Abstract
To establish a series of objective parameters to predict the risk of relapse from axillary lymph node-negative (ANN) breast cancer, and evaluate the patterns of recurrence according to molecular subtypes, we collected information on 2126 consecutive breast cancer patients operated between 2002 and 2006. In this case-control study, 212 patients experiencing recurrence or breast cancer related death were defined as ‘poor group’. Another 212 patients were selected from the remaining cases with stratified sampling method to comprise the ‘good group’. Significant differences were found in vascular invasion, grade and molecular subtype between the two groups. Expression of ER and PR in the ‘poor group’ was lower (P < 0.05). However, positive rates of Ki67, p53 and VEGF in the ‘poor group’ were higher (P < 0.05). Multivariate analysis revealed that molecular subtype, expression of VEGF, tumor grade, and vascular invasion were closely correlated with bad outcome. Analysis of the ‘poor group’ demonstrated that ‘HER2 positive’ and ‘triple negative’ subtypes more commonly suffered from distant metastases and death. No metastasis was found in patients with pure invasive papillary carcinoma, invasive cribriform carcinoma or adenoid cystic carcinoma, whereas the diagnoses of invasive micropapillary carcinoma, invasive apocrine carcinoma, invasive papillary carcinoma mixed with invasive ductal carcinoma, or metaplastic carcinoma were correlated with distant metastasis and death. In conclusion, molecular subtype and expression of VEGF are useful markers for predicting prognosis of ANN breast cancer patients. ‘Luminal A-like’ subtype has better outcome than others. Moreover, molecular subtypes have different recurrence patterns.
Keywords: Breast cancer, molecular subtype, p53, VEGF, recurrence
Introduction
Approximately 65-70% of breast cancers are localized in the breast without involvement of axillary lymph nodes or other sites [1]. Although the prognosis of patients with axillary lymph node-negative (ANN) breast cancer is relatively good, approximately one-third of them experience disease recurrence after initial diagnosis [2,3].
The choice of utilizing chemotherapy for ANN women is paramount. Not all the patients derive same degree of benefit from the same chemotherapy regimen. Several problems still exist, for example, which kind of patients needs more powerful adjuvant therapy to minimize recurrence rate? Is there any simple and efficient marker in this field? Those mean searches for more effective and adequate prognosis evaluation tools. Although the Oncotype DX™ assay analyzing the levels of 21 genes, recurrence score (RS), is a potentially powerful tool for stratification of HR-positive patients, it is too expensive to use in routine clinical practice [4]. Developing an alternative assay which is inexpensive and convenient to use becomes the urgent need. The features in previous studies indicating increased risk of relapse include young age [5], extensive vascular invasion [6], presence of high grade, and overexpression of human epidermal growth factor receptor 2 (HER2) [7]. Breast cancer is a collection of diseases demonstrating heterogeneity at the molecular, histopathologic and clinical levels. Gene expression studies have identified molecularly distinct subtypes with prognostic implications across multiple treatment settings [8,9]. These subtypes include ‘luminal A-like’, ‘luminal B-like (HER2 negative)’, ‘luminal B-like (HER2 positive)’, ‘HER2 positive’ and ‘triple negative’ [10]. Although treatment has been shown to vary according to these subtypes, limited information is available for each subtype with regard to patterns of disease recurrence especially in ANN patients.
The p53 tumor suppressor gene has a regulatory function in defense against various kinds of cancer, including breast cancer [11]. The mutant type of p53 protein accumulates within malignant cells. Sequencing studies have shown strong prognostic significance of p53 mutations in breast cancer [12,13]. In addition, vascular endothelial growth factor (VEGF) and angiogenesis are important to tumor growth and metastasis in breast cancer [14]. The combination detection of p53 and VEGF may provide clues to predict prognosis.
In the current case-control study, we used a cohort of ANN breast cancer patients from a single institution in China and made a detailed comparison between cases with good prognosis and those with poor prognosis to determine the potential indicators predicting outcome. Then we analyzed the recurrence pattern according to subtypes and recurrent patterns of special types of invasive breast cancer. The aim of the study was to find a limited number of objective parameters, choose a simple and validated method of determination and then use them to predict the risk of local recurrence or distant metastases in ANN breast cancer.
Materials and methods
Study population
We conducted a retrospective chart review of ANN breast cancer patients between January 1, 2002 and December 31, 2006 treated in the Tianjin Medical University Cancer Institute and Hospital, whose tumors were primary, invasive, without involvement in other sites, and were followed up appropriately. All the patients underwent preoperative breast mammography and ultrasound of the breast and abdomen, and X-ray or computed tomography (CT) scan of the thorax. The surgically removed breast lesions were thoroughly sampled for pathological examination. All the patients were diagnosed as invasive carcinoma based on paraffin-embedded slices after operation by two pathologists. The pathological stage of tumor was assessed according to the criteria established by the 7th edition of the American Joint Committee on Cancer (AJCC) staging manual. Histological grade of the tumors were classified into I-III according to Elston and Ellis’ criterion [15]. Peritumoural vascular invasion was assessed following the recommendation by Rosen and Obermann [16]. During the follow-up time, physical examinations and radiographic inspections were applied every 6-12 months for 5 years, and then annually. Among 2126 eligible ANN patients, 212 patients experienced local recurrence or distant metastasis or breast cancer related death during the follow-up interval, who were defined as ‘poor group’. A 1:1 case-control group was designed by stratified sampling method [17], depending on the patients’ age (pre-menopause and post-menopause), the clinical stage and the pathologic types of the ‘poor group’. Another 212 patients were selected from the remaining cases to comprise the ‘good group’. We also gathered data on tumor grade, estrogen-receptor (ER), progesterone-receptor (PR), HER2 status, Ki67 labeling index, p53, VEGF positivity, operation mode, treatment application, and recurrence status. The exclusion criteria were: male patients, females with previous cancer history, non-invasive breast cancers, bilateral tumors, the patients treated with neoadjuvant chemotherapy, and the patients lost follow-up.
Detection methods and standardization assessment
Immunostaining for the localization of ER, PR, HER2 protein, Ki67 antigen, p53 and VEGF was performed on consecutive tissue sections from primary disease. ER and PR were categorized as negative (< 1%) and positive (≥ 1%), in accordance with recent guidelines [18]. HER2-positive cases were defined as immunohistochemistry (IHC) score of 3+ or IHC score of 2+ plus fluorescent in situ hybridization with amplification ratio ≥ 2.0. Ki67 status was expressed in terms of percentage of positive cells, with a threshold of 14% of positive cells [19]. For p53, positive staining of fewer than 10% of the tumor cells was defined as negative expression and staining of 10% or more of the tumor cells as positive expression [20]. As for VEGF, at least 10% of cells needed to be stained to be considered positive [21].
Molecular subtype criteria
The criteria for subtype classification were as follows [10]: ‘luminal A-like’ (ER positive and PR ≥ 20% and HER2 negative and Ki-67 < 14%), ‘luminal B-like (HER2 negative)’ (ER positive and HER2 negative and at least one of: Ki-67 ≥ 14% or PR < 20%), ‘luminal B-like (HER2 positive)’ (ER and HER2 positive, with any Ki67 and PR), ‘HER2 positive’ (ER and PR negative and HER2 positive), and ‘triple negative’ (ER and PR negative and HER2 negative).
Statistical analysis
The SPSS software (Version 17.0 for Windows) was used to carry out the statistical analyses. The correlation analyses between different prognosis groups and subtypes were examined by the χ2 test. The multivariate analysis using a logistic multiple regression model P < 0.05 was considered statistically significant.
Results
Patient cohort
212 cases (9.5%) were assembled in the ‘poor group’, with the median follow-up of 76.6 months for patients who were alive. All cases developed recurrence, consisting of local (confined to ipsilateral breast or chest wall including mastectomy scars), contralateral, regional-nodal (including ipsilateral axillary, supraclavicular, and internal mammary lymph node metastases), or distant metastases (including bone, bone marrow, lung, liver, brain, and other organs). These recurrent foci were detected and proven through roentgenography, sonography, computed tomography, radioisotope scanning, magnetic resonance imaging, or puncture biopsy. 108 cases among them had died from the disease. For 212 patients who were defined as ‘good group’, median follow-up time was 77.8 months.
Clinicopathologic features were presented in Table 1. Among all patients, significant differences were found in peritumoural vascular invasion, grade and molecular subtype between poor and good groups (P < 0.05). Invasion of peritumoural vascular vessels and poor differentiation were strictly correlated with recurrence (P < 0.05). Distribution of molecular subtypes in ‘poor’ and ‘good’ groups was different with statistical significance (P < 0.05). ‘Luminal A-like’ subtype accounted for 41.5% in the ‘good group’, but only 24.1% in the ‘poor group’. There was no significant difference in age, tumor size, menopausal status, histological type, or treatment (P > 0.05).
Table 1.
Characteristic of patients with poor and good prognosis
| Characteristic | Poor group | % | Good group | % | P value |
|---|---|---|---|---|---|
| All | 212 | 212 | |||
| Age at diagnosis, years | |||||
| Mean (range) | 54.0 (29-83) | 54.3 (29-84) | 0.762 | ||
| Menopausal status | |||||
| Premenopausal | 102 | 48.1 | 102 | 48.1 | |
| Postmenopausal | 110 | 51.9 | 110 | 51.9 | 1.0 |
| Tumor size | |||||
| ≤ 2 cm | 64 | 30.2 | 64 | 30.2 | |
| 2-5 cm | 142 | 67.0 | 142 | 67.0 | |
| > 5 cm | 8 | 3.8 | 8 | 3.8 | 1.0 |
| Histological type | |||||
| Infiltrative ductal carcinoma | 189 | 89.2 | 189 | 89.2 | |
| Infiltrative lobular carcinoma | 6 | 2.8 | 6 | 2.8 | |
| Other infiltrative carcinoma | 17 | 8.0 | 17 | 8.0 | 1.0 |
| Family history | |||||
| Yes | 31 | 14.6 | 27 | 12.7 | |
| No | 181 | 85.4 | 185 | 87.3 | 0.572 |
| Peritumoural vascular invasion | |||||
| Absent | 180 | 84.9 | 200 | 94.3 | |
| Present | 32 | 15.1 | 12 | 5.7 | 0.001 |
| Tumor grade | |||||
| Low | 6 | 2.8 | 25 | 11.8 | |
| Intermediate | 159 | 75.0 | 158 | 74.5 | |
| High | 43 | 20.3 | 24 | 11.3 | |
| Unknown | 4 | 1.9 | 5 | 2.4 | 0.001 |
| Molecular subtype | |||||
| ‘Luminal A-like’ | 51 | 24.1 | 88 | 41.5 | |
| ‘Luminal B-like (HER2 negative)’ | 38 | 17.9 | 37 | 17.5 | |
| ‘Luminal B-like (HER2 positive)’ | 23 | 10.8 | 19 | 9.0 | |
| ‘HER2 positive’ | 33 | 15.6 | 22 | 10.4 | |
| ‘Triple negative’ | 67 | 31.6 | 46 | 21.7 | 0.003 |
| Chemotherapy | |||||
| Yes | 173 | 81.6 | 180 | 84.9 | |
| No | 39 | 18.4 | 32 | 15.1 | 0.363 |
| Radiotherapy | |||||
| Yes | 52 | 24.5 | 55 | 25.9 | |
| No | 160 | 75.5 | 157 | 74.1 | 0.737 |
| Endocrine therapy | |||||
| No. of Luminal subtype | 112 | 144 | |||
| Yes | 88 | 78.6 | 126 | 87.5 | |
| No | 24 | 11.3 | 18 | 12.5 | 0.063 |
Abbreviation: HER2, human epidermal growth factor receptor 2.
ER, PR, HER2, Ki67, p53 and VEGF detection
Expression rates of ER (50.5%) and PR (45.3%) in the ‘poor group’ were lower than that in the ‘good group’ (χ2 = 8.107, P < 0.05 and χ2 = 8.509, P < 0.05, respectively). Positive rates of Ki67, p53 and VEGF in the ‘poor group’ were 67.5%, 37.3% and 35.8%, respectively, while in the ‘good group’ those were 49.1%, 24.1% and 17.5%, respectively, with significant difference (P < 0.05). However, expression of HER2 did not demonstrate significant difference (P > 0.05) (Figure 1).
Figure 1.
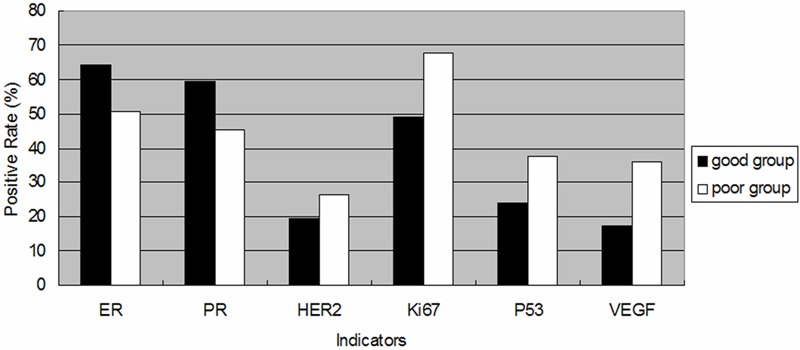
Histograms of six parameters in two groups based on different prognosis. Black block: positive rate of ER, PR, HER2, Ki67, p53 and VEGF in patients with ‘good group’; white block: positive rate of n ER, PR, HER2, Ki67, p53 and VEGF in patients with ‘poor group’. There is statistically significant difference in five indicators expression within the groups (P < 0.05).
Correlations among six candidates and clinicopathologic indicators
Expression of ER or PR was associated with three following indicators as age, tumor size, and tumor grade significantly (P < 0.05). In addition, expression of HER2, Ki67, p53 or VEGF was associated closely with one or more of these indicators, respectively (P < 0.05). The details were shown in Table 2.
Table 2.
Correlation of six parameters with clinicopathologic factors in two groups
| Factors | ER | PR | HER2 | Ki67 | p53 | VEGF |
|---|---|---|---|---|---|---|
| Age | ** | * | - | * | - | - |
| Tumour size | * | * | - | - | - | * |
| Tumour grade | * | * | * | * | * | ** |
| Peritumoural vascular invasion | - | - | * | * | * | ** |
| Molecular subtype | n/a | n/a | n/a | ** | - | * |
P < 0.001;
P < 0.05;
-, no correlation; n/a, not applicable.
Multiple factor analysis by logistic multiple regression analysis
Univariate analysis showed that protein expression of five indicators was associated with different prognosis respectively (P < 0.05). Besides, clinicopathologic indicators such as tumor grade, peritumoural vascular invasion and molecular subtypes were correlated with recurrence or death (P < 0.05). In order to study further their mutual interaction, and to select more effective potential predicting markers, these indicators were analyzed with a multivariate logistic regression model. In analytical process, the interconnected factors were avoided to be calculated simultaneously (such as ER, PR and Ki67). Results of the multivariate analysis revealed that ‘HER2 positive’ and ‘triple negative’ subtypes were demonstrated to be independent risk factors for relapse. The probability of recurrent risk for ‘HER2 positive’ vs. ‘luminal A-like’ was 1.956 (95% CI: 1.004-3.846, P = 0.049) and for ‘triple negative’ vs. ‘luminal A-like’ was 2.304 (95% CI: 1.342-3.955, P = 0.002). Besides, patients with vascular invasion (P = 0.023), poor differentiation (intermediate vs. low, P = 0.002; high vs. low, P = 0.001), and high expression of VEGF (P = 0.040) were useful markers predicting prognosis (Table 3).
Table 3.
Logistic multiple regression analysis comparing two groups
| Factors | β | SE | Sig (P) | Expβ | 95% CI | |
|---|---|---|---|---|---|---|
|
| ||||||
| Lower | Lower | |||||
| Peritumoural vascular invasion | ||||||
| Invasion vs no invasion | 0.865 | 0.381 | 0.023 | 2.376 | 1.125 | 5.015 |
| Tumor grade | ||||||
| Intermediate vs low | 1.507 | 0.486 | 0.002 | 4.513 | 1.742 | 11.691 |
| High vs Low | 1.737 | 0.542 | 0.001 | 5.682 | 1.964 | 16.434 |
| P53 | ||||||
| P53+ vs. p53- | 0.225 | 0.258 | 0.383 | 1.252 | 0.756 | 2.074 |
| VEGF | ||||||
| VEGF+ vs VEGF- | 0.572 | 0.279 | 0.040 | 1.772 | 1.026 | 3.060 |
| Molecular subtype | ||||||
| ‘Luminal B-like (HER2 negative)’ vs. ‘luminal A-like’ | 0.444 | 0.305 | 0.145 | 1.559 | 0.858 | 2.835 |
| ‘Luminal B-like (HER2 positive)’ vs. ‘luminal A-like’ | 0.555 | 0.371 | 0.134 | 1.742 | 0.842 | 3.605 |
| ‘HER2 positive’ vs. ‘luminal A-like’ | 0.675 | 0.343 | 0.049 | 1.965 | 1.004 | 3.846 |
| ‘Triple negative’ vs. ‘luminal A-like’ | 0.835 | 0.276 | 0.002 | 2.304 | 1.342 | 3.955 |
Abbreviations: β, regression coefficient; SE, standard error; Sig (P), probability value; 95% CI, confidence interval.
Effect of breast cancer subtype on recurrence
To explore the difference in rates of relapse and death caused by molecular subtype, an analysis, presented in Table 4, was undertaken among patients in ‘poor group’. High rates of distant metastases were demonstrated among ‘HER2 positive’ (75.8%), ‘triple negative’ (71.6%), and ‘luminal B-like (HER2 positive)’ (65.2%) subtypes, whereas were less frequently seen in ‘luminal A-like’ (49.0%) and ‘luminal B-like (HER2 negative)’ (55.3%, P =0.044) subtypes. In addition, death rates were higher in ‘triple negative’ (62.7%) and ‘HER2 positive’ (57.6%), in contrast with ‘luminal A-like’ (31.4%) and ‘luminal B-like (HER2 positive)’ (43.5%, P = 0.005) subtypes. There was no significant difference among subtypes in rates of contralateral breast cancer, local and regional-nodal recurrence (P > 0.05).
Table 4.
Frequency of recurrence and death among patients with poor prognosis
| Molecular subtypes | Patients NO. | CLBC | Loco-relapse | Regional nodal | Distant metastases | DFBC | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| NO. | % | NO. | % | NO. | % | NO. | % | NO. | % | ||
| ‘Luminal A-like’ | 51 | 11 | 21.6 | 10 | 19.6 | 7 | 13.7 | 25 | 49.0 | 16 | 31.4 |
| ‘Luminal B-like (HER2 negative)’ | 38 | 6 | 15.8 | 6 | 15.8 | 5 | 13.2 | 21 | 55.3 | 21 | 55.3 |
| ‘Luminal B-like (HER2 positive)’ | 23 | 5 | 21.7 | 6 | 26.1 | 6 | 26.1 | 15 | 65.2 | 10 | 43.5 |
| ‘HER2 positive’ | 33 | 4 | 12.1 | 7 | 21.2 | 6 | 18.2 | 25 | 75.8 | 19 | 57.6 |
| ‘Triple negative’ | 67 | 11 | 16.4 | 10 | 14.9 | 7 | 10.4 | 48 | 71.6 | 42 | 62.7 |
| Total | 212 | P value | P value | P value | P value | P value | |||||
| 0.797 | 0.765 | 0.435 | 0.044 | 0.005 | |||||||
Abbreviations: HER2, human epidermal growth factor receptor 2, CLBC, contralateral breast cancer; DFBC, death from breast cancer. Note: Recurrence may occur in one or more of the sites listed. Individual patients may be counted more than once.
Recurrent patterns of special types of invasive breast cancer
Among the cases in ‘poor group’, seventeen patients were diagnosed as special types of invasive breast cancer including invasive micropapillary carcinoma (IMPC) (Figure 2A), invasive apocrine carcinoma (IAC) (Figure 2B), invasive papillary carcinoma (IPC) (Figure 2C), invasive cribriform carcinoma (ICC) (Figure 2D), glycogen-rich clear cell carcinoma (GRCC) (Figure 2E), mucoid carcinoma (MC) (Figure 2F), MBC (metaplastic breast cancer), adenoid cystic carcinoma (ACC) and carcinosarcoma. Among patients who developed distant metastasis and death, one was diagnosed as IMPC+IDC, one was IAC, one was IPC+IDC, and one was MBC. No metastasis was found in patients who were diagnosed as pure IPC, ICC or ACC (Table 5).
Figure 2.
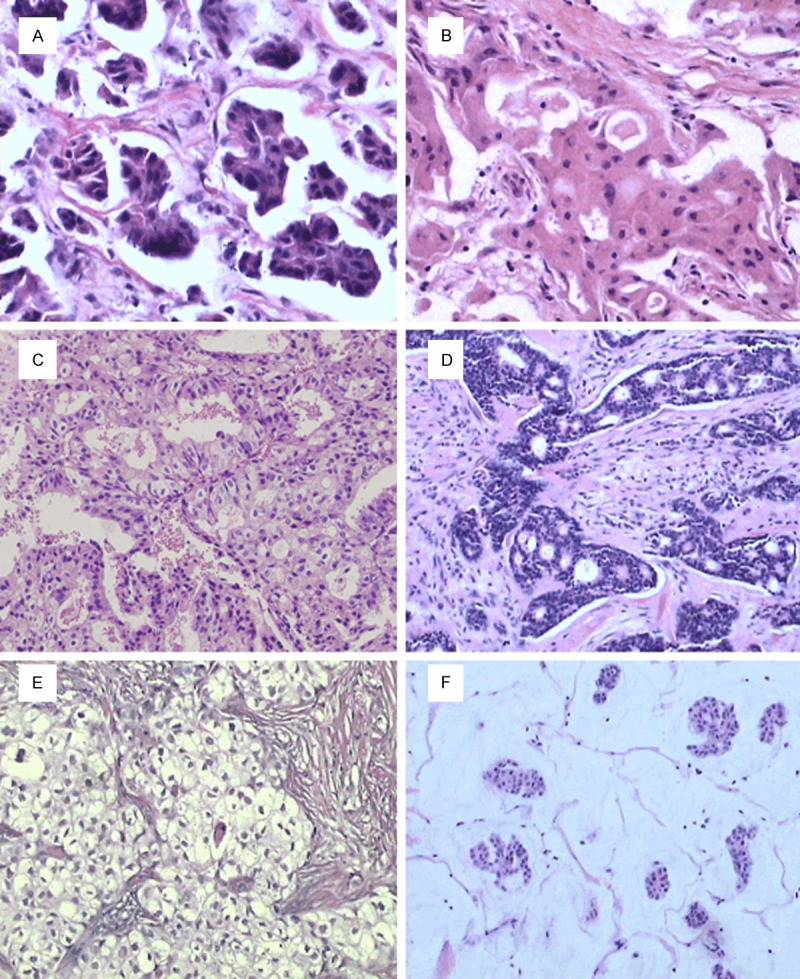
A: Invasive micropapillary carcinoma of the breast. HE staining; magnification ×400. B: Apocrine carcinoma of the breast. HE staining; magnification ×400. C: Invasive papillary carcinoma of the breast. HE staining; magnification ×200. D: Invasive cribriform carcinoma of the breast. HE staining; magnification ×200. E: Glycogen-rich clear cell carcinoma of the breast. HE staining; magnification ×200. F: Mucoid carcinoma of the breast. HE staining; magnification ×100.
Table 5.
Outcomes of special types of invasive breast cancer who developed recurrence
| Patient no. | Type of infiltrative carcinoma | Adjuvant chemotherapy | First site(s) of recurrence | Time to recurrence (months) | Following site(s) of recurrence | Time to DFBC (months) |
|---|---|---|---|---|---|---|
| 1 | IMPC | CMF | CLBC | 14 | No | No |
| 2 | IMPC+IDC | CMF | Regional-nodal | 33 | Lung | 74 |
| 3 | IAC | CAF | Regional-nodal | 42 | No | No |
| 4 | IAC | CMF | Bone | 46 | Liver, regional-nodal | 68 |
| 5 | IPC | 0 | CLBC+regional-nodal | 30 | No | No |
| 6 | IPC | CMF | CLBC | 22 | No | No |
| 7 | IPC | CMF | Regional-nodal | 46 | No | No |
| 8 | IPC | 0 | Loco-relapse | 30 | No | No |
| 9 | IPC+IDC | CMF | Lung | 24 | Liver, bone | 50 |
| 10 | ICC | 0 | CLBC | 42 | NO | No |
| 11 | GRCC | CMF | Unknown | Unknown | Unknown | 80 |
| 12 | MC | CMF | Bone | 50 | No | No |
| 13 | MC+IDC | CMF | Lung | 54 | No | No |
| 14 | MC | CMF | Loco-relapse | 45 | No | No |
| 15 | MBC | CAF | Lung | 34 | Brain | 40 |
| 16 | ACC | CMF | Loco-relapse+regional-nodal | 46 | No | No |
| 17 | carcinosarcoma | CAF | Lung | 35 | No | No |
Abbreviations: IMPC, Invasive micropapillary carcinoma; IDC, invasive ductal carcinoma; IAC, Invasive apocrine carcinoma; IPC, Invasive papillary carcinoma; ICC, Invasive cribriform carcinoma; GRCC, Glycogen-rich clear cell carcinoma; MC, Mucoid carcinoma; MBC, Metaplastic breast cancer; ACC, adenoid cystic carcinoma; CMF, cyclophosphamide, methotrexate and fluorouracil; CAF, cyclophosphamide, doxorubicin and fluorouracil; CLBC, contralateral breast cancer; DFBC, death from breast cancer.
Discussion
Up to now, there was relatively few study performed to pick out patients with poor prognosis from a large cohort of ANN breast cancer patients to do a case-control study. In this study, we analyzed the distinctions, including clinicopathologic characteristics and molecular subtypes, between two groups of patients with ANN breast cancer of different prognosis in China, investigating the clinical benefits provided by these candidate factors, choosing a simple and validated method of predicting the risk of recurrence. An IHC profile, based on the degree of expression of ER, PR, HER2, and Ki67 was used to identify subgroups of breast cancer patients with different outcomes [22,23]. P53 and VEGF were also evaluated to validate the ability to distinguish prognosis.
In our study, lymphovascular invasion were considered to elevate risk, which was similar with Abi-Raad’s report, which showed that lymphovascular invasion was associated with increased risk of loco-regional recurrences on multivariate analysis (P = 0.002) [24]. Moreover, tumor grade of the ‘good group’ mostly appeared low while the ‘poor group’ showed intermediate and high grade. Likewise, in an analysis [25], factor as low-grade was significantly associated with lower recurrence free survival (HR 1.74, 95% CI 1.14-2.66, P = 0.01).
Our study also found that the prognosis was optimistic with ‘luminal A-like’ subtype. In this study, ‘luminal A-like’ tumors occupied the highest percentage (41.5%) among ‘good’ outcome patients. On the contrary, this subtype had fallen by half for ‘poor’ patients (24.1%). We confirm here that the ‘luminal A-like’ phenotype is an excellent marker of prognosis. Similarly, subtypes of ‘luminal B-like (HER2 positive)’, ‘HER2 positive’ and ‘triple negative’ were more common in patients who experienced relapse or death. These results were consistent with that of Hage et al’s study [26]. Similarly, Theriault et al evaluated the risk of recurrence in 1012 small size ANN breast cancer patients by different tumor subtypes. He reported that patients with ‘HER2 positive’ breast cancer had 4.98 (95% CI, 2.91-8.53) times risk of relapse compared to patients with HR-positive diseases [5]. Wong et al’s research on 541 patients found that the combination of HR and HER2 status could be used as surrogates for gene defined molecular subtypes in Asian breast cancer patients with ANN diseases to predict prognosis [27]. Likewise, the study on 136 ‘triple negative’ breast cancer and 529 non-‘triple negative’ ANN breast cancer patients showed that, the relapse rates were 14.7% for the former group and 6.6% for the latter (P = 0.004), and 4-year recurrent free survival was significantly shorter among patients with ‘triple negative’ subtype compared with those with non-‘triple negative’ diseases (85.5% vs. 94.2%, respectively; P = 0.001) [28]. It could be concluded that the ‘HER2 positive’ and ‘triple negative’ subtypes had more aggressive clinical characteristics in ANN breast cancer patients.
Other markers which were found to have the ability of prediction of prognosis were Ki67, p53and VEGF, which were known to behave aggressively [29]. In the univariate analysis in our study, significant expression of Ki67, p53 and VEGF were observed correlated with poor survival. Ki67 expression is the most common criterion of proliferative index. Studies have shown a strong, statistically significant correlation between Ki67 and clinical outcomes in breast cancer [30,31]. In E. Munzone’s study, 5-year cumulative incidence of breast cancer related death were 2.3% and 9.0% in Ki67 low (≤ 35%) group and Ki67 high (> 35%) group, respectively, with an adjusted HR of 2.5 (95% CI, 1.0-6.0, P = 0.046) [32]. Studies have confirmed that p53 mutations were associated with worse overall and disease-free survival in breast cancer cases. In a comprehensive meta-analysis of 16 studies including over 3,500 patients, the relative hazard of dying of breast cancer for unselected patients with a p53 mutation in their tumor was 2.0 (95% CI, 1.7-2.5) [33]. VEGF and angiogenesis are important to tumor growth and metastasis across a range of solid tumor types [34]. VEGF has been implicated as a key mediator of angiogenesis in breast cancer [35]. In Linderholm et al’s study of 219 patients with ANN breast cancer patients, significantly shorter distant disease free survival was observed in patients with high VEGF (P = 0.006) [36].
In the multivariate analysis in our study, VEGF, ‘HER2 positive’ and ‘triple negative’ subtypes were high-risk factors in the ‘poor group’, as well as clinical indicators including peritumoural vascular invasion and grade. Their combined use will improve screening and treatment for high-risk cases of ANN breast cancer. It should be pointed out that, molecular subtype of breast cancer is classified on the basis of ER, PR, HER2 and Ki67, so these indicators were not included in the multivariate analysis to reduce adverse interference. P53 did not present statistical significance in the multivariate analysis, may be due to some factors covering and concealing the function of others as a result of a complex relationship.
In addition, this study demonstrated that breast cancer subtypes were associated with unique patterns of relapse. Patients with ‘triple negative’ subtype were likely to suffer from distant metastasis, while ‘HER2 positive’ subtype had significantly more distant metastasis and local recurrence rates. The results was consisted with Lin’s study that visceral relapse was more frequently to occur in ‘triple negative’ and ‘HER2 positive’ patients in Chinese breast cancer patients [37]. According to Rodriguez-Pinilla’s reports, a higher percentage of local recurrence and visceral metastasis arose in ‘triple negative’ tumors with ANN breast cancer, which was somewhat partial similarity with our analysis [38].
In the last part of our study, special types of invasive breast cancer which developed recurrence were analyzed with regard to the adjuvant chemotherapy type, recurrence time and recurrence sites. IMPC has been described as a poorly recognized aggressive. Pettinato et al. reported that 49% of patients with IMPC died of distant metastasis at a mean of 5.2 years (range, 1-10.5 years) after initial diagnosis [39]. In our study, there were two patients with IMPC experiencing recurrence and one of them died from the disease, demonstrating a poor prognosis. One patient presenting with mixture of IPC and IDC developed distant metastasis and died, while the other 4 patients with pure IPC did not. This probably potentially indicated that the mortality of pure IPC was exceptionally low, might due to less biologically aggressive than other forms of cancer [40]. Even though MC was less likely to spread to lymph nodes than other types of breast cancer, and tended to be a less aggressive type, there were 2 patients with MC experiencing distant metastasis in our study. Luckily both of them were still alive probably because MC progressed less rapidly than other types. MBC was a highly heterogeneous group of tumors that were characterized by an admixture of adenocarcinoma and dominant areas of spindle cells, with squamous and/or mesenchymal differentiation. MBC could easily spread to lymph nodes and other areas of the body, especially the lungs. In our study, the MBC patient’s first metastasis site was lung, subsequently developing to brain, and then died in a few months. The result might indicate that MBC was aggressive carcinoma predicting poor prognosis.
A limitation of this part is that conventional imaging would not necessarily detect all metastatic disease, with subclinical metastases being missed. In addition, local recurrence may be ignored when serious distant metastasis happened. However, this bias would be present for all breast cancer subtypes and would not affect the results we reported.
In conclusion, this study highlights the need for better and simple predictive tools in treating ANN breast cancer patients who are at high risk of relapse. Utilizing various simple independent indicators, including vascular invasion, grade, expression of VEGF, and molecular subtype, the risk of recurrence could be predicted to guide individualized therapeutic regimen. In addition, defining the pattern of breast cancer recurrence can provide useful information for guiding the development of surveillance recommendations and for designing future therapeutic approaches.
Acknowledgements
This work was financially supported by National Science Foundation of China (81172532); Program for Changjiang Scholars and Innovative Research Team in University (TRT0743). The authors gratefully acknowledge Mrs Xiumin Ding and Ying Wang for technology assistance.
Disclosure of conflict of interest
None.
References
- 1.Harbeck N, Thomssen C. A new look at node-negative breast cancer. Oncologist. 2011;16(Suppl 1):51–60. doi: 10.1634/theoncologist.2011-S1-51. [DOI] [PubMed] [Google Scholar]
- 2.Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, Gelber RD, Castiglione-Gertsch M, Coates AS, Goldhirsch A, Cardoso F. Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J. Clin. Oncol. 2013;31:3083–3090. doi: 10.1200/JCO.2012.46.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher CS, Cole DJ, Mitas M, Garrett-Meyer E, Metcalf JS, Gillanders WE, Mikhitarian K, Urist MM, Mann GB, Doherty G, Herrmann VM, Hill AD, Eremin O, El-Sheemy M, Orr RK, Valle AA, Henderson MA, Dewitty RL, Sugg SL, Frykberg E, Yeh K, Bell RM, Baker MK. Molecular detection of micrometastatic breast cancer in histopathology-negative axillary lymph nodes fails to predict breast cancer recurrence: a final analysis of a prospective multi-institutional cohort study. Ann Surg Oncol. 2010;17(Suppl 3):312–320. doi: 10.1245/s10434-010-1258-y. [DOI] [PubMed] [Google Scholar]
- 4.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 5.Theriault RL, Litton JK, Mittendorf EA, Chen H, Meric-Bernstam F, Chavez-Macgregor M, Morrow PK, Woodward WA, Sahin A, Hortobagyi GN, Gonzalez-Angulo AM. Age and survival estimates in patients who have node-negative T1ab breast cancer by breast cancer subtype. Clin Breast Cancer. 2011;11:325–331. doi: 10.1016/j.clbc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, Montagna E, Dellapasqua S, Iorfida M, Cardillo A, Veronesi P, Luini A, Intra M, Gentilini O, Scarano E, Goldhirsch A, Colleoni M. Prognosis in women with small (T1mic,T1a,T1b) node-negative operable breast cancer by immunohistochemically selected subtypes. Breast Cancer Res Treat. 2011;127:713–720. doi: 10.1007/s10549-011-1465-7. [DOI] [PubMed] [Google Scholar]
- 7.Bull SB, Ozcelik H, Pinnaduwage D, Blackstein ME, Sutherland DA, Pritchard KI, Tzontcheva AT, Sidlofsky S, Hanna WM, Qizilbash AH, Tweeddale ME, Fine S, McCready DR, Andrulis IL. The combination of p53 mutation and neu/erbB-2 amplification is associated with poor survival in node-negative breast cancer. J. Clin. Oncol. 2004;22:86–96. doi: 10.1200/JCO.2004.09.128. [DOI] [PubMed] [Google Scholar]
- 8.Sorlie T. Introducing molecular subtyping of breast cancer into the clinic? J. Clin. Oncol. 2009;27:1153–1154. doi: 10.1200/JCO.2008.20.6276. [DOI] [PubMed] [Google Scholar]
- 9.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 10.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9:714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 12.Olivier M, Langerod A, Carrieri P, Bergh J, Klaar S, Eyfjord J, Theillet C, Rodriguez C, Lidereau R, Bieche I, Varley J, Bignon Y, Uhrhammer N, Winqvist R, Jukkola-Vuorinen A, Niederacher D, Kato S, Ishioka C, Hainaut P, Borresen-Dale AL. The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin Cancer Res. 2006;12:1157–1167. doi: 10.1158/1078-0432.CCR-05-1029. [DOI] [PubMed] [Google Scholar]
- 13.Jung SY, Jeong J, Shin SH, Kwon Y, Kim EA, Ko KL, Shin KH, Ro J, Lee KS, Park IH, Lee S, Kim SW, Kang HS. Accumulation of p53 determined by immunohistochemistry as a prognostic marker in node negative breast cancer; analysis according to St Gallen consensus and intrinsic subtypes. J Surg Oncol. 2011;103:207–211. doi: 10.1002/jso.21819. [DOI] [PubMed] [Google Scholar]
- 14.Shivakumar S, Prabhakar BT, Jayashree K, Rajan MG, Salimath BP. Evaluation of serum vascular endothelial growth factor (VEGF) and microvessel density (MVD) as prognostic indicators in carcinoma breast. J Cancer Res Clin Oncol. 2009;135:627–636. doi: 10.1007/s00432-008-0497-9. [DOI] [PubMed] [Google Scholar]
- 15.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 16.Rosen PP, Oberman H. Tumors of the mammary gland. Washington, DC: Armed Forces Institute of Pathology; 1993. [Google Scholar]
- 17.Niu Y, Fu X, Lv A, Fan Y, Wang Y. Potential markers predicting distant metastasis in axillary node-negative breast carcinoma. Int J Cancer. 2002;98:754–760. doi: 10.1002/ijc.10136. [DOI] [PubMed] [Google Scholar]
- 18.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi T, Iwaya K, Moriya T, Yamasaki T, Tsuda H, Yamamoto J, Matsubara O. A simple immunohistochemical panel comprising 2 conventional markers, Ki67 and p53, is a powerful tool for predicting patient outcome in luminal-type breast cancer. BMC Clin Pathol. 2013;13:5. doi: 10.1186/1472-6890-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox SB, Generali D, Berruti A, Brizzi MP, Campo L, Bonardi S, Bersiga A, Allevi G, Milani M, Aguggini S, Mele T, Dogliotti L, Bottini A, Harris AL. The prolyl hydroxylase enzymes are positively associated with hypoxia-inducible factor-1alpha and vascular endothelial growth factor in human breast cancer and alter in response to primary systemic treatment with epirubicin and tamoxifen. Breast Cancer Res. 2011;13:R16. doi: 10.1186/bcr2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J. Clin. Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 23.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 24.Abi-Raad R, Boutrus R, Wang R, Niemierko A, Macdonald S, Smith B, Taghian AG. Patterns and risk factors of locoregional recurrence in T1-T2 node negative breast cancer patients treated with mastectomy: implications for postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81:e151–157. doi: 10.1016/j.ijrobp.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blancas I, Garcia-Puche JL, Bermejo B, Hanrahan EO, Monteagudo C, Martinez-Agullo A, Rouzier R, Hennessy BT, Valero V, Lluch A. Low number of examined lymph nodes in node-negative breast cancer patients is an adverse prognostic factor. Ann Oncol. 2006;17:1644–1649. doi: 10.1093/annonc/mdl169. [DOI] [PubMed] [Google Scholar]
- 26.van der Hage JA, Mieog JS, van de Velde CJ, Putter H, Bartelink H, van de Vijver MJ. Impact of established prognostic factors and molecular subtype in very young breast cancer patients: pooled analysis of four EORTC randomized controlled trials. Breast Cancer Res. 2011;13:R68. doi: 10.1186/bcr2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong FY, Chin FK, Lee KA, Soong YL, Chua ET. Hormone receptors and HER-2 status as surrogates for breast cancer molecular subtypes prognosticate for disease control in node negative Asian patients treated with breast conservation therapy. Ann Acad Med Singapore. 2011;40:90–96. [PubMed] [Google Scholar]
- 28.Rhee J, Han SW, Oh DY, Kim JH, Im SA, Han W, Park IA, Noh DY, Bang YJ, Kim TY. The clinicopathologic characteristics and prognostic significance of triple-negativity in node-negative breast cancer. BMC Cancer. 2008;8:307. doi: 10.1186/1471-2407-8-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taneja P, Maglic D, Kai F, Zhu S, Kendig RD, Fry EA, Inoue K. Classical and Novel Prognostic Markers for Breast Cancer and their Clinical Significance. Clin Med Insights Oncol. 2010;4:15–34. doi: 10.4137/cmo.s4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milde-Langosch K, Karn T, Muller V, Witzel I, Rody A, Schmidt M, Wirtz RM. Validity of the proliferation markers Ki67, TOP2A, and RacGAP1 in molecular subgroups of breast cancer. Breast Cancer Res Treat. 2012;137:57–67. doi: 10.1007/s10549-012-2296-x. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Yin Q, Yu Q, Zhang J, Liu Z, Wang S, Lv S, Niu Y. A retrospective study of breast cancer subtypes: the risk of relapse and the relations with treatments. Breast Cancer Res Treat. 2011;130:489–498. doi: 10.1007/s10549-011-1709-6. [DOI] [PubMed] [Google Scholar]
- 32.Munzone E, Botteri E, Sciandivasci A, Curigliano G, Nole F, Rotmensz N, Colleoni M, Viale G, Esposito A, Luini A, Mastropasqua MG, Goldhirsch A European Institute of Oncology. Prognostic significance of Ki-67 in node-negative (pN0), triple-negative (TN) breast cancer (BC) J. Clin. Oncol. 2011;29 abstr 1056. [Google Scholar]
- 33.Pharoah PD, Day NE, Caldas C. Somatic mutations in the p53 gene and prognosis in breast cancer: a meta-analysis. Br J Cancer. 1999;80:1968–1973. doi: 10.1038/sj.bjc.6690628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 35.Queiroga FL, Pires I, Parente M, Gregorio H, Lopes CS. COX-2 over-expression correlates with VEGF and tumour angiogenesis in canine mammary cancer. Vet J. 2011;189:77–82. doi: 10.1016/j.tvjl.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Linderholm BK, Gruvberger-Saal S, Ferno M, Bendahl PO, Malmstrom P. Vascular endothelial growth factor is a strong predictor of early distant recurrences in a prospective study of premenopausal women with lymph-node negative breast cancer. Breast. 2008;17:484–491. doi: 10.1016/j.breast.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Lin Y, Yin W, Yan T, Zhou L, Di G, Wu J, Shen Z, Shao Z, Lu J. Site-specific relapse pattern of the triple negative tumors in Chinese breast cancer patients. BMC Cancer. 2009;9:342. doi: 10.1186/1471-2407-9-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Pinilla SM, Sarrio D, Honrado E, Hardisson D, Calero F, Benitez J, Palacios J. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res. 2006;12:1533–1539. doi: 10.1158/1078-0432.CCR-05-2281. [DOI] [PubMed] [Google Scholar]
- 39.Pettinato G, Manivel CJ, Panico L, Sparano L, Petrella G. Invasive micropapillary carcinoma of the breast: clinicopathologic study of 62 cases of a poorly recognized variant with highly aggressive behavior. Am J Clin Pathol. 2004;121:857–866. doi: 10.1309/XTJ7-VHB4-9UD7-8X60. [DOI] [PubMed] [Google Scholar]
- 40.Liu ZY, Liu N, Wang YH, Yang CC, Zhang J, Lv SH, Niu Y. Clinicopathologic characteristics and molecular subtypes of invasive papillary carcinoma of the breast: a large case study. J Cancer Res Clin Oncol. 2013;139:77–84. doi: 10.1007/s00432-012-1302-3. [DOI] [PubMed] [Google Scholar]


