Abstract
alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors that lack the glutamate receptor GluR2 subunit are Ca(2+)-permeable and exhibit inwardly rectifying current responses to kainate and AMPA. A proportion of cultured rat hippocampal neurons show similar Ca(2+)-permeable inwardly rectifying AMPA receptor currents. Inward rectification in these neurons was lost with intracellular dialysis and was not present in excised outside-out patches but was maintained in perforated-patch whole-cell recordings, suggesting that a diffusible cytoplasmic factor may be responsible for rectification. Inclusion of the naturally occurring polyamines spermine and spermidine in the recording pipette prevented loss of rectification in both whole-cell and excised-patch recordings; Mg2+ and putrescine were without effect. Inward rectification of Ca(2+)-permeable AMPA receptors may reflect voltage-dependent channel block by intracellular polyamines.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai A., Baudry M., Staubli U., Lynch G., Gall C. Induction of ornithine decarboxylase by subseizure stimulation in the hippocampus in vivo. Brain Res Mol Brain Res. 1990 Feb;7(2):167–169. doi: 10.1016/0169-328x(90)90094-t. [DOI] [PubMed] [Google Scholar]
- Araneda R. C., Zukin R. S., Bennett M. V. Effects of polyamines on NMDA-induced currents in rat hippocampal neurons: a whole-cell and single-channel study. Neurosci Lett. 1993 Apr 2;152(1-2):107–112. doi: 10.1016/0304-3940(93)90495-7. [DOI] [PubMed] [Google Scholar]
- Baudry M., Najm I. Kainate-induced seizure activity stimulates the polyamine interconversion pathway in rat brain. Neurosci Lett. 1994 Apr 25;171(1-2):151–154. doi: 10.1016/0304-3940(94)90627-0. [DOI] [PubMed] [Google Scholar]
- Bennett J. A., Dingledine R. Topology profile for a glutamate receptor: three transmembrane domains and a channel-lining reentrant membrane loop. Neuron. 1995 Feb;14(2):373–384. doi: 10.1016/0896-6273(95)90293-7. [DOI] [PubMed] [Google Scholar]
- Benveniste M., Mayer M. L. Multiple effects of spermine on N-methyl-D-aspartic acid receptor responses of rat cultured hippocampal neurones. J Physiol. 1993 May;464:131–163. doi: 10.1113/jphysiol.1993.sp019627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochet P., Audinat E., Lambolez B., Crépel F., Rossier J., Iino M., Tsuzuki K., Ozawa S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994 Feb;12(2):383–388. doi: 10.1016/0896-6273(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992 Jan;8(1):189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Dingledine R., Hume R. I., Heinemann S. F. Structural determinants of barium permeation and rectification in non-NMDA glutamate receptor channels. J Neurosci. 1992 Oct;12(10):4080–4087. doi: 10.1523/JNEUROSCI.12-10-04080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donevan S. D., Jones S. M., Rogawski M. A. Arcaine blocks N-methyl-D-aspartate receptor responses by an open channel mechanism: whole-cell and single-channel recording studies in cultured hippocampal neurons. Mol Pharmacol. 1992 Apr;41(4):727–735. [PubMed] [Google Scholar]
- Donevan S. D., Rogawski M. A. GYKI 52466, a 2,3-benzodiazepine, is a highly selective, noncompetitive antagonist of AMPA/kainate receptor responses. Neuron. 1993 Jan;10(1):51–59. doi: 10.1016/0896-6273(93)90241-i. [DOI] [PubMed] [Google Scholar]
- Fakler B., Brändle U., Glowatzki E., Weidemann S., Zenner H. P., Ruppersberg J. P. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995 Jan 13;80(1):149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- Ficker E., Taglialatela M., Wible B. A., Henley C. M., Brown A. M. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994 Nov 11;266(5187):1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Hattori Y., Moriwaki A., Lu Y. F., Hori Y. Increases in brain polyamine concentrations in chemical kindling and single convulsion induced by pentylenetetrazol in rats. Neurosci Lett. 1993 Jan 4;149(1):63–66. doi: 10.1016/0304-3940(93)90348-o. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Hattori Y., Moriwaki A., Saeki K., Hori Y. Changes in polyamine concentrations in amygdaloid-kindled rats. J Neurochem. 1989 Sep;53(3):986–988. doi: 10.1111/j.1471-4159.1989.tb11805.x. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Single F. N., Köhler M., Sommer B., Sprengel R., Seeburg P. H. RNA editing of AMPA receptor subunit GluR-B: a base-paired intron-exon structure determines position and efficiency. Cell. 1993 Dec 31;75(7):1361–1370. doi: 10.1016/0092-8674(93)90622-w. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Hartley M., Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991 May 10;252(5007):851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Maron C., Heinemann S. N-glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1. Neuron. 1994 Dec;13(6):1331–1343. doi: 10.1016/0896-6273(94)90419-7. [DOI] [PubMed] [Google Scholar]
- Holz R., Finkelstein A. The water and nonelectrolyte permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J Gen Physiol. 1970 Jul;56(1):125–145. doi: 10.1085/jgp.56.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume R. I., Dingledine R., Heinemann S. F. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991 Aug 30;253(5023):1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- Iino M., Mochizuki S., Ozawa S. Relationship between calcium permeability and rectification properties of AMPA receptors in cultured rat hippocampal neurons. Neurosci Lett. 1994 May 23;173(1-2):14–16. doi: 10.1016/0304-3940(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Racca C., Sakmann B., Seeburg P. H., Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994 Jun;12(6):1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Laschet J., Trottier S., Grisar T., Leviel V. Polyamine metabolism in epileptic cortex. Epilepsy Res. 1992 Jul;12(2):151–156. doi: 10.1016/0920-1211(92)90035-r. [DOI] [PubMed] [Google Scholar]
- Lerma J., Morales M., Ibarz J. M., Somohano F. Rectification properties and Ca2+ permeability of glutamate receptor channels in hippocampal cells. Eur J Neurosci. 1994 Jul 1;6(7):1080–1088. doi: 10.1111/j.1460-9568.1994.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Lopatin A. N., Makhina E. N., Nichols C. G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994 Nov 24;372(6504):366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- McBain C. J., Mayer M. L. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994 Jul;74(3):723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- Najm I., el-Skaf G., Massicotte G., Vanderklish P., Lynch G., Baudry M. Changes in polyamine levels and spectrin degradation following kainate-induced seizure activity: effect of difluoromethylornithine. Exp Neurol. 1992 Jun;116(3):345–354. doi: 10.1016/0014-4886(92)90013-g. [DOI] [PubMed] [Google Scholar]
- Otis T. S., Raman I. M., Trussell L. O. AMPA receptors with high Ca2+ permeability mediate synaptic transmission in the avian auditory pathway. J Physiol. 1995 Jan 15;482(Pt 2):309–315. doi: 10.1113/jphysiol.1995.sp020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
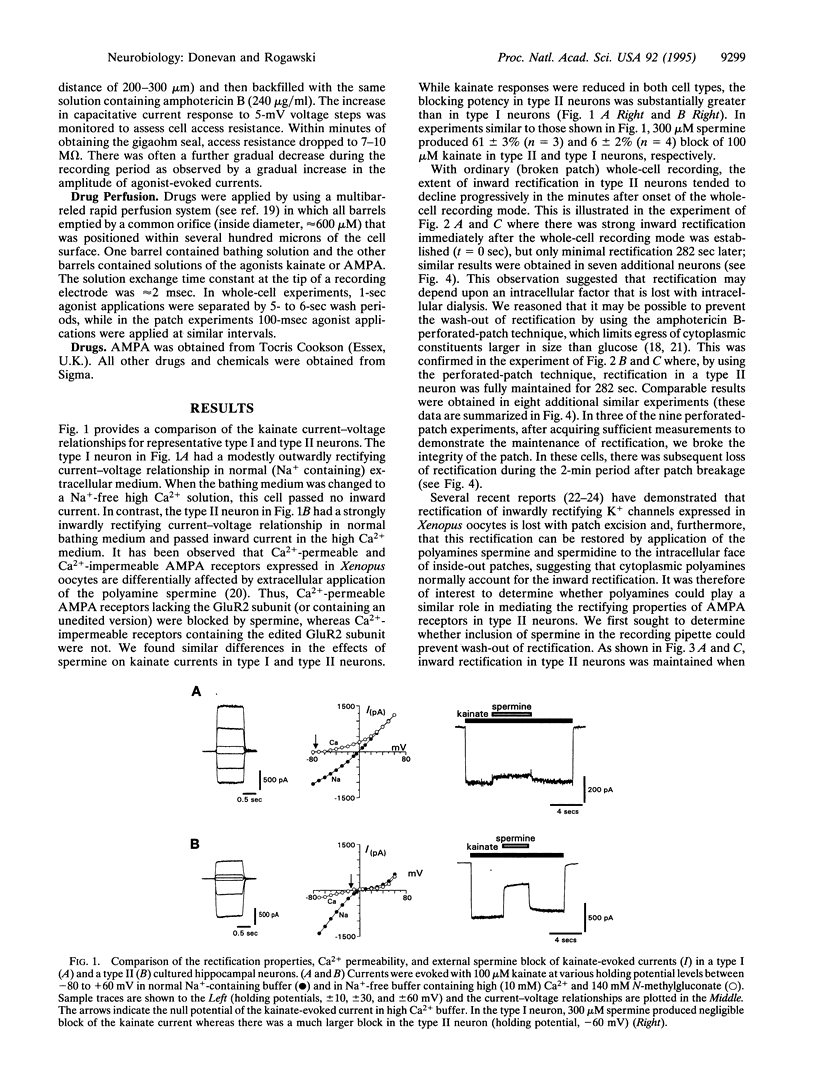
- Ozawa S., Iino M., Tsuzuki K. Two types of kainate response in cultured rat hippocampal neurons. J Neurophysiol. 1991 Jul;66(1):2–11. doi: 10.1152/jn.1991.66.1.2. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Sommer B., Keinänen K., Verdoorn T. A., Wisden W., Burnashev N., Herb A., Köhler M., Takagi T., Sakmann B., Seeburg P. H. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990 Sep 28;249(4976):1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
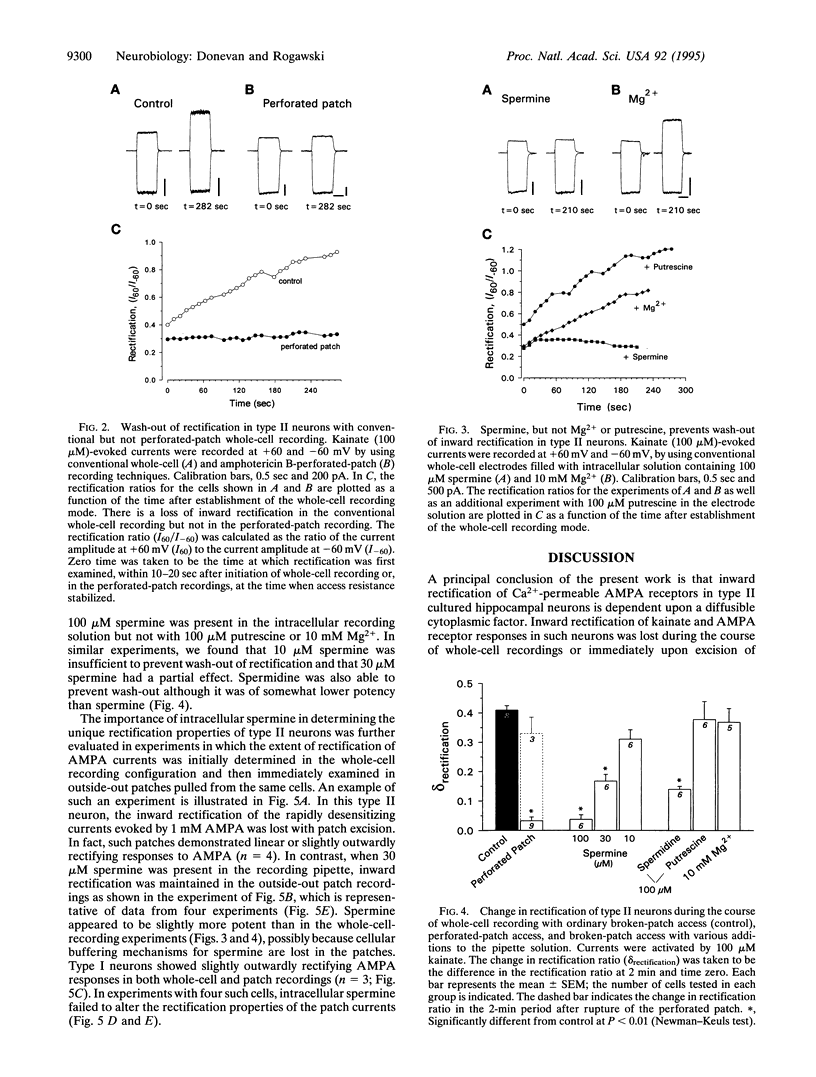
- Sommer B., Köhler M., Sprengel R., Seeburg P. H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991 Oct 4;67(1):11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Donevan S. D., Rogawski M. A. Hydrophobic interactions of n-alkyl diamines with the N-methyl-D-aspartate receptor: voltage-dependent and -independent blocking sites. Mol Pharmacol. 1994 Jan;45(1):117–124. [PubMed] [Google Scholar]
- Verdoorn T. A., Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991 Jun 21;252(5013):1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Kusama-Eguchi K., Kobayashi H., Igarashi K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J Biol Chem. 1991 Nov 5;266(31):20803–20809. [PubMed] [Google Scholar]
- Williams K., Romano C., Dichter M. A., Molinoff P. B. Modulation of the NMDA receptor by polyamines. Life Sci. 1991;48(6):469–498. doi: 10.1016/0024-3205(91)90463-l. [DOI] [PubMed] [Google Scholar]




