenotypic and clinical correlates. Am J Obstet Gynecol. 1997;176:158–165. doi: 10.1016/s0002-9378(97)80029-4. [DOI] [PubMed] [Google Scholar]
- 117.Anderson DJ, Politch JA, Tucker LD, Fichorova R, Haimovici F, Tuomala RE, Mayer KH. Quantitation of mediators of inflammation and immunity in genital tract secretions and their relevance to HIV type 1 transmission. AIDS Res Hum Retro-viruses. 1998;14(Suppl 1):S43–S49. [PubMed] [Google Scholar]
- 118.Olmsted SS, Khanna KV, Ng EM, Whitten ST, Johnson ON, 3rd, Markham RB, et al. Low pH immobilizes and kills human leukocytes and prevents transmission of cell-associated HIV in a mouse model. BMC Infect Dis. 2005;5:79. doi: 10.1186/1471-2334-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levine WC, Pope V, Bhoomkar A, Tambe P, Lewis JS, Zaidi AA, et al. Increase in endocervical CD4 lymphocytes among women with nonulcerative sexually transmitted diseases. J Infect Dis. 1998;177:167–174. doi: 10.1086/513820. [DOI] [PubMed] [Google Scholar]
- 120.Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988;158:819–828. doi: 10.1016/0002-9378(88)90078-6. [DOI] [PubMed] [Google Scholar]
- 121.Cook RL, Redondo-Lopez V, Schmitt C, Meriwether C, Sobel JD. Clinical, microbiological, and biochemical factors in recurrent bacterial vaginosis. J Clin Microbiol. 1992;30:870–877. doi: 10.1128/jcm.30.4.870-877.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clemetson DB, Moss GB, Willerford DM, Hensel M, Emonyi W, Holmes KK, et al. Detection of HIV DNA in cervical and vaginal secretions. Prevalence and correlates among women in Nairobi, Kenya. JAMA. 1993;269:2860–2864. [PubMed] [Google Scholar]
- 123.Cowan FF, Pascoe SJ, Barlow KL, Langhaug LF, Jaffar S, Hargrove JW, et al. Association of genital shedding of herpes simplex virus type 2 and HIV-1 among sex workers in rural Zimbabwe. AIDS. 2006;20:261–267. doi: 10.1097/01.aids.0000198086.39831.4a. [DOI] [PubMed] [Google Scholar]
- 124.John GC, Nduati RW, Mbori-Ngacha D, et al. Genital shedding of human immunodeficiency virus type 1 DNA during pregnancy: association with immunosuppression, abnormal cervical or vaginal discharge, and severe vitamin A deficiency. J Infect Dis. 1997;175:57–62. doi: 10.1093/infdis/175.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kreiss J, Willerford DM, Hensel M, Emonyi W, Plummer F, Ndinya-Achola J, et al. Association between cervical inflammation and cervical shedding of human immunodeficiency virus DNA. J Infect Dis. 1994;170:1597–1601. doi: 10.1093/infdis/170.6.1597. [DOI] [PubMed] [Google Scholar]
- 126.McClelland RS, Wang CC, Overbaugh J, Richardson BA, Corey L, Ashley RL, et al. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS. 2002;16:2425–2430. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 127.McClelland RS, Wang CC, Mandaliya K, Overbaugh J, Reiner MT, Panteleeff DD, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS. 2001;15:105–110. doi: 10.1097/00002030-200101050-00015. [DOI] [PubMed] [Google Scholar]
- 128.Mostad SB, Kreiss JK, Ryncarz AJ, Mandaliya K, Chohan B, Ndinya-Achola J, et al. Cervical shedding of herpes simplex virus in human immunodeficiency virus-infected women: effects of hormonal contraception, pregnancy, and vitamin A deficiency. J Infect Dis. 2000;181:58–63. doi: 10.1086/315188. [DOI] [PubMed] [Google Scholar]
- 129.Mostad SB, Overbaugh J, DeVange DM, Welch MJ, Chohan B, Mandaliya K, et al. Hormonal contraception, vitamin A deficiency, and other risk factors for shedding of HIV-1 infected cells from the cervix and vagina. Lancet. 1997;350:922–927. doi: 10.1016/S0140-6736(97)04240-2. [DOI] [PubMed] [Google Scholar]
- 130.Spinillo A, Zara F, Gardella B, Preti E, Mainini R, Maserati R. The effect of vaginal candidiasis on the shedding of human immunodeficiency virus in cervicovaginal secretions. Am J Obstet Gynecol. 2005;192:774–779. doi: 10.1016/j.ajog.2004.10.609. [DOI] [PubMed] [Google Scholar]
- 131.Spinillo A, Debiaggi M, Zara F, Maserati R, Polatti F, De Santolo A. Factors associated with nucleic acids related to human immunodeficiency virus type 1 in cervicovaginal secretions. BJOG. 2001;108:634–641. doi: 10.1111/j.1471-0528.2001.00141.x. [DOI] [PubMed] [Google Scholar]
- 132.Wang CC, McClelland RS, Reilly M, Overbaugh J, Emery SR, Mandaliya K, et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J Infect Dis. 2001;183:1017–1022. doi: 10.1086/319287. [DOI] [PubMed] [Google Scholar]
- 133.Manhart LE, Mostad SB, Baeten JM, Astete SG, Mandaliya K, Totten PA. High mycoplasma genitalium organism burden is associated with shedding of HIV-1 DNA from the cervix. J Infect Dis. 2008;197:733–736. doi: 10.1086/526501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang CC, McClelland RS, Overbaugh J, Reilly M, Panteleeff DD, Mandaliya K, et al. The effect of hormonal contraception on genital tract shedding of HIV-1. AIDS. 2004;18:205–209. doi: 10.1097/00002030-200401230-00009. [DOI] [PubMed] [Google Scholar]
- 135.Baeten JM, McClelland RS, Richardson BA, Bankson DD, Lavreys L, Wener MH, et al. Vitamin A deficiency and the acute phase response among HIV-1-infected and -uninfected women in Kenya. J Acquir Immune Defic Syndr. 2002;31:243–249. doi: 10.1097/00126334-200210010-00016. [DOI] [PubMed] [Google Scholar]
- 136.Baeten JM, Mostad SB, Hughes MP, Overbaugh J, Bankson DD, Mandaliya K, et al. Selenium deficiency is associated with shedding of HIV-1-infected cells in the female genital tract. J Acquir Immune Defic Syndr. 2001;26:360–364. doi: 10.1097/00126334-200104010-00013. [DOI] [PubMed] [Google Scholar]
- 137.McClelland RS, Baeten JM, Overbaugh J, Richardson BA, Mandaliya K, Emery S, et al. Micronutrient supplementation increases genital tract shedding of HIV-1 in women: results of a randomized trial. J Acquir Immune Defic Syndr. 2004;37:1657–1663. doi: 10.1097/00126334-200412150-00021. [DOI] [PubMed] [Google Scholar]
- 138.Graham SM, Holte SE, Peshu NM, Richardson BA, Panteleeff DD, Jaoko WG, et al. Initiation of antiretroviral therapy leads to a rapid decline in cervical and vaginal HIV-1 shedding. AIDS. 2007;21:501–507. doi: 10.1097/QAD.0b013e32801424bd. [DOI] [PubMed] [Google Scholar]
- 139.Nunnari G, Sullivan J, Xu Y, Nyirjesy P, Kulkosky J, Cavert W, et al. HIV type 1 cervicovaginal reservoirs in the era of HAART. AIDS Res Hum Retroviruses. 2005;21:714–718. doi: 10.1089/aid.2005.21.714. [DOI] [PubMed] [Google Scholar]
- 140.Panther LA, Tucker L, Xu C, Tuomala RE, Mullins JI, Anderson DJ. Genital tract human immunodeficiency virus type 1 (HIV-1) shedding and inflammation and HIV-1 env diversity in perinatal HIV-1 transmission. J Infect Dis. 2000;181:555–563. doi: 10.1086/315230. [DOI] [PubMed] [Google Scholar]
- 141.Mbopi-Keou FX, Gresenguet G, Mayaud P, Weiss HA, Gopal R, Matta M, et al. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J Infect Dis. 2000;182:1090–1096. doi: 10.1086/315836. [DOI] [PubMed] [Google Scholar]
- 142.Iversen AK, Larsen AR, Jensen T, Fugger L, Balslev U, Wahl S, et al. Distinct determinants of human immunodeficiency virus type 1 RNA and DNA loads in vaginal and cervical secretions. J Infect Dis. 1998;177:1214–1220. doi: 10.1086/515266. [DOI] [PubMed] [Google Scholar]
- 143.Debiaggi M, Zara F, Spinillo A, De Santolo A, Maserati R, Bruno R, et al. Viral excretion in cervicovaginal secretions of HIV-1-infected women receiving antiretroviral therapy. Eur J Clin Microbiol Infect Dis. 2001;20:91–96. doi: 10.1007/s100960000442. [DOI] [PubMed] [Google Scholar]
- 144.Benki S, McClelland RS, Emery S, Baeten JM, Richardson BA, Lavreys L, et al. Quantification of genital human immunodeficiency virus type 1 (HIV-1) DNA in specimens from women with low plasma HIV-1 RNA levels typical of HIV-1 non-transmitters. J Clin Microbiol. 2006;44:4357–4362. doi: 10.1128/JCM.01481-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Andreoletti L, Chomont N, Gresenguet G, Matta M, de Dieu Longo J, Carreno MP, et al. Independent levels of cell-free and cell-associated human immunodeficiency virus-1 in genital-tract secretions of clinically asymptomatic, treatment-naive African women. J Infect Dis. 2003;188:549–554. doi: 10.1086/377104. [DOI] [PubMed] [Google Scholar]
- 146.Zara F, Nappi RE, Brerra R, Migliavacca R, Maserati R, Spinillo A. Markers of local immunity in cervico-vaginal secretions of HIV infected women: implications for HIV shedding. Sex Transm Infect. 2004;80:108–112. doi: 10.1136/sti.2003.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Henin Y, Mandelbrot L, Henrion R, Pradinaud R, Coulaud JP, Montagnier L. Virus excretion in the cervicovaginal secretions of pregnant and nonpregnant HIV-infected women. J Acquir Immune Defic Syndr. 1993;6:72–75. [PubMed] [Google Scholar]
- 148.Vogt MW, Witt DJ, Craven DE, Byington R, Crawford DF, Hutchinson MS, et al. Isolation patterns of the human immunodeficiency virus from cervical secretions during the menstrual cycle of women at risk for the acquired immunodeficiency syndrome. Ann Intern Med. 1987;106:380–382. doi: 10.7326/0003-4819-106-3-380. [DOI] [PubMed] [Google Scholar]
- 149.Vogt MW, Witt DJ, Craven DE, Byington R, Crawford DF, Schooley RT, Hirsch MS. Isolation of HTLV-III/LAV from cervical secretions of women at risk for AIDS. Lancet. 1986;1:525–527. doi: 10.1016/s0140-6736(86)90884-6. [DOI] [PubMed] [Google Scholar]
- 150.Wofsy CB, Cohen JB, Hauer LB, Padian NS, Michaelis BA, Evans LA, Levy JA. Isolation of AIDS-associated retrovirus from genital secretions of women with antibodies to the virus. Lancet. 1986;1:527–529. doi: 10.1016/s0140-6736(86)90885-8. [DOI] [PubMed] [Google Scholar]
- 151.Saracino A, Di Stefano M, Fiore JR, Lepera A, Raimondi D, Angarano G, Pastore G. Frequent detection of HIV-1 RNA but low rates of HIV-1 isolation in cervicovaginal secretions from infected women. New Microbiol. 2000;23:79–83. [PubMed] [Google Scholar]
- 152.Cummins JE, Jr, Villanueva JM, Evans-Strickfaden T, Sesay SM, Abner SR, Bush TJ, et al. Detection of infectious human immunodeficiency virus type 1 in female genital secretions by a short-term culture method. J Clin Microbiol. 2003;41:4081–4088. doi: 10.1128/JCM.41.9.4081-4088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hillier SL. Normal genital flora. In: Holmes KK, Sparling PF, Stamm W, et al., editors. Sexually transmitted diseases. 4th. New York: McGraw Hill Medical Publications; 2008. pp. 298–308. [Google Scholar]
- 154.Parkhurst MR, Saltzman WM. Leukocytes migrate through three-dimensional gels of midcycle cervical mucus. Cell Immunol. 1994;156:77–94. doi: 10.1006/cimm.1994.1154. [DOI] [PubMed] [Google Scholar]
- 155.Masters WH, Johnson VE. The artificial vagina: anatomic, physiologic, psychosexual function. West J Surg Obstet Gynecol. 1961;69:192–212. [PubMed] [Google Scholar]
- 156.Masters WH, Johnson VE. The physiology of the vaginal reproductive function. West J Surg Obstet Gynecol. 1961;69:105–120. [PubMed] [Google Scholar]
- 157.Pearce-Pratt R, Phillips DM. Studies of adhesion of lympho-cytic cells: implications for sexual transmission of human immunodeficiency virus. Biol Reprod. 1993;48:431–445. doi: 10.1095/biolreprod48.3.431. [DOI] [PubMed] [Google Scholar]
- 158.Tan X, Phillips DM. Cell-mediated infection of cervix derived epithelial cells with primary isolates of human immunodeficiency virus. Arch Virol. 1996;141:1177–1189. doi: 10.1007/BF01718823. [DOI] [PubMed] [Google Scholar]
- 159.Dezzutti CS, Guenthner PC, Cummins JE, Jr, Cabrera T, Marshall JH, Dillberger A, Lal RB. Cervical and prostate primary epithelial cells are not productively infected but sequester human immunodeficiency virus type 1. J Infect Dis. 2001;183:1204–1213. doi: 10.1086/319676. [DOI] [PubMed] [Google Scholar]
- 160.Maher D, Wu X, Schacker T, Horbul J, Southern P. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc Natl Acad Sci U S A. 2005;102:11504–11509. doi: 10.1073/pnas.0500848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu Z, Chen Z, Phillips DM. Human genital epithelial cells capture cell-free human immunodeficiency virus type 1 and transmit the virus to CD4+ Cells: implications for mechanisms of sexual transmission. J Infect Dis. 2003;188:1473–1482. doi: 10.1086/379248. [DOI] [PubMed] [Google Scholar]
- 162.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 164.Alfsen A, Yu H, Magerus-Chatinet A, Schmitt A, Bomsel M. HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol Biol Cell. 2005;16:4267–4279. doi: 10.1091/mbc.E05-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pudney J, Anderson DJ. Immunobiology of the human penile urethra. Am J Pathol. 1995;147:155–165. [PMC free article] [PubMed] [Google Scholar]
- 166.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 167.Howell AL, Edkins RD, Rier SE, Yeaman GR, Stern JE, Fanger MW, Wira CR. Human immunodeficiency virus type 1 infection of cells and tissues from the upper and lower human female reproductive tract. J Virol. 1997;71:3498–3506. doi: 10.1128/jvi.71.5.3498-3506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Phillips DM, Zacharopoulos VR. Nonoxynol-9 enhances rectal infection by herpes simplex virus in mice. Contraception. 1998;57:341–348. doi: 10.1016/s0010-7824(98)00040-7. [DOI] [PubMed] [Google Scholar]
- 169.Fichorova RN, Tucker LD, Anderson DJ. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;184:418–428. doi: 10.1086/322047. [DOI] [PubMed] [Google Scholar]
- 170.Hillier SL, Moench T, Shattock R, Black R, Reichelderfer P, Veronese F. In vitro and in vivo: the story of nonoxynol 9. J Acquir Immune Defic Syndr. 2005;39:1–8. doi: 10.1097/01.qai.0000159671.25950.74. [DOI] [PubMed] [Google Scholar]
- 171.Stafford MK, Ward H, Flanagan A, Rosenstein IJ, Taylor-Robinson D, Smith JR, et al. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:327–331. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- 172.Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13:491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- 173.Thompson LA, Barratt CL, Bolton AE, Cooke ID. The leuko-cytic reaction of the human uterine cervix. Am J Reprod Immunol. 1992;28:85–89. doi: 10.1111/j.1600-0897.1992.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 174.Moore JP, Shattock RJ. Preventing HIV-1 sexual transmission: not sexy enough science, or no benefit to the bottom line? J Antimicrob Chemother. 2003;52:890–892. doi: 10.1093/jac/dkh011. [DOI] [PubMed] [Google Scholar]
- 175.Frost SD, Liu Y, Pond SL, Chappey C, Wrin T, Petropoulos CJ, et al. Characterization of human immunodeficiency virus type 1 (HIV-1) envelope variation and neutralizing antibody responses during transmission of HIV-1 subtype B. J Virol. 2005;79:6523–6527. doi: 10.1128/JVI.79.10.6523-6527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Frost SD, Wrin T, Smith DM, Kosakovsky Pond SL, Liu Y, Paxinos E, et al. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc Natl Acad Sci U S A. 2005;102:18514–18519. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gottlieb GS, Heath L, Nickle DC, Wong KG, Leach SE, Jacobs B, et al. HIV-1 variation before seroconversion in men who have sex with men: analysis of acute/early HIV infection in the multicenter AIDS cohort study. J Infect Dis. 2008;197:1011–1015. doi: 10.1086/529206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhu T, Mo H, Wang N, Nam DS, Cao Y, Koup RA, Ho DD. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 179.Zhang LQ, MacKenzie P, Cleland A, Holmes EC, Brown AJ, Simmonds P. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J Virol. 1993;67:3345–3356. doi: 10.1128/jvi.67.6.3345-3356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303:2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 181.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sagar M, Laeyendecker O, Lee S, Gamiel J, Wawer MJ, Gray RH, et al. Selection of HIV variants with signature genotypic characteristics during heterosexual transmission. J Infect Dis. 2009;199:580–589. doi: 10.1086/596557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Long EM, Martin HL, Jr, Kreiss JK, Rainwater SM, Lavreys L, Jackson DJ, et al. Gender differences in HIV-1 diversity at time of infection. Nat Med. 2000;6:71–75. doi: 10.1038/71563. [DOI] [PubMed] [Google Scholar]
- 184.Haaland RE, Hawkins PA, Salazar-Gonzalez J, Johnson A, Tichacek A, Karita E, et al. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 2009;5:e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ritola K, Pilcher CD, Fiscus SA, Hoffman NG, Nelson JA, Kitrinos KM, et al. Multiple V1/V2 env variants are frequently present during primary infection with human immunodeficiency virus type 1. J Virol. 2004;78:11208–11218. doi: 10.1128/JVI.78.20.11208-11218.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sagar M, Kirkegaard E, Long EM, Celum C, Buchbinder S, Daar ES, Overbaugh J. Human immunodeficiency virus type 1 (HIV-1) diversity at time of infection is not restricted to certain risk groups or specific HIV-1 subtypes. J Virol. 2004;78:7279–7283. doi: 10.1128/JVI.78.13.7279-7283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pillai SK, Good B, Pond SK, Wong JK, Strain MC, Richman DD, Smith DM. Semen-specific genetic characteristics of human immunodeficiency virus type 1 env. J Virol. 2005;79:1734–1742. doi: 10.1128/JVI.79.3.1734-1742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ping LH, Cohen MS, Hoffman I, Vernazza P, Seillier-Moiseiwitsch F, Chakraborty H, et al. Effects of genital tract inflammation on human immunodeficiency virus type 1 V3 populations in blood and semen. J Virol. 2000;74:8946–8952. doi: 10.1128/jvi.74.19.8946-8952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhu T, Wang N, Carr A, Nam DS, Moor-Jankowski R, Cooper DA, Ho DD. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Byrn RA, Zhang D, Eyre R, McGowan K, Kiessling AA. HIV-1 in semen: an isolated virus reservoir. Lancet. 1997;350:1141. doi: 10.1016/S0140-6736(97)24042-0. [DOI] [PubMed] [Google Scholar]
- 191.Delwart EL, Mullins JI, Gupta P, Learn GH, Jr, Holodniy M, Katzenstein D, et al. Human immunodeficiency virus type 1 populations in blood and semen. J Virol. 1998;72:617–623. doi: 10.1128/jvi.72.1.617-623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Paranjpe S, Craigo J, Patterson B, Ding M, Barroso P, Harrison L, et al. Subcompartmentalization of HIV-1 quasispecies between seminal cells and seminal plasma indicates their origin in distinct genital tissues. AIDS Res Hum Retroviruses. 2002;18:1271–1280. doi: 10.1089/088922202320886316. [DOI] [PubMed] [Google Scholar]
- 193.Peters B, Whittall T, Babaahmady K, Gray K, Vaughan R, Lehner T. Effect of heterosexual intercourse on mucosal alloimmunisation and resistance to HIV-1 infection. Lancet. 2004;363:518–524. doi: 10.1016/S0140-6736(04)15538-4. [DOI] [PubMed] [Google Scholar]
- 194.Anderson DJ, Bach DL, Yunis EJ, DeWolf WC. Major histo-compatibility antigens are not expressed on human epididy-mal sperm. J Immunol. 1982;129:452–454. [PubMed] [Google Scholar]
- 195.Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, Neutra MR. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol. 2002;169:566–574. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 196.Burkhard MJ, Dean GA. Transmission and immunopathogenesis of FIV in cats as a model for HIV. Curr HIV Res. 2003;1:15–29. doi: 10.2174/1570162033352101. [DOI] [PubMed] [Google Scholar]
- 197.Bendinelli M, Pistello M, Lombardi S, Poli A, Garzelli C, Matteucci D, et al. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clin Microbiol Rev. 1995;8:87–112. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gardner MB, Luciw PA. Animal models of AIDS. FASEB J. 1989;3:2593–2606. doi: 10.1096/fasebj.3.14.2556312. [DOI] [PubMed] [Google Scholar]
- 199.Moench TR, Whaley KJ, Mandrell TD, Bishop BD, Witt CJ, Cone RA. The cat/feline immunodeficiency virus model for transmucosal transmission of AIDS: nonoxynol-9 contraceptive jelly blocks transmission by an infected cell inoculum. AIDS. 1993;7:797–802. doi: 10.1097/00002030-199306000-00006. [DOI] [PubMed] [Google Scholar]
- 200.Bishop SA, Stokes CR, Gruffydd-Jones TJ, Whiting CV, Harbour DA. Vaginal and rectal infection of cats with feline immunodeficiency virus. Vet Microbiol. 1996;51:217–227. doi: 10.1016/0378-1135(96)00038-7. [DOI] [PubMed] [Google Scholar]
- 201.D'Cruz OJ, Waurzyniak B, Uckun FM. Antiretroviral spermicide WHI-07 prevents vaginal and rectal transmission of feline immunodeficiency virus in domestic cats. Antimicrob Agents Chemother. 2004;48:1082–1088. doi: 10.1128/AAC.48.4.1082-1088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lecollinet S, Richardson J. Vaccination against the feline immunodeficiency virus: the road not taken. Comp Immunol Microbiol Infect Dis. 2008;31:167–190. doi: 10.1016/j.cimid.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 203.Yamamoto JK, Pu R, Sato E, Hohdatsu T. Feline immunodeficiency virus pathogenesis and development of a dual-subtype feline-immunodeficiency-virus vaccine. AIDS. 2007;21:547–563. doi: 10.1097/QAD.0b013e328013d88a. [DOI] [PubMed] [Google Scholar]
- 204.Ibata B, Parr EL, King NJ, Parr MB. Migration of foreign lymphocytes from the mouse vagina into the cervicovaginal mucosa and to the iliac lymph nodes. Biol Reprod. 1997;56:537–543. doi: 10.1095/biolreprod56.2.537. [DOI] [PubMed] [Google Scholar]
- 205.Zacharopoulos VR, Perotti ME, Phillips DM. A role for cell migration in the sexual transmission of HIV-1? Curr Biol. 1997;7:534–537. doi: 10.1016/s0960-9822(06)00225-9. [DOI] [PubMed] [Google Scholar]
- 206.Di Fabio S, Giannini G, Lapenta C, Spada M, Binelli A, Germinario E, et al. Vaginal transmission of HIV-1 in hu-SCID mice: a new model for the evaluation of vaginal microbicides. AIDS. 2001;15:2231–2238. doi: 10.1097/00002030-200111230-00003. [DOI] [PubMed] [Google Scholar]
- 207.Khanna KV, Whaley KJ, Zeitlin L, Moench TR, Mehrazar K, Cone RA, et al. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J Clin Invest. 2002;109:205–211. doi: 10.1172/JCI13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.D'Cruz OJ, Uckun FM. Limitations of the human-PBL-SCID mouse model for vaginal transmission of HIV-1. Am J Reprod Immunol. 2007;57:353–360. doi: 10.1111/j.1600-0897.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 209.Berges BK, Akkina SR, Folkvord JM, Connick E, Akkina R. Mucosal transmission of R5 and X4 tropic HIV-1 via vaginal and rectal routes in humanized Rag2−/− gammac −/− (RAG-hu) mice. Virology. 2008;373:342–351. doi: 10.1016/j.virol.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Denton PW, Estes JD, Sun Z, Othieno FA, Wei BL, Wege AK, et al. Antiretroviral preexposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hofer U, Baenziger S, Heikenwalder M, Schlaepfer E, Gehre N, Regenass S, et al. RAG2−/− gamma(c)−/− mice transplanted with CD34+ cells from human cord blood show low levels of intestinal engraftment and are resistant to rectal transmission of human immunodeficiency virus. J Virol. 2008;82:12145–12153. doi: 10.1128/JVI.01105-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Girard M, Mahoney J, Wei Q, van der Ryst E, Muchmore E, Barre-Sinoussi F, Fultz PN. Genital infection of female chimpanzees with human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1998;14:1357–1367. doi: 10.1089/aid.1998.14.1357. [DOI] [PubMed] [Google Scholar]
- 213.Sodora DL, Gettie A, Miller CJ, Marx PA. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S119–S123. [PubMed] [Google Scholar]
- 214.Weiler AM, Li Q, Duan L, Kaizu M, Weisgrau KL, Friedrich TC, et al. Genital ulcers facilitate rapid viral entry and dissemination following intravaginal inoculation with cell-associated simian immunodeficiency virus SIVmac239. J Virol. 2008;82:4154–4158. doi: 10.1128/JVI.01947-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kaizu M, Weiler AM, Weisgrau KL, Vielhuber KA, May G, Piaskowski SM, et al. Repeated intravaginal inoculation with cell-associated simian immunodeficiency virus results in persistent infection of nonhuman primates. J Infect Dis. 2006;194:912–916. doi: 10.1086/507308. [DOI] [PubMed] [Google Scholar]
- 216.Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.LeGrand R. Microbicides 2008. New Delhi, India: 2008. Infection of macaques after vaginal exposure to cell-associated SIV. [Google Scholar]
- 218.Cohen MS, Pilcher CD. Amplified HIV transmission and new approaches to HIV prevention. J Infect Dis. 2005;191:1391–1393. doi: 10.1086/429414. [DOI] [PubMed] [Google Scholar]
- 219.Tan X, Pearce-Pratt R, Phillips DM. Productive infection of a cervical epithelial cell line with human immunodeficiency virus: implications for sexual transmission. J Virol. 1993;67:6447–6452. doi: 10.1128/jvi.67.11.6447-6452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chancey CJ, Khanna KV, Seegers JF, Zhang GW, Hildreth J, Langan A, Markham RB. Lactobacilli-expressed single-chain variable fragment (scFv) specific for intercellular adhesion molecule 1 (ICAM-1) blocks cell-associated HIV-1 transmission across a cervical epithelial monolayer. J Immunol. 2006;176:5627–5636. doi: 10.4049/jimmunol.176.9.5627. [DOI] [PubMed] [Google Scholar]
- 221.Van Herrewege Y, Michiels J, Waeytens A, De Boeck G, Salden E, Heyndrickx L, et al. A dual chamber model of female cervical mucosa for the study of HIV transmission and for the evaluation of candidate HIV microbicides. Antiviral Res. 2007;74:111–124. doi: 10.1016/j.antiviral.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 222.Tudor D, Derrien M, Diomede L, Drillet AS, Houimel M, Moog C, et al. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4(+) cell infection: an IgA gene and functional analysis. Mucosal Immunol. 2009;2:412–426. doi: 10.1038/mi.2009.89. [DOI] [PubMed] [Google Scholar]
- 223.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, Gallay PA. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol. 2007;81:395–405. doi: 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Buffa V, Stieh D, Mamhood N, Hu Q, Fletcher P, Shattock RJ. Cyanovirin-N potently inhibits human immunodeficiency virus type 1 infection in cellular and cervical explant models. J Gen Virol. 2009;90:234–243. doi: 10.1099/vir.0.004358-0. [DOI] [PubMed] [Google Scholar]
- 226.Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med. 2000;6:475–479. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- 227.Blaskewicz C, Nadolski A, Pudney J, Ayehunie S, Anderson DJ. Role of integrin receptors and junctional proteins in macro-phage migration through a vaginal epithelial tissue model. 14th International Congress of Mucosal Immunology; Boston, Massachusetts, USA. 2009. [Google Scholar]
- 228.Shieh CC, Sadasivan BK, Russell GJ, Schon MP, Parker CM, Brenner MB. Lymphocyte adhesion to epithelia and endothelia mediated by the lymphocyte endothelial-epithelial cell adhesion molecule glycoprotein. J Immunol. 1999;163:1592–1601. [PubMed] [Google Scholar]
- 229.Pearce-Pratt R, Phillips DM. Sulfated polysaccharides inhibit lymphocyte-to-epithelial transmission of human immunodeficiency virus-1. Biol Reprod. 1996;54:173–182. doi: 10.1095/biolreprod54.1.173. [DOI] [PubMed] [Google Scholar]
- 230.Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci. 2009;122:215–225. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 231.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- 232.Parr MB, Parr EL. Interferon-gamma up-regulates intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 and recruits lymphocytes into the vagina of immune mice challenged with herpes simplex virus-2. Immunology. 2000;99:540–545. doi: 10.1046/j.1365-2567.2000.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Carreno MP, Chomont N, Kazatchkine MD, Irinopoulou T, Krief C, Mohamed AS, et al. Binding of LFA-1 (CD11a) to intercellular adhesion molecule 3 (ICAM-3; CD50) and ICAM-2 (CD102) triggers transmigration of human immunodeficiency virus type 1-infected monocytes through mucosal epithelial cells. J Virol. 2002;76:32–40. doi: 10.1128/JVI.76.1.32-40.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang JH, Kwas C, Wu L. Intercellular adhesion molecule 1 (ICAM-1), but not ICAM-2 and -3, is important for dendritic cell-mediated human immunodeficiency virus type 1 transmission. J Virol. 2009;83:4195–4204. doi: 10.1128/JVI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sanders RW, de Jong EC, Baldwin CE, Schuitemaker JH, Kapsenberg ML, Berkhout B. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J Virol. 2002;76:7812–7821. doi: 10.1128/JVI.76.15.7812-7821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dunehoo AL, Anderson M, Majumdar S, Kobayashi N, Berk-land C, Siahaan TJ. Cell adhesion molecules for targeted drug delivery. J Pharm Sci. 2006;95:1856–1872. doi: 10.1002/jps.20676. [DOI] [PubMed] [Google Scholar]
- 237.Severson EA, Jiang L, Ivanov AI, Mandell KJ, Nusrat A, Parkos CA. Cis-dimerization mediates function of junctional adhesion molecule A. Mol Biol Cell. 2008;19:1862–1872. doi: 10.1091/mbc.E07-09-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zen K, Babbin BA, Liu Y, Whelan JB, Nusrat A, Parkos CA. JAM-C is a component of desmosomes and a ligand for CD11b/CD18-mediated neutrophil transepithelial migration. Mol Biol Cell. 2004;15:3926–3937. doi: 10.1091/mbc.E04-04-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Shacklett BL, Cox CA, Sandberg JK, Stollman NH, Jacobson MA, Nixon DF. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J Virol. 2003;77:5621–5631. doi: 10.1128/JVI.77.10.5621-5631.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Schon MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- 241.Higgins JM, Mandlebrot DA, Shaw SK, Russell GJ, Murphy EA, Chen YT, et al. Direct and regulated interaction of integrin alphaEbeta7 with E-cadherin. J Cell Biol. 1998;140:197–210. doi: 10.1083/jcb.140.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 243.Quayle AJ, Kourtis AP, Cu-Uvin S, Politch JA, Yang H, Bowman FP, et al. T-lymphocyte profile and total and virus-specific immunoglobulin concentrations in the cervix of HIV-1-infected women. J Acquir Immune Defic Syndr. 2007;44:292–298. doi: 10.1097/QAI.0b013e31802c5b3a. [DOI] [PubMed] [Google Scholar]
- 244.Harmsen AG, Muggenburg BA, Snipes MB, Bice DE. The role of macrophages in particle translocation from lungs to lymph nodes. Science. 1985;230:1277–1280. doi: 10.1126/science.4071052. [DOI] [PubMed] [Google Scholar]
- 245.Wells CL, Maddaus MA, Simmons RL. Role of the macrophage in the translocation of intestinal bacteria. Arch Surg. 1987;122:48–53. doi: 10.1001/archsurg.1987.01400130054008. [DOI] [PubMed] [Google Scholar]
- 246.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 249.Fichorova RN, Anderson DJ. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod. 1999;60:508–514. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- 250.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 251.Maxion HK, Kelly KA. Chemokine expression patterns differ within anatomically distinct regions of the genital tract during Chlamydia trachomatis infection. Infect Immun. 2002;70:1538–1546. doi: 10.1128/IAI.70.3.1538-1546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Herold S, von Wulffen W, Steinmueller M, Pleschka S, Kuziel WA, Mack M, et al. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J Immunol. 2006;177:1817–1824. doi: 10.4049/jimmunol.177.3.1817. [DOI] [PubMed] [Google Scholar]
- 253.Rasmussen SJ, Eckmann L, Quayle AJ, Shen L, Zhang YX, Anderson DJ, et al. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7:238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 256.Jolly C, Sattentau QJ. Retroviral spread by induction of virological synapses. Traffic. 2004;5:643–650. doi: 10.1111/j.1600-0854.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 257.Piguet V, Sattentau Q. Dangerous liaisons at the virological synapse. J Clin Invest. 2004;114:605–610. doi: 10.1172/JCI22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, et al. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 260.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol. 2000;1:23–29. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 261.Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, et al. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- 262.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 263.Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol. 2007;9:310–315. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 265.Perotti ME, Pirovano A, Phillips DM. Carrageenan formulation prevents macrophage trafficking from vagina: implications for microbicide development. Biol Reprod. 2003;69:933–939. doi: 10.1095/biolreprod.102.014555. [DOI] [PubMed] [Google Scholar]
- 266.Di Fabio S, Van Roey J, Giannini G, van den Mooter G, Spada M, Binelli A, et al. Inhibition of vaginal transmission of HIV-1 in hu-SCID mice by the nonnucleoside reverse transcriptase inhibitor TMC120 in a gel formulation. AIDS. 2003;17:1597–1604. doi: 10.1097/00002030-200307250-00003. [DOI] [PubMed] [Google Scholar]
- 267.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Isola P, Merico A, et al. AIDS vaccination studies using feline immunodeficiency virus as a model: immunisation with inactivated whole virus suppresses viraemia levels following intravaginal challenge with infected cells but not following intravenous challenge with cell-free virus. Vaccine. 1999;18:119–130. doi: 10.1016/s0264-410x(99)00189-9. [DOI] [PubMed] [Google Scholar]
- 268.Willits RK, Saltzman WM. The effect of synthetic polymers on the migration of monocytes through human cervical mucus. Biomaterials. 2004;25:4563–4571. doi: 10.1016/j.biomaterials.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 269.Matoba N, Magerus A, Geyer BC, Zhang Y, Muralidharan M, Alfsen A, et al. A mucosally targeted subunit vaccine candidate eliciting HIV-1 transcytosis-blocking Abs. Proc Natl Acad Sci U S A. 2004;101:13584–13589. doi: 10.1073/pnas.0405297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hocini H, Belec L, Iscaki S, Garin B, Pillot J, Becquart P, Bomsel M. High-level ability of secretory IgA to block HIV type 1 transcytosis: contrasting secretory IgA and IgG responses to glycoprotein 160. AIDS Res Hum Retroviruses. 1997;13:1179–1185. doi: 10.1089/aid.1997.13.1179. [DOI] [PubMed] [Google Scholar]
- 271.Hocini H, Bomsel M. Infectious human immunodeficiency virus can rapidly penetrate a tight human epithelial barrier by transcytosis in a process impaired by mucosal immunoglo-bulins. J Infect Dis. 1999;179(Suppl 3):S448–S453. doi: 10.1086/314802. [DOI] [PubMed] [Google Scholar]
- 272.Bomsel M, Heyman M, Hocini H, Lagaye S, Belec L, Dupont C, Desgranges C. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9:277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- 273.Chomont N, Hocini H, Gody JC, Bouhlal H, Becquart P, Krief-Bouillet C, et al. Neutralizing monoclonal antibodies to human immunodeficiency virus type 1 do not inhibit viral transcytosis through mucosal epithelial cells. Virology. 2008;370:246–254. doi: 10.1016/j.virol.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 274.Cone RA, Hoen T, Wong X, Abusuwwa R, Anderson DJ, Moench TR. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect Dis. 2006;6:90. doi: 10.1186/1471-2334-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Peterson L, Nanda K, Opoku BK, Ampofo WK, Owusu-Amoako M, Boakye AY, et al. SAVVY (C31G) gel for prevention of HIV infection in women: a Phase 3, double-blind, randomized, placebo-controlled trial in Ghana. PLoS One. 2007;2:e1312. doi: 10.1371/journal.pone.0001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Feldblum PJ, Adeiga A, Bakare R, Wevill S, Lendvay A, Obadaki F, et al. SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomized controlled trial in Nigeria. PLoS One. 2008;3:e1474. doi: 10.1371/journal.pone.0001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Karim SA, Colletti A, Richardson B, et al. Safety and effectiveness of vaginal microbicides BufferGel and 0.5% PRO2000/5 gel for the prevention of HIV infection in women: results of the HPTN 035 trial. 16th Conference on Retro-viruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 278.Chang TL, Chang CH, Simpson DA, Xu Q, Martin PK, Lagenaur LA, et al. Inhibition of HIV infectivity by a natural human isolate of Lactobacillus jensenii engineered to express functional two-domain CD4. Proc Natl Acad Sci U S A. 2003;100:11672–11677. doi: 10.1073/pnas.1934747100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Pusch O, Kalyanaraman R, Tucker LD, Wells JM, Ramratnam B, Boden D. An anti-HIV microbicide engineered in commensal bacteria: secretion of HIV-1 fusion inhibitors by lactobacilli. AIDS. 2006;20:1917–1922. doi: 10.1097/01.aids.0000247112.36091.f8. [DOI] [PubMed] [Google Scholar]
- 280.Liu X, Lagenaur LA, Lee PP, Xu Q. Engineering of a human vaginal Lactobacillus strain for surface expression of two-domain CD4 molecules. Appl Environ Microbiol. 2008;74:4626–4635. doi: 10.1128/AEM.00104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kremer J, Jager S. The significance of antisperm antibodies for sperm-cervical mucus interaction. Hum Reprod. 1992;7:781–784. doi: 10.1093/oxfordjournals.humrep.a137737. [DOI] [PubMed] [Google Scholar]
- 282.Whaley KJ, Zeitlin L. Preventing transmission: plant-derived microbicides and mucosal vaccines for reproductive health. Vaccine. 2005;23:1819–1822. doi: 10.1016/j.vaccine.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 283.Isojima S. Sperm and seminal plasma antigens relevant to contraceptive vaccine development. Curr Opin Immunol. 1989;2:752–756. doi: 10.1016/0952-7915(90)90045-i. [DOI] [PubMed] [Google Scholar]
- 284.Scordi-Bello IA, Mosoian A, He C, Chen Y, Cheng Y, Jarvis GA, et al. Candidate sulfonated and sulfated topical microbicides: comparison of antihuman immunodeficiency virus activities and mechanisms of action. Antimicrob Agents Chemother. 2005;49:3607–3615. doi: 10.1128/AAC.49.9.3607-3615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Romer D, Brighty DW, Robson CL, Sattentau QJ. Candidate polyanionic microbicides inhibit human T-cell lymphotropic virus type 1 receptor interactions, cell-free infection, and cell-cell spread. Antimicrob Agents Chemother. 2009;53:678–687. doi: 10.1128/AAC.01550-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Fletcher PS, Wallace GS, Mesquita PM, Shattock RJ. Candidate polyanion microbicides inhibit HIV-1 infection and dissemination pathways in human cervical explants. Retrovirology. 2006;3:46. doi: 10.1186/1742-4690-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Patton DL, Cosgrove Sweeney YT, McCarthy TD, Hillier SL. Preclinical safety and efficacy assessments of dendrimer-based (SPL7013) microbicide gel formulations in a nonhuman primate model. Antimicrob Agents Chemother. 2006;50:1696–1700. doi: 10.1128/AAC.50.5.1696-1700.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mumper RJ, Bell MA, Worthen DR, Cone RA, Lewis GR, Paull JR, Moench TR. Formulating a sulfonated antiviral dendrimer in a vaginal microbicidal gel having dual mechanisms of action. Drug Dev Ind Pharm. 2009;35:515–524. doi: 10.1080/03639040802488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Witvrouw M, Fikkert V, Pluymers W, Matthews B, Mardel K, Schols D, et al. Polyanionic (i.e., polysulfonate) dendrimers can inhibit the replication of human immunodeficiency virus by interfering with both virus adsorption and later steps (reverse transcriptase/integrase) in the virus replicative cycle. Mol Pharmacol. 2000;58:1100–1108. [PubMed] [Google Scholar]
- 290.Jiang YH, Emau P, Cairns JS, Flanary L, Morton WR, McCarthy TD, Tsai CC. SPL7013 gel as a topical micro-bicide for prevention of vaginal transmission of SHIV89. 6P in macaques. AIDS Res Hum Retroviruses. 2005;21:207–213. doi: 10.1089/aid.2005.21.207. [DOI] [PubMed] [Google Scholar]
- 291.Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 292.Keller MJ, Zerhouni-Layachi B, Cheshenko N, John M, Hogarty K, Kasowitz A, et al. PRO 2000 gel inhibits HIV and herpes simplex virus infection following vaginal application: a double-blind placebo-controlled trial. J Infect Dis. 2006;193:27–35. doi: 10.1086/498533. [DOI] [PubMed] [Google Scholar]
- 293.Teleshova N, Chang T, Profy A, Klotman ME. Inhibitory effect of PRO 2000, a candidate microbicide, on dendritic cell-mediated human immunodeficiency virus transfer. Antimi-crob Agents Chemother. 2008;52:1751–1758. doi: 10.1128/AAC.00707-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sachdev DD, Zerhouni-Layachi B, Ortigoza M, Profy AT, Tuen M, Hioe CE, Klotman ME. The differential binding and activity of PRO 2000 against diverse HIV-1 envelopes. J Acquir Immune Defic Syndr. 2009;51:125–129. doi: 10.1097/QAI.0b013e31819f9e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lederman MM, Jump R, Pilch-Cooper HA, Root M, Sieg SF. Topical application of entry inhibitors as ‘virustats’ to prevent sexual transmission of HIV infection. Retrovirology. 2008;5:116. doi: 10.1186/1742-4690-5-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Princen K, Schols D. HIV chemokine receptor inhibitors as novel anti-HIV drugs. Cytokine Growth Factor Rev. 2005;16:659–677. doi: 10.1016/j.cytogfr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 297.Veazey RS, Ling B, Green LC, Ribka EP, Lifson JD, Piatak M, Jr, et al. Topically applied recombinant chemokine analogues fully protect macaques from vaginal simian–human immunodeficiency virus challenge. J Infect Dis. 2009;199:1525–1527. doi: 10.1086/598685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Genesca M, Mc Chesney MB, Miller CJ. Antiviral CD8+Tcells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6. J Intern Med. 2009;265:67–77. doi: 10.1111/j.1365-2796.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 300.Lehner T, Shearer GM, Hackett CJ, Schultz A, Sharma OK. Alloimmunization as a strategy for vaccine design against HIV/AIDS. AIDS Res Hum Retroviruses. 2000;16:309–313. doi: 10.1089/088922200309188. [DOI] [PubMed] [Google Scholar]
- 301.Wang Y, Abel K, Lantz K, Krieg AM, McChesney MB, Miller CJ. The Toll-like receptor 7 (TLR7) agonist, imiquimod, and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. J Virol. 2005;79:14355–14370. doi: 10.1128/JVI.79.22.14355-14370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Garcia-Lerma JG, Otten RA, Qari SH, Jackson E, Cong ME, Masciotra S, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5:e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Cranage M, Sharpe S, Herrera C, Cope A, Dennis M, Berry N, et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local preexposure application of tenofovir gel. PLoS Med. 2008;5:e157. doi: 10.1371/journal.pmed.0050157. discussion e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kunjara-Na-Ayudhya R, Hopkins N, Cost M, Billitto N, Rooney J, Dezzutti CS. Microbicide, tenofovir 1% gel, efficacy determined for pre and postcoital use. Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 305.Parikh UM, Dobard C, Sharma S, Cong ME, Jia H, Martin A, et al. Complete protection from repeated vaginal SHIV Exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol. 2009;83:10358–10365. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Mayer KH, Maslankowski LA, Gai F, El-Sadr WM, Justman J, Kwiecien A, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20:543–551. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- 307.Peterson L, Taylor D, Roddy R, Belai G, Phillips P, Nanda K, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS Clin Trials. 2007;2:e27. doi: 10.1371/journal.pctr.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Fletcher P, Harman S, Azijn H, Armanasco N, Manlow P, Perumal D, et al. Inhibition of human immunodeficiency virus type 1 infection by the candidate microbicide dapivirine, a nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother. 2009;53:487–495. doi: 10.1128/AAC.01156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Romano J. Multiple dosage forms of the NNRTI microbicide dapivirine: product development and evaluation. Retrovirology. 2006;3:S54. [Google Scholar]
- 310.Fletcher P, Kiselyeva Y, Wallace G, Romano J, Griffin G, Margolis L, Shattock R. The nonnucleoside reverse transcriptase inhibitor UC-781 inhibits human immunodeficiency virus type 1 infection of human cervical tissue and dissemination by migratory cells. J Virol. 2005;79:11179–11186. doi: 10.1128/JVI.79.17.11179-11186.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Hossain MM, Parniak MA. In vitro microbicidal activity of the nonnucleoside reverse transcriptase inhibitor (NNRTI) UC781 against NNRTI-resistant human immunodeficiency virus type 1. J Virol. 2006;80:4440–4446. doi: 10.1128/JVI.80.9.4440-4446.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Yang H, Parniak MA, Isaacs CE, Hillier SL, Rohan LC. Characterization of cyclodextrin inclusion complexes of the anti-HIV nonnucleoside reverse transcriptase inhibitor UC781. AAPS J. 2008;10:606–613. doi: 10.1208/s12248-008-9070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Patton DL, Sweeney YT, Balkus JE, Rohan LC, Moncla BJ, Parniak MA, Hillier SL. Preclinical safety assessments of UC781 antihuman immuno deficiency virus topical microbicide formulations. Antimicrob Agents Chemother. 2007;51:1608–1615. doi: 10.1128/AAC.00984-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Liu S, Lu H, Neurath AR, Jiang S. Combination of candidate microbicides cellulose acetate 1,2-benzenedicarboxylate and UC781 has synergistic and complementary effects against human immunodeficiency virus type 1 infection. Antimicrob Agents Chemother. 2005;49:1830–1836. doi: 10.1128/AAC.49.5.1830-1836.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Fernandez-Romero JA, Thorn M, Turville SG, Titchen K, Sudol K, Li J, et al. Carrageenan/MIV-150 (PC-815), a combination microbicide. Sex Transm Dis. 2007;34:9–14. doi: 10.1097/01.olq.0000223287.46097.4b. [DOI] [PubMed] [Google Scholar]
- 316.Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;59:455–471. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- 317.Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect Dis. 2008;8:685–697. doi: 10.1016/S1473-3099(08)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


