Abstract
Langerhans cell histiocytosis (LCH) is a rare disorder characterized by the proliferation of abnormal Langerhans cells. Previous studies mainly focused on children with LCH. However, there is limited information on the clinical and pathological aspects of LCH in adults. Therefore, this study aimed to investigate the clinical and pathological aspects of LCH in Chinese adults. The results showed that the average age of 18 LCH patients was 35.22 ± 16.57 years old. The ratio of male to female was 3.5:1.14 patients (77.8%) had single-system involvement and 4 patients (22.2%) had multi-system diseases. The skin (38.9%) and lungs (44.4%) were the mainly affected organs. No BRAF mutations were detected in the lesions of 18 cases. The number of FOXP3+ Tregs was significantly increased in LCH. In conclusion, clinical features of LCH in adults are distinct from those in children. Adult LCH has a relatively good prognosis and presents as a benign disease. Immune regulation plays an important role in the pathogenesis of adult LCH.
Keywords: Langerhans cell histiocytosis, BRAF, mutations, FOXP3+ regulatory T cells
Introduction
Langerhans cell histiocytosis (LCH) is a rare disorder characterized by the proliferation of abnormal Langerhans cells. While LCH patients range in age from newborn to elderly, LCH has a peak incidence in very young children aged 1-3 years old [1]. The annual incidence of LCH is five cases per million children. The ratio of male and female LCH patients is approximately 2:1. The clinical manifestations of LCH vary depending on the sites, numbers and severity of the organs involved. The clinical spectrum of LCH ranges from single isolated lesions to disseminated malignant diseases. The course of LCH is either spontaneous remission or aggressive, even lethal [2]. LCH can involve any organs, but most often the bones, followed by the skin, lungs, liver, spleen and lymph nodes. In the past, LCH were divided into three kinds of traditional types, namely Litterer-Siwe disease, Hand-Schuller-Christian disease and eosinphilic granuloma [3]. Now it is clear that LCH includes a spectrum of these disorders with different courses and variable clinical symptoms, and LCH is classified as single-system involvement and multi-system disease based on the extent of the organ involved.
The etiology of LCH is unclear. Many studies suggest that LCH is a reactive proliferative disease. Abnormal expression of cytokines and chemokines and immune dysregulation play an important role in the pathogenesis of LCH [4]. Pathologic LCH cells and T cells produce high levels of cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), granulocyte macrophage-colony stimulating factor (GM-CSF) and tumor necrosis factor-α (TNF-α). The interaction of T cells and LCH cells in a cytokine amplification cascade may lead to the proliferation of LCH cells and explain the characteristic features of LCH [5].
Recently, the finding that pathologic Langerhans cells (LCs) in LCH are monoclonal has provided a strong support for the idea that LCH is a neoplastic disease [6,7]. BRAF is an important component of the signaling cascade that is regulated by its upstream RAS protein kinase and involved in cell proliferation, differentiation, migration and apoptosis [8]. BRAF mutation can activate MEK, ERK kinase and transmit mitosis signals through MAPK signaling pathways, ultimately leading to tumor formation. A recent study reported oncogenic BRAF V600E mutations in the paraffin tissues of LCH patients [9], indicating that LCH is a neoplasm.
Previous studies mainly focused on children with LCH. However, there is limited information on the clinical and pathological aspects of LCH in adults. In this study, we aimed to investigate the clinical and pathological aspects of LCH in Chinese adults, especially the status of BRAF mutation and the immune regulation of FOXP3+ regulatory T cells (Tregs) infiltration in adult LCH.
Materials and methods
Patients and samples
A total of 18 patients who were diagnosed as LCH at our hospital were enrolled in this study, including 14 males and 4 females. The age of onset ranged from 18 to 78 years old. Formalin-fixed, paraffin-embedded sections were confirmed as LCH by pathological and immunohistochemical examination (positive staining for CD1a, CD68, S100, CD207). This study was approved by the Ethics Committee of Beijing Chao-Yang Hospital, and informed consent was obtained from all patients before the study. Patients received a systemic examination to determine the extent of disease, including physical examination, X-ray or chest computed tomography, skeletal survey and abdominal ultrasonography.
PCR and direct sequencing
DNA was extracted from paraffin-embedded LCH tissue samples, among which 7 cases from the skin, 8 cases from the lungs and 3 cases from the bones. Blocks were cored in areas of the highest histiocyte density, and DNA was extracted using QIAmp DNA FFPE tissue kit (QIAGEN) according to the instructions [9]. BRAF exons 11 and 15 were amplified by PCR with the following primers. BRAF exon 15, Forward 5’-TACTATCTGCAGCATCTTCATTCC-3’; Reverse 5’-TACTATAGTTGAGACCTTCAATGAC-3’; BRAF exon 11, Forward 5’-ATCTCTTCCTGTATCCCTCTCAG-3’, Reverse 5’-TTGAGGACTAGTTAACCTGGAGG-3’. PCR was performed on PCR System 9700 (ABI) with the following cycling conditions: an initial denaturation at 95°C for 5 min, followed by 40 cycles with denaturation at 94°C for 15 s, annealing at 50°C for 20 s, and extension at 72°C for 60 s, then a final extension at 72°C for 5 min. Negative controls were included in each set of amplifications. PCR products were detected by 2% agarose gel electrophoresis, and then purified and sequenced using the Big Dye Terminator kit and analyzed on ABI 3730xl DNA Analyzer (Applied Biosystems). Chromas software was applied to analyze the sequencing results.
Immunohistochemistry
Immunohistochemistry was performed to detect FoxP3 regulatory T cells (Tregs) on formalin-fixed, paraffin-embedded tissue sections. 4 um thickness serial sections were dewaxed and rehydrated. Heat-induced antigen retrieval was followed by the blockade of endogenous peroxidase activity with hydrogen peroxide. The sections were incubated overnight at 4°C with primary antibodies: mouse monoclonal anti-FOXP3 antibody (Ab22510, Abcam), mouse monoclonal anti-CD3 antibody (ZM-0417, Zymed), then incubated with horseradish peroxidase (HRP) labeled streptavidin biotin complex (SABC) and diaminobenzidine (DAB) as the substrate. The sections were counterstained with Harris Haematoxylin and then mounted. As the negative controls, mouse IgG isotypes were used instead of primary antibody.
Positive FoxP3 regulatory T cells were observed as the cells with the brown staining in the nucleus under light microscope. Positive CD3 cells were observed with the brown staining of the membrane. The number of positive cells in ten randomly selected areas was analyzed using image analysis software (Image Pro Plus) at a magnification of × 400. FOXP3+ labeling index (LI) was defined as the percentage of FOXP3+ cells among total CD3+ cells [10].
Statistical analysis
Statistical analysis was performed using SPSS version 19.0 for Windows. Data were expressed as mean ± standard deviation (x̅ ± s). Comparisons were performed by t test. A value of P < 0.05 was considered statistically significant.
Results
General information
The characteristics of 18 LCH patients were summarized in Table 1. Their average age was 35.22 ± 16.57 years old. The predominance of male patients was prominent (77.8%). The ratio of male to female was 3.5:1.14 patients (77.8%) had single-system involvement while 4 patients (22.2%) had multi-system diseases, including 3 cases associated with diabetes insipidus, 2 cases with lymph node involvement, 1 case with pituitary involvement, 1 case with bone and lung involvement. Our data showed that the skin (38.9%) and lungs (44.4%) were the mainly affected organs in adult LCH, and bone involvement accounted for 22.2% of LCH patients (4/18) (Figure 1).
Table 1.
Clinical characteristics of LCH patients
| Patient no. | Age (year) | Gender | Smoking | Site | Stage |
|---|---|---|---|---|---|
| 1 | 78 | M | -- | Skin | Unifocal |
| 2 | 23 | M | -- | Skin | Unifocal |
| 3 | 58 | F | -- | Skin | Unifocal |
| 4 | 39 | M | + | Skin | Multifocal |
| 5 | 22 | F | -- | Skin | Unifocal |
| 6 | 40 | F | -- | Skin | Unifocal |
| 7 | 63 | M | -- | Skin | Unifocal |
| 8 | 18 | M | + | Lung | Unifocal |
| 9 | 26 | M | + | Lung | Multifocal |
| 10 | 27 | M | + | Lung | Unifocal |
| 11 | 30 | M | + | Lung | Multifocal |
| 12 | 23 | M | + | Lung | Unifocal |
| 13 | 45 | M | + | Lung | Unifocal |
| 14 | 20 | M | + | Lung | Unifocal |
| 15 | 24 | M | + | Lung | Multifocal |
| 16 | 33 | M | + | Bone | Unifocal |
| 17 | 26 | F | -- | Bone | Unifocal |
| 18 | 39 | M | + | Bone | Unifocal |
Figure 1.
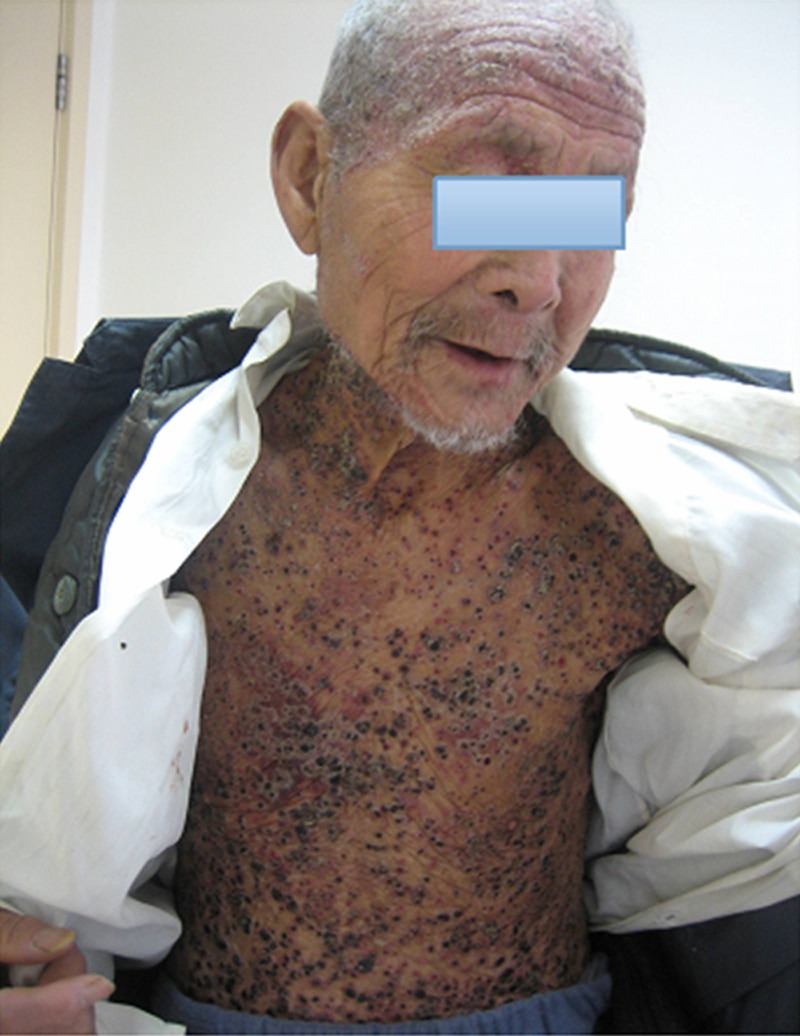
Clinical manifestation of cutaneous lesions in a male adult LCH patient.
BRAF mutations in LCH patients
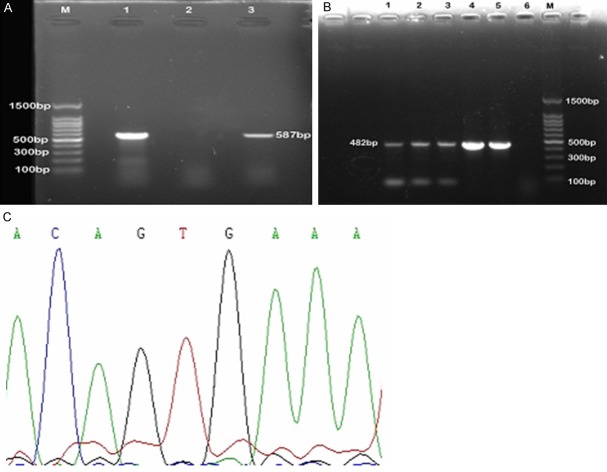
Agarose gel electrophoresis showed that PCR product of BRAF exon 15 was 587 bp, and BRAF exon 11 was 482 bp. Bidirectional sequencing of PCR products showed that no BRAF mutations on exon 11 or 15 were detected in 18 cases of adult LCH (Figure 2).
Figure 2.
Analysis of BRAF mutations in exon 15 and exon 11. A. PCR product of BRAF exon 15. M: 100 bp DNA ladder; 1 and 3: patinet samples. 2: negative control. B. PCR product of BRAF exon 11. M: 100 bp DNA ladder; 1-5: patient samples. 6: negative control. C. Sequencing of BRAF exon 15. No BRAF V600E mutation was detected.
FOXP3+ Treg in LCH patients
Immunohistochemical results showed that a large number of FoxP3-positive cells were detected among T lymphocytes in LCH lesions, which represented T-regulatory lymphocytes (Tregs). Tregs were located in close proximity to dendritic cells (DCs). The number of Treg was correlated with the infiltration density of lymphocyte. Tregs represented 22-30% of CD3+ cells in different lesions. In contrast, the number of FOXP3-positive cells was low in control tissues (Figure 3). There were remarkable differences in the number of FOXP3+ cells between LCH groups and control groups (P < 0.05) (Table 2).
Figure 3.
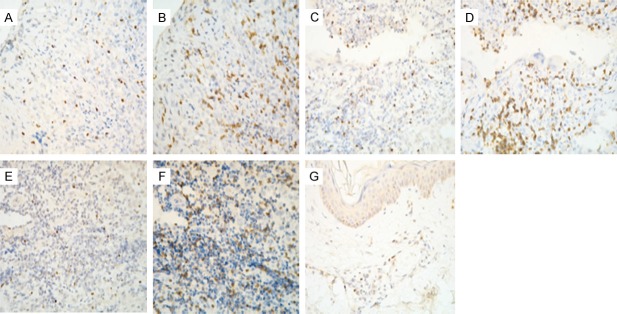
Immunohistochemical staining of FOXP3+ and CD3+ cells in LCH and control tissues. A. Distribution of FOXP3+ in skin lesion. B. Distribution of CD3+ in skin lesion. C. Distribution of FOXP3+ in lung lesion. D. Distribution of CD3+ in lung lesion. E. Distribution of FOXP3+ in bone lesion. F. Distribution of CD3+ in bone lesion. G. Distribution of FOXP3+ in normal skin.
Table 2.
The number of FOXP3+ cells in different LCH tissues (x̅ ± s)
| Group | Patients | Number of CD3+ | Number of FOXP3+ | FOXP3+/CD3+ (%) |
|---|---|---|---|---|
| Skin LCH | 7 | 159 ± 88.55 | 49.14 ± 30.98 | 30.20 ± 8.75* |
| Normal skin | 6 | 76.33 ± 12.26 | 7.17 ± 5.77 | 8.75 ± 5.46 |
| Lung LCH | 8 | 180.88 ± 31.66 | 41.63 ± 16.43 | 22.59 ± 7.03** |
| Bronchiectasis | 6 | 70.83 ± 67.19 | 7.33 ± 8.73 | 8.00 ± 4.89 |
| Bone LCH | 3 | 157.33 ± 24.11 | 38.00 ± 14.73 | 24.03 ± 8.46*** |
| Plasmacell myeloma | 3 | 123.33 ± 15.27 | 3.67 ± 1.53 | 2.90 ± 0.96 |
P < 0.01 vs. normal skin;
P < 0.01 vs. bronchiectasis;
P < 0.05 vs. Plasmacell myeloma.
Treatment and follow-up of LCH patients
Treatment strategies included local surgical excision, radiotherapy, oral or topical corticosteroids and nitrogen mustard, given alone or in combination. Among patients with cutaneous LCH, 4 cases were treated with topical corticosteroids, 1 with local lesion excision, 1 with topical nitrogen mustard, 1 with oral corticosteroids. All three patients with bone LCH underwent surgical curettage, and one of them received postoperative radiotherapy. Patients with pulmonary LCH were treated with partial lung excision, and two of them received oral prednisone.
All patients gave up smoking after the operation. 18 cases of LCH received follow-up for 1 to 5 years after treatment. Three patients were lost, and the remaining 15 patients were alive. 2 patients had reactive diseases while the other 13 patients were in good condition.
Discussion
Our results showed that the clinical features of LCH in adults are different from those in children. The incidence of male patients (77.8%) is significantly high, which is consistent with the reported male predominance in LCH [11]. We found that all pulmonary LCH patients were male and had a history of smoking. Meanwhile two cases of male patients with bone LCH had a history of smoking. The duration time of smoking history ranged from 3 to 20 years, and the number of cigarettes was ≥ 10 per day among these patients. Therefore, the high incidence of LCH in male patients may be related to their frequent history of smoking. Single-system involvements are more common and the lungs and skin are the preferably involved organs in adult LCH, which are distinct from those of LCH in children [12]. Follow-up of 1 to 5 years showed that none of 15 patients in this group died, suggesting that adult LCH usually has a good prognosis.
BRAF is the most frequently mutated protein kinase in human cancer, and plays an important role in cancer induction, maintenance and progression [13]. BRAF gene mutations occur in a variety of tumors and cancer cell lines, especially in malignant melanoma, papillary thyroid carcinoma, lung adenocarcinoma, colorectal cancer [14]. The incidence of BRAF mutation is 3% in lung cancer of European, but only l% in lung adenocarcinoma of Japanese patients [15]. There are two main types of BRAF mutations: 11% of the mutations are located in exon 11; 89% of the mutations occur in exon 15, especially BRAF V600E mutation. Badalian-Very et al. reported that the incidence of BRAF V600E mutation was 57% in 61 LCH patients, mainly in patients with bone involvement, and the mutation rate was higher in young patients with 67.7% of children under age 15 had BRAF mutation [9]. BRAF V600E or 600DLAT mutations were detected in 11 cases among 16 LCH granuloma samples from children patients [16]. Furthermore, BRAF V600E mutation was detected in 18 of 46 (39%) LCH lesions, V600K mutation in 1 of 46 (2%) lesions, and wild-type status in 27 of 46 (59%) lesions. The patients with BRAF mutation were basically those with bone lesions (14/19) [17]. However, BRAF V600E mutation was detected in two of the five pulmonary LCH patients [18]. Kansal et al. identified the rare, variant BRAF V600D mutation in congenital, benign LCH patient, and no BRAF V600E mutation was detected [19].
Surprisingly, our study showed that no BRAF mutations in exon 11 and 15 were detected in all 18 cases of adult LCH. The first possible reason may be due to a relatively small number of enrolled patients, the age of the patients and different disease sites. Previously reported LCH patients are mainly children, and the younger are patients, the higher are BRAF mutation rates. In comparison, all of the patients enrolled in this study are adults over 18 years old. As for the disease site, previous studies have shown that LCH BRAF mutation rate was higher in LCH with bone and multi-system involvements. In our study, the mainly involved site is the skin and lungs, and 77.8% of the patients had single-system involvement. Consists with our result, previous study showed that 69-72% of adult LCH only had a single-system involvement, mainly the bone, lungs and skin [20]. The clinical feature of pulmonary LCH (PLCH) is different from that of other tissues involved in LCH. PLCH occurs almost exclusively in adult smokers, and has a number of bilateral pulmonary nodules and, for the most part, little systemic damage. It has an unpredictable course, with some cases progressing to fibrosis, while others remaining stable or even spontaneously regressing. This suggests a good prognosis in PLCH. In this study, 8 patients with pulmonary LCH were cigarette smokers, with 3 to 20 years of smoking history. Second, due to ethnic and geographic differences, the sites, types and proportion of BRAF mutation may be distinct. In addition, we only focused on exon 11 and exon 15 of BRAF, mutations in other exons could not be excluded.
To explore the immune mechanisms of adult LCH, we detected FOXP3+ Tregs in LCH tissues by immunohistochemistry. We found that the population of FOXP3+ Tregs was significantly increased in LCH tissues. Tregs are a subgroup of T lymphocytes that suppress immune responses and maintain immunologic tolerance [21]. Tregs suppress the activation of CD4+ CD8+ T cells through the secretion of inhibitory cytokines transforming growth factor beta (TGF-β), interleukin-10 (IL-10) and cell-cell direct contact [10]. In addition, Tregs inhibit anti-tumor immune responses and promote tumor development. As a specific marker for regulatory T cells, FOXP3+ may reflect the number and function of Tregs [22].
In abnormal proliferative Langerhans cells of LCH, the expression of CD54, CD68, chemokine receptor CCR6 is upregulated, while CD83, CD86, and dendritic cell (DC)-LAMP, CCR7 is downregulated [23], suggesting that LCH cells are functionally immature dendritic cells (DC). The maturation status of DC determines the outcome of the immune response. Mature DC stimulates effector T cells and promotes immune reactions, whereas immature DC can induce the production of Tregs and promote immune tolerance [24]. Senechal et al. reported that the numbers of Tregs in peripheral blood and granuloma tissues of children LCH were higher compared to controls. In addition, the number of Tregs in blood was normal after the remission of LCH [25]. In addition, CD3+ cells isolated from LCH lesions had increased expression of FOXP3, CTLA4 (an activation marker associated with Treg and inhibition of inflammation) [26]. Our data showed that FOXP3+ Tregs were located in close proximity to DC and the number of Tregs was correlated with the infiltration density of lymphocyte. The higher number of Tregs may suppress immune response against LCs, resulting in increased survival of LCs and granuloma formation. Therefore, Tregs may prevent the immune system from clearing the abnormal Langerhans cells via immunosuppressant function, leading to the pathogenesis of LCH.
In conclusion, we found that clinical features of LCH in adults are distinct from those of LCH in children. The incidence of LCH is significantly increased in adult males, and the majority of them have a long history of smoking. The major sites of LCH are the lungs and skin, accompanied by a relatively good prognosis. No BRAF mutations were detected in adult LCH granuloma. In addition, the population of FOXP3+ Tregs was significantly increased in LCH. Our data suggest that adult LCH may be a benign, reactive proliferative disease. Immune regulation has an important role in the pathogenesis of adult LCH. Measures to reduce the population of Tregs would improve the treatment of adult LCH.
Acknowledgements
We thank Hongying Zhao and Youzhi Yu for technical support and image analyses. We thank Department of Respiratory Medicine, Beijing Chao-Yang Hospital for the recruitment of LCH patients.
Disclosure of conflict of interest
None.
References
- 1.Abla O, Egeler RM, Weitzman S. Langerhans cell histiocytosis: Current concepts and treatments. Cancer Treat Rev. 2010;36:354–359. doi: 10.1016/j.ctrv.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Egeler RM, D’Angio GJ. Langerhans cell histiocytosis. J Pediatr. 1995;127:1–11. doi: 10.1016/s0022-3476(95)70248-2. [DOI] [PubMed] [Google Scholar]
- 3.Azouz EM, Saigal G, Rodriguez MM, Podda A. Langerhans cell histiocytosis: pathology, imaging and treatment of skeletal involvement. Pediatr Radiol. 2005;35:103–115. doi: 10.1007/s00247-004-1262-0. [DOI] [PubMed] [Google Scholar]
- 4.Garabedian L, Struyf S, Opdenakker G, Sozzani S, Van Damme J, Laureys G. Langerhans cell histiocytosis: a cytokine/chemokine-mediated disorder? Eur Cytokine Netw. 2011;22:148–153. doi: 10.1684/ecn.2011.0290. [DOI] [PubMed] [Google Scholar]
- 5.Laman JD, Leenen PJ, Annels NE, Hogendoorn PC, Egeler RM. Langerhans cell histiocytosis “insight into DC biology”. Trends Immunol. 2003;24:190–196. doi: 10.1016/s1471-4906(03)00063-2. [DOI] [PubMed] [Google Scholar]
- 6.Willman CL, Busque L, Griffith BB, Favara BE, McClain KL, Duncan MH, Gilliland DG. Langerhans’ cell histiocytosis (histiocytosis X)-a clonal proliferative disease. N Engl J Med. 1994;331:154–160. doi: 10.1056/NEJM199407213310303. [DOI] [PubMed] [Google Scholar]
- 7.Yu RC, Chu C, Buluwela L, Chu AC. Clonal proliferation of Langerhans cells in Langerhans cell histiocytosis. Lancet. 1994;343:767–768. doi: 10.1016/s0140-6736(94)91842-2. [DOI] [PubMed] [Google Scholar]
- 8.Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009;283:125–134. doi: 10.1016/j.canlet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, Kuo FC, Ligon AH, Stevenson KE, Kehoe SM, Garraway LA, Hahn WC, Meyerson M, Fleming MD, Rollins BJ. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919–1923. doi: 10.1182/blood-2010-04-279083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Boer OJ, van der Loos CM, Teeling P, van der Wal AC, Teunissen MB. Immunohistochemical analysis of regulatory T cell markers FOXP3 and GITR on CD4+CD25+ T cells in normal skin and inflammatory dermatoses. J Histochem Cytochem. 2007;55:891–898. doi: 10.1369/jhc.6A7119.2007. [DOI] [PubMed] [Google Scholar]
- 11.Islinger RB, Kuklo TR, Owens BD, Horan PJ, Choma TJ, Murphey MD, Temple HT. Langerhans cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res. 2000;379:231–235. doi: 10.1097/00003086-200010000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Margo CE, Goldman DR. Langerhans Cell Histiocytosis. Surv Ophthalmol. 2008;53:332–358. doi: 10.1016/j.survophthal.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Dhomen N, Marais R. New insight into BRAF mutations in cancer. Curr Opin Genet Dev. 2007;17:31–39. doi: 10.1016/j.gde.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Kotoula V, Sozopoulos E, Litsiou H, Fanourakis G, Koletsa T, Voutsinas G, Tseleni-Balafouta S, Mitsiades CS, Wellmann A, Mitsiades N. Mutational analysis of the BRAF, RAS and EGFR genes in human adrenocortical carcinomas. Endocr Relat Cancer. 2009;16:565–572. doi: 10.1677/ERC-08-0101. [DOI] [PubMed] [Google Scholar]
- 15.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 16.Satoh T, Smith A, Sarde A, Lu HC, Mian S, Trouillet C, Mufti G, Emile JF, Fraternali F, Donadieu J, Geissmann F. B-RAF mutant alleles associated with Langerhans cell histiocytosis, a granulomatous pediatric disease. PLoS One. 2012;7:e33891. doi: 10.1371/journal.pone.0033891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahm F, Capper D, Preusser M, Meyer J, Stenzinger A, Lasitschka F, Berghoff AS, Habel A, Schneider M, Kulozik A, Anagnostopoulos I, Müllauer L, Mechtersheimer G, von Deimling A. BRAFV600E mutant protein is expressed in cells of variable maturation in Langerhans cell histiocytosis. Blood. 2012;120:e28–34. doi: 10.1182/blood-2012-06-429597. [DOI] [PubMed] [Google Scholar]
- 18.Yousem SA, Dacic S, Nikiforov YE, Nikiforova M. Pulmonary Langerhans cell histiocytosis: profiling of multifocal tumors using next-generation sequencing identifies concordant occurrence of BRAF V600E mutations. Chest. 2013;143:1679–1684. doi: 10.1378/chest.12-1917. [DOI] [PubMed] [Google Scholar]
- 19.Kansal R, Quintanilla-Martinez L, Datta V, Lopategui J, Garshfield G, Nathwani BN. Identification of the V600D mutation in Exon 15 of the BRAF oncogene in congenital, benign langerhans cell histiocytosis. Genes Chromosomes Cancer. 2013;52:99–106. doi: 10.1002/gcc.22010. [DOI] [PubMed] [Google Scholar]
- 20.Satter EK, High WA. Langerhans cell histiocytosis: a review of the current recommendations of the histiocyte society. Ped Dermatol. 2008;25:291–295. doi: 10.1111/j.1525-1470.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- 21.Grauer OM, Nierkens S, Bennink E, Toonen LW, Boon L, Wesseling P, Sutmuller RP, Adema GJ. CD4+ Foxp3 regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007;121:95–105. doi: 10.1002/ijc.22607. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S. Naturally arising Foxp3 expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 23.Geissmann F, Lepelletier Y, Fraitag S, Valladeau J, Bodemer C, Debré M, Leborgne M, Saeland S, Brousse N. Differentiation of Lang erhans cells in Langerhans cell histiocytosis. Blood. 2001;97:1241–1248. doi: 10.1182/blood.v97.5.1241. [DOI] [PubMed] [Google Scholar]
- 24.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: The importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senechal B, Elain G, Jeziorski E, Grondin V, Patey-Mariaud de Serre N, Jaubert F, Beldjord K, Lellouch A, Glorion C, Zerah M, Mary P, Barkaoui M, Emile JF, Boccon-Gibod L, Josset P, Debré M, Fischer A, Donadieu J, Geissmann F. Expansion of regulatory T cells in patients with langerhans cell histiocytosis. PLoS Med. 2007;4:e253. doi: 10.1371/journal.pmed.0040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen CE, Li L, Peters TL, Leung HC, Yu A, Man TK, Gurusiddappa S, Phillips MT, Hicks MJ, Gaikwad A, Merad M, McClain KL. Cell-Specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared to epidermal Langerhans cells. J Immunol. 2010;184:4557–4567. doi: 10.4049/jimmunol.0902336. [DOI] [PMC free article] [PubMed] [Google Scholar]