Abstract
In this study, we aimed to clarify the expression profiles of Yes-associated protein (YAP) and phosphorylated YAP (pYAP) protein and to verify the clinical implication of the expression of YAP protein in human breast cancer. We selected 678 cases of formalin-fixed paraffin-embedded (FFPE) breast cancer tissue to construct tissue microarray (TMA) blocks. We performed immunohistochemical staining of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth receptor-2 (HER-2) and Ki-67 and fluorescent in situ hybridization (FISH) assay for HER-2 on the TMA sections and divided breast cancers into molecular subtypes: luminal A, luminal B, HER-2, triple negative breast cancer (TNBC). Then, we examined YAP and pYAP expression status using immunohistochemical analysis according to the molecular subtypes of breast cancer. We found that HER-2 type breast cancer demonstrated elevated expression level in tumoral cytoplasmic YAP (P = 0.011) and pYAP (P = 0.049). Expressions of stromal YAP (P = 0.002) and pYAP (P < 0.001) were higher in luminal B and HER-2 type breast cancer but lower in TNBC. In univariate analysis, nuclear YAP expression of tumor cells was associated with shorter overall survival (OS) (P = 0.024). Cytoplasmic YAP expression of HER-2 type breast cancer cells negatively affected disease-free survival (DFS) (P = 0.034). In conclusion, we concluded that there was a significant difference in YAP and pYAP expression status according to molecular subtypes and tumoral and cellular components of breast cancers. Finally, we found that nuclear and cytoplasmic YAP expression could be a prognostic marker for breast cancer patients.
Keywords: Breast cancer, molecular subtype, YAP
Introduction
Yes-Associated Protein (YAP) is a recently identified oncogenic transcription coactivator. As a causative oncogene found in the 11q22 amplicon that is frequently observed in human cancer, YAP enhances invasion and proliferation, suppresses apoptosis and is sufficient for transformation [1]. Finally, YAP is the downstream effector molecule of a newly emerging tumor suppressor pathway called the Hippo pathway [2]. Thus, understanding the function of YAP may provide insight to universally conserved control mechanisms of tumorigenesis and tumor progression.
When YAP is phosphorylated on serine 127 by Lats kinase, phosphorylated YAP is sequestered from the nucleus by 14-3-3 and transcription activities of target genes are thus decreased [3]. In contrast, YAP overexpression, or nuclear localization of YAP, was frequently found in many cancers [4]. Recently, many researchers reported the functions of YAP in breast cancers, but these mechanisms still remain poorly understood. Several studies demonstrated YAP as a tumor suppressor in breast cancer, showing decreased level of YAP expression in human breast cancer tissue relative to normal breast tissue [5], increased cell migration and invasiveness of YAP-downregulated breast cancer cells in vitro, and increased tumor growth in the YAP-knockout mouse in vivo [6]. On the contrary, other researchers have asserted that YAP acts as an oncogene in breast cancer, demonstrating that YAP overexpression enhanced cellular proliferation [7], and xenograft mice transplanted with a YAP-overexpressing breast cancer cell line promoted tumor formation and growth in comparison to the control tumor group [8]. Therefore, it is necessary to clarify the expression status of YAP in human breast cancer tissue and its clinical implications in breast cancer.
In this study, we compared YAP and phosphorylated YAP (pYAP) expression profiles in breast cancers according to their molecular subtypes and cellular compartments, and then evaluated YAP as a prognostic factor in breast cancer patients.
Materials and methods
Patient selection
Patients diagnosed with invasive breast cancer and treated by surgical resection during January 2002 to December 2006 were included in this study. Patients who received preoperative neoadjuvant chemotherapy or hormonal treatment were excluded. This study was approved by the Institutional Review Board of Yonsei University Severance Hospital. A breast pathologist (Koo JS) retrospectively reviewed the histology of all cases using hematoxylin-eosin (H&E) stained slides. The histological grade was assessed using the Nottingham grading system [9]. Clinicopathologic parameters evaluated in each case included patient age at initial diagnosis, lymph node metastasis, tumor recurrence, distant metastasis, and patient survival.
Tissue microarray
On H&E stained slides of tumors, a representative area was selected and the corresponding spot was marked on the surface of the paraffin block. Using a biopsy needle, the selected area was punched out and a 3-mm tissue core was placed into a 6 × 5 recipient block. Tissue from the invasive tumor was then extracted. Two tissue cores were extracted to minimize extraction bias. Each tissue core was assigned a unique tissue microarray (TMA) location number that was linked to a database containing other clinicopathologic data.
Immunohistochemistry
The antibodies used for immunohistochemistry in this study are shown in Table 1. We performed immunohistochemical staining on formalin-fixed paraffin-embedded (FFPE) tissue using TMA. Briefly, 5-μm-thick sections were obtained with a microtome, transferred onto adhesive slides, and dried at 62°C for 30 minutes. After incubation with primary antibodies (Table 1), immunodetection was performed with biotinylated antimouse immunoglobulin, followed by peroxidase-labeled streptavidin using a labeled streptavidin biotin kit with 3,3’-diaminobenzidine chromogen as substrate. The primary antibody incubation step was omitted in the negative control. Positive control tissue was used per manufacturer’s recommendation. Slides were counterstained with Harris hematoxylin.
Table 1.
Source, clone, and dilution of antibodies used in this study
| antibody | Company | clone | dilution |
|---|---|---|---|
| YAP related | |||
| YAP | Santa Cruz Biotechnology, INC., California, USA | 9A1 | 1:100 |
| Phosphate YAP (ser127) | Abcam, Cambridge, UK | EP1675Y | 1:100 |
| Molecular subtype related | |||
| ER | Thermo Scientific, San Siego, CA, USA | SP1 | 1:100 |
| PR | DAKO, Glostrup, Denmark | PgR | 1:50 |
| HER-2 | DAKO, Glostrup, Denmark | Polyclonal | 1:1500 |
| Ki-67 | Abcam, Cambridge, UK | MIB | 1:1000 |
Interpretation of immunohistochemical staining
All immunohistochemical markers were assessed by light microscopy. A cut-off value of 1% or more positively stained nuclei was used to define estrogen receptor (ER) and progesterone receptor (PR) positivity [10]. Human epidermal growth receptor-2 (HER-2) staining was analyzed according to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines using the following categories: 0 = no immunostaining; 1+ = weak incomplete membranous staining, less than 10% of tumor cells; 2+ = complete membranous staining, either uniform or weak in at least 10% of tumor cells; and 3+ = uniform intense membranous staining in at least 30% of tumor cells [11]. HER-2 immunostaining was considered positive when strong (3+) membranous staining was observed whereas cases with 0 to 1+ were regarded as negative. Cases showing 2+ HER-2 expression were evaluated for HER-2 amplification by fluorescent in situ hybridization (FISH).
Expression status of YAP and pYAP in tumor cells and stromal cells was assessed according to the cellular compartments such as nucleus and cytoplasm. Immunostaining result was graded 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). We determined positive reactivity when moderate (grade 2) or strong (grade 3) positive cancer cells were identified over 10% of the tumor area.
Ki-67 labeling indices (LI) were scored by counting the number of positively stained nuclei and expressed as a percentage of total tumor cells.
Tumor phenotype classification
In this study, we classified breast cancer phenotypes according to the immunohistochemistry results for ER, PR, HER-2, and Ki-67 LI. FISH results for HER-2 were as follows [12]: luminal A type: ER and/or PR positive, HER-2 negative, and Ki-67 LI <14%; luminal B type: (HER-2 negative) ER and/or PR positive, HER-2 negative, and Ki-67 LI ≥14% and (HER-2 positive) ER and/or PR positive and HER-2 overexpressed and/or amplified; HER-2 type: ER and PR negative and HER-2 overexpressed and/or amplified; TNBC type: ER, PR, and HER-2 negative.
Statistical analysis
Data were analyzed using SPSS for Windows, Version 12.0 (SPSS Inc., Chicago, IL, USA). To determine statistical significance, Student’s t and Fisher’s exact tests were used for continuous and categorical variables, respectively. Statistical significance was reached when P < 0.05. Kaplan-Meier survival curves and log-rank statistics were employed to evaluate time to tumor recurrence and overall survival. Multivariate regression analysis was performed using the Cox proportional hazards model.
Results
Patients’ clinicopathologic characteristics
First, we investigated clinical features of breast cancer patients from the medical records and reviewed pathologic features of patients’ breast cancer tissue (Table 2). In total, included cases of breast cancers were 280 cases of luminal A type (41.3%), 156 cases of luminal B type (23.0%), 67 cases of HER-2 type (9.9%), and 175 cases of triple negative breast cancer (TNBC) type (25.8%). TNBC-type breast cancers demonstrated higher histologic grade (P < 0.001), higher T stage (P = 0.014), and higher Ki-67 LI (P < 0.001) than other molecular subtypes. HER-2 type breast cancer was associated with older age at diagnosis (P = 0.022), more frequent tumor recurrence (P = 0.018) and lower survival rate (P = 0.007) than other molecular subtypes.
Table 2.
Clinicopathologic characteristics of patients according to breast cancer phenotype
| Parameters | Total (N = 678) (%) | Luminal A (n = 280) (%) | Luminal B (n = 156) (%) | HER-2 (n = 67) (%) | TNBC (n = 175) (%) | P-value |
|---|---|---|---|---|---|---|
| Age (years) | 0.022 | |||||
| ≤50 | 403 (59.4) | 160 (57.1) | 105 (67.3) | 31 (46.3) | 107 (61.1) | |
| >50 | 275 (40.6) | 120 (42.9) | 51 (32.7) | 36 (53.7) | 68 (38.9) | |
| Histologic grade | <0.001 | |||||
| I/II | 453 (66.8) | 253 (90.4) | 106 (67.9) | 35 (52.2) | 59 (33.7) | |
| III | 225 (33.2) | 27 (9.6) | 50 (32.1) | 32 (47.8) | 116 (66.3) | |
| Tumor stage | 0.014 | |||||
| T1 | 323 (47.6) | 149 (53.2) | 77 (49.4) | 31 (46.3) | 66 (37.7) | |
| T2/T3 | 355 (52.4) | 131 (46.8) | 79 (50.6) | 36 (53.7) | 109 (62.3) | |
| Nodal metastasis | 0.163 | |||||
| Absent | 400 (59.0) | 158 (56.4) | 86 (55.1) | 41 (61.2) | 115 (65.7) | |
| Present | 278 (41.0) | 122 (43.6) | 70 (44.9) | 26 (38.8) | 60 (34.3) | |
| Estrogen receptor status | <0.001 | |||||
| Negative | 252 (37.2) | 5 (1.8) | 5 (3.2) | 67 (100.0) | 175 (100.0) | |
| Positive | 426 (62.8) | 275 (98.2) | 151 (96.8) | 0 (0.0) | 0 (0.0) | |
| Progesterone receptor status | <0.001 | |||||
| Negative | 336 (49.6) | 46 (16.4) | 48 (30.8) | 67 (100.0) | 175 (100.0) | |
| Positive | 342 (50.4) | 234 (83.6) | 108 (69.2) | 0 (0.0) | 0 (0.0) | |
| HER-2 status | <0.001 | |||||
| Negative | 531 (78.3) | 280 (100.0) | 76 (48.7) | 0 (0.0) | 175 (100.0) | |
| Positive | 147 (21.7) | 0 (0.0) | 80 (51.3) | 67 (100.0) | 0 (0.0) | |
| Ki-67 LI (%) | <0.001 | |||||
| ≤14 | 380 (56.0) | 280 (100.0) | 46 (29.5) | 27 (40.3) | 27 (15.4) | |
| >14 | 298 (44.0) | 0 (0.0) | 110 (70.5) | 40 (59.7) | 148 (84.6) | |
| Tumor recurrence | 60 (8.8) | 15 (5.4) | 13 (8.3) | 9 (13.4) | 23 (13.1) | 0.018 |
| Patients’ death | 58 (8.6) | 13 (4.6) | 13 (8.3) | 9 (13.4) | 23 (13.1) | 0.007 |
| Duration of clinical follow-up (months, mean ± SD) | 70 ± 31 | 72 ± 29 | 70 ± 31 | 66 ± 35 | 67 ± 33 | 0.390 |
TNBC, triple negative breast cancer.
Expression of YAP and pYAP according to molecular subtypes of breast cancer
Next, we performed immunohistochemical staining for identification of YAP and pYAP expression status according to the molecular subtype of breast cancer (Table 3 and Figure 1). We further evaluated expression profiles of YAP and pYAP dividing cellular components into the nucleus and cytoplasm. Our results showed increased tumoral cytoplasmic YAP expression (P = 0.011) and tumoral cytoplasmic pYAP expression (P = 0.049) in HER-2 type breast cancer tissue compared to other molecular subtypes. In stromal component, YAP (P = 0.002) and pYAP (P < 0.001) were increased in luminal B and HER-2 type breast cancer tissues but relatively decreased in TNBC type breast cancer tissue. However, there was no significant difference in nuclear YAP expression according to the molecular subtype of breast cancer.
Table 3.
Expression of YAP and pYAP according to breast cancer phenotype
| Parameters | Total (N = 678) (%) | Luminal A (n = 280) (%) | Luminal B (n = 156) (%) | HER-2 (n = 67) (%) | TNBC (n = 175) (%) | P-value |
|---|---|---|---|---|---|---|
| YAP (T-Nu) | 0.181 | |||||
| Negative | 636 (93.8) | 261 (93.2) | 152 (97.4) | 62 (92.5) | 161 (92.0) | |
| Positive | 42 (6.2) | 19 (6.8) | 4 (2.6) | 5 (7.5) | 14 (8.0) | |
| YAP (T-Cy) | 0.011 | |||||
| Negative | 646 (95.3) | 265 (94.6) | 151 (96.8) | 59 (88.1) | 171 (97.7) | |
| Positive | 32 (4.7) | 15 (5.4) | 5 (3.2) | 8 (11.9) | 4 (2.3) | |
| YAP (S) | 0.002 | |||||
| Negative | 574 (84.7) | 246 (87.9) | 121 (77.6) | 51 (76.1) | 156 (89.1) | |
| Positive | 104 (15.3) | 34 (12.1) | 35 (22.4) | 16 (23.9) | 19 (10.9) | |
| pYAP (T-Cy) | 0.049 | |||||
| Negative | 541 (79.8) | 224 (80.0) | 129 (82.7) | 45 (67.2) | 143 (81.7) | |
| Positive | 137 (20.2) | 56 (20.0) | 27 (17.3) | 22 (32.8) | 32 (18.3) | |
| pYAP (T-Nu) | 0.449 | |||||
| Negative | 672 (99.1) | 279 (99.6) | 155 (99.4) | 66 (98.5) | 172 (98.3) | |
| Positive | 6 (0.9) | 1 (0.4) | 1 (0.6) | 1 (1.5) | 3 (1.7) | |
| pYAP (S) | <0.001 | |||||
| Negative | 615 (90.7) | 261 (93.2) | 129 (82.7) | 57 (85.1) | 168 (96.0) | |
| Positive | 63 (9.3) | 19 (6.8) | 27 (17.3) | 10 (14.9) | 7 (4.0) |
TNBC, triple negative breast cancer.
Figure 1.
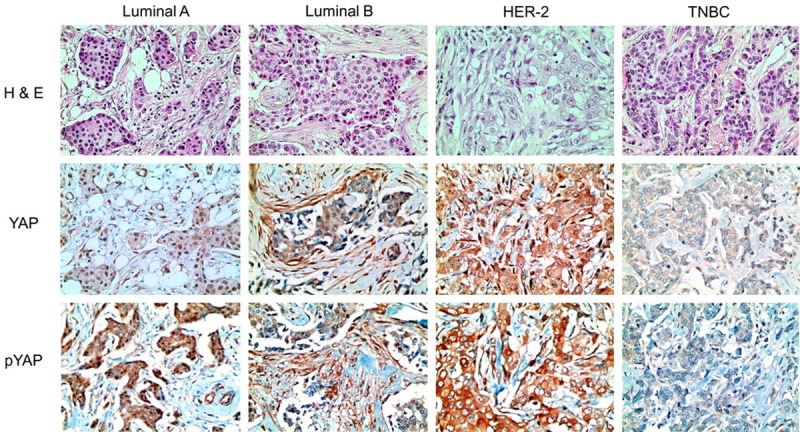
Expression of YAP and pYAP according to the molecular subtype of breast cancer. HER-2 type breast cancer demonstrated cytoplasmic YAP and pYAP in cancer cells. Luminal B and HER-2 type breast cancer revealed a higher expression rate of stromal YAP and pYAP, but TNBC did not. Microscopic magnification, 200X.
Correlation between expression of YAP and pYAP proteins and clinicopathologic factors
We investigated various clinicopathologic parameters known to affect patients’ prognosis according to expression status of YAP and pYAP: age at diagnosis, tumor stage, nodal metastasis, histologic grade, ER status, PR status, HER-2 status, and Ki-67 L.I. (Tables 4 and 5). Both YAP expression (P = 0.001) and pYAP expression (P = 0.001) were increased in the stromal cells of HER-2 type breast cancer. Cytoplasmic pYAP expression was associated lower tumor stage (P = 0.003). However, nuclear YAP expression in tumor cells was not associated with any significant differences in various clinicopathologic parameters.
Table 4.
Correlations between the expression of YAP and clinicopathologic parameters
| Parameters | YAP (T-Nu) | YAP (T-Cy) | YAP (S) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Negative n = 636 (%) | Positive n = 42 (%) | P-value* | Negative n = 646 (%) | Positive n = 32 (%) | P-value* | Negative n = 574 (%) | Positive n = 104 (%) | P-value* | |
| Age (years) | 0.737 | 0.138 | 0.046 | ||||||
| ≤50 | 377 (59.3) | 26 (61.9) | 388 (60.1) | 15 (46.9) | 332 (57.8) | 71 (68.3) | |||
| >50 | 259 (40.7) | 16 (38.1) | 258 (39.9) | 17 (53.1) | 242 (42.2) | 33 (31.7) | |||
| Histologic grade | 0.512 | 0.812 | 0.908 | ||||||
| I/II | 423 (66.5) | 30 (71.4) | 431 (66.7) | 22 (68.8) | 383 (66.7) | 70 (67.3) | |||
| III | 213 (33.5) | 12 (28.6) | 215 (33.3) | 10 (31.2) | 191 (33.3) | 34 (32.7) | |||
| Tumor stage | 0.522 | 0.784 | 0.756 | ||||||
| T1 | 305 (48.0) | 18 (42.9) | 307 (47.5) | 16 (50.0) | 272 (47.4) | 51 (49.0) | |||
| T2/T3 | 331 (52.0) | 24 (57.1) | 339 (52.5) | 16 (50.0) | 302 (52.6) | 53 (51.0) | |||
| Nodal metastasis | 0.564 | 0.435 | 0.938 | ||||||
| Absent | 377 (59.3) | 23 (54.8) | 379 (58.7) | 21 (65.6) | 339 (59.1) | 61 (58.7) | |||
| Present | 259 (40.7) | 19 (45.2) | 267 (41.3) | 11 (34.4) | 235 (40.9) | 43 (41.3) | |||
| Estrogen receptor status | 0.264 | 0.678 | 0.715 | ||||||
| Negative | 233 (36.6) | 19 (45.2) | 239 (37.0) | 13 (40.6) | 215 (37.5) | 37 (35.6) | |||
| Positive | 403 (63.4) | 23 (54.8) | 407 (63.0) | 19 (59.4) | 359 (62.5) | 67 (64.4) | |||
| Progesterone receptor status | 0.706 | 0.959 | 0.743 | ||||||
| Negative | 314 (49.4) | 22 (52.4) | 320 (49.5) | 16 (50.0) | 286 (49.8) | 50 (48.1) | |||
| Positive | 322 (50.6) | 20 (47.6) | 326 (50.5) | 16 (50.0) | 288 (50.2) | 54 (51.9) | |||
| HER-2 status | 0.230 | 0.074 | 0.001 | ||||||
| Negative | 495 (77.8) | 36 (85.7) | 510 (78.9) | 21 (65.6) | 462 (80.5) | 69 (66.3) | |||
| Positive | 141 (22.2) | 6 (14.3) | 136 (21.1) | 11 (34.4) | 112 (19.5) | 35 (33.7) | |||
| Ki-67 LI (%) | 0.639 | 0.138 | 0.177 | ||||||
| ≤14 | 355 (55.8) | 25 (59.5) | 358 (55.4) | 22 (68.8) | 328 (57.1) | 52 (50.0) | |||
| >14 | 281 (44.2) | 17 (40.5) | 288 (44.6) | 10 (31.2) | 246 (42.9) | 52 (50.0) | |||
P-value was corrected by the Bonferroni method.
Table 5.
Correlations between the expression of pYAP and clinicopathologic parameters
| Parameters | pYAP (T-Cy) | pYAP (T-Nu) | pYAP (S) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Negative n = 541 (%) | Positive n = 137 (%) | P-value* | Negative n = 672 (%) | Positive n = 6 (%) | P-value* | Negative n = 615 (%) | Positive n = 63 (%) | P-value* | |
| Age (years) | 0.066 | 0.691 | 0.882 | ||||||
| ≤50 | 331 (61.2) | 72 (52.6) | 400 (59.5) | 3 (50.0) | 365 (59.3) | 38 (60.3) | |||
| >50 | 210 (38.8) | 65 (47.4) | 272 (40.5) | 3 (50.0) | 250 (40.7) | 25 (39.7) | |||
| Histologic grade | 0.755 | 0.994 | 0.087 | ||||||
| I/II | 363 (67.1) | 90 (65.7) | 449 (66.8) | 4 (66.7) | 417 (67.8) | 36 (57.1) | |||
| III | 178 (32.9) | 47 (34.3) | 223 (33.2) | 2 (33.3) | 198 (32.2) | 27 (42.9) | |||
| Tumor stage | 0.003 | 0.688 | 0.113 | ||||||
| T1 | 242 (44.7) | 81 (59.1) | 321 (47.8) | 2 (33.3) | 287 (46.7) | 36 (57.1) | |||
| T2/T3 | 299 (55.3) | 56 (40.9) | 351 (52.2) | 4 (66.7) | 328 (53.3) | 27 (42.9) | |||
| Nodal metastasis | 0.723 | 1.000 | 0.753 | ||||||
| Absent | 321 (59.3) | 79 (57.7) | 396 (58.9) | 4 (66.7) | 364 (59.2) | 36 (57.1) | |||
| Present | 220 (40.7) | 58 (42.3) | 276 (41.1) | 2 (33.3) | 251 (40.8) | 27 (42.9) | |||
| Estrogen receptor status | 0.161 | 0.202 | 0.227 | ||||||
| Negative | 194 (35.9) | 58 (42.3) | 248 (36.9) | 4 (66.7) | 233 (37.9) | 19 (30.2) | |||
| Positive | 347 (64.1) | 79 (57.7) | 424 (63.1) | 2 (33.3) | 382 (62.1) | 44 (69.8) | |||
| Progesterone receptor status | 0.864 | 0.447 | 0.264 | ||||||
| Negative | 269 (49.7) | 67 (48.9) | 332 (49.4) | 4 (66.7) | 309 (50.2) | 27 (42.9) | |||
| Positive | 272 (50.3) | 70 (51.1) | 340 (50.6) | 2 (33.3) | 306 (49.8) | 36 (57.1) | |||
| HER-2 status | 0.090 | 1.000 | 0.001 | ||||||
| Negative | 431 (79.7) | 100 (73.0) | 526 (78.3) | 5 (83.3) | 492 (80.0) | 39 (61.9) | |||
| Positive | 110 (20.3) | 37 (27.0) | 146 (21.7) | 1 (16.7) | 123 (20.0) | 24 (38.1) | |||
| Ki-67 LI (%) | 0.815 | 0.413 | 0.251 | ||||||
| ≤14 | 302 (55.8) | 78 (56.9) | 378 (56.2) | 2 (33.3) | 349 (56.7) | 31 (49.2) | |||
| >14 | 239 (44.2) | 59 (43.1) | 294 (43.8) | 4 (66.7) | 266 (43.3) | 32 (50.8) | |||
P-value was corrected by the Bonferroni method.
Impact of expression of YAP and pYAP proteins on patient prognosis
We analyzed overall survival (OS) rate and disease-free survival (DFS) rate of breast cancer patients according to YAP and pYAP expression using Kaplan-Meier survival curve (Figure 2). Distinctively, patients with breast cancer who showed nuclear YAP expression in tumor cells had inferior OS compared to patients with nuclear YAP-negative breast cancer (Figure 2A, P = 0.024). When we analyzed OS and DFS according to the molecular subtype of breast cancer, cytoplasmic YAP expression of tumor cells in HER-2 type negatively affected DFS of breast cancer patients (Figure 2B, P = 0.034). However, nuclear YAP expression of tumor cells did not affect either OS or DFS in each molecular subtype of breast cancer.
Figure 2.
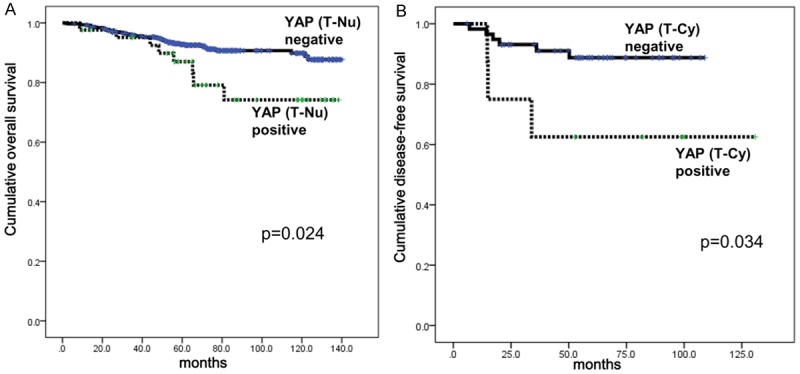
Survival analysis of breast cancer patients according to the expression status of YAP protein. A. Overall survival curve according to the nuclear expression status of YAP in breast cancer cells. B. Disease-free survival curve according to the cytoplasmic expression status of YAP in HER-2 type breast cancer cells. T-Nu, nuclear expression of tumor cells. T-Cy, cytoplasmic expression of tumor cells.
Next, we investigated predictable variables associated with patients’ survival according to YAP expression status using Cox regression analysis (Table 6). In univariate analysis, only nuclear YAP expression of tumor cells was correlated with shorter OS (P = 0.024) and demonstrated a tendency of negative correlation with DFS (P = 0.079).
Table 6.
Univariate analysis of the impact of the expression of YAP and pYAP in breast cancers on disease-free survival or overall survival by log-rank test
| Parameters | Number of patients/recurrence/death | Disease-free survival | Overall survival | ||
|---|---|---|---|---|---|
|
|
|
||||
| Mean survival (95% CI) months | P-value | Mean survival (95% CI) months | P-value | ||
| YAP (T-Nu) | 0.079 | 0.024 | |||
| Negative | 636/53/50 | 126 (123-130) | 130 (127-133) | ||
| Positive | 42/7/8 | 111 (99-123) | 116 (103-130) | ||
| YAP (T-Cy) | 0.579 | 0.924 | |||
| Negative | 646/56/55 | 127 (124-130) | 129 (126-132) | ||
| Positive | 32/4/3 | 117 (105-129) | 124 (115-134) | ||
| YAP (S) | 0.163 | 0.110 | |||
| Negative | 574/55/54 | 124 (120-128) | 128 (125-131) | ||
| Positive | 104/5/4 | 132 (126-138) | 134 (130-139) | ||
| pYAP (T-Cy) | 0.920 | 0.894 | |||
| Negative | 541/49/47 | 127 (123-130) | 129 (126-132) | ||
| Positive | 137/11/11 | 117 (110-124) | 127 (120-134) | ||
| pYAP (T-Nu) | n/a | n/a | |||
| Negative | 672/60/58 | n/a | n/a | ||
| Positive | 6/0/0 | n/a | n/a | ||
| pYAP (S) | 0.981 | 0.894 | |||
| Negative | 615/55/53 | 126 (122-130) | 129 (126-132) | ||
| Positive | 63/5/5 | 98 (92-103) | 121 (114-128) | ||
When the predictable parameters were adjusted for by multivariate analysis (Table 7), a tendency for nuclear YAP expression of tumor cells to independently affect inferior OS was revealed (Hazard ratio: 2.073, 95% CI: 0.973-4.416, P = 0.059). As expected, lymph node metastasis was associated with shorter DFS (Hazard ratio: 2.210, 95% CI: 1.295-3.771, P = 0.004) and shorter OS (Hazard ratio: 1.879, 95% CI: 1.098-3.216, P = 0.021). Higher tumor stage was also associated with shorter DFS (Hazard ratio: 2.024, 95% CI: 1.109-3.693, P = 0.022).
Table 7.
Multivariate analysis of breast-cancer survival
| Included parameters | Disease-free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| T stage | 0.022 | 0.100 | ||||
| T1 versus T2-3 | 2.024 | 1.109-3.693 | 1.629 | 0.910-2.914 | ||
| N stage | 0.004 | 0.021 | ||||
| N0 versus N1-3 | 2.210 | 1.295-3.771 | 1.879 | 1.098-3.216 | ||
| Histologic grade | 0.507 | 0.726 | ||||
| I/II versus III | 1.213 | 0.686-2.144 | 0.900 | 0.500-1.621 | ||
| ER status | 0.291 | 0.223 | ||||
| Negative versus Positive | 1.496 | 0.709-3.158 | 1.575 | 0.758-3.272 | ||
| PR status | 0.274 | 0.086 | ||||
| Negative versus Positive | 1.534 | 0.713-3.300 | 1.976 | 0.909-4.296 | ||
| HER-2 status | 0.531 | 0.618 | ||||
| Negative versus Positive | 1.208 | 0.669-2.183 | 1.164 | 0.640-2.115 | ||
| YAP (T-Nu) | 0.125 | 0.059 | ||||
| Negative versus Positive | 1.867 | 0.841-4.147 | 2.073 | 0.973-4.416 | ||
Discussion
We investigated the expression profiles of YAP and pYAP in various molecular subtypes of breast cancers. Previous studies have reported the expression frequency of YAP in breast cancer to range from 45-75%, which is much higher than our results showing 6.2% of tumors having nuclear expression and 4.7% of tumors having cytoplasmic expression [5,8]. We believe that this difference may be explained by the cut-off value of positive reactivity. In this study, we determined positive reactivity of YAP protein when moderately (grade 2) or strongly (grade 3) positive cancer cells were identified over 10% of tumor area, because normal luminal cells or myoepithelial cells also had weak positivity of YAP protein. Based on the same criteria, a previous report indicated that 62% of breast cancers had moderate positivity and only 6% had strong positivity [13]; and in another study, 29% of breast cancers demonstrated YAP [8].
As YAP protein functions as a transcription coactivator in the nucleus and phosphorylated YAP protein is sequestered in the cytoplasm, it is needed to localize YAP and pYAP protein to identify the oncogenic function of YAP in breast cancer cells. We divided cellular components such as the nucleus and cytoplasm and sorted through the expression status of YAP and pYAP in each component using immunohistochemical assay. Our results showed that the expression of YAP protein differed between each molecular subtype of breast cancer. We discovered that HER-2 type breast cancer demonstrated increased expression level of cytoplasmic YAP and pYAP among subtypes, although previous studies reported that YAP expression of breast cancer cells was associated with invasive lobular carcinoma [14] and ER/PR positivity [5]. As we mentioned above, YAP protein is phosphorylated in the cytoplasm by a kinase cascade associated with the Hippo signaling pathway. Because HER-2 is a transmembrane receptor which has an intrinsic tyrosine kinase [15], we suggest that cytoplasmic overexpression of YAP and pYAP, but not nuclear overexpression, in HER-2 type breast cancer could be due to the possibility that HER-2 amplification/overexpression increases intrinsic kinase activity and, thus, a significant amount of YAP protein can be phosphorylated in the cytoplasm. However, the precise mechanism of increased phosphorylation of YAP in HER-2 type breast cancer should be studied further. Unexpectedly, the nuclear pYAP expression in a few cases of breast cancer ranged from 0.4 to 1.7% according to molecular subtype. This result may be due to non-specific or over staining and nuclear pYAP expression may not, in fact, be associated with any clinicopathologic features of breast cancer.
This is the first study to focus on the stromal expression of YAP and pYAP proteins in breast cancer. YAP and pYAP expression of stromal cells were increased in luminal B type and HER-2 type breast cancers but decreased in TNBC. We struggled to understand this phenomenon and considered the possibility of mechanotransduction of tumor cells in surrounding stromal cells regulated by the Hippo signaling pathway. It is known that the actin cytoskeleton could decrease the phosphorylation of YAP as a key molecule of the Hippo signaling pathway and de-phosphorylated YAP is translocated into the nucleus, increasing transcripts of downstream growth factors such as CCN1/CYR61 and CCN2/CTGF [16,17]. In breast cancer, the concept of cancer associated fibroblasts consisting of tumor stroma is explained by several types of cells with a well-known component being the myofibroblast in which α-smooth muscle actin is expressed [18]. To sum up these findings, we believe that YAP and pYAP expression of tumor stromal cells composed of cancer-associated fibroblasts may be affected by cancer cells.
In this study, we investigated the prognosis of patients with nuclear YAP-expressing breast cancer. When the survival analysis included all subtypes of breast cancer, nuclear YAP expression of the cancer cells was associated with inferior OS. A detailed analysis of molecular subtypes showed cytoplasmic YAP expression of tumor cells in HER-2 type breast cancer was related to shorter DFS. In the same manner, previous studies have suggested that YAP expression is associated with poor prognosis in various solid tumors including ovarian cancer [19], urinary bladder cancer [20], colorectal cancer [21], esophageal cancer [22], stomach cancer [23], and lung cancer [24]. In breast cancer, it has been thought that YAP was not an independent prognostic marker [13]. Accordingly, we also did not consider YAP expression to be an independent predictable parameter in Cox multivariate analysis. Although only one report has demonstrated that decreased levels of YAP expression in luminal A type breast cancer was associated with decreased DFS [25], it is necessary to verify the effect of YAP expression of each molecular subtype on patients’ prognosis.
To conclude, YAP and pYAP proteins were expressed differently according to the molecular subtypes of breast cancer and its cellular components. In this study, we show that increased nuclear YAP expression correlated with decreased OS in breast cancer and overexpression of cytoplasmic YAP in HER-2 type breast cancer was associated with shorter DFS.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A1002886). This study was supported by a faculty research grant from Yonsei University College of Medicine for 2013 (6-2013-0146).
Disclosure of conflict of interest
None.
References
- 1.Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 4.Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tufail R, Jorda M, Zhao W, Reis I, Nawaz Z. Loss of Yes-associated protein (YAP) expression is associated with estrogen and progesterone receptors negativity in invasive breast carcinomas. Breast Cancer Res Treat. 2012;131:743–750. doi: 10.1007/s10549-011-1435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T, Gangeswaran R, Manson-Bishop C, Smith P, Danovi SA, Pardo O, Crook T, Mein CA, Lemoine NR, Jones LJ, Basu S. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–1759. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- 7.Zhi X, Zhao D, Zhou Z, Liu R, Chen C. YAP promotes breast cell proliferation and survival partially through stabilizing the KLF5 transcription factor. Am J Pathol. 2012;180:2452–2461. doi: 10.1016/j.ajpath.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Su L, Ou Q. Yes-associated protein promotes tumour development in luminal epithelial derived breast cancer. Eur J Cancer. 2012;48:1227–1234. doi: 10.1016/j.ejca.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 10.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 12.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheen-Chen SM, Huang CY, Tsai CH, Liu YW, Wu SC, Huang CC, Eng HL, Chan YC, Ko SF, Tang RP. Yes-associated protein is not an independent prognostic marker in breast cancer. Anticancer Res. 2012;32:3321–3325. [PubMed] [Google Scholar]
- 14.Vlug EJ, van de Ven RA, Vermeulen JF, Bult P, van Diest PJ, Derksen PW. Nuclear localization of the transcriptional coactivator YAP is associated with invasive lobular breast cancer. Cell Oncol (Dordr) 2013;36:375–384. doi: 10.1007/s13402-013-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Fiore PP, Segatto O, Lonardo F, Fazioli F, Pierce JH, Aaronson SA. The carboxy-terminal domains of erbB-2 and epidermal growth factor receptor exert different regulatory effects on intrinsic receptor tyrosine kinase function and transforming activity. Mol Cell Biol. 1990;10:2749–2756. doi: 10.1128/mcb.10.6.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan T, Xu Y, Qin Z, Robichaud P, Betcher S, Calderone K, He T, Johnson TM, Voorhees JJ, Fisher GJ. Elevated YAP and Its Downstream Targets CCN1 and CCN2 in Basal Cell Carcinoma: Impact on Keratinocyte Proliferation and Stromal Cell Activation. Am J Pathol. 2014;184:937–43. doi: 10.1016/j.ajpath.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy P, Deguchi M, Cheng Y, Hsueh AJ. Actin cytoskeleton regulates Hippo signaling. PLoS One. 2013;8:e73763. doi: 10.1371/journal.pone.0073763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 19.Jeong W, Kim SB, Sohn BH, Park YY, Park ES, Kim SC, Kim SS, Johnson RL, Birrer M, Bowtell DS, Mills GB, Sood A, Lee JS. Activation of YAP1 Is Associated with Poor Prognosis and Response to Taxanes in Ovarian Cancer. Anticancer Res. 2014;34:811–817. [PMC free article] [PubMed] [Google Scholar]
- 20.Liu JY, Li YH, Lin HX, Liao YJ, Mai SJ, Liu ZW, Zhang ZL, Jiang LJ, Zhang JX, Kung HF, Zeng YX, Zhou FJ, Xie D. Overexpression of YAP 1 contributes to progressive features and poor prognosis of human urothelial carcinoma of the bladder. BMC Cancer. 2013;13:349. doi: 10.1186/1471-2407-13-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Shi S, Guo Z, Zhang X, Han S, Yang A, Wen W, Zhu Q. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One. 2013;8:e65539. doi: 10.1371/journal.pone.0065539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Yeo MK, Kim SH, Kim JM, Huang SM, Kim MR, Song KS, Kim KH. Correlation of expression of phosphorylated and non-phosphorylated Yes-associated protein with clinicopathological parameters in esophageal squamous cell carcinoma in a Korean population. Anticancer Res. 2012;32:3835–3840. [PubMed] [Google Scholar]
- 23.Song M, Cheong JH, Kim H, Noh SH. Nuclear expression of Yes-associated protein 1 correlates with poor prognosis in intestinal type gastric cancer. Anticancer Res. 2012;32:3827–3834. [PubMed] [Google Scholar]
- 24.Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010;101:1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehn S, Tobin NP, Sims AH, Stal O, Jirstrom K, Axelson H, Landberg G. Decreased expression of Yes-associated protein is associated with outcome in the luminal A breast cancer subgroup and with an impaired tamoxifen response. BMC Cancer. 2014;14:119. doi: 10.1186/1471-2407-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]


