Abstract
We comparatively analyzed the difference between three-dimensional arterial spin labeling (3D-ASL) and the conventional dynamic susceptibility contrast (DSC) perfusion imaging in the setting of assessing brain tumor perfusion in 28 patients with proved brain tumors. All patients were scheduled with standard MRI, 3D-ASL and DSC scannings on a GE DISCOVERY MR 750 system. Maximal relative tumor perfusion was obtained based on the region of interest (ROI) method. A close correlation between 3D-ASL and DSC perfusion imaging was noted as manifested by the absence of significant differences between ASL nTBF and DSC nTBF when normalized to M (mirror region) and GM (contralateral gray matter). However, ASL nTBF was found to be highly correlated with DSC nTBF and DSC nTBV when normalized to M, GM and WM (contralateral normal white matter). Together, our data support that 3D-ASL possesses the potential to be a noninvasive alternate for DSC-MRI in assessing brain tumor perfusion in the setting of treatment prognosis and metastasis, particularly for those patients with renal failure and patients required for collection of follow up information.
Keywords: Arterial spin labeling, dynamic susceptibility contrast perfusion imaging, brain tumor, magnetic resonance perfusion imaging, correlation
Introduction
It has been well recognized that angiogenesis plays an essential role in tumor growth, invasion and metastasis [1], while magnetic resonance perfusion imaging serves as a novel tool to assess angiogenesis and capillary permeability, which provides the feasibility for grading diagnosis and therapeutic follow-up of brain tumors. Currently, two major types of perfusion imaging methods are available to evaluate brain tumor perfusion. One depends on the exogenous tracers such as dynamic susceptibility contrast (DSC) perfusion imaging, which allows simultaneous measurement of cerebral blood flow (CBF) and cerebral blood volume (CBV), while the other relies on the endogenous tracers such as water molecules in arterial blood applied in arterial spin labeling (ASL) [2].
During the process for arterial spin labeling perfusion imaging, water is used as a freely diffusible intrinsic tracer, while arterial blood outside the imaging section is labeled by an inversion pulse. After a transit time from the labeling region to the imaging section, blood spins exchange with tissue water at the capillaries. Subtraction of a control image without prior labeling leaves the transported magnetization control image without prior labeling only, which results in a perfusion-weighted image, and CBF can be next quantified by using the general kinetic model. The major advantages of ASL are its noninvasiveness (lack of contrast agent requirement), less scanning duration, higher SNR, and potential for CBF quantification. Furthermore, it can be repeated in patients with failed control of sedation or motion. As a result, ASL has been used to assess cerebral perfusion in patients with acute ischemic stroke [3], Alzheimer disease [4], and posterior visual pathway diseases [5]. Altogether, these data suggest that ASL could be feasible for assessing cerebral blood perfusion in patients with brain tumors. Indeed, there is suggestive evidence that ASL could be an alternative for DSC perfusion imaging in assessing perfusion in brain tumors [1,6-10]. We, thus, in the present report, conducted studies to evaluate the potential application of three-dimensional ASL in quantitative CBF measurements of brain tumors.
Materials and methods
Patient population
A total of 34 patients with brain tumors were enrolled in this prospective study. The following inclusion criteria were applied: 1) the patient underwent both 3D-ASL and DSC examinations at the same 3T scanner; 2) the patient had no previous history of surgical resection, biopsy, or treatment of the tumor; and 3) the size for the tumor solid component (except for cyst, necrosis, calcification or hemorrhage) was greater than 3 cm in its shortest diameter, which was subsequently undergone resection or biopsy. Patients with motion artifacts or susceptibility artifacts that degrade ASL imaging, as well as those with underlying heart disease, hypertension, or vasculopathy which could alter ASL perfusion were excluded. Six patients were excluded because of severe susceptibility or motion artifacts. Therefore, 28 patients (15 female and 13 male patients with age from 5 to 67 years old) were selected for the study, which include 15 meningiomas, 6 low-grade astrocytomas (WHO grade I and II), 4 high-grade astrocytomas (WHO grade III and IV) and 3 metastases pathologically. The study was approved by the Tongji Hospital Ethnic Review Board, and informed consents were obtained from all study subjects.
Imaging protocol
All MR examinations were performed at 3T MR imaging (Discovery 750, GE Healthcare, Milwaukee, Wisconsin) with a 32-channel phased array head coil. Vacuum cushions were put into the coil to hold the patient’s head still and reduce the noise in some way. The MR imaging protocols included axial T1-weighted imaging, T2-weighted imaging, 3D-ASL, DSC perfusion imaging and contrast-enhanced (CE) T1WI. Three-D ASL was performed by use of a pseudocontinuous labeling period of 1500 ms with a post labeling delay time of 1525 ms. Whole-brain images were obtained with an interleaved 3D stack of spirals fast spin echo (FSE) sequence and background suppression. Multiarm spiral imaging was used, with 8 arms and 1024 points acquired on each arm. A high level of background suppression was achieved by use of 4 separate inversion pulses spaced around the pseudocontinuous labeling pulse. The entire process took 4 min 38 seconds to complete which included proton attenuation.
All scans were performed in the axial plane. For accurate graphic prescription of 3D ASL, the sagittal image was obtained following the 3-plane localizer. Other 3D ASL parameters were as follows: 34 axial slices; TR 4787 ms; TE 14.6 ms; slice thickness 4.0 mm; FOV 24×24 cm; Matrix 1024×8; NEX 3 and bandwidth 62.5 kHz. DSC-MRI employed gradient echo echo-planar imaging (GRE-EPI) sequence and the imaging parameters were: 20 axial slices; TR 1500 ms; TE minimum; FOV 24×24 cm; slice thickness 5 mm; spacing 1.5 mm; FOV 24 cm×24 cm; Matrix 128×96; Flip Angle 60; NEX 1; bandwidth 62.5 kHz and acquisition time 1 min 20 seconds. DSC-MRI was performed during the administration of a standard 0.2 mmol/kg dose of a Gd-based contrast agent (Magnevist, Bayer Schering Pharma AG, Berlin, Germany) at a rate of 3 ml/s with an MRI compatible power injector, followed by a flush of 20 mL saline injected at the same rate.
Imaging processing and analysis
Data postprocessing was performed on the 4.5 workstation, while 3D ASL and Brainstat softwares were used for 3D ASL and DSC-MRI image analysis, respectively. ROIs (Regions of interest) were located in the maximum signal enhancement of the tumor (T) (at least 3 ROIs, showing the highest color levels of the solid portion of a tumor on semi-automatically obtained CBV and CBF maps and the values were averaged) and their mirror regions (M). ROI of the contralateral normal white matter (WM) was located at posterior limb of internal capsule, whereas ROI of the contralateral gray matter (GM) was located at the frontal cortex. Standard MR imaging including T2WI and CE-T1WI was used to cross-reference solid portions of the tumor to the 3D-ASL and DSC-MRI tumor blood flow maps. Effort was made to localize ROI on ASL CBF and on DSC-MRI maps (rCBF and rCBV) at the same position. Areas of cyst, necrosis, calcification, hemorrhage and large vessels were avoided. A board-certified experienced neuroradiologist (6 years of experience) with a Certificate of Added Qualification blinded to clinical and pathologic data selected ROIs. Another blinded board-certified neuroradiologist with a Certificate of Added Qualification (20 years of experience) independently confirmed the appropriate ROI placement.
To correct for age-dependent and patient-dependent variations of mean cerebral perfusion [11], maximal tumor blood flow was normalized to a 150-mm2 ROI in the contralateral gray matter to produce the maximal relative tumor blood flow (rTBF) [12]. Previous studies in tumor perfusion have used various brain regions as the internal references, including contralateral white matter [13,14], gray matter [15-18], or the cerebellum [10]. In the present study, we used all three above mentioned regions as the reference regions to reach the best normalization.
A quantitative analysis was performed with mean value of ROI for T divided by the mean value of reference ROIs for M (or WM, GM). Values for normalized tumor blood flow (nTBF) [14] were thus estimated based on ASL CBF (ASL nTBF) and DSC rCBF maps (DSC nTBF). Values for normalized tumor blood volume (DSC nTBV) were produced by the corresponding procedure on DSC rCBV maps.
Statistical analysis
Paired t test was applied to compare ASL and DSC measurements. Linear regression and Pearson’s correlation were employed to evaluate the correlations among quantitative results. Statistical analyses were carried out using software SPSS for Windows (version 17.0, SPSS, Chicago, Ill), and in all cases, p < 0.05 was considered with statistical significance.
Results
Quantitative perfusion measurement between ASL and DSC
We first sought to demonstrate the values normalized to M, WM and GM for tumor blood flow and blood volume using ASL (ASL nTBF) and DSC perfusion imaging (DSC nTBF and DSC nTBV), respectively. Comparative studies were next conducted, and we failed to observe a statistical difference between ASL nTBF and DSC nTBF when the mirror and the contralateral normal gray matter regions were used as the reference standards (Table 1). Perfusion examples for different types of brain tumors are shown in Figures 1, 2, 3 and 4.
Table 1.
Quantitative perfusion analysis between ASL and DSC
| Reference Region | ASL nTBF | DSC nTBF | DSC nTBV |
|---|---|---|---|
| M | 2.36 ± 1.27**,o | 2.64 ± 1.50Δ,o | 2.98 ± 1.77Δ,** |
| GM | 1.83 ± 1.00o | 1.60 ± 0.865Δ,o | 1.92 ± 1.210Δ,o |
| WM | 2.67 ± 1.48*,** | 4.82 ± 2.51*,Δ | 5.70 ± 3.33Δ,** |
statistical difference between ASL nTBF and DSC nTBF;
statistical difference between DSC nTBF and DSC nTBV;
significant difference between ASL nTBF and DSC nTBV;
significant difference between ASL nTBF and DSC nTBF or between DSC nTBF and DSC nTBV.
Figure 1.
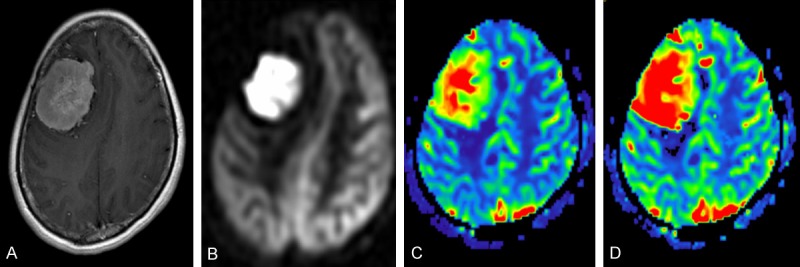
A 45-year-old woman with meningioma in the right frontal region. CE-T1WI (A), ASL perfusion map (B), DSC rCBF map (C), and DSC rCBV map (D) demonstrated that meningioma was homogeneously hyperperfused on both ASL perfusion map and DSC maps.
Figure 2.
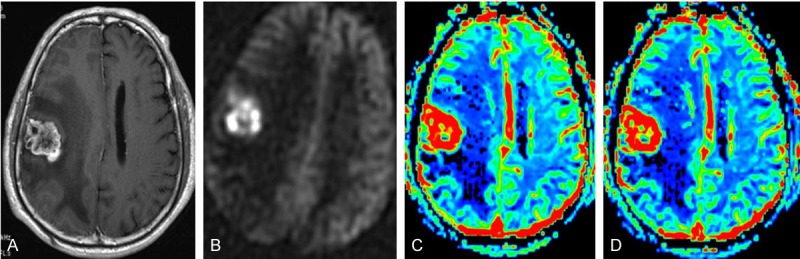
A 56-year-old man with tumor metastasis (primary lesion was renal clear cell carcinoma) in the right frontal lobe. CE-T1WI (A), ASL perfusion map (B), DSC rCBF map (C), and DSC rCBV map (D) demonstrated that the lesion was mainly hyperperfused, but hypoperfusion was noted in necrotic region in the central on both ASL perfusion map and DSC maps.
Figure 3.
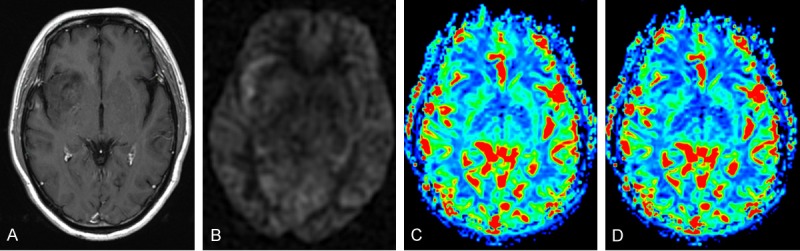
Grade II astrocytoma in a 55-year-old man located in the right insular lobe. CE-T1WI (A), ASL perfusion map (B), DSC rCBF map (C), and DSC rCBV map (D) showed that the lesion was predominantly hypoperfused with linear hyperfusion near the right sylvian fissure on the ASL perfusion map, and was homogeneously hypoperfused on DSC maps.
Figure 4.
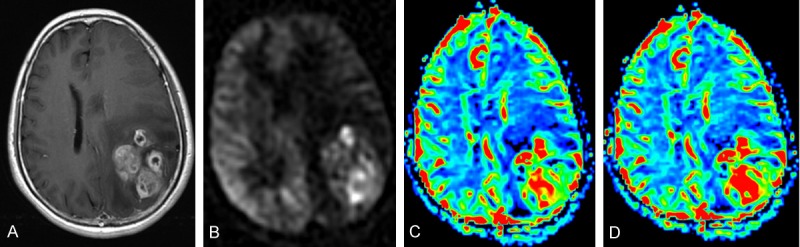
Grade IV astrocytoma in a 60-year-old woman located in the left parietal lobe. CE-T1WI (A), ASL perfusion map (B), DSC rCBF map (C), and DSC rCBV map (D) indicated a hyperperfused mass with hypoperfused necrosis and peripheral edema.
Correlations between ASL and DSC quantitative measurements
The relationship between the mean ASL nTBF and DSC nTBF values in all cases is shown in Figure 5. A highly significant correlation between ASL nTBF and DSC nTBF was noted by Pearson’s correlation analysis when all selected three regions (M, GM, WM) were used as the reference standards. Particularly, when the mirror region was employed as a reference, much higher correlation coefficient (R=0.807) was obtained as compared with that of other two reference regions [R=0.737 (GM) and 0.729 (WM), respectively].
Figure 5.
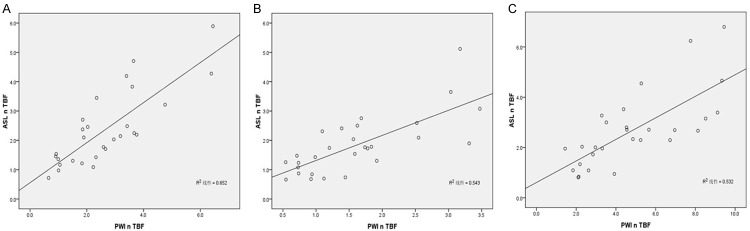
Scatter diagram of ASL nTBF and DSC nTBF values normalized to M (A), GM (B) and WM (C). Significant correlations (R=0.807, 0.737 and 0.729, respectively) were noted between ASL nTBF and DSC nTBF.
Similarly, Pearson’s correlation analysis was employed to demonstrate the relationship between the mean ASL nTBF and DSC nTBV values in all cases. A significant correlation was detected when all three selected regions (M, GM, WM) were used as the reference standards. However, when the mirror region was used as a reference, the correlation coefficient was much more significantly (R=0.780) than that of GM (R=0.621) or WM region (R=0.616) as the reference standards (Figure 6).
Figure 6.
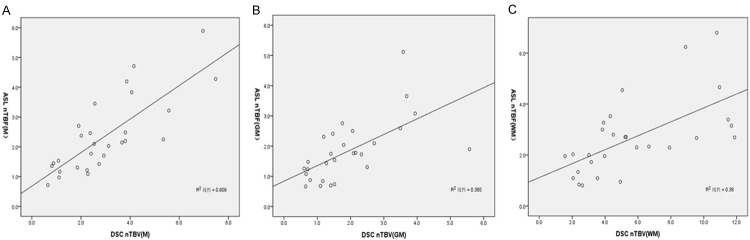
Scatter diagram of ASL nTBF and DSC nTBV values normalized to M (A), GM (B) and WM (C). Significant correlations (R=0.780, 0.621 and 0.616, respectively) were detected between ASL nTBF and DSC nTBV.
Finally, we conducted analysis for the scatter diagram of the mean DSC nTBF and DSC nTBV values in all cases, and assessed Pearson’s correlation coefficient between DSC nTBF and DSC nTBV. Remarkably, very significant correlations between DSC nTBF and DSC nTBV values were detected once our data normalized to M (a, R=0.984), GM (b, R=0.959) and WM (c, R=0.962), respectively (Figure 7). Taken all data together, our studies support that 3D-ASL could be a viable tool to serve as a non-invasive alternate for DSC-MRI in the setting of assessing treatment prognosis and metastasis of brain tumors.
Figure 7.
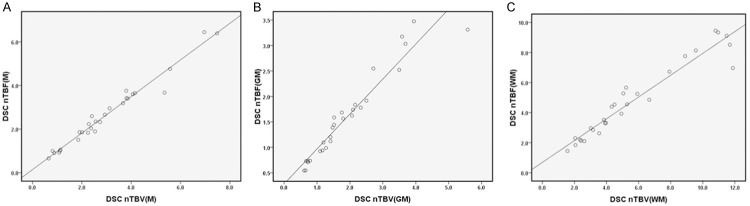
Scatter diagram of DSC nTBF and DSC nTBV values normalized to M (A), GM (B) and WM (C). Very significant correlations (R=0.984, 0.959 and 0.962, respectively) were obtained between DSC nTBF and DSC nTBV.
Discussion
Our data demonstrated evidence supporting a close correlation between ASL and DSC perfusion imaging approach. Comparison was carried out between measurements of normalized CBF from ASL and the corresponding CBF from DSC in 28 tumors covering three types of intracranial tumors, and the results were comparable in terms of normalized CBF measurements in brain tumors. These data suggest that ASL-based normalized CBF could be served as a viable biomarker for quantitative analysis of blood perfusion in patients with brain tumors. Our results are consistent with previous studies in which characterization of cerebral perfusion by ASL and DSC-MRI were comparable in brain tumors [6-9].
To improve SNR and the accuracy of tumor blood flow measurements, we normalized our data to correct age-dependent and patient-dependent effect, and to reduce the effect caused by the elevated intracranial pressure from brain tumors. It is worthy of note, when all three selected regions (M, GM and WM) were used as the reference standards, a significant correlation was detected between ASL nTBF and DSC nTBF, and no statistical difference for ASL nTBF and DSC nTBF was noted when M and GM were served as the internal references. Together, these findings suggest that ASL nCBF could be an alternative for DSC nCBF, which might be a marker of quantitative perfusion parameters in patients with brain tumors.
We also found that the mean CBF values derived from DSC was significantly higher than that from ASL when normalized to WM. This result is possibly caused by the underestimation of WM CBF in ASL due to relatively long transit times and perfusion values in the lower range of measurable flow [19,20]. A previous study demonstrated a higher ASL nCBF value of tumor to GM ratio obtained with ASL as compared with that obtained from DSC method, which renders detection and delineation of tumors feasible [21]. A similar trend was noted in our study, and this phenomenon could be demonstrated on some 3D-ASL CBF maps compared to DSC-MRI maps, since a larger difference in signal enhancement was indeed seen between the tumor and brain cortex (Figures 1, 2 & 4), but it was undetectable in all of our ASL images (Figure 3). In one patient with WHO grade II astrocytoma in the right insular lobe, the difference for signal enhancement between tumor and brain cortex was not so prominent, and a small part of hyperfusion near the right sylvian fissure in this predominantly hypoperfused tumor was noted in 3D-ASL perfusion map, which might be caused by the intravascular spin-labeling or elevated mean transit time [22].
Of note, a significant correlation between ASL nTBF and DSC nTBF and nearly a complete correlation between DSC nTBF and DSC nTBV can be reached once T, GM and WM were taken as the reference region. Specifically, when normalized to M, a highly significant correlation (R=0.780) was detected, while only moderate correlations could be noted when normalized to GM and WM (R=0.621 and 0.616, respectively). In general, a relatively normalized perfusion value of brain tumor perfusion is sufficient in clinical setting, and in fact, many published MRI studies employed DSC nCBV in brain tumor perfusion evaluation [14,23,24]. The R value listed above confirms that ASL nCBF and DSC nCBF may be as good in the assessment of brain tumor perfusion as DSC nCBV. Therefore, ASL nTBF and DSC nTBF may possess equal predictive effect as DSC nTBV on the evaluation of tumor angiogenesis. Indeed, both techniques demonstrated similar hemodynamic characteristics of meningiomas, gliomas and metastases, and were able to detect heterogeneous blood flow distribution within an individual tumor.
The low cost, low risk of nephrogenic systemic sclerosis, immediate availability, and easy quantification without extensive postprocessing have rendered ASL a promising tool in tumor angiogenesis assessment and glioma grading [7,9,18,25]. Particularly, its higher tumor to gray matter contrast and absence of susceptibility signal loss made tumor detection and delineation much easier. On the other hand, in order to avoid repeated contrast injections, 3D-ASL perfusion MRI could be a better choice to monitor and follow-up brain tumor treatment. The potential application of 3D-ASL perfusion MRI in distinguishing pediatric high-grade and low-grade tumors [10] would benefit pediatric patients with brain tumors because of no requirement for contrast media, high SNR, high labeling efficiency [26], and CBF quantification. Just as one coin has two sides, 3D-ASL perfusion MR has several limitations manifested by longer scanning time than that of DSC perfusion MRI and lower spatial resolution.
Although data resulted from our current study are exciting and encouraging, several limitations should be considered. The study only included a small number of patients, especially for those patients with high-grade and low-grade gliomas and patients with metastases. As a result, a larger sample size would be necessary to confirm our data and to establish the perfusion differences of different types of brain tumors. It is possible that underestimation of ASL tumor blood flow in the case of tortuous vasculature from angiogenesis could be due to the delay of signal arrival. Indeed, underestimation of ASL CBF in brain regions with delayed flow such as the white matter has been noted in previous investigations [20]. Alternatively, overestimation of tumor blood flow could also be present in the case of vascular shunting.
In summary, we demonstrated the correlation between 3D-ASL and DSC-MRI by employing multi-parametric normalized methods of brain tumor perfusion. The correlation between 3D-ASL and DSC-MRI suggests that blood volume and flow in tumors is coupled. Given that no contrast injection is required, 3D-ASL could be a viable tool to serve as a non-invasive alternate for DSC-MRI in the evaluation of perfusion in brain tumors, particularly for those patients with renal failure and patients required for collection of follow up information.
Acknowledgements
This work was supported by the Chinese National 12th Five-year Support Program for Science and Technology (2011BAI08B10), the National Natural Science Foundation of China (30870702 and 81130014), and the Natural Science Foundation of Hubei Province (2010CDA034).
Disclosure of conflict of interest
The authors declare no competing financial interests.
References
- 1.Troprès I, Grimault S, Vaeth A, Grillon E, Julien C, Payen JF, Lamalle L, Décorps M. Vessel size imaging. Magn Reson Med. 2001;45:397–408. doi: 10.1002/1522-2594(200103)45:3<397::aid-mrm1052>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Järnum H, Steffensen EG, Knutsson L, Fründ ET, Simonsen CW, Lundbye-Christensen S, Shankaranarayanan A, Alsop DC, Jensen FT, Larsson EM. Perfusion MRI of brain tumours: a comparative study of pseudo-continuous arterial spin labelling and dynamic susceptibility contrast imaging. Neuroradiology. 2010;52:307–317. doi: 10.1007/s00234-009-0616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang DJ, Alger JR, Qiao JX, Hao Q, Hou S, Fiaz R, Gunther M, Pope WB, Saver JL, Salamon N, Liebeskind DS UCLA Stroke Investigators. The value of arterial spin-labeled perfusion imaging in acute ischemic stroke: comparison with dynamic susceptibility contrast-enhanced MRI. Stroke. 2012;43:1018–1024. doi: 10.1161/STROKEAHA.111.631929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binnewijzend MA, Kuijer JP, Benedictus MR, van der Flier WM, Wink AM, Wattjes MP, van Berckel BN, Scheltens P, Barkhof F. Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology. 2013;267:221–230. doi: 10.1148/radiol.12120928. [DOI] [PubMed] [Google Scholar]
- 5.Huang D, Wu B, Shi K, Ma L, Cai Y, Lou X. Reliability of three-dimensional pseudo-continuous arterial spin labeling MR imaging for measuring visual cortex perfusion on two 3T scanners. PLoS One. 2013;8:e79471. doi: 10.1371/journal.pone.0079471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Westen D, Petersen ET, Wirestam R, Siemund R, Bloch KM, Ståhlberg F, Björkman-Burtscher IM, Knutsson L. Correlation between arterial blood volume obtained by arterial spin labelling and cerebral blood volume in intracranial tumours. MAGMA. 2011;24:211–223. doi: 10.1007/s10334-011-0255-x. [DOI] [PubMed] [Google Scholar]
- 7.Warmuth C, Gunther M, Zimmer C. Quantification of blood flow in brain tumors: comparison of arterial spin labeling and dynamic susceptibility-weighted contrast-enhanced MR imaging. Radiology. 2003;228:523–532. doi: 10.1148/radiol.2282020409. [DOI] [PubMed] [Google Scholar]
- 8.White CM, Pope WB, Zaw T, Qiao J, Naeini KM, Lai A, Nghiemphu PL, Wang JJ, Cloughesy TF, Ellingson BM. Regional and voxel-wise comparisons of blood flow measurements between dynamic susceptibility contrast magnetic resonance imaging (DSC-MRI) and arterial spin labeling (ASL) in brain tumors. J Neuroimaging. 2014;24:23–30. doi: 10.1111/j.1552-6569.2012.00703.x. [DOI] [PubMed] [Google Scholar]
- 9.Kimura H, Takeuchi H, Koshimoto Y, Arishima H, Uematsu H, Kawamura Y, Kubota T, Itoh H. Perfusion imaging of meningioma by using continuous arterial spin-labeling: comparison with dynamic susceptibility-weighted contrast-enhanced MR images and histopathologic features. AJNR Am J Neuroradiol. 2006;27:85–93. [PMC free article] [PubMed] [Google Scholar]
- 10.Yeom KW, Mitchell LA, Lober RM, Barnes PD, Vogel H, Fisher PG, Edwards MS. Arterial spin-labeled perfusion of pediatric brain tumors. AJNR Am J Neuroradiol. 2014;35:395–401. doi: 10.3174/ajnr.A3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med. 2004;51:736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- 12.Wolf RL, Wang J, Wang S, Melhem ER, O’Rourke DM, Judy KD, Detre JA. Grading of CNS neoplasms using continuous arterial spin labeled perfusion MR imaging at 3 Tesla. J Magn Reson Imaging. 2005;22:475–482. doi: 10.1002/jmri.20415. [DOI] [PubMed] [Google Scholar]
- 13.Law M, Yang S, Babb JS, Knopp EA, Golfinos JG, Zagzag D, Johnson G. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol. 2004;25:746–755. [PMC free article] [PubMed] [Google Scholar]
- 14.Emblem KE, Nedregaard B, Nome T, Due-Tonnessen P, Hald JK, Scheie D, Borota OC, Cvancarova M, Bjornerud A. Glioma grading by using histogram analysis of blood volume heterogeneity from MR-derived cerebral blood volume maps. Radiology. 2008;247:808–817. doi: 10.1148/radiol.2473070571. [DOI] [PubMed] [Google Scholar]
- 15.Law M, Oh S, Babb JS, Wang E, Inglese M, Zagzag D, Knopp EA, Johnson G. Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging--prediction of patient clinical response. Radiology. 2006;238:658–667. doi: 10.1148/radiol.2382042180. [DOI] [PubMed] [Google Scholar]
- 16.Hirai T, Kitajima M, Nakamura H, Okuda T, Sasao A, Shigematsu Y, Utsunomiya D, Oda S, Uetani H, Morioka M, Yamashita Y. Quantitative blood flow measurements in gliomas using arterial spin-labeling at 3T: intermodality agreement and inter- and intraobserver reproducibility study. AJNR Am J Neuroradiol. 2011;32:2073–2079. doi: 10.3174/ajnr.A2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulmer S, Helle M, Jansen O, Mehdorn HM, Nabavi A. Intraoperative dynamic susceptibility contrast weighted magnetic resonance imaging (iDSC-MRI) - Technical considerations and feasibility. Neuroimage. 2009;45:38–43. doi: 10.1016/j.neuroimage.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi T, Yoshiura T, Hiwatashi A, Togao O, Yamashita K, Nagao E, Shono T, Mizoguchi M, Nagata S, Sasaki T, Suzuki SO, Iwaki T, Kobayashi K, Mihara F, Honda H. Perfusion imaging of brain tumors using arterial spin-labeling: correlation with histopathologic vascular density. AJNR Am J Neuroradiol. 2008;29:688–693. doi: 10.3174/ajnr.A0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen ET, Zimine I, Ho YC, Golay X. Non-invasive measurement of perfusion: a critical review of arterial spin labelling techniques. Br J Radiol. 2006;79:688–701. doi: 10.1259/bjr/67705974. [DOI] [PubMed] [Google Scholar]
- 20.van Gelderen P, de Zwart JA, Duyn JH. Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magn Reson Med. 2008;59:788–795. doi: 10.1002/mrm.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong ET, Appignani B, Hackney DB, Alsop DC. Comparison of arterial spin labeling and dynamic susceptibility contrast imaging in glioma. Proc Intl Soc Mag Reson Med. 2006;14:3157. [Google Scholar]
- 22.Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, part 1: technique and artifacts. AJNR Am J Neuroradiol. 2008;29:1228–1234. doi: 10.3174/ajnr.A1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Rödiger LA, Shen T, Miao J, Oudkerk M. Preoperative subtyping of meningiomas by perfusion MR imaging. Neuroradiology. 2008;50:835–840. doi: 10.1007/s00234-008-0417-3. [DOI] [PubMed] [Google Scholar]
- 24.Jenkinson MD, Smith TS, Joyce KA, Fildes D, Broome J, du Plessis DG, Haylock B, Husband DJ, Warnke PC, Walker C. Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumours. Neuroradiology. 2006;48:703–713. doi: 10.1007/s00234-006-0122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HS, Kim SY. A prospective study on the added value of pulsed arterial spin-labeling and apparent diffusion coefficients in the grading of gliomas. AJNR Am J Neuroradiol. 2007;28:1693–1699. doi: 10.3174/ajnr.A0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Licht DJ, Jahng GH, Liu CS, Rubin JT, Haselgrove J, Zimmerman RA, Detre JA. Pediatric perfusion imaging using pulsed arterial spin labeling. J Magn Reson Imaging. 2003;18:404–413. doi: 10.1002/jmri.10372. [DOI] [PubMed] [Google Scholar]


