Abstract
Acute kidney injury (AKI) predicts high mortality in severely burned patients. Apoptosis plays a significant role during AKI; however, the apoptotic mechanisms underlying AKI induced by burn injury are not clear. Here, we report a critical role for tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-Death receptor 5 (DR5) signaling in the pathogenesis of AKI. C57BL/6 male mice were subjected to full thickness scald burn. Apoptosis was significantly up-regulated in mouse kidney 24 h after the burn. Meanwhile, the TRAIL and DR5 expression levels were significantly increased in the kidney 24 h after the burn. Soluble DR5 treatment reduced apoptotic cell death and alleviated kidney injury induced by the burn through blocking the interaction of endogenous TRAIL with DR5. These results demonstrated that TRAIL plays a deleterious role in AKI pathogenesis induced by scald burns. Inhibition of TRAIL function in the kidney may represent a novel protective strategy to treat AKI in patients with burns.
Keywords: TRAIL, DR5, apoptosis, acute kidney injury, burn
Introduction
Acute kidney injury (AKI) is a common severe burn injury complication and one major cause of death in septic burn patients [1,2]. Compelling evidences have suggested an important role for apoptosis in AKI [3-5]. Depending on the experimental or clinical conditions, apoptosis occurs in different segments of the renal tubule, particularly the proximal and distal tubules [6]; however, the detailed molecular mechanisms that underlie this pathogenesis are still poorly understood.
As a member of the tumor necrosis factor (TNF) family, TNF-related apoptosis-inducing ligand (TRAIL) selectively induces apoptosis in tumor cells but not in most normal cells, which makes it distinguished from other members of TNF family [7]. In humans, five TRAIL receptors have been identified. As death receptors, both TRAIL receptor 1 (TRAIL-R1, DR4) and TRAIL-R2 (DR5) contain cytoplasmic death domains to transduce apoptotic signals to activate downstream caspases [8-10]. As decoy receptors, TRAIL-R3 (DcR1, TRID) and TRAIL-R4 (DcR2, TRUNDD) contain truncated death domains, which make them could not mediate apoptosis [11]. Additionally, soluble receptor osteoprotegerin (OPG) is not a apoptosis related receptor, whose function is to inhibit osteoclastogenesis and increase bone density [12]. In mice, only one TRAIL death receptor has been identified [13], which shares 79% sequence homology with human DR5 [14]. Additionally, mice also have two decoy receptors that lack intracellular death domains [15].
TRAIL induces apoptosis via death receptor pathway in tumor cells [16]. By cross-linking with death receptor DR4 or DR5, TRAIL causes the recruitment of Fas-associated death domain (FADD) adaptor molecule, the cleavage of caspase 8, and the activation of downstream caspases 3, 7, and 6, leading to apoptosis [17]. During the TRAIL-mediated apoptotic process, the TRAIL/death receptor interaction is the first step in the initiation of apoptotic signal transduction. Among the five receptors, DR5 has the highest affinity for TRAIL at 37°C and is preferentially bound by TRAIL [18].
Both TRAIL and its receptors are expressed in the kidneys [19]. Many cancer or inflammation-related kidney disease studies evaluating TRAIL have been conducted in renal diseases. Changes in endogenous TRAIL and TRAIL receptor expression in renal diseases, such as renal cell carcinoma [20,21], lupus nephritis [22] and diabetic nephropathy [23], have suggested that the TRAIL signaling pathway might participate in many renal diseases. The role of the TRAIL signaling pathway in AKI caused by burn injury remains unclear, however.
In this study, the contribution of the TRAIL-DR5 signaling pathway to apoptosis in severe-burn-injury-induced AKI was investigated in a mouse model. Furthermore, the potential of sDR5 to target the TRAIL-DR5 signaling pathway for the treatment of AKI was explored.
Materials and methods
Full thickness scald burn mouse model
Male C57BL/6 mice (22.9 ± 0.7 g, 6-8 weeks) were purchased from SLAC Laboratory Animal Co. Ltd. (Shanghai, China). This study was performed according to the National Institute of Health Guidelines for the Use of Laboratory Animals. The animal protocol was approved by the Shandong University Animal Use Committee. All efforts were made to minimize suffering.
The full thickness scald burn model was established as previously described [24]. Briefly, male C57BL/6 mice (n = 10 per group) were anesthetized using sodium pentobarbital (60 mg/kg intraperitoneally) and 2% lidocaine with 1:100000 epinephrine. Then, the back and flank skin were shaved, and the mice were placed on a special template. The skin was immersed in 98°C water for 12 s to produce a full-thickness dermal burn comprising 30% of the total body surface area (TBSA). Sham-operated mice were exposed to room temperature (25°C) water. After immersion, all animals were immediately resuscitated with lactated ringer solution (4 mL/kg intraperitoneally). The breathing and heart rates of the burned mice were carefully monitored to ensure that all mice were under anesthetic and without pain before they were recovered from anesthesia.
Recombinant human sDR5 expression and purification
The recombinant human sDR5 protein was expressed by the Pichia pastoris (Pp) GS115 strain (pGAPZaA-sDR5) [25] and purified with ProBondTM affinity chromatography columns (Invitrogen, Carlsbad, CA). The sDR5 protein is a human truncated DR5, which can effectively block TRAIL induced apoptosis in both mouse and human systems [25-27].
TRAIL blockade in vivo
To investigate the effects of TRAIL blockade on AKI in severely burned mice, soluble DR5 (sDR5) was applied to block the TRAIL apoptotic signaling pathway. The mice subjected to a dermal burn were randomized to vehicle (equivalent PBS intraperitoneally), or soluble DR5 protein (20 mg/kg in PBS intraperitoneally). These treatments were immediately given after burn treatment. The second injection was performed 1 h after the first treatment.
Animals receiving vehicle treatment were sacrificed at 3 h, 6 h, and 24 h following burn injury (n = 6 for each time point), while those receiving sDR5 treatment were sacrificed 24 h following burn injury (n = 6 for each treatment). Blood and kidney tissues were collected for biochemical assay, TUNEL assay, histological analysis, and TRAIL and DR5 expression assays.
Renal function monitoring
Creatinine and BUN serum levels were measured with a Hitachi 7060 Fully Automated Biochemistry Analyzer (Tokyo, Japan) using a clinical chemistry analyzer system and kits (Prochem-V, Drew Scientific, Dallas, TX).
Histological examination for tubular damage
The harvested kidneys were fixed in 10% formalin. The tissues were dehydrated, embedded with paraffin wax, cut into 6 mm sections and mounted. After the tissues were removed from the paraffin, they were stained with hematoxylin and eosin. Histological feature of kidney tubular epithelial injury includes the proximal tubular brush border loss, apical membrane blebbing, tubular cell detachment, tubular epithelium vacuolation, and intraluminal aggregation [28]. Five visual fields of each section were randomly chosen. Histological injury was scored based on the percentage of tubules with epithelial injury: 0, normal; 1, < 10%; 2, 10 to 25%; 3, 26 to 75%; and 4, > 75%.
Terminal deoxyribonucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay
A TUNEL assay was performed using the in situ Cell Death Detection Kit (Roche Molecular Biochemicals, Mannheim, Germany). The paraffin sections were attached to glass slides, dewaxed with xylene, and rehydrated through a series of decreasing concentrations of ethanol. Then, the slides were placed in a plastic jar containing 200 mL of 0.1 M citrate buffer (pH 6.0), and 350 W microwave irradiation was applied for 5 min, after which the slides were washed four times with PBS. Thereafter, the sections were incubated for 60 min at 37°C with the TUNEL reaction mixture (50 μL), followed by three PBS rinses. The stained sections were observed by fluorescence microscopy.
Apoptosis in the paraffin-embedded tumor tissues was detected by the Dead End Fluorimetric system following the manufacturer’s protocol as previously reported [29]. The samples were immediately analyzed under a fluorescence microscope (Olympus Optical Co LTD, Tokyo, Japan) using a standard fluorescent filter set to view the green fluorescence at 520 nm. Renal tissue apoptosis was quantitatively determined by grinding renal tissues into a single cell suspension; the cell suspension was then TUNEL stained, and apoptosis was detected by flow cytometry (Beckman Coulter, Miami, FL).
Western blot analysis
Frozen kidney tissue samples were incubated with SDS sample buffer containing 62.5 mM Tris-HCl (pH 6.8), 2% W/V SDS, 10% glycerol, 50 mM DTT, 0.01% W/V bromophenol blue, 1 mM PMSF, 1 μg/mL leupeptin, and 2 μg/mL aprotinin for 10 min on ice. After sonication, the lysates boiled for 5 min and then cooled on ice. After centrifugation, equivalent amounts of proteins were loaded onto 10% SDS-PAGE gels on a Minigel apparatus. Proteins were transferred onto nitrocellulose membranes by electrophoresis and then were incubated overnight at 4°C with the following primary antibodies: anti-TRAIL (eBioscience, Mountain View, CA), anti-DR5 (eBioscience, Mountain View, CA), and anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were washed with TBS-T and incubated with a secondary antibody at room temperature for 1 h. Protein bands were visualized by ECL-Plus reagent (GE Healthcare, Piscataway, NJ). The results were normalized to β-actin.
Statistical analysis
The results are presented as the mean ± SD. The data were analyzed by one-way ANOVA (San Diego, CA, USA). A value of P < 0.05 was considered to be significant.
Results
Burn injury caused AKI in mice
To study the time dependent effect of burn-induced AKI, two well-known renal function indexes, the BUN and serum creatinine levels, were measured at 3 h, 6 h, and 24 h after burn injury. Both BUN and creatinine increased significantly in a time-dependent manner during the first 24 h after burn injury (Figure 1). Furthermore, histological examinations of the mice 24 h following burn injury revealed that the burn injuries caused epithelial cell necrosis, vacuolation, and desquamation in the renal tubules of the vehicle-treated mice with higher tubular damage score (2.0 ± 0.5; P < 0.05) than the sham-burned group (0.25 ± 0.02) (Figure 2). All of these above results indicated that severe burn injury (30% of TBSA) resulted in significant renal dysfunction and tubular damage at 24 h in the mice; therefore, we selected 24 h following burn injury as the time point for this study.
Figure 1.

BUN and creatinine levels in mice postburn. Analysis of BUN and creatinine (A, B) at different time points (3 h, 6 h, and 24 h) after burn injury and (C, D) with different treatments 24 h after burn injury. These data demonstrated that burn caused significant renal dysfunction at 24 h, which was improved by blocking the TRAIL-DR5 pathway with sDR5 at 24 h. *, P < 0.05 Vehicle vs. Sham; #, P < 0.05 Vehicle vs. sDR5. n = 10 mice for each group.
Figure 2.
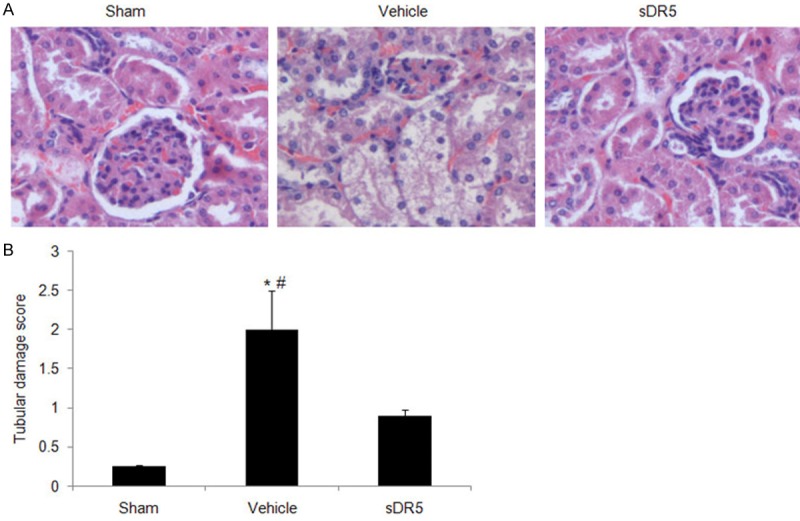
Renal histopathological injury in the mice 24 h after burn injury. A. Representative images (magnification = 200 ×) demonstrate the tubular damage with different treatments after burn using hematoxylin and eosin staining. B. Tubular damage score was got based on the histological examinations. These data showed that the burn caused significant renal damage at 24 h, which was improved by blocking the TRAIL-DR5 pathway with sDR5 at 24 h. *, P < 0.05 Vehicle vs. Sham; #, P < 0.05 Vehicle vs. sDR5. n = 10 mice for each group.
Burn injury induced renal tubular cell apoptosis
TUNEL staining in situ showed that tubular apoptotic cells increased significantly 24 h after burn injury compared with the sham-burned group (Figure 3A). The TUNEL positive cells were further quantified by flow cytometry. As shown in Figure 3B, burn injury induced significant apoptosis at the 24 h time point (21.6 ± 4.25% vs. 1.8 ± 0.5% in the sham-burned group; P < 0.05). Additionally, an increase in apoptosis of approximately 12-fold was observed in the burned mice at 24 h compared with the sham-burned group (P < 0.05). All of these results indicated that the severe burns induced obvious apoptotic kidney injury in the mice 24 h after burn injury.
Figure 3.
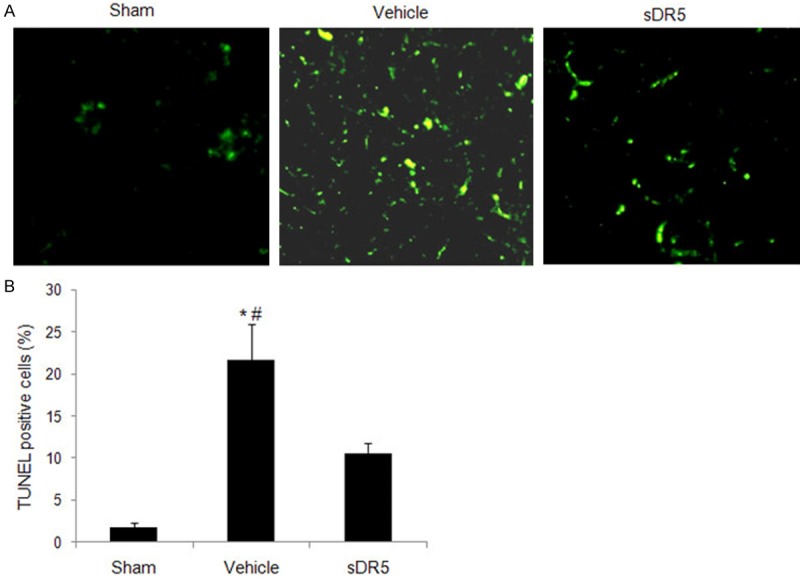
Renal cell apoptosis in mice at 24 h after burn injury. Burn induced renal cell apoptosis represented by renal TUNEL staining (magnification = 400 ×) was observed by fluorescence microscopy (A) and flow cytometry (B) with different treatments at 24 h post-burn injury. The data showed that the burns induced increased renal cell apoptosis at 24 h after burn injury. Blocking the TRAIL-DR5 pathway with sDR5 alleviated the renal apoptotic injury. *, P < 0.05 Vehicle vs. Sham; #, P < 0.05 Vehicle vs. sDR5. n = 10 mice for each group.
TRAIL and DR5 expression increased in the mouse kidneys after full-thickness scald burn
TRAIL induced apoptosis plays a prominent role in many diseases, including cancer [21]. In pathological conditions, the expression patterns of TRAIL and its receptors are usually changed [22]. Whether TRAIL induced apoptosis is involved in the kidney-injury-associated apoptosis induced by burn injury is not clear. Because the TRAIL-DR5 interaction is the first step in the TRAIL signaling pathway, we investigated TRAIL and DR5 protein expression levels in mouse kidneys by western blotting following full thickness scald burn injury. As shown in Figure 4, both TRAIL and DR5 proteins were detected in both sham-operated and vehicle-treated burn mice. Furthermore, burn injury significantly increased TRAIL and DR5 expression 24 h following burn injury (Figure 4). These data suggest that TRAIL induced apoptosis might be involved in kidney injury associated apoptosis in mice induced by burn injury.
Figure 4.
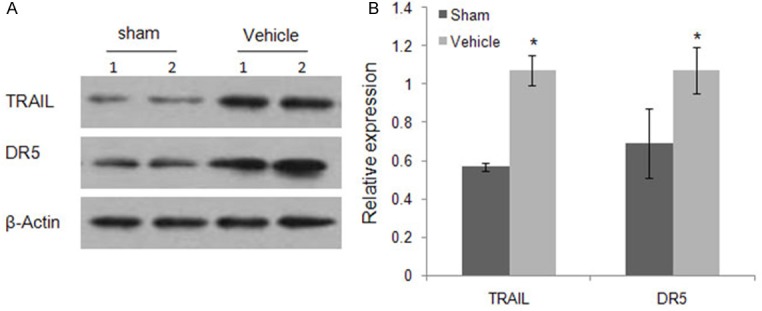
TRAIL and DR5 expression in the kidneys of mice post-burn injury. Burn injury induced elevated TRA-IL and DR5 expression in the kidneys of mice at 24 h after the burn was determined by western blot. Anti-actin blots show protein loading. A. Equal amounts of immunoblotted proteins from two representative kidney lysates of the sham control and vehicle treatment groups were assayed. B. Western blotting bands from three independent measurements were quantified with Image J. The relative expression of each band = (density of each band/density of the actin band). Mean ± SD was from three independent measurements. *, P < 0.05 Vehicle vs. Sham. n = 10 mice for each group.
sDR5 administration alleviated kidney apoptotic cell death, histological damage, and renal dysfunction induced by severe scald burn
To investigate whether TRAIL-DR5 interactions played a role in severe scald-burn-mediated kidney injury, we used a soluble DR5 protein to block TRAIL activity in vivo. Previously, we showed that sDR5 could bind TRAIL with a high affinity and inhibit TRAIL-stimulated cellular apoptosis in vitro and in vivo [25-27,30]. In this study, sDR5 protein or PBS vehicle was administered intraperitoneally, once immediately and once 1 h after the burn.
sDR5 treatment significantly decreased cellular apoptosis in the mouse kidneys compared with the vehicle-treated group, as shown by TUNEL staining in situ (Figure 3A) and flow cytometry (Figure 3B). The therapeutic effects of sDR5 were also observed by histological scoring. As shown in Figure 2, renal histopathological injury in the burned mice was significantly reduced in the sDR5-treated mice compared with the vehicle-treated mice. Additionally, as an important renal function biomarker, the BUN and serum creatinine levels were also significantly decreased compared with the vehicle-treated mice, which provide further support for the alleviated renal damage by sDR5 administration in the mouse scald burn injury model.
Taken together, these data demonstrate that the TRAIL-DR5 interactions played a role in the observed severe scald-burn-mediated kidney injury in mice and that sDR5 had therapeutic AKI benefits.
Discussion
AKI is a common complication in patients with severe burn injury. Apoptosis is gradually gaining importance in the understanding of AKI development [3,31-33]. Apoptosis can be triggered by ischemia, exogenous toxins, or endogenous mediators. Understanding the molecular mechanisms underlying apoptosis is crucial for effective therapeutic strategy development. However, the mechanisms underlying apoptosis during AKI are not well clarified. In this study, TRAIL-DR5 apoptosis pathway was found to play an important role in AKI induced by burn injury in a mouse severe burn injury model. Specifically, we showed that: (1) Apoptosis was up-regulated in the kidney after burn injury; (2) TRAIL and DR5 expression levels were up-regulated in the kidney after burn injury, and; (3) Functional TRAIL blockade by sDR5 effectively alleviated apoptotic renal cell death and kidney damage, indicating TRAIL is a contributing factor in the development of AKI induced by burn injury.
As members of the TNF superfamily, TNFα and FasL, have been reported to be involved in burn pathogenesis [34,35]. Furthermore, TNFα was detected in burn acute renal failure plasma [36], indicating that it might be involved in the AKI induced by burn injury. As the third member of TNF superfamily, TRAIL is expressed in glomerular tubules [37], while TRAIL-R2 (DR5) is additionally expressed in Henle’s loop [38] in the normal kidney, indicating that TRAIL is not toxic to kidney tissue under physiological conditions. Although TRAIL is not required during normal kidney development and physiology [39], the TRAIL apoptotic signaling pathway was reported to be involved in many renal diseases [4], such as renal cell carcinoma [20,21], lupus nephritis [22], and diabetic nephropathy [23]. In these pathological conditions, the expression pattern of TRAIL and its receptors were usually changed. So far, there is no report regarding the role of the TRAIL signaling pathway in AKI induced by burn injury.
In this study, we observed that apoptosis was increased in mouse kidneys following burn injury. Simultaneously, TRAIL and DR5 expression were up-regulated in the kidneys of mice induced by burn injury. Furthermore, intraperitoneal sDR5 protein injection significantly reduced apoptotic cell death in the kidney and alleviated renal dysfunction. These data indicate that TRAIL is acting as a toxic factor in the AKI induced by burn injury. Essentially, organ injury induced by burn, like AKI, was caused by ischemia. TRAIL and DR5 expression were also up-regulated during neuronal damage after transient global cerebral ischemia [27]. This last report is consistent with our findings regarding AKI induced by burn. All of these studies suggested that the TRAIL mediated apoptotic pathway was involved in the pathologic process caused by ischemia.
TRAIL [37] and DR5 [38] are normally expressed in the kidneys [40], but no toxicity has been reported under normal physiological conditions. The important consequences induced by TRAIL in the kidneys need to be considered in the context of renal cell sensitivity to TRAIL in normal and pathologic conditions. Cellular DR5 expression was considered to be the crucial step in TRAIL-mediated killing [41]. In this study, up-regulated DR5 expression in renal tissues induced by burn injury could enhance their sensitivity to TRAIL-induced apoptosis. Additionally, a recent report indicated that the IFN-α cytokine can make renal cell carcinoma more sensitive to TRAIL-induced apoptosis [20]. Accumulating evidence suggests that inflammatory mediators and inflammatory cells, are all participated in AKI pathogenesis [42,43]. These findings may explain why TRAIL can induce apoptotic renal cell death during AKI induced by burn injury but illicit no toxic effect on normal kidneys.
In summary, out results suggest that TRAIL-DR5 apoptotic pathway plays a great role in AKI induced by burn injury. Blocking TRAIL apoptosis pathway with soluble DR5 might be effective for AKI therapy in burn patients.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21307077), Doctoral Fund of the Ministry of Education of China (20130131120007), China Postdoctoral Science Foundation funded project (2013M541920) and Natural Science Foundation of Shandong Province (ZR2013BQ026).
Disclosure of conflict of interest
None.
References
- 1.Schneider DF, Dobrowolsky A, Shakir IA, Sinacore JM, Mosier MJ, Gamelli RL. Predicting acute kidney injury among burn patients in the 21st century: a CART analysis. J Burn Care Res. 2011;33:242–251. doi: 10.1097/BCR.0b013e318239cc24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmieri T, Lavrentieva A, Greenhalgh DG. Acute kidney injury in critically ill burn patients. Risk factors, progression and impact on mortality. Burns. 2010;36:205–211. doi: 10.1016/j.burns.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Kockara A, Kayatas M. Renal cell apoptosis and new treatment options in sepsis-induced acute kidney injury. Renal Failure. 2013;35:291–294. doi: 10.3109/0886022X.2012.744040. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Niño MD, Benito-Martin A, Gonçalves S, Sanz AB, Ucero AC, Izquierdo MC, Ramos AM, Berzal S, Selgas R, Ruiz-Ortega M. TNF superfamily: a growing saga of kidney injury modulators. Mediators Inflamm. 2010;2010:182958. doi: 10.1155/2010/182958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonegio R, Lieberthal W. Role of apoptosis in the pathogenesis of acute renal failure. Curr Opin Nephrol Hypertens. 2002;11:301–308. doi: 10.1097/00041552-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Pabla N, Wei Q, Dong Z. Essentials of Apoptosis. Springer: 2009. Apoptosis in acute kidney injury; pp. 565–579. [Google Scholar]
- 7.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 8.Pan G, Ni J, Wei YF, Yu Gl, Gentz R, Dixit VM. An antagonist decoy receptor and a death domaincontaining receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 9.Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Screaton GR, Mongkolsapaya J, Xu XN, Cowper AE, McMichael AJ, Bell JI. TRICK2, a new alternatively spliced receptor that transduces the cytotoxic signal from TRAIL. Curr Biol. 1997;7:693–696. doi: 10.1016/s0960-9822(06)00297-1. [DOI] [PubMed] [Google Scholar]
- 11.Mundt B, Kühnel F, Zender L, Paul Y, Tillmann H, Trautwein C, Manns MP, Kubicka S. Involvement of TRAIL and its receptors in viral hepatitis. FASEB J. 2003;17:94–96. doi: 10.1096/fj.02-0537fje. [DOI] [PubMed] [Google Scholar]
- 12.Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 13.Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 14.Wu GS, Burns TF, Zhan Y, Alnemri ES, El-Deiry WS. Molecular cloning and functional analysis of the mouse homologue of the KILLER/ DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 1999;59:2770–2775. [PubMed] [Google Scholar]
- 15.Schneider P, Olson D, Tardivel A, Browning B, Lugovskoy A, Gong D, Dobles M, Hertig S, Hofmann K, Van Vlijmen H. Identification of a new murine tumor necrosis factor receptor locus that contains two novel murine receptors for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Biol Chem. 2003;278:5444–5454. doi: 10.1074/jbc.M210783200. [DOI] [PubMed] [Google Scholar]
- 16.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 17.Özören N, El-Deiry WS. Cell surface Death Receptor signaling in normal and cancer cells. Semin Cancer Biol. 2003;13:135–147. doi: 10.1016/s1044-579x(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 18.Truneh A, Sharma S, Silverman C, Khandekar S, Reddy MP, Deen KC, Mclaughlin MM, Srinivasula SM, Livi GP, Marshall LA. Temperature-sensitive differential affinity of TRAIL for its receptors DR5 is the highest affinity receptor. J Biol Chem. 2000;275:23319–23325. doi: 10.1074/jbc.M910438199. [DOI] [PubMed] [Google Scholar]
- 19.Lorz C, Benito A, Ucero AC, Santamaria B, Ortiz A. Trail and kidney disease. Front Biosci (Landmark Ed) 2009;14:3740–3749. doi: 10.2741/3485. [DOI] [PubMed] [Google Scholar]
- 20.Clark PE, Polosukhina DA, Gyabaah K, Moses HL, Thorburn A, Zent R. TRAIL and Interferon-α Act Synergistically to Induce Renal Cell Carcinoma Apoptosis. J Urol. 2010;184:1166–1174. doi: 10.1016/j.juro.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voelkel-Johnson C. TRAIL-mediated signaling in prostate, bladder and renal cancer. Nature Rev Urol. 2011;8:417–427. doi: 10.1038/nrurol.2011.81. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen V, Cudrici C, Zernetkina V, Niculescu F, Rus H, Drachenberg C, Rus V. TRAIL, DR4 and DR5 are upregulated in kidneys from patients with lupus nephritis and exert proliferative and proinflammatory effects. Clin Immunol. 2009;132:32–42. doi: 10.1016/j.clim.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Candido R. The osteoprotegerin/tumor necrosis factor related apoptosis-inducing ligand axis in the kidney. Curr Opin Nephrol Hypertens. 2013;23:69–74. doi: 10.1097/01.mnh.0000437611.42417.7a. [DOI] [PubMed] [Google Scholar]
- 24.Gao C, Huan J, Li W, Tang J. Protective effects of ulinastatin on pancreatic and renal damage in rats following early scald injury. Burns. 2009;35:547–552. doi: 10.1016/j.burns.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Song K, Chen Y, Göke R, Wilmen A, Seidel C, Göke A, Hilliard B, Chen Y. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J Exp Med. 2000;191:1095–1104. doi: 10.1084/jem.191.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu YG, Liu SX, Liang XH, Zhang Q, Gao LF, Han LH, Cao YL, Hou N, Du J, Sun WS. Blockade of TRAIL pathway ameliorates HBV-induced hepatocyte apoptosis in an acute hepatitis model. Biochem Biophys Res Comm. 2007;352:329–334. doi: 10.1016/j.bbrc.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Cui M, Wang L, Liang X, Ma X, Liu Y, Yang M, Liu K, Wei X, Zhou Z, Chen YH. Blocking TRAIL-DR5 signaling with soluble DR5 reduces delayed neuronal damage after transient global cerebral ischemia. Neurobiol Dis. 2010;39:138–147. doi: 10.1016/j.nbd.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Bátkai S, Gao B, Haskó G, Pacher P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Rad Biol Med. 2010;48:457–467. doi: 10.1016/j.freeradbiomed.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollinedo F, Gajate C. Microtubules, microtubule-interfering agents and apoptosis. Apoptosis. 2003;8:413–450. doi: 10.1023/a:1025513106330. [DOI] [PubMed] [Google Scholar]
- 30.Liang X, Liu Y, Zhang Q, Gao L, Han L, Ma C, Zhang L, Chen YH, Sun W. Hepatitis B virus sensitizes hepatocytes to TRAIL-induced apoptosis through Bax. J Immunol. 2007;178:503–510. doi: 10.4049/jimmunol.178.1.503. [DOI] [PubMed] [Google Scholar]
- 31.Wan L, Bellomo R, Di Giantomasso D, Ronco C. The pathogenesis of septic acute renal failure. Curr Opin Crit Care. 2003;9:496–502. doi: 10.1097/00075198-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80:29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furuichi K, Kokubo S, Hara A, Imamura R, Wang Q, Kitajima S, Toyama T, Okumura T, Matsushima K, Suda T, Mukaida N, Kaneko S, Wada T. Fas Ligand Has a Greater Impact than TNF-alpha on Apoptosis and Inflammation in Ischemic Acute Kidney Injury. Nephron Extra. 2012;2:27–38. doi: 10.1159/000335533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada Y, Endo S, Nakae H, Makabe H, Sato N, Wakabayashi G, Kitamura M, Inada K, Sato S. Examination of soluble Fas (sFas) and soluble Fas ligand (sFasL) in patients with burns. Burns. 2003;29:799–802. doi: 10.1016/s0305-4179(03)00201-8. [DOI] [PubMed] [Google Scholar]
- 35.Marano M, Fong Y, Moldawer L, Wei H, Calvano S, Tracey K, Barie P, Manogue K, Cerami A, Shires G. Serum cachectin/tumor necrosis factor in critically ill patients with burns correlates with infection and mortality. Surg Gynecol Obst. 1990;170:32. [PubMed] [Google Scholar]
- 36.Oudemans-van Straaten HM. Circulating pro-apoptotic mediators in burn septic acute renal failure. Crit Care. 2008;12:126. doi: 10.1186/cc6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorz C, Benito-Martín A, Boucherot A, Ucero AC, Rastaldi MP, Henger A, Armelloni S, Santamaría B, Berthier CC, Kretzler M. The death ligand TRAIL in diabetic nephropathy. J Am Soc Nephrol. 2008;19:904–914. doi: 10.1681/ASN.2007050581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spierings DC, de Vries EG, Vellenga E, van den Heuvel FA, Koornstra JJ, Wesseling J, Hollema H, de Jong S. Tissue distribution of the death ligand TRAIL and its receptors. J Histochem Cytochem. 2004;52:821–831. doi: 10.1369/jhc.3A6112.2004. [DOI] [PubMed] [Google Scholar]
- 39.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 40.Malyszko J, Przybylowski P, Koc-Zorawska E, Mysliwiec M. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Is a Marker of Kidney Function and Inflammation in Heart and Kidney Transplant Recipients. Transplant Proc. 2011;43:1877–1880. doi: 10.1016/j.transproceed.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 41.Ransohoff RM, Trapp BD. Taking two TRAILS. Neuron. 2005;46:355–356. doi: 10.1016/j.neuron.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 42.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2010;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009;130:41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


