Abstract
Introduction
Erectile function recovery (EFR) rates after radical prostatectomy (RP) vary greatly based on a number of factors, such as erectile dysfunction (ED) definition, data acquisition means, time-point postsurgery, and population studied.
Aim
To conduct a meta-analysis of carefully selected reports from the available literature to define the EFR rate post-RP.
Main Outcome Measures
EFR rate after RP.
Methods
An EMBASE and MEDLINE search was conducted for the time range 1985–2007. Articles were assessed blindly by strict inclusion criteria: report of EFR data post-RP, study population ≥50 patients, ≥1 year follow-up, nerve-sparing status declared, no presurgery ED, and no other prostate cancer therapy. Meta-analysis was conducted to determine the EFR rate and relative risks (RR) for dichotomous subgroups.
Results
A total of 212 relevant studies were identified; only 22 (10%) met the inclusion criteria and were analyzed (9,965 RPs, EFR data: 4,983 subjects). Mean study population size: 226.5, standard deviation = 384.1 (range: 17–1,834). Overall EFR rate was 58%. Single center series publications (k = 19) reported a higher EFR rate compared with multicenter series publications (k = 3): 60% vs. 33%, RR = 1.82, P = 0.001. Studies reporting ≥18-month follow-up (k = 10) reported higher EFR rate vs. studies with <18-month follow-up (k = 12), 60% vs. 56%, RR = 1.07, P = 0.02. Open RP (k = 16) and laparoscopic RP (k = 4) had similar EFR (57% vs. 58%), while robot-assisted RP resulted in a higher EFR rate (k = 2), 73% compared with these other approaches, P = 0.001. Patients <60 years old had a higher EFR rate vs. patients ≥60 years, 77% vs. 61%, RR = 1.26, P = 0.001.
Conclusions
These data indicate that most of the published literature does not meet strict criteria for reporting post-RP EFR. Single and multiple surgeon series have comparable EFR rates, but single center studies have a higher EFR. Younger men have higher EFR and no significant difference in EFR between ORP and LRP is evident.
Keywords: Erectile Function, Radical Prostatectomy, Meta-Analysis, Factors Affecting Erection Recovery Rate
Introduction
Prostate cancer is the second most common cancer and the third most common cause of cancer-related death among men. According to the American Cancer Society data, it was estimated that in 2008, 186,320 new cases would be diagnosed (25% of all new cancer cases) and 28,660 deaths (10% of cancer-related deaths) would be attributed to prostate cancer [1]. Radical prostatectomy (RP) is a curative treatment for early prostate cancer, with a proven long-term survival benefit [2]. Optimal outcomes of RP are not limited to cancer control, but also include urinary continence and preservation of erectile function (EF) [3]. Preservation of EF has become a goal in prostate cancer surgery with advances in our understanding of the prostate and cavernous nerve anatomy and with the introduction of nerve-sparing RP by Walsh and Donker in 1982 [4]. Currently, with prevalent early prostate-specific antigen (PSA) testing, the significance of EF preservation has grown: prostate cancer is diagnosed at an earlier age, at a lower disease stage with excellent long-term cure rates, thus men have many years of potential sexual activity ahead.
Erectile dysfunction (ED) has a negative effect on the quality of life of men and their sexual partners, and not uncommonly, the burden of ED persists long after cancer cure concerns have subsided [5]. The reported incidence of long-term ED after RP ranges from approximately 14–90%, a too wide a range to permit proper patient counseling and treatment selection decision-making [6]. In recent years, new treatment options have emerged, such as laparoscopic and robotic-assisted RP techniques, which have established their role in prostate cancer surgery, with a comparable or even lower reported ED incidences of only 7–33% [7–9]. Interpretation and comparison of EF outcomes data are hindered by publication of studies of diverse methodological quality, use of non-unified EF assessment methods, inconsistent definitions of ED, different surgical techniques, selective favorable subgroup analysis, reporting on heterogeneous and ill-defined patient populations, and other methodological differences [6].
With advances in clinical research, methods and assessment tools for evaluation of ED have evolved; however, randomized-controlled studies are not feasible, as curative treatment cannot be withheld, and active surveillance is an option only for highly selected patients with minimal disease. Thus, an ideal study to define the incidence of ED after RP will probably never be designed.
The present study employs meta-analytic methodology to analyze highly selected quality publications and addresses the question of the actual incidence of ED after RP for prostate cancer.
Methods
Publication Selection
To retrieve publications addressing ED after RP, we conducted a literature search of the PubMED/MEDLINE and EMBASE databases, looking for publications in the English language, published between the years 1985 and present (accessed April 2008). Publications prior to 1985 were not considered, so as to include only contemporary publications describing surgical prostate cancer treatment after the introduction of PSA testing for early diagnosis of prostate cancer and after the introduction of cavernous nerve sparing surgery. Search keywords used were: erectile dysfunction OR sexual function OR impotence AND radical prostatectomy. Initial screening process excluded publications reporting on a study group of less than 50 subjects and/or presenting EF outcomes after less than a year of postoperative follow-up. To further select high-quality publications, we established selection criteria (inclusion and exclusion) to assess studies’ design and methodology (Table 1). Each article had authors removed and was assigned a code, so that application of the inclusion and exclusion criteria and publication selection was performed in a blinded fashion by two independent investigators. In case of repeated analysis of the same database, we selected a single report with the best methodological quality, after carefully assessing publication year, population study size, accuracy of nerve-sparing status report, quality of pre- and postsurgery EF assessment, and quality of statistical methods.
Table 1.
Publication selection criteria
Inclusion criteria
|
EF = erectile function; RP = radical prostatectomy; ORP = open radical prostatectomy; LRP = laparoscopic radical prostatectomy; RARP = robot-assisted radical prostatectomy; EFR = erectile function recovery; PSA prostate-specific antigen; PDE5i = phosphodiesterase type 5 inhibitors; ED = erectile dysfunction.
Erectile function recovery (EFR)-related and study design-related data were retrieved from the selected publications and were analyzed to identify patterns in assessment and reporting of EF outcome after RP. Specifically, we recorded EFR rate, whether the study was a single center or multicenter report; the instruments used to assess EF, whether EF was surgeon- or patient-reported; time interval from surgery to EF assessment; and definition of successful outcome after RP.
Effect Size Determination
The proportion of patients recovering EF, EFR rate, was the main effect size outcome. Effect sizes and standard errors were calculated directly from the EFR rate and sample size reported in each study [10]. This technique produces reliable estimates of effect size and standard error when most proportions fall between 0.2 and 0.8, as was the case for all the included studies. Each obtained effect size was weighted by its inverse variance weight prior to combination.
Variance Modeling
We employed a fixed effects variance model because this model assumes the existence of only study-level variance and thus increases statistical power for detecting moderators. Variability of the fixed effects variance component was tested with the Q test for homogeneity, as defined by Lispey and Wilson [10]. A significant Q test (interpreted as a chi-square using k-1 degrees of freedom where k equals the number of studies) indicates that additional variance beyond that expected for the given sample size exists in the scores and implies the existence of moderators.
Moderator Analysis
Moderators were examined using Q test, a statistical test appropriate for meta-analysis that employs weighted data and compares within and between groups heterogeneity, similar to that of the analysis of variance. This produces a between and within groups Q statistic that is distributed and tested as a chi-square. The following hypotheses were examined: (i) single center series would result in higher EFR than multicenter series; (ii) EFR rates reported ≥18 months from surgery would be higher than those <18 months from surgery; (iii) robot-assisted RP (RARP), laparoscopic RP (LRP), and open RP (ORP) would result in the same EFR; (iv) bilateral nerve-sparing surgery would result in a higher EFR than unilateral; and (v) older patients (≥60) would have lower reported EFR than those <60 years.
Sources of Bias
Mean effects were assessed for degree of publication bias using two techniques: Examination of the funnel plot and “trim and fill”. Trim and fill is a technique developed by Duval and Tweedie and assesses the symmetry of the funnel plot [11]. It assumes that small-N studies with negative effects are not likely to get published and would fall on the lower left of the funnel plot. When publication bias exists, a disproportionate number of studies will fall to the lower right of the plot. This technique determines the number of asymmetrical studies, imputes their counterparts to the left, and calculates a new mean effect size. All analyses were performed using Comprehensive Meta-Analysis Version 2 (2005) software package (Biostat, Engle-wood, NJ).
Results
Initial search of the PubMED/MEDLINE and EMBASE databases retrieved 1,326 publications, 796 from the PubMED/MEDLINE database and 1,156 from the EMBASE database. Initial exclusion of publications reporting on a study group of less than 50 subjects and/or presenting EF data collected after less than 12-month follow-up after RP yielded 212 publications, eligible for further full review and 22 studies (10%) met full inclusion criteria (Figures 1 and 2) [12–33]. Of the 9,965 total subjects, appropriate ED data were available for 4,983. Mean patient age was 61 years (range = 55–67). The average study sample size was 226.5, standard deviation = 384.1 (range = 17–1,834). Sixteen studies reported on ORP, four LRP, and two RARP. The definition of a favorable EF outcome varied considerably, as we found 22 different definitions (Table 2).
Figure 1.
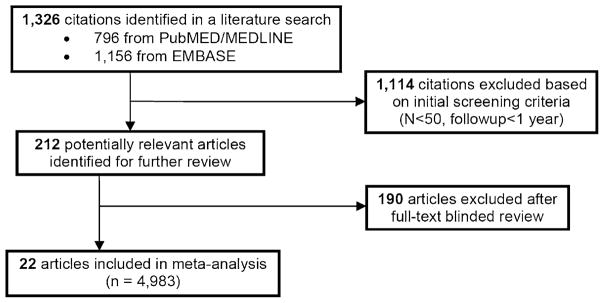
Publication selection algorithm.
Figure 2.
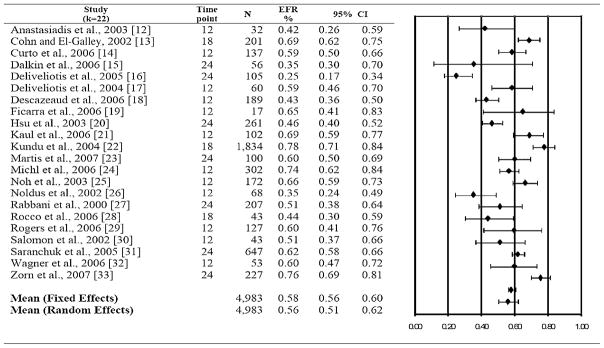
Erectile function recovery (EFR) rates (95% confidence interval [CI]), study time-point, and sample size by study. On the right: a forest plot, a graphic representation of the mean erectile function recovery and 95% CI.
Table 2.
Variation in definition of favorable erectile function outcome
|
SHIM = Sexual Health Inventory for Men; IIEF = International Index of Erectile Function; EPIC = Expanded Prostate Cancer Index Composite; UCLA-PCI = University of California, Los Angeles, prostate cancer index.
The overall fixed effects EFR rate was 58% (95% confidence interval = 56–60%), with significant heterogeneity among effect sizes where Q(21) = 164.5, P = 0.001 (Figure 2). Trim and fill imputed no values and examination of the funnel plot suggested appropriate variation in reported EFR rates among the present sample of studies (Figure 3).
Figure 3.
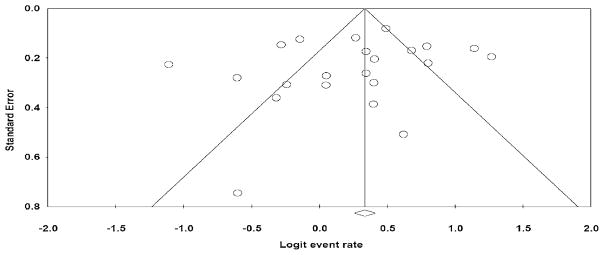
Funnel plot of standard error by logit event rate: a funnel plot is a scatter plot of treatment effect by a measure of sample size. In an unbiased study, there should be greater variability among effect sizes based on small samples than those based on large samples, and therefore, the plot should take the shape of a funnel. An asymmetric funnel with more positive effects than negative, especially for small sample sizes, usually indicates publication bias.
In terms of moderators (Table 3), single center series publications EFR rates (60%, k = 19) were higher compared with multicenter series publications EFR rates (33%, k = 3), relative risks (RR) = 1.82, P = 0.001. Studies reporting ≥18-month follow-up post-RP reported somewhat higher EFR (60%, k = 10) rates than studies with <18-month follow-up (56%, k = 12), RR = 1.07, P = 0.02. Differences were found in EFR among ORP (57%, k = 16), LRP (58%, k = 4), and RARP (73%, k = 2), P = 0.001. Bilateral nerve-sparing surgery (60%, k = 21) was associated with higher EFR than unilateral (47%, k = 12), RR = 1.28, P = 0.001. Eight studies reported specific outcomes for identified age ranges, and patients <60 years of age reported a higher rate of EFR than those ≥60 years of age (77% vs. 61%, RR = 1.26, P = 0.001).
Table 3.
Erectile function recovery rate, 95% CIs, and Q test results by predictor
| Comparison | No. of studies (k) | Erectile function recovery (%; 95% CI) | Q (df)* | P value |
|---|---|---|---|---|
| Center | ||||
| Single | 19 | 60 (58–62) | 43.45 (1) | 0.001 |
| Multiple | 3 | 33 (26–40) | ||
| Follow-up | ||||
| <18 months | 12 | 56 (53–59) | 5.29 (1) | 0.02 |
| ≥18 months | 10 | 60 (58–62) | ||
| Surgical approach | ||||
| Open | 16 | 57 (54–59) | 30.54 (2) | 0.001 |
| Laparoscopic | 4 | 58 (52–64) | ||
| Robotic | 2 | 73 (68–78) | ||
| Nerve sparing | ||||
| Bilateral | 21 | 60 (58–62) | 16.96 (1) | 0.001 |
| Unilateral | 12 | 47 (42–53) | ||
| Age group | ||||
| <60 years old | 8 | 77 (74–79) | 68.11 (1) | 0.001 |
| ≥60 years old | 8 | 61 (58–64) | ||
The Q test is a statistical test appropriate for meta-analysis that employs weighted data and compares within and between groups heterogeneity, similar to that of the analysis of variance.
CI = confidence interval; df = degrees of freedom.
Discussion
ED is a concern for patients undergoing RP for localized prostate cancer, an important consideration in treatment selection, and a significant determinant of treatment satisfaction [34]. Despite the abundance of literature reporting RP outcomes, EFR rates vary greatly, ranging approximately 20–90%, too great a variation to provide patients with proper preoperative counseling [6]. With the introduction of LRP and RARP, treatment options have expanded, making treatment selection process even more complex. The variability in reported EF outcomes originates in the discrepancy in subjects’ age, presurgery EF level, comorbidity profiles, surgical techniques, definition of a favorable EF outcome, data collection methods, duration of postsurgery follow-up, utilization of additional prostate cancer treatments, and use of erectogenic therapies [6]. A recent publication by Mulhall reviewed the medical literature in 2000–2008 and identified patterns in defining and reporting EF outcomes after RP. Mulhall found that the incidence of reported ED after RP is extremely discrepant, ranging from 12% to 96%, with a higher incidence in multi-center, multi-surgeon series compared with single center, single surgeon studies. There was great variation in study population, data acquisition, and EF definitions. Mulhall summarized the minimal requirements for adequate reporting to include comorbidity profile, patient selection process, data acquisition methods, questionnaires used, baseline EF data, long-term EF data (>24 months), definition of adequate EF, proportions of men returning to normal, proportion of men returning to preoperative EF level, erectogenic medications use, and extent to which rehabilitation strategy was used [35]. However, this comprehensive review of the current literature did not employ a meta-analysis methodology.
In the present study, we conducted a meta-analysis to analyze the present literature and applied rigorous inclusion criteria to select for the highest quality publications available in the literature. Of the 212 publications reporting on EF outcomes after RP available after initial selection (≥1-year follow-up and ≥50 subjects), only 22 publications (in the time period selected, 1985 and later) eventually fulfilled the inclusion criteria and passed the blinded review process by two of the authors. Worthy of mention is that all selected publications were published in 2000 and thereafter. Indeed, recent advances in clinical research including patient-reported outcomes, development of validated questionnaires, risk stratification, and other methodologies have improved the quality of EF outcomes research. Despite these methodological improvements, there is still considerable heterogeneity among studies and therefore there is a need for a meta-analysis. To ascertain a high quality meta-analysis methodology, we used the proposal for reporting of meta-analyses studies published in 2000 by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group, as the basis for the present study design and results reporting [36].
A key point in meta-analysis studies is a potential selection bias in the publication selection process, possibly leading to skewed results. To assess our selection process, we employed a funnel plot analysis of the selected publications, which revealed appropriate variation, confirming that the selection was based on quality (narrow standard error) rather than biased selection based on the reported EFR. Interestingly, while the 22 selected publications reported on 9,965 subjects, information on EFR was available for only 4,983. This finding highlights the difficulty involved in clinical research of EFR but also raises the possibility of a potential bias of reporting on selected groups of patients, thus potentially enriching the results. The overall EFR rate was 58%, however, and this incidence of favorable EF outcome should be viewed in the light of the definitions used in the individual studies and the effect of erectogenic therapy use should also be considered. In 13 of the publications included in the present analysis, it was stated clearly that subjects used erectogenic therapy but none of these publications presented a detailed report on the extent of erectogenic therapy use and its impact on their results. As we hypothesized, single center studies, younger age at surgery, bilateral nerve sparing, and having ≥18-month follow-up were associated with statistically significant higher EFR rates. Comparing EFR after surgery using different approaches, ORP, LRP and RARP, as expected we found no significant difference between open and laparoscopic techniques. However, the robot-assisted studies reported remarkably higher EFR rate. This difference in EFR rate after RARP should be interpreted with caution for the following reasons: only two robotic surgery studies were included in this meta-analysis, neither of which included an open or laparoscopic surgery comparison groups; and a total of only 343 patients was analyzed. At this point, we cannot explain this difference in EFR.
In recent years we, have witnessed the emergence of LRP, followed by the RARP. These innovative surgical approaches attracted much attention from patients exploring treatment options for prostate cancer. The superiority of a certain surgical approach is a pivotal question, faced not only by prostate cancer patients, but also by prostate cancer surgeons. Comparative radical prostatectomy outcomes review articles of unselected publications failed to find any significant differences between ORP, LRP, and RARP in EFR. A recent review article by Ficarra et al. looked at studies comparing outcomes of different surgical approaches and found only two articles that used a validated questionnaire to assess EF outcomes. Of six articles with EFR data, three studies compared open and laparoscopic surgery, none of which showed any advantage of specific approach: one study compared open and laparoscopic surgery reporting on shorter median time for EFR with laparoscopic surgery, one study compared open and robotic-assisted surgery showing no significant difference, and one study compared laparoscopic and robotic–assisted surgery, showing no significant difference [37]. Another review article by Berryhill et al. compared outcomes of robotic RP with laparoscopic and open surgery and reported EFR rates of 14–61% for unilateral nerve sparing and 24–97% for bilateral nerve-sparing robotic surgery, 35–64% for unilateral nerve sparing and 43–79% for bilateral nerve-sparing laparoscopic surgery at 12 months, and 17–53% for unilateral nerve sparing and 37–86% for bilateral nerve-sparing open surgery at 12–24 months [38]. These review articles, however, were not focused entirely on EF outcomes and included other outcomes; the publication selection process was not detailed and most importantly, meta-analytic statistical methods were not employed to integrate the weighted contribution of each individual study or to verify non-biased publication selection. In essence, the Ficarra et al. and the Berryhill et al. articles were both typical review articles. In both articles, the authors concluded that the available literature comparing EF outcomes of the different surgical approaches is scarce and insufficient to demonstrate any clear advantage of a certain approach.
A meta-analysis study design is a valuable, yet controversial, tool to integrate medical publications, in this era of medical information wealth. According to evidence-based medicine principles, randomized controlled studies provide the highest level of evidence. A meta-analysis of randomized controlled studies is a widely used methodology to identify, appraise, and combine the results of individual studies. However, not uncommonly randomized controlled studies are not feasible and only data from observational studies are available, as is the case for EFR after RP, and a meta-analysis must rely exclusively on observational studies. Despite limitations of a meta-analysis approach to synthesize results of observational studies, it has clear advantages over an individual observational study and therefore a meta-analysis methodology for observational studies is increasingly used in the current medical literature to assess intervention effect [36]. Walker et al. summarized the critical issues to address in a meta-analysis study to include: (i) identification and selection of studies; (ii) heterogeneity of results; (iii) availability of information; and (iv) analysis of the data [39]. An inherent limitation of the meta-analysis methodology and any meta-analysis study is the dependency on the quality of the individual available studies. In addition, an important limitation of the present study is the relatively small number of publications reporting on laparoscopic and robotic surgery, which is a result of the rigorous selection process to allow the inclusion of publications of only the highest methodological quality, to reduce heterogeneity, and to improve availability of information. Despite our efforts, there is a considerable heterogeneity among the selected studies in study design, erectile function definition, duration of follow-up, population selection, and other factors among the individual selected studies. This heterogeneity may have had an impact on our results; however, this is inherent to the present medical literature and stems from the lack of standardization in post-RP EFR outcomes reporting. A carefully designed meta-analysis study is a powerful tool to critically select publication and facilitate integration of the available knowledge, aiding clinicians and patients in decision-making. The present study has a number of strengths, mainly its thorough search process, the employment of strict selection criteria to identify the highest methodological quality studies available in the contemporary medical literature, and the use of an advanced meta-analytic methodology, based on the MOOSE group proposal [36].
Despite the high prevalence of RP utilization for localized prostate cancer, improved studies are needed. The ideal study to describe EFR rate after RP should be designed prospectively, with an age-, comorbidity profile-, and baseline EF-matched control group, using an accurate measurement tool to assess EF pre- and post-RP, and adjusted for variability in other factors, such as variation in surgical technique and use of erectogenic therapies after surgery. With the well-established place of ORP in the management of localized prostate cancer and the attractiveness of the newer laparoscopic and robot-assisted approach to patients, such a well-designed comparative study would be necessary to draw conclusions regarding the impact of a particular surgical approach on EFR.
Conclusions
Numerous publications have analyzed RP outcomes and specifically, EFR outcomes. This wealth of literature does not essentially facilitate treatment decision-making, as despite the abundance of literature on EFR after RP, the present analysis demonstrates that most of the published literature does not meet strict criteria for EFR reporting. We found that single center studies reported a higher EFR rate. Patient age at surgery <60 years, nerve-sparing status, and study follow-up ≥18 months were associated with higher EFR rates. The superiority of a certain surgical approach, open, laparoscopic, or robotic-assisted surgery is not established and yet to be defined.
Acknowledgments
This study was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers.
Footnotes
Conflict of Interest: None declared.
Statement of Authorship
-
Conception and DesignJohn P. Mulhall; Raanan Tal; Christian J. Nelson
-
Acquisition of DataRaanan Tal; Hannah H. Alphs
-
Analysis and Interpretation of DataRaanan Tal; Christian J. Nelson; John P. Mulhall; Paul Krebs
-
Drafting the ArticleRaanan Tal; Christian J. Nelson; John P. Mulhall; Paul Krebs
-
Revising It for Intellectual ContentJohn P. Mulhall; Raanan Tal; Christian J. Nelson
-
Final Approval of the Completed ArticleJohn P. Mulhall; Raanan Tal; Christian J. Nelson
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bill-Axelson A, Holmberg L, Ruutu M, Haggman M, Andersson SO, Bratell S, Spangberg A, Busch C, Nordling S, Garmo H, Palmgren J, Adami HO, Norlen BJ, Johansson JE. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–84. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 3.Eastham JA, Scardino PT, Kattan MW. Predicting an optimal outcome after radical prostatectomy: the trifecta nomogram. J Urol. 2008;179:2207–10. doi: 10.1016/j.juro.2008.01.106. discussion 10–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–7. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Cruz JJ, Cabrera-Leon A, Martin-Morales A, Fernandez A, Burgos R, Rejas J. Male erectile dysfunction and health-related quality of life. Eur Urol. 2003;44:245–53. doi: 10.1016/s0302-2838(03)00215-x. [DOI] [PubMed] [Google Scholar]
- 6.Burnett AL, Aus G, Canby-Hagino ED, Cookson MS, D’Amico AV, Dmochowski RR, Eton DT, Forman JD, Goldenberg SL, Hernandez J, Higano CS, Kraus S, Liebert M, Moul JW, Tangen C, Thrasher JB, Thompson I. Erectile function outcome reporting after clinically localized prostate cancer treatment. J Urol. 2007;178:597–601. doi: 10.1016/j.juro.2007.03.140. [DOI] [PubMed] [Google Scholar]
- 7.Rassweiler J, Wagner AA, Moazin M, Gozen AS, Teber D, Frede T, Su LM. Anatomic nerve-sparing laparoscopic radical prostatectomy: comparison of retrograde and antegrade techniques. Urology. 2006;68:587–91. doi: 10.1016/j.urology.2006.03.082. discussion 91–2. [DOI] [PubMed] [Google Scholar]
- 8.Menon M, Shrivastava A, Kaul S, Badani KK, Fumo M, Bhandari M, Peabody JO. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol. 2007;51:648–57. doi: 10.1016/j.eururo.2006.10.055. discussion 57–8. [DOI] [PubMed] [Google Scholar]
- 9.Tewari A, Rao S, Martinez-Salamanca JI, Leung R, Ramanathan R, Mandhani A, Vaughan ED, Menon M, Horninger W, Tu J, Bartsch G. Cancer control and the preservation of neurovascular tissue: how to meet competing goals during robotic radical prostatectomy. BJU Int. 2008;101:1013–8. doi: 10.1111/j.1464-410X.2008.07456.x. [DOI] [PubMed] [Google Scholar]
- 10.Lipsey MW, Wilson DB. Practical Meta-analysis. Thousand Oaks CA: SAGE Publications; 2001. [Google Scholar]
- 11.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 12.Anastasiadis AG, Salomon L, Katz R, Hoznek A, Chopin D, Abbou CC. Radical retropubic versus laparoscopic prostatectomy: a prospective comparison of functional outcome. Urology. 2003;62:292–7. doi: 10.1016/s0090-4295(03)00352-2. [DOI] [PubMed] [Google Scholar]
- 13.Cohn JH, El-Galley R. Radical prostatectomy in a community practice. J Urol. 2002;167:224–8. [PubMed] [Google Scholar]
- 14.Curto F, Benijts J, Pansadoro A, Barmoshe S, Hoepffner JL, Mugnier C, Piechaud T, Gaston R. Nerve sparing laparoscopic radical prostatectomy: our technique. Eur Urol. 2006;49:344–52. doi: 10.1016/j.eururo.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Dalkin BL, Christopher BA, Shawler D. Health related quality of life outcomes after radical prostatectomy: attention to study design and the patient-based importance of single-surgeon studies. Urol Oncol. 2006;24:28–32. doi: 10.1016/j.urolonc.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Deliveliotis C, Delis A, Papatsoris A, Antoniou N, Varkarakis IM. Local steroid application during nerve-sparing radical retropubic prostatectomy. BJU Int. 2005;96:533–5. doi: 10.1111/j.1464-410X.2005.05679.x. [DOI] [PubMed] [Google Scholar]
- 17.Deliveliotis C, Liakouras C, Delis A, Skolarikos A, Varkarakis J, Protogerou V. Prostate operations: long-term effects on sexual and urinary function and quality of life. Comparison with an age-matched control population. Urol Res. 2004;32:283–9. doi: 10.1007/s00240-004-0411-0. [DOI] [PubMed] [Google Scholar]
- 18.Descazeaud A, Debre B, Flam TA. Age difference between patient and partner is a predictive factor of potency rate following radical prostatectomy. J Urol. 2006;176:2594–8. doi: 10.1016/j.juro.2006.07.145. discussion 8. [DOI] [PubMed] [Google Scholar]
- 19.Ficarra V, Novara G, Galfano A, Stringari C, Baldassarre R, Cavalleri S, Artibani W. Twelve-month self-reported quality of life after retropubic radical prostatectomy: a prospective study with Rand 36-Item Health Survey (Short Form-36) BJU Int. 2006;97:274–8. doi: 10.1111/j.1464-410X.2005.05893.x. [DOI] [PubMed] [Google Scholar]
- 20.Hsu EI, Hong EK, Lepor H. Influence of body weight and prostate volume on intraoperative, perioperative, and postoperative outcomes after radical retropubic prostatectomy. Urology. 2003;61:601–6. doi: 10.1016/s0090-4295(02)02422-6. [DOI] [PubMed] [Google Scholar]
- 21.Kaul S, Savera A, Badani K, Fumo M, Bhandari A, Menon M. Functional outcomes and oncological efficacy of Vattikuti Institute prostatectomy with Veil of Aphrodite nerve-sparing: an analysis of 154 consecutive patients. BJU Int. 2006;97:467–72. doi: 10.1111/j.1464-410X.2006.05990.x. [DOI] [PubMed] [Google Scholar]
- 22.Kundu SD, Roehl KA, Eggener SE, Antenor JA, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retro-pubic prostatectomies. J Urol. 2004;172:2227–31. doi: 10.1097/01.ju.0000145222.94455.73. [DOI] [PubMed] [Google Scholar]
- 23.Martis G, Diana M, Ombres M, Cardi A, Mastrangeli R, Mastrangeli B. Retropubic versus perineal radical prostatectomy in early prostate cancer: eight-year experience. J Surg Oncol. 2007;95:513–8. doi: 10.1002/jso.20714. [DOI] [PubMed] [Google Scholar]
- 24.Michl UH, Friedrich MG, Graefen M, Haese A, Heinzer H, Huland H. Prediction of postoperative sexual function after nerve sparing radical retro-pubic prostatectomy. J Urol. 2006;176:227–31. doi: 10.1016/S0022-5347(06)00632-X. [DOI] [PubMed] [Google Scholar]
- 25.Noh C, Kshirsagar A, Mohler JL. Outcomes after radical retropubic prostatectomy. Urology. 2003;61:412–6. doi: 10.1016/s0090-4295(02)02147-7. [DOI] [PubMed] [Google Scholar]
- 26.Noldus J, Michl U, Graefen M, Haese A, Hammerer P, Huland H. Patient-reported sexual function after nerve-sparing radical retropubic prostatectomy. Eur Urol. 2002;42:118–24. doi: 10.1016/s0302-2838(02)00219-1. [DOI] [PubMed] [Google Scholar]
- 27.Rabbani F, Stapleton AM, Kattan MW, Wheeler TM, Scardino PT. Factors predicting recovery of erections after radical prostatectomy. J Urol. 2000;164:1929–34. [PubMed] [Google Scholar]
- 28.Rocco F, Carmignani L, Acquati P, Gadda F, Dell’Orto P, Rocco B, Bozzini G, Gazzano G, Morabito A. Restoration of posterior aspect of rhab-dosphincter shortens continence time after radical retropubic prostatectomy. J Urol. 2006;175:2201–6. doi: 10.1016/S0022-5347(06)00262-X. [DOI] [PubMed] [Google Scholar]
- 29.Rogers CG, Su LM, Link RE, Sullivan W, Wagner A, Pavlovich CP. Age stratified functional outcomes after laparoscopic radical prostatectomy. J Urol. 2006;176:2448–52. doi: 10.1016/j.juro.2006.07.153. [DOI] [PubMed] [Google Scholar]
- 30.Salomon L, Anastasiadis AG, Katz R, De La Taille A, Saint F, Vordos D, Cicco A, Hoznek A, Chopin D, Abbou CC. Urinary continence and erectile function: a prospective evaluation of functional results after radical laparoscopic prostatectomy. Eur Urol. 2002;42:338–43. doi: 10.1016/s0302-2838(02)00360-3. [DOI] [PubMed] [Google Scholar]
- 31.Saranchuk JW, Kattan MW, Elkin E, Touijer AK, Scardino PT, Eastham JA. Achieving optimal outcomes after radical prostatectomy. J Clin Oncol. 2005;23:4146–51. doi: 10.1200/JCO.2005.12.922. [DOI] [PubMed] [Google Scholar]
- 32.Wagner A, Link R, Pavlovich C, Sullivan W, Su L. Use of a validated quality of life questionnaire to assess sexual function following laparoscopic radical prostatectomy. Int J Impot Res. 2006;18:69–76. doi: 10.1038/sj.ijir.3901376. [DOI] [PubMed] [Google Scholar]
- 33.Zorn KC, Gofrit ON, Orvieto MA, Mikhail AA, Zagaja GP, Shalhav AL. Robotic-assisted laparoscopic prostatectomy: functional and pathologic outcomes with interfascial nerve preservation. Eur Urol. 2007;51:755–62. doi: 10.1016/j.eururo.2006.10.019. discussion 63. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman RM, Hunt WC, Gilliland FD, Stephenson RA, Potosky AL. Patient satisfaction with treatment decisions for clinically localized prostate carcinoma. Results from the Prostate Cancer Outcomes Study. Cancer. 2003;97:1653–62. doi: 10.1002/cncr.11233. [DOI] [PubMed] [Google Scholar]
- 35.Mulhall JP. Defining and reporting erectile function outcomes after radical prostatectomy: challenges and misconceptions. J Urol. 2009;181:462–71. doi: 10.1016/j.juro.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 36.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 37.Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, Guazzoni G, Guillonneau B, Menon M, Montorsi F, Patel V, Rassweiler J, Van Poppel H. Retropubic, Laparoscopic, and Robot-Assisted Radical Prostatectomy: A Systematic Review and Cumulative Analysis of Comparative Studies. Eur Urol. 2009 Jan 25; doi: 10.1016/j.eururo.2009.01.036. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Berryhill R, Jr, Jhaveri J, Yadav R, Leung R, Rao S, El-Hakim A, Tewari A. Robotic prostatectomy: a review of outcomes compared with laparoscopic and open approaches. Urology. 2008;72:15–23. doi: 10.1016/j.urology.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 39.Walker E, Hernandez AV, Kattan MW. Meta-analysis: Its strengths and limitations. Cleve Clin J Med. 2008;75:431–9. doi: 10.3949/ccjm.75.6.431. [DOI] [PubMed] [Google Scholar]


