Abstract
This study is to investigate the effects of vitamin D on renal fibrosis in rat diabetic nephropathy models, as well as the changes and interactions in the expressions of renal fibrogenesis- and inflammation-related genes. Rat diabetic nephropathy models were established by high-fat diets, which were subjected to TGF-β1 manipulation, as well as vitamin D treatment. H&E staining, Masson staining, and TEM detection were performed to assess the effects of vitamin D treatment and/or TGF-β1 manipulation on pathological changes in the renal tissues in these rat diabetic nephropathy models. Immunohistology and real-time PCR were used to evaluate the expressions of TGF-β1, MCP-1, CTGF, and VDR. Histological staining and TEM detection showed that, in both TGF-β1 over-expressed and interfered groups, vitamin D administration alleviated the renal fibrosis, compared with the vehicle treatment. Similar results were observed with the immunohistological staining. Real-time PCR analysis indicated that, when TGF-β1 was over-expressed in diabetic nephropathy, the expressions of MCP-1 and CTGF were also up-regulated, which would be decreased by the treatment of vitamin D. On the other hand, when TGF-β1 was interfered in DN, the expressions of MCP-1 and CTGF were relatively down-regulated, which would be further lowered by vitamin D administration. The mRNA expression of VDR was elevated by vitamin D treatment in these diabetic nephropathy models. Active vitamin D3 and lentivirus-mediated TGF-β1 interference could effectively reduce the renal fibrosis and protect the renal function in diabetic nephropathy rat models, which makes a promising therapeutic strategy for the disease.
Keywords: Diabetic nephropathy, vitamin D, TGF-β1, CTGF, MCP-1, VDR
Introduction
Diabetic nephropathy (DN) is a common microvascular complication of diabetes, and the main pathological changes include capillary basement membrane thickening and mesangial matrix hyperplasia, resulting in glomerular sclerosis [1]. Currently, DN is the leading cause of end-stage renal disease (ESRD) in Europe and other developed countries; in China, DN ranked second as the causes of ESRD, accounting for 25% of all the ERSD cases [2,3], which continues to go up. Studies have found that inflammatory cytokines and pro-inflammatory factors are closely linked with DN pathogenesis and development, therefore DN has been considered as an inflammatory disease [4,5]. In recent years, the protective effects of active vitamin D3 (also known as 1, 25-dihydroxyvitamin D3, or calcitriol) on kidney functions attract more and more attentions.
Active vitamin D is an important hormone regulating calcium homeostasis and phosphorus metabolism. Clinical studies have shown that active vitamin D can improve the survival rates of patients with chronic kidney disease, and the mechanisms are not only dependent on its modulation of blood levels of calcium, phosphorus, and PTH [6,7]. Furthermore, 1,25-(OH)2D3 can reduce glomerulosclerosis and interstitial fibrosis [8,9]. However, the therapeutic effects of vitamin D3, especially together with the manipulation of transforming growth factor-β1 (TGF-β1), on renal fibrosis in DN, have not been fully understood.
In the present study, we investigated the effects of vitamin D on renal fibrosis in rat DN models, as well as the changes and interactions in the expressions of TGF-β1, monocyte chemoattractant protein-1 (MCP-1), connective tissue growth factor (CTGF), and vitamin D receptor (VDR) during the disease pathogenesis. The possible roles of vitamin D and these related genes in the pathogenesis and development of renal fibrosis in DN were also discussed.
Materials and methods
Animal modeling and grouping
A total of 108 male SD rats (SPF), weighing 280 ± 10 g, were provided by the Xinjiang Disease Control and Prevention Center. All the animal experiments were approved by Xinjiang management committee for medical laboratory animal sciences. Rat DN models were established by feeding with high-fat and high-sugar diets, containing 10% refining lard, 20% sucrose, 2% cholesterol, 8% custard powder, and 60% of normal diet, for indicated duration. Then these rats were subjected to the peritoneal injection of streptozotocin (35 mg/kg, STZ; Sigma, St. Louis, MO, USA). One week later, the fasting plasma glucose (FPG) and 2-h plasma glucose (2hPG) were tested, and rats with FPG ≥ 7.0 mmol/L and/or 2hPG ≥ 11.1 mmol/L were considered as DN models.
These DN model rats were randomly divided into the following groups: the control model group (n=18), TGF-β1 over-expressed groups either treated with vitamin D (0.25 μg/tablet, J20100056; Shanghai Roche Pharmaceuticals, Shanghai, China) (n=18) or vehicle (peanut oil) (n=18), and TGF-β1 interfered groups either treated with vitamin D (n=18) or vehicle (n=18). Another group transfected with lenti-GFP was used as lentivirus control. For drug treatment, 0.03 μg/kg vitamin D in 0.05 mL peanut oil was administrated once daily via gavage, and equivalent administration of peanut oil was used as vehicle control.
Lentivirus preparation and injection
For the over-expression and interference of TGF-β1, the lentiviral vector of pLVX-mCMV-ZsGreen was used (Biowit Technologies Co., Ltd., Shenzhen, China). Vectors of pLVX-hTGFB1-mCMV-ZsGreen and pLVX-ShRNA2-hTGFB1-1,2,3 were prepared and titrated as described previously [10-12]. Lentivirus injections (150 μL) were made in the left kidney of rat DN models. Animals were killed at indicated time points.
Tissue sampling and preparation
These rats were sacrificed on day 3, 14, and 37, respectively, after the lentiviral injection. Four-six milliliters of venous blood and 24h-urine prior to sacrifice were collected, and the kidneys on the virus-injected side were removed. The tissues were cut into smaller pieces (0.5 cm), immersed in RNAlater solution at room temperature, and then stored at -20°C. Renal cortex of 1 mm × 1 mm × 1 mm was obtained from the kidney, and then fixed in 2.5% glutaraldehyde. Remaining kidney tissues were fixed in 4% paraformaldehyde.
Histopathological staining
Fresh kidney tissue was washed with saline, and then fixed in 10% formalin. After dehydrated, these tissues were embedded with paraffin, and then cut into 3 μm sections on a microtome (Leica, Nussloch, Germany). H&E staining and Masson staining (with Masson staining kits from Maxim-Bio, Fuzhou, Fujian, China) were performed.
Transmission electron microscopy (TEM) examination
A small piece of samples was double fixed with glutaraldehyde and osmic acid. After acetone gradient dehydration, the sample piece was embedded with Epon812 epoxy resin, and then cut into ultramicrocuts on a Leica UC6 ultramicrotome (Leica). The sections were stained with lead-uranium, and then subjected to transmission electron microscopy on a standard JEOL 1230 electron microscope (JEOL, Tokyo, Japan).
Immunohistochemistry
Paraffin sections were de-waxed and re-hydrated through a graded alcohol series. The endogenous peroxidase was removed, and the sections were exposed to antigen retrieval. The samples were incubated with Rabbit anti-rat MCP-1 polyclonal antibody (l:50 dilution; Boster Biological Technology, Wuhan, Hubei, China), rabbit anti-rat CTGF polyclonal antibody (l:5 dilution; Bioss Biotechnology, Beijing, China), rabbit anti-rat collagen type I polyclonal antibody (1:100 dilution; Bioss Biotechnology), and rabbit anti-rat VDR polyclonal antibody (1:200 dilution; Bioss Biotechnology), respectively, at 4°C overnight. Then secondary antibodies were added to incubate the sections. After stained with DAB chromogenic reagent (ZSGB-BIO, Beijing, China) and counterstained with hematoxylin for 5-10 min, these sections were sealed and then visualized under microscopy with the CM-2000B biomedicine image analysis system (Beihang, Beijing, China). Brown staining was considered as positive. Five fields were randomly selected under high magnification (×400), and the averaged number of positive cells were counted and calculated.
Real-time PCR
Total RNA was extracted with RNeasy Mini kits (Qiagen, Hilden, Germany). QuantiTect Rev Transcription Kits (Qiagen) were used to perform reverse transcription with oligo (dT) primers, according to manufactures’ instructions. The real-time quantitative PCR assays were performed with QuantiFast SYBR green PCR Master Mix containing ROX as a passive reference (Qiagen), on Bio-Rad iQ5 system (Bio-Rad, Hercules, CA, USA). Primer sequences used were listed in Table 1. Quantitative PCR amplification conditions were as follows: melt at 95°C for 5 min; and at 95°C for 50 s, 60°C for 30 s, for 40 cycles. β-actin was used as control, and the relative expression level of TGF-β1 was calculated by the ΔΔCt method.
Table 1.
Primer sequences for real-time PCR analysis
| Primers | Sequences |
|---|---|
| TGF-β1 forward | 5’-AGAAGTCACCCGCGTGCTAAT-3’ |
| TGF-β1 reverse | 5’-CACTGCTTCCCGAATGTCTGA-3’ |
| MCP-1 forward | 5’-CAGCCAGATGCAGTTAATGCC-3’ |
| MCP-1 reverse | 5’-AGCCGACTCATTGGGATCAT-3’ |
| CTGF forward | 5’-GCCTGTTCCAAGACCTGT-3’ |
| CTGF reverse | 5’-GGATGCACTTTTTGCCCTTCTTA-3’ |
| VDR forward | 5’-GCCCCTCATAAAGTTCCAGGTG-3’ |
| VDR reverse | 5’-GGATAGGCGGTCCTGAATGG-3’ |
Note: TGF, transforming growth factor; MCP, monocyte chemoattractant protein; CTGF, connective tissue growth factor; VDR, vitamin D receptor.
Statistical analysis
Data were expressed as mean ± SD. SPSS 17.0 software was used for statistical analysis. Two-way analysis of variance was used for the group comparison, and LSD was performed for pairwise comparison. P < 0.05 was considered statistically significant.
Results
Histopathological staining of the renal tissues in rat DN models
In order to investigate the effects of vitamin D3 on the renal fibrosis in DN rat models, H & E staining and Masson staining of these renal tissues were performed, on day 37 after lentivirus injection. As shown in Figure 1A, H&E staining showed that, that there were significant differences between the control model group and the lenti-GFP-treated group. In these groups, mesangial cell hyperplasia, adhesion between glomerular capillaries and Bowman’s capsules (with walls), and occlusion of the capillary network were observed. When TGF-β1 was over-expressed in these models, with the vehicle (peanut oil) treatment, the mesangial cell proliferation and the adhesion between glomerular capillaries and Bowman’s capsules (without walls) were both significantly enhanced, while in vitamin D3-treated group, there were slight mesangial cell hyperplasia, and adhesion between glomerular capillaries and Bowman’s capsules (with intact walls). In neither group, the occlusion of the capillary network was observed. On the other hand, in shRNA-TGF-β1 groups treated with vehicle, mesangial cell hyperplasia and renal tubule atrophy and dilation were obvious, and there was also massive inflammatory cell infiltration. When shRNA-TGF-β1 models were treated with vitamin D3, mesangial cell hyperplasia, renal tubule dilation, and inflammatory cell infiltration were alleviated effectively, and there was neither adhesion between glomerular capillaries and Bowman’s capsules (with intact walls) nor occlusion of the capillary network.
Figure 1.
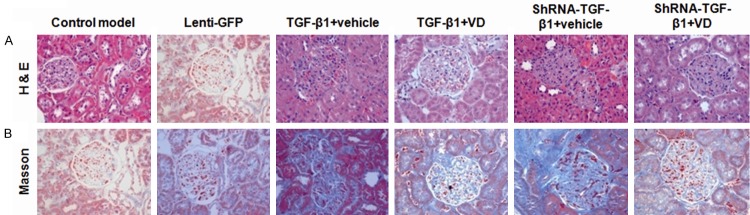
Histopathological staining of the renal tissues in rat DN models. H&E staining (A) and Masson staining (B) were performed to assess the pathological changes in renal tissues in these rat DN models, on day 37 after the lentiviral injection.
Similar results were obtained with the Masson staining (Figure 1B). There were no significant differences between the control model group and the lenti-GFP-treated group, where normal structures of glomeruli and kidney tubules, as well as the blue-staining basement membrane were observed, with mild hyperplasia of glomerular capillaries. In TGF-β1 over-expressed groups, compared with the treatment of vehicle, vitamin D3 administration led to lighter blue staining, visible capillary lumen, reduced tubular atrophy, as well as less extensive glomerular and/or interstitial fibrosis. In TGF-β1 interfered groups, compared with vehicle, vitamin D3 treatment also resulted in lighter blue staining, visible capillary lumen, and restoring occlusion of the capillary network. These results suggest that the lentiviral vectors per se cannot influence DN pathology, which make the system an appropriate tool to deliver or manipulate the expression of genes in these models. Accordingly, only control model group was used as control in the following experiments. Most importantly, no matter TGF-β1 is over-expressed or interfered, vitamin D3 could mitigate the pathological changes in DN. Furthermore, treatment of vitamin D3 in shRNA interfering group provides the most beneficial therapeutic effects in these DN models.
TEM examination of the renal tissues in rat DN models
To further confirm the therapeutic effects of vitamin D3 on renal fibrosis in DN, TEM detection was performed, on day 37 after lentivirus injection. As shown in Figure 2, in the control model group, our results indicated increased density and partial shrinkage of the basement membrane, with a few domal uplifts. There was also local edema in renal tubular epithelial cells, with abundant mitochondria, increased gap between cytoplasmic membrane folds, sporadic vacuoles, as well as occasional epithelial cell necrosis. In TGF-β1 over-expressed group treated with vehicle, there were obvious epithelial cell swelling and severe shrinkage of basement membrane, with plenty of domal uplifts. The gaps between cytoplasmic membrane folds in renal tubular epithelial cells were dramatically increased, and the microvilli were irregularly arranged. However, when treated with vitamin D3, normal epithelial cell structure, with abundant mitochondria, was observed. Basement membrane was slightly shrunken, and wax-like small bodies with low electron density were seen in epithelial cells. The arrangement of microvilli was ordered and uniform. In TGF-β1 interfered groups, when treated with vehicle, there was significant swelling in epithelial cells, as well as the mitochondria within these cells. The gaps between cytoplasmic membrane folds were widened, and the basement membrane was shrunken. In contrast, after the treatment of vitamin D3, the structures of the renal tubules and the epithelial cells were basically normal, with plenty of mitochondria. The gaps between cytoplasmic membrane folds were slightly increased, and smooth basement membrane was observed. These results confirm the therapeutic effects of vitamin D3 on renal fibrosis in DN, and the combination of shRNA-TGF-β1 interference and vitamin D3 make the most effective therapy.
Figure 2.
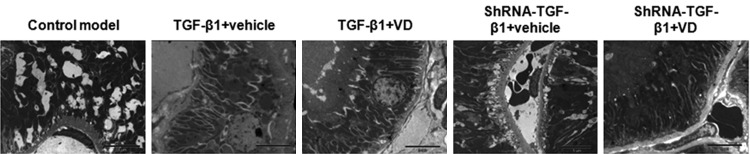
TEM detection of the renal tissues in rat DN models. Renal tissues in these rat DN models were subjected to TEM detection to assess the therapeutic effects of vitamin D3 on renal fibrosis, on day 37 after the lentiviral injection.
Immunohistochemical staining of the renal tissues in rat DN models
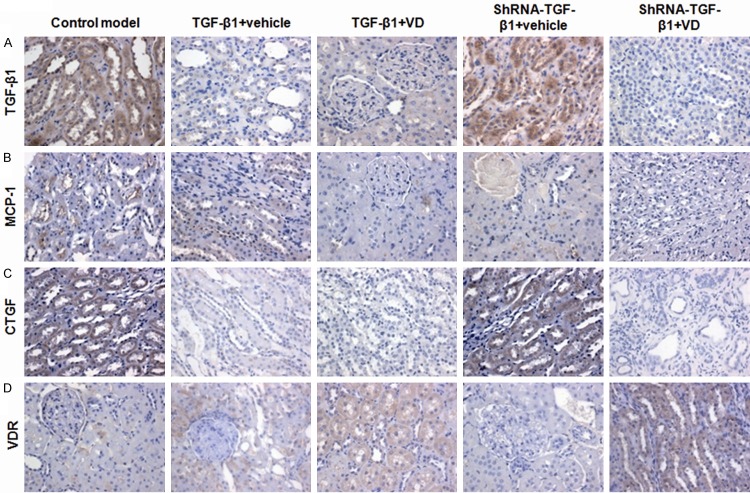
To investigate the molecular bases for the therapeutic effects of vitamin D3 on renal fibrosis, immunohistochemical staining was performed to detect the expressions of TGF-β1, MCP-1, VDR, and CTGF in renal tissues, on day 37after lentivirus injection. As shown in Figure 3, these molecules were mainly expressed in the renal tubules and interstitial cytoplasm. In the control model group, for TGF-β1 (Figure 3A), MCP-1 (Figure 3B), and CTGF (Figure 3C), there were obvious brown staining of the granules in renal tissues. In TGF-β1 over-expressed groups, compared with the vehicle treatment, vitamin D3 administration significantly decreased the brown granules (Figure 3A-C; P < 0.05). In TGF-β1 interfered groups, similar results were obtained; the brown granules were significantly decreased in the vitamin D3-treated group, compared with the vehicle-treated group (Figure 3A-C; P < 0.05). For VDR, the amount of brown staining granules and the covered area in vitamin D3-treated groups were significantly higher than the vehicle-treated group (Figure 3D; P < 0.05). These results suggest that vitamin D3 could regulate the expression of TGF-β1, MCP-1, CTGF, and VDR, which would contribute to its therapeutic effects on renal fibrosis in DN.
Figure 3.
Immunohistological staining of fibrogenesis- and inflammation-related factors in the renal tissues in rat DN models. Immunohistological staining was performed to assess the expression of TGF-β1 (A), MCP-1 (B), CTGF (C), and VDR (D), in the renal tissues in DN, on day 37 after the lentiviral injection.
Real-time PCR analysis of the renal tissues in rat DN models
The mRNA expressions of TGF-β1, MCP-1, CTGF, and VDR in rat DN models were further investigated with real-time PCR analysis (Figure 4). To further find out the changes in the expressions of these genes along with the disease progression, their mRNA expressions were examined on day 3, 14, and 37, respectively, after lentivirus injection. For TGF-β1, its expressions in the control model group were relatively low, without significant differences between different time points within the same treatment group. As expected, the expressions of TGF-β1 were dramatically elevated in TGF-β1 over-expressed groups. Compared with vehicle treatment, vitamin D3 administration significantly decreased TGF-β1 expressions, at all detected time points (Figure 4A; P < 0.05). In TGF-β1 interfered groups, the expression levels of TGF-β1 were dramatically decreased, and its expressions were even lower in the vitamin D3-treated group than the vehicle-treated group (Figure 4A; P < 0.05). For MCP-1 (Figure 4B) and CTGF (Figure 4C), TGF-β1 over-expression resulted in significant increase in their mRNA levels in these models, while TGF-β1 interference led to decrease in their mRNA expression levels. Compared with the treatment of vehicle, vitamin D3 dramatically declined the mRNA expression levels of MCP-1 and CTGF, in both situations of TGF-β1 over-expression and interference (Figure 4B and 4C; P < 0.05). For VDR, TGF-β1 over-expression significantly increased the mRNA levels of VDR, while TGF-β1 interference dramatically decreased VDR mRNA expression (Figure 4D). Compared with the treatment of vehicle, vitamin D3 dramatically up-regulated the mRNA expression levels of VDR, in both situations of TGF-β1 over-expression and interference (Figure 4D; P < 0.05). These results suggest that vitamin D3 could down-regulate the mRNA expressions of TGF-β1, CTGF, and MCP-1, and up-regulate VDR mRNA expression in DN models, which probably contribute to its protective effects on kidney.
Figure 4.
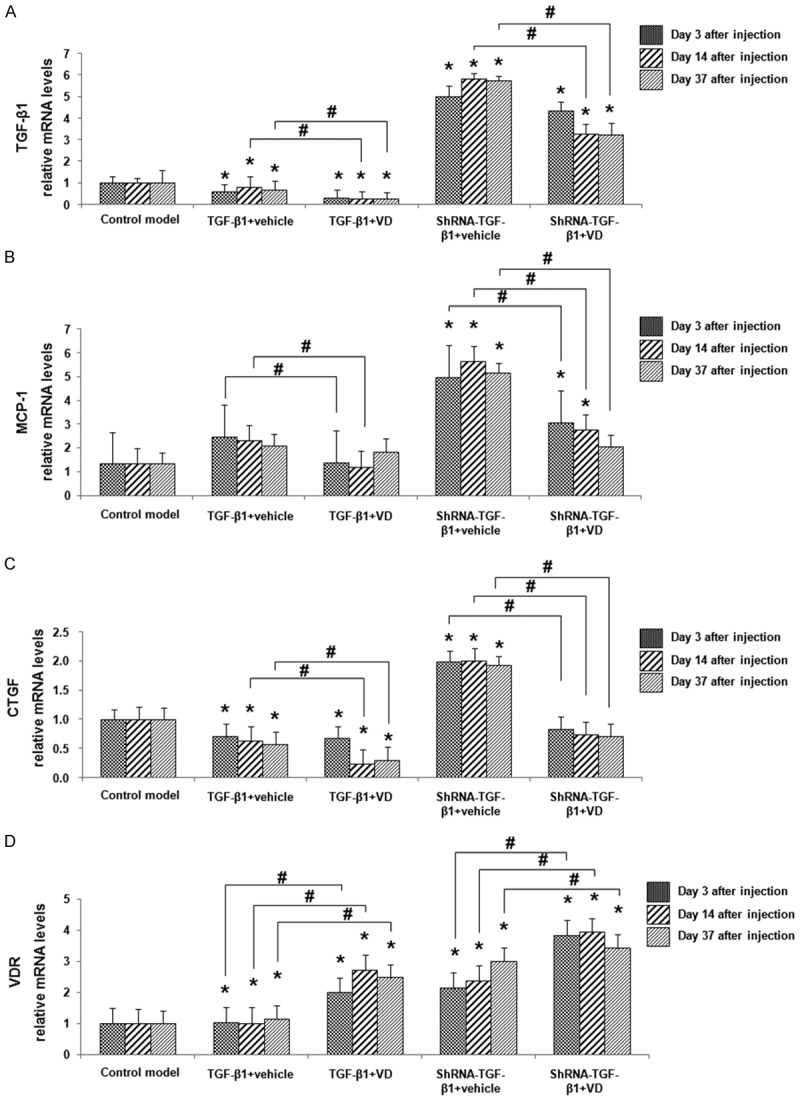
The mRNA expressions of fibrogenesis- and inflammation-related factors in the renal tissues in rat DN models. Quantitative real-time PCR was performed to assess the mRNA expression levels of TGF-β1 (A), MCP-1 (B), CTGF (C), and VDR (D), in the renal tissues in DN, on day 3, 14, and 37, after the lentiviral injection. Compared with control models at the same time point, *P < 0.05; compared with vehicle-treated group at the same time point, #P < 0.05.
Discussion
In recent years, studies reveal that the pathogenesis of diabetic nephropathy (DN) may result from metabolic disorders and hemodynamic impairment. Studies have also shown that the release of inflammatory cytokines and chemokines will lead to kidney damage [13-15]. The actions of cytokines in renal interstitial fibrosis and related mechanisms have gradually become a new hot spot in related research field, especially concerning the roles of TGF-β1, MCP-l, CTGF, and VDR, in DN pathogenesis and development.
TGF-β1 is a potent fibrogenic factor, which contributes to the glomerular hypertrophy and the progressive accumulation of extra cellular matrix (ECM) through promoting fibronectin synthesis and inhibiting ECM-degrading enzymes, ultimately resulting in glomerulosclerosis. Diamond et al. [16] found that, in unilateral ureteral obstruction model rats, mononuclear macrophage infiltration in the obstructed kidney cortex was significantly correlated with the expression of TGF-β1 mRNA in short periods, meanwhile the expression of MCP-1 was also obviously enhanced in the tubular epithelial cells.
On the other hand, CTGF, a cysteine-rich peptide, is one of the anti-fibrosis factors, which was first discovered by Bradham et al. in 1991 [17,18]. Under physiological conditions, there are small amounts of CTGF in renal interstitial cells; under certain pathological conditions, CTGF expression would be significantly enhanced. It has been also shown that CTGF expressions are elevated in varying animal models of fibrosis [19,20]. Since the biological effects of CTGF are always simple, blocking its expression or inhibiting its activity may be effective ways to prevent fibrosis.
VDR has been shown to be mainly expressed in distal convoluted tubules and collecting ducts of the kidney, which may also be found in mesangial cells, podocytes, as well as juxtaglomerular cells [21,22]. Tan et al. [8] found that, in mouse models of renal interstitial fibrosis, the expression of VDR in kidney tissue was significantly decreased, and the down-regulation of VDR could be restored to the normal level by the treatment of paricalcitol, an analog of active vitamin D. Furthermore, the therapeutic effects of paricalcitol on renal interstitial fibrosis have also been positively correlated with its regulation of VDR expression [21,23]. Vitamin D levels are higher in diabetic patients than non-diabetics, lower in females than males, which might be associated with insulin resistance and sex hormone levels [24,25]. Wood et al. [26] demonstrated that active vitamin D deficiency is one of the risk factors in patients with chronic kidney disease, establishing a vicious positive feedback loop. In our study, results from real-time PCR revealed that the expressions of VDR were elevated by the treatment of vitamin D, in both combinations with over-expressed- and shRNA-TGF-β1, compared with corresponding group treated with vehicle. In line with this, Bikle et al. [27] indicated that 1, 25-(OH)2D3 could up-regulate the expressions of VDR in various tissues of bones and muscles.
In the present study, we have constructed the lentiviral systems for TGF-β1 over-expression and interference, and the expressions of TGF-β1 were manipulated in rat DN models. H&E staining, Masson staining, immunohistochemistry, and real-time PCR analysis were performed to assess the effects of vitamin D on the renal fibrosis in these models. Our results showed that the injection of lentiviral vectors expressing GFP did not induce significant differences on renal morphology and gene expressions in these models. Compared with the vehicle-treated groups, the expressions of TGF-β1 were decreased in vitamin D-treated groups along with the time, either with TGF-β1-over-expression or -interference. Following the over-expression of TGF-β1, both CTGF and MCP-1 were elevated in the DN models, and vice versa. Based on these results, we conclude that vitamin D exerts protective effects on kidney, probably by down-regulating the expressions of TGF-β1, CTGF, and MCP-1, and up-regulating the VDR expression.
Acknowledgements
This work was supported by Natural science foundation of China grant (No. 8160116). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
All authors declare no financial and non-financial competing interests.
References
- 1.Tuttle KR. Protein kinase C-beta inhibition for diabetic kidney disease. Diabetes Res Clin Pract. 2008;82:S70–S74. doi: 10.1016/j.diabres.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 2.Huang JF, Liao ZH, Xiao HP, Qian RL. Records of the First National Conference on Diabetes and Metabolic Diseases and Nephrology in 2009. Chinese Journal Diabetes. 2009;17:54–55. [Google Scholar]
- 3.Chen XM. Nephrology in china. Nat Rev Nephrol. 2013;9:523–528. doi: 10.1038/nrneph.2013.146. [DOI] [PubMed] [Google Scholar]
- 4.Radford MG Jr, Donadio JV Jr, Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8:199–207. doi: 10.1681/ASN.V82199. [DOI] [PubMed] [Google Scholar]
- 5.Hastings MC, Moldoveanu Z, Suzuki H, Berthoux F, Julian BA, Sanders JT, Renfrow MB, Novak J, Wyatt RJ. Biomarkers in IgA nephropathy: relationship to pathogenetic hits. Expert Opin Med Diagn. 2013;7:615–627. doi: 10.1517/17530059.2013.856878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andress DL. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int. 2006;69:33–43. doi: 10.1038/sj.ki.5000045. [DOI] [PubMed] [Google Scholar]
- 7.Essien U, Goel N, Melamed ML. Role of vitamin D receptor activation in racial disparities in kidney disease outcomes. Semin Nephrol. 2013;33:416–424. doi: 10.1016/j.semnephrol.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2006;17:3382–3393. doi: 10.1681/ASN.2006050520. [DOI] [PubMed] [Google Scholar]
- 9.Firrincieli D, Braescu T, Housset C, Chignard N. Illuminating liver fibrosis with vitamin D. Clin Res Hepatol Gastroenterol. 2014;38:5–8. doi: 10.1016/j.clinre.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 11.Geraerts M, Willems S, Baekelandt V, Debyser Z, Gijsbers R. Comparison of lentiviral vector titration methods. BMC Biotechnol. 2006;6:34. doi: 10.1186/1472-6750-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dismuke D, Samulski RJ. Hepatic gene therapy using lentiviral vectors: has safety been established? Hepatology. 2013;58:13–14. doi: 10.1002/hep.26460. [DOI] [PubMed] [Google Scholar]
- 13.Matavelli LC, Huang J, Siragy HM. (Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin Exp Pharmacol Physiol. 2010;37:277–282. doi: 10.1111/j.1440-1681.2009.05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoi H, Mukoyama M, Mori K, Kasahara M, Suganami T, Sawai K, Yoshioka T, Saito Y, Ogawa Y, Kuwabara T, Sugawara A, Nakao K. Over expression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73:446–455. doi: 10.1038/sj.ki.5002722. [DOI] [PubMed] [Google Scholar]
- 15.Jaffa AA, Usinger WR, McHenry MB, Jaffa MA, Lipstiz SR, Lackland D, Lopes-Virella M, Luttrell LM, Wilson PW. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Group. Connective tissue growth factor and susceptibility to renal and vascular disease risk in type 1 diabetes. J Clin Endocrinol Metab. 2008;93:1893–1900. doi: 10.1210/jc.2007-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond JR, Kees-Folts D, Ding G, Frye JE, Restrepo NC. Macrophages, monocyte chemoattractant peptide-1, and TGF-beta 1 in experimental hydronephrosis. Am J Physiol. 1994;266:F926–F933. doi: 10.1152/ajprenal.1994.266.6.F926. [DOI] [PubMed] [Google Scholar]
- 17.Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- 18.Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letterio JJ, Böttinger EP. TGF-beta knockout and dominant-negative receptor transgenic mice. Miner Electrolyte Metab. 1998;24:161–167. doi: 10.1159/000057365. [DOI] [PubMed] [Google Scholar]
- 20.Sakai N, Chun J, Duffield JS, Wada T, Luster AD, Tager AM. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J. 2013;27:1830–1846. doi: 10.1096/fj.12-219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doorenbos CR, van den Born J, Navis G, de Borst MH. Possible renoprotection by vitamin D in chronic renal disease: beyond mineral metabolism. Nat Rev Nephrol. 2009;5:691–700. doi: 10.1038/nrneph.2009.185. [DOI] [PubMed] [Google Scholar]
- 22.Inda Filho AJ, Melamed ML. Vitamin D and kidney disease: what we know and what we do not know. J Bras Nefrol. 2013;35:323–331. doi: 10.5935/0101-2800.20130051. [DOI] [PubMed] [Google Scholar]
- 23.Tang J, Chonchol MB. Vitamin D and kidney stone disease. Curr Opin Nephrol Hypertens. 2013;22:383–389. doi: 10.1097/MNH.0b013e328360bbcd. [DOI] [PubMed] [Google Scholar]
- 24.Nashold FE, Spach KM, Spanier JA, Hayes CE. Estrogen controls vitamin D3-mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression. J Immunol. 2009;183:3672–3681. doi: 10.4049/jimmunol.0901351. [DOI] [PubMed] [Google Scholar]
- 25.Luong KV, Nguyen LT. The Role of Vitamin D in Autoimmune Hepatitis. J Clin Med Res. 2013;5:407–415. doi: 10.4021/jocmr1505w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood C, González EA, Martin KJ. Challenges in the therapy of secondary hyperparathyroldism. Ther Apher Dial. 2005;9:4–8. doi: 10.1111/j.1774-9987.2005.00208.x. [DOI] [PubMed] [Google Scholar]
- 27.Bikle DD. Vitamin D: an ancient hormone. Exp Dermatol. 2011;20:7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]