Abstract
Early growth response gene-1 (Egr1) has a crucial function in the development and progression of prostate cancer. However, whether Egr1 contributes to the transition of advanced androgen-independent prostate cancer (AIPC) from androgen-dependent prostate cancer (ADPC) remains largely unknown. To the best of our knowledge, through immunohistochemical staining methods, we were the first to identify that Egr1 is more highly expressed in AIPC clinical specimens than in androgen-dependent prostate cancer (ADPC). An in vitro study with quantitative RT-PCR and Western blot demonstrated that Egr1 also has a higher expression in androgen-independent PC3 cells than in the androgen-dependent LNCaP cells. Egr1 expression in LNCaP cells was significantly upregulated during the androgen deprivation treatment (ADT) and was re-downregulated through the addition of dihydrotestosterone. Although no variation in PC3 cells was identified, Egr1 responded to dihydrotestosterone and flutamide in the androgen receptor (AR)-transfected PC3 cells. Further investigation with Egr1 agonist and specific siRNA-targeting Egr1 revealed that Egr1 upregulation or downregulation was accompanied by a change in inhibitors of differentiation and DNA binding-1 (Id1) in the same direction in both LNCaP and PC3 cells. The variation is shown to be negatively regulated by androgen through AR during ADT. Our data suggested that upregulated Egr1 might partially contribute to the emergence of AIPC after prolonged ADT. This study also elucidated the potential mechanism underlying Id1 participation in the progression of prostate cancer. Understanding the key molecular events in the transition from ADPC to AIPC may provide new therapeutic intervention strategies for patients with AIPC.
Keywords: Prostate cancer, Egr1, Id1, androgen deprivation treatmen, androgen receptor
Introduction
Prostate cancer is the most common malignancy in American men and the second leading cause of male cancer mortality [1]. In 2012, prostate cancer was expected to account for 29% (241,740) of all newly diagnosed cancers and 9% (28,170) of all male cancer deaths [2]. The development and progression of prostate cancer are initially androgen-dependent. Hence, androgen deprivation therapy (ADT) is generally employed to treat advanced or metastatic prostate cancer. However, prolonged androgen deprivation generally results in relapse and androgen-independent tumor growth [3]. No effective treatment strategy for advanced androgen-independent prostate cancer [AIPC, also known as castration-resistant prostate cancer (CRPC)] is available. The molecular events that enable prostate cancer cells to proliferate in reduced androgen conditions are poorly understood. Therefore, at present, the most challenging issue in the clinical treatment of prostate cancer is the emerging AIPC [4]. A more in-depth understanding of the variations in gene expression during the transition from androgen-dependent prostate cancer (ADPC) to AIPC may lead to new paradigms and possible improvements in the treatment of AIPC.
The family of early growth response (Egr) transcription factors has a highly conserved DNA-binding domain consisting of three zinc finger motifs, which bind to a GC-rich region in the promoters of their target genes [5]. The induction of Egr1 by external stimuli is generally transient but sustained in a large fraction of prostate tumors, suggesting that Egr1 has a critical function in the initiation and/or progression of prostate cancer [6,7]. These previous findings suggest that Egr1 protein may be an important candidate molecular marker for aggressive human prostate cancer. However, to date, whether Egr1 participates in AIPC remains unknown.
Previous studies have suggested the critical role of Egr1 in cancer metastasis and tumor invasion [8]. Our recent evidence shows that inhibitors of differentiation and DNA binding-1 (Id1) are negatively regulated by androgen through androgen receptors (AR) which contribute to the development of AIPC [9]. In addition, Id1 transcription was at least partially regulated by a protein complex containing the Egr1 [10]. Combining the present results with those from previous studies, we hypothesize that Egr1 might contribute to the negative regulation of AR on Id1 and participate in the formation of AIPC or CRPC.
Materials and methods
Tissue specimens
Study protocols involving human materials were approved by the institutional ethics committee of Changhai Hospital (Shanghai, China). Formalin-fixed and paraffin-embedded tissue specimens were obtained from the archives of the hospital’s Department of Pathology. ADPC specimens were obtained from 10 ADPC patients by puncture biopsy, and AIPC specimens were obtained from 5 AIPC patients during transurethral resection of the prostate (TURP), who received ADT for 11 to 17 months before TURP. Clinicopathologic details of the 15 cases are described in Table 1.
Table 1.
Clinicopathologic details of the 15 case studies
| Stage | ADPC | AIPC |
|---|---|---|
| Number of cases | 10 | 5 |
| Age (years) | 53 to 81 (median, 68) | 61 to 74 (median, 69) |
| Time of ADT before operation (month) | 0 | 11 to 17 (median, 15) |
| PSA (Pre-ADT, ng/ml) | 6.13 to 86.14 (median, 18.72) | > 100 |
Footnotes: ADPC: androgen-dependent prostate cancer; AIPC: androgen-independent prostate cancer; ADT: androgen deprivation therapy; PSA: prostate-specific antigen.
Immunohistochemistry
Paraffin-embedded prostate tissue specimens were sliced to 5 μm sections, deparaffinized with xylene, rehydrated with decreasing concentrations of ethanol, blocked with methanol/hydrogen peroxide, and incubated overnight with 0.66 Ag/ml rabbit polyclonal Egr1 antibody (588; Santa Cruz Biotechnology, Santa Cruz, CA). Egr1 immunocomplexes were detected using a biotinylated-labeled anti-rabbit secondary antibody and streptavidin-peroxidase with the Vector Nova Red substrate kit for peroxidase (Vector Laboratories, Burlingame, CA). Tissue sections were counterstained with hematoxylin. Semi-quantitative determination of Egr1 was performed. The proportion of positively stained cells was judged as follows: 0 = no cells were stained, 1 = 0% to 1% cells, 2 = 1% to 10% cells, 3 = 10% to 33% cells, 4 = 33% to 66% cells, and 5 = 66% to 100% cells. In addition to the proportion score, an intensity score was obtained based on the mean intensity of staining: 0 = negative, 1 = weakly positive, 2 = intermediately positive, and 3 = strongly positive. The intensity and the proportion scores were added to obtain a total score. The scoring values were analyzed and grouped as follows: 7 to 8, high Egr1 expression; 3 to 6, low Egr1 expression; and 0 to 2, negative Egr1 expression. Slides were blindly evaluated by two investigators independently, who were unaware of the pathologic characteristics.
Cell culture
Human prostate cancer cell line PC-3 was obtained from the Institution of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). Human prostate cancer LNCaP cells were provided by Prof. Klaus Jung (Department of Urology, University Hospital Charité, Humboldt University, Germany). All cell lines were maintained in RPMI-1640 medium (Gibco, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco) at 37°C in 5% CO2. Phenol-red free RPMI-1640 medium (Gibco) with 10% charcoal-stripped FBS (CS-FBS, Biological Industries, M.P. Ashrat, Israel) was used as the androgen deprivation condition.
Quantitative RT-PCR
Total RNA was extracted from the prostate cancer cells using Trizol (Invitrogen Life Technologies, Carlsbad, CA, USA), and reversely transcribed to cDNA using PrimeScriptTM Reagent Kit (Takara, Tokyo, Japan). One milligram cDNA was used as the template for quantitative PCR (SYBR Green, Takara), which was performed using the Lightcycler Detection System (Roche, Basel, Switzerland) with ΔΔCt method, according to the manufacturer’s instructions. The expression level of housekeeping gene GAPDH was used to normalize the Egr1 and Id1 mRNA expression. The PCR was set at 95°C (15 s) and 60°C (30 s) in a total of 40 cycles with a final extension step at 60°C for 5 min. The primers used in this study were as follows: 5’-TGA CCG CAG AGT CTT TTC CT-3’ and 5’-TGG GTT GGT CAT GCT CAC TA-3’ for Egr1; 5’-GTA AAC GTG CTG CTC TAC GAC ATG-3’ and 5’-AGC TCC AAC TGA AGG TCC CTG-3’ for Id1; and 5’-GAC AAC TTT GGC ATC GTG GA-3’ and 5’-ATG CAG GGA TGA TGT TCT GG-3’ for GAPDH. Values represent the mean ± SD from at least three independent experiments. Each experiment was performed in triplicates.
Western blot
Changes in the Egr1 expression in the LNCap and PC3 cells, as well as the AR expression in AR-transfected PC-3 cells, were detected by Western blot analysis. Cells were collected and lyzed in 100 μl M-PER Mammalian Protein Extraction Reagent (Pierce, Rockford, IL, USA) containing 3% Halt Phosphatase inhibitor Cocktail (Pierce). All samples were normalized according to the protein concentration, separated in 10% SDS-PAGE gel, and then transferred to polyvinylidene difluoride membranes (Gelman Pall Life. Sciences, Ann Arbor, MI USA) using the wet transfer blotting system (Bio-Rad, Hercules, CA, USA). After blocking in Tris-buffered saline (TBS: 120 mmol/l NaCl, 50 mmol/l Tris-HCl, pH 7.4) containing 0.05% Tween 20 and 1% BSA, the blot was probed with Egr1 polyclone antibodies (1:500, Santa Cruz, 588) or AR monoclonal antibodies (1:1000, Santa Cruz, sc-7305) and incubated overnight at 4°C. Subsequently, the membranes were washed extensively, incubated with horseradish peroxidase-conjugated secondary antibodies (1:2000, Cell Signaling Technology, Danvers, MA, USA) for 1.5 h at room temperature, and washed thrice with TBS containing 0.05% Tween-20 for enhanced chemiluminescence detection.
Plasmid transfection
PC-3 cells were seeded at a density of 2.5×105 cells/well into a six-well culture plate (Nalge Nunc, Rochester, NY, USA) with RPMI-1640 medium containing 10% FBS or phenol-red free RPMI-1640 medium containing 10% CS-FBS. After adherence, PC-3 cells were transfected with full-length human AR expression plasmid pSGAR2 packaged by Lipofectin 2000 as described [9]. Then, 48 h post-transfection, cells were collected and prepared for further Western blot and/or qPCR analysis.
Short interfering RNA (siRNA) preparation and transfection
The sequence of siRNA oligonucleotide-target Egr1 and the control oligonucleotides were as previously described [11] and synthesized by Shanghai Shenggong Co. LNCaP and PC3 cells were cultured 24 h before transfection, resulting in an 80% confluence of the cell monolayer. Specific Egr1 and control siRNA were transfected to the cells mixed with LipofectamineTM 2000 (Invitrogen) according to the manufacturer’s recommendation. After 6 h at 37°C, the medium was changed, and the cells were cultivated in RPMI-1640 supplemented with 10% CS-FBS. The expression inhibition rates of Egr1 in both cells were approximately 70% at mRNA levels (data not shown).
Statistical analysis
Variation in the results obtained between two groups was calculated by Student’s t-test. Variation in the results obtained from two different group tissue specimens were analyzed with Wilcoxon’s Signed Rank Sum test. SAS 9.1.3 (SAS Institute, Cary, NC, USA) was used to complete data analyses. All of the above hypothesis tests were two-sided and two-tailed. A P value of 0.05 or less was considered to indicate statistical significance.
Results
High Egr-1 expression was confirmed in AIPC patients
To understand the potential biological and clinical role of Egr1 protein in prostate cancer of ADPC and AIPC stages, the Egr1 protein expression level was detected in 10 ADPC patients and 5 AIPC patients. Immunohistochemistry showed that Egr1 protein in prostate cancer was located mainly in the cytoplasm, which was consistent with previous studies (Figure 1) [12,13]. High Egr1 protein levels was found in all AIPC specimens with the highest total scores of 8, whereas 9 of 10 cases in ADPC specimens exhibited low expression with total scores ranging from 4 to 6. Only one ADPC specimen was classified as a high expression group, having the total score of 7 (Table 2, P < 0.001). Thus, Egr1 was identified to be significantly more highly expressed in AIPC patients than in ADPC patients.
Figure 1.
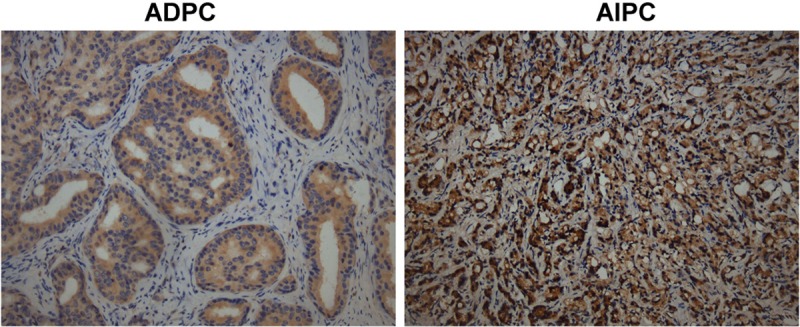
Egr1 was significantly upregulated in clinical AIPC samples. Egr1 expression was determined by immunohistochemistry in the representative paired slides of the prostate cancer in ADPC or AIPC groups, respectively (×200).
Table 2.
Egr1 expression in AIPC and ADPC patients
| Egr1 level | Total score | Distribution of included cases | |
|---|---|---|---|
|
| |||
| ADPC (10) | AIPC (5) | ||
| Negative | ≤2 | 0 | 0 |
| Low | 3 | 0 | 0 |
| 4 | 2 | 0 | |
| 5 | 6 | 0 | |
| 6 | 1 | 0 | |
| High | 7 | 1 | 0 |
| 8 | 0 | 5 | |
Footnotes: ADPC: androgen-dependent prostate cancer; AIPC: androgen-independent prostate cancer.
Androgen downregulates the Egr1 expression in prostate cancers in vitro
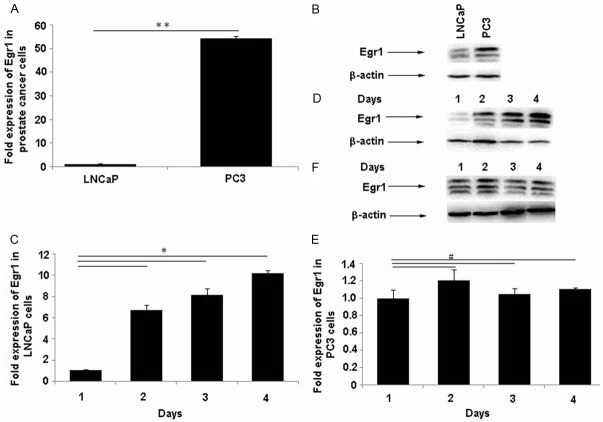
To further investigate the possible correlation of Egr1 expression with the formation of AIPC, we first determined Egr-1 expression in vitro in ADPC (LNCaP) and AIPC (PC-3) cells. Quantitative real-time-PCR analysis showed that Egr1 mRNA expression was higher in PC-3 cells than in LNCaP cells, which was consistent with the result of the Western blot (Figure 2A and 2B). Considering that these two cancer cell lines represent the difference in androgen dependence, the different Egr1 expression patterns might correlate with the biological function of androgen during the transition from ADPC to AIPC. To further elucidate how Egr1 expression was regulated after androgen deprivation in ADPC cells in vitro, LNCaP and PC-3 cells were cultured in phenol-red free RPMI-1640 medium containing 10% CS-FBS for 4 d, respectively, and Egr1 mRNA and protein level expression was measured. Egr1 mRNA and protein expression was gradually upregulated in LNCaP cells cultured in androgen deprivation medium (Figure 2C and 2D), whereas no significant difference was identified in the PC-3 cells (Figure 2E and 2F). These data suggest that Egr1 was downregulated by androgen, and the gradual upregulation of Egr1 in prostate cancer during ADT might contribute to the transition of prostate cancers from ADPC to AIPC.
Figure 2.
Egr1 was more highly expressed in PC3 cells and was evidently upregulated in LNCaP cells after ADT in vitro. LNCaP and PC3 cells were seeded and cultured in vitro in RPMI-1640 containing 10% FBS for 48 h, and were subsequently collected and detected for Egr1 mRNA (A) and protein (B) levels using qRT-PCR and Western blot. Egr1 was more highly expressed in PC3 than in LNCaP cells (**P < 0.001); (C to F) After incubation in phenol-red free RPMI-1640 containing 10% CS-FBS for 1 d to 4 d, LNCaP and PC3 cells were collected and these cells underwent qRT-PCR and Western blot analysis. Expression of Egr1 mRNA (C) and protein (D) were both gradually upregulated in LNCaP cells, whereas no significant difference was identified in PC-3 cells (E and F) (*P < 0.01, #P > 0.05).
AR mediated the downregulation of Egr1 by androgen
As shown above, no significant difference in Egr1 expression was found when AR-negative PC-3 cells were cultured in androgen-free conditions. This phenomenon indicates the possibility that the variation in Egr1 expression might be mediated by androgen through AR. DHT and AR antagonist flutamide (Sigma-Aldrich) were then used to probe the potential correlation between the Egr1 expression and androgen-AR pathway. One group of LNCaP cells were cultured for 2 d in phenol-red free RPMI-1640 medium containing 10% CS-FBS, followed by administration of different concentrations of DHT for 2 d. The other group of LNCaP cells was cultured for 4 d in RPMI-1640 medium containing 10% FBS medium after administration of different concentrations of flutamide. As shown in Figure 3A and 3B, DHT administration significantly downregulated Egr1 mRNA expression, whereas flutamide significantly upregulated its expression; both regulations were in a dose-dependent manner. As expected, these phenomena could not be repeated in AR-negative PC-3 cells (Figure 3C and 3D).
Figure 3.
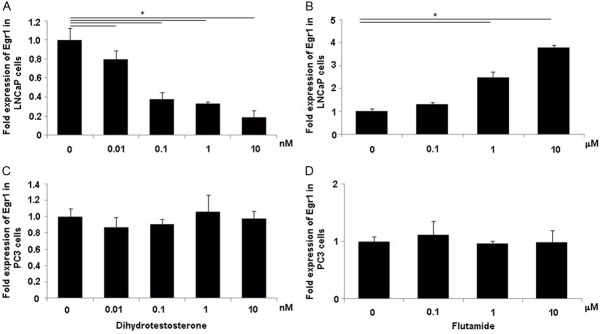
Androgenic regulation of Egr1 in LNCaP or PC-3 cells. LNCaP (A) and PC-3 (C) cells were seeded in six-well culture dishes and incubated in phenol-red free RPMI-1640 medium containing 10% CS-FBS for 48 h. The cells were then treated with indicated concentrations of dihydrotestosterone for another 48 h. LNCaP (B) and PC-3 (D) cells were seeded in six-well culture dishes, incubated in RPMI-1640 medium containing 10% FBS and the indicated concentrations of flutamide for 4 d. After incubation, total RNA of all these cells was isolated and subjected to qRT-PCR analysis. All data have been normalized to GAPDH RNA and plotted relative to the Egr1 expression levels in LNCaP cells incubated in medium without drugs (*P < 0.01). Egr1 was down-regulated by DHT and up-regulated by flutamide in LNCaP cells, but was not affected by the two drugs in PC3 cells.
To further confirm whether AR mediates the regulation of Egr1 expression, AR-negative PC-3 cells were transfected with AR expression plasmid pSGAR2 containing EGFP expression sequence to investigate the effect of DHT and flutamide on Egr1 mRNA expression during the course of androgen deprivation treatment. AR expression in PC-3 cells after transfection was confirmed by highly coexpressed EGFP (Figure 4A) and by specific AR protein bands using Western blot analysis, compared with control cells transfected with empty plasmid (Figure 4B). The group of PC-3 cells transfected in phenol-red free RPMI-1640 medium containing 10% CS-FBS was administered with different DHT concentrations for 2 d. The other group of cells transfected in RPMI-1640 medium containing 10% FBS were administered with different flutamide concentrations for 4 d. Similar to the LNCaP cells, Egr1 mRNA expression was downregulated by DHT (Figure 4C) and upregulated by flutamide (Figure 4D). These data confirmed that the upregulation of Egr1 expression in prostate cancer cells after androgen deprivation treatment was mediated by AR.
Figure 4.
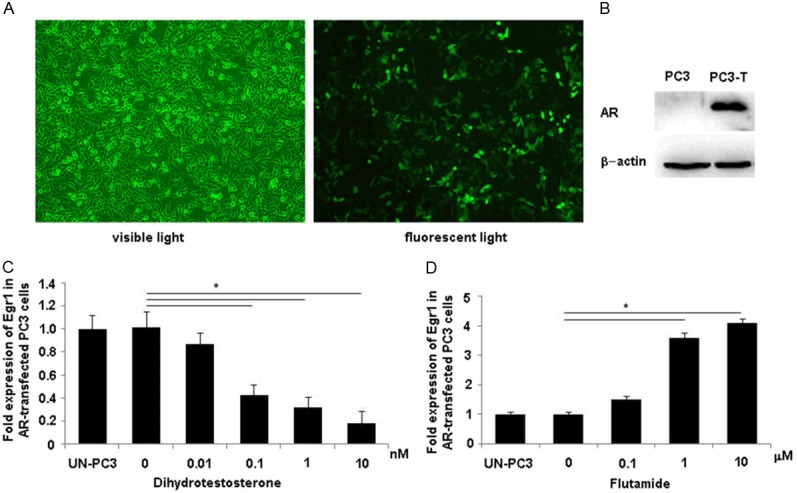
Egr1 was negatively regulated by AR. PC-3 cells were transiently transfected with AR expression plasmid pSGAR2 containing EGFP expression sequence, and 48 h later the cells were detected for EGFP expression using fluorescence microscopy. Most of the transfected cells expressed EGFP, suggesting high AR expression (A); (B) The transfected PC3 cells were collected and analyzed for the AR protein expression using Western blot assay, and a specific AR protein band was detected as compared with the empty plasmid control. PC-3-T: plasmid pSGAR2 transfected PC-3 cells. (C) PC-3 cells were transiently transfected with plasmid pSGAR2 and cultured in phenol-red free RPMI-1640 medium containing 10% CS-FBS for 48 h. Then, the cells were treated with different DHT concentrations for another 48 h before the cell lysates were prepared and measured using qRT-PCR. (D) PC3 cells were transiently transfected in RPMI-1640 medium containing 10% FBS for 48 h. Then, the cells were treated with different flutamide concentrations for another 4 d before mRNA levels were measured using qRT-PCR. All data were normalized to GAPDH RNA and plotted relative to the Egr1 expression levels in PC-3 cells transfected with empty plasmid (*P < 0.01).
Id1 is regulated by Egr1 in prostate cancer cells
Id1 was also found to be negatively regulated by androgen through AR [9], and the transcription of Id1 was at least partially regulated by a protein complex containing the Egr1 [10]. To investigate whether the negative regulation of Id1 by androgen was mediated by Egr1 in prostate cancer cells, we used Egr1 agonist bombesin to upregulate Egr1 and investigate the Id1 mRNA expression in prostate cancer cells. LNCaP and PC3 cells were both cultured in phenol-red free RPMI-1640 medium containing 10% CS-FBS for 2 d, and were administered with bombesin for 3 d. As shown in Figure 5, bombesin administration significantly upregulated Egr1 expression in both LNCaP and PC3 cells (Figure 5A and 5B), and simultaneously upregulated Id1 mRNA expression in a dose-dependent manner (Figure 5C and 5D).
Figure 5.
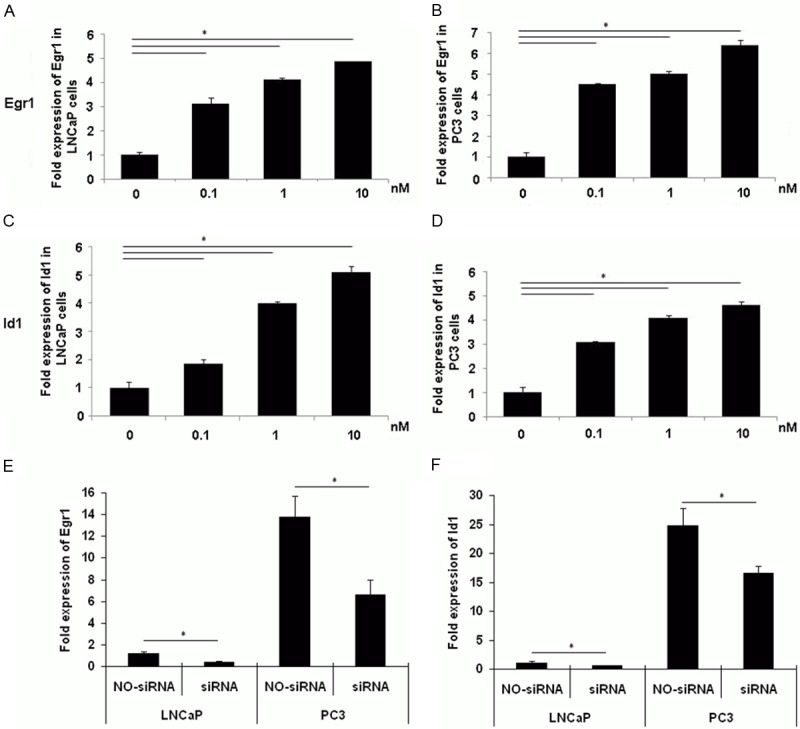
Egr1 directly regulates the Id1 expression in prostate cancer. LNCaP and PC3 cells were first incubated in phenol-red free RPMI-1640 medium containing 10% FBS for 48 h. The cells were then treated with indicated concentrations of bombesin for another 72 h before mRNA levels were measured using qRT-PCR. Bombesin induced a dose-dependent upregulation of Egr1 both in LNCaP (A) and PC3 (B) cells. Id1 was also upregulated in the same pattern in LNCaP (C) and PC3 (D) cells. When specific siRNA targeting at Egr1, or the control siRNA were used before 1 nM of bombesin was supplemented, the upregulation of Id1 became withdrawn following the silencing of Egr1 in both cells (E, F, *P < 0.01).
To further confirm the direct regulation of Egr1 on Id1, we used siRNA to silence Egr1 and investigated the Id1 changes in LNCaP and PC3 cells. LNCaP and PC3 cells were both transfected with specific or with control siRNA cultured in phenol-red free RPMI-1640 medium containing 10% CS-FBS for 2 d and were administered with 1 nM bombesin for 3 d. Cells were then collected, and Egr1 and Id1 mRNA expression was quantified using qRT-PCR. As shown in Figure 5E and 5F, Egr1 expression was downregulated significantly after transfection with specific Egr1 siRNA in both LNCaP and PC3 cells and not very surprisingly, Id1 expression was also downregulated in the experiment groups. Therefore, Egr1 might have participated in the AIPC formation through the Id1 regulation during ADT in patients with prostate cancers.
Discussion
ADT has been used to shrink androgen-dependent tumors for many years. However, prolonged androgen deprivation often results in relapse and AIPC, which progresses and undergoes metastasis. The evolution of prostate cancer from an androgen-dependent state to AIPC is always a lethal progression. The AR is essential in both, although its function in androgen-independent cancers is poorly understood [14]. Developing diagnostic and therapeutic approaches that target AIPC, therefore, has significant potential for improving survival and quality of life of prostate cancer patients [15]. Earlier research performed to understand hormone-refractory prostate cancer focused on molecular alterations, such as AR overexpression or mutations, which could allow AR to respond to low levels of androgens or be directly activated by other ligands. More recently, AR has been suggested to be indirectly activated by cell-surface receptors, such as HER2, interleukin-6 receptor, and G-protein-coupled receptors [16]. Despite awareness on the important function of AR signaling in prostate cancer, the exact effect of ADT on AR signaling has not been well characterized [17]. In this study, we expanded our understanding of Egr1 expression regulation in prostate cancer after androgen deprivation and have provided new insights into the importance of the Egr1 action in the Id1 regulation by androgen through AR.
Egr1 is a prototypic member of the zinc finger transcription factor family that also includes Egr2, Egr3, and Egr4. These four proteins have a high degree of homology at their DNA-binding zinc finger domains, but they have divergent sequences outside the DNA-binding domains. In normal tissues, Egr1 expression is generally low or undetectable. In response to growth factors and cytokine signaling, Egr1 regulates cell growth, differentiation, and apoptosis. Aberrant Egr1 expression is linked to human diseases, such as ischemic injury, cancers, inflammation, atherosclerosis, and cardiovascular pathogenesis [17]. The function of Egr1 in cancer pathogenesis is complex, because it functions both in cell proliferation and apoptosis [18]. In several types of human tumor cells, Egr1 exhibits prominent suppressor gene activity by binding to and direct transactivation of major tumor suppressor factors, including transforming growth factor-β1, p53, p73, fibronectin, and PTEN. The downstream pathways of these factors display multiple nodes of interaction with each other. This condition suggests the existence of a functional network of suppressor factors that serve to maintain normal growth regulation and resist the emergence of transformed variants. Suppression of Egr1 expression is common in non-small cell lung cancers, breast cancer, glioblastomas, and acute myelogenous leukemia [18-20]. Paradoxically, increased Egr1 expression is consistently observed in epithelial ovarian and prostate cancers, where growing evidence indicates that Egr1 is oncogenic [13,21,22]. The basis of the oncogenic function of Egr1 in prostate cancer is not well known. Egr1 has been suggested to directly regulate genes that function in the development of prostate cancer, including IGF-II, TGF-β1, and PDGF-A, which were previously implicated in enhancing tumor progression [7]. In mouse prostate cancer cells, Egr1 was found to promote cell growth, cell cycle progression, and increased cell resistance to apoptotic signals by upregulating cyclin D2, inhibiting P19ink4d, and FAS expression [23]. Insufficient Egr1 significantly impaired tumor progression but did not affect the tumor initiation and tumor growth rate in prostate cancers [24]. Egr1 is also upregulated in structurally intact adjacent prostate tissues and defines the field cancerization. Field cancerization at prostatectomy specimen surgical margins may be a predictor of cancer recurrence [25]. More recently, Yang et al. showed that Egr1 expression enhanced the androgen-independent growth of prostate carcinoma cells both in vitro and in vivo. Egr1 partially mediated these effects through the AR signaling pathway [26]. However, how Egr1 was regulated after androgen deprivation in prostate cancer and whether the elevated Egr1 expression contributes to the transition of ADPC to AIPC (or CRPC) remains unknown. Whether Id1 is involved and regulated by Egr1 during the transformation is also undetermined.
To the best of our knowledge, we were the first to identify that Egr1 was significantly more highly expressed in well-characterized AIPC patients than in ADPC patients. This result, together with the aforementioned previous research, identified the possibility that Egr1 is involved in the transition from ADPC to AIPC during ADT in prostate cancers. Further in vitro investigation of Egr1 expression in ADPC (LNCaP) and AIPC (PC-3) cells confirmed the results obtained from clinical samples. The gradual upregulation of Egr1 in androgen-dependent LNCaP cells during ADT, and downregulation after retreatment with DHT, suggested that Egr1 was negatively regulated by androgen in prostate cancers. AR antagonist and agonist experiments, especially the androgen-dependent variation of Egr1 in PC-3 cells before and after the knock in of AR, further confirmed that Egr1 was negatively mediated by the androgen-AR pathway. Upregulation of Egr1 in ADPC cells after prolonged androgen deprivation in vitro and the relatively high expression of Egr1 in both AIPC model system and in clinical cases suggest that Egr1 might have an essential function during the transition of prostate cancers from ADPC to AIPC.
Helix-loop-helix (HLH) proteins are transcriptional factors that control a variety of developmental pathways, including hematopoiesis, myogenesis, neurogenesis, and mammary gland development [5]. The Id family proteins, including Id1, Id2, Id3, and Id4, belong to the family of HLH proteins and function as dominant negative regulators of basic HLH transcriptional factors. Of all the members of the Id protein family, Id1 is the one mostly linked to tumorigenesis, moreover regulating cellular senescence as well as cell proliferation and survival. Id1 is overexpressed in prostate, lung, breast, gastric, esophageal, and cervical cancers; especially in the poorly- differentiated cancers [27]. The intensity of Id1 staining in carcinomas was significantly stronger than that in the normal prostate and benign prostatic hyperplasia, and it was significantly increased with increasing malignancy of carcinomas, as measured by the Gleason score [28]. Overexpression of Id1 was observed in multidrug resistant cells in prostate cancer [29] and could result in an androgen-independent phenotype of prostate cancer cells (LNCaP) [30]. We have examined the potential role of Id1 in the malignant progression of prostate cancer and have shown that Id1 is significantly overexpressed in all prostate cancer cell lines and clinical specimens examined. The observed upregulation during ADT both in vivo and in vitro indicated that Id1 might have an important function in the transition from ADPC to AIPC. Meanwhile, Id1 expression in ADPC was negatively regulated by androgen in a receptor-dependent way [9]. However, the potential molecular mechanism of Id1 negative regulation by AR is still poorly understood. Previous studies have found a 210-bp enhancer element containing a consensus Egr1 binding site in the upstream region of the Id1 gene. The regulation of the Id1 response to serum has been suggested to be mediated in part by the Egr1 early response gene in muscle cells and fibroblasts [9]. In fibroblast growth factor-2 stimulated human neuroblastoma SK-N-MC cell line, Egr1 was found to directly bind to the Id1 promoter [31]. To further confirm whether Egr1 is a key element in the regulation of Id1 by AR signal during ADT in our experiment system, we investigated the Id1 variation after the Egr1 pathway was activated or inactivated in vitro. Here, we demonstrated that upregulation of Egr1 with bombesin, an agonist of Egr1, was accompanied by a significant upregulation of Id1 in both LNCaP and PC3 cells. Moreover, silencing Egr1 with specific siRNA also down-regulated the Id1 expression. Therefore, this study is the first to confirm that Egr1 was involved in the negative regulation of androgen on Id1 through AR in prostate cancers.
In summary, our data suggest that Egr1 might at least partially contribute to the negative regulation of AR on Id1 and participate in the formation of AIPC (or CRPC). The present study further expands our understanding of the regulation of Id-1 expression after androgen deprivation in prostate cancer. Additionally, new insights into the action of AR in the regulation of Id1 and Egr1 by androgen are provided. Further studies are needed to clarify the underlying mechanism of the AR signaling pathway in AIPC and to identify the cross-talk between Egr1, Id1, and AR, which may finally lead to new strategies to neutralize aggressive androgen-independent progression of prostate cancer.
Acknowledgements
This work was supported by a National Natural Science Foundation Research Grant, China (No: 30901502), a Grant from Shanghai’s Education Committee Chenguang Fund (No: 2010CG039), and PLA Medical Science Youth Training Project (13QNP092).
Disclosure of conflict of interest
None.
References
- 1.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–864. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Dreicer R, Gleave M, Kibel AS, Thrasher JB, Moul JW. Targeting the androgen receptor--theory and practice. Urology. 2011;78:S482–484. doi: 10.1016/j.urology.2011.05.052. [DOI] [PubMed] [Google Scholar]
- 4.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, Asangani IA, Ateeq B, Chun SY, Siddiqui J, Sam L, Anstett M, Mehra R, Prensner JR, Palanisamy N, Ryslik GA, Vandin F, Raphael BJ, Kunju LP, Rhodes DR, Pienta KJ, Chinnaiyan AM, Tomlins SA. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HJ, Hong JM, Yoon KA, Kim N, Cho DW, Choi JY, Lee IK, Kim SY. Early growth response 2 negatively modulates osteoclast differentiation through upregulation of Id helix-loop-helix proteins. Bone. 2012;51:643–650. doi: 10.1016/j.bone.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Abdulkadir SA, Carbone JM, Naughton CK, Humphrey PA, Catalona WJ, Milbrandt J. Frequent and early loss of the EGR1 corepressor NAB2 in human prostate carcinoma. Hum Pathol. 2001;32:935–939. doi: 10.1053/hupa.2001.27102. [DOI] [PubMed] [Google Scholar]
- 7.Svaren J, Ehrig T, Abdulkadir SA, Ehrengruber MU, Watson MA, Milbrandt J. EGR1 target genes in prostate carcinoma cells identified by microarray analysis. J Biol Chem. 2000;275:38524–38531. doi: 10.1074/jbc.M005220200. [DOI] [PubMed] [Google Scholar]
- 8.Zheng L, Pu J, Jiang G, Weng M, He J, Mei H, Hou X, Tong Q. Abnormal expression of early growth response 1 in gastric cancer: association with tumor invasion, metastasis and heparanase transcription. Pathol Int. 2010;60:268–277. doi: 10.1111/j.1440-1827.2010.02512.x. [DOI] [PubMed] [Google Scholar]
- 9.Xu B, Sun Y, Tang G, Xu C, Wang L, Zhang Y, Ji J. Id-1 expression in androgen-dependent prostate cancer is negatively regulated by androgen through androgen receptor. Cancer Lett. 2009;278:220–229. doi: 10.1016/j.canlet.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Tournay O, Benezra R. Transcription of the dominant-negative helix-loop-helix protein Id1 is regulated by a protein complex containing the immediate-early response gene Egr-1. Mol Cell Biol. 1996;16:2418–2430. doi: 10.1128/mcb.16.5.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parra E, Ortega A, Saenz L. Down-regulation of Egr-1 by siRNA inhibits growth of human prostate carcinoma cell line PC-3. Oncol Rep. 2009;22:1513–1518. [PubMed] [Google Scholar]
- 12.Mora GR, Olivier KR, Cheville JC, Mitchell RF Jr, Lingle WL, Tindall DJ. The cytoskeleton differentially localizes the early growth response gene-1 protein in cancer and benign cells of the prostate. Mol Cancer Res. 2004;2:115–128. [PubMed] [Google Scholar]
- 13.Eid MA, Kumar MV, Iczkowski KA, Bostwick DG, Tindall DJ. Expression of early growth response genes in human prostate cancer. Cancer Res. 1998;58:2461–2468. [PubMed] [Google Scholar]
- 14.Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, Chen Z, Beroukhim R, Wang H, Lupien M, Wu T, Regan MM, Meyer CA, Carroll JS, Manrai AK, Janne OA, Balk SP, Mehra R, Han B, Chinnaiyan AM, Rubin MA, True L, Fiorentino M, Fiore C, Loda M, Kantoff PW, Liu XS, Brown M. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw G, Price AM, Ktori E, Bisson I, Purkis PE, McFaul S, Oliver RT, Prowse DM. Hedgehog signalling in androgen independent prostate cancer. Eur Urol. 2008;54:1333–1343. doi: 10.1016/j.eururo.2008.01.070. [DOI] [PubMed] [Google Scholar]
- 16.Wolff DW, Xie Y, Deng C, Gatalica Z, Yang M, Wang B, Wang J, Lin MF, Abel PW, Tu Y. Epigenetic repression of regulator of G-protein signaling 2 promotes androgen-independent prostate cancer cell growth. Int J Cancer. 2012;130:1521–1531. doi: 10.1002/ijc.26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharyya S, Fang F, Tourtellotte W, Varga J. Egr-1: new conductor for the tissue repair orchestra directs harmony (regeneration) or cacophony (fibrosis) J Pathol. 2013;229:286–297. doi: 10.1002/path.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang HS, Ock J, Lee HJ, Lee YJ, Kwon BM, Hong SH. Early growth response protein 1 upregulation and nuclear translocation by 2’-benzoyloxycinnamaldehyde induces prostate cancer cell death. Cancer Lett. 2013;329:217–227. doi: 10.1016/j.canlet.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Arora S, Wang Y, Jia Z, Vardar-Sengul S, Munawar A, Doctor KS, Birrer M, McClelland M, Adamson E, Mercola D. Egr1 regulates the coordinated expression of numerous EGF receptor target genes as identified by ChIP-on-chip. Genome Biol. 2008;9:R166. doi: 10.1186/gb-2008-9-11-r166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baron V, Adamson ED, Calogero A, Ragona G, Mercola D. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther. 2006;13:115–124. doi: 10.1038/sj.cgt.7700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kataoka F, Tsuda H, Arao T, Nishimura S, Tanaka H, Nomura H, Chiyoda T, Hirasawa A, Akahane T, Nishio H, Nishio K, Aoki D. EGRI and FOSB gene expressions in cancer stroma are independent prognostic indicators for epithelial ovarian cancer receiving standard therapy. Genes Chromosomes Cancer. 2012;51:300–312. doi: 10.1002/gcc.21916. [DOI] [PubMed] [Google Scholar]
- 22.Gregg J, Fraizer G. Transcriptional Regulation of EGR1 by EGF and the ERK Signaling Pathway in Prostate Cancer Cells. Genes Cancer. 2011;2:900–909. doi: 10.1177/1947601911431885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virolle T, Krones-Herzig A, Baron V, De Gregorio G, Adamson ED, Mercola D. Egr1 promotes growth and survival of prostate cancer cells. Identification of novel Egr1 target genes. J Biol Chem. 2003;278:11802–11810. doi: 10.1074/jbc.M210279200. [DOI] [PubMed] [Google Scholar]
- 24.Abdulkadir SA, Qu Z, Garabedian E, Song SK, Peters TJ, Svaren J, Carbone JM, Naughton CK, Catalona WJ, Ackerman JJ, Gordon JI, Humphrey PA, Milbrandt J. Impaired prostate tumorigenesis in Egr1-deficient mice. Nat Med. 2001;7:101–107. doi: 10.1038/83231. [DOI] [PubMed] [Google Scholar]
- 25.Jones AC, Trujillo KA, Phillips GK, Fleet TM, Murton JK, Severns V, Shah SK, Davis MS, Smith AY, Griffith JK, Fischer EG, Bisoffi M. Early growth response 1 and fatty acid synthase expression is altered in tumor adjacent prostate tissue and indicates field cancerization. Prostate. 2012;72:1159–1170. doi: 10.1002/pros.22465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang SZ, Eltoum IA, Abdulkadir SA. Enhanced EGR1 activity promotes the growth of prostate cancer cells in an androgen-depleted environment. J Cell Biochem. 2006;97:1292–1299. doi: 10.1002/jcb.20736. [DOI] [PubMed] [Google Scholar]
- 27.Cheng YJ, Tsai JW, Hsieh KC, Yang YC, Chen YJ, Huang MS, Yuan SS. Id1 promotes lung cancer cell proliferation and tumor growth through Akt-related pathway. Cancer Lett. 2011;307:191–199. doi: 10.1016/j.canlet.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Forootan SS, Wong YC, Dodson A, Wang X, Lin K, Smith PH, Foster CS, Ke Y. Increased Id-1 expression is significantly associated with poor survival of patients with prostate cancer. Hum Pathol. 2007;38:1321–1329. doi: 10.1016/j.humpath.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Lin JC, Chang SY, Hsieh DS, Lee CF, Yu DS. Modulation of mitogen-activated protein kinase cascades by differentiation-1 protein: acquired drug resistance of hormone independent prostate cancer cells. J Urol. 2005;174:2022–2026. doi: 10.1097/01.ju.0000176476.14572.39. [DOI] [PubMed] [Google Scholar]
- 30.Ling MT, Wang X, Lee DT, Tam PC, Tsao SW, Wong YC. Id-1 expression induces androgen-independent prostate cancer cell growth through activation of epidermal growth factor receptor (EGF-R) Carcinogenesis. 2004;25:517–525. doi: 10.1093/carcin/bgh047. [DOI] [PubMed] [Google Scholar]
- 31.Passiatore G, Gentilella A, Rom S, Pacifici M, Bergonzini V, Peruzzi F. Induction of Id-1 by FGF-2 involves activity of EGR-1 and sensitizes neuroblastoma cells to cell death. J Cell Physiol. 2011;226:1763–1770. doi: 10.1002/jcp.22505. [DOI] [PMC free article] [PubMed] [Google Scholar]